Abstract
Background and Aim
Immune checkpoint inhibitors (ICIs) have been widely used in hepatocellular carcinoma (HCC), while only a subset of patients experience clinical benefit. We aimed to investigate the effects of viral etiology on response to ICIs in HCC and depict the tumor immune microenvironment (TIME) of virally infected and uninfected HCC.
Methods
A systematic search was conducted in PubMed, Web of Science, Embase, and the Cochrane central register of controlled trials up to August 2021. Clinical trials reporting the efficacy of ICIs in HCC were eligible. Baseline characteristics including first author, year of publication, National Clinical Trials (NCT) registry number, study region, sample sizes, interventions, line of treatment, and viral status were extracted. Meta-analysis was conducted to generate combined odds ratios (ORs) with 95% confidence intervals (CI) based on random or fixed effect model, depending on heterogeneity. Tumor immune microenvironment was depicted using ESTIMATE and CIBERSORT algorithm.
Results
Eight studies involving 1,520 patients were included. Combined data suggested that there was no significant difference of objective response rate (ORR) between virally infected HCC and non-viral HCC patients [OR = 1.03 (95% CI, 0.77–1.37; I2 = 30.9%, pH = 0.152)]. Similarly, difference was not observed on ORR between HBV-HCC and HCV-HCC patients [OR = 0.74 (95% CI, 0.52–1.06; I2 = 7.4%, pH = 0.374)]. The infiltration of immune cells in the tumor microenvironment did not differ by etiology except for M0 macrophages, M2 macrophages, regulatory T cells, naive B cells, follicular helper T cells, activated dendritic cells, activated mast cells, and plasma cells. Despite differences in infiltration observed in specific cell types, the immune score and stromal score were generally comparable among etiology groups.
Conclusion
Viral etiology may not be considered as the selection criteria for patients receiving ICIs in HCC, and viral status has little impact on TIME remodeling during HCC tumorigenesis.
Keywords: viral status, immune checkpoint inhibitor, tumor microenvironment, hepatocellular carcinoma, meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is ranked as the sixth most frequent malignancy, and the third most common cause of cancer-related mortality worldwide (1). Hepatic resection remains the major treatment option for early-stage HCC patients with well-preserved liver function. Sorafenib, a multityrosine kinase inhibitor (mTKI) that targets serine–threonine kinases Raf-1 and B-Raf, vascular endothelial growth factor receptors (VEGFRs), and other kinases including platelet-derived growth factor receptor β (PDGFR-β), has been considered the mainstay of treatment for advanced hepatocellular carcinoma (aHCC) in the past decade (2, 3). The treatment landscape of aHCC has changed dramatically over the past few years with the advent of immune checkpoint inhibitors (ICIs). Combination of atezolizumab with bevacizumab is now considered the standard of care for aHCC patients as the first-line treatment (3). Currently, the combination of different ICIs or ICI-based combination with mTKIs or antiangiogenic monoclonal antibodies are under exploration and may further revolutionize the first-line treatment scenario (4, 5). On the other hand, even though dual VEGF/PD-L1 blockade nearly doubled the objective response rate (ORR) of ICI monotherapy, still more than half of the patients did not respond (6). Therefore, it is of utmost importance to identify the subsets of HCC that are most likely to benefit from immunotherapies.
Approximately 13% of new cancer cases worldwide are associated with infections (7). It appears that viral-associated carcinoma has distinct biological and clinical features compared with viral-independent tumors. Previous studies revealed that human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC) presents a higher response rate and more lymphocyte infiltration than HPV-negative HNSCC (8–10). The etiology of HCC varies substantially by geographic region. Globally, approximately 54% cases of HCC are attributed to chronic hepatitis B virus (HBV) infection, especially in Asia and sub-Saharan Africa (11), while chronic hepatitis C virus (HCV) infection is likely the predominant cause of HCC in Italy and Japan (1). Hepatitis viral infection disrupts normal signaling pathways; leads to sustained hepatic inflammation, fibrosis, and aberrant hepatocyte regeneration; and exerts complex biological effects on the tumor microenvironment (TME) (12). It is still controversial whether there is a difference in clinical response rate for ICIs between HBV- and HCV-associated HCC. Some reported that responses occurred regardless of HCC etiology, while some others demonstrated that clinical activity was observed predominantly in uninfected or HCV-infected cohorts (13, 14).
