Abstract
Pharmacological treatments for opioid use disorders (OUDs) may have mixed efficacy across diverse groups (i.e., sex/gender, race/ethnicity, socioeconomic status [SES]). The present systematic review aims to examine how diverse groups have been included in U.S. randomized clinical trials examining pharmacological treatments (i.e., methadone, buprenorphine, or naltrexone) for OUDs. PubMed was systematically searched according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The initial search yielded 567 articles. After exclusion of ineligible articles, 50 remained for the present review. Of the included articles, 14.0% (n = 7) reported both full (i.e., accounting for all participants) sex/gender and race/ethnicity information; only two of those articles also included information about any SES indicators. Moreover, only 22.0% (n = 11) reported full sex/gender information, and 42.0% (n = 21) reported full racial/ethnic information. Furthermore, only 10.0% (n = 5) reported that their lack of subgroup analyses or diverse samples was a limitation to their studies. Particularly underrepresented were American Indian/Alaska Native, Asian, Native Hawaiian/Other Pacific Islander, and multiracial individuals. These results also varied by medication type; Black individuals were underrepresented in buprenorphine RCTs but were well represented in RCTs for methadone and/or naltrexone. In conclusion, it is critical that all people receive efficacious pharmacological care for OUDs given the ongoing opioid epidemic. Findings from the present review, however, support that participants from diverse or marginalized backgrounds are underrepresented in treatment trials, despite being at increased risk for disparities related to OUDs. Suggestions for future research are advanced.
Keywords: opioid use disorder, methadone, buprenorphine, naltrexone, diversity
Of the 20.5 million Americans who met criteria for any substance use disorder in 2015, 10% specifically had an opioid use disorder (OUD); for example, misuse of opioids for purposes other than prescribed, or non-prescription use of opioids (SAMHSA, 2019). Opioid misuse is associated with numerous negative consequences including health (e.g., HIV, [Willner-Reid et al., 2008], coma, [Khansari et al., 2013], and premature death [APA, 2018]), legal (e.g., criminal justice involvement; Winkelman et al., 2018), and economic consequences (Birnbaum et al., 2011). While the “opioid epidemic” (DEA, 2017) has affected all races/ethnicities, age groups, sexes/genders, and socioeconomic statuses (SES; Pletcher et al., 2008), specific demographic groups are disproportionately affected. For instance, prevalence rates of OUDs tend to be highest among individuals ages 18–25 and younger (0.9–1.3%) and these rates decrease with age across the lifespan, with the lowest rates seen among adults age 65 years and older (0.09%; APA, 2013; SAMHSA, 2019). OUD prevalence rates are also higher among those who are unemployed or of a low socioeconomic status (SES; Han et al., 2017). Moreover, research reports mixed findings in terms of rates of OUD across gender groups; however, findings support that women tend to progress from use to dependence more quickly than men and have more use-related social and psychological consequences (Back et al., 2011). Nationally representative data also suggest there are differences by race/ethnicity; American Indian/Alaska Native (AI/AN) and multiracial individuals are most likely to report opioid misuse, followed closely by White and Hispanic/Latinx individuals, while Black and Asian individuals report less misuse (Nalven et al., 2020; SAMHSA, 2019). Of additional concern, research suggests that minoritized groups (e.g., racial/ethnic, low SES) may be the most likely to experience negative consequences and health disparities related to opioid misuse (King et al., 2014; Singh et al., 2019); these disparities may be related to access to and utilization of pharmacological treatments.
Pharmacological Treatments for OUD
There are currently three medications approved by the U.S. Food and Drug Administration (FDA) for use in OUD treatment: methadone, buprenorphine, and naltrexone (FDA, 2019). Methadone, an opioid agonist, prevents or reduces withdrawal, craving, and opioid misuse relapse by occupying opioid mu receptors and diminishing euphoric effects (Salsitz & Wiegand, 2016). Methadone must be administered daily at specialty clinics by personnel who monitor an oral liquid or pill administration (Samet at al., 2018). Buprenorphine, a partial mu-opioid agonist and kappa-opioid receptor antagonist, is thought to be as effective as methadone but has lower misuse and overdose potential (Johnson et al., 1992; Ling et al., 1994). Buprenorphine typically results in less analgesia, euphoria, and respiratory depression than methadone (Whelan & Remski, 2012) and, therefore, may be a preferred treatment for OUDs. Buprenorphine has several methods of administration including as buccal film (in the cheek), sublingual film or tablet (under the tongue), as a subdermal implant (under the skin), or as a subcutaneous injection; it can be prescribed such that physician visits are progressively less frequent over an individuals’ course of use (i.e., rather than requiring daily, or even weekly visits), thus increasing access compared to methadone (SAMHSA, 2020). Finally, naltrexone, an opioid antagonist, functions by blocking opioid postsynaptic receptors and the related euphoric effect from opioids but does not assist with withdrawal symptoms. Naltrexone is typically administered daily as an oral pill or every four weeks as an intramuscular injection and can be prescribed by health care providers that can prescribe medication (e.g., nurse practitioners, physician assistants, physicians) as it has less misuse potential then methadone and buprenorphine (SAMHSA, 2020). Though found to be effective and safe for treatment of OUDs, naltrexone has poor adherence rates because it can result in severe withdrawal and concomitantly blocks the desired effects of opioid use (e.g., euphoria or pain relief; Jarvis et al., 2018).
It is important to note that pharmacological treatments utilized for OUDs have been found to differ in efficacy across demographic subgroups; however, many of these findings are preliminary and require further exploration and replication. These differences are likely due to biological, environmental, and psychosocial factors, among others. Younger individuals, those with lower SES, and females tend to report worse outcomes following pharmacological treatment for OUD compared to older individuals, those with higher SES, and males (Barbosa-Leiker et al., 2018; Hillhouse et al., 2013; Parran et al., 2010). Although some research suggests there may be specific gene polymorphisms (i.e., variations in the formation of genes) of the OPRD1 gene that are associated with worse buprenorphine treatment outcomes among females but not males (Clarke et al., 2014), these findings are equivocal and need to be replicated in larger, more diverse samples. Further, a systematic review of 26 articles focused on buprenorphine concluded that findings were inconsistent across studies and small sample sizes (with only 26% female participants in included articles) did not allow for definitive statements regarding sex differences in outcomes (Ling et al., 2019). Regarding racial differences, Pro et al., (2020) conducted a retrospective, cross-sectional study and found that the effects of medication for OUD varied widely based on race, such that AI/AN women were most likely to benefit from medication while White men were the least likely. Their study did not account for differing treatment mechanisms making it impossible to determine precise explanations for their findings; however, the authors theorized that AI/AN individuals may be participating in culturally adapted OUD treatment that may have better success within their communities. Moreover, Crist et al., (2013) found a specific polymorphism on the OPRD1 gene was associated with worse outcomes following buprenorphine treatment for Black (but not White) individuals, despite the fact that the polymorphism occurred among both racial groups. It is important to note, however, that these genetic findings are correlational in nature and inconclusive.
While further exploration is necessary concerning the efficacy of pharmacological treatments for OUDs, it is evident that certain demographic groups have barriers to accessing and receiving efficacious treatment. For example, Wu and colleagues (2016) found that, among the National Surveys on Drug Use and Health dataset that is considered to be nationally representative, adolescents, uninsured people, and Black or Asian individuals underutilized opioid pharmacological treatment. Other studies have found that Black individuals with an OUD were only half as likely to enroll in a methadone or buprenorphine maintenance treatment (Potter et al., 2015) and Hispanic/Latinx and incarcerated individuals were less likely to utilize OUD pharmacological treatment (Evans et al., 2019) than their White or non-incarcerated counterparts. Beyond lack of recruitment, Black, Hispanic/Latinx, low-income individuals, and women with an OUD are prescribed opioid pharmacological treatment less often than White, higher-income individuals, and men (Lagisetty et al., 2019; Lapham et al., 2021). Rates of retention in pharmacological opioid treatment have also been found to be lower for patients who were younger, Black, Hispanic/Latinx, or unemployed (Weinstein et al., 2017). Other possible barriers to treatment for marginalized groups may include a lack of trust in research and/or medical professionals, stigma involved in accessing care (Harris et al., 1996; Pacheco et al., 2013; Schick et al., 2020), or limited access and poor quality of care (Stein et al., 2018); however, it is clear that there are disparities in ability to be successfully treated with pharmacological treatment for an OUD among people from certain demographic groups. Moreover, while there is evidence to support these disparities among relatively larger marginalized racial groups (e.g., Black, Asian, Latinx), there is a lack of research to support or refute the evidence that disparities exist among groups less-well represented in research, such as AI/AN, Native Hawaiian/Other Pacific Islander (NH/OPI), and multiracial groups; this lack of support suggests more research is necessary. In summary, the limited body of existing literature suggests there are demographic group differences in OUD prevalence, disparities, and pharmacological treatment and efficacy; yet, it remains unclear the extent to which diverse sample have been represented in trials of medication treatments for OUDs.
