Abstract
Urinary mutagenicity reflects systemic exposure to complex mixtures of genotoxic/carcinogenic agents and is linked to tumor development. Coal combustion emissions (CCE) and diesel engine exhaust (DEE) are associated with cancers of the lung and other sites, but their influence on urinary mutagenicity is unclear. We investigated associations between exposure to CCE or DEE and urinary mutagenicity. In two separate cross-sectional studies of non-smokers, organic extracts of urine were evaluated for mutagenicity levels using strain YG1041 in the Salmonella (Ames) mutagenicity assay. First, we compared levels among 10 female bituminous (smoky) coal users from Laibin, Xuanwei, China, and 10 female anthracite (smokeless) coal users. We estimated exposure-response relationships using indoor air concentrations of two carcinogens in CCE relevant to lung cancer, 5-methylchrysene (5MC) and benzo[a]pyrene (B[a]P). Second, we compared levels among 20 highly exposed male diesel factory workers and 15 unexposed male controls; we evaluated exposure-response relationships using elemental carbon (EC) as a DEE-surrogate. Age-adjusted linear regression was used to estimate associations. Laibin smoky coal users had significantly higher average urinary mutagenicity levels compared to smokeless coal users (28.4±14.0 SD vs. 0.9±2.8 SD rev/ml-eq, p=2 x 10−5) and a significant exposure-response relationship with 5MC (p=7 x 10−4). DEE-exposed workers had significantly higher urinary mutagenicity levels compared to unexposed controls (13.0±10.1 SD vs. 5.6±4.4 SD rev/ml-eq, p=0.02) and a significant exposure-response relationship with EC (p-trend=2 x 10−3). Exposure to CCE and DEE is associated with urinary mutagenicity, suggesting systemic exposure to mutagens, potentially contributing to cancer risk and development at various sites.
Keywords: Complex mixtures, coal combustion, smoky coal, diesel exhaust, Salmonella mutagenicity, urinary genotoxicity biomarkers
INTRODUCTION
Indoor coal combustion emissions (CCE) and diesel engine exhaust (DEE) are known human carcinogens based largely on findings from epidemiologic and mechanistic studies of lung cancer (IARC 1989, 2012, 2014; Silverman 2018). These environmental exposures are among the most prominent non-smoking related risk factors for lung cancer (Samet et al., 2009; IARC 2014) and contribute to air pollution-associated lung carcinogenesis (IARC 2016). Furthermore, there is evidence that exposure to CCE and DEE may be related to risk of tumor development at other sites besides the lung (Sharpe et al., 1989; Kim et al., 2016; Koutros et al., 2020; Sapkota et al., 2008).
With respect to indoor CCE, we have shown that female lifetime users of bituminous (smoky) coal had substantially elevated lung cancer mortality compared to anthracite (smokeless) coal users in a cohort study conducted in the Xuanwei region of China (Barone-Adesi et al., 2012), which was further supported by evidence from parallel case-control studies (Lan et al., 2008; Kim et al., 2014; Seow et al., 2014; Vermeulen et al., 2019; Wong et al., 2019). The smokeless coal users were largely from the southwestern region of Xuanwei near Reshui. Additionally, we found that exposure to a specific type of smoky coal sourced from geologic deposits near Laibin township of Xuanwei had a particularly strong relationship with lung cancer risk (Lan et al., 2008; Wong et al., 2019). Much of the evidence for the carcinogenicity of DEE was derived from the Diesel Exhaust in Miners Study and a study of trucking industry workers, which found significant increasing trends in lung cancer risk with increasing cumulative DEE exposure (Garshick et al., 2012; Silverman et al., 2012; Silverman 2017, 2018) and average intensity (Silverman et al., 2012; Silverman 2017, 2018).
Polycyclic aromatic hydrocarbons (PAHs) and their methyl-derivatives play an important role in CCE-associated lung cancer (IARC 2012; Vermeulen et al., 2019). Nitroarenes (nitro-PAHs) and aromatic amines appear to contribute substantially to the carcinogenicity and mutagenicity of DEE (IARC 1989, 2014). Studies conducted in Xuanwei found that residents were highly exposed to PAHs at near occupational levels in their homes from domestic coal combustion (Mumford et al., 1995; Downward et al., 2014). In a subsequent epidemiologic study, we found strong associations between a cluster of 25 PAHs and lung cancer risk in this region (Vermeulen et al., 2019). Among these PAHs, a particularly carcinogenic methyl-derivative, 5-methylchrysene (5MC) (Hecht et al., 1974), was associated with the highest risk (Vermeulen et al., 2019).
There is evidence supporting the role of PAHs in CCE-induced lung cancer. For example, we have found that this malignancy is linked to the nucleotide excision repair (NER) pathway (Shen et al., 2005), which repairs PAH-induced DNA damage; the aldo-keto reductase (AKR) pathway, which activates PAH dihydrodiols to form redox-active o-quinones (Lan et al., 2004); and the base-excision repair pathway, which plays a role in the generation or repair of oxidative damage (Lan et al., 2004). Additional supporting evidence comes from mutation spectra studies of lung tumors from women exposed to smoky coal emissions, notably from the patterns of TP53 mutations (DeMarini et al., 2001; Granville et al., 2003; Keohavong et al., 2005).
Urinary mutagenicity is an integrated, overall measure that reflects systemic exposure to complex mixtures of genotoxic/carcinogenic agents. Urinary mutagenicity has been linked to colorectal adenoma risk (Peters et al., 2003) and correlates with biomarkers among those exposed to a variety of pollutants, including cigarette smoke, benzidine dyes, heterocyclic amines, nitrotoluenes, and woodsmoke (Yamasaki and Ames 1977; De Meo et al., 1987; Clonfero et al., 1995; Cerná et al., 1997; DeMarini et al., 1997; Kato et al., 2004; Sabbioni et al., 2006; Shaughnessy et al., 2011; Long et al., 2014; Keir et al., 2017; Adetona et al., 2019; Wu et al., 2021).
The reported associations between exposure to coal products, DEE, and urinary mutagenicity have been inconsistent, which could be due to low exposure levels and/or limited exposure variation. Although Mielzynska and Snit (1992) found an association with coal, other studies did not (Moller and Dybing 1980; Recio et al., 1984). We have found high concentrations of the PAH metabolite 9-hydroxybenzo[a]pyrene, a known mutagen (Lubet et al., 1979), in urine samples collected from smoky coal-exposed subjects (Mumford et al., 1995); however, these samples were not assessed for mutagenicity. Furthermore, although DEE is highly mutagenic (IARC 2014), two studies of individuals occupationally exposed to DEE did not detect relationships with urinary mutagenicity (Williems et al., 1989; Schenker et al., 1992).
To explore further, we performed two separate air monitoring studies for relevant pollutants and determined urinary mutagenicity levels among non-smokers with high indoor exposure to either CCE or DEE. One study was conducted among women from the general population who domestically burned either smokeless coal or a particularly carcinogenic form of smoky coal sourced from geologic deposits near Laibin township of Xuanwei, China (Laibin smoky coal). We examined exposure-response relationships with two prominent carcinogenic PAH-constituents of CCE, namely 5MC and benzo[a]pyrene (B[a]P). We measured 5MC because of a priori evidence for its association with lung cancer from our previous study conducted in Xuanwei (Vermeulen et al., 2019), and B[a]P was analyzed because it is a known human carcinogen that is used as a surrogate for other PAHs (IARC 2010a). The second study was among men who worked in a diesel truck engine-testing facility in China, where we examined an exposure-response relationship with elemental carbon (EC), a well-recognized surrogate for exposure to DEE (IARC 2014).
MATERIALS AND METHODS
Indoor coal burning study
We analyzed a subset of non-smoking women who domestically burned particularly carcinogenic Laibin smoky coal or smokeless coal from a community-based cross-sectional study conducted in Xuanwei, China, in August-September 1993. We investigated these women because we found that smoky coal sourced from geologic deposits near Laibin township were associated with a high risk of lung cancer, even greater than smoky coal from other regions of Xuanwei (Lan et al., 2008; Wong et al., 2019).
