Abstract
Objective.
Subglottic stenosis (SGS) is a known complication of granulomatosis with polyangiitis (GPA). We investigated the impact of medical and surgical interventions on the surgical dilation interval and characterized patients with glottic involvement.
Study Design.
A retrospective chart review of patients with GPA-associated SGS was performed from 2010 to 2019.
Setting.
Tertiary academic medical center.
Methods.
The impact of medical and surgical interventions on dilation interval was assessed. The prevalence of glottic involvement was assessed, and clinical characteristics and outcomes were compared with patients without glottic involvement.
Results.
A total of 39 patients with GPA-associated SGS were analyzed. Dilation intervals in patients receiving leflunomide (n = 4; median, 484 days; 95% CI, 405–1099) were greater than in those not receiving leflunomide (median, 155 days; 95% CI, 48–305; P = .033). The surgical technique used did not affect dilation interval. Patients with glottic involvement (n = 13) had a greater incidence of dysphonia (13/13 vs 15/26 [58%], P = .007) and a shorter dilation interval with involvement (median, 91 days; interquartile range, 70–277) versus without involvement (median, 377 days; interquartile range, 175–1148; hazard ratio, 3.38; 95% CI, 2.26–5.05; P < .001). Of 13 patients, 8 (62%) did not have glottic involvement on first presentation.
Conclusion.
Although GPA is classically thought to affect the subglottis, it also involves the glottis in a subset of patients. These patients have greater complaints of dysphonia and require more frequent surgery. Systemic therapy may increase dilation intervals. In this preliminary study, patients taking leflunomide demonstrated an improvement, highlighting the need for further study of immunosuppression regimens in the treatment of GPA-associated SGS.
Keywords: granulomatosis with polyangiitis, laryngotracheal stenosis, subglottic stenosis, vasculitis, glottic stenosis, dysphonia
Granulomatosis with polyangiitis (GPA) is a rare autoimmune small-vessel vasculitis with an annual incidence of 5 to 10 cases per million.1,2 The head, neck, and airway are commonly affected, with up to 70% to 100% of patients developing otolaryngologic or respiratory tract symptoms.1,3–6 Subglottic stenosis (SGS) is the most common laryngeal manifestation in GPA, occurring in 10% to 23% of patients, and can lead to life-threatening airway obstruction.3,7–10 Diagnosis is challenging: SGS may develop in the absence of active signs of GPA inflammation; subglottic biopsy results are usually not revealing of necrotizing granulomas pathognomonic for the disease; and 16% to 25% of patients with GPA do not test positive for the serologic marker c-ANCA.5,7,8,11–15 After diagnosis, treatment for GPA-associated SGS typically involves immunosuppressive therapy and surgical intervention.7 Patients typically undergo multiple surgical procedures due to restenosis, which is used as a clinical outcome that may be tracked to monitor disease relapse.16,17
While GPA is classically thought to affect the subglottis, there are rare reports that it involves the glottis, supraglottis, and distal airways.8,9,18–20 Glottic involvement especially places patients at risk for aspiration and airway obstruction secondary to vocal cord immobility and may reduce the efficacy of surgical procedures.18,21,22 While several factors have been speculated to contribute to the pathogenesis of SGS in GPA, including small-vessel vasculitis complicating a delicate blood supply of the subglottic region, similar etiologies for the development of glottic stenosis have not been described.3,13 It is also not known if these patients present with glottic involvement simultaneous with the onset of subglottic disease, if it develops later in the disease course, or if it occurs as a complication of surgical treatment for SGS.
Systemic immunosuppression is the primary treatment for GPA. Many classes of immunosuppressive medications are used, including cyclophosphamide, steroids, and biologics (eg, rituximab), as well as disease-modifying antirheumatic drugs, including methotrexate, leflunomide, azathioprine, and mycophenolate mofetil. The previous first-line therapy, cyclophosphamide with systemic steroids, has been largely replaced by rituximab due to a more favorable side effect profile and noninferior efficacy.11,12,23–25 In less severe presentations, drugs such as methotrexate, azathioprine, mycophenolate mofetil, and leflunomide are still used.25–28 However, the majority of studies investigating immunosuppressant therapies in patients with GPA do not study the specific benefits of these medications for SGS.11,29–31 Treatment of SGS in patients with GPA is challenged by persistence of otolaryngologic symptoms in patients despite being classified as in clinical remission.11,17 Up to 50% of patients with GPA who develop SGS do so while being treated with immunosuppressants.32 When compared with involvement of organs such as the kidney, airway manifestations have been reported to have resistance to immunosuppressant therapy.11 Several small cohort studies suggest prolonged dilation intervals for GPA-associated SGS with increased systemic steroid dosing at time of surgery, as well as with use of azathioprine, rituximab, and cyclophosphamide.8,19,20,33
To better understand clinical outcomes of GPA-associated airway stenosis, we assessed the effects of clinical and surgical indicators on time between dilation procedures or on dilation interval. We hypothesized that a larger-than-reported subgroup of patients with GPA and SGS also develop glottic involvement and that these patients require more frequent surgery. We also hypothesized that systemic immunosuppression is effective in prolonging the dilation interval.
Materials and Methods
Patient Selection
This study was approved by the Johns Hopkins University institutional review board. Records were reviewed for patients ≥18 years old diagnosed with GPA-associated SGS and seen within the Department of Otolaryngology–Head and Neck Surgery between January 1, 2010, and December 31, 2019. Between January 1, 2010, and September 30, 2015, individuals were identified with ICD-9 diagnostic codes (GPA-446.4; laryngotracheal stenosis, 478.74/478.79). From October 1, 2015, through December 31, 2019, ICD-10 codes were used (GPA-M31.30/M31.31; laryngotracheal stenosis, J38.6/J39.8). Patients with infraglottic/subcordal involvement were excluded from the study.
Recorded Variables and Outcome Measures
Clinical Data.
Data were obtained for baseline demographics, clinical notes, tracheostomy history, operative notes, medication use, spirometry, and serology. Individual comorbidities were recorded and Charlson Comorbidity Index scores calculated.34 All patients with SGS were diagnosed clinically due to subjective complaints of dyspnea with the finding of SGS on laryngoscopy/bronchoscopy or computed tomography imaging. An open procedure was defined as any open surgery of the trachea except tracheostomy. Serology and spirometry were assessed at time of first dilation. Quality of life was assessed with the following measures: Voice-Related Quality of Life (VRQOL), EAT-10 (Eating Assessment Tool), and Clinical COPD Questionnaire (chronic obstructive pulmonary disease).35–37 The presence or absence of dysphonia was a binary variable based on chief complaint from clinical notes.
Surgical Data.
Cotton-Myer grade was assessed at the initial dilation procedure. When the degree of stenosis was described by airway diameter alone, Cotton-Myer grade was estimated by comparing the diameter of stenosis with the average diameter of the subglottis (17 mm in men and 14 mm in women).38 Data on surgical interventions were collected, including steroid injections (Decadron [4, 10, 12 mg]; Kenalog [10, 40 mg]; Depo-Medrol [25, 40 mg]), topical mitomycin C, balloon dilation (semi- or noncompliant), rigid dilation (rigid scopes or metal dilators), and methods of excision (cold knife or sickle, CO2 laser, cryoexcision, or microdebrider).
A dilation procedure was defined as a suspension microlaryngoscopy with dilation of the airway. The dilation treatment window was defined as the duration between the first and last dilation procedures or until last follow-up in patients receiving only 1 dilation. Time to next dilation was modeled and stratified by glottic involvement. Dilations performed in patients with tracheostomy or indwelling airway stent and procedures performed immediately following an open surgery were excluded, as these interventions were not necessarily performed due to dyspnea and did not reflect the natural course of disease.
Immunosuppressant and Surgical Interventions.
