Abstract
Photoredox catalysis is a powerful means to generate odd-electron species under mild reaction conditions from a wide array of radical precursors. Herein, we present the application of this powerful catalytic manifold to address the hydroalkylation and hydroaminoalkylation of electronically diverse vinylarenes. This reaction allows for generalized alkene hydroalkylation leveraging common alkyl radical precursors, such as organotrifluoroborate salts and carboxylic acids. Furthermore, utilizing easily accessible α-silyl amine reagents or tertiary amines directly, secondary and tertiary amine moieties can be installed onto monoaryl and diaryl alkenes to access valuable products, including γ,γ-diarylamines pharmacophores. Thus, under a unified system, both hydroalkylation and hydroaminoalkylation of alkenes are achieved. The substrate scope is evaluated through 57 examples, the synthetic utility of the method is demonstrated, and preliminary mechanistic insights are presented.
Subject terms: Synthetic chemistry methodology, Photocatalysis
Many useful chemical scaffolds include carbon or nitrogen substitutions at two or three atoms away from benzene. Here, the authors show a unified hydroalkylation and hydroaminoalkylation protocol to access these structures via a regioselective photocatalytic addition to simple styrenes.
Introduction
Olefin functionalization is a routinely applied strategy in organic synthesis1. Feedstock alkenes such as vinylarenes are attractive templates for a variety of reaction manifolds, including polar and radical reactions, cycloadditions, and transition metal-catalyzed functionalizations2. In particular, the conversion of the π-bond into polar groups, such as alcohols3, ethers4, thioethers5,6, amines7,8, halogens9, or carbonyls10,11 has seen substantial development in recent decades. The identification of such desirable functional group targets in medicinal chemistry has been an important motivating force in the development of these methods12.
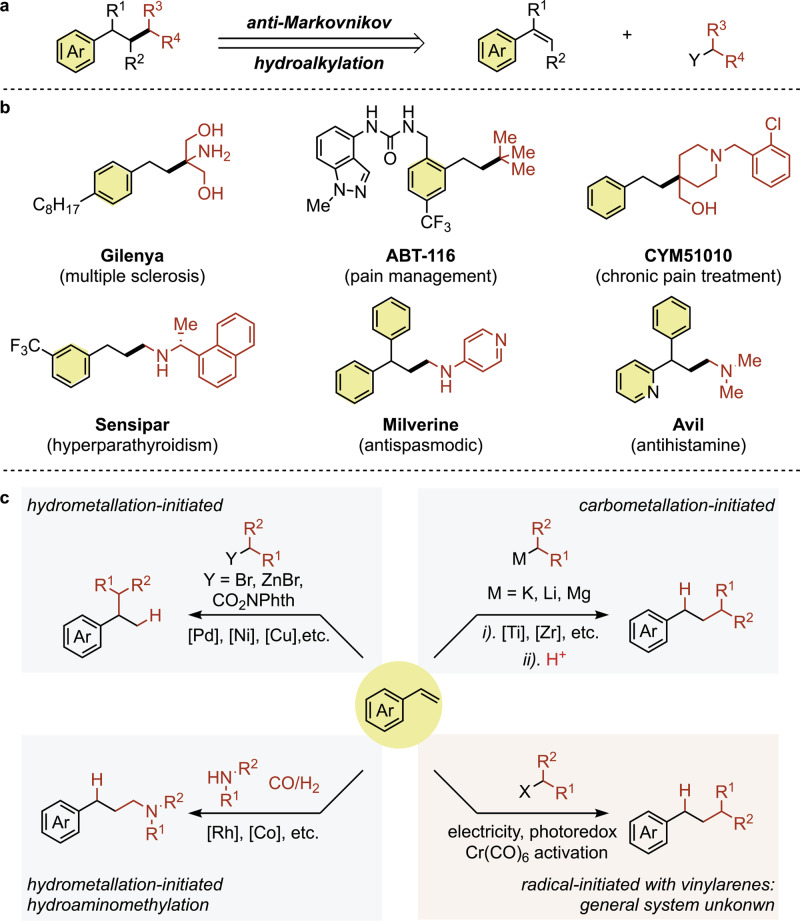
In evaluating the occurrence of functionalities found broadly in organic chemistry, alkylated arene fragments emerge as prominent features in many important classes of molecules, particularly in pharmaceutical agents13. Hydro(amino)alkylation reactions offer a complementarity for the synthesis of these important moieties to traditional alkyl–alkyl cross-couplings catalyzed by transition metals for the synthesis of these important moieties (Fig. 1)14,15. First, the requirement for water-sensitive organometallic coupling partners, such as Grignard reagents, organolithiums, zincates, or cuprates can be avoided, expanding overall practicality16,17. In addition, kinetic challenges that commonly plague transition metal catalysis, such as sluggish activation of alkyl halides or competitive β-hydride elimination, can be entirely circumvented18. Given this complementarity, the development and refinement of general and catalytic platforms for alkene hydroalkylations is of significant interest.
Fig. 1. Significance and syntheses of (amino)alkylated arenes.
a Accessing valuable (amino)alkylated arenes via anti-Markovnikov hydro(amino)alkylation of simple styrene derivatives. b Biologically active molecules containing (γ-amino) alkylated arenes. c Literature reports for hydroalkylation of styrenes: hydrometallation-initiated Markovnikov hydroalkylation; carbometallation-initiated anti-Markovnikov hydroalkylation; hydrometallation-initiated anti-Markovnikov hydroaminomethylation using syngas; and radical-initiated anti-Markovnikov hydroalkylation.
