Abstract
Background
As the coronavirus disease 2019 (COVID-19) pandemic has progressed, there has been a growing awareness of the long-term impacts of the COVID-19 infection. However, until recently, there was no published study that investigated COVID-19-related sequelae and related factors for greater than six months from the onset of COVID-19 symptoms or the time of COVID-19 diagnosis in Korea.
Materials and Methods
Online survey and statistical analysis were conducted by Kyungpook National University Hospital on 5,252 patients diagnosed as COVID-19 between February 18, 2020 and March 14, 2020. Responders aged between 16 and 70 years were included. Long-term sequelae were defined as persistent symptoms or signs ≥ 6 months after acute COVID-19 infection. The survey was conducted from September 8, 2020 to September 10, 2020. Clinical characteristics and self-reported clinical sequelae of the responders were analyzed to investigate the prevalence and factors associated with sequelae using descriptive and multivariate logistic regression analysis.
Results
The median period from the date of the first symptom onset or COVID-19 diagnosis to the time of the survey was 195 (interquartile range [IQR] 191 - 200) days. The response rate was 17.1% (900 out of 5,252). The median age was 31 (IQR 24.0 - 47.0) years old, and 627 responders were female (69.7%). Regarding the disease severity, 29 (3.2%) were asymptomatic, 763 (84.8%) mild, 86 (9.6%) moderate, 17 (1.9%) severe, and 5 (0.6%) critical. In total, 591 (65.7%) responders suffered from COVID-19-related long-term sequelae and 78 (8.6%) responders were receiving outpatient treatment for COVID-19-related long-term sequelae. The most common symptoms identified during the isolation period were anosmia and ageusia at 44.5% and 43.5%, respectively. Fatigue was the most common long-term sequelae, accounting for 253 (26.2%) responders, followed by concentration difficulty, amnesia, cognitive dysfunction, anxiety, and depression, which accounted for over 20%. Female gender was identified as the factor associated with mental and psychological long-term sequelae (P <0.05).
Conclusion
The results showed that the rate of COVID-19-related long-term sequelae was 65.7%. The most common long-term sequela was fatigue. The risk factor identified was female gender. It was found that the long-term sequelae had various manifestations, including mental and psychological aspects. To improve the care of COVID-19 recovered patients with COVID-19-related long-term sequelae, the participation of a comprehensive and an interdisciplinary group of researchers is required.
Keywords: COVID-19, SARS-CoV-2, Sequelae, Long-term consequences, Clinical sequelae
INTRODUCTION
With the progression of the novel coronavirus disease 2019 (COVID-19) pandemic, there has been a growing awareness about the long-term impacts of COVID-19 infection, including cardiac, neurological, metabolic, and respiratory long-term sequelae. The clinical characteristics, treatment, and prognosis of patients who recovered from COVID-19 infection have been extensively studied [1,2,3]; however, to our knowledge, there are limited studies on the prevalence, nature, duration, and factors related to sequelae in convalescent COVID-19 patients.
Increasing evidence suggests that COVID-19 patients can experience long-lasting symptoms [4,5], irrespective of the severity of the initial infection. Hence, detailed studies on COVID-19 disease severity are required to establish the optimal management of such individuals.
We know from the previous studies that severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) from previous coronavirus epidemics were associated with a significant psychiatric burden in the acute and postillness stages. In SARS and MERS, after recovery from the infection, fatigue, sleep disorder, and impaired concentration and memory were reported in more than 15% of patients at a follow-up period ranging between 6 weeks and 39 months [6,7,8]. It is identified that individuals with COVID-19 experience psychiatric symptoms persisting months after the initial infection [9]. In particular, significant psychological symptoms, such as depression and anxiety, were reported in up to 40% of patients following COVID-19, similar to patients with previous coronavirus infections [10,11]. However, few studies have investigated the psychological sequelae [12] and associated factors in recovered COVID-19 patients. Furthermore, because COVID-19 is a new disease, much about the clinical course, including the long-term sequelae, remains uncertain.
Daegu is a city with a population of 2.4 million and is the first city in Korea to experience a severe COVID-19 outbreak that affected > 5,000 COVID-19 patients in early 2020. Hence, a large number of patients were cured and recovered from COVID-19 infection, and the follow-up duration for COVID-19 survivors from the Korea was longer than that of patients from other countries around the world. To our knowledge, no study has assessed the COVID-19-related sequelae in Korea. Therefore, we investigated the sequelae in Korean patients to determine whether multiple relevant symptoms subsided after recovering from the COVID-19 infection, and to identify the factors related to the occurrence and persistence of long-term symptoms.
MATERIALS AND METHODS
1. Study design and population
Google online survey with information of the researcher’s name and analyses were conducted by infectious disease physicians at the Kyungpook National University Hospital located in Daegu city. Among patients who were diagnosed with COVID-19 between February 18, 2020 and March 14, 2020, 5,252 recovered patients aged between 16 and 70 years were identified from the clinical data registry provided by the Daegu Center for Infectious Diseases Control and Prevention. The survey duration was 3 days and the survey period was from September 8, 2020 to September 10, 2020.
