Abstract
Objective
Recent studies have found aristaless-related homeobox gene (ARX)/pancreatic and duodenal homeobox 1 (PDX1), alpha-thalassemia/mental retardation X-linked (ATRX)/death domain-associated protein (DAXX) and alternative lengthening of telomeres (ALT) to be promising prognostic biomarkers for non-functional pancreatic neuroendocrine tumours (NF-PanNETs). However, they have not been comprehensively evaluated, especially among small NF-PanNETs (≤2.0 cm). Moreover, their status in neuroendocrine tumours (NETs) from other sites remains unknown.
Design
An international cohort of 1322 NETs was evaluated by immunolabelling for ARX/PDX1 and ATRX/DAXX, and telomere-specific fluorescence in situ hybridisation for ALT. This cohort included 561 primary NF-PanNETs, 107 NF-PanNET metastases and 654 primary, non-pancreatic non-functional NETs and NET metastases. The results were correlated with numerous clinicopathological features including relapse-free survival (RFS).
Results
ATRX/DAXX loss and ALT were associated with several adverse prognostic findings and distant metastasis/recurrence (p<0.001). The 5-year RFS rates for patients with ATRX/DAXX-negative and ALT-positive NF-PanNETs were 40% and 42% as compared with 85% and 86% for wild-type NF-PanNETs (p<0.001 and p<0.001). Shorter 5-year RFS rates for ≤2.0 cm NF-PanNETs patients were also seen with ATRX/DAXX loss (65% vs 92%, p=0.003) and ALT (60% vs 93%, p<0.001). By multivariate analysis, ATRX/DAXX and ALT status were independent prognostic factors for RFS. Conversely, classifying NF-PanNETs by ARX/PDX1 expression did not independently correlate with RFS. Except for 4% of pulmonary carcinoids, ATRX/DAXX loss and ALT were only identified in primary (25% and 29%) and NF-PanNET metastases (62% and 71%).
Conclusions
ATRX/DAXX and ALT should be considered in the prognostic evaluation of NF-PanNETs including ≤2.0 cm tumours, and are highly specific for pancreatic origin among NET metastases of unknown primary.
Keywords: neuroendocrine tumors, pancreatic endocrine tumour, pancreatic islet cell, pancreatic pathology, pancreatic surgery
Significance of this study.
What is already known on this subject?
The frequent detection of non-functional pancreatic neuroendocrine tumours (NF-PanNETs), especially those measuring ≤2.0 cm, represents a treatment dilemma.
While most NF-PanNETs are clinically indolent, a subset may behave aggressively and metastasise widely.
Advancements in molecular techniques have identified several prognostic biomarkers for PanNETs and include the status of aristaless-related homeobox gene (ARX), pancreatic and duodenal homeobox 1 (PDX1), alpha-thalassemia/mental retardation X-linked (ATRX), death domain-associated protein (DAXX) and alternative lengthening of telomeres (ALT).
The evaluation of these putative biomarkers has largely been restricted to single-centre studies, limited in study cohort size and assessed in diverse patient populations, such as those with functional PanNETs, synchronous distant metastases and germline predisposition syndromes.
The prognostic utility of ARX, PDX1, ATRX, DAXX and ALT in small NF-PanNETs (≤2.0 cm) and their status in neuroendocrine tumours (NETs) from other sites are unknown.
What are the new findings?
Within a multi-institutional, international cohort of NF-PanNETs, loss of ATRX/DAXX and the presence of ALT correlated with several adverse prognostic pathological features and distant metastases/recurrence on follow-up.
Relapse-free survival (RFS) rates were shorter for patients, and, in particular, for those patients with small NF-PanNETs (≤2.0 cm) with ATRX/DAXX loss and/or ALT positivity.
The status of ATRX/DAXX and ALT were independent, negative prognostic biomarkers for NF-PanNETs.
Classifying NF-PanNETs by ARX/PDX1 expression did not independently correlate with RFS.
In addition to primary NF-PanNETs and NF-PanNET metastases, only a small subset of pulmonary carcinoids exhibited ATRX/DAXX loss and ALT.
How might it impact on clinical practice in the foreseeable future?
Incorporating the status of ATRX/DAXX by immunohistochemistry and ALT by telomere-specific fluorescence in situ hybridisation as prognostic biomarkers for NF-PanNETs is clinically feasible and inexpensive.
In the setting of an NET metastasis of unknown origin, ATRX/DAXX and ALT are highly specific diagnostic biomarkers to indicate the possibility of a pancreatic primary.
Introduction
Pancreatic neuroendocrine tumours (PanNETs) are the second most common malignancy of the pancreas and comprise a heterogeneous group of neoplasms. Historically, the incidence of PanNETs has been low, and patients often presented with hormonal hypersecretion (functional PanNETs). However, with increasing use of abdominal imaging, the incidence of PanNETs, especially non-functional PanNETs (NF-PanNETs), has risen rapidly.1 The 5-year survival of patients with NF-PanNETs is reported to be as low as 54% and is highly dependent on the presence of distant metastases.2 Patients with localised disease have a reported 5-year survival of 93% as compared with 27% for patients with metastatic disease. Many patients develop infiltrative and widely metastatic neoplasms, while others may present with slow growing, indolent tumours. In fact, in recent years, the overtreatment of NF-PanNETs has been a subject of debate and an observational approach may be warranted for a subset of patients.3 4
Routinely used prognostic biomarkers for NF-PanNETs are tumour size, grade and stage. Current recommendations by the National Comprehensive Cancer Network, European Neuroendocrine Tumor Society and North American Neuroendocrine Tumor Society are to surgically resect NF-PanNETs >2.0 cm in size with negative margins and regional lymphadenectomy due to their association with metastatic spread.5–7 A limited surgical procedure, or even surveillance, can be considered in NF-PanNETs ≤2.0 cm as these neoplasms often do not metastasise. Nevertheless, NF-PanNETs ≤2.0 cm can be aggressive, including those initially classified as clinically indolent.8–11 In addition to tumour size, WHO advocates grading of NF-PanNETs based on proliferative activity that includes mitotic index and Ki-67 immunohistochemistry.12 Many studies suggest tumours of at least WHO grade 2 are associated with a worse clinical behaviour.13 However, both mitotic index and Ki-67 measurements are susceptible to sampling issues, interpretation errors, and may not truly reflect the clinical behaviour of these neoplasms as WHO grade 1 tumours can also develop distant metastases.14 15 Thus, additional biomarkers are needed to improve the prognostic classification and management of patients.
Advancements in molecular technologies have identified several putative prognostic biomarkers for NF-PanNETs. Whole exome and whole-genome sequencing studies have found recurrent alterations in alpha-thalassemia/mental retardation X-linked chromatin remodeler (ATRX) and death domain-associated protein (DAXX) that are associated with metastatic disease.16–20 Mutations in these genes often result in loss of nuclear expression of their respective proteins by immunohistochemistry. Furthermore, ATRX/DAXX loss frequently coincides with the presence of alternative lengthening of telomeres (ALT), a telomerase-independent telomere maintenance mechanism, which can reliably be assayed using telomere-specific fluorescence in situ hybridisation (FISH).21 Loss of ATRX/DAXX and the presence of ALT correlate with a shorter relapse-free survival (RFS).22–24 Additionally, whole transcriptome and epigenome studies have found the differential expression of the transcription factors, aristaless-related homeobox gene (ARX) and pancreatic and duodenal homeobox 1 (PDX1), can also determine the risk of metastatic disease.25 26 PDX1 expression is typically associated with an indolent clinical behaviour, while the expression of ARX or the lack of both proteins correlates with an aggressive disease course. The status of these transcription factors in PanNETs was recently reported to be a prognostic biomarker for RFS and independent of tumour size, WHO grade and ALT.26
However, the prognostic significance of ARX and PDX1 in NF-PanNETs was assessed in a study cohort limited in number and enriched towards patients with germline predisposition syndromes.26 In contrast, loss of ATRX/DAXX and the presence of ALT in NF-PanNETs have been evaluated by multiple investigators, but their clinical utility among patients with NF-PanNETs of ≤2.0 cm has not been comprehensively analysed. Moreover, the status of ARX, PDX1, ATRX/DAXX and ALT in NF-PanNET metastases and neuroendocrine tumours (NETs) from other organ sites remains largely unexplored and may be of clinical and prognostic relevance within these neoplasms. In fact, in addition to the pancreas, the expression of ARX and PDX1 are key factors for endocrine progenitor cells of other organ sites.27 Therefore, the objectives of this study were to (1) determine the correlation of ARX and PDX1 expression with clinicopathological features in a large, international, multi-institutional cohort of sporadic NF-PanNETs without distant metastatic disease at presentation, (2) validate the prognostic significance of these transcription factors, ATRX/DAXX, and ALT in NF-PanNETs and include a separate analysis among tumours of ≤2.0 cm and (3) comprehensively evaluate the status of all five biomarkers in NF-PanNETs metastases and NETs from other organ sites.