The efficacy of immunotherapies is attuned by multiple immunosuppressive signals within the TME (15). Therefore, there exists intense interest in uncovering the underlying mechanisms leading to the immunosuppressive milieu. With the widespread use of high-throughput omics data, it is now possible to take an in-depth view of the global gene expression pattern and its involvement in HCC tumorigenesis and progression (16). Computational algorithms, including CIBERSORT and ESTIMATE, have also been developed for assessments on abundance of infiltrated immune cells based on gene expression profiles (14, 15). Altogether, these techniques provided reliable and economical methods to depict detailed tumor immune microenvironment (TIME) landscape.
HCC remains an extremely lethal disease worldwide, with poor prognosis and limited therapeutic options for patients in advanced stage (17). The mechanisms by which chronic hepatitis induces HCC may differ by etiology, and it remains unclear whether HCC etiology may be considered as selection criteria for immunotherapy. To our knowledge, this is the first study that conducted a comprehensive analysis on the etiology of HCC and the efficacy of ICIs. The current study first conducted a meta-analysis to investigate effects of viral etiology on response to ICIs in HCC. The TIME of virally infected and uninfected HCC was further depicted using bioinformatic methods.
Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines to design, analyze, and report our meta-analytic findings (18).
Data Source and Searching Strategy
Online databases including PubMed, Web of Science, Embase, and the Cochrane central register of controlled trials were systematically reviewed up to August 2020. Meeting abstracts from European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO), and American Association of Cancer Research (AACR) from 2015 onwards were also reviewed. Related studies were identified using the following terms, including hepatocellular carcinoma, immune checkpoint inhibitors, nivolumab, pembrolizumab, atezolizumab, avelumab, camrelizumab, SHR-1210, and durvalumab. Detailed searching strategy is presented in Supplementary Table S1 . Additional papers were identified by a manual search of the references from the eligible articles.
Criteria for Inclusion and Exclusion
The inclusion criteria were as follows: (i) clinical trials including anti-programmed cell death-1 (anti-PD-1)/programmed death-ligand 1 (PD-L1) monotherapy or in combination with other monoclonal antibodies (e.g., anti-CTLA-4 antibodies, anti-VEGFR antibodies) or TKIs; (ii) articles in English and present the data for any of the efficacy outcomes including ORR, complete response (CR), partial response (PR), stable disease (SD), progression of disease (PD), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS). We did not apply any restrictions on phase of study, line of treatment, treatment duration, or drug dosage. Studies were excluded if they were (i) studies on conditions other than HCC, (ii) studies including combination therapies other than above mentioned, (iii) sharing the same participants completely or partially, and (iv) preclinical studies or case reports.
Data Extraction and Quality Assessment
Included trials were reviewed in detail, and data extraction were independently performed by two investigators (ZD and LY). Disagreements were resolved by discussion and consensus with a third author (ZD). The following information including first author, year of publication, National Clinical Trials (NCT) registry number, study region, sample sizes, interventions, line of treatment, and viral status were extracted. Quality of eligible studies were assessed using a 20-criterion quality appraisal checklist reported previously (19). Criteria regarding study design, demographic characteristics, intervention, follow-up and outcomes, competing interest, and sources of financial support were incorporated in the checklist.
Bioinformatics Analysis
Gene expression data and corresponding clinicopathological information of 729 HCC samples (GSE9843, GSE78737, GSE107170, and GSE121248) were acquired from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/repository) and Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo) databases. Viral statuses in TCGA-LIHC cohort were extracted from the supplemental material from the TCGA-LIHC marker paper (20). The fragments per kilobase million (FPKM) values of TCGA-LIHC datasets were transformed into transcripts per kilobase million (TPM) as previous described, which was believed to be identical to those from microarrays (21). Single-sample gene-set enrichment analysis (ssGSEA) is a unique GSEA method used to calculate separate enrichment scores for each sample (22). The infiltration level of different immune cell types in HCC samples was quantified using ssGSEA based on 29 immune-associated gene sets reported previously (23). The immune score, stromal score, and ESTIMATE score of each single HCC sample were estimated using the ESTIMATE algorithm to validate the effect of ssGSEA immune clustering (24). CIBERSORT deconvolution algorithm was then utilized to precisely measure the fractions of 22 human immune cell subsets in HCC samples (25).
