Abstract
Backgound
The members of the so-called ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) are a frequent cause of severe infection, ranking among the most relevant causes of hospital infections. In Peru, few studies, often focused in a single ESKAPE microorganism, have been performed, but none providing an overall and comprehensive long-time analysis of the antibiotic resistance of ESKAPE microorganisms. In the present study, the evolution of antimicrobial resistance levels of ESKAPE microorganisms isolated during 2009 - 2010 (Period 1) and 2012 - 2014 (Period 2) in a IV-level hospital in Lima was analyzed.
Materials and Methods
ESKAPE microorganisms were isolated from inpatients clinical samples. Bacterial identification, as well as antimicrobial susceptibility levels for up to 29 antimicrobial agents and presence of Extended-Spectrum β-Lactamases (only established in K. pneumoniae) were determined using automatic methods.
Results
Of 9,918 clinical isolates, 1,917/3,777 (50.8%) [JAN/2009-JUN/2010 (Period 1)] and 4764/6141 (46.4%) [JAN/2012-DEC/2014 (Period 2)] belonged to the ESKAPE group (P <0.0001). ESKAPE were more frequent in the intensive care unit (ICU) (P <0.0001). E. faecium decreased from 5.1% to 4.1% (P <0.5), S. aureus from 10.5% to 7.0% (P <0.05), and P. aeruginosa from 12.9% to 11.6% (P <0.05), while, A. baumannii increased from 5.0% to 6.7% (P <0.05), mainly related to an increase in ICU isolates (8.4% vs. 17.1%; P <0.05). Overall, high levels of antimicrobial resistance were detected, but with few exceptions (e.g. vancomycin in E. faecium), antibiotic resistance levels remained stable or lower in Period 2. Contrarily, A. baumannii showed significantly increased resistance to different cephalosporins, carbapenems and amoxicillin plus sulbactam.
Conclusion
The introduction of a successful extensively drug-resistant A. baumannii clone in the ICU is suspected. The isolation of ESKAPE and levels of antibiotic resistance levels have reduced over time.
Keywords: Multidrug resistance, Intensive Care Unit, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa
Introduction
The description of infections related to pathogenic microorganisms showing resistance to various antimicrobial agents is continuously rising. Accordingly, multidrug resistant (MDR) and extensively drug-resistant microorganisms (XDR) are often described as a cause of infections [1,2,3], with pan-resistant microorganisms also being reported as a cause of severe hospital-acquired infections [4]. These finding have led to antibiotic resistance currently being considered as one of the most relevant challenges in public health [5].
Antibiotic resistance has a direct impact on human health and behavior. Recent data on the impact of antibiotic resistance in the European Union (EU) have shown that >33,000 deaths and 874,541 disability-adjusted life-years (DALYS) were attributed to infections caused by antibiotic-resistant microorganisms in 2015 [6] and these antibiotic-resistant microorganisms have a direct impact on treatment costs. In the EU, in 2007, these costs were estimated at more than 1.5 billion euros/year [7]. Regarding future antibiotic resistance scenarios, the World Bank has predicted the risk of a deficit in the annual gross domestic product of $3.4 trillion by 2030 rising to $6.1 trillion annually by 2050 [8]. Indeed, antibiotic resistance is considered one of the most relevant health challenges of the present century, directly threating the Sustainable Development Goals [4,8,9].
In this context, a series of pathogenic microorganisms are especially notorious for both their extremely high levels of antibiotic resistance, arriving to the above-mentioned status of pan-resistance and their ability to survive in hospital environments [4,9]. Among these pathogenic microorganisms, members of the so-called ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) are of special concern [4,9], resulting in worse patient outcomes and increased treatment costs [4,6,8,10]. This problem is further compounded by ESKAPE microorganisms often accounting for more than 50% of hospital acquired infections [11].
Peru is a middle-income country with over-the-counter access to medications, including antibiotics [12,13]. Although data on antibiotic resistance in Peru are scarce, the panorama is discouraging, with high levels of antibiotic resistance to carbapenems, third-generation cephalosporins and fluoroquinolones, among others having been reported [1,2,3,14], in addition to emerging resistance to last-resort antibiotics such as colistin or tigecycline [2,15]. Despite this, there is practically no comprehensive surveillance of the evolution of antibacterial resistance of microorganisms, such as those of the ESKAPE group, over different time periods.
In this context, the evolution of antimicrobial resistance levels of ESKAPE microorganisms isolated during 2009 - 2010 and 2012 - 2014 in a IV-level hospital in Lima using data from the internal database was analyzed.
Material and Methods
1. Study area
All isolates belonging to one of the ESKAPE species isolated from patients admitted to the medicine and surgery departments, and the intensive care unit (ICU) of the Hospital Nacional Guillermo Almenara Irigoyen (HNGAI) of Lima (Peru) during 2009 - 2010 (Period 1) and 2012 - 2014 (Period 2) were included in the study. The study did not involve humans and no personal data was accessible for the researchers.
The HNGAI is a IV-level hospital with 1,051 beds in the departments studied: Medicine (477 beds), Surgery (453 beds) and ICU (21 beds). Samples from the Gynecology, Psychiatry and the Emergency and Outpatient (mainly involving community-acquired infections) Departments (overall accounting for 100 beds) were excluded from the analysis.
2. Microorganisms
The microorganisms were isolated from different clinical samples following standard microbiology protocols at the HNGAI Microbiology Laboratory. The identification, antibiotic susceptibility and presence of Extended-Spectrum β-Lactamases (ESBL) (only K. pneumoniae) were performed using the automatic MicroScan system (Siemens Medical Solutions Diagnostics, Camberley, UK) in Period 1 and with Microscan and Vitek 2 (BioMérieux, Marcy l'Etoile, France) in Period 2.