Materials and methods
Study design and case selection
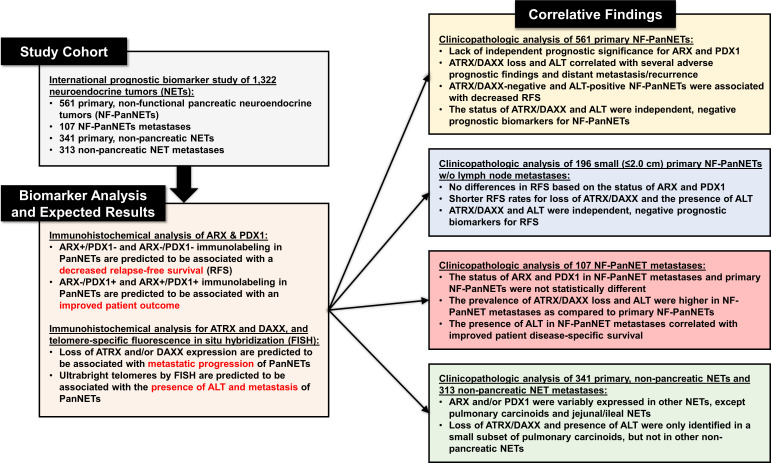
The study design is summarised in figure 1 and includes the overall patient cohort, biomarker staining and expected results, and correlative clinicopathological findings. The surgical pathology archives from Amsterdam University Medical Center (UMC), UMC Utrecht, ARC-Net Research Center, Asan Medical Center, Barnes-Jewish Hospital and University of Pittsburgh Medical Center (UPMC) were queried for neuroendocrine neoplasms of the pancreas between 1991 and 2017 that underwent enucleation, central pancreatectomy, pancreaticoduodenectomy or distal pancreatectomy. Cases were cross-referenced with clinical and follow-up data obtained from patient paper and/or electronic medical records. The study inclusion criteria consisted of the following: a solitary, clinically non-functional, well-differentiated NET (confirmed with positive immunolabelling for neuroendocrine markers: synaptophysin and/or chromogranin A) centred within the pancreas; absence of a confirmed or suspected genetic syndrome associated with pancreatic neuroendocrine neoplasms: multiple endocrine neoplasia type 1 (MEN1) syndrome, von Hippel-Lindau syndrome, neurofibromatosis type 1 syndrome, familial atypical multiple mole melanoma syndrome and tuberous sclerosis complex syndrome; surveillance and survival data; and cases with sufficient material for ancillary studies. Patients with a confirmed or suspected genetic syndrome associated with PanNETs were excluded considering these patients may develop multiple primary PanNETs, and, within these scenarios, sampling of each primary from the same patient is impractical for prognostication purposes. It is important to underscore that exclusion of patients was based on both clinical and germline data; however, formal germline testing for all patients included within this study was not performed.
Figure 1.
A summary of the study design to include details of individual patient cohorts, biomarker staining with expected results and correlative clinicopathological findings on individual biomarker analysis. ALT, alternative lengthening of telomeres; ARX, aristaless-related homeobox gene; ATRX, alpha-thalassemia/mental retardation X-linked; DAXX, death domain-associated protein; PDX1, pancreatic and duodenal homeobox 1.
In total, 561 patients with a resected NF-PanNET fulfilled the aforementioned criteria and consisted of the following: 28 patients from Amsterdam UMC, 27 patients from UMC Utrecht, and 82 patients from ARC-Net Research Center (online supplemental table S1), 168 patients from Asan Medical Center (online supplemental table S2) and 79 patients from Barnes-Jewish Hospital and 177 patients from UPMC (online supplemental table S3). Within the same timeframe, the surgical pathology archives at Barnes-Jewish Hospital and UPMC were also queried to identify NF-PanNET distant organ metastases and yielded 107 patients with sufficient pathological material available of their metastatic specimen for ancillary studies. Further, NETs from other organ sites to include primaries (n=341) and distant organ metastases (n=313) were identified within the UPMC surgical pathology archives for subsequent analysis. Archival pathological material from all 1322 neoplasms was used to create high-density tissue microarrays (TMAs) as previously described.18 23 24 28 29 While the TMAs were used for telomere-specific FISH, whole tumour sections for 256 primary NF-PanNETs, 63 NF-PanNET metastases and 132 primary, non-pancreatic NETs and 62 non-pancreatic NET metastases were evaluated immunohistochemically for ARX, PDX1, ATRX and DAXX.
gutjnl-2020-322595supp001.pdf (150.4KB, pdf)
Clinical and demographic data were reviewed for each case. Corresponding pathology gross reports and H&E stained slides were also reviewed for the following pathological features: tumour size, location, lymphovascular invasion, perineural invasion, extension outside of the pancreas and regional lymph node metastasis. Each primary NF-PanNET was graded using the 2017/2019 WHO classification system for pancreatic neuroendocrine neoplasms on the basis of mitotic rate and Ki-67 immunohistochemistry as Grade 1 (G1), <2 mitoses/2 mm2 and Ki-67 of <3%; Grade 2 (G2), 2 to 20 mitoses/2 mm2 or Ki-67 of 3%–20%; or grade 3 (G3), >20 mitoses/2 mm2 or Ki-67 of >20%.12 30 For cases with discordant mitotic rate and Ki-67 measurements, the highest grade was assigned. Pathological primary tumour classification was determined according to the eighth edition of the American Joint Committee on Cancer Staging Manual, which is based on tumour size and extent of invasion.31 Follow-up information was extracted from the patient’s medical records to include data on surveillance, RFS, and disease-specific survival (DSS). Pancreatic and non-pancreatic neuroendocrine carcinomas were excluded from this study.
Immunohistochemistry
TMAs or whole section tumour slides were deparaffinised with serial xylene treatments and subjected to antigen retrieval using heated citrate solution. Immunolabelling for ARX (MABN102 mouse monoclonal, Millipore), PDX1 (AB134150 rabbit monoclonal, Abcam), ATRX (HPA001906 rabbit polyclonal, Sigma Aldrich) and DAXX (HPA008736 rabbit polyclonal, Sigma Aldrich or AB32140 rabbit monoclonal, Abcam) were performed either on an automated Ventana Benchmark XT system using the biotin-free Ventana OptiView DAB IHC Detection Kit (Ventana Medical Systems) or manually (online supplemental materials and methods).
gutjnl-2020-322595supp002.pdf (23.6KB, pdf)
Assessment of ARX, PDX1, ATRX and DAXX was done blinded to any patient data including outcome. For ARX and PDX1, ‘positive’ expression was defined as nuclear staining of >10% of neoplastic cells as previously described.28 32 Tumour tissue with ≤10% of nuclear expression was scored as ‘negative’ expression. Surrounding islets within the uninvolved pancreas were used as a positive internal control. Immunolabelling for ATRX and DAXX was considered ‘positive’ or preserved if >5% of neoplastic cells had nuclear staining, as previously described.22 23 Neoplasms were scored as ‘negative’ or loss of staining for ATRX or DAXX if the pattern was that of cytoplasmic accumulation with nuclear clearing, as long as adequate internal controls (eg, nuclear labelling of adjacent endothelial cells, lymphocytes and/or islets of Langerhans) were present. Intratumoural heterogeneity or heterogeneous staining of ATRX and DAXX was defined as the clear presence of two distinct populations of tumour cells demonstrating preserved and loss of nuclear staining.24 For subsequent statistical analysis, cases with heterogeneous staining were scored as loss or negative staining.
Fluorescence in situ hybridisation
Telomere-specific FISH was performed as previously described using a fluorescently tagged telomeric-C PNA probe for each TMA (online supplemental materials and methods).33 Scoring for ALT was performed by assessing at least 250 nuclei from all for each case. Using previously described criteria, ALT-positive cases were defined by the presence of large, ultrabright intranuclear foci consistent with telomere FISH signals in at least 1% of tumour nuclei and the total signal intensity for individual foci>10 fold than telomere signals from stromal cells.23 24 Importantly, areas of necrosis were excluded from evaluation.
Statistical analysis
χ2 analysis or Fisher’s exact tests were used to compare categorical data, and Mann-Whitney U test was used to compare continuous variables. Survival curves were constructed using the Kaplan-Meier method and differences between groups were evaluated by the log-rank test. RFS was calculated from the date of surgery to the date of first distant metastasis/local recurrence after surgery and censored at the date of last follow-up or death. The prognostic significance of clinical and pathological characteristics was determined using univariate Cox regression analysis. Multivariate analyses of significant risk factors by univariate analysis were performed using Cox proportional hazard regression to identify independent risk factors for RFS. DSS for patients with NF-PanNETs metastases was calculated from the date of primary diagnosis and date of metastatic diagnosis to the date of death due to disease or date of last follow-up (if death did not occur). All statistical analyses were performed using the SPSS Statistical software, V.25 (IBM) and statistical significance was defined as a p<0.05. The Cox proportional hazard assumptions were inspected for each covariate and graphed using scaled Schoenfeld residuals. For each covariate, and each model as a whole, none of the Cox proportional hazard assumptions were violated. These analyses were performed with the survival and survminer packages for R V.4.0.2.