Statistical Analysis
The association between viral status and response to ICIs in HCC was assessed by odd ratios (ORs) with an estimate of 95% CIs. A synthesized OR >1 suggests a higher response rate to ICIs. Heterogeneity was measured by the Cochran Q statistic and the I2 statistic [100% (Q − df)/Q]. The p-value for heterogeneity was represented as pH. Random-effects model will be utilized once the I2 >50% and reach statistical significance. Otherwise, the fixed-effects model was used to generate the pooled meta-analysis. In addition, Labbe’ plot and Galbraith plot were generated to visually evaluate the heterogeneity among studies. The influence of a single study on the overall meta-analysis estimate was investigated by metainf Stata command. Publication bias was assessed by inspecting the symmetry of the funnel plot and tested with Egger’s test. All the statistical analysis conducted in the current meta-analysis were performed by STATA version 12.0 (Stata Corporation, College Station, TX).
Results
Study Selection
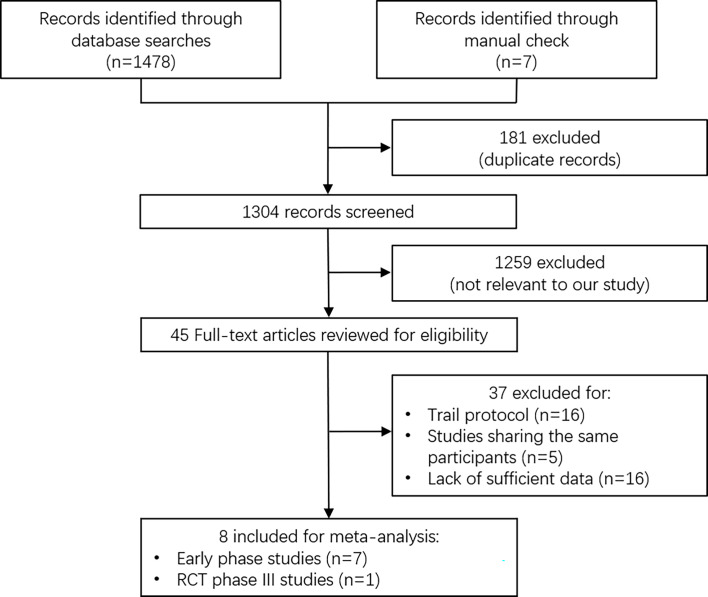
A total of 1,485 articles were retrieved based on the search terms given above, among which 181 duplicated records were removed. After initial screening, 1,259 articles not relevant to our study were removed. In total, 45 studies were eligible for a full-text assessment, of which 37 were removed, as they were trail protocol (n = 16), studies sharing the same participants (n = 5), or studies lack of sufficient data (n = 16). Finally, 8 studies with 1,520 patients were included for meta-analysis, among which 7 were early phase trails and 1 was phase III randomized controlled trial (RCT) ( Figure 1 ) (6, 13, 14, 16, 26–29). These included studies were trails on anti-PD-1/L1 monotherapy (nivolumab, pembrolizumab, and durvalumab), anti-PD-1/L1 combined with anti-CTLA-4 (tremelimumab and ipilimumab), and anti-PD-1 in combination with anti-VEGFR therapy (atezolizumab plus bevacizumab) Table 1 . In the study conducted by Kelly et al., patients were treated with different immunotherapy agents, and we considered each to be a single study arm and analyzed it accordingly (16).
Figure 1.
Flow diagram for study selection.
Table 1.
Basic characteristics of eligible studies.