Briefly, the subjects of the parent community-based cross-sectional study were administered questionnaires and 120 participants aged 45-60 years were classified into four groups: (1) 30 women from Chengguan or Laibin who burned smoky coal in their homes without chimneys, (2) 30 women from Chengguan or Laibin who burned smoky coal with chimneys, (3) 30 women from Reshui who burned smokeless coal without chimneys, and (4) 30 women from Yilihe who used electricity. We excluded those with (a) unknown demographic, occupational, smoking, reproductive, and dietary information; (b) known occupational exposure to toxicants; (c) serious infectious diseases such as AIDS, hepatitis, or active tuberculosis; and (d) treatment with chemotherapy, radiation, or other medications that could affect PAH levels in blood or urine. In the current study, we analyzed 10 Laibin smoky coal users without chimneys who had the highest possible indoor air pollution exposure levels and 10 smokeless coal users, all of whom had urine samples with sufficient volume for the urinary mutagenicity assay. This study was conducted in accordance with the World Medical Association Declaration of Helsinki’s recommendations for human subject protection. The research protocol was approved by a United States Environmental Protection Agency Human Subjects Research Review Official.
Detailed exposure assessments have been performed in previous studies of subjects exposed to indoor CCE (Mumford et al., 1993; Mumford et al., 1995; Downward et al., 2014; Hu et al., 2014; Wong et al., 2017). Here, we collected indoor area air samples from the participants’ homes for 3 days, 24 h/day, at a flow rate of 4 L/min. There were four indoor air samplers per study site and one for quality assurance. Air filter samples were collected and placed in desiccators for 24 h, weighed, and stored in polyethylene bags. PAHs (ng/m3) from air samples were identified using gas chromatograph connected to a mass spectrometer (GC/MS) as described by Wong et al. (2017). Urine samples (~75 ml) were collected twice a day (in the morning after the subject cooked breakfast and in the evening before dinner) from the participants on the same days as the air sampling and stored at below −10°C until processing. We pooled the repeated urine samples for each subject to obtain sufficient volume for the urinary mutagenicity assay.
Diesel biomarker study
We assessed the influence of DEE exposure among non-smoking participants sampled from a previously described occupational study (Lan et al., 2015). Briefly, we recruited 54 male workers highly exposed to DEE in a truck engine-testing facility in China. These workers spent most of their shift in direct proximity to the engines being tested. The exposure assessment survey was conducted from October 2012 to March 2013 and included all workers in the testing facility. Repeated full-shift personal air samples for EC exposure were collected using a cyclone attached to the lapel near the breathing zone, with an aerodynamic cut-off of 2.5 μm and a flow rate of 3.5 L/min using quartz filters. EC was measured on the quartz filters using NIOSH Method 5040 (Birch 2002). Additionally, 55 unexposed male workers, frequency-matched to the exposed workers by age ±5 years and smoking status (never, former, current) were recruited from control workplaces (brewery, water treatment plant, meat packing facility, and an administrative facility) in the same local region of China, with work processes that did not involve exposure to DEE, other types of particulates, or any known or suspected genotoxic, hematotoxic, or immunotoxic chemicals. Overnight urine samples (~450 ml) were collected from the subjects following their work shift. For the current study, we analyzed a study sample of 20 non-smoking diesel engine testing workers and 15 non-smoking unexposed controls who provided urine samples with sufficient volume to conduct the mutagenicity assay.
The study was approved by Institutional Review Boards at the U.S. National Cancer Institute and the National Institute of Occupational Health and Poison Control, China Center for Disease Control. Participation was voluntary and all subjects provided written informed consent.
Urinary mutagenicity assay
Deconjugated urine contains both un-conjugated and formerly conjugated mutagens, resulting in mutagenic potencies ~2- to 15-fold higher than those of urines not deconjugated (Shaughnessy et al. 2011), increasing the ability to detect urinary mutagenicity. Thus, we performed the urinary mutagenicity assay as described by Kato et al. (2004) using enzymatic deconjugation. Briefly, 50 ml of urine were thawed, filtered through 0.25-μm filters, and de-conjugated enzymatically in 0.2-M (10% v/v) sodium acetate buffer (pH 5.0) (Sigma, St. Louis, MO) containing β-glucuronidase (6 U/ml urine; Cat. No. G-7017, Sigma, St. Louis, MO) and sulphatase (2 U/ml urine, Cat. No. S-9751, Sigma, St. Louis, MO) for 16 h at 37°C. The urine was then poured through a C-18 silica-gel column (Waters Corp, Milford, MA), and the organics were eluted from the column with 10 ml of methanol, which was then solvent-exchanged into dimethyl sulfoxide (DMSO) to produce an organic concentrate at 150X; concentrates were stored at 4°C until used for bioassays. Methanol/C-18 blanks were also prepared and tested for mutagenicity.
We evaluated the extracts in the Salmonella (Ames) plate-incorporation mutagenicity assay (Maron and Ames 1983) with strain YG1041, which is a frameshift strain derived from strain TA98 that over-expresses both O-acetyltransferase and nitro-reductase, enhancing the detection of mutagenic aromatic amines, nitroarenes, and PAHs (Hagiwara et al., 1993), all of which are present in CCE and DEE. The extracts were tested at 0.3, 0.6, 1.2, 3, 6, and 12 ml-equivalents (ml-eq) of urine per plate at one plate per dose. Duplicate experiments were performed for all 35 DEE samples, but for only 19 of the 20 CCE samples due to limited amounts of urine. All experiments were performed with metabolic activation (S9) mix made from Aroclor-induced, Sprague-Dawley rat-liver S9 (Moltox, Boone, NC). Plates were incubated at 37°C for 3 days, after which the colonies were counted on an automatic colony counter (ProtoCOL 3, Synbiosis, Frederick, MD). We used DMSO at 100 μl/plate as the negative control, along with 2-nitrofluorene in DMSO at 3 μg/plate in the absence of S9 and 2-aminioanthracene in DMSO at 0.5 μg/plate in the presence of S9 as positive controls.
We used Prism (GraphPad, San Diego, CA) to construct mutagenicity dose-response curves using data from either single experiments or else replicate experiments. Linear regressions over the linear portion of the dose-response curves were used to determine the mutagenic potency, which was the slope of the curve expressed as rev/ml-eq. The linear portion of the curve was defined by the initial doses that gave the highest r2-value. A dose-related response of an experimental sample that approached or exceeded a 2-fold increase in rev/plate relative to the DMSO control and that had a p-value ≤ 0.05 based on a trend test (Prism, GraphPad, San Diego, CA) was considered a mutagenic response. Samples that did not achieve these requirements were assigned mutagenic potencies of zero. The archived urines from the CCE study were collected 27 years ago and were not assessed for creatinine concentrations. In the DEE study, urinary creatinine concentrations were only available for post-shift samples, but not the overnight samples used for the mutagenicity assay. As such, direct comparisons between the CCE and DEE studies could be made based only on rev/ml-eq.
To assess reproducibility between replicate experiments, intraclass correlations (ICC) were calculated separately for the CCE and DEE studies using linear mixed models with revertant counts as the dependent variable, replicate experiment (i.e., time 1 and 2) as the independent variable, along with random intercepts for study subject and dose (ml-eq/plate). The estimated ICC for the CCE and DEE studies were 84% and 92%, respectively.
Statistical analyses
In the CCE study, unadjusted and age-adjusted linear regression models were used to assess associations between type of coal used (Laibin Smoky vs. Smokeless) and continuous urinary mutagenicity levels. We considered education as a covariate; however, its inclusion did not change the findings >10%, and a parsimonious model was chosen. Additionally, we considered exposure to secondhand smoke as a covariate, but it lacked statistical variation and was not included in the models. We also evaluated exposure-response relationships between 5MC and B[a]P from indoor area air samples and urinary mutagenicity using single pollutant models. Additional sensitivity analysis was conducted using a bi-pollutant model. Spearman correlations were estimated among the PAHs. B[a]P and 5MC were specified a priori for analysis based on previous findings (IARC 2010a; Vermeulen et al., 2019).
In the DEE study, unadjusted and age-adjusted linear regression models were used to assess associations between diesel exposure (DEE-exposed workers vs. unexposed controls) and continuous urinary mutagenicity levels (rev/ml-eq). We also analyzed exposure-response relationships using the unexposed controls and personal EC measurements from the DEE-exposed workers. EC was analyzed as: 1) geometric means (μg/m3) corrected for background levels among the unexposed measured during initial walkthrough surveys of control workplaces, 2) tertiles as specified previously (Lan et al., 2015): low (6.1–39.0 μg/m3), medium (39.1–54.5 μg/m3), and high (54.6–107.7 μg/m3), and 3) categories based on the new European Union (EU) occupational exposure limit (OEL) of 50 μg/m3: (<25 μg/m3), (25 - <50 μg/m3), and (≥50 μg/m3). We considered body mass index, work years, and packyears of past smoking as potential covariates; however, these factors were not associated with urine mutagenicity in our analyses and were not included in the final models. Additionally, we conducted sensitivity analyses restricted to subjects exposed to EC levels below the new OEL of 50 μg/m3 to determine if exposure-response trends could be detected among those with lower DEE exposure. All analyses were conducted using SAS v9.4, and a two-sided p < 0.05 was considered statistically significant.