The antibiotics in each analysis varied according to the bacterial species and included: ampicillin, penicillin, oxacillin, amoxicillin plus clavulanic acid, piperacillin-tazobactam (TZP), ampicillin-sulbactam (SAM), cefazolin, cefotaxime, ceftriaxone, ceftazidime, cefepime, imipenem, meropenem, ertapenem, aztreonam, trimethoprim-sulfamethoxazole (TMP/SMX), amikacin, gentamicin, streptomycin, tetracycline, ciprofloxacin, vancomycin, rifampicin, erythromycin and clindamycin. Susceptibility patterns were established in agreement with the Clinical and Laboratory Standards Institute [16]. A minimal inhibitory concentration ≤2 µg/ml was considered the tigecycline breakpoint in accordance with the US Food and Drug Administration (https://www.fda.gov/drugs/development-resources/tigecycline-injection-products) and Nicolau et al. [17].
In addition, chloramphenicol was included in the determination of S. aureus susceptibility during Period 1, while linezolid was tested in 39 E. faecium and 54 S. aureus in Period 2. Similarly, in Period 2, tigecycline susceptibility was also tested in 177 K. pneumoniae and 37 A. baumannii, while colistin susceptibility levels were established in 31 P. aeruginosa and 20 A. baumannii.
3. Statistical analysis
The Fisher exact test was used to determine statistical associations. A P-value <0.05 was considered significant.
In all cases intermediate and resistant isolates were classified together as “non-susceptible”. Coagulase negative Staphylococcus (CoNS) were considered as contamination [18] and were not included in the statistical analyses.
4. Ethics Approval and Consent to Participate
The data extracted and the manuscript was reviewed with Scientific and Ethics Committee. No experimental intervention was performed. It did not require any specification of guidelines, legislations, or permissions.
Results
1. Analysis of the time and ESKAPE isolation according to hospital department
Overall, 10,948 isolates were recovered from clinical samples of admitted patients. Of these, 1,030 belonged to CoNS and were classified as sample contamination (contamination rate 9.4%). Excluding CoNS, 3,777 bacterial isolates were recovered in Period 1 (2,227, 887, 663 from medicine, surgery and ICU, respectively) and 6,141 in Period 2 (3,271, 1,860, 1,010 from medicine, surgery and ICU respectively). Of these, 4,764 (48.0%) microorganisms belonged to the ESKAPE group (1,917 - Period 1 and 2,848 - Period 2), representing 50.8% of the isolates of Period 1 and 46.4% of Period 2 (P <0.05). Analysis of the evolution of ESKAPE isolates in Periods 1 and 2 showed a reduction in the isolation of E. faecium from 5.1% to 4.1% (P <0.05), S. aureus from 10.5% to 7.0% (P <0.05), and P. aeruginosa from 12.9% to 11.6% (P <0.05), and an increase in A. baumannii from 5.0% to 6.7% (P <0.05) (Table 1).
Table 1. ESKAPE isolates, 2009 - 2010 and 2012 - 2014.
| ESKAPE | Medicine | Surgery | ICU | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 - 2010 (2,227) | 2012 - 2014 (3,271) | P | 2009 - 2010 (887) | 2012 - 2014 (1,860) | P | 2009 - 2010 (663) | 2012 - 2014 (1,010) | P | 2009 - 2010 (3,777) | 2012 - 2014 (6,141) | P | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Enterococcus faecium | 119 (5.3) | 153 (4.7) | 0.3101 | 40 (4.5) | 71 (3.8) | 0.4075 | 35 (5.3) | 31 (3.1) | 0.0289 | 194 (5.1) | 255 (4.1) | 0.0251 |
| Staphylococcus aureus | 193 (8.7) | 214 (6.5) | 0.0039 | 104 (11.7) | 117 (6.3) | <0.0001 | 99 (14.9) | 101 (10.0) | <0.0001 | 396 (10.5) | 432 (7.0) | <0.0001 |
| Klebsiella pneumoniae | 301 (13.5) | 448 (13.7) | 0.8728 | 114 (12.8) | 239 (12.8) | 0.8557 | 82 (12.4) | 129 (12.8) | 0.8218 | 497 (13.2) | 816 (13.3) | 0.8788 |
| Acinetobacter baumannii | 100 (4.5) | 147 (4.5) | 1.000 | 33 (3.7) | 95 (5.0) | 0.1212 | 56 (8.4) | 173 (17.1) | <0.0001 | 189 (5.0) | 415 (6.7) | 0.0004 |
| Pseudomonas aeruginosa | 271 (12.2) | 357 (10.9) | 0.0038 | 106 (11.9) | 201 (10.8) | 0.3999 | 112 (16.9) | 152 (15.0) | 0.3372 | 489 (12.9) | 710 (11.6) | 0.0423 |
| Enterobacter | 92 (4.1) | 118 (3.6) | 0.3515 | 31 (3.5) | 69 (3.7) | 0.8281 | 29 (4.4) | 33 (3.3) | 0.2300 | 152 (4) | 220 (3.6) | 0.2764 |
| ESKAPE | 1,076 (48.3) | 1,437 (43.9) | 0.0014 | 428 (48.2) | 792 (42.6) | <0.0001 | 413 (62.3) | 619 (61.3) | 0.6813 | 1,917 (50.8) | 2,848 (46.4) | <0.0001 |
| PM+S/I | <0.0001 | <0.0001 | ||||||||||
ESKAPE, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.; ICU, intensive care unit; M, medicine; S, surgery; I, intensive care unit.
In Periods 1 and 2, ESKAPE microorganisms were more frequently isolated as a cause of infection in the ICU (62.3% and 61.3%, respectively; P <0.05) (Table 1).
K. pneumoniae was the most common member of ESKAPE group, accounting for 1,313 isolates (497 and 816 in the period 1 and 2 respectively; 13.2% of total isolates). Accordingly, the analysis by hospital areas, showed that K. pneumoniae was the most frequent ESKAPE microorganism in medicine and surgery departments (749 and 353 isolates respectively). The microorganisms most frequently isolated in the ICU in Period 1 were P. aeruginosa (112 isolates) followed by S. aureus (99 isolates), being A. baumannii (173 isolates) and P. aeruginosa (152 isolates) in Period 2 (Table 1). Among the ESKAPE group Enterobacter spp. was the least frequently isolated accounting for 3.7% of all the microorganisms analyzed and 7.8% of the ESKAPE group.