Common to any olefin functionalization process, the regioselectivity of the addition is a critical parameter. The majority of transition metal-catalyzed hydroalkylation reactions, such as those promoted by Pd19, Ni20–22, and Cu23, initiate with a hydrometallation step, which engenders exclusive Markovnikov selectivity with vinylarenes given the stability of the π-benzyl intermediate (Fig. 1c, top left). Correspondingly, anti-Markovnikov hydroalkylation of styrenes remains underdeveloped. Carbometallation-initiated strategies have emerged to address this regiochemical challenge24,25. However, the majority of this methodology is applicable only to electronically activated Michael acceptors. When applied to vinylarenes, reactive organometallic (Mg26, Li27,28, K29) reagents are required, and chemical yields vary significantly with the electron properties of the π-system (Fig. 1c, top right). Anti-Markovnikov hydroaminoalkylations of vinylarenes have been met with greater success, albeit requiring privileged ligand scaffolds and high pressures of syngas to promote reactivity and regioselectivity (Fig. 1c, bottom left)30–35. Alternatively, these aliphatic amines can be accessed through intermolecular anti-Markovnikov hydroamination, a complementary disconnection, which has been a great success recently using photoredox catalysis36,37.
Anti-Markovnikov-selective hydro(amino)alkylations of electron-deficient alkenes initiating with the addition of a carbon-centered radical to the π-system have been well-developed. However, given the nucleophilic character of (amino)alkyl radicals, such reactions have largely been restricted to highly polarized alkenes, such as Michael acceptors38–44. To date, few select examples have emerged that demonstrate reactivity with vinylarenes. The scope is entirely restricted to highly nucleophilic radicals45,46 or electrophilic styrenes47–50, strongly reducing conditions51, or by stoichiometric activation of the arene by metal carbonyl complexes (Fig. 1c, bottom right)52. At present, a system that exhibits a generality of scope under practical conditions remains unknown.
Herein, we report a unified catalytic system for anti-Markovnikov hydroalkylation and hydroaminoakylation of various vinylarenes through photoredox catalysis. We demonstrate that the modular access to high-value alkylated arene fragments is readily accessed via the intermolecular hydroalkylation or hydroaminoalkylation of feedstock vinylarenes (Fig. 1a). This strategy enables the direct synthesis of valuable amine pharmacophores well-represented in top-selling pharmaceuticals like Sensipar, Milverine, or Avil and their derivatives53.
Results
Reaction discovery and optimization
To address this important synthetic gap in the otherwise well-developed suite of hydroalkylation reactions, we sought to harness the regioselectivity of a single-electron mechanism. Visible light photocatalysis has been established as a means for the facile production of the requisite open-shell alkyl species from a diverse array of precursors54. Oxidizable substrates, such as organoborons, organosilicons, or carboxylic acids are a particularly attractive suite of reagents; these inexpensive and shelf-stable substrates undergo facile activation via reductive quenching of common photoredox catalysts. In accordance with our proposed mechanistic outline, the addition of the generated radical to the vinylarene generates a benzylic radical (Fig. 2). Processing of this intermediate by stepwise or concerted electron and proton transfer would deliver the hydroalkylated product. However, such intermediates tend to have large negative reduction potentials (E = −1.60 V vs. saturated calomel electrode (SCE))55; identification of a hydrogen atom transfer reagent, therefore, represents a key reaction parameter.
Fig. 2. Mechanistic working hypothesis.
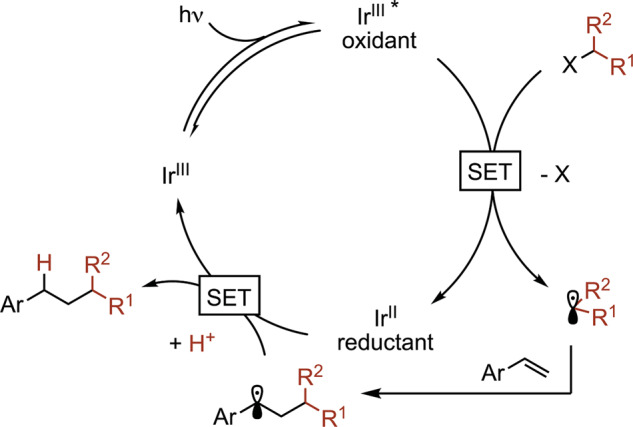
A proposed catalytic cycle for a general hydroalkylation of vinylarenes.
Considering these design principles, we initiated our study of the hydroalkylation reaction of 1,1-diphenylethylene with benzyl potassium trifluoroborate to form 1. Surveying common H-sources, alcohols were found to perform poorly in this hydroalkylation reaction (Table 1, Entries 1–2). Thiophenol, a common H-atom transfer reagent, produces the thiol-ene adduct exclusively (Table 1, Entry 4). Ultimately, phenol derivatives were found to be uniquely effective (Table 1, Entries 5–13). This could be a consequence of the lower pKa or the lower O–H bond dissociation energy, depending on the mode of H-transfer (vide infra). Alternatively, it could be related to the ability of the phenoxide anion to capture liberated BF3, which is known to inhibit similar transformations56,57. An excess of the phenol reagent is found to be beneficial (Table 1, Entry 6).
Table 1.
Select optimization results for the hydroalkylation reactiona.
| Entry | H-source | Equiv. H-source | t (h) | In situ yield 1 (%) |
|---|---|---|---|---|
| 1 | IPA | 3 | 24 | 16 |
| 2 | MeOH | 3 | 24 | 11 |
| 3 | AcOH | 3 | 24 | 15 |
| 4 | PhSH | 1 | 24 | 0 |
| 5 | PhOH | 1 | 24 | 16 |
| 6 | PhOH | 3 | 24 | 23 |
| 7 | PhOH | 3 | 36 | 42 |
| 8 | 2-MeO-PhOH | 3 | 36 | 84 |
| 9 | 2-MeO-PhOH | 3 | 48 | >99 |
| 10 | 4-CF3-PhOH | 3 | 48 | 51 |
| 11 | 4-Cl-PhOH | 3 | 48 | 70 |
| 12 | 4-Me-PhOH | 3 | 48 | >99 |
| 13 | 4-MeO-PhOH | 3 | 48 | >99 |
aConditions: 1,1-diphenylethylene (0.1 mmol, 1.0 equiv), BnBF3K (1.5 equiv), [Ir(dF(CF3)ppy)2(dtbbpy)][PF6] (1 mol%), H-source, DMF, r.t., 24 W blue LED light; GC yield reported with reference to 1-methylnaphthalene as an internal standard.