Results
The clinicopathological features of ARX, PDX1, ATRX/DAXX and ALT in NF-PanNETs
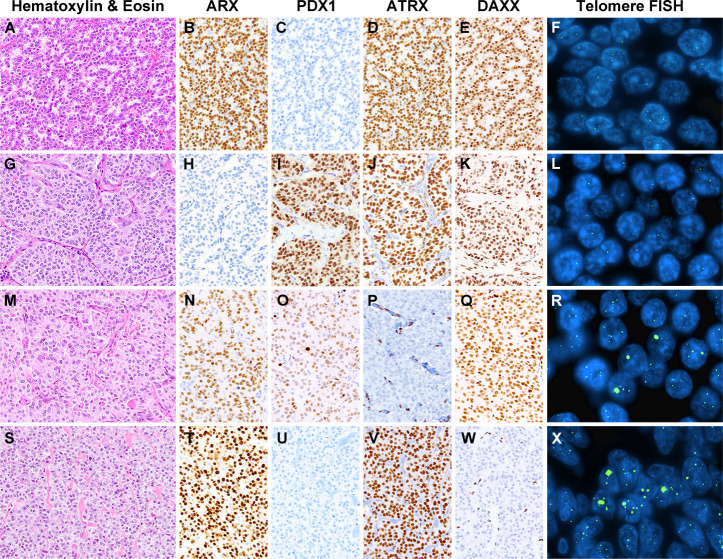
Among an international, multi-institutional cohort of 561 non-syndromic and NF-PanNETs without distant metastases at surgical resection, nuclear expression for ARX and PDX1 was identified in 406 (72%) and 239 (43%) cases (figure 2), respectively, and summarised in online supplemental table S1 and online supplemental data. Expression of both proteins was present in 126 (22%) NF-PanNETs and absent in 42 (8%) NF-PanNETs. In comparison to ARX-negative NF-PanNETs, ARX expression correlated with larger tumour size, pancreatic body/tail location, high WHO grade, negative regional lymph node involvement, loss of ATRX/DAXX immunolabelling and ALT (p<0.050 for all). PDX1-positive NF-PanNETs were more frequently found in female patients and associated with pancreatic head/uncinate/neck location, preserved immunolabelling for ATRX/DAXX, and an absence of ALT (p<0.050 for all).
Figure 2.
Representative examples of non-functional pancreatic neuroendocrine tumours (NF-PanNETs) that have been assessed by immunolabelling for ARX, PDX1, ATRX and DAXX, and telomere-specific fluorescence in situ hybridisation (FISH) for ALT. (A) NF-PanNET with positive expression for ARX (B) and negative expression for PDX1 (C), while both ATRX (D) and DAXX (E) exhibited preserved nuclear expression and an absence of alternative lengthening of telomeres (ALT) (F). (G) NF-PanNET with negative expression for ARX (H) and positive expression for PDX1 (I), while both ATRX (J) and DAXX (K) had preserved expression and an absence of ALT (L). (M) NF-PanNET with expression for both ARX (N) and PDX1 (O), but loss of nuclear ATRX (P) and preserved nuclear expression for DAXX (Q). the loss of ATRX expression correlated with the presence of large, ultrabright intranuclear foci by telomere-specific FISH, consistent with ALT (R). (S) NF-PanNET with positive ARX expression (T), negative PDX1 expression (U), preserved ATRX expression (V) and loss of DDAXX expression (W). Similar to loss of ATRX, DAXX-negative NF-PanNETs were associated with large, ultrabright telomere signals, consistent with ALT (X). ARX, aristaless-related homeobox gene; ATRX, alpha-thalassemia/mental retardation X-linked; DAXX, death domain-associated protein; PDX1, pancreatic and duodenal homeobox 1.
gutjnl-2020-322595supp003.pdf (41.1KB, pdf)
Based on ARX and PDX1 status, the NF-PanNET study cohort was subdivided into the following four immunophenotypic groups: 280 (50%) ARX-positive/PDX1-negative, 113 (20%) ARX-negative/PDX1-positive, 126 (22%) ARX-positive/PDX1-positive (‘double positive’, DP), and 42 (8%) ARX-negative/PDX1-negative (‘double negative’, DN) cases (online supplemental table S4). Further, in accordance with Cejas et al,26 NF-PanNETs classified as ARX-positive/PDX1-negative, and ‘DN’ were combined into a single group (ARX++DN, n=322 (57%)) and summarised in online supplemental table S5. Likewise, ARX-negative/PDX1-positive, and ‘double positive’ NF-PanNETs were categorised together (PDX1++DP, n=239 (43%)). In relation to PDX1++DP NF-PanNETs, ARX++DN NF-PanNETs were more likely to occur in male patients, show pancreatic body/tail location, have loss of ATRX/DAXX immunolabelling and exhibit ALT (p<0.050 for all).
The presence of ALT and ATRX/DAXX loss in NF-PanNETs was identified in 160 (29%) and 142 (25%) cases, respectively (table 1 and online supplemental table S6). Further, among the entire study cohort, ALT positivity and/or loss of ATRX/DAXX were detected in 170 (30%) NF-PanNETs. ALT-positive NF-PanNETs, as compared with ALT-negative NF-PanNETs, were associated with a male predilection, and loss of ATRX/DAXX immunolabelling (p<0.001 for all). In addition, ALT-positive NF-PanNETs exhibited larger tumour size, high WHO grade, lymphovascular invasion, perineural invasion, advanced pathological T-stage, regional lymph node metastases, and metachronous (postoperative) distant metastases/recurrences (p<0.001 for all). Similar associations were seen with ATRX/DAXX loss (online supplemental table S5) and the combination of ALT positivity and/or loss of ATRX/DAXX.
Table 1.
Clinical and pathological comparison of ARX, PDX1 and ALT status in 561 non-syndromic, primary NF-PanNETs
| Patient or tumour characteristics | Total, n=561 | ARX-positive, n=406 (72%) |
ARX-negative, n=155 (28%) |
P value | PDX1-positive, n=239 (43%) |
PDX1-negative, n=322 (57%) |
P value | ALT-positive, n=160 (29%) |
ALT-negative, n=401 (71%) |
P value |
| Gender | ||||||||||
| Female | 275 (49%) | 191 (47%) | 84 (54%) | 0.132 | 131 (55%) | 144 (45%) | 0.021 | 59 (37%) | 216 (54%) | <0.001 |
| Male | 286 (51%) | 215 (53%) | 71 (46%) | 108 (45%) | 178 (55%) | 101 (63%) | 185 (46%) | |||
| Mean age (range), years | 56.9 (20–93) | 56.5 (23–83) | 57.9 (20–93) | 0.265 | 56.2 (20–93) | 57.4 (22–85) | 0.233 | 58.2 (26–93) | 56.4 (20–85) | 0.270 |
| Mean tumour size (range), cm | 3.3 (0.6–18.0) | 3.4 (0.7–18.0) | 2.9 (0.6–10.0) | 0.017 | 3.0 (0.6–18.0) | 3.5 (0.7–15.5) | 0.063 | 4.7 (0.7–18.0) | 2.7 (0.6–10.0) | <0.001 |
| Location | ||||||||||
| Head, neck and uncinate | 233 (41%) | 153 (38%) | 80 (52%) | 0.006 | 117 (49%) | 116 (36%) | 0.002 | 61 (38%) | 172 (43%) | 0.490 |
| Body and tail | 325 (58%) | 250 (61%) | 75 (48%) | 120 (50%) | 205 (64%) | 98 (61%) | 227 (56%) | |||
| Diffuse | 3 (1%) | 3 (1%) | 0 (0%) | 2 (1%) | 1 (<1%) | 1 (1%) | 2 (1%) | |||
| WHO grade | ||||||||||
| Low (G1) | 362 (65%) | 256 (63%) | 106 (68%) | 0.020 | 163 (68%) | 199 (62%) | 0.050 | 58 (36%) | 304 (76%) | <0.001 |
| Intermediate (G2) | 189 (33%) | 146 (36%) | 43 (28%) | 75 (31%) | 114 (35%) | 99 (62%) | 90 (22%) | |||
| High (G3) | 10 (2%) | 4 (1%) | 6 (4%) | 1 (1%) | 9 (3%) | 3 (2%) | 7 (2%) | |||
| Lymphovascular invasion | n=424 | n=299 | n=125 | n=163 | n=261 | n=112 | n=312 | |||
| Absent | 282 (67%) | 199 (67%) | 83 (66%) | 1.000 | 111 (68%) | 171 (66%) | 0.599 | 43 (38%) | 239 (77%) | <0.001 |
| Present | 142 (33%) | 100 (33% | 42 (34%) | 52 (32%) | 90 (34%) | 69 (62%) | 73 (23%) | |||
| Perineural invasion | n=424 | n=299 | n=125 | n=163 | n=261 | n=112 | n=312 | |||