On comparing the evolution of the microorganisms isolated in the two study periods, the most relevant finding was the significant increase in A. baumannii isolates (5.0% vs. 6.7%; P <0.05) and the parallel decrease in E. faecium (5.1% vs. 4.1%; P <0.05), P. aeruginosa (12.9% vs. 11.6%; P <0.05) and S. aureus (10.5% vs. 7.0%; P <0.05). (Table 1).
2. Analysis of antimicrobial resistance
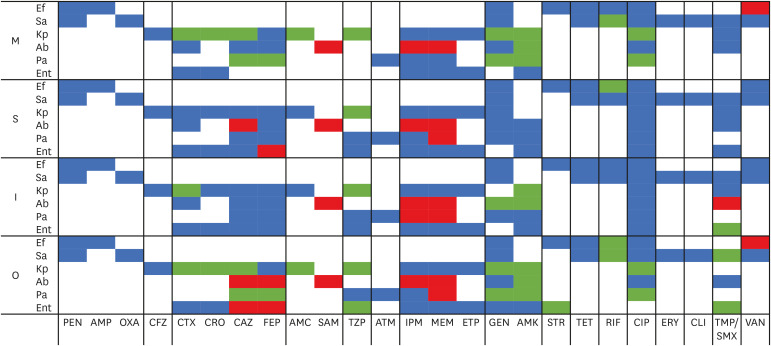
In general, we found high levels of antimicrobial resistance to most of the antibiotics tested (Fig. 1, Table 2, 3, 4). Fortunately, although only a reduced number of microorganisms were tested, the isolates of the ESKAPE group showed high susceptibility to last-resort antibacterial agents. Thus, E. faecium was only susceptible to linezolid, S. aureus to linezolid and vancomycin, A. baumannii to colistin and tigecycline, P. aeruginosa to colistin. Regarding Enterobacteriaceae, both K. pneumoniae and Enterobacter spp. were highly susceptible to carbapenems (Table 2, 3, 4). Surprisingly, the antibiotic resistance levels tended to remain stable or decrease in Period 2, except for A. baumannii, in which resistance to different cephalosporins, carbapenems and SAM significant increased over time (Fig. 1).
Figure 1. Evolutive trends between the two study periods.
Blue, No significant differences; Green, Significant decrease; Red, Significant increase.
M, medicine department; S, surgery department; I, intensive care unit; O, overall data; Ef, Enterococcus faecium; Sa, Staphylococcus aureus; Kp, Klebsiella pneumoniae; Ab, Acinetobacter baumannii; Pa, Pseudomonas aeruginosa; Ent, Enterobacter spp.; PEN, penicillin; AMP, ampicillin; OXA, oxacillin; CFZ, cefazolin; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; FEP, cefepime; AMC, amoxicillin-clavulanic; SAM, ampicillin-sulbactam; TZP, piperacillin-tazobactam; ATM, aztreonam; IPM, imipenem; MEM, meropenem; ETP, ertapenem; GEN, gentamicin; AMK, amikacin; STR, streptomycin; TET, tetracycline; RIF: rifampicin; CIP, ciprofloxacin; ERY, erythromycin; CLI, clindamycin; TMP/SMX, trimethoprim-sulfamethoxazole; VAN, vancomycin.
Table 2. Antimicrobial resistance levels of Enterococcus faecium and Staphylococcus aureus isolates.
| A) Enterococcus faecium | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PEN | AMP | VAN | GENa | STRa | TET | CIP | RIF | ||||||||||||
| % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | |||||
| M+S+I | P1 | 194 | 90 | NS | 90 | NS | 42 | <0.05 | 74 | NS | 67 | NS | 56 | NS | 93 | NS | 88 | <0.05 | ||
| P2 | 255 | 93 | 91 | 53 | 74 | 68 | 63 | 95 | 78 | |||||||||||
| O | 449 | 91 | 90 | 48 | 74 | 67 | 60 | 94 | 82 | |||||||||||
| M | P1 | 119 | 93 | NS | 92 | NS | 36 | <0.05 | 80 | NS | 67 | NS | 60 | NS | 96 | NS | 86 | NS | ||
| P2 | 153 | 95 | 93 | 52 | 76 | 72 | 65 | 95 | 79 | |||||||||||
| O | 272 | 94 | 92 | 45 | 78 | 70 | 62 | 95 | 82 | |||||||||||
| S | P1 | 40 | 90 | NS | 90 | NS | 47 | NS | 72 | NS | 75 | NS | 47 | NS | 95 | NS | 95 | <0.05 | ||
| P2 | 71 | 89 | 88 | 46 | 71 | 60 | 59 | 94 | 77 | |||||||||||
| O | 111 | 89 | 89 | 46 | 71 | 66 | 55 | 94 | 84 | |||||||||||
| I | P1 | 35 | 80 | NS | 80 | NS | 54 | NS | 54 | NS | 57 | NS | 49 | NS | 93 | NS | 86 | NS | ||
| P2 | 31 | 94 | 90 | 74 | 71 | 71 | 58 | 97 | 74 | |||||||||||
| O | 66 | 86 | 85 | 64 | 62 | 64 | 53 | 95 | 80 | |||||||||||
| P1 | PM+S/I | 0.06 | 0.07 | NS | <0.05 | NS | NS | NS | NS | |||||||||||
| P2 | PM+S/I | NS | NS | NS | NS | NS | NS | NS | NS | |||||||||||
| O | PM+S/I | NS | NS | <0.05 | <0.05 | NS | NS | NS | NS | |||||||||||
aHigh level resistance.
In the Table are only reported significant differences (P <0.05), as well as those P-values ≤0.09.
In addition, susceptibility to linezolid was tested in 39 E. faecium and 53 S. aureus isolates from Period 2 with all being susceptible.
All S. aureus isolates were susceptible to vancomycin. In addition, chloramphenicol was tested in S. aureus from Period 1 showing an overall resistance rate of 16% with a maximum among intensive care unit isolates of 21%.
M, medicine; S, surgery; I, intensive care unit; P1, Period 1 (2009 - 2010); P2, Period 2 (2012 - 2014); O, overall; PEN, penicillin; AMP, ampicillin; VAN, vancomycin; GEN, gentamicin; STR, streptomycin; TET, tetracycline; CIP, ciprofloxacin; RIF, rifampicin; NS, non significant; OXA, oxacillin; ERY, erythromycin; CLI, clindamycin; TMP/SMX, trimethoprim-sulfamethoxazole.