RESULTS
Indoor coal combustion emissions
The demographic characteristics are shown in Table 1. Laibin smoky coal users were slightly more advanced in age (47.9±5.4 SD vs. 45.4±4.1 SD years) and had a higher proportion of illiteracy (80% vs. 50%) than smokeless coal users. However, there was no considerable difference in exposure to secondhand smoke.
TABLE 1.
Characteristics of subjects from the cross-sectional indoor coal burning study
| Characteristic | Laibin smoky coal users (n = 10) |
Smokeless coal users (n = 10) |
|---|---|---|
| Age, years, mean (SD) | 47.9 (5.4) | 45.4 (4.1) |
| Educationa, n, % | ||
| Illiterate | 8 (80%) | 5 (50%) |
| Elementary | 2 (20%) | 4 (40%) |
| Exposed to ≥1 person who smoked at home, n, % | 9 (90%) | 10 (100%) |
| Smoking, n, % | ||
| Current | 0 | 0 |
| Former | 0 | 0 |
| Never | 10 (100%) | 10 (100%) |
| Pack-years, mean (SD) | No data | No data |
One smokeless coal user had missing education data.
Urinary mutagenicity levels by coal use, along with categories of 5MC and B[a]P, are shown in Table 2. We found that Laibin smoky coal users were exposed to high concentrations of B[a]P (Median (IQR)=134.3 (99.7-152.4) ng/m3) and 5MC (Median (IQR)=71.3 (54.8-96.3) ng/m3) in household air, whereas smokeless coal users had relatively lower exposure to B[a]P (Median (IQR)=37.0 (28.4-46.4) ng/m3) and 5MC (Median (IQR)=4.5 (3.2-6.2) SD ng/m3). Those who domestically burned carcinogenic Laibin smoky coal had strikingly higher urinary mutagenicity compared with smokeless coal users: 28.4±14.0 SD vs. 0.9±2.8 SD rev/ml-eq; βunadjusted = 26.6 (95%CI: 18.1-37.0, p=9 x 10−6); βage-adjusted = 28.3 (95%CI: 18.3-38.4, p=2 x 10−5) (Figure 1A).
TABLE 2.
Urinary biomarkers in subjects from the cross-sectional indoor coal burning study
| Comparison Group |
Laibin smoky coal user
exposure category |
|||
|---|---|---|---|---|
| Biomarker | Smokeless coal users¥ n=10 |
Overall Laibin smoky coal users n=10 |
Low PAH subgroup 5MC <71.3 ng/m3 B[a]P <134.3 ng/m3 n=5 |
High PAH subgroup 5MC ≥71.3 ng/m3 B[a]P ≥134.3 ng/m3 n=5 |
| Mutagenicity, mean rev/ml-eq (SD) | 0.9 (2.8) ¥ | 28.4 (14.0) | ||
| Difference %, compared to smokeless coal | Reference | 3055.6 | ||
| Wilcoxon p-value | Reference | 1.7 x 10−4 | ||
| 5MC | ||||
| Urinary mutagenicity, mean rev/ml-eq (SD) | 0.9 (2.8) ¥ | 26.7 (12.3) | 30.2 (16.8) | |
| Difference %, compared to smokeless coal | Reference | 2866.7 | 3255.6 | |
| Wilcoxon p-value | Reference | 1.2 x 10−3 | 1.2 x 10−3 | |
| B[a]P | ||||
| Urinary mutagenicity, mean rev/ml-eq (SD) | 0.9 (2.8) ¥ | 24.0 (11.1) | 32.9 (16.4) | |
| Difference %, compared to smokeless coal | Reference | 2566.7 | 3555.6 | |
| Wilcoxon p-value | Reference | 1.2 x 10−3 | 1.2 x 10−3 | |
p < 0.05 was considered statistically significant.
A common reference/comparison group of smokeless coal users was used for the three separate analyses of: 1) Laibin smoky coal users, 2) 5MC concentrations, and 3) B[a]P concentrations.
Figure 1.
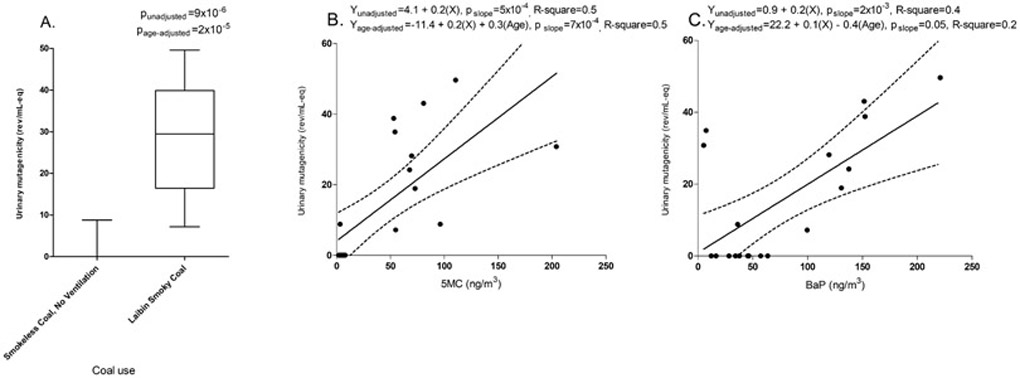
(Panel A) Urinary mutagenicity levels among female non-smokers who used particularly carcinogenic Laibin smoky coal or smokeless coal in the 1993 community-based cross-sectional study in Xuanwei, China. Differences between exposure groups were tested with unadjusted and age-adjusted linear regression models. Exposure-response relationships between urinary mutagenicity and air 5-methylchrysene (5MC) (Panel B) and benzo[a]pyrene (B[a]P) (Panel C). Associations were tested with unadjusted and age-adjusted linear regression models.
There was a significant exposure-response relationship between 5MC and urinary mutagenicity in the single pollutant model: βage-adjusted = 0.2 (95%CI: 0.1-0.3, p=7 x 10−4), along with a suggestive association with B[a]P (Figure 1A and 1B). In the bi-pollutant model with 5MC and B[a]P, the effect of 5MC remained significant: βage-adjusted = 0.2 (95%CI: 0.1-0.3, p=5 x 10−3). Furthermore, 5MC and B[a]P concentrations were modestly correlated (Spearman rho=0.36).
Diesel engine exhaust
The demographic and anthropometric characteristics are shown in Table 3. DEE-exposed subjects were more advanced in age (43.2±6.5 SD vs. 39.4±8.8 SD years), worked more years (18.7±7.9 vs. 12.8±9.1 years), and had a higher proportion of never-smokers (45% vs. 33%) than unexposed controls.
TABLE 3.
Characteristics of subjects from the cross-sectional diesel biomarker study
| Characteristic | DEE-Exposed (n = 20) | Unexposed Controls (n = 15) |
|---|---|---|
| Age, years, mean (SD) | 43.2 (6.5) | 39.4 (8.8) |
| Body mass index, kg/m2 (SD) | 25.4 (3.8) | 25.4 (4.0) |
| Work years, mean (SD) | 18.7 (7.9) | 12.8 (9.1) |
| Geometric mean of elemental carbon (EC) exposure among individuals, corrected for background levels, μg/m3, mean (SD) | 44.5 (24.9) | Not applicable |
| Smoking, n, % | ||
| Current | 0 | 0 |
| Former | 11 (55%) | 10 (67%) |
| Never | 9 (45%) | 5 (33%) |
| Pack-years, mean (SD) | 5.2 (8.3) | 3.3 (10.6) |
Abbreviations: diesel engine exhaust (DEE).
Urinary mutagenicity levels by categories of DEE are shown in Table 4. We found that DEE-exposed factory workers had significantly higher urinary mutagenicity levels compared to unexposed controls: 13.0±10.1 SD vs. 5.6±4.4 SD rev/ml-eq; βunadjusted = 7.4 (95%CI:1.7-13.1, p=0.01); βage-adjusted = 7.2 (95%CI:1.2-13.2, p=0.02) (Figure 2A). Moreover, we found a significant DEE exposure-response relationship with urinary mutagenicity, which further supported the association: mean urinary mutagenicity levels for unexposed subjects were 5.6±4.4 SD, ECtertile-1=6.7±4.8 SD, ECtertile-2=15.5±9.4 SD, and ECtertile-3=19.6±12.9 SD rev/ml-eq (Figure 2B); p-trendage-adjusted=2 x 10−3 (Figure 2C). Subjects who were exposed to 25 - <50 μg/m3 of EC, which was below the new EU OEL of 50 μg/m3, had significantly higher urinary mutagenicity compared to the unexposed (p=0.03, Figure 3A). Further, positive exposure-response trends between EC and urinary mutagenicity (pslope age-adjusted = 0.01) were detected among subjects exposed to EC concentrations below the new EU OEL (Figure 3B).