The questionnaire survey list included sex, birth year, birth date, residential address, COVID-19 diagnosis date, symptom onset date, health-care utilization during and after the infection, quarantine end date, oxygen treatment history, symptoms during the quarantine period with the option of an open text field to add other symptoms, symptoms that persisted beyond the quarantine period, newly diagnosed diseases after COVID-19, exacerbation of underlying diseases after COVID-19, undergoing outpatient treatment for COVID-19-related sequelae, and wanting to connect for medical treatment of sequelae.
Symptoms during the isolation period from acute COVID-19 included fever (≥37.5°C), febrile sense, myalgia, arthralgia, chilly sense, fatigue, cough, sore throat, nasal congestion or rhinorrhea, sputum, dyspnea, chest discomfort, chest tightness, palpitation, arrhythmia, headache, depression, anxiety, nausea or vomiting, diarrhea, gastrointestinal discomfort, anosmia, ageusia (loss of taste), and hypoacusis.
The sequelae were categorized into 45 symptoms and signs and included fever (≥37.5°C), febrile sense, myalgia, arthralgia, chilly sense, fatigue, cough, sore throat, nasal congestion or rhinorrhea, sputum, dyspnea, chest discomfort, chest tightness, palpitation, arrhythmia, headache, brain fog, cognitive dysfunction, concentration difficulty, amnesia, dizziness, abnormal directional sensibility, insomnia including poor sleep quality, hyperemia, hallucination, seizure, depression, anxiety, social phobia, posttraumatic stress disorder, obsessive thinking, nausea or vomiting, anorexia, diarrhea, gastrointestinal discomfort, anosmia, ageusia, hypoacusis, tinnitus, alopecia, skin rashes, itchy skin, COVID toes, swollen fingers, and paresthesia.
Underlying diseases included hypertension, diabetes mellitus, heart diseases (heart failure, arrhythmia, valvular heart disease, and coronary heart disease), chronic pulmonary disease, chronic kidney disease, cerebrovascular disease, psychiatric disorders, etc.
In order to confirm the reliability of the questionnaire survey results, we reaffirmed the clinical data (COVID-19 diagnosis date, first symptom onset date, and severity) of the respondents provided by the Daegu Center for Infectious Diseases Control and Prevention and compared it with the clinical information entered in the individual questionnaire. All the data were reviewed by infectious disease physicians.
2. Definitions
The presence of SARS-CoV-2 infection was confirmed using real-time reverse transcription polymerase chain reaction (RT-PCR) assay performed using nasopharyngeal swabs or other upper respiratory tract specimens [13]. Symptom duration was defined as the period from the first COVID-19-related symptom onset to the online survey date in symptomatic COVID-19 responders, and COVID-19 diagnosis date to the online survey date in asymptomatic COVID-19 responders. End of quarantine period was defined as having two consecutive negative PCR results with a 24-hour interval. Long-term sequelae in this study was defined as any remaining or newly identified symptoms or signs related with COVID-19 that persisted ≥6 months after COVID-19 infection.
Asymptomatic patients were defined as those who had no symptoms or signs, including feeling feverish or chills, cough, shortness of breath or difficulty in breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea. Fatigue was defined as a different decline in physical strength compared with the predisease state. Brain fog was defined as lack of sharp memory or sharp focus. Cognitive dysfunction was defined as loss of intellectual functions, such as thinking, remembering, and reasoning, severely enough to interfere with daily functioning. Amnesia refers to loss of memory and inability to recall facts, information, and experiences. COVID toe was characterized as painful red or purple lesions that typically form on the fingers or toes.
Illness severity scores were assigned as per the following clinical information: 1) asymptomatic: no symptoms or discomfort throughout the entire disease period, with body temperature <37.5°C; 2) mild: presence of symptoms with or without fever (≥37.5°C), but not manifesting or identified pneumonia; 3) moderate: pneumonia diagnosed by a clinician, but not requiring oxygenation other than room air; 4) severe: pneumonia diagnosed by a clinician, requiring oxygenation therapy (nasal prong, facial mask, or high-flow oxygen therapy); and 5) critical: pneumonia diagnosed by a clinician and need for mechanical ventilation therapy and/or extracorporeal membrane oxygenation or death.
3. Statistical analyses
A descriptive analysis was conducted. Continuous variables are presented as median (interquartile range, IQR) values, and categorical variables are presented as numbers (percentage, %). Categorical variables were compared using Fisher’s exact test or chi-square test and noncategorical variables were analyzed using Student’s t-test or Mann-Whitney U-test to compare the differences between the respondents with and without sequelae and for the comparison of the age, severity group, and sex differences according to the sequelae. Multivariate regression analysis was performed to control the confounding factors and to determine the factors associated with the prevalence of long-term sequelae. All the tests were considered to be statistically significant at P <0.05. Statistical analyses were performed using R statistics version 4.0.2 (The R Foundation; https://www.r-project.org).
4. Ethics statement
This study was reviewed and approved by the Institutional Review Board of Kyungpook National University Hospital (approval no.: 2020-03-044). All the respondents provided digital informed consent before the questionnaire was administered. Without the informed consent, the remaining questionnaire could not be completed. All methods were performed in accordance with relevant guidelines and regulations.