| Absent | 341 (80%) | 248 (83%) | 93 (74%) | 0.060 | 129 (79%) | 212 (81%) | 0.616 | 73 (65%) | 268 (86%) | <0.001 |
| Present | 83 (20%) | 51 (17%) | 32 (26%) | 34 (21%) | 49 (19%) | 39 (35%) | 44 (14%) | |||
| Primary tumour (pT) stage | ||||||||||
| T1 | 179 (32%) | 120 (30%) | 59 (38%) | 0.115 | 79 (33%) | 100 (31%) | 0.404 | 13 (8%) | 166 (41%) | <0.001 |
| T2 | 228 (41%) | 168 (41%) | 60 (39%) | 103 (43%) | 125 (39%) | 69 (43%) | 159 (40%) | |||
| T3 | 147 (26%) | 111 (27%) | 36 (23%) | 55 (23%) | 92 (28%) | 71 (44%) | 76 (19%) | |||
| T4 | 7 (1%) | 7 (2%) | 0 (0%) | 2 (1%) | 5 (2%) | 7 (5%) | 0 (0%) | |||
| Regional node (pN) stage | n=502 | n=368 | n=134 | n=213 | n=289 | n=155 | n=347 | |||
| N0 | 362 (72%) | 277 (75%) | 85 (63%) | 0.010 | 146 (69%) | 216 (75%) | 0.132 | 85 (55%) | 277 (80%) | <0.001 |
| N1 | 140 (28%) | 91 (25%) | 49 (37%) | 67 (31%) | 73 (25%) | 70 (45%) | 70 (20%) | |||
| Metachronous metastases/recurrences | ||||||||||
| Absent | 423 (75%) | 298 (70%) | 125 (81%) | 0.080 | 189 (79%) | 234 (73%) | 0.092 | 71 (44%) | 352 (88%) | <0.001 |
| Present | 138 (25%) | 108 (30%) | 30 (19%) | 50 (21%) | 88 (27%) | 89 (56%) | 49 (12%) | |||
| ARX expression | ||||||||||
| Negative | 155 (28%) | 113 (47%) | 42 (13%) | <0.001 | 18 (11%) | 137 (34%) | <0.001 | |||
| Positive | 406 (72%) | 126 (53%) | 280 (87%) | 142 (89%) | 264 (66%) | |||||
| PDX1 expression | ||||||||||
| Negative | 322 (57%) | 280 (69%) | 42 (27%) | <0.001 | 115 (72%) | 207 (52%) | <0.001 | |||
| Positive | 239 (43%) | 126 (31%) | 113 (73%) | 45 (28%) | 194 (48%) | |||||
| ATRX/DAXX expression | ||||||||||
| Preserved | 419 (75%) | 283 (70%) | 136 (88%) | <0.001 | 197 (82%) | 222 (69%) | <0.001 | 28 (18%) | 391 (98%) | <0.001 |
| Loss | 142 (25%) | 123 (30%) | 19 (12%) | 42 (18%) | 100 (31%) | 132 (82%) | 10 (2%) | |||
| ALT status | ||||||||||
| Negative | 401 (71%) | 264 (65%) | 137 (88%) | <0.001 | 194 (81%) | 207 (64%) | <0.001 | |||
| Positive | 160 (29%) | 142 (35%) | 18 (12%) | 45 (19%) | 115 (36%) |
ALT, alternative lengthening of telomeres; ARX, aristaless-related homeobox; ATRX, alpha-thalassemia/mental retardation X-linked; DAXX, death domain-associated protein; NF-PanNET, non-functional pancreatic neuroendocrine tumour; PDX1, pancreatic and duodenal homeobox 1.
The prognostic significance of ARX, PDX1, ATRX/DAXX and ALT in NF-PanNETs
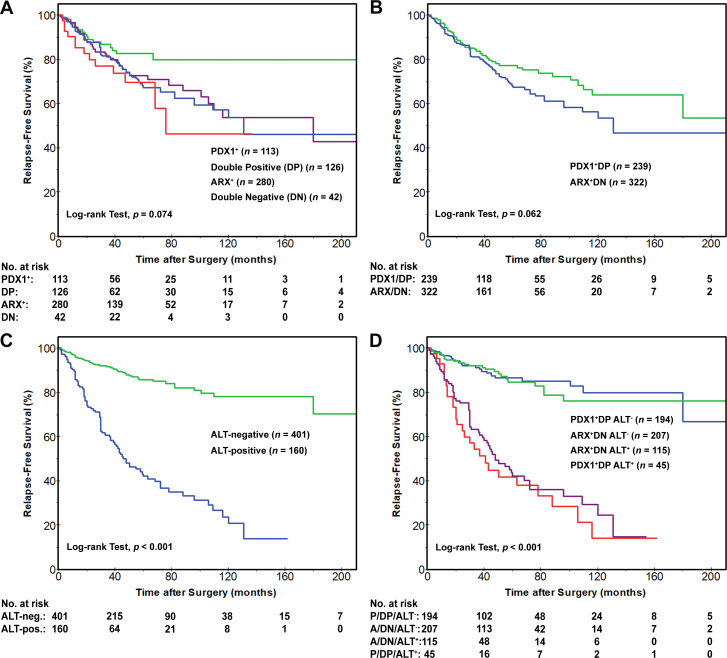
An analysis of RFS revealed no statistically significant differences based on the status of ARX, PDX1 or among the four immunophenotypic groups (figure 3A). Furthermore, no RFS differences were observed between patients with ARX++DN NF-PanNETs and patients with PDX1++DP NF-PanNETs (figure 3B). In contrast, the RFS for patients with ALT-positive NF-PanNETs was significantly shorter than patients with ALT-negative NF-PanNETs (figure 3C). Patient RFS rates associated with ALT-positive NF-PanNETs were 86%, 42% and 21% at 1, 5 and 10 years, respectively, as compared with the ALT-negative NF-PanNETs with 96%, 86% and 78% at 1, 5 and 10 years, respectively (p<0.001). However, the combination of ALT status and the immunophenotypic subgroups, ARX++DN and PDX1++DP, did not demonstrate any additional prognostic relevance over ALT status alone (figure 3D). Of note, a longer RFS was identified among patients with ARX-negative/PDX1-positive NF-PanNETs as compared with the remaining patients (p=0.014); however, there was a lack of additional prognostic significance on accounting for ALT (online supplemental figure S1). Loss of ATRX/DAXX immunolabelling was also associated with shorter RFS with 1-year, 5-year and 10-year rates of 90%, 40% and 20%, respectively, as compared with 96%, 85% and 78%, respectively, for preserved ATRX/DAXX immunolabelling (p<0.001). The combination of ALT-positive and/or ATRX/DAXX-negative NF-PanNETs correlated with shorter RFS rates of 90%, 42% and 21% at 1, 5 and 10 years, respectively, as compared with ALT-negative and ATRX/DAXX positive NF-PanNETs with 97%, 87% and 80% at 1, 5 and 10 years, respectively (p<0.001).
Figure 3.
Kaplan-Meier curves comparing relapse-free survival (RFS) after surgical resection for patients with NF-PanNETs. (A) No statistically significant differences in RFS were identified between patients with NF-PanNETs (n=561) that were classified into four immunophenotypic groups: ARX-positive/PDX1-negative (ARX+), ARX-negative/PDX1-positive (PDX+), ARX-positive/PDX1-positive (DP) and ARX-negative/PDX1-negative (DN). (B) Similarly, no RFS difference was seen between patients with ARX++DN NF-PanNETs and patients with PDX1++DP NF-PanNETs. however (C) the RFS for patients with ALT-positive NF-PanNETs was significantly shorter than patients with ALT-negative NF-PanNETs. In addition, (D) the combination of ALT status and the immunophenotypic subgroups, ARX++DN and PDX1++DP, did not demonstrate any prognostic benefit over ALT status alone. ALT, alternative lengthening of telomeres; ARX, aristaless-related homeobox gene; NF-PanNETs, non-functional pancreatic neuroendocrine tumours; PDX1, pancreatic and duodenal homeobox 1.
gutjnl-2020-322595supp004.pdf (939.5KB, pdf)
Results of univariate and multivariate Cox regression analysis for RFS in relation to various clinicopathological features including ARX, PDX1, ATRX/DAXX and ALT status are shown in table 2. By univariate analysis, shorter RFS was associated with tumour size >2.0 cm, tumour size as a continuous variable, G2-to-G3 WHO grade, lymphovascular invasion, perineural invasion, advanced tumour stage (pT3 and pT4 vs pT1 and pT2), regional lymph node metastasis, loss of ATRX/DAXX immunolabelling and the presence of ALT (p<0.001 for all). Multivariate analysis was used to determine the prognostic significance of ALT for RFS and included tumour size >2.0 cm, WHO grade, lymphovascular invasion, perineural invasion and regional lymph node metastasis. The presence of ALT was an independent prognostic factor for RFS (p<0.001), and similar results were observed with loss of ATRX/DAXX immunolabelling when substituted for ALT or combined with ALT (p<0.001 for both).