TABLE 4.
Urinary mutagenicity levels among subjects from the cross-sectional diesel biomarker study
| Comparison Group |
DEE Exposure Category |
||||
|---|---|---|---|---|---|
| Measurements | Unexposed Controls (n=15) |
Overall DEE- Exposed (n=20) |
Low DEE subgroup 6.1-39.0 μg/m3 (n=8) |
Medium DEE subgroup 39.1-54.5 μg/m3 (n=7) |
High DEE subgroup 54.6-107.7 μg/m3 (n=5) |
| Mutagenicity, mean rev/ml-eq (SD) | 5.6 (4.4) | 13.0 (10.1) | 6.7 (4.8) | 15.5 (9.4) | 19.6 (12.9) |
| Difference %, compared to controls | Reference | 132.1 | 19.6 | 176.8 | 250 |
| Wilcoxon p-value | Reference | 0.02 | 0.58 | 0.01 | 0.01 |
Exposure tertile categories are based on elemental carbon (EC) concentrations from Lan et al. (2015). Abbreviations: diesel engine exhaust (DEE).
Figure 2.
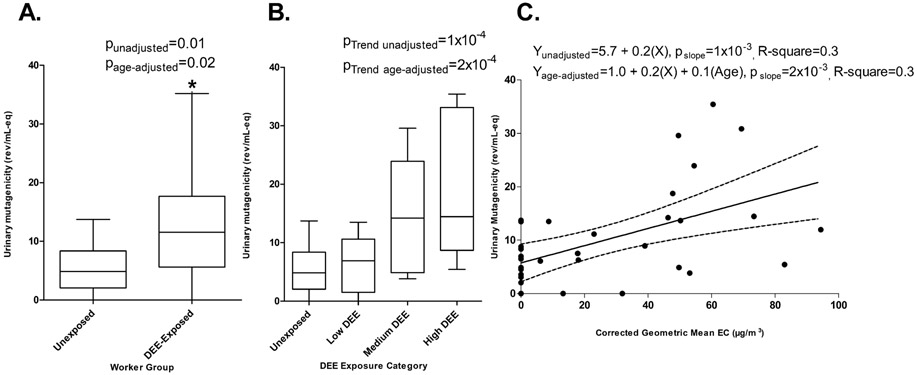
(Panel A) Urinary mutagenicity levels among male engine testing factory workers exposed to diesel engine exhaust and unexposed controls. Differences between exposure groups were tested with unadjusted and age-adjusted linear regression models. (Panel B) DEE exposure was categorized as tertiles by EC concentrations: low (6.1–39.0 μg/m3), medium (39.1–54.5 μg/m3), and high (54.6–107.7 μg/m3). (Panel C) DEE exposure as reflected by EC concentrations was analyzed continuously as geometric mean (μg/m3) corrected for background levels among the controls.
Figure 3.
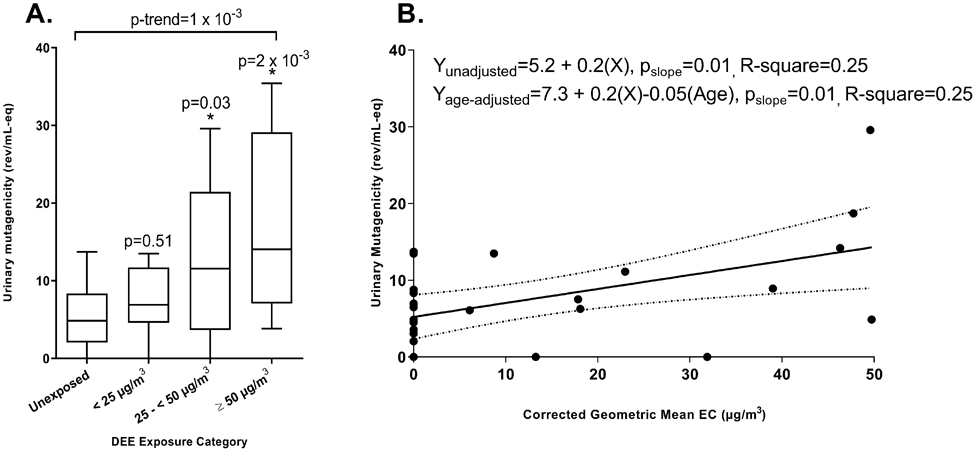
(Panel A) Associations between exposure to elemental carbon (EC) and urinary mutagenicity using EC categories defined by the new European Union occupational exposure limit of <50 μg/m3: unexposed controls (n = 15), <25 μg/m3 (n = 6), 25 - <50 μg/m3 (n = 6), and ≥50 μg/m3 (n = 8). (Panel B) Associations between exposure to elemental carbon (EC) and urinary mutagenicity among subjects below the new European Union occupational exposure limit of <50 μg/m3 of EC. EC concentrations were analyzed continuously as geometric mean (μg/m3) corrected for background levels among the controls.
DISCUSSION
Urinary mutagenicity reflects systemic exposure to complex mixtures of genotoxic agents or probable carcinogens (DeMarini et al., 1997; Kato et al., 2004; Sabbioni et al., 2006; Wu et al., 2021; Peters et al., 2004). Further, urinary mutagenicity has been linked to tumor development (Peters et al., 2003) and urinary metabolites of carcinogenic PAHs were found to be associated with lung and gastric cancer risk (Yuan et al., 2014; Liao et al., 2014) in epidemiologic studies. Our findings show, for the first time, strong evidence for systemic exposure to mutagens among people exposed to CCE or DEE, as well as significant associations between the levels of urinary mutagenicity and the concentrations of relevant marker pollutants in the air for CCE or DEE. Exposure to CCE and DEE has been found to be associated with lung cancer risk as well as development at malignancies of other sites (Kim et al., 2016; Koutros et al., 2020; IARC 2014; IARC 2010c). Indoor CCE has been associated with renal cell carcinoma (kidney cancer) (Sharpe et al., 1989) and esophageal cancer (Pan et al., 1999), and associated non-significantly with cervical (Wu et al., 2004) and salivary gland cancer (Zheng et al., 1996). DEE has been associated with cancers of the bladder (Koutros et al., 2020), colon/colorectum (Siemiatycki et al., 1988), prostate (Seidler et al., 1998), larynx (Elci et al., 2003), and non-Hodgkin lymphoma (Karunanyake et al., 2008), and suggestively associated with kidney cancer (Peters et al., 2018) and leukemia (IARC 2014). Our findings provide further evidence that systemic exposure to mutagens from CCE or DEE could be involved in the etiologic mechanisms of lung cancer as well as tumors at other sites.
Indoor coal combustion emissions
This is the first report showing that non-smoking women who domestically burned a particularly carcinogenic form of smoky coal sourced from geologic deposits near Laibin township of Xuanwei, China, had significantly elevated urinary mutagenicity levels compared to those who used less toxic smokeless coal. We found positive exposure-response relationships for the carcinogenic 5MC constituent of Laibin smoky coal. 5MC had higher and more consistent mutagenic potency than B[a]P in our analyses and has been found to be highly carcinogenic (IARC 2010a), even compared to other PAH species (Hecht et al., 1974).
Several human studies have examined the relationship between coal products and urinary mutagenicity, most of which were conducted among coke oven workers. De Méo et al. (1987) found that among non-smokers, steel workers exposed to coke oven emissions had higher urinary mutagenicity compared to unexposed controls. This finding was further supported by another study of non-smokers (Simioli et al., 2004), along with a repeated-measures study that found that highly exposed coke oven workers had higher overall urinary mutagenicity levels compared to those with lower exposure over the work week (Chao et al., 2008). Although Recio et al. (1984) found no clear evidence among non-smokers that urinary mutagenicity was higher among U.S. coal liquefaction pilot plant workers compared with controls, they did find differences by smoking status. Our investigation expands upon the findings from these occupational studies by examining subjects from a general population who had indoor air pollution exposure from domestic coal combustion at levels comparable to those of occupational studies.