Table 3. Antimicrobial resistance levels of Klebsiella pneumoniae and Enterobacter spp. isolates.
| A) Klebsiella pneumoniae | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Antibiotic resistance levels | |||||||||||||||||||||||||
| AMC | CFZ | CTX | CRO | CAZ | FEP | ETP | TZP | AMK | GEN | CIP | TMP/SMX | |||||||||||||||
| % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | |||
| M+S+I | P1 | 497 | 76 | <0.05 | 86 | NS | 84 | <0.05 | 83 | <0.05 | 83 | <0.05 | 78 | NS | 3 | NS | 48 | <0.05 | 21 | <0.05 | 66 | <0.05 | 81 | <0.05 | 69 | 0.06 |
| P2 | 816 | 68 | 86 | 76 | 78 | 77 | 74 | 2 | 22 | 10 | 54 | 73 | 74 | |||||||||||||
| O | 1,313 | 71 | 86 | 79 | 80 | 79 | 2 | 32 | 14 | 59 | 76 | 72 | ||||||||||||||
| M | P1 | 301 | 76 | <0.05 | 87 | NS | 85 | <0.05 | 84 | <0.05 | 84 | <0.05 | 78 | NS | 3 | NS | 49 | <0.05 | 24 | <0.05 | 67 | <0.05 | 80 | <0.05 | 67 | NS |
| P2 | 448 | 65 | 87 | 75 | 77 | 75 | 73 | 2 | 24 | 10 | 52 | 71 | 72 | |||||||||||||
| O | 749 | 69 | 87 | 79 | 80 | 79 | 75 | 2 | 34 | 16 | 58 | 75 | 70 | |||||||||||||
| S | P1 | 114 | 69 | NS | 82 | 81 | NS | 78 | NS | 80 | NS | 77 | NS | 2 | NS | 46 | <0.05 | 19 | NS | 64 | NS | 79 | NS | 69 | NS | |
| P2 | 239 | 71 | 86 | 80 | 80 | 79 | 75 | 3 | 20 | 14 | 57 | 75 | 76 | |||||||||||||
| O | 353 | 70 | 84 | 80 | 79 | 79 | 76 | 3 | 29 | 16 | 59 | 77 | 74 | |||||||||||||
| I | P1 | 82 | 85 | 0.09 | 89 | 85 | <0.05 | 85 | 0.09 | 85 | NS | 80 | NS | 3 | NS | 45 | <0.05 | 13 | <0.05 | 65 | 0.06 | 85 | NS | 76 | NS | |
| P2 | 129 | 75 | 82 | 71 | 75 | 76 | 73 | 3 | 22 | 4 | 55 | 76 | 74 | |||||||||||||
| O | 211 | 79 | 85 | 77 | 79 | 80 | 76 | 3 | 31 | 8 | 59 | 80 | 75 | |||||||||||||
| P1 | PM+S/I | <0.05 | NS | NS | NS | NS | NS | NS | NS | 0.08 | NS | NS | NS | |||||||||||||
| P2 | PM+S/I | 0.08 | NS | NS | NS | NS | NS | NS | NS | <0.05 | NS | NS | NS | |||||||||||||
| O | PM+S/I | <0.05 | NS | NS | NS | NS | NS | NS | NS | <0.05 | NS | NS | NS | |||||||||||||
In the Table are only reported significant differences (P <0.05), as well as those P-values ≤0.09.
All K. pneumoniae isolates were susceptible to meropenem and imipenem. In the second period was established the tigecycline susceptibility in 117 K. pneumoniae isolates, with an overall resistance of 12% being observed (Medicine 18%; Surgery: 0%; Intensive Care Unit: 12%).
M, medicine; S, surgery; I, intensive care unit; P1, Period 1 (2009 - 2010); P2, Period 2 (2012 - 2014); O, overall; AMC, amoxicillin-clavulanic acid; CFZ, cefazolin; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; FEP, cefepime; ETP, ertapenem; TZP, piperacillin-tazobactam; AMK, amikacin; GEN, gentamicin; CIP, ciprofloxacin; TMP/SMX, trimethoprim-sulfamethoxazole; NS, non significant; IPM, imipenem; MEM, meropenem.
Table 4. Antimicrobial resistance levels of Acinetobacter baumannii and Pseudomonas aeruginosa isolates.
| A) Acinetobacter baumannii | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Antibiotic resistance levels | |||||||||||||||||||||
| SAM | CTX | CAZ | FEP | IPM | MEM | AMK | GEN | CIP | TMP/SMX | |||||||||||||
| % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | % | P | |||
| M+S+I | P1 | 189 | 65 | <0.05 | 91 | 0.06 | 85 | <0.05 | 85 | <0.05 | 63 | <0.05 | 61 | <0.05 | 84 | <0.05 | 78 | NS | 89 | NS | 86 | NS |
| P2 | 414 | 91 | 97 | 93 | 93 | 90 | 88 | 69 | 72 | 93 | 90 | |||||||||||
| O | 603 | 83 | 95 | 91 | 91 | 81 | 80 | 74 | 74 | 92 | 89 | |||||||||||
| M | P1 | 100 | 61 | <0.05 | 87 | NS | 81 | NS | 81 | NS | 61 | <0.05 | 61 | <0.05 | 81 | <0.05 | 78 | NS | 87 | NS | 84 | NS |
| P2 | 147 | 89 | 92 | 88 | 88 | 86 | 78 | 59 | 71 | 88 | 84 | |||||||||||
| O | 247 | 78 | 90 | 85 | 85 | 76 | 72 | 68 | 74 | 88 | 84 | |||||||||||
| S | P1 | 33 | 68 | <0.05 | 88 | NS | 82 | <0.05 | 82 | 0.08 | 63 | <0.05 | 64 | <0.05 | 82 | NS | 73 | NS | 88 | NS | 91 | NS |
| P2 | 94 | 91 | 95 | 94 | 93 | 87 | 90 | 67 | 76 | 94 | 89 | |||||||||||
| O | 127 | 86 | 93 | 91 | 90 | 81 | 83 | 71 | 75 | 93 | 90 | |||||||||||
| I | P1 | 56 | 61 | <0.05 | 100 | NS | 95 | NS | 93 | NS | 67 | <0.05 | 71 | <0.05 | 91 | <0.05 | 80 | <0.05 | 93 | NS | 86 | <0.05 |
| P2 | 173 | 91 | 99 | 96 | 98 | 95 | 95 | 74 | 72 | 97 | 96 | |||||||||||
| O | 229 | 83 | 99 | 96 | 97 | 88 | 89 | 78 | 76 | 95 | 93 | |||||||||||
| P1 | PM+S/I | NS | <0.05 | <0.05 | <0.05 | NS | NS | NS | NS | NS | NS | |||||||||||
| P2 | PM+S/I | NS | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | NS | <0.05 | <0.05 | |||||||||||
| O | PM+S/I | NS | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | NS | <0.05 | <0.05 | |||||||||||
In the Table are only reported significant differences (P <0.05), as well as those P-values ≤0.09.