The effects of immunosuppressant and surgical therapies on dilation interval were assessed. A crossover design was used to compare average dilation intervals in patients receiving and not receiving an intervention. As no patients in our study had immunosuppression-free dilation intervals, dilation intervals receiving a specific immunosuppressant were evaluated against intervals receiving other immunosuppression agents. Follow-up intervals following the last dilation procedure were included in analysis if they were longer than the preceding interval. Patients who did not start a medication until after their last dilation were included for descriptive purposes but excluded from statistical analysis. Only patients with at least 1 dilation interval in each category were included for analysis. Surgical interventions to open the airway were performed by otolaryngology or interventional pulmonology with an approach based on surgeon preference.
Statistical Analysis
All analyses were conducted with STATA SE software (version 15.1; StataCorp) with the significance level set at α = 0.05. Clinical indicators were stratified by glottic involvement and compared with a Student’s t test for parametric continuous variables, Wilcoxon rank sum for nonparametric continuous variables, and Fisher’s exact test for categorical variables. A conditional Cox proportional hazards model accounting for recurrent events and within-patient correlations was used to model time to next dilation for consecutive events, with adjustments for age and sex.39,40 For immunosuppressant and surgical interventions, dilation intervals for patients with available data were compared with paired t tests for parametric values or Wilcoxon signed rank tests for nonparametric values.
Results
Study Cohort
Thirty-nine patients with GPA-associated SGS were identified (62% female) and presented at a mean 6 SD age of 39.6 6 13.9 years (Table 1). Glottic involvement was present in 13 (33%) patients. In those 13 patients, 2 (15%) had glottic involvement at the initial SGS presentation while 8 (62%) did not. The remaining 3 (23%) were classified as undetermined due to a lack of records. Glottic involvement presented as posterior commissure scarring (n = 6, 46%), cricoarytenoid joint fixation only (n = 6, 46%), and vocal fold scarring (n = 1, 8%).
Table 1.
Clinical Characteristics of Patients With GPA-Associated SGS, With and Without Glottic Involvement.
| Patients, mean ± SD or No. (%) | ||||
|---|---|---|---|---|
| Characteristic | All GPA (N = 39) | SGS without glottic involvement (n = 26)a | SGS with glottic involvement (n = 13)b | P valuec |
| Age at SGS diagnosis, y | 39.6 ± 13.9 | 39.8 ± 14.8 | 39.3 ± 12.3 | .92 |
| Female sex | 24 (62) | 18 (69) | 6 (46) | .19 |
| Race/ethnicity | .79 | |||
| Caucasian | 35 (90) | 22 (85) | 13 (100) | |
| African American | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 1 (3) | 1 (4) | 0 (0) | |
| Hispanic | 2 (5) | 2 (8) | 0 (0) | |
| Other | 1 (3) | 1 (4) | 0 (0) | |
| Family history of autoimmune disease | 7 (18) | 5 (19) | 2 (15) | >.99 |
| Smoking history | .38 | |||
| Never | 33 (85) | 23 (8) | 10 (77) | |
| Former | 6 (15) | 3 (12) | 3 (23) | |
| Active | 0 (0) | 0 (0) | ||
| Comorbidities | ||||
| CCI score | 0.79 ± 1.2 | 0.88 ± 1.3 | 0.62 ± 1.2 | .53 |
| GERD | 18 (46) | 10 (38) | 8 (62) | .20 |
| Diabetes mellitus type 2 | 3 (8) | 2 (8) | 1 (8) | >.99 |
| Hypertension | 16 (41) | 10 (38) | 6 (46) | .74 |
| COPD | 4 (10) | 1 (4) | 3 (23) | .099 |
| Obstructive sleep apnea | 2 (5) | 1 (4) | 1 (8) | >.99 |
| Cerebrovascular accident | 0 (0) | 0 (0) | 0 (0) | — |
| Myocardial infarction | 1 (3) | 0 (0) | 1 (8) | .33 |
| Allergies | 13 (33) | 8 (31) | 5 (38) | .72 |
| Asthma | 10 (26) | 9 (35) | 1 (8) | .12 |
| Symptoms and organ involvement | ||||
| Dyspnea | 39 (100) | 26 (100) | 13 (100) | — |
| Stridor | 32 (82) | 22 (85) | 10 (77) | .67 |
| Dysphonia | 28 (72) | 15 (58) | 13 (100) | .007 |
| Sinonasal | 34 (87) | 23 (88) | 11 (85) | >.99 |
| Pulmonary | 18 (46) | 12 (46) | 6 (46) | >.99 |
| Ear | 26 (67) | 17 (65) | 9 (69) | >.99 |
| Ocular | 10 (26) | 7 (27) | 3 (23) | >.99 |
| Skin | 8 (21) | 5 (19) | 3 (23) | >.99 |
| Musculoskeletal | 14 (36) | 8 (31) | 6 (46) | .48 |
| Renal | 9 (23) | 5 (19) | 4 (31) | .45 |
| Neurologic | 2 (5) | 0 (0) | 2 (15) | .11 |
| Other areas of stenosis | ||||
| Supraglottic | 6 (15) | 2 (8) | 4 (31) | .15 |
| Tracheal | 14 (36) | 9 (35) | 5 (38) | >.99 |
| Bronchial | 2 (5) | 1 (4) | 1 (8) | >.99 |
| ANCA type | 37 | 25 | 12 | .16 |
| c-ANCA positive | 23 (62) | 18 (72) | 5 (42) | |
| p-ANCA positive | 5 (14) | 2 (8) | 3 (25) | |
| ANCA negative | 9 (24) | 5 (20) | 4 (33) | |
| History of tracheostomy | 8 (21) | 3 (12) | 5 (38) | .090 |
| Tracheostomy at last follow-up | 2 (5) | 0 (0) | 2 (15) | .11 |
| History of open laryngotracheal surgery | 5 (13) | 2 (8) | 3 (23) | .31 |
| Cotton-Myer graded | 33 | 22 | 11 | .23 |
| 1 | 16 (49) | 10 (45) | 6 (55) | |
| 2 | 12 (36) | 10 (45) | 2 (18) | |
| 3 | 5 (15) | 2 (10) | 3 (27) | |
| 4 | 0 (0) | 0 (0) | 0 (0) | |
| History of dilation procedure | 32 (82) | 21 (81) | 11 (85) | >.99 |
| Dilation treatment, mo, median (IQR) | 57 (20–100) | 36 (12–70) | 74 (57–113) | .054 |
Abbreviations: CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; GPA, granulomatosis with polyangiitis; IQR, interquartile range; SGS, subglottic stenosis.
Two patients also had lupus.
One patient also had sarcoidosis, and 1 had relapsing polychondritis.
Bold indicates a significant difference (P < .05, Fisher’s exact test) between patients with and without glottic involvement.
Cotton-Myer scores at first dilation used. For patients who did not have any dilations, Cotton-Myer grade at earliest presentation was used.
All patients with glottic involvement reported dysphonia (13/13) as opposed to 58% (15/26, P = .007) of patients without (Table 1). Thirty-two patients with GPA-associated SGS required at least 1 dilation during their treatment course. The time to next dilation was significantly shorter in patients with glottic involvement (median, 91 days; interquartile range [IQR], 70–277) than in those without (median, 377 days; IQR, 175–1148; hazard ratio, 3.38; 95% CI, 2.26–5.05; P < .001; Figure 1).
Figure 1.