Our findings of associations between urinary mutagenicity and the concentrations of carcinogenic PAHs in the air among CCE-exposed women are concordant with studies of other exposures in which a variety of genotoxicity biomarkers were associated with concentrations of PAHs in the air (DeMarini 2013). 5MC has been evaluated by IARC (2010a) as a Group 2B (possible) human carcinogen, and B[a]P is a well-studied Group 1 (known) human carcinogen (IARC 2010a). Although some studies for urinary mutagenicity reported null or equivocal findings (Venier et al., 1985; Clonfero et al., 1995; Mielyńska et al., 1997), others found positive associations between urinary mutagenicity and PAH exposure, such as in bus drivers (Hansen et al., 2004), coke oven workers (Simioli et al., 2004; Siwińska et al., 2004), rubber manufacturers (Peters et al., 2008), policeman (Ledda et al., 2018), and municipal firefighters (Keir et al., 2017).
Women in our study with CCE exposure had the highest increase in urinary mutagenicity relative to controls (31.5-fold) compared with subjects exposed to other types of agents (Table 5). Although their level of urinary mutagenicity was approximately an order-of-magnitude lower than that of subjects exposed to benzidine, charcoal production emissions, or nitrotoluenes (Table 5), women using smoky coal in Xuanwei, China, have an extremely elevated risk for lung cancer due to the emissions from smoky coal (IARC 2010a). Because urinary mutagenicity has been linked to increased colorectal cancer risk (Peter et al., 2003), the systemic exposure to mutagens, including mutagenic/carcinogenic PAHs, by women in this study suggests that exposure to CCE might pose risks for cancers to additional organs (Kim et al., 2016; IARC 2010c; Sharpe et al., 1989; Pan et al., 1999) along with the lung.
TABLE 5.
Comparisons of urinary mutagenicity from various exposuresa
| Exposure | Mean rev/ml-eq | Fold increase | Reference |
|---|---|---|---|
| Smoky coal combustion emissions | 24.8 | 31.5 | Present study |
| Diesel engine exhaust | 19.6 | 3.5 | Present study |
| Benzidine | 496.0 | 20.7 | DeMarini et al. (1997) |
| Charcoal production emissions | 276.0 | 2.3 | Kato et al. (2004) |
| Nitrotoluenes | 127.0 | Not reported | Sabbioni et al. (2006) |
| Wildfire | 11.0 | 2.6 | Wu et al. (2021) |
Other than for benzidine, all other data were generated in Salmonella frameshift strain YG1041, which over-expresses both O-acetyltransferase and nitroreductase. The benzidine data were generated in the related strain YG1024, which over-expresses only O-acetyltransferase. Data for benzidine were extrapolated from published dose-response curves, and data for charcoal emissions were estimated from published data.
Diesel engine exhaust
Our results contribute evidence to an inconclusive body of literature on urinary mutagenicity from DEE-exposed subjects, showing that male DEE-exposed workers had significantly higher (3.5-fold) levels of urinary mutagenicity compared to unexposed controls (Table 5). Further, we observed a positive exposure-response relationship between EC and urinary mutagenicity. Previous human studies have shown that exposure to DEE or atmospheres containing DEE induces a range of genotoxicity biomarkers, including DNA adducts, DNA damage, HPRT mutations, and micronuclei (DeMarini 2013; IARC 2014). However, relative to controls, increased levels of urinary mutagenicity were not found in occupational studies of diesel-exposed car mechanics (Willems et al., 1989), railroad workers (Schenker et al., 1992), or road tanker drivers (Nylander and Berg 1991). These null findings may have been due to limited sample size, low diesel exposure levels, and/or limited exposure variation. In contrast, our study of diesel engine testing workers was conducted in a high-exposure workplace, and the controls were from workplaces determined empirically to have negligible DEE exposure based on walkthrough surveys (Lan et al., 2015), which likely improved the likelihood of detecting effects. DEE levels in our study were considerably higher than those found in a study of U.S. trucking industry workers (Davis et al., 2006, 2007; Smith et al., 2006; Garshick et al. 2008).
In addition to lung cancer, bladder cancer has also been positively associated with DEE exposure (IARC 2014; Koutros et al., 2020). The most plausible mechanism is that nitroarenes (nitro-PAHs), which account for much of the mutagenic activity of diesel exhaust (DeMarini et al., 2004), are metabolized to aromatic amines (IARC 2014), which are well-known bladder carcinogens (IARC 2010b). DEE induced a significant 3.5-fold increase in urinary mutagenicity relative to controls (Table 5), although the level (19.6 rev/ml-eq) was more than an order-of-magnitude lower than that of subjects exposed to benzidine (496 rev/ml-eq), a known bladder carcinogen (IARC 2010b). However, we used Salmonella strain YG1041, which we have shown to be the most sensitive of all the Salmonella strains for detecting the mutagenicity of organic extracts of diesel exhaust particles (Mutlu et al., 2015a,b). This increased sensitivity is because YG1041 over-expresses both nitroreductase, which metabolizes nitroarenes to aromatic amines, as well as O-acetyltransferase, which metabolizes activated aromatic amines (S9 was used in the mutagenicity assay) to acetylamines, which can bind covalently to DNA to form mutagenic DNA adducts (Hagiwara et al., 1993). Our finding of elevated levels of urinary mutagenicity among subjects exposed to DEE supports the association between exposure to DEE and bladder cancer (IARC 2014; Koutros et al., 2020).
The United States (US) has not set a permissible exposure limit for diesel exhaust for general industry; however, the US Mine Safety and Health Administration (MSHA) currently enforces diesel particulate matter standards at underground metal/nonmetal mines and at underground coal mines. In the US, a miner's personal exposure must not exceed an 8-h time-weighted average of 160 μg/m3 of total carbon (TC; EC conversion factors range from 1.19-1.44 (MSHA 2001; Noll et al., 2015). The EU adopted an OEL of 50 μg/m3 for EC in December 2018, which will take effect in 2026 for underground mines and tunnel construction and in 2023 for other industries (Directive 2019). There has been concern that this new EU OEL for EC leaves a high residual risk of DEE-related cancers. Even at EC concentrations below 50 μg/m3, we detected a marked biological effect on urinary mutagenicity in our study. As such, the need for lower OELs for EC may be justified to protect workers’ health. In 2001, the American Conference of Governmental Industrial Hygienists (ACGIH) proposed a recommended threshold limit value of 20 μg/m3 of EC. Although ACGIH recommendations are not legal standards, a number of regulatory authorities consider these recommendations when drafting legislation. In July 2020, the Netherlands adopted an even lower OEL for EC of 10 μg/m3, which is the most stringent in Europe (Diesel Engine Exhaust 2019).
Strengths and limitations of studies
Our study had notable strengths. First, our study subjects were exposed to high levels of CCE or DEE, which increased the likelihood of detecting effects. Further, we were able to estimate a DEE exposure-response using EC measurements from personal air samples, which are correlated with diesel particulate matter (Noll et al., 2007). Second, the study subjects were current non-smokers, which reduced potential confounding by smoking. Current active smoking has been found to influence short-term urinary mutagenicity levels (Yamasaki and Ames 1977; Recio et al., 1984; De Meo et al., 1987; Kawano et al., 1987; Clonfero et al., 1995). Third, we used a well-established method to detect urinary mutagenicity, which improved the comparability of our results to other similar studies.
Our study had some limitations. First, our sample size was small; however, the statistical power to detect effects was improved by analyzing subjects with very high versus low exposure levels. Second, we had limited information on potential confounders and did not have the sample size to accommodate more covariates. However, we assessed the influence of some potentially influential variables, e.g., body mass index, work years, and packyears of past smoking in the diesel study, and these factors had little effect on the findings. Third, our lack of creatinine measurements precluded the expression of urinary mutagenicity data in terms of rev/creatinine concentration, which prevented comparisons of our data to a wider literature. However, expression of urinary mutagenicity as rev/ml-eq is sufficient to conclude if subjects have mutagenic urine or not, which was the key issue addressed in our study.
Diesel exhaust and coal emissions are composed of both organic and inorganic compounds. Although inorganics such as inorganic carbon nanoparticles might contribute to the carcinogenicity of both diesel exhaust (IARC 1989, 2014) and coal emissions (IARC 2010C, 2012), inorganic carbon would not have contributed to the urinary mutagenicity because we evaluated organic extracts of the urine, which did not contain inorganic carbon.
Conclusions
In summary, we found that exposure to indoor smoky coal combustion emissions or diesel engine exhaust can result in systemic exposure to mutagens. Further confirmation of these results with larger sample sizes, measurement of additional exposure biomarkers, and comprehensive identification of chemical constituents of air samples would improve our understanding of the potential risk that these exposures present to organs other than the lung. The established carcinogenicity to the lung of coal combustion emissions (IARC 2012) and diesel engine exhaust (IARC 2014) may extend to other organs given the observed elevated urinary mutagenicity caused by these exposures. Indeed, coal combustion emissions and diesel engine exhaust have been linked to risk of cancer of several sites in addition to lung (Sharpe et al., 1989; Pan et al., 1999; Kim et al., 2016; Koutros et al., 2020; IARC 2014; IARC 2010c), and our findings suggest that systemic exposure to mutagens could be potentially involved in carcinogenesis at these sites.