Additional tuning of the phenol reagent and reaction time led to the optimized conditions (Table 1, Entry 9): 1.0 equivalent of the alkene, 1.5 equivalents of the alkyl-BF3K salt undergo the coupling in 48 h with 3.0 equivalents of 2-methoxyphenol and 1 mol% of [Ir(dF(CF3)ppy)2(dtbbpy)][PF6] in dimethylformamide (DMF) (0.10 M) with irradiation by 24 W blue light-emitting diodes (LEDs). Notably, other phenol derivatives were found to provide good to excellent yields of 1 (Table 1, Entries 10–13); however, 2-methoxyphenol (guaiacol) is a naturally occurring and inexpensive feedstock.
Substrate scope
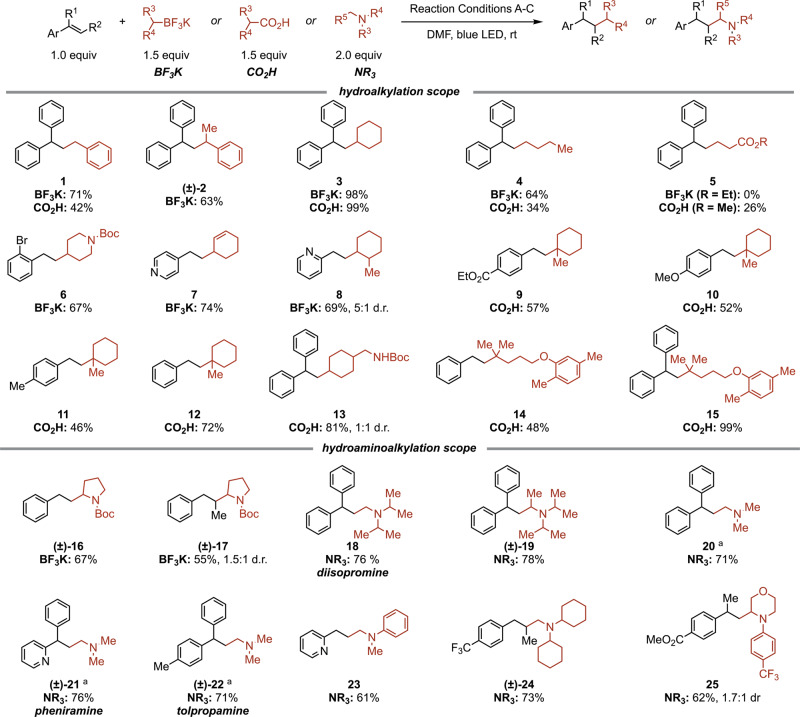
With optimized conditions in hand, the scope of hydroalkylation reaction was investigated (Fig. 3). Both 1° and 2° alkyl trifluoroborate salts (1–8) are reactive to afford the desired products in good yields. The efficiency of n-butyl-BF3K (4) is noteworthy due to the significantly higher oxidation potential of this substrate (+1.81 V vs. SCE for phenethyl-BF3K vs. +1.05 V vs. SCE for Bn-BF3K)48. With respect to the trifluoroborate carbon components, the reaction efficiency was found to be sensitive to the structure of the phenol derivative. For instance, 3 can be obtained in good yield with 2-methoxyphenol (79%); however, phenol itself was found to be more effective, providing 98% yield. Nitrogen-containing alkyl fragments can be incorporated into the products utilizing 4-methoxyphenol, such as the 1-boc-piperidinyl moiety (6). Formal hydroaminoalkylation can also be achieved α-amino-BF3K salts (16–17). In addition to benzylic moieties (1–2), allylic fragments can also be installed (7). The synthesis of 7 was particularly sensitive to the phenol equivalencies, with lower loadings proving more effective (see Supplementary information for details). This could be a consequence of a slower radical addition by the stabilized allylic species. Importantly, the alkene remains intact, leaving this valuable functionality available for further elaboration. Steric hindrance in the incorporated alkyl fragment is tolerated, as demonstrated by 8, which is furnished in 74% yield with 5:1 diastereomeric ratio (d.r.).
Fig. 3. Scope of hydro(amino)alkylation.
Conditions A: trifluoroborate salt (1.5 equiv), PC (1.0 mol%), 2-methoxylphenol (3.0 equiv), DMF (0.1 M), r.t., 48 h; Conditions B: carboxylic acid (1.5 equiv), PC (1.0 mol%), K3PO4 (1.5 equiv), DMF (0.2 M), r.t., 24 h; Conditions C: tertiary amine (2.0 equiv), PC (0.5 mol%), DMF (0.2 M), r.t., 24 h. a Me3N⋅HCl (3.0 equiv) and DBU (3.0 equiv) were used. See Supplementary Methods for experimental details. d.r. diastereomeric ratio, PC [Ir(dF(CF3)ppy)2(dtbbpy)][PF6].