Present Study
The gold standard of research methodologies for interventions, randomized controlled trials (RCTs) are a scientifically robust method of testing intervention efficacy. Participation in RCTs by members of diverse groups is important as it allows for a better understanding of potential differences in efficacy of treatments that may be important for targeted pharmacological interventions. Furthermore, participation in such trials provides opportunities for marginalized and underserved groups to receive potentially greater efficacious medical care (Klein et al., 2015). Concerningly, reviews examining pharmacological treatment for other conditions have found that inclusion of individuals from underrepresented groups is lacking (Chen Jr et al., 2014; Schick et al., 2020). Such underrepresentation is of concern, as it is difficult to conclude how effective a given treatment is within a group for which it has not yet been adequately assessed. Yet, it is important to also note that overrepresentation of vulnerable groups (e.g., those who are economically disadvantaged) is exploitative, hence, it is critical that studies employ fair and equitable recruitment procedures (DHEW, 1979). It remains unclear, however, the extent to which diverse demographic groups have been included in pharmacological treatment trials for opioid use disorders.
Gatzke-Kopp (2016), Clark and colleagues (2019) and others have called for more diverse representation in psychophysiological and pharmacological research, and a clearer understanding of the current state of diversity inclusion in the OUD literature is needed. Therefore, the present study aims to examine the extent to which diverse groups (i.e., age, sex/gender, race/ethnicity, SES) have been included in U.S. RCTs examining the efficacy of pharmacological treatment for OUDs (i.e., methadone, buprenorphine, and naltrexone). We specifically focus on the U.S. because people of color and of lower SES have experienced historical and contemporary inequities (e.g., access to resources and adequate healthcare) that have resulted in notable health disparities (Kreps, 2006; Williams, 2012). Moreover, while the authors recognize that sex is a biological variable and gender is a social construct encompassing the characteristics and roles of femininity and masculinity, these variables are analyzed together in the present review because no articles reported on both variables or provided information on how the data was collected so that we could ascertain whether sex and gender were being accurately represented.
Materials and Method
This systematic review followed guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (PROSPERO registration # CRD42020192077), which ensures the accuracy and quality of conducting and reporting a systematic review (Liberati et al., 2009; Moher et al., 2009). The PRISMA statement includes a 27-item checklist specifying information required to be reported and a four-phase flow diagram that details the identification, screening, eligibility, and inclusion of relevant articles.
Search Strategy
PubMed, selected to collect articles representing trials funded by the National Institutes of Health, was searched for peer-reviewed manuscripts in the English language on March 31, 2020 using the search terms: (“opioid use disorder” OR “opioid dependence”) AND (“randomized controlled” OR “randomized trial” OR “clinical trial”) AND (“methadone” OR “buprenorphine” OR “naltrexone”). All papers generated using search criteria were compiled into a Microsoft Excel database. Abstracts were screened by two independent coders from the initial search to assess inclusionary criteria, and a third coder resolved any discrepancies in coding. The search strategy is illustrated in the flow diagram (see Figure 1).
Figure 1.
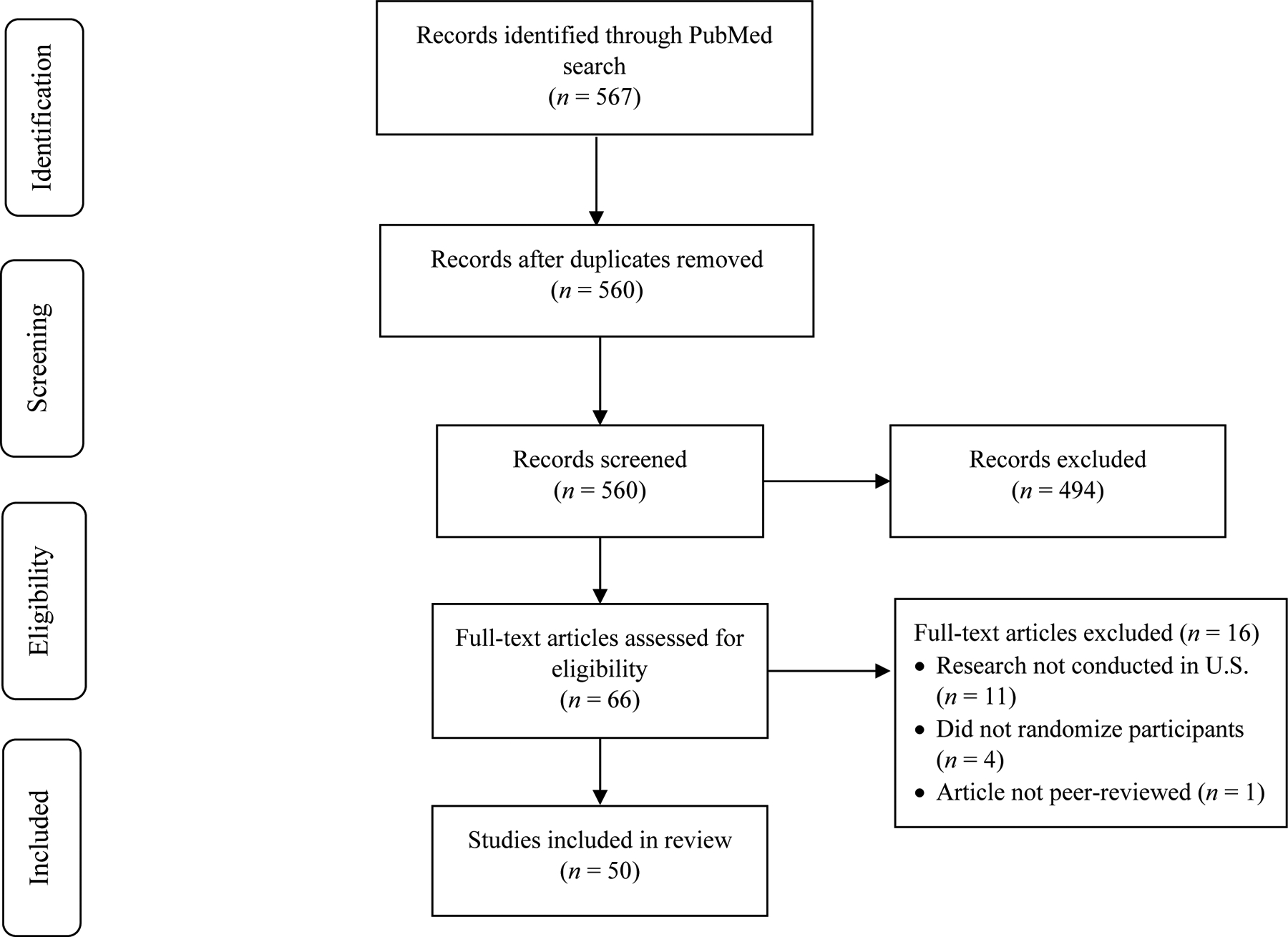
Flow Diagram for PRISMA Systematic Review Procedure
Eligibility Criteria
Articles were restricted on the basis of six pre-defined criteria: (1) reporting in English language; (2) conducted in the U.S.; (3) assignment of at least five participants per condition (consistent with prior systematic review methodology; Bouza et al., 2004); (4) intent of the paper was to test the efficacy of methadone, buprenorphine, or naltrexone for opioid dependence or OUD; and (5) assessment of at least one opioid use outcome (i.e., uranalysis, self-report; Moeller et al., 2008; Palamar et al., 2019).
Data Extraction and Synthesis
The remaining full-length articles were reviewed and information relevant to study goals was extracted and compiled into a table (see Table 1). The following information was extracted from each article: (1) funding source; (2) sample size; (3) reporting of sample demographics including age, sex/gender, race/ethnicity, SES; (4) sample demographic information (e.g., number and percent of participants that were members of various demographic groups); (5) subgroup analysis by demographic group; and (6) discussion of the inclusion/diversity in article limitations. Variables that were coded to represent SES include employment status (e.g., full-time, part-time, unemployed, etc.), income, and living situation (e.g., living with homelessness, unstable living situation, etc.).
Table 1.