In Period 2 susceptibility to colistin was established in 20 A. baummanni and 31 P. aeruginosa isolates, and that of tigecycline was established in 37 A. baumanni isolates, all being susceptible.
M, medicine; S, surgery; I, intensive care unit; P1, Period 1 (2009 - 2010); P2, Period 2 (2012 - 2014); O, overall; SAM, ampicillin-sulbactam; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; IPM, imipenem; MEM, meropenem; AMK, amikacin; GEN, gentamicin; CIP: ciprofloxacin; TMP/SMX, trimethoprim-sulfamethoxazole; NS, non significant; ATM, aztreonam; TZP, piperacillin-tazobactam.
Regarding other antibacterial agents, the antibiotic resistance levels of E. faecium ranged from 48% (vancomycin) to 94% (ciprofloxacin). Overall, the antibiotic resistance levels remained unaltered during the two periods, except for resistance to vancomycin, which significantly increased, and rifampicin which significantly decreased. In addition, a non-significant increase in tetracycline resistance was observed. Analysis by hospital department showed that resistance to vancomycin was higher in isolates from the ICU in both periods, being significant when all the isolates were considered together. Meanwhile the resistance of high level to gentamicin was significantly lower among ICU isolates while that to β-lactam tend to be lower in ICU isolates (Table 2A).
Regarding S. aureus, resistance levels to TMP/SMX (13%), tetracycline (18%) and rifampicin (12%) were low irrespective of the period or department. In addition, while the levels of resistance to most of the antibiotics tested remained unaltered in both periods, resistance to TMP/SMX and rifampicin decreased over time. In the case of TMP/SMX, a trend to decreasing levels of resistance was observed in all departments, while a decrease in rifampicin resistance was observed in isolates from the medicine department and the ICU. The presence of methicillin-resistant S. aureus (MRSA) was higher among ICU isolates in Periods 1 and 2, being lower in the Medicine Department. In general, antibiotic resistance was higher among ICU isolates (Table 2B).
In addition to carbapenems, tigecycline (for which only partial data from Period 2 were available) and amikacin showed good activity against K. pneumoniae (12% and 14%, of antibiotic resistance respectively. Unexpectedly, a trend towards higher levels of susceptibility to all the antibacterial agents tested, except cefepime was observed, being especially notable in the Medicine Department and the ICU. This finding was especially relevant in regard to TZP, with overall resistance levels decreasing from 48% to 22%, related to a significant decrease in resistance levels in all the departments analyzed. Regarding cephalosporins, a slight overall, albeit non-significant, decrease was observed in the frequency of ESBL in Periods 1 and 2 (80% vs. 76%), being especially notable among ICU isolates (81% vs. 74%) (Table 3A).
A. baumannii isolates presented high levels of antimicrobial resistance, with a trend to an increase in Period 2, except for aminoglycosides (amikacin and gentamicin) which showed a significant antimicrobial resistance levels decrease. These trends were observed in the three departments analyzed, with most antibiotics reaching resistance levels higher than 90%. In general, isolates from the ICU showed significantly higher levels of resistance to the antimicrobial agents analyzed, being greater than 80% to all antibiotics (except colistin and tigecycline) (Table 4A).
In contrast to A. baumannii, the overall levels of antibiotic resistance of P. aeruginosa tended to decrease or remain unaltered, with most of the antibiotics showing levels of resistance of around 50%. The most notable exception was the level of resistance to carbapenems which increased in both periods analyzed and were significant with meropenem, largely fueled by the increase in carbapenem resistance levels in ICU isolates (Table 4B).
Finally, Enterobacter spp. showed an overall trend to decreased resistance to antibiotics tested, achieving significance in relation to TZP and TMP/SMX. On the other hand, cephalosporins tended to show higher levels of antibiotic resistance, with ceftazidime and cefepime presenting significant increases. This increase in cephalosporin resistance was mainly found in isolates from medicine and surgery departments (Table 3B).
Discussion
Antibiotic resistance is a growing problem worldwide [5,6,19,20]. This phenomenon is fueled by different factors beyond the obligation to adequately treat human or animal infections and includes social, economic, and cultural factors, which severely impact the final levels of antibiotic consumption and subsequent selective pressure of antibiotic-resistant microorganisms [20]. Furthermore, current trade and travel facilities play a key role in the rapid dispersion of antibiotic-resistant microorganisms which may be visible and produce pathogenic processes, or silent and occult, when related to commensal or non-pathogenic microorganisms [20,21,22].