Alkyl potassium trifluoroborate salts are versatile substrates for this reaction. However, we sought to expand substrate generality to include other common radical precursors. Alkyl carboxylic acids are an abundant feedstock and the ability to engage these directly would greatly enhance the practicality and utility of the transformation. With these substrates, external H-sources are not required, and the conditions could be easily adjusted. An evaluation of carboxylic acid substrates reveals that secondary and tertiary carboxylic acids are more reactive under optimized conditions compared to primary ones (e.g., 3 vs. 4), likely due to the lower oxidation potential of the substrate and the more stabilized alkyl radical intermediate that is formed. Tertiary acids can be used to construct fully substituted carbon centers, which can be challenging to assemble by other means (9–12, 14–15). Importantly, electron-rich monoaryl alkenes, which fail in related reactions, readily participate in this hydroalkylation protocol (10–11). The incorporation of the phenethyl group into a substrate is readily achieved with styrene and unactivated alkyl fragments (12). Finally, gemfibrozil and tranexamic acid both couple readily with mono- and diarylalkenes to afford 13-15, demonstrating the tolerance of aryl ethers and secondary carbamates in the hydroalkylation reaction.
To expand the purview of this hydroalkylation platform to generally access aminoalkylated products, we investigated the reactivity of tertiary amines. Such substrates are known to undergo a H-atom shift following SET oxidation to form the α-amino radical at the least hindered α position58. Following minor adjustments to the reaction conditions, tertiary aliphatic amines including diisopropylmethylamine (18), diisopropylethylamine (Hünig’s base, (±)-19), trimethylamine (20), dicyclohexylmethylamine (±)-24, phenyldimethylamine (23), and cyclic (±)-25 anilines all afford the hydroaminoalkylation products with very good yields. Biologically active diisopromine (18), pheniramine (±)-21, and tolpropamine (±)-22 were all synthesized using this method.
The ability to use tertiary amines directly significantly expands the hydroaminoalkylation scope. However, only tertiary amines are reactive under the optimized conditions, and an excess of the amine substrate (2 equiv) paired with long reaction times (≥24 h) are required to achieve high conversion. This is likely due to the relatively high oxidation potential of 3° alkyl amines (>+1.0 V vs. SCE), which is even higher for 2° alkyl amines (>+1.5 V vs. SCE)59. The highly oxidizing excited state of the catalyst, [Ir(dF(CF3)ppy)2(dtbbpy)+]*, also oxidizes the products in some cases. For example, when triethylamine was utilized under standard conditions, both the desired tertiary amine product and dealkylated secondary amine side products were obtained, complicating the purification process.
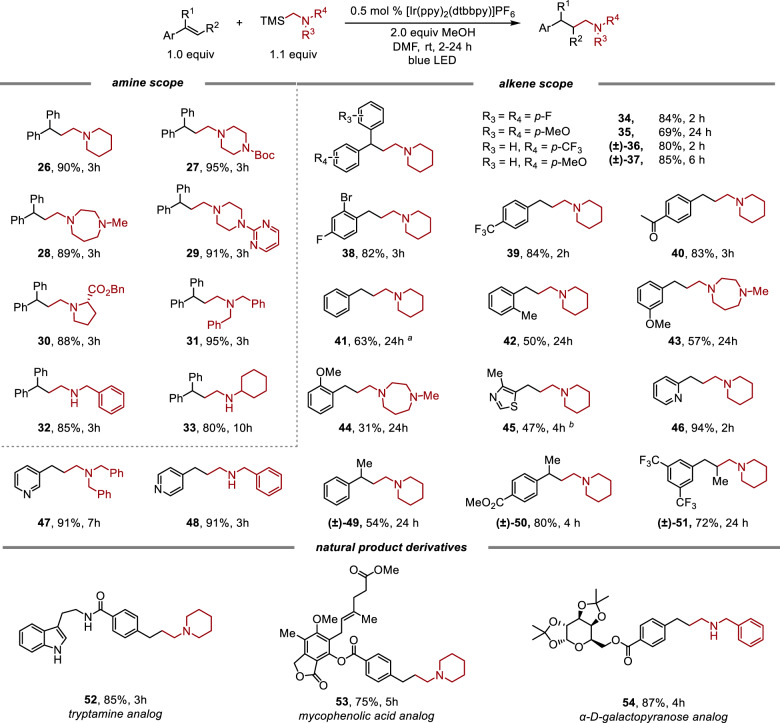
To overcome this constraint, we envisioned that the introduction of a silyl group alpha to the amine, which leads to a significant decrease in the oxidation potential (+0.71 V for α-TMS methylpiperidine, compared to +1.31 V for N-benzylpiperidine vs. SCE)60, would facilitate SET to the photoexcited catalyst thereby increasing the reaction rate. This redox auxiliary approach has been extensively studied in modern electroorganic synthesis61. Indeed, when applying MeOH as proton source, this SET process is further promoted (see Stern–Volmer quenching experiments in the Supplementary information). Moreover, a less oxidizing photocatalyst can be applied, eliminating the unproductive dealkylation pathway. Thus, a significantly broadened scope for hydroaminoalkylation can be achieved with α-trimethylsilyl (TMS) amines, including the introduction of a secondary amine moiety into the products.
As shown in Fig. 4, α-TMS amines derived from 2° cyclic and acyclic dialkyl amines, such as piperidine (26) or N,N-dibenzylamine (31) all afford the desired tertiary amine products in excellent yields. A variety of potentially sensitive functionalities were well-tolerated, including Boc-protected amine (27), basic tertiary amine (28), pyrimidine (29), and α-amino ester (30). Secondary α-TMS amines also proved to be effective under these conditions to give the 2° amine products in yields of 85% and 80% with the N–H bonds remaining intact (32, 33).
Fig. 4. Scope of hydroaminomethylation with α-TMS amine.
Standard conditions (Conditions D) are alkene (0.2 mmol, 1.0 equiv), amine (1.1 equiv), [Ir(ppy)2(dtbbpy)]PF6 (0.5 mol%), DMF (1.0 mL, 0.1 M), r.t., 24 W blue LED light; isolated yields, average of two runs. aThe 2.0 equiv amine used. b1.0 equiv amine and 2.0 equiv alkene used.