Summary of Included Articles
| Citation | Sample Size | Mean Age (Years) | Sex/Gender Reporting | Sex/Gender Inclusion | Race/Ethnicity Reporting | Racial/Ethnic Inclusion | SES Inclusion | Subgroup Analyses | Discussed Diversity in Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Methadone | |||||||||
| Fiellin et al., 2001 | 46 | 41 | Partial | 65% men | Partial | 78% White | 67% employed full-time Monthly income, M = $1541 | No | None |
| Gruber et al., 2008 | 111 | 42 | Full | 61% male 39% female |
Full | 41% White 30% Black 5% Asian 4% AI/AN 20% Hispanic/Latinx |
Monthly income, M = $1398 | No | None |
| Masson et al., 2004 | 91 | Not reported | None | None | None | No | Yes: SES | ||
| Schwartz et al., 2017 | 295 | 43 | Partial | 59% male | Full | 41% White 58% Black 1% Hispanic/Latinx |
37% employed | No | Yes: race, SES |
| Schwartz et al., 2020 | 225 | 38 | Partial | 80% male | Full | 25% White 62% Black 13% other 3% Hispanic/Latinx |
44% employed | Yes: sex | None |
| Sees et al., 2000 | 179 | 39 | Partial | 59% men | Full | 51% White 30% Black 13% Hispanic/Latinx 6% other |
47% employed full-time 9% no stable living arrangement Monthly income, M = $855 | No | None |
| Strain et al., 1999 | 192 | 38 | Partial | 65% men | Partial | 49% White | 25% employed | Yes: sex | None |
| Strain et al., 1993 | 95 | 35 | Partial | 68% male | Partial | 52% Black | 60% unemployed | No | None |
| Buprenorphine | |||||||||
| Collins et al., 2005 | 106 | 36 | Partial | 72% male | Full | 53% White 12% Black 29% Hispanic/Latinx 6% other |
56% employed | No | None |
| Cushman et al., 2016 | 113 | 40 | Partial | 69% male | Full | 49% White 23% Black 20% Hispanic/Latinx 8% other |
35% homeless | No | None |
| D’Onofrio et al., 2015 | 329 | 31 | Partial | 76% men | Full | 75% White 7% Black 16% Hispanic/Latinx 1% other |
52% employed full-time 26% employed part-time 9% no stable living arrangement | No | None |
| Fiellin et al., 2006 | 166 | 36 | Partial | 78% male | Partial | 77% White | 48% employed full-time Monthly income, m = $1368 | No | None |
| Fiellin et al., 2014 | 113 | 30 | Partial | 58% male | Partial | 96% White 7% Hispanic/Latinx |
44% employed full-time | No | None |
| Gunderson et al., 2010 | 20 | 45 | Partial | 90% male | Full | 45% White 20% Black 35% Hispanic/Latinx |
25% employed | No | None |
| Haight et al., 2019 | 489 | 40 | Full | 66% male | Full | 71% White 27% Black/AA 2% other |
None | No | Full |
| Hopper et al. 2005 | 20 | 47 | Partial | 65% male | Partial | 85% Black/AA | 20% employed | No | None |
| Johnson et al., 1995a | 99 | 34 | Partial | 29% female | Partial | 50% non-White | 36% employed | No | None |
| Johnson et al., 1995b | 150 | 33 | Partial | 38% female | Partial | 47% non-White | 23% employed | Yes: gender | None |
| Ling et al., 2010 | 163 | 37 | Partial | 47% male | Full | 75% White 12% Black 13% other 15% Hispanic/Latinx |
None | No | None |
| Ling et al., 1998 | 736 | 36 | Partial | 33% female | Full | 49% White 22% Black 28% Hispanic/Latinx |
33% unemployed | No | None |
| Ling et al., 2009 | 516 | 36 | Partial | 33% female | Partial | 76% White 12% Black 6% Hispanic/Latinx |
35% unemployed | No | None |
| Lofwall et al., 2018 | 428 | 38 | Full | 61% men 39% women |
Partial | 75% White | 35% employed | No | None |
| Lucas et al., 2010 | 93 | 46 | Partial | 28% female | Partial | 98% Black/AA | 30% employed 48% own/rent home 39% staying with others 4% group home 9% homeless | No | None |
| Marsch et al., 2005a | 134 | 33 | Partial | 63% male | Partial | 98% White | 46% employed full-time Monthly income, m = $871 | Yes: SES, gender | None |
| Marsch et al., 2005b | 36 | 17 | Partial | 39% male | Partial | 97% White | None | No | None |
| Marsch et al., 2016 | 53 | 21 | Full | 58% male 42% female |
Partial | 76% White | 9% homeless | No | None |
| Potter et al., 2015 | 252 | 33 | Partial | 43% female | Partial | 89% White | 67% employed full-time | Yes: SES, gender, race, age | None |
| Rosenthal et al., 2013 | 287 | 36 | Partial | 61% male | Full | 83% White 13% Black 5% other 18% Hispanic/Latinx |
None | No | None |
| Rosenthal et al., 2016 | 177 | 39 | Full | 59% male 41% female |
Full | 95% White 3% Black 1% Asian 1% AI/AN 1% other 3% Hispanic/Latinx 97% non-Hispanic/Latinx |
55% employed full-time 10% employed part-time 18% unemployed 7% retired/disability | No | Yes: race, SES |
| Sigmon et al., 2016 | 50 | 35 | Partial | 58% male | Partial | 88% White | 52% employed | No | None |
| Stein et al., 2020 | 110 | 32 | Partial | 68% male | Partial | 79% White 11% Black 12% other 10% Hispanic/Latinx |
15% employed | Yes: gender, race | None |
| Woody et al., 2008 | 152 | 19 | Partial | 59% male | Partial | 74% White 2% Black 1% Filipino 25% Hispanic/Latinx |
N/A* | No | Yes: race |
| Naltrexone | |||||||||
| Comer et al., 2006 | 60 | 41 | Full | 77% male 24% female |
Full | 37% White 35% Black 3% Asian 18% Hispanic/Latinx 7% other |
None | No | None |
| Coviello et al., 2010 | 111 | 34 | Partial | 82% male | Full | 47% White 26% Black 27% Hispanic/Latinx |
23% employed | No | None |
| Friedmann et al., 2018a | 15 | 37 | Partial | 93% male | Partial | 83% White | 20% employed | No | None |
| Friedmann et al., 2018b | 308 | 44 | Partial | 85% male | Partial | 20% White 50% Black 27% Hispanic/Latinx |
18% employed | Yes: sex, SES, race | None |
| Jarvis et al., 2019 | 84 | 43 | Partial | 71% men | Partial | 80% Black | 53% unemployed | No | None |
| Lee et al., 2016 | 308 | 44 | Partial | 85% male | Partial | 20% White 50% Black 27% Hispanic/Latinx |
18% employed | No | None |
| Lee et al., 2015 | 33 | 44 | Full | 100% male | None | 18% employed 33% homeless | No | Yes: sex | |
| Mannelli et al., 2009 | 96 | 33 | Partial | 61% male | Full | 71% White 29% Black |
53% unemployed | No | None |
| Sullivan et al., 2013 | 57 | 41 | Partial | 77% men | Partial | 37% White 35% Black 18% Hispanic/Latinx |
None | No | None |
| Methadone and Buprenorphine | |||||||||
| Johnson et al., 2000 | 220 | 36 | Partial | 34% female | Partial | 60% non-White | 30% employed | Yes: sex, age, race | None |
| Johnson et al., 1992 | 162 | 33 | Full | 70% men 30% women |
Full | 58% White 40% Black 2% other |
None | No | None |
| Kosten et al., 1993 | 125 | 32 | Partial | 73% male | Partial | 69% White | 55% employed full-time | No | None |
| Ling et al., 1996 | 225 | 41 | Full | 80% male 20% female |
Full | 14% White 20% Black 65% Hispanic/Latinx 1% other |
None | No | None |
| Schottenfeld et al., 2005 | 162 | 36 | Partial | 66% male | Full | 52% White 36% Black 11% Hispanic/Latinx |
42% employed | No | None |
| Schottenfeld et al., 1997 | 116 | 33 | Partial | 69% male | Partial | 78% White | 71% employed | No | None |
| Strain et al., 1994 | 164 | 32 | Partial | 71% male | Full | 49% White 51% Black/AA |
35% employed | No | None |
| Buprenorphine and Naltrexone | |||||||||
| Bisaga et al., 2018 | 378 | 36 | Full | 66% male 34% female |
Full | 74% White 20% Black/AA 6% other |
None | No | other |
| Lee et al., 2018 | 474 | 34 | Full | 70% male 30% female |
Partial | 76% White 10% Black 17% Hispanic/Latinx |
18% employed 63% unemployed | No | None |
Note. SES = socioeconomic status; AI/AN = American Indian/Alaska Native; AA = African American.
Years of education and employment were assessed but are not strong indicators of SES among youth.
Results
Search Results
The search strategy yielded 567 articles. After removing duplicates, the search resulted in 560 unique articles. After the initial abstract review, 494 were excluded for failing to meet inclusion criteria. Following the procedures outlined previously, the remaining 66 articles were reviewed. Of those 66, 16 articles were deemed to be ineligible after full-text review (i.e., did not randomize participants to conditions, were not peer-reviewed, or were not conducted in the U.S.). The remaining 50 full-text articles were reviewed and included in the present study. These final articles were subsequently examined, and relevant information pertaining to study goals was extracted. Information regarding reported sample demographic breakdowns, subgroup analyses conducted, and mention of cultural inclusion in limitations sections for each article were then compiled into a table (see Table 1).