RESULTS
1. Demographics and characteristics
Of the total 5,252 subjects followed up past the initial 6 months after COVID-19 symptom onset or diagnosis, the response rate was 17.1% (900 out of 5,252). The female sex distribution was 63.1% (2,748 out of 4,352) in nonrespondents and 69.7% (627 out of 900) in respondents (P <0.001). Median age was 42.0 (IQR 25.0 - 55.0) in nonrespondents and 30.5 (IQR 24.0 - 47.0) in respondents (P <0.001). Moderate disease severity or higher was found in 16.2% (704 out of 4,352) of non-respondents and 12.0% (108 out of 900) of respondents (P = 0.002). Days from COVID-19 diagnosis to survey were 194 (IQR 191 - 200) in nonrespondents and 195 (IQR 191 - 200) in respondents (P = 0.002). The median age of the 900 respondents was 31 years (IQR 24.0 - 47.0 years), ranging between 16 and 70 years, and 627 (69.7%) of the respondents were female. Regarding the age distribution, 433 (48.1%) respondents were aged 16 - 29 years, 130 (14.4%) were aged 30 - 39 years, 149 (16.6%) were aged 40 - 49 years, 141 (15.7%) were aged 50 - 59 years, and 47 (5.2%) were aged 60 - 69 years. Those aged 16 - 29 years accounted for the largest proportion (48.1%), whereas those aged ≥50 years accounted for 20.9% of the study population. Regarding disease severity, 29 (3.2%) patients were asymptomatic, 763 (84.8%) had mild disease, 86 (9.6%) had moderate disease, 17 (1.9%) had severe disease, and 5 (0.6%) were in a critical condition. The majority of the respondents (84.8%) had mild disease and 12.0% had moderate or more severe disease. Results of the comparative analysis between those with and without symptoms or signs identified during acute COVID-19 infection showed that 822 (91.3%) of the respondents had at least one symptom and 78 respondents (8.7%) had no other symptoms. The prevalence of symptoms was higher in women (596, 72.5%) than in men (226, 27.5%; P <0.001). The median age was higher in the presence of symptom group (P <0.001). Furthermore, none of the asymptomatic respondents showed severe or more disease severity (Table 1).
Table 1. Clinical characteristics of 900 respondents who had recovered from COVID-19 infection, based on the presence of symptoms or signs identified during the acute COVID-19 infection.
| Characteristics | No symptom (N = 78) | Presence of symptoms (N = 822) | Total (N = 900) | P-value | |
|---|---|---|---|---|---|
| Days from COVID-19 diagnosis to survey [IQR] (days) | 194.0 [191.0;199.0] | 196.0 [192.0;200.0] | 195.0 [191.0;200.0] | 0.027 | |
| Days from COVID-19-related symptom onset to diagnosis [IQR] (days) | 0.0 [0.0;3.0] | 0.0 [0.0;4.0] | 0.0 [0.0;4.0] | 0.087 | |
| Sex | <0.001 | ||||
| Male | 47 (60.3%) | 226 (27.5%) | 273 (30.3%) | ||
| Female | 31 (39.7%) | 596 (72.5%) | 627 (69.7%) | ||
| Age, median [IQR] (years) | 24.0 [21.0;36.0] | 32.0 [24.0;48.0] | 30.5 [24.0;47.0] | <0.001 | |
| Age distribution (years) | 0.002 | ||||
| 16 – 29 | 50 (64.1%) | 383 (46.6%) | 433 (48.1%) | ||
| 30 – 39 | 14 (17.9%) | 116 (14.1%) | 130 (14.4%) | ||
| 40 – 49 | 10 (12.8%) | 139 (16.9%) | 149 (16.6%) | ||
| 50 – 59 | 4 (5.1%) | 137 (16.7%) | 141 (15.7%) | ||
| 60 – 70 | 0 (0.0%) | 47 (5.7%) | 47 (5.2%) | ||
| Age (years) | 0.001 | ||||
| <50 | 74 (94.9%) | 638 (77.6%) | 712 (79.1%) | ||
| ≥50 | 4 (5.1%) | 184 (22.4%) | 188 (20.9%) | ||
| Disease severity | <0.001 | ||||
| Asymptomatic | 25 (32.1%) | 4 (0.5%) | 29 (3.2%) | ||
| Mid | 51 (65.4%) | 712 (86.6%) | 763 (84.8%) | ||
| Moderate | 2 (2.6%) | 84 (10.2%) | 86 (9.6%) | ||
| Severe | 0 (0.0%) | 17 (2.1%) | 17 (1.9%) | ||
| Critical | 0 (0.0%) | 5 (0.6%) | 5 (0.6%) | ||
| Disease severity | 0.012 | ||||
| < Moderate | 76 (97.4%) | 716 (87.1%) | 792 (88.0%) | ||
| ≥ Moderate | 2 (2.6%) | 106 (12.9%) | 108 (12.0%) | ||
| Isolation period [IQR] (days) | 28.5 [22.0;37.0] | 27.0 [21.0;37.0] | 27.0 [21.0;37.0] | 0.409 | |
| Isolation period | 0.479 | ||||
| <3 weeks | 16 (20.5%) | 204 (24.8%) | 220 (24.4%) | ||
| ≥3 weeks | 62 (79.5%) | 618 (75.2%) | 680 (75.6%) | ||
| Isolated place | <0.001 | ||||
| Tertiary hospital | 14 (17.9%) | 364 (44.3%) | 378 (42.0%) | ||
| Therapeutic living center | 64 (82.1%) | 441 (53.6%) | 505 (56.1%) | ||
| Home isolation | 0 (0.0%) | 17 (2.1%) | 17 (1.9%) | ||
COVID-19, coronavirus disease 2019; IQR, interquartile range.