Table 2.
Univariate and multivariate Cox regression analyses for relapse-free survival among 561 primary NF-PanNETs and 196 primary NF-PanNETs of ≤2.0 cm and without lymph node metastases
| Patient or tumour characteristics | Entire NF-PanNET cohort | NF-PanNETs ≤2.0 cm without lymph node metastases | ||||||
| Univariate Cox regression analysis | Multivariate Cox regression analysis | Univariate Cox regression analysis | Multivariate Cox regression analysis | |||||
| Relapse-free survival HR (95% CI) | P value | Relapse-free survival HR (95% CI) | P value | Relapse-free survival HR (95% CI) | P value | Relapse-free survival HR (95% CI) | P value | |
| Gender, female versus male (ref.) | 1.354 (0.964 to 1.900) | 0.080 | 1.501 (0.559 to 4.028) | 0.420 | ||||
| Age, years* | 1.013 (0.999 to 1.027) | 0.067 | 1.034 (0.992 to 1.078) | 0.113 | ||||
| Tumour size, >2.0 cm vs ≤2.0 cm (ref.) | 3.036 (1.954 to 4.719) | <0.001 | 2.214 (1.114 to 4.399) | 0.023 | ||||
| Tumour size* | 1.198 (1.142 to 1.257) | <0.001 | 4.352 (1.15 to 16.454 | 0.030 | 2.251 (0.556 to 9.107) | 0.255 | ||
| Location, head, neck and uncinate vs body and tail/diffuse (ref.) | 1.219 (0.873 to 1.704) | 0.245 | 2.244 (0.884 to 5.695) | 0.089 | ||||
| WHO grade, G2 or G3 vs G1 (ref.) | 3.282 (2.335 to 4.612) | <0.001 | 1.492 (0.901 to 2.471) | 0.120 | 1.854 (0.596 to 5.772) | 0.286 | ||
| Lymphovascular invasion, presence vs absence (ref.)† | 3.562 (2.311 to 5.491) | <0.001 | 1.057 (0.615 to 1.815) | 0.841 | ||||
| Perineural invasion, presence vs absence (ref.)† | 3.042 (1.971 to 4.695) | <0.001 | 1.158 (0.689 to 1.946) | 0.580 | ||||
| Tumour stage (pT), pT3 and pT4 vs pT1 and pT2 (ref.) | 2.825 (2.020 to 3.951) | <0.001 | ||||||
| Lymph node metastasis (pN), pN1 vs pN0 (ref.)‡ | 4.041 (2.853 to 5.721) | <0.001 | 2.150 (1.276 to 3.621) | 0.004 | ||||
| ARX expression, positive vs negative (ref.) | 1.381 (0.921 to 2.071) | 0.118 | 2.584 (0.748 to 8.931) | 0.133 | ||||
| PDX1 expression, positive vs negative (ref.) | 0.720 (0.508 to 1.019) | 0.064 | 0.880 (0.346 to 2.239) | 0.788 | ||||
| Immunophenotype, ARX1+ + DN vs. PDX1+ + DP (ref.) | 1.390 (0.981 to 1.968) | 0.064 | 1.137 (0.447 to 2.893) | 0.788 | ||||
| ATRX/DAXX expression, loss versus preserved (ref.) | 5.144 (3.647 to 7.257) | <0.001 | 4.199 (1.490 to 11.830) | 0.007 | ||||
| ALT, positive versus negative (ref.) | 5.650 (3.964 to 8.055) | <0.001 | 3.514 (2.152 to 5.739) | <0.001 | 5.629 (2.171 to 14.595) | <0.001 | 4.155 (1.428 to 12.087) | 0.009 |
*The patient or tumour characteristic of interest was evaluated as a continuous variable.
†Cox regression analysis is based on data available for 424 of 561 (76%) patients.
‡Cox regression analysis is based on data available for 502 of 561 (90%) patients.
ALT, alternative lengthening of telomeres; ARX, aristaless-related homeobox; ATRX, alpha-thalassemia/mental retardation X-linked; DAXX, death domain-associated protein; DN, double negative for ARX and PDX1; DP, double positive for ARX and PDX1; NF-PanNET, non-functional pancreatic neuroendocrine tumour; PDX1, pancreatic and duodenal homeobox 1; ref, reference.
Prognostic biomarkers for NF-PanNETs ≤2.0 cm and without regional lymph node metastases
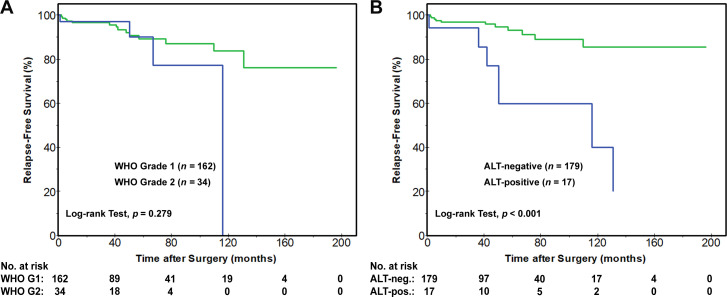
Considering the difficulties in predicting the clinical behaviour of NF-PanNETs≤2.0 cm and without regional lymph node metastases, a separate subanalysis was performed for 196 patients with small (≤2.0 cm) NF-PanNETs (online supplemental table S7). No statistically significant differences in RFS were identified based on the status of ARX, PDX1, four immunophenotypic groups, ARX++DN and PDX1++DP and WHO grade (figure 4A). Shorter RFS rates were seen with loss of ATRX/DAXX immunolabelling and the presence of ALT. The 1-year, 5-year and 10-year RFS rates were 92%, 65% and 32%, respectively, for patients with loss of ATRX/DAXX as compared with 97%, 92% and 85%, respectively, for patients with preserved ATRX/DAXX (p=0.003). Similarly, 1-year, 5-year and 10-year RFS rates for patients with ALT-positive NF-PanNETs were 94%, 60% and 40%, respectively, as compared with 97%, 93% and 86%, respectively, for patients with ALT-negative NF-PanNETs (p<0.001) (figure 4B).
Figure 4.
Kaplan-Meier curves for RFS among patients with NF-PanNETs of ≤2.0 cm and without regional lymph node metastases (n=196). Based on the 2017/2019 WHO grading system for NF-PanNETs, (A) no statistically significant difference in RFS was identified; however (B) the presence of ALT was associated with shorter RFS. ALT, alternative lengthening of telomeres; NF-PanNETs, non-functional pancreatic neuroendocrine tumours; RFS, relapse-free survival.
By univariate Cox regression analysis of cases ≤2.0 cm, tumour size as a continuous variable, loss of ATRX/DAXX immunolabelling, and the presence of ALT (p<0.050 for all) were the only tumour characteristics associated with shorter RFS. Moreover, within a multivariate model that included tumour size and ALT status, the presence of ALT was an independent prognostic factor for RFS (p=0.009) and interchangeable with loss of ATRX/DAXX immunolabelling (p=0.042). Recursive partitioning for tumour size was attempted to determine an optimal prognostic threshold within this subgroup and yielded an optimal ≤1.6 cm cut-off (p=0.017) (online supplemental table S8). The 1-year, 5-year and 10-year RFS rates for patients with NF-PanNETs of ≤1.6 cm were 98%, 92% and 92%, respectively, as compared with 95%, 85% and 61%, respectively, for patients with NF-PanNETs of >1.6 cm. However, by multivariate analysis, the presence of ALT (p=0.011) and the loss of ATRX/DAXX immunolabelling (p=0.044) were independent prognostic factors, but tumour size of ≤1.6 cm was not significant in either multivariate model.
The status of ARX, PDX1, ATRX/DAXX and ALT in NF-PanNET metastases, other primary NETs and NET metastases
Within a separate cohort of 107 NF-PanNET metastases from various distant sites, nuclear expression for ARX and PDX1 expression was identified in 85 (79%) and 36 (34%) cases, respectively (table 3 and online supplemental data). In comparison to 561 primary NF-PanNETs, the status of ARX and PDX1 in NF-PanNET metastases was not statistically different. Conversely, the presence of ALT was detected in a higher proportion of NF-PanNET metastases (n=76, 71%) as compared with primary NF-PanNETs (n=160, 29%, p<0.001). A similar correlation was found for ATRX/DAXX loss among 66 (62%) NF-PanNET metastases and 142 (25%) primary NF-PanNETs (p<0.001).