Summary of Included Articles
In total, 50 articles met all criteria; a summary of reporting and inclusion by demographic factors, subgroup analyses inclusion, and lack of inclusion as a limitation are presented in Table 2 for the full sample and by medication. The total number of participants included in all articles was 9,124, with a weighted average age of 36.2 years old. Three articles were conducted with adolescents or young adults (i.e., ages 13–24 years old), while the others reported average ages between 30 and 47 years old. Most articles (80.0%, n = 40) were funded, at least in part, by the NIH and of the 10 remaining articles, six were funded by pharmaceutical companies, four were funded by United States Public Health Service grants, and one article had no reported funding source. While 98.0% (n = 49) of the articles included at least some information on sex/gender and 96.0% (n = 48) included some information of race/ethnicity, only 14.0% (n = 7) of the articles reported both full (i.e., accounting for all of the participants) sex/gender and race/ethnicity information and only two of those articles (i.e., 4.0% of the articles included in the present review) also included information about any SES indicators. Moreover, 22.0% (n = 11) included full sex/gender information and 42.0% (n = 21) included full racial/ethnic information. Furthermore, only 16.0% (n = 8) of articles conducted subgroup analyses by any demographic factor and 10.0% (n = 5) reported that their lack of subgroup analyses or diverse samples was a limitation of their study.
Table 2.
Summary of Reporting and Inclusion of Diverse Demographic Groups, Subgroup Analyses, and Limitations
| Total Sample* (articles, n = 50) (participants, n = 9,124) | Methadone only (articles, n = 8) (participants, n = 1,234) | Buprenorphine only (articles, n = 24) (participants, n = 4,792) | Naltrexone only (articles, n = 9) (participants, n = 1,072) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Full | 11 (22.0%) | 1 (12.5%) | 4 (16.7%) | 2 (22.2%) |
| Partial | 38 (76.0%) | 6 (75.0%) | 20 (83.3%) | 7 (77.8%) |
| None | 1 (2.0%) | 1 (12.5%) | 0 | 0 |
| Sex Inclusion | ||||
| Reported on inclusion of female/women participants | 17 (34.0%) | 1 (12.5%) | 10 (41.7%) | 1 (11.1%) |
| Females/women includeda | 1,380 (22.3%) | 43 (5.4%) | 883 (31.8%) | 12 (1.3%) |
| Race/Ethnicity Reporting | ||||
| Full | 21 (42.0%) | 4 (50.0%) | 9 (37.5%) | 3 (33.3%) |
| Partial | 27 (54.0%) | 3 (37.5%) | 15 (62.5%) | 5 (55.6%) |
| None | 2 (4.0%) | 1 (12.5%) | 0 | 1 (11.1%) |
| Racial/Ethnic Inclusion | ||||
| Reported on inclusion of Black participants | 32 (64.0%) | 5 (62.5%) | 14 (58.3%) | 7 (77.8%) |
| Black participants includedb | 1,909 (27.3%) | 446 (49.6%) | 614 (16.0%) | 475 (61.4%) |
| Reported on inclusion of Asian participants | 3 (6.0%) | 1 (12.5%) | 1 (4.2%) | 1 (11.1%) |
| Asian participants includedb | 8 (0.1%) | 5 (0.6%) | 1 (0.03%) | 2 (0.3%) |
| Reported on inclusion of AI/AN participants | 2 (4.0%) | 1 (12.5%) | 1 (4.2%) | 0 |
| AI/AN participants includedb | 6 (0.1%) | 4 (0.4%) | 2 (0.1%) | 0 |
| Reported on inclusion of Hispanic/Latinx participants | 24 (48.0%) | 4 (50.0%) | 12 (50.0%) | 5 (55.6%) |
| SES Inclusion | ||||
| Reported on employment | 36 (72.0%) | 6 (75.0%) | 17 (70.8%) | 7 (77.8%) |
| Reported on income | 5 (10.0%) | 3 (37.5%) | 2 (8.3%) | 0 |
| Reported on living arrangement or homelessness | 6 (12.0%) | 1 (12.5%) | 4 (16.7%) | 1 (11.1%) |
| None | 10 (20.0%) | 1 (12.5%) | 4 (16.7%) | 2 (22.2%) |
| Other Inclusion | ||||
| Reported on HIV status | 3 (6.0%) | 1 (12.5%) | 2 (8.3%) | 0 |
| Reported on Hepatitis C status | 5 (10.0%) | 0 | 5 (20.8%) | 0 |
| Reported on people on parole/probation or incarcerated/in court ordered treatment | 7 (14.0%) | 1 (12.5%) | 0 | 5 (55.6%) |
| Subgroup Analyses Conducted | ||||
| Race/Ethnicity | 4 (8.0%) | 0 | 2 (8.3%) | 1 (11.1%) |
| Sex/Gender | 8 (16.0%) | 2 (25.0%) | 4 (16.7%) | 1 (11.1%) |
| SES | 3 (6.0%) | 0 | 2 (8.3%) | 1 (11.1%) |
| Included as Limitations | ||||
| Race/Ethnicity | 3 (6.0%) | 1 (12.5%) | 2 (8.3%) | 0 |
| Sex/Gender | 1 (2.0%) | 0 | 0 | 1 (11.1%) |
| SES | 3 (6.0%) | 2 (25.0%) | 1 (4.2%) | 0 |
Note. The numbers presented in this table do not necessarily reflect the full counts and percentages of individuals included but reflect representation based on information given in the articles. Percentages may not total 100 due to partial reporting; SES = socioeconomic status; AI/AN = American Indian/Alaska Native.
The total sample sizes and percentages are based off all articles including those for methadone, buprenorphine, naltrexone, and articles including two medications of interest.
The percentage of females/women included is based on the combined sample size of articles reporting any sex/gender information (n = 6,192; 790; 2,779; and 915 participants for the total sample, methadone, buprenorphine, and naltrexone, respectively).
The percentage of individuals included is based on the combined sample size of articles reporting any racial/ethnic information (n = 6,994; 900; 3,843; and 774 participants for the total sample, methadone, buprenorphine, and naltrexone, respectively).
Regarding sex/gender, of the 9,124 total participants in included articles, 6,192 (67.9%) participants’ sex/gender were reported. Only 34.0% (n = 17) of articles mentioned the inclusion of females/women, while 86.0% (n = 43) indicated the number or percent of male/men participants. Of the 6,192 participants whose sex/gender were reported, male/men individuals were the largest included group, representing 77.7% (n = 4,812) of all individuals whose sex/gender was reported in the articles, while females/women represented only 22.3% (n = 1,380). None of the articles differentiated between biological sex and gender as unique factors in their demographics and many used them interchangeably. Moreover, no articles mentioned the inclusion of transgender or non-binary gender individuals in their samples.
Regarding race, of the 9,124 total participants in included articles, 6,994 (76.7%) participants’ races were reported (excluding ethnicity and “other” categories). Of these 6,994 participants, White individuals were the largest included group, representing 72.5% (n = 5,071) of all individuals whose race was reported in the articles, followed by individuals who identified as Black (n = 1,909, 27.3%). Less than 1% were identified as Asian (n = 8) or AI/AN (n = 6). While some articles categorized non-White individuals into an “other” category, no articles included mention of participants that were either Native Hawaiian/Other Pacific Islander or multiracial. Moreover, while 64.0% (n = 32) of articles reported on the inclusion of Black participants, less than 1% of articles reported on the inclusion of Asian or AI/AN participants, and no articles mentioned the inclusion of NH/OPI or multiracial participants. Regarding ethnicity, less than half (n = 24, 48.0%) of the articles reported on participant ethnicity or described the percent of their population that was Hispanic/Latinx.
Regarding SES, 72.0% (n = 36) of articles reported on participants’ employment status, 10.0% (n = 5) reported some measure of income (typically sample mean income), and 12.0% (n = 6) reported on number/percent of participants who were experiencing homelessness or without a stable living arrangement. Only 20.0% (n = 10) did not report on any variables related to SES (e.g., employment, income, living situation). Of the 36 articles that reported on the percent of their participants that were employed (versus not; n = 24, 48.0%), 35.5% (n = 1,491) of participants were employed. Of the five articles that reported a mean income, the weighted mean monthly income was $1,136.70.
Methadone Findings
A total of eight articles specifically assessed outcomes for methadone treatment, with 1,234 participants included. Only one article reported on the number of female/women participants (the rest reported only on the percent male/men). Only two of the methadone articles reviewed reported on issues of inclusion and diversity in their limitations section (i.e., two mentioned SES and one mentioned race). Notably, in one of the two articles, the authors stated that having predominately Black and unemployed participants in their sample may mean their findings are limited in their generalizability (this article reported to have 58% Black participants, 41% White participants, and 1% Hispanic participants, while 37% of participants were reported to have been employed in the past 30 days).
Buprenorphine Findings
A total of 24 articles specifically assessed outcomes for buprenorphine treatment, with 4,792 participants included. Three of these articles specifically focused on adolescents or young adults (notably, no articles focused on other medications used samples of adolescents/young adults). Only 16.1% (n = 617) of participants with an identified race in these articles were reported to be Black, Asian, or AI/AN.