2. Characteristics of clinical sequelae
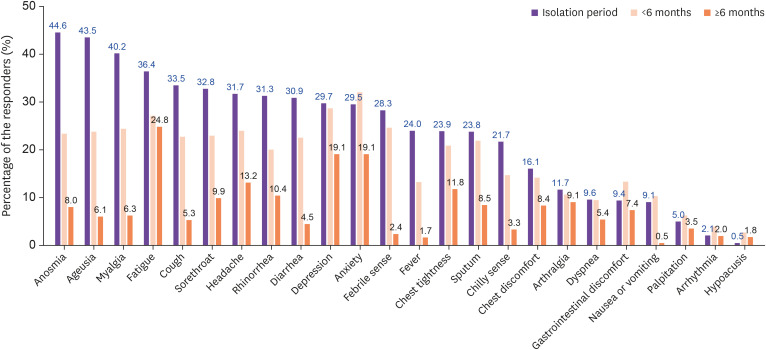
The distribution of persistent symptoms or signs was identified in 822 respondents. The most common symptoms identified during the isolation period were anosmia and ageusia at 44.5% and 43.5%, respectively. However, the respondents suffering from anosmia and ageusia decreased to 8.0% and 6.1%, respectively, after at least 6 months from the symptom onset. Among the identified symptoms during isolation period, fatigue, depression, and anxiety accounted for 36.4%, 29.7% and 29.5%, respectively. It was found that respondents with fatigue, depression, and anxiety decreased over time, but these symptoms remained persistently high after 6 months from acute COVID-19 infection, with fatigue accounting for 24.8%, and both depression and anxiety 19.1% (Fig. 1).
Figure 1. Distribution of persistent symptoms or signs including identified symptoms during the isolation period according to the symptom persistent period.
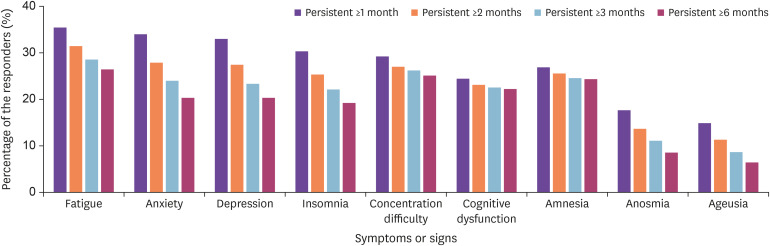
Overall, the frequency of key long-term sequelae including anosmia and ageusia decreased; however, the number of people suffering from concentration difficulty, cognitive dysfunction, and amnesia remained high, near the baseline level (Fig. 2).
Figure 2. Duration of key persistent symptoms or signs according to the symptom persistent period 1, 2, 3, and 6 months after acute COVID-19 infection.
The most common persistent symptoms after 1 and 2 months from acute COVID-19 infection were fatigue, anxiety, and depression (Supplementary Fig. 1A, 1B). After 3 and 6 months from acute COVID-19 infection, the most common persistent symptoms were fatigue, concentration difficulties, and amnesia (Supplementary Fig. 1C, 1D).
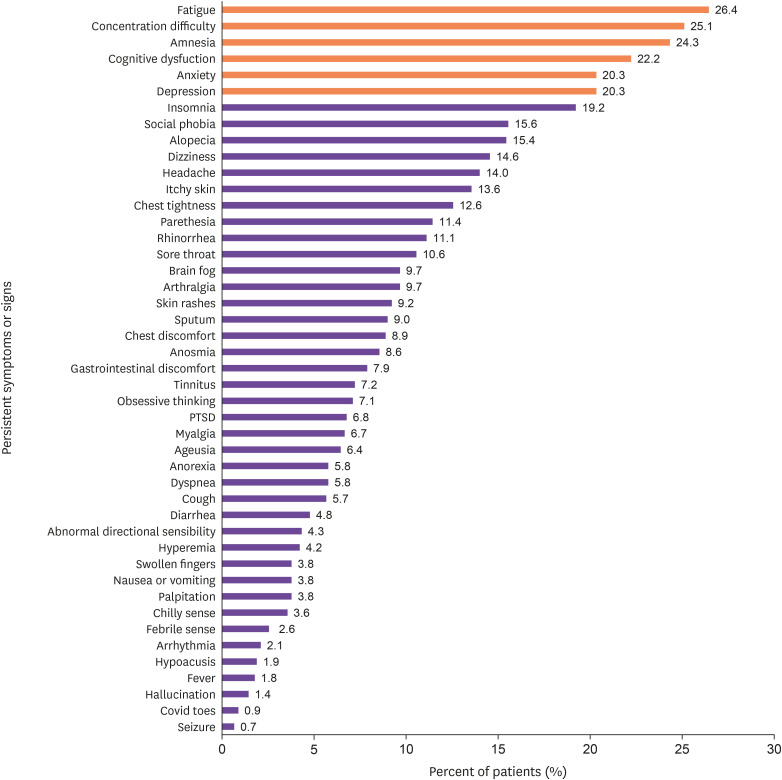
The 45 long-term sequelae were identified in 591 (65.7%) respondents. In this study, fatigue was the most common persistent symptom (238, 26.4%), followed by concentration difficulty (226, 25.1%), amnesia (219, 24.3%), cognitive dysfunction (200, 22.2%), anxiety (183, 20.3%), depression (183, 20.3%), insomnia (173, 19.2%), hair loss (139, 15.4%), and long-term persistent respiratory symptoms, such as dyspnea (52, 5.8%) (Fig. 3).