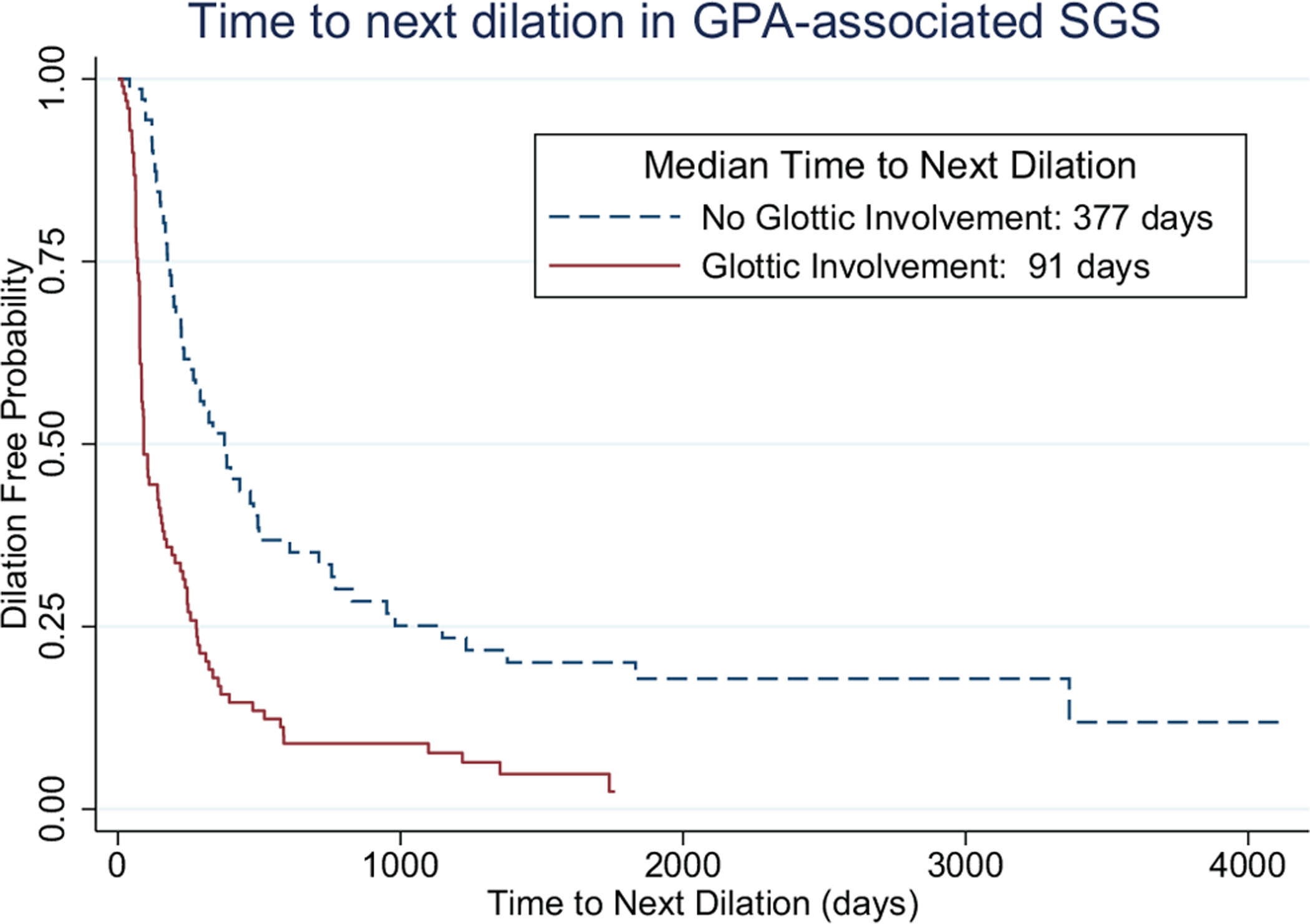
Predicted time to next dilation by glottic involvement: median, 91 days with glottic involvement (IQR, 70–277) vs 377 days without (IQR, 175–1148). Cox hazard ratio, 3.38; 95% CI, 2.26–5.05; P<.001. IQR, interquartile range.
Serology, Patient-Reported Outcomes, and Spirometry
Erythrocyte sedimentation rate (ESR) near the time of surgery was greater in patients with glottic involvement (median, 29.5 mm/h; IQR, 7–42) than without (median, 10.0 mm/h; IQR, 3–20; P = .047; Table 2). Other serology (ANCA, PR3, MPO), patient-reported outcomes (VRQOL, EAT-10, and Clinical COPD Questionnaire; Table 3), and spirometry did not differ between groups.
Table 2.
Serology Values for Patients With GPA at Time of First Dilation, Stratified by Glottic Involvement.
| Patients, No. (%) or No. (median; IQR) | ||||
|---|---|---|---|---|
| Laboratory test | All GPA (N = 39) | SGS without glottic involvement (n = 26) | SGS with glottic involvement (n = 13) | P valuea |
| c-ANCA positive | 20/30 (67) | 15/20 (75) | 5/10 (50) | .23 |
| c-ANCA titer | .57 | |||
| >40 | 7/15 (47) | 6/11 (55) | 1/4 (25) | |
| ≤40 | 8/15 (53) | 5/11 (45) | 3/4 (75) | |
| p-ANCA positive | 4/30 (13) | 1/20 (5) | 3/10 (30) | .095 |
| p-ANCA titer | — | |||
| >80 | 0 (0) | 0/0 | 0/1 (0) | |
| ≤80 | 1/1 (100) | 0/0 | 1/1 (100) | |
| Antibody, units | ||||
| PR3 | 16 (29.0; 10.7–68.5) | 12 (29.0; 10.7–73.5) | 4 (31.8; 16.6–65.1) | >.99 |
| MPO | 10 (2.4; 1.9–3.5) | 7 (2.1; 1.7–2.8) | 3 (7.9; 1.9–32.6) | .21 |
| Level | ||||
| CRP, mg/dL | 14 (0.5; 0.1–0.9) | 10 (0.5; 0.2–0.8) | 4 (1.8; 0.1–4.5) | .89 |
| ESR, mm/h | 21 (12.0; 4–23) | 15 (10.0; 3–20) | 6 (29.5; 7–42) | .047 |
| Positive | ||||
| ANA | 3/4 (75) | 2/3 (67) | 1/1 (100) | >.99 |
| RF | 1/6 (17) | 1/5 (20) | 0/1 (0) | >.99 |
| RDW, % | 26 (13.6; 13.3–14.0) | 17 (13.7; 13.4–14.0) | 9 (13.3; 12.7–13.7) | .099 |
Abbreviations: ANA, antineutrophil antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GPA, granulomatosis with polyangiitis; IQR, interquartile range; RDW, red blood cell distribution width; RF, rheumatoid factor; SGS, subglottic stenosis.
Bold indicates a significant difference (P < .05, Fisher’s exact test) between patients with and without glottic involvement.
Table 3.
Patient-Reported Quality of Life Measures Stratified by Laryngeal Involvement.
| SGS, No. (mean ± SD) | |||
|---|---|---|---|
| Outcome | Without glottic involvement | With glottic involvement | P value |
| VRQOL, raw | 13 (18.5 ± 9.7) | 10 (25.8 ± 11.0) | .11 |
| EAT-10 | 13 (0.85 ± 1.3) | 9 (4.7 ± 8.5) | .12 |
| CCQ | 11 (2.4 ± 1.1) | 5 (3.1 ± 1.5) | .28 |
Abbreviations: CCQ, Clinical COPD Questionnaire (chronic obstructive pulmonary disease); EAT-10, Eating Assessment Tool; SGS, subglottic stenosis; VRQOL, Voice Related Quality of Life.
Immunosuppressive Therapy
Patients receiving leflunomide (n = 4) had a prolonged dilation interval (median, 484 days; 95% CI, 405–1099) when compared with the same individuals while not receiving the medication (median, 155 days; 95% CI, 48–305; P = .033; Figure 2). Rituximab induction was given prior to leflunomide in 2 of 4 patients, and a third patient received rituximab maintenance therapy throughout the treatment course. The fourth patient started taking methotrexate and switched to leflunomide maintenance therapy.
Figure 2.