For the alkene scope, various electronically differentiated 1,1-diaryl alkenes were evaluated. Electron-rich derivatives, such as 4,4′-dimethoxylphenylethylene, require a longer reaction time (24 h) to reach completion (35) with diminished yield (69%); however, hydroaminomethylation of electron-poor 4,4′-difluorophenylethylene (34) only requires 2 h for full conversion with an 84% isolated yield. Various monoaryl alkene derivatives were also examined. Vinylarenes bearing electron-withdrawing groups such as fluoro and bromo (38), trifluoromethyl (39), and ketone (40), as well as electron-donating methyl (42) and methoxy (43, 44) groups, all afford the desired amine products with moderate to excellent yields (31–84%). Heterocycles, such as thiazole (45) and pyridine (ortho, meta, and para, 46–48) are well-tolerated under standard conditions for the synthesis of either secondary or tertiary amine products. Moreover, aryl, alkyl-disubstituted alkenes are amenable to the functionalization to access β- or γ-branched amine products (49–51) in good yields (54–72%). Finally, aryl alkenes derived from natural products, such as tryptamine (52), mycophenolic acid (53), and α-d-galactopyranose (54) are reactive under standard conditions to form the desired products with many functional groups remaining unaffected, including 2° amides, indoles, esters, lactones, internal alkenes, and acetals.
Synthetic application
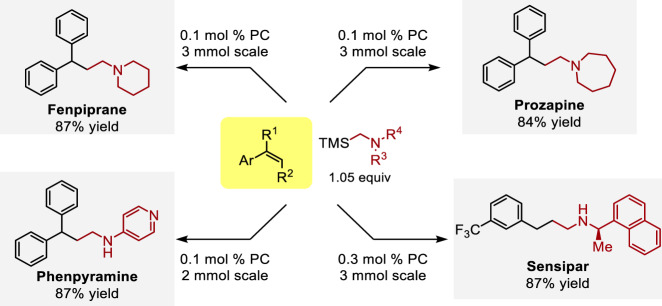
Having developed a general methodology that enables hydroalkylation and hydroaminoalkylation of diverse vinylarene substrates, we sought to demonstrate its application in the syntheses of biologically active γ-arylated amines as illustrated in Fig. 5. Starting from readily available α-TMS amines, synthesized via nucleophilic substitution of the corresponding chloromethyl silane, several γ-diphenyl amines were obtained in excellent yield (>80%) on gram-scale with only 0.1 mol% catalyst loading, thus demonstrating the synthetic potential of this method. It is worth noting that phenpyramine, a challenging substrate for Rh-catalyzed hydroaminomethylation (35% yield)35, was prepared in 87% yield in 2.0 mmol scale employing our method. Sensipar, a top-selling pharmaceutical agent, is obtained from readily accessible m-CF3 styrene and corresponding α-TMS amine utilizing this method in 3 mmol scale, without any erosion of enantiopurity.
Fig. 5. Synthetic application hydroaminoalkylaltion of vinylarenes.
Synthese of four bioactive molecules using this photoredox method with low catalyst loadings. PC [Ir(ppy)2(dtbbpy)]PF6 (photocatalyst). See Supplementary Methods for details.
Mechanism investigations
To provide support for our working mechanistic hypothesis outlined above (Fig. 2), a series of control reactions were carried out under optimized conditions for the hydroaminoalkylation (Fig. 6). In the presence of 2,2,6,6-tetramethylpiperidinyloxy (TEMPO), the reaction was found to be completely inhibited, and the expected TEMPO-alkyl adduct was detected. To confirm the source of the H-atom in the product, deuterium-labeling studies were carried out. When the reaction is conducted in DMF-d7, no deuterium incorporation at the benzylic site is observed, confirming that H-atom transfer from the solvent is not operative. To corroborate this result, the application of MeOD under the standard conditions leads to a product with 93% D incorporation at the benzylic position.
Fig. 6. Mechanistic investigations.
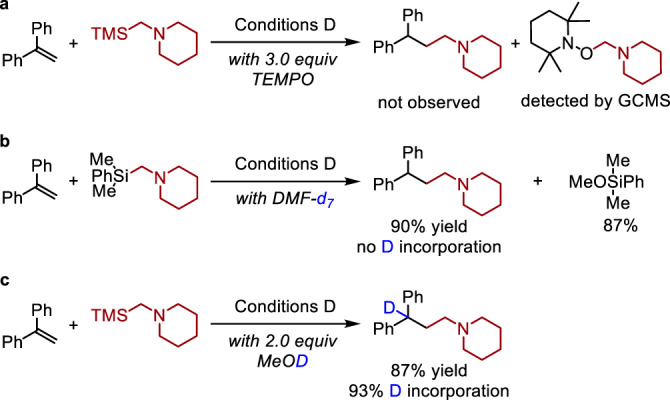
a Control experiments with TEMPO. b Deuterium-labeling studies in DMF-d7. c Deuterium-labeling studies with MeOD. See Fig. 4 legend for Conditions D.
In the case of diphenylethylene studied above, the reduction potential of the radical intermediate (E = –1.34 V vs. SCE) is within range of the Ir photocatalyst (E = –1.37 V vs. SCE), which supports a sequential ET/PT mechanism involving proton transfer following prior reduction to the carbanion. However, with monoaryl alkene substrates, the benzylic radicals feature more negative reduction potentials that fall outside the redox window of the photocatalyst (E ≈ –1.45 V vs. SCE for the styrene-derived intermediate). Although slightly endergonic, this electron transfer could be kinetically feasible if cage escape and protonation of the subsequently formed carbanion competes with back electron transfer62,63. Alternatively, a concerted ET/PT mechanism64 could render this step thermodynamically feasible. Although either mechanism cannot be strictly refuted based on current evidence, it is likely that different mechanisms may be operative for different substrates based on electronic properties. Similar mechanistic studies were conducted for the hydroalkylation reaction with carboxylic acids and provide additional supporting evidence (see Supplementary information for more details).