Figure 3. Distribution of 45 long-term sequelae (persistent symptoms or signs over 6 months after acute COVID-19 infection).
The most common long-term sequelae according to age distribution were as follows: concentration difficulties in 16 - 29 age groups, fatigue in 30 - 59 age groups, and amnesia in 60–70 age groups (Supplementary Fig. 2A-E). The most common long-term sequelae based on disease severity during acute COVID-19 is shown in supplementary Fig. 3. Fatigue and concentration difficulties were the most frequent long-term sequelae in disease severity mild to severe groups. In the critical disease severity group, amnesia and concentration difficulties were identified as the most frequent symptoms (Supplementary Fig. 3). The most common long-term sequelae based on sex were identified as fatigue, concentration difficulties, and amnesia. No differences were observed in most frequent long-term sequelae in both females and males (Supplementary Fig. 4).
According to age distribution, key long-term sequelae were found to be higher in the 50 years or over age group than in the less than 50 years old age group (Supplementary Fig. 5). Distribution of key long-term sequelae based on the disease severity showed that the higher the disease severity, the higher the frequency of concentration difficulties, cognitive dysfunction, and amnesia (Supplementary Fig. 6).
In total, 291 of the 900 respondents (32.3%) developed a new disease because of COVID-19. The number of respondents in whom the underlying disease got aggravated because of COVID-19 was 202 (22.4%, 202/900). Aggravated underlying diseases were identified as 10.4% psychological disease, 5.1% hypertension, 2.6% chronic lung disease, 2.0% heart disease, 2.0% diabetes, 1.4% kidney disease, 0.6% cerebral vascular disease, and 1.7% others. Thus far, 77 (8.6%) patients receiving constant outpatient medical care due to COVID-19-related long-term persistent symptoms were identified. In addition, 120 (13.3%) respondents expressed the desire to connect for medical care and to follow-up the long-term sequelae.
3. Factors associated with the prevalence of long-term sequelae
After observing the prevalence of COVID-19-related sequelae among the respondents, factors that could affect the prevalence of the sequelae were investigated. Multivariate analysis was performed for the most frequent long-term sequelae. The occurrence of long-term sequelae was significantly associated with moderate to severe disease, age ≥50 years, and female sex. In particular, fatigue, cognitive dysfunction, and amnesia were significantly associated with all moderate to severe disease, age ≥50 years, and female sex. Long-term sequelae of anosmia and ageusia were associated with age ≥50 years (Table 2).
Table 2. Univariate and multivariate logistic regression analysis for the factors associated with long-term sequelae.
| Sequelae | Factors | Univariate OR | Multivariate OR | ||
|---|---|---|---|---|---|
| 95% CI | P-value | 95% CI | P-value | ||
| Fatigue | ≥ Moderate severity | 1.94 (12.7 – 2.93) | 0.002 | 1.60 (1.03 – 2.48) | <0.001 |
| ≥50 years old | 1.65 (1.16 – 2.32) | 0.005 | 1.51 (1.05 – 2.17) | 0.025 | |
| Female | 2.41 (1.69 – 3.52) | <0.001 | 2.36 (1.65 – 3.45) | <0.001 | |
| Anxiety | ≥ Moderate severity | 2.09 (1.33 – 3.22) | 0.001 | 1.89 (1.17 – 2.92) | 0.008 |
| ≥50 years old | 1.36 (0.92 – 1.98) | 0.114 | 1.20 (0.80 – 1.78) | 0.370 | |
| Female | 2.19 (1.48 – 3.32) | <0.001 | 2.12 (1.43 – 3.22) | <0.001 | |
| Depression | ≥ Moderate severity | 2.09 (1.33 – 3.22) | 0.001 | 1.74 (0.95 – 2.75) | 0.018 |
| ≥50 years old | 1.58 (1.08 – 2.28) | 0.017 | 1.42 (0.95 – 2.10) | 0.079 | |
| Female | 3.02 (1.98 – 4.76) | <0.001 | 2.94 (1.92 – 4.64) | <0.001 | |
| Insomnia | ≥ Moderate severity | 1.65 (1.03 – 2.60) | 0.033 | 1.67 (0.84 – 2.19) | 0.200 |
| ≥50 years old | 1.67 (1.14 – 2.43) | 0.008 | 1.57 (1.06 – 2.32) | 0.024 | |
| Female | 1.92 (1.30 – 2.90) | 0.001 | 1.89 (1.27 – 2.86) | 0.002 | |
| Concentration difficulty | ≥ Moderate severity | 2.52 (1.66 – 3.82) | <0.001 | 2.22 (1.44 – 3.43) | <0.001 |
| ≥50 years old | 1.44 (1.01 – 2.05) | 0.042 | 1.24 (0.85 – 1.79) | 0.263 | |
| Female | 2.46 (1.70 – 3.62) | <0.001 | 2.36 (1.63 – 3.49) | <0.001 | |
| Cognitive dysfunction | ≥ Moderate severity | 2.32 (1.51 – 3.55) | <0.001 | 1.89 (1.20 – 2.94) | 0.005 |
| ≥50 years old | 1.79 (1.24 – 2.56) | 0.002 | 1.60 (1.09 – 2.34) | 0.015 | |
| Female | 3.01 (2.01 – 4.65) | <0.001 | 2.94 (1.95 – 4.55) | <0.001 | |
| Amnesia | ≥ Moderate severity | 2.42 (1.59 – 3.68) | <0.001 | 1.87 (1.20 – 2.89) | 0.005 |
| ≥50 years old | 2.24 (1.58 – 3.17) | <0.001 | 2.03 (1.41 – 2.91) | <0.001 | |
| Female | 2.51 (1.73 – 3.73) | <0.001 | 2.47 (1.69 – 3.68) | <0.001 | |
| Anosmia | ≥ Moderate severity | 1.10 (0.52 – 2.12) | 0.781 | - | - |