1. Analysis of the period and ESKAPE isolation according to clinical department
In the departments analyzed, the role of ESKAPE microorganisms as infectious agents was relevant, overall accounting for ~50% of all recorded microorganisms isolated as a cause of infection in the HNGAI. This high relevance is in accordance with that described in other reports, in which ESKAPE rates in different clinical settings were around 40 - 60%, being together Escherichia coli the most frequent pathogen involved in hospital-acquired infections [2,10,11]. It was of note that while the number of A. baumannii increased, ESKAPE were significantly less frequently recovered during Period 2. There is no clear explanation for the reduction in ESKAPE. A Program for Optimization of Antibiotic use (PROA) was not established until 2018, and there is no information about changes in internal procedures. The increase of A. baumannii might be related to the presence of a successful hospital clone causing an undetected outbreak. This suggestion is supported by the increase in cephalosporin, carbapenem and SAM resistance levels detected, and with the overall increase mainly in the ICU. Inter-hospital dissemination of successful A. baumannii clones exhibiting high levels of antibiotic resistance, similar to those reported in the present study, has been previously described in Lima [2], the most relevant difference being the lower levels of resistance to SAM (62.5%) compared to our study (91%).
2. Analysis of antimicrobial resistance
While the present data showed a clear trend to maintained or reduced antibiotic resistance levels, overall, high levels of antibiotic resistance were detected in both study periods similar to previous reports in Peru [1,2,3,14]. This trend to decreasing resistance levels is intriguing, and taking into account the lack of specific antibiotic management related to PROA, it is not the result of a series of structured countermeasures.
High levels of antibiotic resistance were especially notable among ICU isolates. The fragility of ICU patients usually requires greater use of antibiotics [23], resulting in enhanced pressure towards the selection of antibiotic-resistant microorganisms, which may explain this phenomenon. As mentioned above, in the ICU, the most serious concerns were related to the increasing presence of vancomycin-resistant Enterococcus faecium (VRE) and the high and increasing levels of resistance to antibiotics shown by A. baumannii.
The presence of VRE is of increasing concern. In 2017, VRE were identified as causing 54,500 infections in the United States, with a lethality rate of ~10% (5,400 deaths), leading the Centers for Disease Control and Prevention (CDC) to classify this pathogen as a serious threat [19]. Another relevant concern is the possible transfer of genetic material from VRE to S. aureus. Thus, while infrequent [24], and to our knowledge, has not been reported in health care settings in Peru, the potential risk of transfer of vancomycin-resistant determinants from VRE to S. aureus cannot be ruled out [24].
Meanwhile, A. baumannii isolates showed antibiotic resistance levels >80% to most of the agents tested, with carbapenems and extended spectrum cephalosporins resistance levels being >90%. This finding indicates the de facto lack of utility of these latter antibiotics for the treatment of A. baumannii infections and the scarcity of treatment options out of the last-resort antibiotics. Thus, the present study showed that in a small subset of isolates, A. baumannii presented 100% of susceptibility to colistin and tigecycline and could therefore, potentially, be useful for treatment. Nevertheless, colistin has not been used in the clinical setting for years because its high levels of toxicity [25], and should be used with caution. Regarding tigecycline, the literature is controversial. Thus, while several studies have reported a higher risk of death in patients receiving this antibiotic [26], other data suggest that is a good candidate for the treatment of infection by A. baumannii [27]. Considering that carbapenem-resistant A. baumannii has been classified as an urgent threat [19], other alternative treatments such as sulbactam/avibactam [28] or cefiderocol [29] might be useful in the treatment of MDR and XDR A. baumannii.
As with other ESKAPE members, S. aureus from the ICU showed higher levels of antibiotic resistance, in agreement with the significantly higher presence of MRSA, which often presents higher levels of antibiotic resistance in ICUs [30]. While several antibiotics, including vancomycin and linezolid, showed excellent activity, the current data are concerning. The prevalence of MRSA in this study is higher than that of other studies performed in Peru. Thus, while Schwalb et al. reported that 65% of S. aureus acquired in hospital settings were MRSA, on analyzing samples from 7 hospitals in Peru, Garcia et al., reported 72.5% of MRSA in ICUs and 45% in the remaining hospital wards [30,31]. Of note, Garcia et al. also reported worrisome levels of antibiotic resistance >90% in several cases, such as ciprofloxacin or erythromycin [30].
Carbapenem-resistant and ESBL-producing Enterobacteriaceae are considered urgent and serious threats, respectively [19]. In the present study carbapenem-resistant K. pneumoniae was practically absent. While different carbapenemases including KPC, NDM, and IMP have been detected among K. pneumoniae from Peru [32,33], other reports described a low prevalence of carbapenem-resistant K. pneumoniae [1,34], in agreement with our results. Notwithstanding, local carbapenem-resistant K. pneumoniae outbreaks have been described in recent years [33,35]. On the other hand, while tending to decrease over time, high levels of ESBL-producer K. pneumoniae were detected. In this sense, ESBL-producer K. pneumoniae are frequent in Peru, accounting for >70% of K. pneumoniae isolates in different studies [1,34]. Of note, a great variety of ESBLs, including different CTX-M and SHV have been described among Peruvian K. pneumoniae, with CTX-M-15 being the most prevalent [1]. Regarding tigecycline, 18% and 16% of resistant isolates were detected in the Medicine Department and ICU. While these are low levels of resistance, alert about the emergence of resistance to most modern agents.
P. aeruginosa is an opportunistic pathogen frequently involved in nosocomial infections, often showing high levels of antibiotic resistance [14,36]. In this sense, the levels of resistance ranging from ~50 to ~75% (except colistin) found in the present study were to be expected and are in the line of previous reports in Peru [14]. The unexpected result was the trend to maintained or decreased antibiotic resistance levels extended to almost all antibiotics, except carbapenems and TZP, which showed an inverse trend in the ICU and Surgery Department. While increasing levels of carbapenems and TZP might be related to the pressure exerted by these agents, resulting in the selection of P. aeruginosa resistant isolates, we have no explanation for the decreasing levels of resistance to other agents, including aminoglycosides, cephalosporins and fluoroquinolones.
In our study, Enterobacter spp. was the least frequent of the ESKAPE members, and this finding may have affected the detection of significant differences between the two study periods analyzed, and probably underlies several differences which were borderline significant. While data for resistance levels of clinical isolates of Enterobacter spp. from Peru are rare, the low levels of carbapenem resistance found agree with the scarcity of carbapenamases detected in Enterobacter spp. isolates from Peru. Thus, a recent systematic review only recorded 7 carbapenemase-producing Enterobacter isolates reported from a total of 313 microorganisms in 2000 - 2019 [37]. Regarding other antimicrobial agents, the present data showed higher overall resistance levels than those found among 433 Enterobacter spp. analyzed in Latin America from 2013 - 2015 [38].