Discussion
The anti-Markovnikov hydro(amino)alkylation of vinylarenes has been demonstrated, proceeding through a radical pathway. A range of valuable (amino)alkyl fragments can be appended onto an alkene substrate to convert abundant feedstocks into valuable linear products. Importantly, electronically diverse vinylarenes are tolerated, representing a breakthrough from the reliance on highly polarized electron-poor alkene substrates for similar reactions. Mechanistic studies have provided valuable insight into the mode of proton transfer in the reaction. Future work will focus on the development of alkene difunctionalization reactions utilizing other radical capture reagents.
Methods
Representative procedure for the hydroalkylation of vinylarenes using organotrifluoroborate salts
In a nitrogen-filled glove box, an oven-dried 4-mL reaction vial equipped with a stir bar was charged with [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (2.3 mg, 2 µmol, 1 mol %) and potassium benzyltrifluoroborate (59.4 mg, 0.30 mmol, 1.5 equiv). This was followed by the addition of anhydrous DMF (2000 µL, 0.10 M), 1,1-diphenylethylene (35.3 µL, 0.20 mmol, 1.0 equiv), and guaiacol (67 µL, 0.60 mmol, 3.0 equiv). The vial was sealed with a Teflon-lined cap, removed from the glove box, and irradiated with two 24 W blue LEDs with stirring at 800 r.p.m. at room temperature (r.t.) for 48 h. The crude mixture was then directly adsorbed onto diatomaceous earth (Celite®) and purified by flash column chromatography on silica (2% ethyl acetate in hexanes). Product 1 was obtained as a colorless oil (38.5 mg, 71%).
Representative procedure for the hydroalkylation of vinylarenes using alkyl carboxylic acids
In a nitrogen-filled glove box, an oven-dried 4-mL reaction vial equipped with a stir bar was charged with [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (1.2 mg, 1 µmol, 1 mol %) and K3PO4 (31.8 mg, 0.15 mmol, 1.5 equiv). This was followed by the addition of 1000 µL of a stock solution of cyclohexane carboxylic acid (0.15 mmol, 1.5 equiv) in anhydrous DMF, and 1,1-diphenylethylene (17.6 µL, 0.10 mmol, 1.0 equiv). The vial was sealed with a Teflon-lined cap, removed from the glove box, and irradiated with two 24 W blue LEDs with stirring at 800 r.p.m. at r.t. for 24 h. The reaction mixture was diluted with ethyl acetate (2.5 mL) and then washed with water (3 × 2 mL) to remove most of the DMF. The organic layer was sampled, and the solvent was removed under a vacuum. Product 3 was obtained in 99% yield by flash column chromatography on silica.
Representative procedure for the hydroaminoalkylation of vinylarenes using α-silyl amine reagents
In a nitrogen-filled glove box, an oven-dried 4-mL reaction vial equipped with a stir bar was charged with [Ir(ppy)2(dtbbpy)]PF6 (0.9 mg, 0.001 mmol, 0.5 mol %), 1,1-diphenylethylene (35.3 µL, 0.2 mmol, 1.0 equiv), 1-((trimethylsilyl)methyl)piperidine (37.6 mg, 0.22 mmol, 1.1 equiv), and 2.0 mL anhydrous DMF. The vial was sealed with a Teflon-lined cap, removed from the glove box, and irradiated with one 24 W blue LED at r.t. for 3 h. The reaction crude was quenched by the addition of DCM, concentrated in vacuo, and then purified by basic alumina chromatography to afford the desired product 26 in 90% yield.
Supplementary information
Acknowledgements
We thank the NIH (1R35GM125029), NSF CAREER (1555337), the Sloan Research Foundation, Novartis, Eli Lilly, Amgen, and the University of Illinois for their generous support of this work.
Author contributions
Z.W., S.N.G. and K.L.H. conceived and designed the project. Z.W. and S.N.G. performed the experiments and analyzed the data. Z.W. and S.N.G. wrote the manuscript with contributions from all authors. K.L.H. directed the project.
Data availability
The data that support the findings of this study are available within the article and its Supplementary information files. Additional data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Jia-Rong Chen and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhao Wu, Samuel N. Gockel.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26170-6.
References
- 1.Corey, E. J. & Cheng, X.-M. The Logic of Chemical Synthesis p. 59 (Wiley, 1989).
- 2.Beller M, Seayad J, Tillack A, Jiao H. Catalytic Markovnikov and anti‐Markovnikov functionalization of alkenes and alkynes: recent developments and trends. Angew. Chem. Int. Ed. 2004;43:3368–3398. doi: 10.1002/anie.200300616. [DOI] [PubMed] [Google Scholar]
- 3.Resch V, Hanefeld U. The selective addition of water. Catal. Sci. Technol. 2015;5:1385–1399. doi: 10.1039/C4CY00692E. [DOI] [Google Scholar]
- 4.Hintermann L. Recent developments in metal-catalyzed additions of oxygen nucleophiles to alkenes and alkynes. Top. Organomet. Chem. 2010;31:123–155. doi: 10.1007/978-3-642-12073-2_6. [DOI] [Google Scholar]
- 5.Ogawa A. Activation and reactivity of Group 16 inter-element linkage—tansition-metal-catalyzed reactions of thiols and selenols. J. Organomet. Chem. 2000;611:463–474. doi: 10.1016/S0022-328X(00)00469-1. [DOI] [Google Scholar]
- 6.Kondo T, Mitsudo T-A. Metal-catalyzed carbon−sulfur bond formation. Chem. Rev. 2000;100:3205–3220. doi: 10.1021/cr9902749. [DOI] [PubMed] [Google Scholar]
- 7.Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Arndt M, Gooßen K, Heydt H, Gooßen LJ. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]
- 9.Petrone DA, Ye J, Lautens M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 2016;116:8003–8104. doi: 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- 10.Takacs JM, Jiang X-T. The Wacker reaction and related alkene oxidation reactions. Curr. Org. Chem. 2003;7:369–396. doi: 10.2174/1385272033372851. [DOI] [Google Scholar]
- 11.Keith JA, Henry PM. The mechanism of the Wacker reaction: a tale of two hydroxypalladations. Angew. Chem. Int. Ed. 2009;48:9038–9049. doi: 10.1002/anie.200902194. [DOI] [PubMed] [Google Scholar]
- 12.Haggin J. Chemists seek greater recognition for catalysis. Chem. Eng. N. 1993;71:23. doi: 10.1021/cen-v071n022.p023. [DOI] [Google Scholar]
- 13.Folkins, H. O. Benzene in Ullmann’s Encyclopedia of Industrial Chemistry, Vol. 5, 237–268 (Wiley, 2012).