| ≥50 years old | 2.22 (1.33 – 3.63) | 0.002 | 2.22 (1.33 – 3.63) | 0.002 | |
| Female | 1.18 (0.71 – 2.03) | 0.542 | - | - | |
| Ageusia | ≥ Moderate severity | 1.38 (0.62 – 2.76) | 0.396 | - | - |
| ≥50 years old | 1.94 (1.07 – 3.40) | 0.240 | 1.94 (1.07 – 3.40) | 0.024 | |
| Female | 1.15 (0.65 – 2.15) | 0.638 | - | - | |
OR, odds ratio; CI, confidence interval.
DISCUSSION
To the best of our knowledge, this is one of the largest long-term studies on COVID-19-related sequelae in patients who recovered from COVID-19 in Korea. In this online survey, we demonstrated that 65.7% of the respondents still suffered from at least one long-term sequelae, although the individual’s condition with respect to the identified sequelae improved over time. Furthermore, 8.6% of the respondents were receiving constant outpatient treatment for COVID-19-related sequelae. Long-term persistent symptoms were manifested as adverse mental and psychological effects.
As COVID-19 continues to spread, the need for research on chronic complications in COVID-19 survivors and on long-term follow-up of patients is emphasized worldwide [14].
A previous study performed in Italy evaluated patients who had recovered from COVID-19 after ~60 days from the first appearance of COVID-19 symptoms; this study consisted of patients with a mean age of 56.5 years (range, 19 - 84 years), while our study consisted of respondents with a mean age of 31 years (range, 24 - 47), relatively younger than those in the Italy study. The Italy study showed that 87.4% of the patients reported persistence of at least one symptom, particularly fatigue (53.1%) and dyspnea (43.4%). Our study showed similar findings in that fatigue was the most common presenting symptom in the post-COVID-19 follow-up. However, only 5.8% of the respondents reported long-term sequelae of dyspnea. This can be attributed to the fact that the Italian study included 104 patients (72.7%) who had pneumonia, including 28 ventilator-supported patients (19.6%), whereas in our study, 84.8% of the respondents were in the mild disease severity group and the time of survey from the COVID-19 related symptom onset was different. The difference in the findings could also be attributed to the fact that the Italian study did not investigate COVID-19-related psychological sequelae, whereas we assessed various types of sequelae, including mental and psychological effects [15].
A cohort study of the 6 months consequences of COVID-19 conducted in Wuhan, China showed that fatigue (63%), sleep difficulties (23%), and anxiety or depression (23%) were the main problems in COVID-19 survivors [16]. Similar results were found in our study that long-term psychological sequelae were identified and it accounted for ≥20% of all sequelae. In our study, particularly, amnesia was the third most common long-term sequelae that persisted for a median period of >6 months. While the China cohort study included survivors with a median age of 57 (47 – 65) years, of which 75% of the total needed oxygen treatment during hospital admission, most of the respondents enrolled in our study were young, did not have any chronic medical condition, and had mild disease severity during the acute COVID-19 infection. Long-term psychological sequelae can seriously threaten not only the overall social well-being, but also the personal day-to-day functional status, resulting in a social burden.
A large array of atypical symptoms appears regardless of disease severity several months after the COVID-19 infection. Therefore, in-depth studies on management during or after the acute stage of COVID-19 infection are crucial for preventing and reducing sequelae.
During and after the SARS and MERS outbreaks, infected patients were commonly reported to experience psychological distress, anxiety or depression symptoms, psychiatric disorders, and chronic fatigue [6,17,18]. The processing of these psychological manifestations were found to be at its peak at one year [19]. However, it is unclear if the risk of developing these can be attributed to the viral infections or to the host immune response [20]. From a biological point of view, the immune response in SARS-CoV-2 infection is associated with proinflammatory cytokines, such as TNF alpha and IL-6, that cause activation of microglia cells and lead to brain damage [21]. Postviral fatigue studies have shown that levels of IL-6 and IL-10 in blood in the acute phase can predict the development of subsequent chronic fatigue [22]. The association of inflammation with depression has been described in the literature and might explain some of the psychiatric morbidities in inflammatory conditions [23]. Therefore, psychological sequelae identified in our study may provide evidence regarding the long-term damage caused by COVID-19 infection to the patient’s central nervous system.