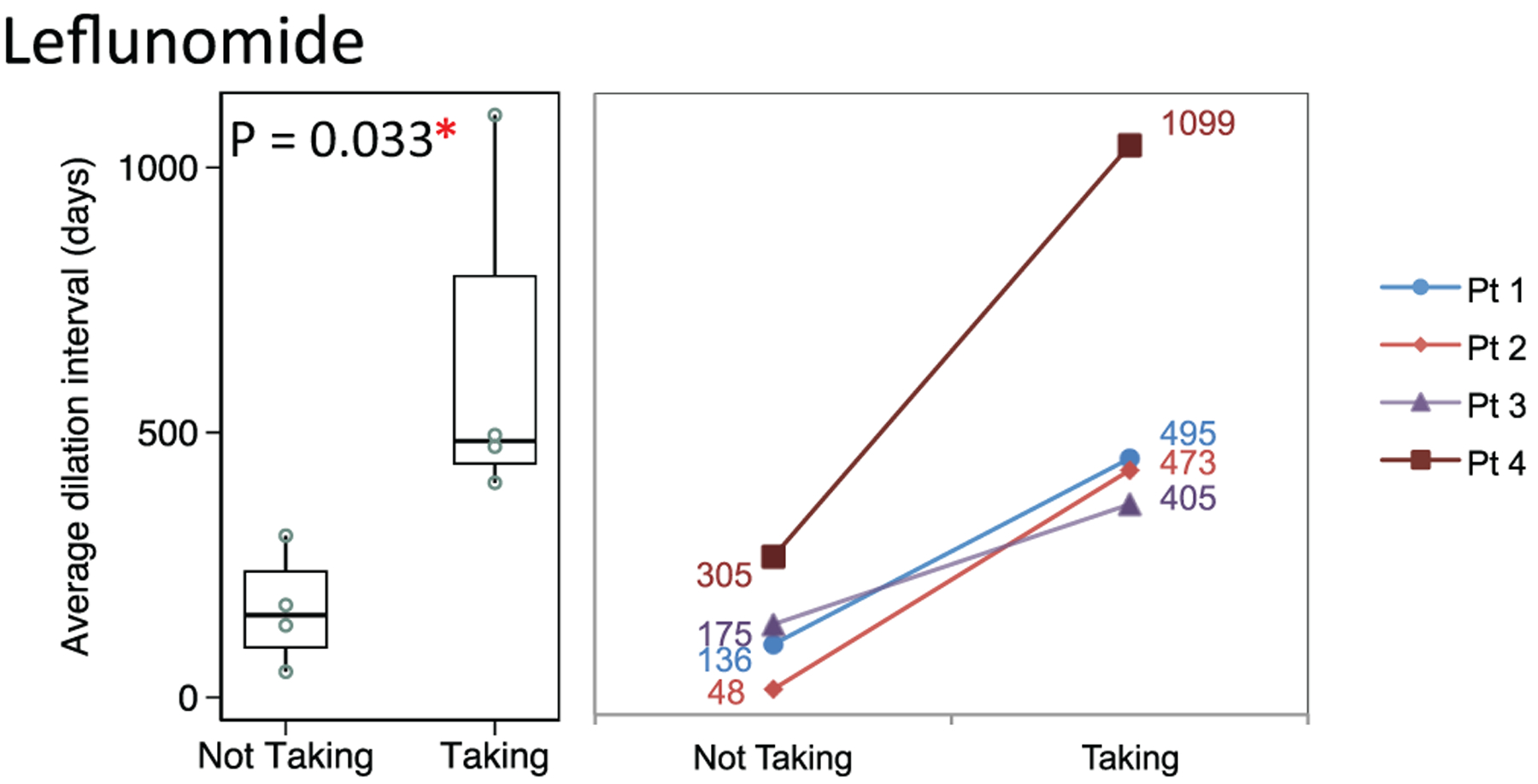
Distribution of dilation intervals in patients receiving and not receiving leflunomide. Box plots (median, interquartile range, range) and accompanying line plots (No.) are shown.
Dilation intervals did not differ significantly in patients taking the following (Figures 3–5):
Rituximab maintenance therapy (n = 3, median, 153 days; 95% CI, 67–295) vs not (80 days; 95% CI, 78–177; P = .26)
Methotrexate (n = 7; median, 259 days; 95% CI, 110–1319) vs not (174 days; 95% CI, 98–422; P = .50)
Azathioprine (n = 4; median, 177 days; 95% CI, 49–405) vs not (394 days; 95% CI, 155–829; P = .34)
Dilation intervals also did not differ significantly in patients receiving immunosuppressive monotherapy (n = 7; median, 270 days; 95% CI, 140–1656) as compared with multitherapy (296 days; 95% CI, 166–703; P = .50; Figure 6).
Figure 3.
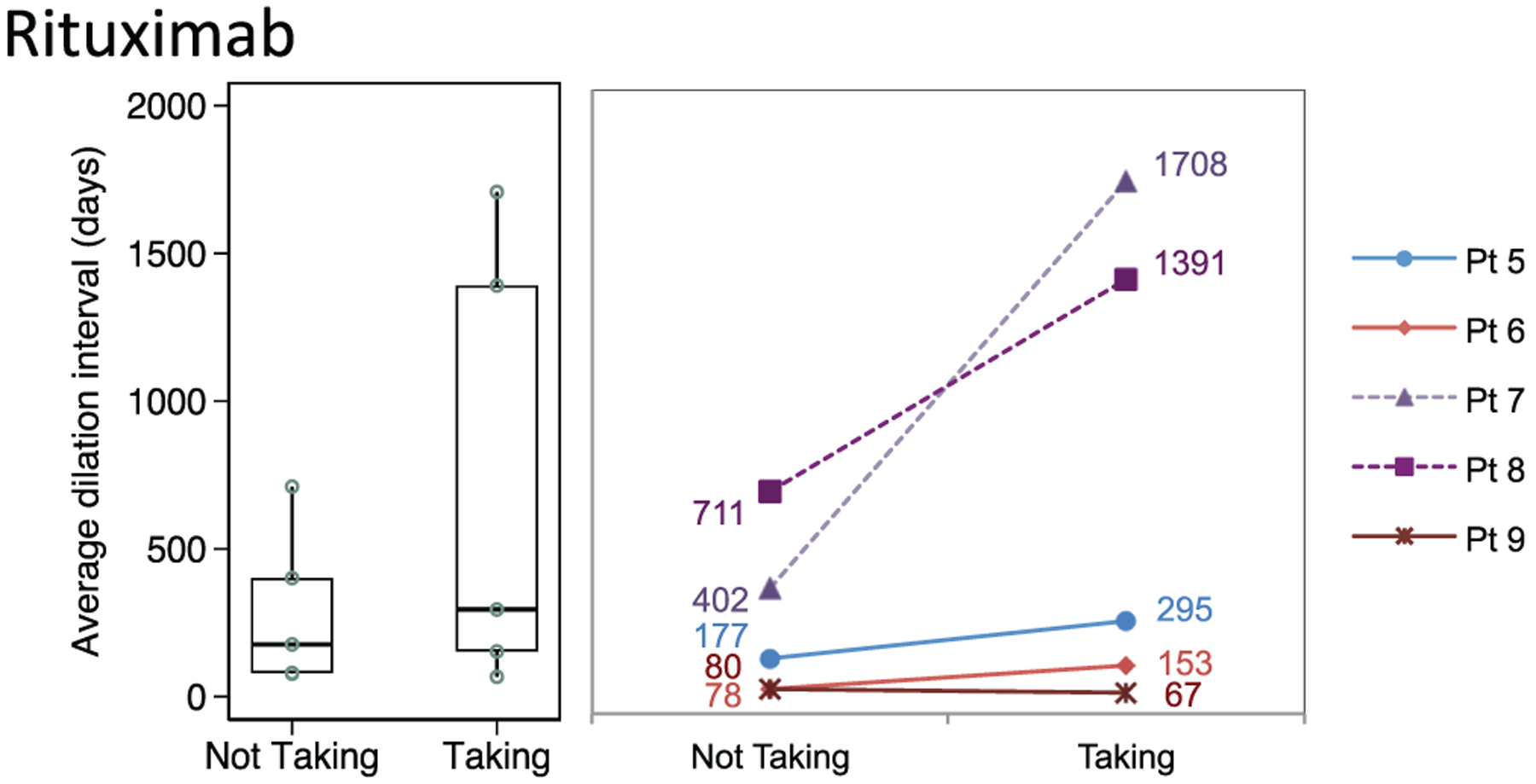
Distribution of dilation intervals in patients receiving and not receiving rituximab. Box plots (median, interquartile range, range) and accompanying line plots (No.) are shown. Dotted lines indicate patients who did not receive the medication until after their last dilation procedure.