- 14.Jana R, Pathak TP, Sigman MS. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011;111:1417–1492. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasker SZ, Standley EA, Jamison TF. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu GC. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 2017;3:692–700. doi: 10.1021/acscentsci.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph A, Lautens M. Secondary alkyl halides in transition-metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]
- 19.Urkalan KB, Sigman MS. Palladium-catalyzed hydroalkylation of styrenes with organozinc reagents to form carbon-carbon sp3-sp3 bonds under oxidative conditions. J. Am. Chem. Soc. 2009;131:18042–18043. doi: 10.1021/ja908545b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, et al. Practical carbon-carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 2016;7:11129. doi: 10.1038/ncomms11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Xiao B, Liu L, Fu Y. Formation of C(sp3)–C(sp3) bonds through nickel-catalyzed decarboxylative olefin hydroalkylation reactions. Chem. Eur. J. 2016;22:11161–11164. doi: 10.1002/chem.201602486. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Zhu J, Zhang Y, Zhu S. NiH-catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)−H alkylation of alkenes. Angew. Chem. Int. Ed. 2018;57:4058–4062. doi: 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y-M, Bruno NC, Placeres AL, Zhu S, Buchwald SL. Enantioselective synthesis of carbo- and heterocycles through a CuH-catalyzed hydroalkylation approach. J. Am. Chem. Soc. 2015;137:10524–10527. doi: 10.1021/jacs.5b07061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marek I. Enantioselective carbometallation of unactivated olefins. J. Chem. Soc. Perkin Trans. 1999;1:535–544. doi: 10.1039/a807060a. [DOI] [Google Scholar]
- 25.Murakami K, Yorimitsu H. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein J. Org. Chem. 2013;9:278–302. doi: 10.3762/bjoc.9.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nii S, Terao J, Kambe N. Titanocene-catalyzed regioselective carbomagnesation of alkenes and dienes. J. Org. Chem. 2004;69:573–576. doi: 10.1021/jo0354241. [DOI] [PubMed] [Google Scholar]
- 27.Wei X, Johnson P, Taylor RJK. Organolithium addition to styrene and styrene derivatives: scope and limitations. J. Chem. Soc. Perkin Trans. 2000;1:1109–1116. doi: 10.1039/a910195k. [DOI] [Google Scholar]
- 28.Norsikian S, Marek I, Normant JF. Enantioselective carbolithiation of β-alkylated styrene. Tetrahedron Lett. 1997;38:7523–7526. doi: 10.1016/S0040-4039(97)10022-3. [DOI] [Google Scholar]
- 29.Zhai D-D, Zhang X-Y, Liu Y-F, Zheng L, Guan B-T. Potassiumamide-catalyzed benzylic C-H bond addition of alkylpyridines to styrenes. Angew. Chem. Int. Ed. 2018;57:1650–1653. doi: 10.1002/anie.201710128. [DOI] [PubMed] [Google Scholar]
- 30.Kalck P, Urrutigoïty M. Tandem hydroaminomethylation reaction to synthesize amines from alkenes. Chem. Rev. 2018;118:3833–3861. doi: 10.1021/acs.chemrev.7b00667. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Dong XQ, Zhang X. Recent progress in rhodium-catalyzed hydroaminomethylation. Org. Chem. Front. 2016;3:1359–1370. doi: 10.1039/C6QO00233A. [DOI] [Google Scholar]
- 32.Ahmed M, Seayad AM, Jackstell R, Beller M. Amines made easily: a highly selective hydroaminomethylation of olefins. J. Am. Chem. Soc. 2003;125:10311–10318. doi: 10.1021/ja030143w. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Huang K, Zhang J, Wu W, Zhang X. Rhodium-catalyzed highly regioselective hydroaminomethylation of styrenes with tetraphosphorus ligands. Org. Lett. 2013;15:3078–3081. doi: 10.1021/ol4012635. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed M, et al. Hydroaminomethylation with novel Rhodium-Carbene complexes: an efficient catalytic approach to pharmaceuticals. Chem. Eur. J. 2007;13:1594–1601. doi: 10.1002/chem.200601155. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Huang K, Zhang J, Wu W, Zhang X. Cascade synthesis of fenpiprane and related pharmaceuticals via rhodium-catalyzed hydroaminomethylation. Org. Lett. 2013;15:1036–1039. doi: 10.1021/ol303483t. [DOI] [PubMed] [Google Scholar]
- 36.Musacchio AJ, et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science. 2017;355:727–730. doi: 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller DC, et al. Anti-Markovnikov hydroamination of unactivated alkenes with primary alkyl amines. J. Am. Chem. Soc. 2019;141:16590–16594. doi: 10.1021/jacs.9b08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giese B, González‐Gómez JA, Witzel T. The scope of radical CC-coupling by the, Tin method. Angew. Chem. Int. Ed. Engl. 1984;23:69–70. doi: 10.1002/anie.198400691. [DOI] [Google Scholar]
- 39.Miyake Y, Nakajima K, Nishibayashi Y. Visible-light-mediated utilization of α-aminoalkyl radicals: addition to electron-deficient alkenes using photoredox catalysts. J. Am. Chem. Soc. 2012;134:3338–3341. doi: 10.1021/ja211770y. [DOI] [PubMed] [Google Scholar]