In addition to the long analysis of the evolution of antibiotic resistance levels of ESKAPE microorganisms in a Peruvian clinical setting, the present data are of special relevance within the context of the coronavirus disease 2019 (COVID-19) pandemic, in which the use of antibiotics and biocides has been extreme in both hospital and community settings, likely contributing to the emergence of XDR and pan-resistant clinical isolates in different areas [13,39]. Thus, these data could contribute to future analyses of the collateral impact of the COVID-19 pandemic on antibiotic resistance levels, providing retrospective pre-pandemic baseline data and trends in the evolution of drug resistance.
The lack of information about clinical outcomes as well as the uncertain role of CoNS as infective pathogens in a small fraction of patients are the main limitations of the present study [11,34]. It should be noted that in 2017 Enterobacter aerogenes was confirmed to be identical to Klebsiella mobilis, was thereby unified as a single species under the genus Klebsiella, and named Klebsiella aerogenes [40]. As databases record isolates pre-2017, it is likely that a number of K. aerogenes were categorized as Enterobacter spp.
In summary, the present study found high levels of antibiotic resistance among ESKAPE microorganisms. While the establishment of a successful XDR A. baumannii clone is suspected in the ICU, the present data provide two intriguing results which are a clear trend to a reduction in the isolation of ESKAPE microorganisms as well as a generalized decrease in the levels of antibiotic resistance. Further studies are needed to determine the evolution of resistance, which is especially important to evaluate the impact of the COVID-19 pandemic on the evolution of antibiotic resistance levels.
ACKNOWLEDGMENTS
We thank Donna Pringle for English editing.
Footnotes
Funding: JR was supported by Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (FONDECYT - Perú) within the “Proyecto de Mejoramiento y Ampliación de los Servicios del Sistema Nacional de Ciencia, Tecnología e Innovación Tecnológica" [contract: 08-2019-FONDECYT-BM-INC-INV"].
Conflicts of interest: No conflict of interest
- Conceptualization: WF-P, NL, MJP, JR.
- Data curation: WF-P, NL, RA, NR, ME.
- Formal analysis: WF-P, NL, JR.
- Investigation: WF-P, NL, RA, NR, ME.
- Visualization: MJP, JR.
- Writing - original draft: JR.
- Writing - review & editing: WF-P, NL, RA, ME, MJP, JR.
References
- 1.García C, Astocondor L, Rojo-Bezares B, Jacobs J, Sáenz Y. Molecular characterization of extended-spectrum β-lactamase-producer Klebsiella pneumoniae isolates causing neonatal sepsis in Peru. Am J Trop Med Hyg. 2016;94:285–288. doi: 10.4269/ajtmh.15-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy-Blitchtein S, Roca I, Plasencia-Rebata S, Vicente-Taboada W, Velásquez-Pomar J, Muñoz L, Moreno-Morales J, Pons MJ, Del Valle-Mendoza J, Vila J. Emergence and spread of carbapenem-resistant Acinetobacter baumannii international clones II and III in Lima, Peru. Emerg Microbes Infect. 2018;7:119. doi: 10.1038/s41426-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma N, Pons MJ, Gomes C, Mateu J, Riveros M, García W, Jacobs J, García C, Ochoa TJ, Ruiz J. Resistance to quinolones, cephalosporins and macrolides in Escherichia coli causing bacteraemia in Peruvian children. J Glob Antimicrob Resist. 2017;11:28–33. doi: 10.1016/j.jgar.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Pons MJ, Ruiz J. Current trends in epidemiology and antimicrobial resistance in intensive care units. J Emerg Crit Care Med. 2019;3:5. [Google Scholar]
- 5.Interagency Coordination Group on Antimicrobial Resistance (IACG) No time to wait: securing the future from drug-resistant infections. Report to the Secretary-General of the United Nations. April 2019. [Accessed 28 January 2021]. Available at: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1.
- 6.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control, European Medicines Agency (ECDC/EMEA) ECDC/EMEA Joint Technical Report. The bacterial challenge: time to react. Stockholm: ECDC; 2009. [Google Scholar]
- 8.World Bank Group. Drug-resistant infections - A threat to our economic future. Washington, DC: International Bank for Reconstruction and Development/The World Bank; 2017. [PubMed] [Google Scholar]
- 9.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 10.Marturano JE, Lowery TJ. ESKAPE pathogens in bloodstream infections are associated with higher cost and mortality but can be predicted using diagnoses upon admission. Open Forum Infect Dis. 2019;6:ofz503. doi: 10.1093/ofid/ofz503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control (ECDC) Healthcare-associated infections acquired in intensive care units. Annual epidemiological report for 2017. [Accessed 28 January 2021]. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-HAI.pdf.