Figure 5.
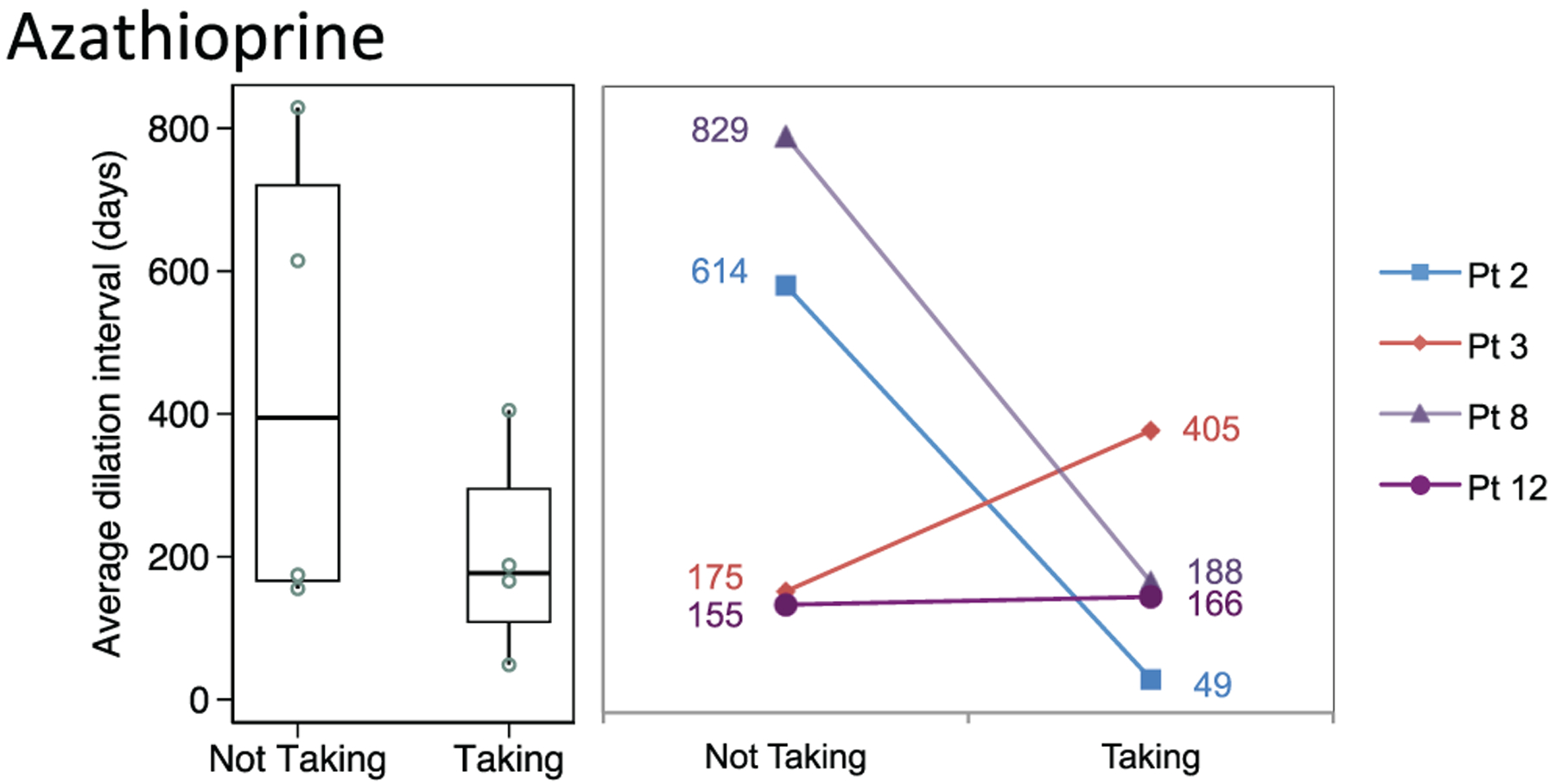
Distribution of dilation intervals in patients receiving and not receiving azathioprine. Box plots (median, interquartile range, range) and accompanying line plots (No.) are shown.
Figure 6.
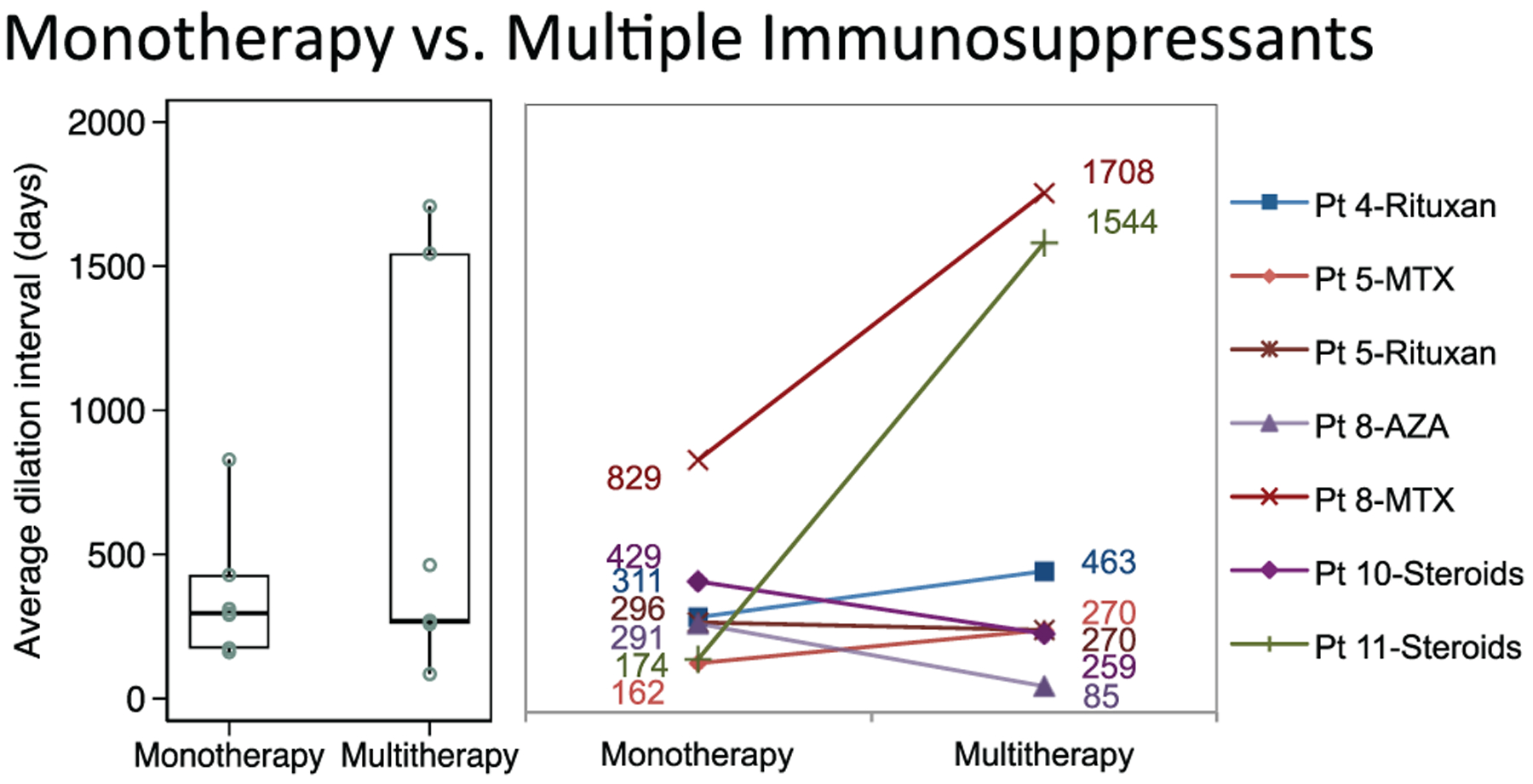
Distribution of dilation intervals in patients receiving immunosuppressive monotherapy compared to multiple immunosuppressants. Box plots (median, interquartile range, range) and accompanying line plots (No.) are shown. AZA, azathioprine; MTX, methotrexate.