| Study | Year | Region | No. of Pts | Study Registration No. | Intervention | Line of Treatment | Phase of Study | HCC Etiology | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HBV | HCV | Uninfected | ||||||||
| El-Khoueiry et al. (26) | 2017 | Global | 262 | NCT01658878 | Nivolumab | Second line | I/II | 66 | 60 | 136 |
| Kelley et al. (16) | 2021 | Global | 332 | NCT02519348 | Durvalumab plus Tremelimumab | Second line | I/II | 123 | 95 | 114 |
| Yau et al. (13) a | 2020 | Global | 148 | NCT01658878 | Nivolumab plus Ipilimumab | Second line | I/II | 75 | 33 | 33 |
| Feun et al. (14) | 2019 | USA | 29 | NCT02658019 | Pembrolizumab | Second line | II | 5 | 9 | 15 |
| Lee et al. (27) | 2020 | Global | 104 | NCT02715531 | Atezolizumab plus Bevacizumab | First line | Ib | 51 | 31 | 22 |
| Zhu et al. (28) | 2018 | Global | 104 | NCT02702414 | Pembrolizumab | Second line | II | 21 | 26 | 57 |
| Finn et al. (6) | 2020 | Global | 501 | NCT03434379 | Atezolizumab plus Bevacizumab | First line | III | 240 | 108 | 153 |
| Wainberg et al. (29) b | 2017 | Global | 40 | NCT01693562 | Duralumab | Second line | I/II | 9 | 8 | 21 |
Seven patients overall were reported as having both HBV and HCV infection.
One HBV-positive patient was non-response evaluable.
Viral Status Has no Impact on Response to Immunotherapy
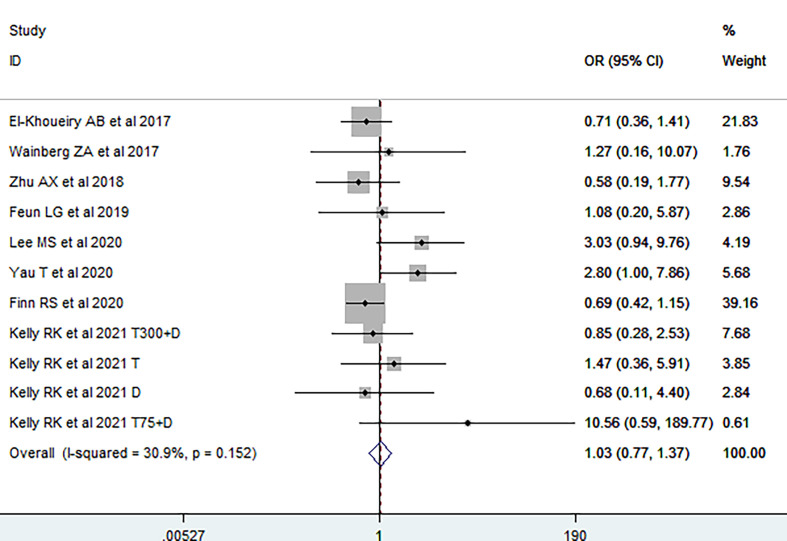
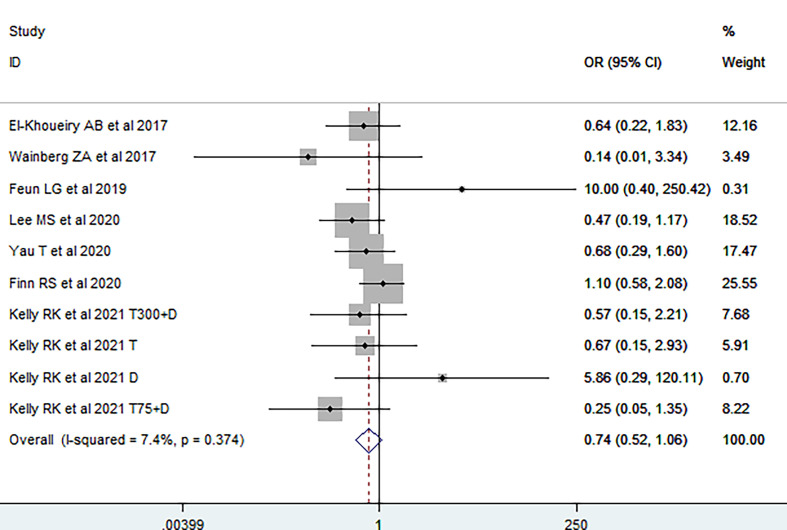
We first investigated the impact of viral etiology on the efficacy of immunotherapy; primary endpoint was ORR. It was demonstrated that no significant difference of ORR was observed between virally infected HCC and non-viral HCC patients [OR = 1.03 (95% CI, 0.77–1.37), Figure 2 ], without significant heterogeneity among studies (I2 = 30.9, pH = 0.152). The funnel plot, sensitivity analysis, LAbbe’ plot, and Galbraith plot were further conducted, and no possible heterogeneity was observed ( Supplementary Figures S1A–D ). Fixed-effects meta-analysis was subsequently utilized to estimate the difference of ORR between HBV-HCC and HCV-HCC. The overall OR was 0.74 (95% CI, 0.52–1.06; I2 = 7.4%, pH = 0.374, Figure 3 ), suggesting no difference on response rate between HBV-HCC and HCV-HCC patients. Although the funnel plot was visually asymmetrical, no publication bias was observed (Egger’s test, p = 0.988, Supplementary Figure S2A ). According to sensitivity analysis, LAbbe’ plot, and Galbraith plot, there was little heterogeneity among the studies ( Supplementary Figures S2B–D ).