Naltrexone Findings
A total of nine articles specifically assessed outcomes for naltrexone treatment, with 1,072 participants included. Only one of these articles reported on the number of females/women included. The only article that discussed demographic composition of the sample as a limitation to the study had enrolled 100.0% males and noted that failure to recruit female participants was a constraint of the study. Of importance, 61.4% (n = 475) of all participants with an identified race in these articles were reported to be Black.
Discussion
The present study systematically examined the inclusion of individuals from diverse backgrounds with respect to sex/gender, race/ethnicity, and SES in RCTs examining methadone, buprenorphine, and naltrexone efficacy for OUDs. Fifty eligible articles were identified, with a majority of articles including at least partial information about sex/gender, race/ethnicity, and SES for their samples; however, participants from diverse backgrounds were not routinely nor representatively included and a majority of participants were male/men and White. These findings are consistent with findings of Gatzke-Kopp (2016) and Clark and colleagues (2019), who reported underrepresentation of diverse participants in psychophysiological research with regard to demographic factors such as sex/gender, race/ethnicity, and SES.
A relative strength of the reviewed articles was that all but two articles reported, to some extent, on both sex/gender and race/ethnicity. Furthermore, the majority of articles reported on some indicator of SES, such as employment status, income, or homelessness. Further, the samples in the reviewed articles may represent a lower than average SES population, which is consistent with the population of individuals with OUDs – a critical factor to consider because this group experiences a high level of health disparities related to OUDs (King et al., 2014; Singh et al., 2019). These findings are important given that research suggests that females, people of low SES, and racially/ethnically minoritized individuals experience significant health disparities related to opioid use and may not respond as well to pharmacological treatment (Barbosa-Leiker et al., 2018; Crist et al., 2013; Parran et al., 2010; Pro et al., 2020). Moreover, people who are both low SES and from a minoritized racial/ethnic background may experience even greater, additive or multiplicative risk for harm related to OUD. Recognition that all people have multiple groups with which they identify underlines the need for subgroup analyses to examine the ways in which identities may interact with each other (Crenshaw, 2017). Such investigations would allow for fine-grained analyses to examine how pharmacological treatments work for people from a range of backgrounds. Reporting on demographic factors and conducting subgroup analyses by such factors would allow readers to better understand the unique sample with whom the study was conducted and may help care providers to determine which medication(s) for OUD is/are appropriate for their patients or client populations.
This review also highlights several weaknesses of the literature with respect to inclusion and diversity in OUD pharmacological treatment studies. Few articles (n = 17, 34.0%) reported the percent of females/women included in their samples, and of the participants with an identified sex/gender, only 22.3% of participants were females/women (compared to 77.7% males/men identified). This finding contrasts with nationally representative data that has found females misuse opioids at similar rates (3.3% past year prevalence) compared to males (4.1% past year prevalence; SAMHSA, 2019). Of particular concern, none of the reviewed studies reported on the inclusion of gender diverse (e.g., transgender or gender non-binary) individuals. It is further problematic that articles did not differentiate between biological sex and socially constructed gender, as biological sex may differ from expressed gender, but sex can affect efficacy of medications for OUD, making it critical to know whether reported samples are reflecting biological sex or gender identity (Barbosa-Leiker et al., 2018).
Another weakness in the existing literature is that of the 6,994 participants with a race reported between all 50 articles, only eight Asian, six AI/AN, and zero NH/OPI or multiracial participants were identified; there may have been additional participants that were included in the reviewed articles, but their races were not identified. These findings are particularly problematic given that AI/AN and multiracial individuals report the highest rates of opioid misuse (Nalven et al., 2020; SAMHSA, 2019) and experience disproportionate health disparities due to OUD compared to other racial groups (King et al., 2014; Singh et al., 2019). Moreover, these findings are inconsistent with nationally representative data that found that 5.2% of multiracial, 5.1% of AI/AN, 3.8% of White, 3.7% of Hispanic/Latinx, 3.4% of Black, 2.8% of NH/OPI, and 1.6% of Asian individuals misuse opioids (SAMHSA, 2019). In contrast, our study found that White individuals are the best represented in clinical trials of medications for OUD, followed by Black participants, while less than one percent or no Asian, AI/AN, NH/OPI, or multiracial individuals were reported to be included.
While the present manuscript is unable to determine the specific reasons for a lack of reporting diverse racial/ethnic inclusion in the reviewed articles, it is plausible that this pattern is consistent with the systemic racism and implicit bias that surrounds the disparities in prescriptions of opioids to marginalized groups (e.g., through racist media portrayal and physician bias; Anderson et al., 2009; Santoro & Santoro, 2018; Tait & Chibnall, 2014). Research has further indicated that Black, compared to White, individuals are significantly less likely to be prescribed an opioid for medical purposes or must present with higher pain levels before being given a prescription (Haq et al., 2021; Johnson-Jennings et al., 2020). While it is possible that more diverse individuals were included in the studies reviewed, the authors of included articles did not indicate clearly whether certain groups are well represented in their clinical trials as made evident in their manuscripts. Therefore, prescribers seeking information about efficacy of a certain medication for OUD would be unable to determine whether specific medications would be useful for specific patients that they were seeing and likely would not have the time to contact authors to determine whether the sample included populations similar to their patient. Similarly, patients looking to access more information about treatment options they are considering would not be able to tell whether individuals whose identities are similar to their own were represented in clinical trials testing the different types of medications. As suggested by others, to address such problems, researchers should strive to carefully collect demographic information and report findings, and journal reviewers and editors should require that the articles they publish include full descriptive information of the relevant demographics of their samples (Schick et al., 2020).
Furthermore, an important weakness in the reviewed literature is that article’s participants were, on average, 36.2 years old; this finding is notable given that OUDs are most prevalent among young adults between ages 18–25 years old (SAMHSA, 2019). With the exception of three trials looking at youth/young adults, the average ages for all articles were between 30 and 47 years old, likely representing an older population than those for whom OUD prevalence rates are especially high. Moreover, the finding that only 16.0% of articles conducted subgroup analyses by any demographic factor and 10.0% indicated that a lack of subgroup analyses or diverse samples was a limitation to their articles is problematic given that there have been multiple calls for more inclusive research (Clark et al., 2019; Gatzke-Kopp, 2016). Given that efficacy may be different among diverse demographic groups (Crist et al., 2013; Pro et al., 2020), it is important that research samples are representative of the diversity that exists in the population most adversely effected by OUDs. It is also noteworthy that many samples were small in size and the average sample size was only 148 participants; therefore, many articles may have been underpowered for subgroup analyses. It may also be that in articles with large samples, authors conducted subgroup analyses by demographic group but found null results and may have decided not to pursue publication, given that nonsignificant results are less likely to be accepted for publication (Dwan et al., 2008).
In terms of articles for specific medications for OUD, there are a few notable differences by medication. First, there were meaningful differences in racial composition by medication type. For example, Black participants made up 49.6% of the samples for the methadone articles, 16.0% for buprenorphine articles, and 61.4% for naltrexone articles. While there is no evidence to suggest how or why samples are chosen for these articles, it is worth noting that buprenorphine has been found to potentially be safer than methadone because it is less susceptible to misuse and overdose (Johnson et al., 1992; Ling et al., 1994), and it is often more accessible than methadone because of its ability to be prescribed with monthly (versus daily) visits (SAMHSA, 2020). By contrast, naltrexone has low adherence rates and is not preferred due to its negative side effects (e.g., dysphoria and severe withdrawl symptoms; Rothenberg et al., 2002). This discrepancy in racial enrollment is problematic given that Black individuals are underrepresented in the medication for OUD with the most RCTs and that is thought to be the safest and more accessible with relatively good adherence (buprenorphine). At the same time, Black individuals are well represented in RCTs for medications with worse adherence (naltrexone) or potentially less safety and accessibility (methadone). This finding is in contrast to young adult and adolescent trials, which were all conducted with buprenorphine. These findings may suggest that there is bias in researcher’s sample selection process or in grants that are being funded for Black individuals who are best represented in trials with a less-preferred medication for OUD (i.e., naltrexone). Overall, it is abundantly evident that more equitable diversity representation is necessary across medications for OUD in RCTs.
Limitations and Future Directions
While the present study provides important information in regard to diverse demographic inclusion in RCTs for pharmacological treatment of OUDs, the results should be considered in the context of their limitations. First, articles included in the present study were limited to those within the U.S. and written in English, which may have omitted articles conducted elsewhere that were more inclusive of particular demographic groups. Second, although the present study covered the medications for OUD currently approved by the FDA, there may be other medications that could be used for OUD treatment that are not included in the present study. Third, while not a limitation of the present review but rather a limitation of the literature and scientific publishing practices, articles that include more diverse groups may not have been accepted for publication, especially if they had small sample sizes. Fourth, it is possible that authors of articles that contained more diverse samples did not perceive the need to report on issues related to inclusion and diversity as a limitation to their study. Thus, the 90.0% percent in the present review that did not include a lack of diverse representation in their limitations may be inflated, although there were few articles that reportedly had diverse representation across all demographics. Finally, articles reviewed herein were located through only one database (PubMed), and while this database should contain all NIH-funded research, it is possible that our review missed some relevant literature; future reviews should also search ClinicalTrials.gov and other relevant databases.