Surgical Procedures
A total of 251 total dilation procedures were performed in 32 patients with GPA-associated SGS. Surgical records were available for 213 (85%) procedures. Steroid injections were used in 153 of 213 dilations (72%), balloon dilators in 114 (54%), rigid dilators in 99 (46%), and mitomycin C in 31 (15%). Surgical techniques used to excise stenosis most commonly included CO2 laser (48 dilations, 23%), followed by microdebrider (46 dilations, 22%), cryoexcision (40 dilations, 19%), and cold knife/sickle (39 dilations, 18%). There were no differences in dilation intervals among surgical techniques. Of 13 patients (38%) with glottic involvement, 5 (38%) received laryngeal dilation, with 1 having also undergone posterior cordotomy with partial arytenoidectomy; the rest (62%) were observed.
Discussion
The treatment of GPA-associated SGS is complex, requiring a combination of systemic therapy and endoscopic surgical excision and dilation to maintain airway patency. While surgical technique did not affect dilation interval, we found that leflunomide and, in certain patients, other systemic immunotherapy prolonged the dilation interval. Our study also identified a unique subgroup of patients with GPA-associated SGS with glottic involvement. The presence of glottic involvement adds to the complexity of care by increasing patient complaints of dysphonia and adds a new level of airway narrowing, which in turn increases the frequency of surgical intervention.
There is currently no standard systemic treatment regimen for GPA-associated SGS.11 Treatment is complex and dependent on physician preference, as seen from the heterogeneity in medications used, often combined as multimodal therapy and with various lengths of treatment. In a small subset of patients in our study, we found leflunomide to prolong dilation intervals, increasing the average dilation interval by almost 330 days. Of 5 patients receiving rituximab, 4 also had lengthening of their dilation interval. Several studies showed that leflunomide is superior to mycophenolate mofetil, methotrexate, and azathioprine in controlling GPA-related complications, including major relapses causing life- or organ-threatening disease activity, as well as minor relapses affecting various organ systems that required increased dosing of immunosuppressant therapy.27,28,41 Henes et al25 suggested that leflunomide may have synergistic effects when used with rituximab induction, as was the case in 2 patients in this study. There is limited evidence in the literature relating to the efficacy of immunosuppression on GPA-associated SGS specifically; the majority of studies reporting successful control of SGS with medications such as rituximab are limited by small sample sizes and heterogenous treatment regimens due to the rare occurrence and complex management of patients with GPA-associated SGS.11,20 With the caveat of these same limitations in our study, the observed increase in dilation interval for the few patients in this study receiving leflunomide serves as an additional example of how immunosuppression may help control the progression of SGS in patients with GPA. Future studies with greater sample numbers are needed to determine the relative efficacy of different medication regimens.
The surgical technique employed in dilation procedures was not found to significantly affect dilation interval in our study. This is consistent with other reports in the literature, where Hseu et al38 and Feinstein et al42 found no associations between dilation interval and perioperative therapies (ie, with balloon vs rigid dilators, cold knife or laser excision techniques, mitomycin C, and steroid injections or a combination of these). While some studies note high rates of recurrence and short dilation intervals for laser monotherapy, others report benefits of using some combination of dilation, laser, mitomycin C, and corticosteroids in reducing symptoms and prolonging dilation interval.5,43–45 Comparisons of the efficacy of surgical technique in SGS are difficult, as studies are usually small and descriptive and lack standard outcomes.5,12,32,38,43,46,47 Furthermore, techniques may be changed in the same patient according to surgeon preference, resulting in additional variability. In our opinion, the optimal endoscopic surgical management of GPA-associated SGS is (1) cold excision to limit adjacent thermal injury and reactive inflammation from the laser, (2) balloon dilation to reduce the risk of sheer injury from rigid dilation, and (3) topical steroid injection to reduce reactive inflammation. In conjunction with rheumatology, we advocate for systemic immunosuppression in combination with surgical intervention.
Glottic involvement in patients with GPA-associated SGS may be more common than previously reported. One prior study described cricoarytenoid joint fixation and vocal cord immobility suggestive of glottic involvement in 20% of its GPA-associated SGS cohort; another described development of posterior glottic stenosis in 28% of their 14 patients with GPA-associated SGS.18,48 Our study showed that 33% of patients with GPA-associated SGS had glottic involvement and a shorter dilation interval and were more likely to have the clinical complaint of dysphonia. Surprisingly, VRQOL scores did not differ between groups, which may be due to limited availability of data. We differentiated whether the glottic involvement was present at the onset of SGS or developed later in the course of disease. The majority of patients developed glottic involvement later in the course of their disease, which is consistent with findings from Guardiani et al,18 who described progressive vocal cord and cricoarytenoid joint involvement as typically occurring after the onset of SGS. Acquired glottic stenosis is most frequently associated with trauma secondary to endotracheal intubation, and risk factors include intubation duration, size of the endotracheal tube, and number of intubations.49,50 While GPA involvement in the cricoarytenoid joint may be responsible for glottic stenosis in certain patients, possibly as a sequelae to the acute inflammatory GPA variant,3,51 the proinflammatory pheno-type of GPA may put these patients at additional risk for developing iatrogenic glottic involvement from subsequent surgical procedures and/or intubation.
The utility of biomarkers in predicting GPA activity is controversial. Biomarkers such as ANCA titers, ESR, and C-reactive protein may be elevated in, but are not correlated with, disease activity and prediction of GPA relapses.52–54 One study found that a combined increase in neutrophil count, C-reactive protein, and anti-PR3 antibodies may successfully predict relapse.52,55 In GPA-associated SGS, the role of systemic inflammatory biomarkers is even more complex because SGS frequently occurs in the absence of systemically active GPA.11,12 In our study, 62% of GPA-associated SGS cases were positive for c-ANCA, which is consistent with reports in the literature ranging between 67% and 70%.7,13 While c-ANCA positivity was not associated with glottic involvement in our cohort, others have shown that c-ANCA may not be predictive of tracheobronchial disease activity altogether.56 In our study, the elevated ESR in patients with glottic involvement suggests that the proinflammatory pheno-type of these patients may predispose them to develop stenosis in the glottic region. Another explanation, given the temporal proximity of serology to surgical dilation procedures, is that their proinflammatory state places them at higher risk for acquiring iatrogenic glottic stenosis from a surgical dilation procedure.
As a retrospective study at a single-study institution, our study has several limitations. As our analyses are limited by availability of the data, unavailable outside hospital records and patients lost to follow-up may result in reporting fewer procedures in some patients.16 We attempted to mitigate this bias by performing statistical analysis only on dilation intervals between known operative dates based on surgical documentation. Due to the heterogeneity and small size of our study cohort, we opted to make within-subject rather than between-subject comparisons when analyzing intervention effects. This crossover design allowed each patient to act as his or her own control, reducing interference from nuanced differences among patients. Finally, immunosuppressive medications have prolonged treatment effects, even after discontinuation. This may affect how intervals “without treatment” are interpreted, as patients may still experience immunosuppressant benefits in the intervals immediately following discontinuation of the treatment. The reverse may be seen in intervals “with treatment, ”as immunosuppressants often take some time to go into effect.24 This would likely result in shorter intervals with therapy and longer intervals without therapy, strengthening our confidence in the significant result seen with leflunomide. While this study had a small sample size, its preliminary findings highlight the need for larger studies investigating the impact of individual immunosuppressant agents on GPA-associated SGS.