Figure 2.
Pooled odds ratio of response rate between virally infected and uninfected HCC.
Figure 3.
Forest plot of response rate in virally infected HCC stratified by etiology.
Impact of Viral Status on HCC Microenvironment
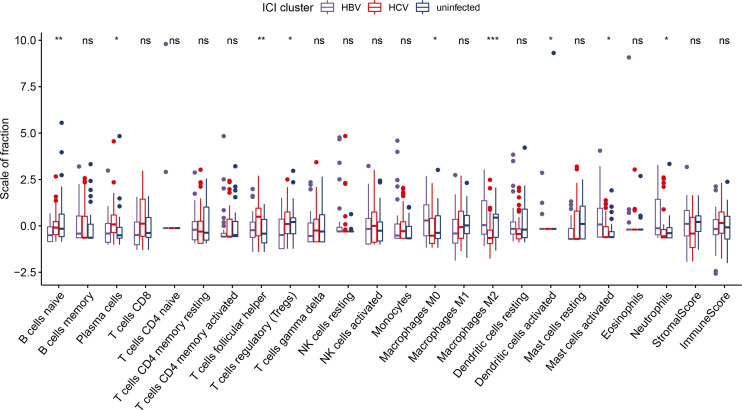
We analyzed the immune cell composition of the TME to further depict the global immune infiltration landscape in HCC with different etiology. Among the three subgroups, patients in the HBV-HCC group were characterized by a significantly higher percentage of M0 macrophages, activated dendritic cells, and activated mast cells. The HCV-HCC group was marked by higher percentage of naive B cells, plasma cells, follicular helper T cells, and regulatory T cells. The non-viral HCC group exhibited a significant increase in the infiltration of M2 macrophages ( Figure 4 ). The ESTIMATE algorithm was utilized to quantitatively evaluate the TIME in HCC subgroups. The stromal score stands for tumor matrix components. The higher the score, the more the matrix around the tumor, while the immune score is closely related to the degree of immune cell infiltration. Although differences in infiltration level were observed in specific cell types, the immune and stromal scores were generally comparable among etiology groups, suggesting that viral infection had minimal impact on tumor microenvironment remodeling during HCC tumorigenesis ( Figure 4 ).
Figure 4.
The fraction of tumor-infiltrating immune cells in HCC stratified by etiology. ns, not significant *p < 0.05; **p < 0.01; ***p < 0.001.
Publication Bias and Sensitivity Analysis
Egger’s s linear regression test was conducted to examine potential publication bias. No publication bias among included studies were observed ( Table 2 ). Besides, funnel plots were generated for visually assessing publication bias ( Supplementary Figures S1A and S2A ).
Table 2.
Egger’s publication bias test.
| Outcomes | No. of study | No. of patients | p for bias | 95% CI for bias |
|---|---|---|---|---|
| Virally infected HCC vs uninfected HCC | 8 | 1,296 | 0.080 | −0.204, 2.694 |
| HBV-HCC vs HCV-HCC | 7 | 771 | 0.988 | −1.739, 1.763 |
CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.
Discussion
ICIs have been used in a wide range of previously untreatable malignancies including viral-associated cancers such as HCC and HNSCC (6, 30). Interestingly, it is observed that some patients with specific viral etiology are more likely to benefit from immunotherapies. A previous study reported that the OS almost doubled in HPV-positive HNSCC compared with HPV-negative patients (31). This is partly because of the synthesis of E6 and E7 oncoproteins in HPV-positive patients that make tumor cells extremely detectable to the immune system (32). A recent pan-cancer analysis of over 10,000 samples from 23 cancer types revealed a significantly higher infiltration of B cells, CD4, and CD8 T cells in HPV-positive HNSCC than HPV-negative HNSCC (10). Besides, bioinformatic analysis of TCGA datasets indicated an increased cytolytic activity in EBV-positive stomach cancer and HPV-positive HNSCC, urothelial cancer, and cervical cancer (33). These observations suggest that proteins from oncogenic viruses may act as immunogenic neoantigens and is associated with an elevated immune response.