Future research should examine ways to enhance motivation or create more guidelines for necessary inclusion of diverse samples. It may be necessary and important to over-sample for groups not well represented in current research. Journal reviewers and editors should also be encouraged to consider the representation of articles they publish and request diverse samples. Furthermore, grant reviewers (especially for NIH funded research) should ensure proposed articles include samples representative of OUD prevalence (and perhaps focus on groups disproportionately affected by related health disparities). It may be that diverse sample are difficult to obtain due to a mistrust by groups historically treated unethically in research (Harris et al., 1996; Pacheco et al., 2013; Schick et al., 2020). Moreover, as Schick at colleagues (2020) suggest, the lack of diverse representation in RCTs may reflect the lack of diverse researchers and individuals holding graduate degrees; therefore, investing resources to support the careers of researchers from marginalized backgrounds is warranted as it may increase representative research. While programs such as certain T-32 NRSA Diversity Supplement Awards, the LEADership, Education and Development (LEAD) program, the McNair Scholars program, and others target these aims, there is still a lack of diverse representation in science and higher education, suggesting the need to continue with such efforts. As the present study attests, there are meaningful gaps in the existing literature on OUD pharmacological treatment, and these steps are important and necessary to ensure diverse groups are represented in RCTs. For instance, despite high prevalence rates of opioid-related overdoses in rural communities (Paulozzi & Xi, 2008), none of the included articles specifically noted or focused on studying populations in rural communities. Therefore, future studies should aim to recruit rural samples, report on community and neighborhood information, and compare the efficacy of medications for OUD in rural versus urban samples. Including such variables will be helpful to contextualize the findings.
Finally, a notable finding, while not a focal point of the present study, was that over half of the naltrexone articles (n = 5) were specifically conducted with populations involved with the criminal justice system, while only one of the methadone articles and none of the buprenorphine articles were conducted specifically with people involved in the criminal justice system. As we noted previously with respect to Black individuals, because naltrexone is a less preferred medication for the treatment of OUDs, this may represent bias in sample selection. It is worth examining in future studies the extent to which, and how equitably, people involved in the criminal justice system are included in pharmacological trials for OUD.
Conclusions
The present study reviewed the inclusion of diverse demographic groups among 50 RCTs examining the efficacy of methadone, buprenorphine, and naltrexone for OUDs. Results demonstrated that some aspects of sex/gender and racial/ethnic information was typically provided in RCTs and people of low SES were generally well represented; however, several groups were not included (e.g., NH/OPI or multiracial individuals) or minimally included (AI/AN or Asian individuals). Furthermore, certain groups (e.g., Black individuals) were better represented in methadone and naltrexone articles than they were in buprenorphine articles. It is important that all people receive efficacious pharmacological care for OUDs in the midst of the current opioid epidemic, particularly participants from diverse or marginalized backgrounds who may already be at increased risk for disparities related to OUDs.
Public Significance Statement:
Opioid use disorders are prevalent in the U.S. and disproportionality affect people from marginalized backgrounds; yet, the present systematic review finds that most trials of pharmacological treatment are conducted with majority White and male participants. Participants from diverse or marginalized backgrounds that may be at increased risk for disparities related to OUDs are currently underrepresented in treatment trials
Acknowledgements:
The authors thank Aayma Jamil, Katie Hostetler, Ben Seebold, and Shannon McKee for their assistance in managing the data and coding abstracts.
Funding:
This work was supported by the National Center for Research Resources under Grant G20RR030883.
Footnotes
Declaration of Interest: The authors report no conflict of interest.
References
- American Psychiatric Association (APA). (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- American Psychiatric Association (APA). (2018). Opioid use disorder. https://www.psychiatry.org/patients-families/addiction/opioid-use-disorder/opioid-use-disorder
- Anderson KO, Green CR, & Payne R (2009). Racial and ethnic disparities in pain: causes and consequences of unequal care. The Journal of Pain, 10(12), 1187–1204. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, & Ling W (2011). Comparative profiles of men and women with opioid dependence: Results from a national multisite effectiveness trial. American Journal of Drug and Alcohol Abuse, 37(5), 313–323. 10.3109/00952990.2011.596982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Leiker C, McPherson S, Layton ME, Burduli E, Roll JM, & Ling W (2018). Sex differences in opioid use and medical issues during buprenorphine/naloxone treatment. American Journal of Drug and Alcohol Abuse, 44(4), 488–496. 10.1080/00952990.2018.1458234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, & Roland CL (2011). Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Medicine, 12(4), 657–667. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Mannelli P, Yu M, Nangia N, Graham CE, Tompkins DA, Kosten TR, Akerman SC, Silverman BL, & Sullivan MA (2018). Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: A phase 3 randomized trial. Drug and Alcohol Dependence, 187, 171–178. 10.1016/j.drugalcdep.2018.02.023 [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, & Amate JM (2004). Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction, 99(7), 811–828. 10.1111/j.1360-0443.2004.00763.x [DOI] [PubMed] [Google Scholar]
- Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, Jamerson B, McCullough C, Pierre C, & Polis AB (2019). Increasing diversity in clinical trials: overcoming critical barriers. Current Problems in Cardiology, 44(5), 148–172. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Crist RC, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, Ling W, Hillhouse MP, Bruce RD, Woody G, & Berrettini WH (2014). Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J, 14(3), 303–308. 10.1038/tpj.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ED, Kleber HD, Whittington RA, & Heitler NE (2005). Anesthesia-assisted vs buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: A randomized trial. JAMA, 294(8), 903–913. 10.1001/jama.294.8.903 [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, & O’Brien CP (2006). Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Archives Of General Psychiatry, 63(2), 210–218. 10.1001/archpsyc.63.2.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello DM, Cornish JW, Lynch KG, Alterman AI, & O’Brien CP (2010). A randomized trial of oral naltrexone for treating opioid-dependent offenders. American Journal on Addictions, 19(5), 422–432. 10.1111/j.1521-0391.2010.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw KW (2017). On intersectionality: Essential writings. The New Press. [Google Scholar]
- Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, Ling W, Hillhouse MP, Bruce RD, Woody G, & Berrettini WH (2013). An intronic variant in OPRD1 predicts treatment outcome for opioid dependence in African-Americans. Neuropsychopharmacology, 38(10), 2003–2010. 10.1038/npp.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman PA, Liebschutz JM, Anderson BJ, Moreau MR, & Stein MD (2016). Buprenorphine initiation and linkage to outpatient buprenorphine do not reduce frequency of injection opiate use following hospitalization. Journal of Substance Abuse Treatment, 68, 68–73. 10.1016/j.jsat.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, & Fiellin DA (2015). Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: A randomized clinical trial. JAMA, 313(16), 1636–1644. 10.1001/jama.2015.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health, Education, and Welfare (DHEW). (1979). The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. [PubMed]
- Drug Enforcement Administration (DEA). (2017). DEA releases 2017 national drug threat assessment https://www.dea.gov/press-releases/2017/10/23/dea-releases-2017-national-drug-threat-assessment
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, Decullier E, Easterbrook PJ, Von Elm E, & Gamble C 2008. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS One, 3(8), e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Yoo C, Huang D, Saxon AJ, & Hser YI (2019). Effects of access barriers and medication acceptability on buprenorphine-naloxone treatment utilization over 2 years: Results from a multisite randomized trial of adults with opioid use disorder. Journal of Substance Abuse Treatment, 106, 19–28. 10.1016/j.jsat.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, O’Connor PG, Chawarski M, Pakes JP, Pantalon MV, & Schottenfeld RS (2001). Methadone maintenance in primary care: A randomized controlled trial. JAMA, 286(14), 1724–1731. 10.1001/jama.286.14.1724 [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, & Schottenfeld RS (2006). Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. New England Journal of Medicine, 355(4), 365–374. 10.1056/NEJMoa055255 [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, & O’Connor PG (2014). Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: A randomized clinical trial. JAMA Internal Medicine, 174(12), 1947–1954. 10.1001/jamainternmed.2014.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). (2019). Information about medication-assisted treatment (MAT). https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat
- Friedmann PD, Wilson D, Hoskinson R Jr., Poshkus M, & Clarke JG (2018a). Initiation of extended release naltrexone (XR-NTX) for opioid use disorder prior to release from prison. Journal of Substance Abuse Treatment, 85, 45–48. 10.1016/j.jsat.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Wilson D, Nunes EV, Hoskinson R Jr., Lee JD, Gordon M, Murphy SM, Bonnie RJ, Chen DT, Boney TY, & O’Brien CP (2018b). Do patient characteristics moderate the effect of extended-release naltrexone (XR-NTX) for opioid use disorder? Journal of Substance Abuse Treatment, 85, 61–65. 10.1016/j.jsat.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp LM (2016). Diversity and representation: Key issues for psychophysiological science. Psychophysiology, 53(1), 3–13. 10.1111/psyp.12566 [DOI] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, & Batki SL (2008). A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug and Alcohol Dependence, 94(1–3), 199–206. 10.1016/j.drugalcdep.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson EW, Wang XQ, Fiellin DA, Bryan B, & Levin FR (2010). Unobserved versus observed office buprenorphine/naloxone induction: A pilot randomized clinical trial. Addictive Behaviors, 35(5), 537–540. 10.1016/j.addbeh.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, Greenwald MK, Nadipelli VR, Ling W, & Heidbreder C (2019). Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 393(10173), 778–790. 10.1016/s0140-6736(18)32259-1 [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, & Jones CM (2017). Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine, 167(5), 293–301. 10.7326/m17-0865 [DOI] [PubMed] [Google Scholar]
- Harris Y, Gorelick PB, Samuels P, & Bempong I (1996). Why African Americans may not be participating in clinical trials. Journal of the National Medical Association, 88, 630–634. [PMC free article] [PubMed] [Google Scholar]
- Haq N, McMahan VM, Torres A, Santos GM, Knight K, Kushel M, & Coffin PO (2021). Race, pain, and opioids among patients with chronic pain in a safety-net health system. Drug and Alcohol Dependence, 222, 108671. 10.1016/j.drugalcdep.2021.108671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, & Ling W (2013). Predictors of outcome after short-term stabilization with buprenorphine. Journal of Substance Abuse Treatment, 44(3), 336–342. 10.1016/j.jsat.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JA, Wu J, Martus W, & Pierre JD (2005). A randomized trial of one-day vs. three-day buprenorphine inpatient detoxification protocols for heroin dependence. Journal of Opioid Management, 1(1), 31–35. [DOI] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, DeFulio A, Koffarnus MN, Leoutsakos JS, Umbricht A, Fingerhood M, Bigelow GE, & Silverman K (2019). The effects of extended-release injectable naltrexone and incentives for opiate abstinence in heroin-dependent adults in a model therapeutic workplace: A randomized trial. Drug and Alcohol Dependence, 197, 220–227. 10.1016/j.drugalcdep.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, & Silverman K (2018). Extended-release injectable naltrexone for opioid use disorder: A systematic review. Addiction, 113(7), 1188–1209. 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Jennings M, Duran B, Hakes J, Paffrath A, & Little MM (2020). The influence of undertreated chronic pain in a national survey: Prescription medication misuse among American indians, Asian Pacific Islanders, Blacks, Hispanics and whites. SSM - Population Health, 11, 100563. 10.1016/j.ssmph.2020.100563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, & Bigelow GE (2000). A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New England Journal of Medicine, 343(18), 1290–1297. 10.1056/nejm200011023431802 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, & Bigelow GE (1995a). Buprenorphine treatment of opioid dependence: Clinical trial of daily versus alternate-day dosing. Drug and Alcohol Dependence, 40(1), 27–35. 10.1016/0376-8716(95)01189-7 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, & Bigelow GE (1995b). A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence, 40(1), 17–25. 10.1016/0376-8716(95)01186-2 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, & Fudala PJ (1992). A controlled trial of buprenorphine treatment for opioid dependence. JAMA, 267(20), 2750–2755. [PubMed] [Google Scholar]
- Khansari M, Sohrabi M, & Zamani F (2013). The useage of opioids and their adverse effects in gastrointestinal practice: A review. Middle East Journal of Digestive Diseases, 5(1), 5–16. https://pubmed.ncbi.nlm.nih.gov/24829664 [PMC free article] [PubMed] [Google Scholar]
- King NB, Fraser V, Boikos C, Richardson R, & Harper S (2014). Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: A systematic review. American Journal of Public Health, 104(8), e32–e42. 10.2105/AJPH.2014.301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Schottenfeld R, Ziedonis D, & Falcioni J (1993). Buprenorphine versus methadone maintenance for opioid dependence. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Kreps GL (2006). Communication and racial inequities in health care. American Behavioral Scientist, 49(6), 760–774. 10.1177/0002764205283800 [DOI] [Google Scholar]
- Lagisetty PA, Ross R, Bohnert A, Clay M, & Maust DT (2019). Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry, 76(9), 979–981. 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham G, Boudreau DM, Johnson EA, Bobb JF, Matthews AG, McCormack J, Liu D, Samet JH, Saxon AJ, Campbell CI, Glass JE, Rossom RC, Murphy MT, Binswanger IA, Yarborough BJH, & Bradley KA (2020). Prevalence and treatment of opioid use disorders among primary care patients in six health systems. Drug and Alcohol Dependence, 207, 107732. 10.1016/j.drugalcdep.2019.107732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA Jr., Wilson D, McDonald R, Rotrosen J, Gourevitch MN, Gordon M, Fishman M, Chen DT, Bonnie RJ, Cornish JW, Murphy SM, & O’Brien CP (2016). Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. New England Journal of Medicine, 374(13), 1232–1242. 10.1056/NEJMoa1505409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, & Gourevitch MN (2015). Opioid treatment at release from jail using extended-release naltrexone: A pilot proof-of-concept randomized effectiveness trial. Addiction, 110(6), 1008–1014. 10.1111/add.12894 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, & Rotrosen J (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet, 391(10118), 309–318. 10.1016/s0140-6736(17)32812-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, & Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine, 151(4), W-65–W-94. [DOI] [PubMed] [Google Scholar]
- Ling S, Mangaoil R, Cleverley K, Sproule B, & Puts M (2019). A systematic review of sex differences in treatment outcomes among people with opioid use disorder receiving buprenorphine maintenance versus other treatment conditions. Drug and Alcohol Dependence, 197, 168–182. 10.1016/j.drugalcdep.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey GL, Rosenthal RN, & Beebe KL (2010). Buprenorphine implants for treatment of opioid dependence: A randomized controlled trial. JAMA, 304(14), 1576–1583. 10.1001/jama.2010.1427 [DOI] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr., Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA Jr., Santos E, Casadonte P, Fye C, Stine S, Wang RI, & Segal D (1998). Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction, 93(4), 475–486. 10.1046/j.1360-0443.1998.9344753.x [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, Jenkins J, Hasson A, Annon J, Saxon A, Selzer J, Boverman J, & Bilangi R (2009). Buprenorphine tapering schedule and illicit opioid use. Addiction, 104(2), 256–265. 10.1111/j.1360-0443.2008.02455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Rawson RA, & Compton MA (1994). Substitution pharmacotherapies for opioid addiction: From methadone to LAAM and buprenorphine. Journal of Psychoactive Drugs, 26(2), 119–128. 10.1080/02791072.1994.10472259 [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, & Klett CJ (1996). A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Archives Of General Psychiatry, 53(5), 401–407. 10.1001/archpsyc.1996.01830050035005 [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Nunes EV, Bailey GL, Sigmon SC, Kampman KM, Frost M, Tiberg F, Linden M, Sheldon B, Oosman S, Peterson S, Chen M, & Kim S (2018). Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: A randomized clinical trial. JAMA Internal Medicine, 178(6), 764–773. 10.1001/jamainternmed.2018.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, Keruly JC, Fiellin DA, Finkelstein R, Barditch-Crovo P, Cook K, & Moore RD (2010). Clinic-based treatment of opioid-dependent hiv-infected patients versus referral to an opioid treatment program: A randomized trial. Annals of Internal Medicine, 152(11), 704–711. 10.7326/0003-4819-152-11-201006010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindl K, Gottheil E, Wu LT, & Gorelick DA (2009). Early outcomes following low dose naltrexone enhancement of opioid detoxification. American Journal on Addictions, 18(2), 109–116. 10.1080/10550490902772785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, & Jacobs EA (2005a). Buprenorphine treatment for opioid dependence: The relative efficacy of daily, twice and thrice weekly dosing. Drug and Alcohol Dependence, 77(2), 195–204. 10.1016/j.drugalcdep.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, & Brooklyn J (2005b). Comparison of pharmacological treatments for opioid-dependent adolescents: A randomized controlled trial. Archives Of General Psychiatry, 62(10), 1157–1164. 10.1001/archpsyc.62.10.1157 [DOI] [PubMed] [Google Scholar]
- Marsch LA, Moore SK, Borodovsky JT, Solhkhah R, Badger GJ, Semino S, Jarrett K, Condon KD, Rossettie K, Vincent P, Hajizadeh N, & Ducat E (2016). A randomized controlled trial of buprenorphine taper duration among opioid-dependent adolescents and young adults. Addiction, 111(8), 1406–1415. 10.1111/add.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson CL, Barnett PG, Sees KL, Delucchi KL, Rosen A, Wong W, & Hall SM (2004). Cost and cost-effectiveness of standard methadone maintenance treatment compared to enriched 180-day methadone detoxification. Addiction, 99(6), 718–726. 10.1111/j.1360-0443.2004.00728.x [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, & Kissack JC (2008). Urine drug screening: practical guide for clinicians. Mayo Clinic Proceedings, 83(1), 66–76. 10.4065/83.1.66 [DOI] [PubMed] [Google Scholar]