Conclusion
There is a significant subset of patients with GPA-associated SGS with glottic involvement who have increased dysphonia and elevated ESR at time of first dilation and require more frequent surgery. Management of GPA-associated SGS is complex and necessarily individualized to the patient and often the treating physician. In this preliminary retrospective series, leflunomide therapy was associated with increased surgical dilation intervals and is an example of how systemic immunosuppression may improve SGS in patients with GPA. Further investigation is necessary to evaluate the efficacy of various immunosuppressive regimens.
Figure 4.
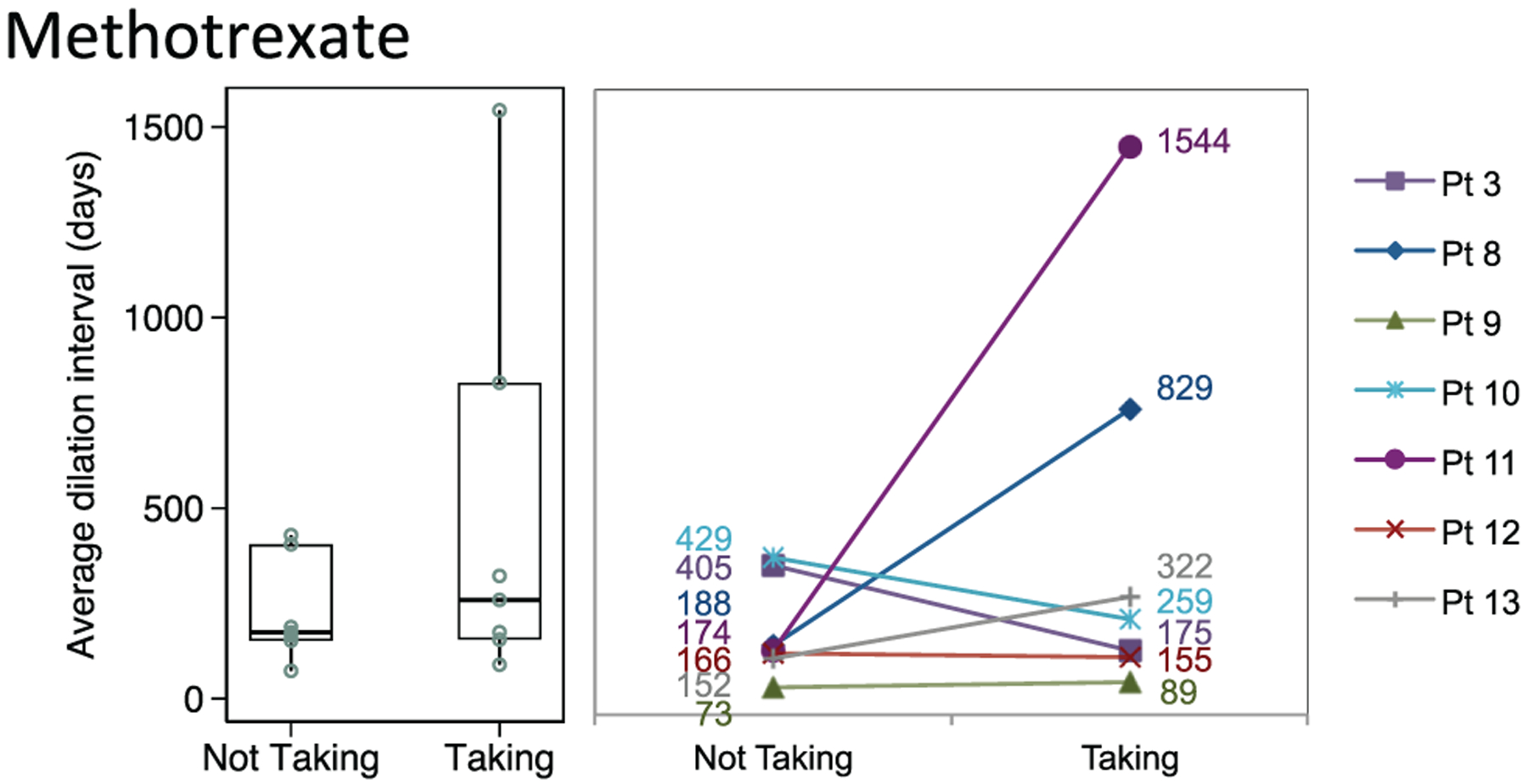
Distribution of dilation intervals in patients receiving and not receiving methotrexate. Box plots (median, interquartile range, range) and accompanying line plots (No.) are shown.
Funding source:
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award numbers 1R01DC018567, 1K23DC014082, and R21DC017225 (Alexander T. Hillel). This study was also financially supported by the Triological Society and American College of Surgeons (Alexander T. Hillel).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: None.
References
- 1.Greco A, Marinelli C, Fusconi M, et al. Clinic manifestations in granulomatosis with polyangiitis. Int J Immunopathol Pharmacol. 2016;29(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutalo PMK, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis). J Autoimmun. 2014;48–49:94–98. [DOI] [PubMed] [Google Scholar]
- 3.Blackabey V, Gan RWC, Buglass H, Kaul V, Ward VMM. Granulomatosis with polyangiitis causing subglottic stenosis—two cases and their management. AME Case Rep. 2018;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor SC, Clayburgh DR, Rosenbaum JT, Schindler JS. Progression and management of Wegener’s granulomatosis in the head and neck. Laryngoscope. 2012;122(8):1695–1700. [DOI] [PubMed] [Google Scholar]
- 5.Martinez Del Pero M, Jayne D, Chaudhry A, Sivasothy P, Jani P. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observational study. JAMA Otolaryngol Head Neck Surg. 2014;140(11):1038–1044. [DOI] [PubMed] [Google Scholar]
- 6.Trimarchi M, Sinico RA, Teggi R, Bussi M, Specks U, Meroni PL. Otorhinolaryngological manifestations in granulomatosis with polyangiitis (Wegener’s). Autoimmun Rev. 2013;12(4):501–505. [DOI] [PubMed] [Google Scholar]
- 7.Jordan NP, Verma H, Siddiqui A, Morrison GA, D’Cruz DP. Morbidity and mortality associated with subglottic laryngotracheal stenosis in granulomatosis with polyangiitis (Wegener’s granulomatosis): a single-centre experience in the United Kingdom. J Laryngol Otol. 2014;128(9):831–837. [DOI] [PubMed] [Google Scholar]
- 8.Marroquín-Fabián E, Ruiz N, Mena-Zúñiga J, Flores-Suárez LF. Frequency, treatment, evolution, and factors associated with the presence of tracheobronchial stenoses in granulomatosis with polyangiitis: retrospective analysis of a case series from a single respiratory referral center. Semin Arthritis Rheum. 2019;48(4): 714–719. [DOI] [PubMed] [Google Scholar]
- 9.Quinn KA, Gelbard A, Sibley C, et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology (Oxford). 2019;58(12):2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez Del Pero M, Rasmussen N, Chaudhry A, Jani P, Jayne D. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur Arch Otorhinolaryngol. 2013;270(1):345–354. [DOI] [PubMed] [Google Scholar]
- 11.Monach PA. L25. Medical treatment of subglottic stenosis in granulomatosis with polyangiitis (Wegener’s). La Presse Médicale. 2013;42(4, pt 2):575–576. [DOI] [PubMed] [Google Scholar]
- 12.Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum. 1996;39(10): 1754–1760. [DOI] [PubMed] [Google Scholar]
- 13.Gluth MB, Shinners PA, Kasperbauer JL. Subglottic stenosis associated with Wegener’s granulomatosis. Laryngoscope. 2003;113(8):1304–1307. [DOI] [PubMed] [Google Scholar]
- 14.Miloslavsky EM, Lu N, Unizony S, et al. Myeloperoxidase-anti-neutrophil cytoplasmic antibody (ANCA)-positive and ANCA-negative patients with granulomatosis with polyangiitis (Wegener’s): distinct patient subsets. Arthritis Rheumatol. 2016; 68(12):2945–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone JH; Wegener’s Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis Rheum. 2003;48(8):2299–2309. [DOI] [PubMed] [Google Scholar]
- 16.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis: risk factors for tracheostomy dependence and dilation interval. Otolaryngol Head Neck Surg. 2017;156(2):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malm I-J, Mener DJ, Kim J, Seo P, Kim YJ. Otolaryngological progression of granulomatosis with polyangiitis after systemic treatment with rituximab. Otolaryngol Head Neck Surg. 2014; 150(1):68–72. [DOI] [PubMed] [Google Scholar]
- 18.Guardiani E, Moghaddas HS, Lesser J, et al. Multilevel airway stenosis in patients with granulomatosis with polyangiitis (Wegener’s). Am J Otolaryngol. 2015;36(3):361–363. [DOI] [PubMed] [Google Scholar]
- 19.Terrier B, Dechartres A, Girard C, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicentre experience. Rheumatology (Oxford). 2015;54(10):1852–1857. [DOI] [PubMed] [Google Scholar]
- 20.Girard C, Charles P, Terrier B, et al. Tracheobronchial stenoses in granulomatosis with polyangiitis (Wegener’s): a report on 26 cases. Medicine (Baltimore). 2015;94(32):e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner GM. Posterior glottic stenosis and bilateral vocal fold immobility: diagnosis and treatment. Otolaryngol Clin North Am. 2000;33(4):855–878. [DOI] [PubMed] [Google Scholar]
- 22.Morcillo A, Wins R, Gómez-Caro A, Paradela M, Molins L, Tarrazona V. Single-staged laryngotracheal reconstruction for idiopathic tracheal stenosis. Ann Thorac Surg. 2013;95(2):433–439. [DOI] [PubMed] [Google Scholar]