- 12.Ecker L, Ruiz J, Vargas M, Del Valle LJ, Ochoa TJ. Prevalence of purchase of antibiotics without prescription and antibiotic recommendation practices for children under five years of age in private pharmacies in peri-urban areas of Lima, Peru. Rev Peru Med Exp Salud Publica. 2016;33:215–223. [PubMed] [Google Scholar]
- 13.Zavala-Flores E, Salcedo-Matienzo J. Pre-hospitalary medication in COVID-19 patients from a public hospital in Lima-Peru. Acta Méd Peru. 2020;37:393–395. [Google Scholar]
- 14.Horna G, Quezada K, Ramos S, Mosqueda N, Rubio M, Guerra H, Ruiz J. Specific type IV pili groups in clinical isolates of Pseudomonas aeruginosa . Int Microbiol. 2019;22:131–141. doi: 10.1007/s10123-018-00035-3. [DOI] [PubMed] [Google Scholar]
- 15.Ugarte Silva RG, Olivo López JM, Corso A, Pasteran F, Albornoz E, Sahuanay Blácido ZP. Resistencia a colistín mediado por el gen mcr-1 identificado en cepas de Escherichia coli y Klebsiella pneumoniae. Primeros reportes en el Perú. An Fac Med. 2018;79:213–217. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Document M100-S24. Wayne, PA: CLSI; 2014. [Google Scholar]
- 17.Nicolau DP, Quintana A, Bradley JMK, Wible M, Dowzicky MJ. A rationale for maintaining current tigecycline breakpoints as established by the USA Food and Drug Administration. Arch Clin Microbiol. 2015;6:7. [Google Scholar]
- 18.Dargère S, Cormier H, Verdon R. Contaminants in blood cultures: importance, implications, interpretation and prevention. Clin Microbiol Infect. 2018;24:964–969. doi: 10.1016/j.cmi.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Center for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 20.Pons MJ, de Toro M, Medina S, Sáenz Y, Ruiz J. Antimicrobianos, resistencia antibacteriana y salud sostenible. South Sustainability. 2020;1:e001 [Google Scholar]
- 21.Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019;26:taz036. doi: 10.1093/jtm/taz036. [DOI] [PubMed] [Google Scholar]
- 22.Ruppé E, Andremont A, Armand-Lefèvre L. Digestive tract colonization by multidrug-resistant Enterobacteriaceae in travellers: An update. Travel Med Infect Dis. 2018;21:28–35. doi: 10.1016/j.tmaid.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz J, Pons MJ. In an inhospitable ICU, not even antibiotic cycling or mixing are the solutions. Ann Infect. 2018;2:2. [Google Scholar]
- 24.Faron ML, Ledeboer NA, Buchan BW. Resistance Mechanisms, Epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J Clin Microbiol. 2016;54:2436–2447. doi: 10.1128/JCM.00211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71:801–810. doi: 10.1007/s00228-015-1865-4. [DOI] [PubMed] [Google Scholar]
- 26.Niu T, Xiao T, Guo L, Yu W, Chen Y, Zheng B, Huang C, Yu X, Xiao Y. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi: 10.2147/IDR.S169432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Chen X, Xu P, Zhu Y, Wang K, Xiang D, Wang F, Banh HL. Clinical experience with tigecycline in the treatment of hospital-acquired pneumonia caused by multidrug resistant Acinetobacter baumannii. BMC Pharmacol Toxicol. 2019;20:19. doi: 10.1186/s40360-019-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez CH, Brune A, Nastro M, Vay C, Famiglietti A. In vitro synergistic activity of the sulbactam/avibactam combination against extensively drug-resistant Acinetobacter baumannii . J Med Microbiol. 2020;69:928–931. doi: 10.1099/jmm.0.001211. [DOI] [PubMed] [Google Scholar]
- 29.Delgado-Valverde M, Conejo MDC, Serrano L, Fernández-Cuenca F, Pascual Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia . J Antimicrob Chemother. 2020;75:1840–1849. doi: 10.1093/jac/dkaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García C, Rijnders MI, Bruggeman C, Samalvides F, Stobberingh EE, Jacobs J. Antimicrobial resistance and molecular typing of Staphylococcus aureus bloodstream isolates from hospitals in Peru. J Infect. 2012;65:406–411. doi: 10.1016/j.jinf.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Schwalb A, Cachay R, de la Flor A, García C, Seas C. Adherence to standards of care and mortality in the management of Staphylococcus aureus bacteraemia in Peru: A prospective cohort study. Int J Infect Dis. 2020;96:601–606. doi: 10.1016/j.ijid.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Horna G, Velasquez J, Fernández N, Tamariz J, Ruiz J. Characterisation of the first KPC-2-producing Klebsiella pneumoniae ST340 from Peru. J Glob Antimicrob Resist. 2017;9:36–40. doi: 10.1016/j.jgar.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Sacsaquispe-Contreras R, Bailón-Calderón H. Identification of carbapenem-resistant genes in enterobacteria from peruvian hospitals, 2013-2017. Rev Peru Med Exp Salud Publica. 2018;35:259–264. doi: 10.17843/rpmesp.25Y.25v25i.3474. [DOI] [PubMed] [Google Scholar]
- 34.Quispe AM, Soza G, Ramos Chirinos M, Quiroz D, Pons MJ. Multidrug resistance bacteremia in neonates and its association with late-onset sepsis and Coagulase-negative Staphylococci. J Infect Dev Ctries. 2020;14:1256–1263. doi: 10.3855/jidc.12568. [DOI] [PubMed] [Google Scholar]
- 35.Resurrección-Delgado C, Montenegro-Idrogo JJ, Chiappe-Gonzalez A, Vargas-Gonzales R, Cucho-Espinoza C, Mamani-Condori DH, Huaroto-Valdivia LM. Klebsiella pneumoniae New Delhi metalo-lactamase in a Peruvian national hospital. Rev Peru Med Exp Salud Publica. 2017;34:261–267. doi: 10.17843/rpmesp.2017.342.2615. [DOI] [PubMed] [Google Scholar]
- 36.Li F, Chen D, Li L, Liang D, Wang F, Zhang B. Analysis of metallo-β-lactamases, oprD mutation, and multidrug resistance of β-lactam antibiotic-resistant strains of Pseudomonas aeruginosa Isolated from Southern China. Curr Microbiol. 2020;77:3264–3269. doi: 10.1007/s00284-020-02148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angles-Yanqui E, Huaringa-Marcelo J, Sacsaquispe-Contreras R. Panorama of carbapenemases in PeruUm panorama das carbapenemases presentes no Peru. Rev Panam Salud Publica. 2020;44:e61. doi: 10.26633/RPSP.2020.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. Resistance among Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013-2015. Braz J Infect Dis. 2017;21:343–348. doi: 10.1016/j.bjid.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz J. Enhanced antibiotic resistance as a collateral COVID-19 pandemic effect? J Hosp Infect. 2021;107:114–115. doi: 10.1016/j.jhin.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tindall BJ, Sutton G, Garrity GM. Enterobacter aerogenes Hormaeche and Edwards 1960 (Approved Lists 1980) and Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980) share the same nomenclatural type (ATCC 13048) on the Approved Lists and are homotypic synonyms, with consequences for the name Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980) Int J Syst Evol Microbiol. 2017;67:502–504. doi: 10.1099/ijsem.0.001572. [DOI] [PubMed] [Google Scholar]