Unlike other human malignancies, the etiological factors of HCC are well established and vary substantially by geographical regions. HBV infection and aflatoxin exposure are likely the predominant causes in Asia and Africa (34, 35), while HCV, alcoholism, and non-alcoholic fatty liver disease (NAFLD) represent major risk factors in other areas of the world, and the latter has become an emerging risk factor for HCC over the past decade (1, 36). Currently, 3.5% of the global population is chronically infected with HBV, up to 40% of which will eventually develop HCC (34). On the other hand, it is estimated that at least 3.5 million people are currently infected with HCV in the United States, and 20% of liver cancer mortality worldwide is associated with HCV infection (37, 38). Chronic hepatitis, either caused by HBV, HCV, or non-alcoholic steatohepatitis (NASH), leads to an immunosuppressive intrahepatic microenvironment, which is marked by an increased expression of inhibitory receptors on the surface of cytotoxic T cells (39). Blockage of these inhibitory receptors and their legends may, on the one hand, reinvigorate tumor-specific T-cell immunity; on the other hand, restore antiviral intrahepatic T-cell responses.
The efficacy of systematic therapy might be affected by different underlying HCC etiologies, with diverse intrahepatic tumor microenvironments distinctly regulating HCC tumorigenesis and immune response. It is still presently controversial whether these etiological differences of oncogenic mechanisms result in difference in response to HCC immunotherapy (40). Different from other viral-associated cancers, responses to ICI were observed irrespective of etiology in HCC. Early phase trails demonstrated that responses to ICIs were generally consistent across HBV-HCC, HCV-HCC, and nonviral-HCC patients, even though the results were not powered for statistical comparisons due to small subgroups (14, 26–28). In line with a previous study with relatively smaller samples, we observed no significant difference in response between virally infected HCC and nonviral-HCC patients (41). Our further analysis suggested that response rates were similar in HBV- and HCV-infected patients. Distinct from other viral-associated malignancies, we observed no etiological difference in the efficacy of immunotherapy in HCC, which may partly result from the unique biological features of hepatitis virus. HBV and HCV are non-cytopathic compared with other tumor-associated viruses (42, 43). With the unique ability to integrate into the host cell genome, persistent viral replication leads to continuous necroinflammation, fibrosis, and aberrant hepatocyte regeneration. High levels of virus and viral antigens together with the naturally immune suppressive environment of the liver contribute to an immunosuppressive tumor microenvironment. We hypothesized that viral-associated HCC is driven by the necroinflammation and cirrhosis due to persistent viral replication, rather than virus-specific immune responses.
Recently, a study provided some new insights into HCC immunotherapy. In this study, based on results from preclinical tests, the authors indicated that tumor immune surveillance was impaired in NASH-induced HCC because CD8+ T cells helped to induce NASH-HCC, rather than invigorating or executing immune surveillance. The authors further conducted a meta-analysis that showed that NASH-HCC was more refractory to ICIs than viral-HCC (44). However, these findings were based on a retrospective study that included a small population of NASH-associated HCC and different lines of treatment. Therefore, additional studies are warranted to confirm the benefit of ICIs in virally infected HCC patients.
The TME is typically a complex and heterogeneous ecosystem with multiple interacting components. Effector cells including CD8 T cells, NK cells, and suppressive immune cells, such as Tregs, M2 macrophages, and myeloid-derived suppressor cells (MDSCs), are the major components of TME. Immune and immunosuppressive cells are abundant in HCC mellitus, balancing the cancer immunity and regulating immune response (45). Generally, macrophages consist of two polarization states, namely, M1 macrophages and M2 macrophages, which exhibit opposing roles in HCC tumorigenesis. M1 macrophages, also known as classically activated macrophages, exert antitumorigenic roles by releasing toxic intermediates including reactive oxygen species (ROS) and stimulating naive T cells to make a Th1/cytotoxic response (46). In contrast, M2 macrophages are polarized by Th2 cytokines and associated with cancer proliferation, angiogenesis, and ECM remodeling (25). Tregs cells are the subset of CD4+ T cells and characterized by the CD25 marker, which are known to exert immunosuppressive effects, and the presence of Tregs is often correlated with poor prognosis in HCC (47, 48). We found that more Tregs were infiltrated in HCV-HCC than in HBV-HCC, consistent with a previous study that demonstrated that Tregs were significantly higher in HCV related HCC, especially in the recurrence subset (49).