- Nalven T, Spillane NS, & Schick MR (2020). Risk and protective factors for opioid misuse in American Indian adolescents. Drug and Alcohol Dependence, 206, 107736. 10.1016/j.drugalcdep.2019.107736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco CM, Daley SM, Brown T, Filippi M, Greiner KA, & Daley CM (2013). Moving forward: Breaking the cycle of mistrust between American Indians and researchers. American Journal of Public Health, 103(12), 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Le A, Guarino H, & Mateu-Gelabert P (2019). A comparison of the utility of urine- and hair testing in detecting self-reported drug use among young adult opioid users. Drug and Alcohol Dependence, 200, 161–167. https://doi-org.uri.idm.oclc.org/10.1016/j.drugalcdep.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parran TV, Adelman CA, Merkin B, Pagano ME, Defranco R, Ionescu RA, & Mace AG (2010). Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug and Alcohol Dependence, 106(1), 56–60. 10.1016/j.drugalcdep.2009.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, & Xi Y (2008). Recent changes in drug poisoning mortality in the United States by urban–rural status and by drug type. Pharmacoepidemiology and Drug Safety, 17(10), 997–1005. [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Kertesz SG, Kohn MA, & Gonzales R (2008). Trends in opioid prescribing by race/ethnicity for patients seeking care in us emergency departments. Journal of the American Medical Association, 299(1), 70–78. 10.1001/jama.2007.64 [DOI] [PubMed] [Google Scholar]
- Potter JS, Dreifuss JA, Marino EN, Provost SE, Dodd DR, Rice LS, Fitzmaurice GM, Griffin ML, & Weiss RD (2015). The multi-site prescription opioid addiction treatment study: 18-month outcomes. Journal of Substance Abuse Treatment, 48(1), 62–69. 10.1016/j.jsat.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro G, Utter J, Cram J, & Baldwin J (2020). Racial/ethnic and gender differences in associations of medication-assisted therapy and reduced opioid use between outpatient treatment admission and discharge. Journal of Psychoactive Drugs. 10.1080/02791072.2020.1717685 [DOI] [PubMed] [Google Scholar]
- Rosenthal RN, Ling W, Casadonte P, Vocci F, Bailey GL, Kampman K, Patkar A, Chavoustie S, Blasey C, Sigmon S, & Beebe KL (2013). Buprenorphine implants for treatment of opioid dependence: Randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction, 108(12), 2141–2149. 10.1111/add.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, & Vocci FJ (2016). Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: A randomized clinical trial. JAMA, 316(3), 282–290. 10.1001/jama.2016.9382 [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, & Nunes EV (2002). Behavioral naltrexone therapy: An integrated treatment for opiate dependence. Journal of Substance Abuse Treatment, 23(4), 351–360. 10.1016/s0740-5472(02)00301-x [DOI] [PubMed] [Google Scholar]
- Salsitz E, & Wiegand T (2016). Pharmacotherapy of opioid addiction: “Putting a real face on a false demon”. Journal of Medical Toxicology, 12(1), 58–63. 10.1007/s13181-015-0517-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Botticelli M, & Bharel M (2018). Methadone in Primary Care — One Small Step for Congress, One Giant Leap for Addiction Treatment. New England Journal of Medicine, 379(1), 7–8. 10.1056/NEJMp1803982 [DOI] [PubMed] [Google Scholar]
- Santoro TN, & Santoro JD (2018). Racial Bias in the US Opioid Epidemic: A Review of the History of Systemic Bias and Implications for Care. Cureus, 10(12), e3733. 10.7759/cureus.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick MR, Spillane NS, & Hostetler KL (2020). A call to action: A systematic review examining the failure to include females and members of minoritized racial/ethnic groups in clinical trials of pharmacological treatments for alcohol use disorder. Alcoholism: Clinical and Experimental Research, 40(10), 1933–1951. 10.1111/acer.14440 [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, & Kosten TR (2005). Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. American Journal of Psychiatry, 162(2), 340–349. 10.1176/appi.ajp.162.2.340 [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, & Kosten TR (1997). Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Archives Of General Psychiatry, 54(8), 713–720. 10.1001/archpsyc.1997.01830200041006 [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Gandhi D, Olsen Y, & Jaffe JH (2017). Patient-centered methadone treatment: A randomized clinical trial. Addiction, 112(3), 454–464. 10.1111/add.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, Mitchell SG, O’Grady KE, Sharma A, & Jaffe JH (2020). Methadone treatment of arrestees: A randomized clinical trial. Drug and Alcohol Dependence, 206, 107680. 10.1016/j.drugalcdep.2019.107680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Banys P, & Hall SM (2000). Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. JAMA, 283(10), 1303–1310. 10.1001/jama.283.10.1303 [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Ochalek TA, Meyer AC, Hruska B, Heil SH, Badger GJ, Rose G, Brooklyn JR, Schwartz RP, Moore BA, & Higgins ST (2016). Interim buprenorphine vs. waiting list for opioid dependence. New England Journal of Medicine, 375(25), 2504–2505. 10.1056/NEJMc1610047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Kim IE, Girmay M, Perry C, Daus GP, Vedamuthu IP, De Los Reyes AA, Ramey CT, Martin EK, & Allender M (2019). Opioid epidemic in the United States: Empirical trends, and a literature review of social determinants and epidemiological, pain management, and treatment patterns. International Journal of Maternal and Child Health and AIDS, 8(2), 89–100. 10.21106/ijma.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, & Pacula RL (2018). A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Substance Abuse, 39(4), 419–425. 10.1080/08897077.2018.1449166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Herman D, Conti M, Anderson B, & Bailey G (2020). Initiating buprenorphine treatment for opioid use disorder during short-term in-patient ‘detoxification’: A randomized clinical trial. Addiction, 115(1), 82–94. 10.1111/add.14737 [DOI] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, & Stitzer ML (1999). Moderate- vs high-dose methadone in the treatment of opioid dependence: A randomized trial. JAMA, 281(11), 1000–1005. 10.1001/jama.281.11.1000 [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, & Bigelow GE (1993). Methadone dose and treatment outcome. Drug and Alcohol Dependence, 33(2), 105–117. 10.1016/0376-8716(93)90052-r [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, & Bigelow GE (1994). Comparison of buprenorphine and methadone in the treatment of opioid dependence. American Journal of Psychiatry, 151(7), 1025–1030. 10.1176/ajp.151.7.1025 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020). Medication-Assisted Treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment [PubMed]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2019). Results from the 2019 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables
- Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD, & Nunes EV (2013). Naltrexone treatment for opioid dependence: Does its effectiveness depend on testing the blockade? Drug and Alcohol Dependence, 133(1), 80–85. 10.1016/j.drugalcdep.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RC, & Chibnall JT (2014). Racial/ethnic disparities in the assessment and treatment of pain: Psychosocial perspectives. American Psychologist, 69(2), 131–141. 10.1037/a0035204 [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services (HHS). (1994). NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Federal Register, 59(14508), 1994–14513. [Google Scholar]
- United States Department of Health and Human Services (HHS). (2006). NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research: Amended, October, 2001. National Institutes of Health. [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, & Cha SS (2014). Medication-assisted therapies — tackling the opioid-overdose epidemic. New England Journal of Medicine, 370(22), 2063–2066. 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- Weinstein ZM, Kim HW, Cheng DM, Quinn E, Hui D, Labelle CT, Drainoni M-L, Bachman SS, & Samet JH (2017). Long-term retention in Office Based Opioid Treatment with buprenorphine. Journal of Substance Abuse Treatment, 74, 65–70. 10.1016/j.jsat.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PJ, & Remski K (2012). Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. Journal of Neurosciences in Rural Practice, 3(1), 45–50. 10.4103/0976-3147.91934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (2012). Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior, 53(3), 279–295. 10.1177/0022146512455804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner-Reid J, Belendiuk KA, Epstein DH, Schmittner J, & Preston KL (2008). Hepatitis C and Human Immunodeficiency Virus risk behaviors in polydrug users on methadone maintenance. Journal of Substance Abuse Treatment, 35(1), 78–86. 10.1016/j.jsat.2007.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman TNA, Chang VW, & Binswanger IA (2018). Health, polysubstance use, and criminal justice involvement among adults with varying levels of opioid use. JAMA Network Open, 1(3), e180558–e180558. 10.1001/jamanetworkopen.2018.0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, Patkar A, Publicker M, McCain K, Potter JS, Forman R, Vetter V, McNicholas L, Blaine J, Lynch KG, & Fudala P (2008). Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: A randomized trial. JAMA, 300(17), 2003–2011. 10.1001/jama.2008.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Zhu H, & Swartz MS (2016). Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence, 169, 117–127. 10.1016/j.drugalcdep.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