A previous study showed that neurologic symptoms are more common in patients with severe COVID-19 infection, given that 84% of the patients admitted to the intensive care unit showed one or more neurologic symptoms [24]. Likewise, our study confirmed that disease severity is an important factor associated with the long-term sequelae, especially psychological sequelae.
Finally, we found that alopecia may be a unique long-term sequela of COVID-19 present in 15.4% of the patients. Moreover, 23.8% of the respondents had experienced alopecia at least once after COVID-19 infection in this research. Alopecia areata has been noted to flare up in the presence of infection, stress of quarantine, and/or fear of infection [25,26]. As per a case report, new-onset alopecia areata forms may be nonspecific cutaneous manifestations of SARS-CoV-2 [27]. Whether inflammation from COVID-19 promotes hair loss has not been reported, and its pathogenesis, clinical course, and treatment require further research. Therefore, if the clinical situations are relevant, physicians need to be aware of the possibility of a recent COVID-19 infection and perform appropriate COVID-19 testing.
In regards to the factors associated with psychological symptoms, the study conducted in China found no association between age, sex, pneumonia severity, and psychological morbidities or fatigue. However, this China study was performed during the acute stage of COVID-19 infection and detect no factors associated with long-term sequelae [28]. A previous study focused on the clinical characteristics of the sequelae using univariate analysis and showed that sequelae were more common in female patients [12]. Our multivariate analysis showed that the difference in long-term sequelae prevalence was associated with age, sex, and disease severity. In particular, for the long-term sequela, fatigue, cognitive dysfunction, and amnesia were found to be all associated with and higher in recovered COVID-19 patients age ≥50 years, female, or having ≥moderate disease severity. Other psychological symptoms, such as anxiety, were found not to be associated with patient’s age ≥50 years. It is possible that other factors, such as social support and background, might exert a stronger impact than the biological age over time.
Susceptibility to symptomatic COVID-19 was found to be higher in the elderly [29]. None of the asymptomatic patients over the age of 60 have been identified in our study. Our study showed that after COVID-19-related symptom onset, long-term sequelae was also associated with older age, especially ≥50 years.
Our study also demonstrated that sequelae can manifest in various forms and be present in a wide range of age groups, similar to the previous study [30]. In particular, we found that various types of long-term sequelae could be present even in young patients with mild symptoms, although the total number of sequelae frequency decreases over time. This was also demonstrated in an earlier study that investigated sequelae for up to the median of 79 days [31]. Continuous research and careful monitoring are necessary for determining whether these sequelae continue for a longer period of time or show full recovery in the majority of patients.
This study has certain limitations. First, respondents with sequelae may have a higher tendency to actively participate in the survey, accounting for the relatively high sequelae in the COVID-19 recovered patients. However, a relatively large number of identical groups were included in the online survey research. Second, only those who could respond to the online survey were enrolled. Hence, our study may have less data from the elderly or respondents with severe sequelae who had difficulty in responding to an online survey. Further research on patients with severe sequelae or in the elderly population is required. Third, investigations on simultaneous infections in the convalescent period have not been conducted, and further studies on simultaneous infections that can affect sequelae will be needed. Fourth, as this study was an online survey study, information of persistent symptoms was reported directly by respondents, and was not based on an objective evaluation.
Despite the limitations, we performed a detailed investigation of 45 symptoms or signs, and, to our knowledge, it represents the largest study on COVID-19-related sequelae in Korea. In addition, this study involved the largest number of young respondents who were diagnosed as mild disease severity, which accounts for most of the COVID-19 patients diagnosed worldwide. Our research provides a reference for the evaluation of symptoms in patients recovering from COVID-19 infection. Moreover, our results highlight the need to enhance the preparedness and competence of health-care professionals in the detection and management of the psychological sequelae related to the current COVID-19 pandemic.
In conclusion, until the survey date, 65.7% of the respondents complained of long-term sequelae. In addition, 8.6% of those who had been constantly receiving outpatient treatment for COVID-19 were found to have related sequelae. The results demonstrated that the rates of psychiatric morbidities and resultant functional disabilities were persistently high as well as clinically significant. To improve the care of patients who have recovered from COVID-19 infection with long-term sequelae, the participation of a comprehensive and an interdisciplinary group of researchers is required.
Footnotes
Funding: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (fund code 2020-ER5334-00)
Conflicts of interest: No conflicts of interest.
- Conceptualization: YJK, SWK.
- Data curation: YJK, SWK.
- Investigation: YJK, SB, SH, KTK, HHC, SWK.
- Supervision: SWK.
- Writing - original draft: YJK, SWK.
- Writing - review & editing: SB, SH, KTK, HHC, SWK.
SUPPLEMENTARY MATERIALS
Clinical characteristics of 5,252 subjects who had recovered from COVID-19 infection, and were followed up past the initial 6 months after COVID-19 symptom onset or diagnosis
Age distribution of 900 respondents who had recovered from COVID-19 infection, based on their sex
Percentage of the responders with persistent symptoms or signs based on a persistent duration of over 1, 2, 3, and 6 months after acute COVID-19 infection.