ACKNOWLEDGEMENTS
We thank all the subjects who volunteered to participate in these studies, as well as Brian Chorley and Rex Pegram (U.S. EPA) for their helpful comments on the manuscript. This study was supported by intramural funding from the National Cancer Institute. WKM was supported by a pre-doctoral traineeship (National Research Service Award T32 ES007126) from the National Institute of Environmental Health Sciences, National Institutes of Health. The urinary mutagenicity analyses were performed at the U.S. Environmental Protection Agency, Research Triangle Park, NC, and were supported by the intramural research program of the Office of Research and Development (ORD) of the U.S. EPA, Research Triangle Park, NC. This paper has been reviewed and approved for publication by the National Cancer Institute (NCI) and the Office of Research and Development and the Chemical Characterization and Exposure Division of the U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA and/or the NCI, nor does mention of trade names or commercial products constitute endorsement or recommendation of use.
Footnotes
CONFLICT OF INTEREST
The authors declare they have no actual or potential competing financial interests.
REFERENCES
- Adetona AM, Martin WK, Warren SH, Hanley NM, Adetona O, Zhang JJ, Simpson C, Paulsen M, Rathbun S, Wang JS, DeMarini DM and Naeher LP (2019) Urinary mutagenicity and other biomarkers of occupational smoke exposure of wildland firefighters and oxidative stress. Inhalation Toxicology, 31, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone-Adesi F, Chapman RS, Silverman DT, He X, Hu W, Vermeulen R, Ning B, Fraumeni JF Jr., Rothman N and Lan Q (2012) Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ, 345, e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch ME (2002) Occupational monitoring of particulate diesel exhaust by NIOSH method 5040. Applied Occupational and Environmental Hygiene, 17, 400–405. [DOI] [PubMed] [Google Scholar]
- Cerná M, Pastorková A, Myers SR, Rössner P and Binková B (1997) The use of a urine mutagenicity assay in the monitoring of environmental exposure to genotoxins. Mutation Research, 391, 99–110. [DOI] [PubMed] [Google Scholar]
- Chao MR, Wang CJ, Wu MT, Pan CH, Kuo CY, Yang HJ, Chang LW, Hu and CW (2008) Repeated measurements of urinary methylated/oxidative DNA lesions, acute toxicity, and mutagenicity in coke oven workers. Cancer Epidemiology Biomarkers and Prevention, 17, 3381–3389. [DOI] [PubMed] [Google Scholar]
- Clonfero E, Granella M, Marchioro M, Barra EL, Nardini B, Ferri G and Foa V (1995) Urinary excretion of mutagens in coke oven workers. Carcinogenesis, 16, 547–554. [DOI] [PubMed] [Google Scholar]
- Davis ME, Smith TJ, Laden F, Hart JE, Blicharz AP, Reaser P, Garshick E (2007) Driver exposure to combustion particles in the U.S. Trucking industry. Journal of Occupational and Environmental Hygiene, 4, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Smith TJ, Laden F, Hart JE, Ryan LM, Garshick E (2006) Modeling particle exposure in U.S. trucking terminals. Environmental Science and Technology, 40, 4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Méo MP, Duménil G, Botta AH, Laget M, Zabaloueff V and Mathias A (1987) Urine mutagenicity of steel workers exposed to coke oven emissions. Carcinogenesis, 8, 363–367. [DOI] [PubMed] [Google Scholar]
- DeMarini DM (2013) Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis, 28, 485–505. [DOI] [PubMed] [Google Scholar]
- DeMarini DM, Brooks LR, Bhatnagar VK, Hayes RB, Eischen BT, Shelton ML, Zenser TV, Talaska G, Kashyap SK, Dosemeci M, Kashyap R, Parikh DJ, Lakshmi V, Hsu F, Davis BB, Jaeger M and Rothman N (1997) Urinary mutagenicity as a biomarker in workers exposed to benzidine: correlation with urinary metabolites and urothelial DNA adducts. Carcinogenesis, 18, 981–988. [DOI] [PubMed] [Google Scholar]
- DeMarini DM, Brooks LR, Warren SH, Kobayashi T, Gilmour MI and Singh P (2004) Bioassay-directed fractionation and salmonella mutagenicity of automobile and forklift diesel exhaust particles. Environmental Health Perspectives, 112, 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini DM, Landi S, Tian D, Hanley NM, Li X, Hu F, Roop BC, Mass MJ, Keohavong P, Gao W, Olivier M, Hainaut P and Mumford JL (2001) Lung tumor KRAS and TP53 mutations in non-smokers reflect exposure to PAH-rich coal combustion emissions. Cancer Research, 61, 6679–6681. [PubMed] [Google Scholar]
- Diesel Engine Exhaust (2019) Health Council of the Netherlands. Diesel Engine Exhaust - Health Council of the Netherlands [Accessed 15 July 2021].
- Directive (2019) Directive (EU) 2019/130 of the European Parliament and of the Council. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019L0130&from=FR [Accessed 15 July 2021].
- Downward GS, Hu W, Rothman N, Reiss B, Wu G, Wei F, Chapman RS, Portengen L, Qing L and Vermeulen R (2014) Polycyclic aromatic hydrocarbon exposure in household air pollution from solid fuel combustion among the female population of Xuanwei and Fuyuan counties, China. Environmental Science & Technology, 48, 14632–14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elci OC, Akpinar-Elci M, Blair A and Dosemeci M (2003) Risk of laryngeal cancer by occupational chemical exposure in Turkey. Journal of Occupational and Environmental Medicine, 45, 1100–1106. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Rosner B, Davis ME, Eisen EA and Smith TJ (2008) Lung cancer and vehicle exhaust in trucking industry workers. Environmental Health Perspectives, 116, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Davis ME, Eisen EA and Smith TJ (2012) Lung cancer and elemental carbon exposure in trucking industry workers. Environmental Health Perspectives, 120, 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granville CA, Hanley NM, Mumford JL and DeMarini DM (2001) Mutation spectra of smoky coal combustion emissions in Salmonella reflect the TP53 and KRAS mutations in lung tumors from smoky coal-exposed individuals. Mutation Research, 525, 77–83. [DOI] [PubMed] [Google Scholar]
- Hagiwara Y, Watanabe M, Oda Y, Sofuni T and Nohmi T (1993) Specificity and sensitivity of Salmonella typhimurium YG1041 and YG1042 strains possessing elevated levels of both nitroreductase and acetyltransferase activity. Mutation Research 291, 171–180. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Wallin H, Binderup ML, Dybdahl M, Autrup H, Loft S and Knudsen LE (2004) Urinary 1-hydroxypyrene and mutagenicity in bus drivers and mail carriers exposed to urban air pollution in Denmark. Mutation Research, 557, 7–17. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Bondinell WE and Hoffmann D (1974) Chrysene and methylchrysenes: presence in tobacco smoke and carcinogenicity. Journal of the Natlional Cancer Institute, 53, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Hu W, Downward GS, Reiss B, Xu J, Bassig BA, Hosgood HD 3rd, Zhang L, Seow WJ, Wu G, Chapman RS, Tian L, Wei F, Vermeulen R and Lan Q (2014) Personal and indoor PM2.5 exposure from burning solid fuels in vented and unvented stoves in a rural region of China with a high incidence of lung cancer. Environmental Science & Technology, 48, 8456–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (1989) Diesel and gasoline engine exhausts and some nitroarenes. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 46. Lyon, France: International Agency for Research on Cancer, p. 458. [Google Scholar]
- IARC (2010a) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 92. Lyon, France: International Agency for Research on Cancer, p. 853. [PMC free article] [PubMed] [Google Scholar]
- IARC (2010b) Some aromatic amines, organic dyes, and related exposures. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 99. Lyon, France: International Agency for Research on Cancer, p. 692. [PMC free article] [PubMed] [Google Scholar]
- IARC (2010c) Household Use of Solid Fuels and High-temperature Frying. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 95. Lyon, France: International Agency for Research on Cancer, p. 144. [PMC free article] [PubMed] [Google Scholar]
- IARC (2012) Personal habits and indoor combustions. A review of human carcinogens. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E. Lyon, France: International Agency for Research on Cancer, p. 538. [PMC free article] [PubMed] [Google Scholar]