Table 3.
Comparison of ARX, PDX1, ATRX/DAXX and ALT status among 1322 neuroendocrine tumours of the pancreas and other organ sites
| Tumour type | Total, n=1322 | ARX-positive/ PDX1-negative |
ARX-negative/ PDX1-positive |
ARX-positive/ PDX1-positive |
ARX-negative/ PDX1-negative |
Loss of ATRX/DAXX | ALT-positive |
| Primary NF-PanNET | 561 | 280 (50%) | 113 (20%) | 126 (22%) | 42 (8%) | 142 (25%) | 160 (29%) |
| NF-PanNET metastases | 107 | 71 (66%) | 22 (21%) | 14 (13%) | 0 (0%) | 66 (62%) | 76 (71%) |
| Primary pulmonary carcinoid/atypical carcinoid | 48 | 0 (0%) | 0 (0%) | 0 (0%) | 48 (100%) | 2 (4%) | 2 (4%) |
| Carcinoid/atypical carcinoid metastases | 28 | 0 (0%) | 0 (0%) | 0 (0%) | 28 (100%) | 0 (0%) | 0 (0%) |
| Primary gastric NET | 32 | 26 (82%) | 2 (6%) | 2 (6%) | 2 (6%) | 0 (0%) | 0 (0%) |
| Gastric NET metastases | 10 | 1 (10%) | 0 (0%) | 0 (0%) | 9 (90%) | 0 (0%) | 0 (0%) |
| Primary duodenal NET | 41 | 3 (7%) | 15 (37%) | 21 (51%) | 2 (5%) | 0 (0%) | 0 (0%) |
| Duodenal NET metastases | 32 | 9 (30%) | 2 (7%) | 19 (56%) | 2 (7%) | 0 (0%) | 0 (0%) |
| Primary ampullary NET | 7 | 3 (42%) | 2 (29%) | 2 (29%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Primary jejunal NET | 35 | 0 (0%) | 0 (0%) | 0 (0%) | 35 (100%) | 0 (0%) | 0 (0%) |
| Jejunal NET metastases | 71 | 0 (0%) | 0 (0%) | 0 (0%) | 71 (100%) | 0 (0%) | 0 (0%) |
| Primary ileal NET | 123 | 0 (0%) | 0 (0%) | 0 (0%) | 123 (100%) | 0 (0%) | 0 (0%) |
| Ileal NET metastases | 151 | 0 (0%) | 0 (0%) | 0 (0%) | 151 (100%) | 0 (0%) | 0 (0%) |
| Primary colonic NET | 8 | 1 (12%) | 0 (0%) | 0 (0%) | 7 (88%) | 0 (0%) | 0 (0%) |
| Colonic NET metastases | 8 | 0 (0%) | 0 (0%) | 0 (0%) | 8 (100%) | 0 (0%) | 0 (0%) |
| Primary rectal NET | 17 | 16 (94%) | 0 (0%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rectal NET metastases | 10 | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Primary appendiceal NET | 28 | 26 (93%) | 0 (0%) | 2 (7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Primary gallbladder NET | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) |
| Gallbladder NET metastases | 3 | 0 (0%) | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | 0 (0%) |
ALT, alternative lengthening of telomeres; ARX, aristaless-related homeobox; ATRX, alpha-thalassemia/mental retardation X-linked; DAXX, death domain-associated protein; NET, neuroendocrine tumour; NF-PanNET, non-functional pancreatic neuroendocrine tumour; PDX1, pancreatic and duodenal homeobox 1.
A clinicopathological analysis of NF-PanNET metastases revealed an association between ALT-positive NF-PanNET metastases and male gender, primary pancreatic body/tail location, positive ARX expression, negative PDX1 expression, loss of ATRX/DAXX expression and more likely to present as metachronous vs synchronous disease (p<0.05) (online supplemental table S9). However, 5-year and 10-year DSS rates were longer for patients with ALT-positive NF-PanNET metastases with respect to the time after primary diagnosis (72% and 45% vs 51% and 24%, respective, p=0.027) and time after diagnosis of metastatic disease (61% and 38% vs 38% and 17%, respectively, p=0.043) (online supplemental figure S2). A similar statistical association between ATRX/DAXX loss and DSS was not observed.
gutjnl-2020-322595supp005.pdf (1,005.4KB, pdf)
In addition to primary NF-PanNETs and NF-PanNET metastases, a cohort of 341 primary, non-pancreatic NETs and 313 NET metastases were evaluated for ARX and PDX1 (table 3 and online supplemental data). Except for lung, jejunal, ileal and gallbladder NETs, both ARX and PDX1 were expressed in NETs from multiple organ sites (online supplemental figure S3) but their status did not demonstrate clinicopathological nor prognostic significance (online supplemental table S10). In contrast, ALT was detected in only 2 (of 48, 4%) non-metastatic carcinoids of the lung and both cases exhibited ATRX loss by immunohistochemistry (online supplemental figure S4). Considering the small number of lung carcinoids that were ALT-positive and showed loss of ATRX, a prognostic analysis could not be performed. None of the remaining primary, non-pancreatic NETs nor NET metastases exhibited ALT or loss of ATRX/DAXX immunolabelling.
gutjnl-2020-322595supp006.pdf (3.5MB, pdf)
gutjnl-2020-322595supp007.pdf (3MB, pdf)
Discussion
Improvements and advancements in pathological assessment, biochemical analysis and molecular techniques have led to the investigation of multiple biomarkers to determine prognosis and guide treatment for NF-PanNETs.11 13 19 34–36 While some biomarkers, such as mitotic count and Ki-67 proliferation index, have been incorporated into the routine evaluation of NF-PanNETs, most are not used in clinical practice as they have been inadequately studied in patient specimens as to demonstrate their prognostic value. Using a large, international, multi-institutional cohort of NF-PanNETs from patients without a germline predisposition syndrome or distant metastases at clinical presentation, we validated the utility of ATRX/DAXX by immunolabelling and ALT by telomere-specific FISH as prognostic biomarkers. Loss of ATRX/DAXX and the presence of ALT correlated with known adverse prognostic features for NF-PanNETs, such as large tumour size, high WHO grade, lymphovascular invasion, perineural invasion, advanced pathological T-stage, and regional lymph node metastases. Importantly, ATRX/DAXX loss and ALT were independent prognostic biomarkers for shorter RFS among patients with NF-PanNETs.
While DNA repair and recombination have been implicated in ALT, the exact mechanisms underlying this telomere maintenance programme are still being elucidated.37 38 However, ALT frequently coincides with alterations in ATRX and/or DAXX.21 Inactivation of ATRX and/or DAXX are late genomic events in the pathogenesis of NF-PanNETs and supported by the identification of intratumoural heterogeneity for both proteins and ALT.24 The prognostic findings presented herein are consistent with previous studies evaluating ATRX/DAXX and ALT.18 22–24 Nonetheless, these reports have included diverse patient populations and, in certain aspects, are potentially of limited clinical relevance in demonstrating the utility of a NF-PanNET prognostic biomarker. For example, prior publications have not specifically addressed patients with NF-PanNETs of ≤2.0 cm and without regional lymph node metastases. These neoplasms are frequently identified incidentally by radiographic imaging and represent a clinical dilemma as to whether surgical management or continued surveillance is indicated. Based on our findings, the loss of ATRX/DAXX and the presence of ALT are associated with shorter RFS for NF-PanNET patients with ≤2.0 cm tumours and without regional lymph node metastases. Moreover, ATRX/DAXX loss and ALT positivity are independent, negative prognostic biomarkers for this subset of neoplasms. Given the large number of patients analysed across multiple geographic regions, our study seems sufficient to consider incorporating the status of ATRX/DAXX and/or ALT to the routine evaluation of NF-PanNETs and potentially the preoperative assessment of patients with small (≤2.0 cm) NF-PanNETs using cytopathological cell block material and/or core needle biopsies.32 39
In support of the prognostic significance of ATRX/DAXX loss and ALT positivity among primary NF-PanNETs, both biomarkers were detected at a higher prevalence in NF-PanNET metastases. While the presence of ALT in NF-PanNETs correlated with the development of distant metastases, an interesting quandary was an improved DSS for patients with ALT-positive NF-PanNET metastases as compared with patients with ALT-negative NF-PanNET metastases. At first glance, these findings are seemingly contradictory; however, the results are in agreement with previous studies. Jiao et al and Dogeas et al both reported PanNET patients with ALT-positive liver metastases had a longer survival time and Kim et al observed that patients with ALT-positive primary tumours had better overall survival.16 23 40 Thus, the prognostic utility of ALT in NF-PanNETs may vary based on the disease context.