- 23.Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43(5): 1021–1032. [DOI] [PubMed] [Google Scholar]
- 24.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henes JC, Fritz J, Koch S, et al. Rituximab for treatment-resistant extensive Wegener’s granulomatosis—additive effects of a maintenance treatment with leflunomide. Clin Rheumatol. 2007;26(10):1711–1715. [DOI] [PubMed] [Google Scholar]
- 26.Luqmani RA. State of the art in the treatment of systemic vasculitides. Front Immunol. 2014;5:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gause A, Metzler C, Reinhold-Keller E, Gross WL. Leflunomide therapy for autoimmune diseases. In: Sticherling M, Christophers E, eds. Treatment of Autoimmune Disorders. Springer; 2003:43–48. [Google Scholar]
- 28.Hazlewood GS, Metzler C, Tomlinson GA, et al. Non-biologic remission maintenance therapy in adult patients with ANCA-associated vasculitis: a systematic review and network meta-analysis. Joint Bone Spine. 2014;81(4):337–341. [DOI] [PubMed] [Google Scholar]
- 29.Grygiel-Górniak B, Limphaibool N, Perkowska K, Puszczewicz M. Clinical manifestations of granulomatosis with polyangiitis: key considerations and major features. Postgrad Med. 2018; 130(7):581–596. [DOI] [PubMed] [Google Scholar]
- 30.Comarmond C, Cacoub P. Granulomatosis with polyangiitis (Wegener): clinical aspects and treatment. Autoimmun Rev. 2014;13(11):1121–1125. [DOI] [PubMed] [Google Scholar]
- 31.Calich AL, Puéchal X, Pugnet G, et al. Rituximab for induction and maintenance therapy in granulomatosis with polyangiitis (Wegener’s): results of a single-center cohort study on 66 patients. J Autoimmun. 2014;50:135–141. [DOI] [PubMed] [Google Scholar]
- 32.Gouveris H, Karaiskaki N, Koutsimpelas D, Chongolwatana C, Mann W. Treatment for adult idiopathic and Wegener-associated subglottic stenosis. Eur Arch Otorhinolaryngol. 2013; 270(3):989–993. [DOI] [PubMed] [Google Scholar]
- 33.Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus. 2008;17(9):832–836. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 35.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–924. [DOI] [PubMed] [Google Scholar]
- 36.van der Molen T, Willemse BWM, Schokker S, ten Hacken NHT, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J Voice. 1999; 13(4):557–569. [DOI] [PubMed] [Google Scholar]
- 38.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124(3):736–741. [DOI] [PubMed] [Google Scholar]
- 39.Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly PJ, Lim LL-Y. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat Med. 2000; 19(1):13–33. [DOI] [PubMed] [Google Scholar]
- 41.Metzler C, Fink C, Lamprecht P, Gross WL, Reinhold-Keller E. Maintenance of remission with leflunomide in Wegener’s granulomatosis. Rheumatology (Oxford). 2004;43(3):315–320. [DOI] [PubMed] [Google Scholar]
- 42.Feinstein AJ, Goel A, Raghavan G, et al. Endoscopic management of subglottic stenosis. JAMA Otolaryngol Head Neck Surg. 2017;143(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavrysen E, Hens G, Delaere P, Meulemans J. Endoscopic treatment of idiopathic subglottic stenosis: a systematic review. Front Surg. 2020;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roediger FC, Orloff LA, Courey MS. Adult subglottic stenosis: management with laser incisions and mitomycin-C. Laryngoscope. 2008;118(9):1542–1546. [DOI] [PubMed] [Google Scholar]
- 45.Shabani S, Hoffman MR, Brand WT, Dailey SH. Endoscopic management of idiopathic subglottic stenosis: factors affecting inter-dilation interval. Ann Otol Rhinol Laryngol. 2017;126(2): 96–102. [DOI] [PubMed] [Google Scholar]
- 46.Volgger V, Mann SL, Englhard AS, Berghaus A. An eleven-year review on treatment outcome after endoscope-based and open surgery for subglottic stenosis. In: Laryngo-rhino-otologie. Vol 97. Georg Thieme Verlag KG; 2018:10159. [Google Scholar]
- 47.Cataneo DC, Ximenes AMG, Cataneo AJM. Mitomycin C in the endoscopic treatment of tracheal stenosis: a prospective cohort study. J Bras Pneumol. 2018;44(6):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dion GR, Chapa JSDL, Bettenhausen W, Dominguez LM, Simpson CB. Differing progression to posterior glottic stenosis in autoimmune and idiopathic subglottic stenosis. Laryngoscope. Published online September 9, 2020. doi: 10.1002/lary.29085 [DOI] [PubMed] [Google Scholar]
- 49.Hoasjoe DK, Franklin SW, Aarstad RF, Day TA, Stucker FJ. Posterior glottic stenosis mechanism and surgical management. Laryngoscope. 1997;107(5):675–679. [DOI] [PubMed] [Google Scholar]
- 50.Hillel AT, Karatayli-Ozgursoy S, Samad I, et al. Predictors of posterior glottic stenosis: a multi-institutional case-control study. Ann Otol Rhinol Laryngol. 2016;125(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen N. L24. Local treatments of subglottic and tracheal stenoses in granulomatosis with polyangiitis (Wegener’s). Presse Med. 2013;42(4, pt 2):571–574. [DOI] [PubMed] [Google Scholar]
- 52.Kucuk H, Tecer D, Goker B, et al. Platelet/lymphocyte ratio and mean platelet volume in patients with granulomatosis with polyangiitis. Adv Rheumatol. 2019;60(1):4. [DOI] [PubMed] [Google Scholar]
- 53.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford). 2012;51(1):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verstockt B, Bossuyt X, Vanderschueren S, Blockmans D. There is no benefit in routinely monitoring ANCA titres in patients with granulomatosis with polyangiitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S-72–76. [PubMed] [Google Scholar]
- 55.Hogan PCP, O’Connell RM, Scollard S, Browne E, Hackett EE, Feighery C. Biomarkers predict relapse in granulomatosis with polyangiitis. J Biomark. 2014;2014:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med. 1995;151(2):522–526. [DOI] [PubMed] [Google Scholar]