- 40.Chu L, Ohta C, Zuo Z, MacMillan DWC. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-pregabalin. J. Am. Chem. Soc. 2014;136:10886–10889. doi: 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo JC, Gui J, Yabe Y, Pan CM, Baran PS. Functionalized olefin cross-coupling to construct carbon-carbon bonds. Nature. 2014;516:343–348. doi: 10.1038/nature14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McManus JB, Onuska NPR, Nicewicz DA. Generation and alkylation of α-carbamyl radicals via organic photoredox catalysis. J. Am. Chem. Soc. 2018;140:9056–9060. doi: 10.1021/jacs.8b04890. [DOI] [PubMed] [Google Scholar]
- 43.Esplt LR, McPherson IS, Wiensch EM, Yoon TP. Enantioselective conjugate additions of α-amino radicals via cooperative photoredox and Lewis acid catalysis. J. Am. Chem. Soc. 2015;137:2452–2455. doi: 10.1021/ja512746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang SB, Wiles RW, Kelly CB, Molander GA. Photoredox generation of carbon-centered radicals enables the construction of 1,1-difluoroalkene carbonyl mimics. Angew. Chem. Int. Ed. 2017;56:15073–15077. doi: 10.1002/anie.201709487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovett GH, Sparling BA. Decarboxylative anti-Michael addition to olefins mediated by photoredox catalysis. Org. Lett. 2016;18:3494–3497. doi: 10.1021/acs.orglett.6b01712. [DOI] [PubMed] [Google Scholar]
- 46.Huang H, et al. Chemo- and regioselective organo-photoredox catalyzed hydroformylation of styrenes via a radical pathway. J. Am. Chem. Soc. 2017;139:9799–9802. doi: 10.1021/jacs.7b05082. [DOI] [PubMed] [Google Scholar]
- 47.Yasu Y, Koike T, Akita M. Visible light‐induced selective generation of radicals from organoborates by photoredox catalysis. Adv. Synth. Catal. 2012;354:3414–3420. doi: 10.1002/adsc.201200588. [DOI] [Google Scholar]
- 48.Diccianni JB, Chin M, Diao T. Synthesis of lactate derivatives via reductive radical addition to α-oxoacrylates. Tetrahedron. 2019;75:4180–4185. doi: 10.1016/j.tet.2019.05.002. [DOI] [Google Scholar]
- 49.Trowbridge A, Reich D, Gaunt MJ. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation. Nature. 2018;561:522–527. doi: 10.1038/s41586-018-0537-9. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava V, Singh PK, Singh PP. Photocatalysed eosin Y mediated C(sp3)–H alkylation of amine substrates via direct HAT. Tetrahedron Lett. 2019;60:1333–1336. doi: 10.1016/j.tetlet.2019.04.016. [DOI] [Google Scholar]
- 51.Leonel E, Paugam JP, Nedelec J-Y, Perichon J. Alkylation and ring formation by electroreductive coupling of arylalkenes and alkyl mono- and dihalides. J. Chem. Res. Synop. 1995;7:278–279. [Google Scholar]
- 52.Byers JH, Janson NJ. Radical additions to (η6-styrene) chromium tricarbonyl. Org. Lett. 2006;8:3453–3455. doi: 10.1021/ol061107f. [DOI] [PubMed] [Google Scholar]
- 53.McGrath NA, Brichacek M, Njardarson JT. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. [DOI] [Google Scholar]
- 54.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6869–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wayner DDM, McPhee DJ, Griller D. Oxidation and reduction potentials of transient free radicals. J. Am. Chem. Soc. 1988;110:132–137. doi: 10.1021/ja00209a021. [DOI] [Google Scholar]
- 56.Karakaya I, Primer DN, Molander GA. Photoredox cross-coupling: Ir/Ni dual catalysis for the synthesis of benzylic ethers. Org. Lett. 2015;17:3294–3297. doi: 10.1021/acs.orglett.5b01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsui JK, Molander GA. Direct α-arylation/heteroarylation of 2-Trifluoroboratochromanones via photoredox/nickel dual catalysis. Org. Lett. 2017;19:436–439. doi: 10.1021/acs.orglett.6b03448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima K, Miyake Y, Nishibayashi Y. Synthetic utilization of α-aminoalkyl radicals and related species in visible light photoredox catalysis. Acc. Chem. Res. 2016;49:1946–1956. doi: 10.1021/acs.accounts.6b00251. [DOI] [PubMed] [Google Scholar]
- 59.Ide T, et al. Regio- and chemoselective Csp3–H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis. Chem. Sci. 2018;9:8453–8460. doi: 10.1039/C8SC02965B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remeur C, Kelly CB, Patel NR, Molander GA. Aminomethylation of aryl halides using α-silylamines enabled by Ni/photoredox dual catalysis. ACS Catal. 2017;7:6065–6069. doi: 10.1021/acscatal.7b01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida J-i, Kataoka K, Horcajada R, Nagaki A. Chem. Rev. 2008;108:2265–2299. doi: 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- 62.Mukherjee S, Maji B, Tlahuext-Aca A, Glorius F. Visible-light-promoted activation of unactivated C(sp3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 2016;138:16200–16203. doi: 10.1021/jacs.6b09970. [DOI] [PubMed] [Google Scholar]
- 63.Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gentry EC, Knowles RR. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 2016;49:1546–1556. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the article and its Supplementary information files. Additional data are available from the corresponding author upon request.