Except for 2 (4%) pulmonary carcinoids, the loss of ATRX/DAXX and the presence of ALT were not seen in other primary non-pancreatic NETs and NET metastases. Among non-pancreatic NETs, a relative paucity of ATRX and/or DAXX genomic alterations have been reported by whole exome sequencing and whole genomic sequencing studies.41–44 Thus, ATRX/DAXX loss and ALT may also serve as diagnostic biomarkers to suggest pancreatic origin for NET metastases where a primary site cannot be clinically determined; but, requires further studies to confirm these findings.45 Additionally, our results indicate that ATRX/DAXX loss and ALT activation play important roles in the pathogenesis of NF-PanNETs, although not in NETs from other organ sites. In contrast, ARX and PDX1 are differentially expressed in gastric, duodenal, ampullary, colonic, rectal and appendiceal NETs. These findings are not surprising as both ARX and PDX1 are critical to the development of not only the islets of Langerhans, but also enteroendocrine cells of the gastrointestinal tract.27 Interestingly, jejunal and ileal NETs were consistently negative for ARX and PDX1, while duodenal and ampullary NETs were positive for either transcription factor in 94% of cases.
In addition to the status of ATRX/DAXX and ALT, ARX and PDX1 have also been reported to be prognostic biomarkers for NF-PanNETs. Recently, Cejas et al reported that locoregional recurrences and distant metastases almost exclusively occurred in patients with ARX+PDX1− and ‘DN’ NF-PanNETs.26 However, the authors’ patient cohort was enriched for patients with MEN1 syndrome. The absence of MEN1 patients within our study may explain the lack of statistical significance observed in the incidence of postoperative distant metastases/recurrences and RFS based on ARX++DN status and PDX1 expression (PDX1++DP). In fact, we found neither subgroup was associated with known adverse prognostic pathological features for NF-PanNETs. Expression of ARX in NF-PanNETs, regardless of PDX1 status, did correlate with large tumour size, high WHO grade, the presence of regional lymph node metastases, ALT positivity and loss of ATRX/DAXX; however, ARX expression was not associated with RFS in neither our full study cohort nor the subcohort of ≤2.0 cm NF-PanNETs without lymph node metastases.
It is worth noting that there are a few limitations to our study. This study represents one of the largest series of NF-PanNETs to be prognostically analysed for molecular biomarkers, but it is still retrospective in design and may suffer from surgical selection bias. In addition, although regional lymph node metastases can be challenging to detect preoperatively, not all patients had sufficient regional lymph nodes for evaluation. Among patients with primary NF-PanNETs, 11% underwent a limited pancreatectomy procedure (eg, enucleation or central pancreatectomy), and, as a result, regional lymphadenectomy was suboptimal for these patients. Enucleation and central pancreatectomy procedures are typically done in the setting of small NF-PanNETs (≤2.0 cm) because most of these tumours have an indolent clinical course. Within our study cohort, 18 of 196 (9%) of small NF-PanNETs developed postoperative distant metastases/recurrences and 28% and 39% of these cases were ATRX/DAXX-negative and ALT-positive, respectively. In comparison, loss of ATRX/DAXX and the presence of ALT were identified in only 5% and 6%, respectively, of non-metastatic, small NF-PanNETs. Thus, incorporating the status of ATRX/DAXX and ALT among ≤2.0 cm NF-PanNETs without regional lymph node metastases could aid in triaging patients for appropriate surgical management to ensure complete regional lymph node dissection. Additionally, the thresholds used for ARX and PDX1 immunolabelling within our study may not be the same as those used by Cejas et al.26 Herein, we defined positive expression at a cut-off of >10% immunolabelling of neoplastic nuclei. While this may partially explain the discrepancy between the results of these two studies, the proportion of ARX+, PDX1+, DP and DN NF-PanNETs within our cohort was 50%, 20%, 22% and 8%, respectively, and similar to the cohort reported by Cejas et al of 43%, 19%, 28% and 10%, respectively.26 Furthermore, ARX and PDX1 cannot be discounted as prognostic markers and may be useful in the setting of MEN1 syndrome. Finally, we used TMAs to evaluate a subset of NF-PanNETs and NETs from other organ sites. As a TMA consists of ‘punches’ of tumour tissue, each sample may not be representative of the entire tumour as compared with whole tissue sections. However, the potentially scant amount of tumour in TMAs can be beneficial at it allows for simulation of needle core biopsies, and, hence, useful in determining the utility of prognostic and diagnostic biomarkers by mimicking preoperative specimens. Moreover, there were no statistically significant differences between NF-PanNETs evaluated using TMAs and NF-PanNETs evaluated using whole sections. It is also important to underscore that immunolabelling for ATRX and DAXX and telomere-specific FISH for ALT were performed at multiple institutions and the results were consistent across individual sites. Hence, our study further demonstrates the broad feasibility of implementing these biomarkers into clinical practice.
In summary, we report the comprehensive assessment of ARX, PDX1, ATRX, DAXX and ALT in a large, international and multi-institutional cohort of non-syndromic NF-PanNETs without synchronous distant metastases. Although the expression of ARX correlated with known adverse prognostic features, such as larger tumour size and high WHO grade, there was a lack of association with RFS. Comparatively, NF-PanNETs with ALT positivity or ATRX/DAXX loss showed several adverse prognostic factors and these patients had shorter RFS. Both the presence of ALT and loss of ATRX/DAXX in NF-PanNETs were negative, independent prognostic biomarkers for RFS including patients with small NF-PanNETs (≤2.0 cm), which represent a clinical dilemma for surgical management. Moreover, analysing a cohort of >1300 pancreatic and non-pancreatic NETs and metastases, the presence of ALT and loss of ATRX/DAXX were highly specific, diagnostic biomarkers of pancreatic origin. While further prospective studies are warranted, our findings underscore the utility of ALT and ATRX/DAXX as prognostic biomarkers for the preoperative and postoperative evaluation of NF-PanNETs, and, in the setting of an NET metastasis of unknown origin, as highly specific diagnostic biomarkers to indicate the possibility of a pancreatic primary.
gutjnl-2020-322595supp008.pdf (3.1MB, pdf)
Acknowledgments
The authors would like to thank Mrs. Kate Smith and Mrs Lynn Wolkenstein for outstanding administrative assistance. In addition, the authors thank Drs Ralph H. Hruban and Matthew H. Kulke for helpful comments and suggestions.
Footnotes
Twitter: @antonio pea, @PancPathologist
WMH, LAAB, CMH and ADS contributed equally.
Contributors: Author contributions, study concept and design: WMH, LAAB, CMH, ADS. Acquisition of clinical and pathological data: WMH, LAAB, JYK, RO, Y-NS, T-CL, DC, MH, JB-C, SA, FHMM, CMH, GDV, MRV, ENVD, GJAO, KMAD, HJZ, AHZ, MH, KL, DG, JWM, AP, MCO, JFP, NB, MA, RB, JC, RD, KEF, AK, KM, SS, HS, AS, MN, XH, MNN, RTL, AM, BR, VC, CL, SB, AP, SC, LL, RS, MiM, MaM, AS, S-MH, LAAB, CMH, ADS. Analysis and interpretation of data: WMH, LAAB, JYK, RO, Y-NS, MH, JB-C, SA, MN, XH, CMH, ADS. Drafting of the manuscript: WMH, LAAB, CMH, ADS.
Funding: This study was supported in part by grants from the Dutch Digestive Foundation/Maag Lever Darm Stichting (CDG 14–020) (to WMH and LAAB); Associazione Italiana Ricerca Cancro (AIRC 5×1000 n. 12 182 and Start up n. 18718), European Community ERANET PMTR-pNET (cod. D18TR5, B46C17000260001), Italian Ministry of Health (FIMPCUP_J38D19000690001), Fondazione Cariverona: Oncology Biobank Project (prot. 203885/2017) (to A. Scarpa); NRF-2016R1A2B4009381 from the National Research Foundation of Korea (to S-MH); the Basic/Translational Science Investigator Award from the North American Neuroendocrine Tumor Society supported by the Neuroendocrine Tumor Research Foundation (to CH); National Institute of Health (NIH/NCI 1R01CA263622), National Pancreas Foundation, Sky Foundation, and the Pittsburgh Liver Research Center at the University of Pittsburgh (NIH/NIDDK P30DK120531) (to ADS).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to this study were included in the article and are deidentified.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Study approval was obtained from the University Medical Center (UMC) Utrecht Biobank Research Ethics Committee, Verona University and Hospital Trust Ethical Committee, Asan Medical Center, and Institutional Review Boards from Washington University and University of Pittsburgh (IRB# PRO13020493).