- IARC (2014) Diesel and gasoline engine exhausts and some nitroarenes. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 105. Lyon, France: International Agency for Research on Cancer, p. 699. [Google Scholar]
- IARC (2016) Outdoor air pollution. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 109. Lyon, France: International Agency for Research on Cancer, p. 448. [Google Scholar]
- Kato M, Loomis D, Brooks LM, Gattas GF, Gomes L, Carvalho AB, Rego MA and DeMarini DM (2004) Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiology Biomarkers and Prevention, 13, 1005–1012. [PubMed] [Google Scholar]
- Kawano H, Inamasu T, Ishizawa M, Ishinishi N and Kumazawa J (1987) Mutagenicity of urine from young male smokers and nonsmokers. Internatinal Archives of Occupational and Environmental Health, 59, 1–9. [DOI] [PubMed] [Google Scholar]
- Karunanayake CP, McDuffie HH, Dosman JA, Spinelli JJ and Pahwa P (2008) Occupational exposures and non-Hodgkin's lymphoma: Canadian case-control study. Environmental Health: A Global Access Science Source, 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir JLA, Akhtar US, Matschke DMJ, Kirkham TL, Chan HM, Ayotte P, White PA and Blais JM (2017) Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in Ottawa firefighters participating in emergency, on-shift fire suppression. Environmental Science & Technology, 51, 12745–12755. [DOI] [PubMed] [Google Scholar]
- Keohavong P, Lan Q, Gao W-M, Zheng K-C, Mady HH, Melhem MF and Mumford JL (2005) Detection of p53 and K-ras mutations in sputum of individuals exposed to smoky coal emissions in Xuan Wei County, China. Carcinogenesis, 26, 303–308. [DOI] [PubMed] [Google Scholar]
- Kim C, Chapman RS, Hu W, He X, Hosgood HD, Liu LZ, Lai H, Chen W, Silverman DT, Vermeulen R, Tian L, Bassig B, Shen M, Zhang Y, Ma S, Rothman N and Lan Q (2014) Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer, 84, 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Seow WJ, Shu XO, Bassig BA, Rothman N, Chen BE, Xiang YB, Hosgood HD, Ji BT, Hu W, Wen C, Chow WH, Cai Q, Yang G, Gao YT, Zheng W and Lan Q (2016) Cooking Coal Use and All-Cause and Cause-Specific Mortality in a Prospective Cohort Study of Women in Shanghai, China. Environmental Health Perspectives, 124, 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutros S, Kogevinas M, Friesen MC, Stewart PA, Baris D, Karagas MR, Schwenn M, Johnson A, Monawar Hosain GM, Serra C, Tardon A, Carrato A, Garcia-Closas R, Moore LE, Nickerson ML, Hewitt SM, Lenz P, Schned AR, Lloreta J, Allory Y, Zhang H, Chatterjee N, Garcia-Closas M, Rothman N, Malats N and Silverman DT (2020) Diesel exhaust and bladder cancer risk by pathologic stage and grade subtypes. Environment International, 135, 105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, He X, Shen M, Tian L, Liu LZ, Lai H, Chen W, Berndt SI, Hosgood HD, Lee KM, Zheng T, Blair A and Chapman RS (2008) Variation in lung cancer risk by smoky coal subtype in Xuanwei, China. International Journal of Cancer, 123, 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Mumford JL, Shen M, DeMarini DM, Bonner MR, He X, Yeager M, Welch R, Chanock S, Tian L, Chapman RS, Zheng T, Keohavong P, Caporaso N and Rothman N (2004) Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis, 11, 2177–2181. [DOI] [PubMed] [Google Scholar]
- Lan Q, Vermeulen R, Dai Y, Ren D, Hu W, Duan H, Niu Y, Xu J, Fu W, Meliefste K, Zhou B, Yang J, Ye M, Jia X, Meng T, Bin P, Kim C, Bassig BA, Hosgood HD 3rd, Silverman D, Zheng Y and Rothman N (2015) Occupational exposure to diesel engine exhaust and alterations in lymphocyte subsets. Occupational and Environmental Medicine, 72, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda C, Loreto C, Bracci M, Lombardo C, Romano G, Cina D, Mucci N, Castorina S and Rapisarda V (2018) Mutagenic and DNA repair activity in traffic policemen: a case-crossover study. Journal of Occupational Medicine and Toxicology, 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao LM, Hofmann JN, Kamangar F, Strickland PT, Ji BT, Yang G, Li HL, Rothman N, Zheng W, Chow WH, Gao YT and Shu XO (2014) Polycyclic aromatic hydrocarbons and risk of gastric cancer in the Shanghai Women's Health Study. International Journal of Molecular Epidemiology and Genetics, 5, 140–144. [PMC free article] [PubMed] [Google Scholar]
- Long AS, Lemieux CL, Yousefi P, Ruiz-Mercado I, Lam NL, Orellana CR, White PA, Smith KR and Holland N (2014) Human urinary mutagenicity after wood smoke exposure during traditional temazcal use. Mutagenesis, 29, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubet RA, Capdevilla J and Prough RA (1979) The metabolic activation of benzo(a)pyrene and 9-hydroxybenzo(a)pyrene by liver microsomal fractions. International Journal of Cancer, 23, 353–357. [DOI] [PubMed] [Google Scholar]
- Maron DM and Ames BN (1983) Revised methods for the Salmonella mutagenicity test. Mutation Research, 113, 173–215. [DOI] [PubMed] [Google Scholar]
- Mielyńska D, Braszcynska Z, Siwińska E, Smolik E, Bubak A and Sokal JA (1997) Exposure of coke-oven workers to polycyclic aromatic hydrocarbons based on biological monitoring results. American Industrial Hygiene Association Journal, 58, 661–666. [DOI] [PubMed] [Google Scholar]
- Mielzyńska D and Snit M (1992) Urine mutagenicity in workers directly employed in coke production. Polish Journal of Occupational Medicine and Environmental Health, 5, 363–371. [PubMed] [Google Scholar]
- Mine Safety and Health Administration (MSHA). (2001) Diesel Particulate Matter Exposure of Underground Metal and Nonmetal Miners. Mine Safety and Health Administration, p. 5706–5910. [Google Scholar]
- Moller M and Dybing E (1980) Mutagenicity studies with urine concentrates from coke plant workers. Scandinavian Journal of Work and Environmental Health, 6, 216–220. [DOI] [PubMed] [Google Scholar]
- Mumford JL, Lee X, Lewtas J, Young TL and Santella RM (1993) DNA adducts as biomarkers for assessing exposure to polycyclic aromatic hydrocarbons in tissues from Xuan Wei women with high exposure to coal combustion emissions and high lung cancer mortality. Environmental Health Perspectives, 99, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JL, Li X, Hu F, Lu XB and Chuang JC (1995) Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis, 16, 3031–3036. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Warren SH, Matthews PP, King C, Walsh L, Kligerman AD, Schmid JE, Janek D, Kooter IM, Linak WP, Gilmour MI and DeMarini DM (2015a) Health effects of soy-biodiesel emissions: mutagenicity-emission factors. Inhalation Toxicology, 27, 858–596. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Warren SH, Matthews PP, Schmid JE, Kooter IM, Linak WP, Gilmour MI and DeMarini DM (2015b) Health effects of soy-biodiesel emissions: bioassay-directed fractionation for mutagenicity. Inhalation Toxicology, 27, 597–612. [DOI] [PubMed] [Google Scholar]
- Noll JD, Bugarski AD, Patts LD, Mischler SE and McWilliams L (2007) Relationship between elemental carbon, total carbon, and diesel particulate matter in several underground metal/non-metal mines. Environmental Science & Technology, 41, 710–716. [DOI] [PubMed] [Google Scholar]
- Noll J, Gilles S, Wu HW, and Rubinstein E (2015) The relationship between elemental carbon and diesel particulate matter in underground metal/nonmetal mines in the United States and coal mines in Australia. Journal of Occupational and Environmental Hygiene, 12, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander G and Berg K (1991) Mutagenicity study of urine from smoking and non-smoking road tanker drivers. International Archives of Occupational and Environmental Health, 63, 229–232. [DOI] [PubMed] [Google Scholar]
- Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T and Goldsmith DF (1999) Nested case‐control study of esophageal cancer in relation to occupational exposure to silica and other dusts. American Journal of Industrial Medicine, 35, 272–280. [DOI] [PubMed] [Google Scholar]