Percentage of the responders with long-term sequelae based on the age range groups.
Percentage of the responders with long-term sequelae based on the four disease severity groups during the acute COVID-19 infection.
Percentage of the responders with long-term sequelae based on sex.
Percentage of the responders with key long-term sequelae based on the 15 - 49 and 50 - 70 years age groups.
Percentage of the responders with long-term sequelae based on disease severity during the acute COVID-19 infection.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JY, Hong SW, Hyun M, Park JS, Lee JH, Suh YS, Kim DH, Han SW, Cho CH, Kim HA. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int J Infect Dis. 2020;98:462–466. doi: 10.1016/j.ijid.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peluso MJ, Kelly JD, Lu S, Goldberg SA, Davidson MC, Mathur S, Durstenfeld MS, Spinelli MA, Hoh R, Tai V, Fehrman EA, Torres L, Hernandez Y, Williams MC, Arreguin MI, Bautista JA, Ngo LH, Deswal M, Munter SE, Martinez EO, Anglin KA, Romero MD, Tavs J, Rugart PR, Chen JY, Sans HM, Murray VW, Ellis PK, Donohue KC, Massachi JA, Weiss JO, Mehdi I, Pineda-Ramirez J, Tang AF, Wenger M, Assenzio M, Yuan Y, Krone M, Rutishauser RL, Rodriguez-Barraquer I, Greenhouse B, Sauceda JA, Gandhi M, Hsue PY, Henrich TJ, Deeks SG, Martin JN. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19. medRxiv. 2021 [Epub ahead of print] [Google Scholar]
- 6.Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, So WY, Fong SY, Lam SP. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 7.Sheng B, Cheng SK, Lau KK, Li HL, Chan EL. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur Psychiatry. 2005;20:236–242. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postolache TT, Benros ME, Brenner LA. Targetable biological mechanisms implicated in emergent psychiatric conditions associated sith SARS-CoV-2 infection. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.2795. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, Won SD, Han W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 14.Cicco S, Vacca A, Cittadini A, Marra AM. Long-term follow-up may be useful in coronavirus disease 2019 survivors to prevent chronic complications. Infect Chemother. 2020;52:407–409. doi: 10.3947/ic.2020.52.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carfì A, Bernabei R, Landi F Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua SE, Cheung V, McAlonan GM, Cheung C, Wong JW, Cheung EP, Chan MT, Wong TK, Choy KM, Chu CM, Lee PW, Tsang KW. Stress and psychological impact on SARS patients during the outbreak. Can J Psychiatry. 2004;49:385–390. doi: 10.1177/070674370404900607. [DOI] [PubMed] [Google Scholar]
- 18.Kim HC, Yoo SY, Lee BH, Lee SH, Shin HS. Psychiatric findings in suspected and confirmed Middle East respiratory syndrome patients quarantined in hospital: a retrospective chart analysis. Psychiatry Investig. 2018;15:355–360. doi: 10.30773/pi.2017.10.25.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins RO, Weaver LK, Pope D, Orme JF, Jr, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 20.Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi S, Moosaie F, Aarabi MH. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57:5263–5275. doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell A, Hepgul N, Nikkheslat N, Borsini A, Zajkowska Z, Moll N, Forton D, Agarwal K, Chalder T, Mondelli V, Hotopf M, Cleare A, Murphy G, Foster G, Wong T, Schütze GA, Schwarz MJ, Harrison N, Zunszain PA, Pariante CM. Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology. 2019;100:276–285. doi: 10.1016/j.psyneuen.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 24.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutlu Ö, Aktaş H, İmren IG, Metin A. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020:1. doi: 10.1080/09546634.2020.1782820. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Jiménez P, Ramirez-Bellver JL, Ruiz-Rodríguez R. Alopecia areata not due by isotretinoin. A thought in COVID-19 time. Dermatol Ther. 2020;33:e13451. doi: 10.1111/dth.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FIvenson D. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2021;60:127. doi: 10.1111/ijd.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi R, Chen W, Liu S, Thompson PM, Zhang LJ, Xia F, Cheng F, Hong A, Surento W, Luo S, Sun ZY, Zhou CS, Li L, Jiang X, Lu GM. Psychological morbidities and fatigue in patients with confirmed COVID-19 during disease outbreak: prevalence and associated biopsychosocial risk factors. medRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 29.Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52:154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van't Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of 5,252 subjects who had recovered from COVID-19 infection, and were followed up past the initial 6 months after COVID-19 symptom onset or diagnosis
Age distribution of 900 respondents who had recovered from COVID-19 infection, based on their sex
Percentage of the responders with persistent symptoms or signs based on a persistent duration of over 1, 2, 3, and 6 months after acute COVID-19 infection.
Percentage of the responders with long-term sequelae based on the age range groups.
Percentage of the responders with long-term sequelae based on the four disease severity groups during the acute COVID-19 infection.
Percentage of the responders with long-term sequelae based on sex.
Percentage of the responders with key long-term sequelae based on the 15 - 49 and 50 - 70 years age groups.
Percentage of the responders with long-term sequelae based on disease severity during the acute COVID-19 infection.