References
- 1. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M. Seer cancer statistics review, 1975-2017. Bethesda, MD: National Cancer Institute, 2019. [Google Scholar]
- 3. Zhang IY, Zhao J, Fernandez-Del Castillo C, et al. Operative versus Nonoperative management of nonfunctioning pancreatic neuroendocrine tumors. J Gastrointest Surg 2016;20:277–83. 10.1007/s11605-015-3043-5 [DOI] [PubMed] [Google Scholar]
- 4. Sadot E, Reidy-Lagunes DL, Tang LH, et al. Observation versus resection for small asymptomatic pancreatic neuroendocrine tumors: a matched case-control study. Ann Surg Oncol 2016;23:1361–70. 10.1245/s10434-015-4986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Network NCC . Neuroendocrine and adrenal tumors (version 1.2019), 2019. [Google Scholar]
- 6. Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153–71. 10.1159/000443171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howe JR, Merchant NB, Conrad C, et al. The North American neuroendocrine tumor Society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 2020;49:1–33. 10.1097/MPA.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011;146:534–8. 10.1001/archsurg.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuo EJ, Salem RR. Population-Level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 2013;20:2815–21. 10.1245/s10434-013-3005-7 [DOI] [PubMed] [Google Scholar]
- 10. Cherenfant J, Stocker SJ, Gage MK, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 2013;154:785–93. 10.1016/j.surg.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 11. Pea A, Yu J, Marchionni L, et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann Surg 2020;271:566–73. 10.1097/SLA.0000000000003022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd RV, Osamura RY, Klöppel G. Who classification of tumours of endocrine organs. Lyon, France: IARC Press, 2017. [Google Scholar]
- 13. McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol 2013;37:1671–7. 10.1097/PAS.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reid MD, Bagci P, Ohike N, et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 2016;29:93. 10.1038/modpathol.2015.124 [DOI] [PubMed] [Google Scholar]
- 15. Hwang HS, Kim Y, An S, et al. Grading by the Ki-67 labeling index of endoscopic ultrasound-guided fine needle aspiration biopsy specimens of pancreatic neuroendocrine tumors can be underestimated. Pancreas 2018;47:1296–303. 10.1097/MPA.0000000000001157 [DOI] [PubMed] [Google Scholar]
- 16. Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199–203. 10.1126/science.1200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scarpa A, Chang DK, Nones K, et al. Whole-Genome landscape of pancreatic neuroendocrine tumours. Nature 2017;543:65–71. 10.1038/nature21063 [DOI] [PubMed] [Google Scholar]
- 18. Roy S, LaFramboise WA, Liu T-C, et al. Loss of chromatin-remodeling proteins and/or CDKN2A associates with metastasis of pancreatic neuroendocrine tumors and reduced patient survival times. Gastroenterology 2018;154:2060–3. 10.1053/j.gastro.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong X, Qiao S, Li F, et al. Whole-Genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: leading to a new classification system. Gut 2020;69:877–87. 10.1136/gutjnl-2018-317233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Domenico A, Pipinikas CP, Maire RS, et al. Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun Biol 2020;3:740. 10.1038/s42003-020-01479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and Daxx mutations. Science 2011;333:425. 10.1126/science.1207313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marinoni I, Kurrer AS, Vassella E, et al. Loss of Daxx and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014;146:453–60. 10.1053/j.gastro.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 23. Kim JY, Brosnan-Cashman JA, An S, et al. Alternative lengthening of telomeres in primary pancreatic neuroendocrine tumors is associated with aggressive clinical behavior and poor survival. Clin Cancer Res 2017;23:1598–606. 10.1158/1078-0432.CCR-16-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singhi AD, Liu T-C, Roncaioli JL, et al. Alternative lengthening of telomeres and loss of DAXX/ATRX expression predicts metastatic disease and poor survival in patients with pancreatic neuroendocrine tumors. Clin Cancer Res 2017;23:600–9. 10.1158/1078-0432.CCR-16-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan CS, Laddha SV, Lewis PW, et al. Atrx, Daxx or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 2018;9:4158. 10.1038/s41467-018-06498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cejas P, Drier Y, Dreijerink KMA, et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med 2019;25:1260–5. 10.1038/s41591-019-0493-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du A, McCracken KW, Walp ER, et al. Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol 2012;365:175–88. 10.1016/j.ydbio.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hackeng WM, Schelhaas W, Morsink FHM, et al. Alternative lengthening of telomeres and differential expression of endocrine transcription factors distinguish metastatic and non-metastatic insulinomas. Endocr Pathol 2020;31:108–18. 10.1007/s12022-020-09611-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corbo V, Dalai I, Scardoni M, et al. Men1 in pancreatic endocrine tumors: analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocr Relat Cancer 2010;17:771–83. 10.1677/ERC-10-0028 [DOI] [PubMed] [Google Scholar]
- 30. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an international agency for research on cancer (IARC) and world Health organization (who) expert consensus proposal. Mod Pathol 2018;31:1770–86. 10.1038/s41379-018-0110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amin MB, Edge SB, Green F. Ajcc cancer staging manual. Springer International Publishing, 2017. [Google Scholar]
- 32. Hackeng WM, Morsink FHM, Moons LMG, et al. Assessment of ARX expression, a novel biomarker for metastatic risk in pancreatic neuroendocrine tumors, in endoscopic ultrasound fine-needle aspiration. Diagn Cytopathol 2020;48:308–15. 10.1002/dc.24368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cesare AJ, Heaphy CM, O'Sullivan RJ. Visualization of telomere integrity and function in vitro and in vivo using immunofluorescence techniques. Current Protocols in Cytometry 2015;73:1–31. 10.1002/0471142956.cy1240s73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellison TA, Wolfgang CL, Shi C, et al. A single institution's 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 2014;259:204–12. 10.1097/SLA.0b013e31828f3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fung AD, Cohen C, Kavuri S, et al. Phosphohistone H3 and Ki-67 labeling indices in cytologic specimens from well-differentiated neuroendocrine tumors of the gastrointestinal tract and pancreas: a comparative analysis using automated image cytometry. Acta Cytol 2013;57:501–8. 10.1159/000351475 [DOI] [PubMed] [Google Scholar]
- 36. Estrella JS, Broaddus RR, Mathews A, et al. Progesterone receptor and PTEN expression predict survival in patients with low- and intermediate-grade pancreatic neuroendocrine tumors. Arch Pathol Lab Med 2014;138:1027–36. 10.5858/arpa.2013-0195-OA [DOI] [PubMed] [Google Scholar]
- 37. Dilley RL, Verma P, Cho NW, et al. Break-Induced telomere synthesis underlies alternative telomere maintenance. Nature 2016;539:54–8. 10.1038/nature20099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J-M, Yadav T, Ouyang J, et al. Alternative lengthening of telomeres through two distinct break-induced replication pathways. Cell Rep 2019;26:955–68. 10.1016/j.celrep.2018.12.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. VandenBussche CJ, Allison DB, Graham MK, et al. Alternative lengthening of telomeres and ATRX/DAXX loss can be reliably detected in FNAs of pancreatic neuroendocrine tumors. Cancer Cytopathol 2017;125:544–51. 10.1002/cncy.21857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dogeas E, Karagkounis G, Heaphy CM, et al. Alternative lengthening of telomeres predicts site of origin in neuroendocrine tumor liver metastases. J Am Coll Surg 2014;218:628–35. 10.1016/j.jamcollsurg.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karpathakis A, Dibra H, Pipinikas C, et al. Prognostic impact of novel molecular subtypes of small intestinal neuroendocrine tumor. Clin Cancer Res 2016;22:250–8. 10.1158/1078-0432.CCR-15-0373 [DOI] [PubMed] [Google Scholar]
- 42. Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet 2013;45:1483–6. 10.1038/ng.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simbolo M, Mafficini A, Sikora KO, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, Rb1, MEN1 and KMT2D. J Pathol 2017;241:488–500. 10.1002/path.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asiedu MK, Thomas CF, Dong J, et al. Pathways impacted by genomic alterations in pulmonary carcinoid tumors. Clin Cancer Res 2018;24:1691–704. 10.1158/1078-0432.CCR-17-0252 [DOI] [PubMed] [Google Scholar]
- 45. Hackeng WM, Dreijerink KMA, de Leng WWJ, et al. Genome methylation accurately predicts neuroendocrine tumor origin: an online tool. Clin Cancer Res 2021;27:1341–50. 10.1158/1078-0432.CCR-20-3281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-322595supp001.pdf (150.4KB, pdf)
gutjnl-2020-322595supp002.pdf (23.6KB, pdf)
gutjnl-2020-322595supp003.pdf (41.1KB, pdf)
gutjnl-2020-322595supp004.pdf (939.5KB, pdf)
gutjnl-2020-322595supp005.pdf (1,005.4KB, pdf)
gutjnl-2020-322595supp006.pdf (3.5MB, pdf)
gutjnl-2020-322595supp007.pdf (3MB, pdf)
gutjnl-2020-322595supp008.pdf (3.1MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to this study were included in the article and are deidentified.