- Peters CE, Parent MÉ, Harris SA, Bogaert L, Latifovic L, Kachuri L, Villeneuve PJ, and Canadian Cancer Registries Epidemiology Group (2018) Occupational Exposure to Diesel and Gasoline Engine Exhausts and the Risk of Kidney Cancer in Canadian Men. Annals of Work Exposures and Health, 62, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Talaska G, Jonsson BA, Kromhout H and Vermeulen R (2008) Polycyclic aromatic hydrocarbon exposure, urinary mutagenicity, and DNA adducts in rubber manufacturing workers. Cancer Epidemiology Biomarkers and Prevention, 17, 1452–1459. [DOI] [PubMed] [Google Scholar]
- Peters U, DeMarini DM, Sinha R, Brooks LR, Warren SH, Chatterjee N and Rothman N (2003) Urinary mutagenicity and colorectal adenoma risk. Cancer Epidemiology Biomarkers and Prevention, 12(11 Pt 1), 1253–1256. [PubMed] [Google Scholar]
- Peters U, Sinha R, Bell DA, Rothman N, Grant DJ, Watson MA, Kulldorff M, Brooks LR, Warren SH and DeMarini DM (2004) Urinary mutagenesis and fried red meat intake: Influence of cooking temperature, phenotype, and genotype of metabolizing enzymes in a controlled feeding study. Environmental and Molecular Mutagenesis, 43, 53–74. [DOI] [PubMed] [Google Scholar]
- Recio L, Enoch HG, Hannan MA and Hill RH (1984) Application of urine mutagenicity to monitor coal liquefaction workers. Mutation Research, 136, 201–207. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Jones CR, Sepai O, Hirvonen A, Norppa H, Jarventaus H, Glatt H, Pomplun D, Yan H, Brooks LR, Warren SH, DeMarini DM and Liu YY (2006) Biomarkers of exposure, effect, and susceptibility in workers exposed to nitrotoluenes. Cancer Epidemiology Biomarkers and Prevention, 15, 559–566. [DOI] [PubMed] [Google Scholar]
- Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ and Rudin CM (2009) Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clinical Cancer Research, 15, 5626–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Gajalakshmi V, Jetly DH, Roychowdhury S, Dikshit RP, Brennan P, Hashibe M, and Boffetta P (2008) Indoor air pollution from solid fuels and risk of hypopharyngeal/laryngeal and lung cancers: a multicentric case-control study from India. International Journal of Epidemiology, 37, 321–328. [DOI] [PubMed] [Google Scholar]
- Schenker MB, Kado NY, Hammond SK, Samuels SJ, Woskie SR and Smith TJ (1992) Urinary mutagenic activity in workers exposed to diesel exhaust. Environmental Research, 57, 133–148. [DOI] [PubMed] [Google Scholar]
- Seidler A, Heiskel H, Bickeböller R, and Elsner G (1998) Association between diesel exposure at work and prostate cancer. Scandinavian Journal of Work, Environment & Health, 24, 486–494. [DOI] [PubMed] [Google Scholar]
- Seow WJ, Hu W, Vermeulen R, Hosgood HD, Downward GS, Chapman RS, He X, Bassig BA, Kim C, Wen C, Rothman N and Lan Q (2014) Household air pollution and lung cancer in China: a review of studies in Xuanwei. Chinese Journal of Cancer, 33, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemiatycki J, Gérin M, Stewart P, Nadon L, Dewar R and Richardson L (1988) Associations between several sites of cancer and ten types of exhaust and combustion products. Results from a case-referent study in Montreal. Scandinavian Journal of Work, Environment & Health, 14, 79–90. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Rochon JE, Adam JM and Suissa S (1989) Case-control study of hydrocarbon exposures in patients with renal cell carcinoma. CMAJ: Canadian Medical Association Journal, 140, 1309–1318. [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy DT, Gangarosa LM, Schliebe B, Umbach DM, Xu Z, MacIntosh B, Knize MG, Matthews PP, Swank AE, Sandler RS, DeMarini DM and Taylor JA (2011) Inhibition of fried meat-induced colorectal DNA damage and altered systemic genotoxicity in humans by crucifera, chlorophyllin, and yogurt. PLoS One, 6(4):e18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Berndt SI, Rothman N, DeMarini DM, Mumford JL, He X, Bonner MR, Tian L, Yeager M, Welch R, Chanock S, Zheng T, Caporaso N and Lan Q (2005) Polymorphisms in the DNA nucleotide excision repair genes and lung cancer risk in Xuan Wei, China. International Journal of Cancer, 116, 768–773. [DOI] [PubMed] [Google Scholar]
- Silverman DT (2017) Diesel exhaust causes lung cancer: now what? Occupational and Environmental Medicine, 74, 233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DT (2018) Diesel Exhaust and Lung Cancer-Aftermath of Becoming an IARC Group 1 Carcinogen. American Journal of Epidemiology, 187, 1149–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DT, Samanic CM, Lubin JH, Blair AE, Stewart PA, Vermeulen R, Coble JB, Rothman N, Schleiff PL, Travis WD, Ziegler RG, Wacholder S and Attfield MD (2012) The Diesel Exhaust in Miners study: a nested case-control study of lung cancer and diesel exhaust. Journal of the Natlional Cancer Institute, 104, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioli P, Lupi S, Gregorio P, Siwińska E, Mielzyńska D, Clonfero E and Pavanello S (2004) Non-smoking coke oven workers show an occupational PAH exposure-related increase in urinary mutagens. Mutation Research, 562, 103–110. [DOI] [PubMed] [Google Scholar]
- Siwińska E, Mielzyńska D and Kapka L (2004) Association between urinary 1-hydroxypyrene and genotoxic effects in coke oven workers. Occupational and Environmental Medicine, 61(3):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Davis ME, Reaser P, Natkin J, Hart JE, Laden F, Heff A and Garshick E (2006) Overview of particulate exposures in the US trucking industry. Journal of Environmental Monitoring, 8, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venier P, Clonfero E, Cottica D, Gava C, Zordan M, Pozzoli L and Levis AG (1985) Mutagenic activity and polycyclic aromatic hydrocarbon levels in urine of workers exposed to coal tar pitch volatiles in an anode plant. Carcinogenesis, 6, 749–752. [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Downward GS, Zhang J, Hu W, Portengen L, Bassig BA, Hammond SK, Wong JYY, Li J, Reiss B, He J, Tian L, Yang K, Seow WJ, Xu J, Anderson K, Ji BT, Silverman D, Chanock S, Huang Y, Rothman N and Lan Q (2019) Constituents of household air pollution and risk of lung cancer among never-smoking women in Xuanwei and Fuyuan, China. Environmental Health Perspectectives, 127, 97001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems MI, de Raat WK, Wesstra JA, Bakker GL, Dubois G and van Dokkum W (1989) Urinary and faecal mutagenicity in car mechanics exposed to diesel exhaust and in unexposed office workers. Mutation Research, 222, 375–391. [DOI] [PubMed] [Google Scholar]
- Wong JYY, Downward GS, Hu W, Portengen L, Seow WJ, Silverman DT, Bassig BA, Zhang J, Xu J, Ji BT, Li J, He J, Yang K, Tian L, Shen M, Huang Y, Vermeulen R, Rothman N and Lan Q (2019) Lung cancer risk by geologic coal deposits: A case-control study of female never-smokers from Xuanwei and Fuyuan, China. International Journal of Cancer, 144, 2918–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JYY, Hu W, Downward GS, Seow WJ, Bassig BA, Ji BT, Wei F, Wu G, Li J, He J, Liu CS, Cheng WL, Huang Y, Yang K, Chen Y, Rothman N, Vermeulen RC and Lan Q (2017) Personal exposure to fine particulate matter and benzo[a]pyrene from indoor air pollution and leukocyte mitochondrial DNA copy number in rural China. Carcinogenesis, 38, 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MT, Lee LH, Ho CK, Wu SC, Lin LY, Cheng BH, Liu CL, Yang CY, Tsai HT and Wu TN (2004) Environmental exposure to cooking oil fumes and cervical intraepithelial neoplasm. Environmental Research, 94, 25–32. [DOI] [PubMed] [Google Scholar]
- Wu CM, Warren SH, DeMarini DM, Song CC, and Adetona O. Urinary mutagenicity and oxidative status of wildland firefighters working at prescribed burns in a Midwestern US forest. Occupational and Environmental Medicine 2021;78:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki E and Ames BN (1977) Concentration of mutagens from urine by absorption with the nonpolar resin XAD-2: cigarette smokers have mutagenic urine. Proceedings of the National Academy of Sciences USA, 74, 3555–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Butler LM, Gao YT, Murphy SE, Carmella SG, Wang R, Nelson HH and Hecht SS (2014) Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis, 35, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Shu XO, Ji BT and Gao YT (1996) Diet and other risk factors for cancer of the salivary glands: a population-based case-control study. International Journal of Cancer, 67, 194–198. [DOI] [PubMed] [Google Scholar]