Characterizing the heterogeneous populations of tumor-infiltrating immune cells in TME may help to deepen our understandings of immune response in HCC tumorigenesis, thus boosting the development of effective immunotherapy (50). Computational algorithms including CIBERSORT and ESTIMATE have been widely utilized to accurately calculate the abundance of 22 immune cell types in TME using gene expression matrix (24, 51). It has previously been demonstrated that HCC etiology was not associated with the expression of genes within a Th1/IFN-γ–related immune signature, which may be predictive of immunotherapy response (41). Here, we demonstrated that specific immune cell types demonstrated different infiltration level, even though no significantly difference was observed in response to ICIs among HCC patients with difference etiology. The reason for this disparity is unclear, as TME remodeling during cancer progression is a complex process that involves a variety of sophisticated regulatory mechanisms, which may not only be determined by a specific cell type. On the other hand, the infiltration of immune cells in TME is calculated based on gene expression matrix using imputation algorithm, which may not fully reflect the real state of TME. TME is considered a complex milieu of intervention with multiple interacting components. The 22 immune cell phenotypes are only a tip of the iceberg, and large portions of immune cells that exert distinct functions are still unknown.
The landscape of novel therapeutic agents and combinations towards aHCC are quickly evolving in the past decade. Inspired by the landmark results of the IMbrave150 Phase III trial, immune-based combinations including durvalumab plus tremelimumab, cabozantinib plus atezolizumab, lenvatinib plus pembrolizumab, and nivolumab plus ipilimumab are under assessment, which may further modify the therapeutic scenario in patients with aHCC in the next 5 years (17, 52). The recent ORIENT-32 study also reported an unprecedented benefit for sintilimab plus bevacizumab biosimilar versus sorafenib monotherapy in the first-line setting for Chinese patients with aHCC (53). We suggest that further efforts should be oriented towards the identification of potential populations who may benefit from immunotherapy and thus help in guiding individualized treatment. In addition, the clinicopathological features of HCC varies among different patient populations. For instance, patients with HBV-associated HCC in China are characterized by younger age, poor ECOG performance status, and increased risk of distant metastasis compared with patients in western countries (53). Thus, additional studies using the real-world data combined with clinical data from different endemic areas are warranted to evaluate the efficacy and safety of ICI-based immunotherapies.
Several limitations should be acknowledged in the current study. First, the current meta-analysis only encompassed a total of eight studies. In fact, the number of studies reported response to ICIs stratified by etiology was relatively small, which may compromise the credibility of the results. Second, most studies included were early phase studies, and more RCTs were needed for higher level evidence. Third, only ORR was illustrated to define the response to ICI. Unfortunately, few studies included in our meta-analysis differentiate between viral and non-viral HCC when they reported OS or PFS; therefore, our ability to distill responses into viral etiology was limited. ORR is the only reliable endpoint to generate statistically powerful meta-data based on the available data. Forth, we failed to differentiate between alcoholic liver disease and NAFLD or NASH in non-virally infected subgroup due to limited data available. Last, the findings from bioinformatics analysis warrant further experimental validation.
Although previous study had investigated the ORR for PD-1/L1 inhibitors in virally infected and uninfected HCC, the number of articles included was relatively small (41). Besides, it has not been well clarified previously whether there is a difference in response to ICIs between HBV-HCC and HCV-HCC patients. The current study comprehensively investigated the etiology of HCC and the efficacy of ICIs and suggested that viral etiology may not be considered as the selection criteria for patients receiving ICIs. We further took an in-depth view of the immune infiltration pattern and suggested that HCC etiology did not have an apparent effect on TME remodeling, which provide new sight for HCC immunotherapy.
Author Contributions
ZND and TL conceived and designed the study. SY, YCY, and CY independently screened the full text of selected studies to confirm eligibility, assess quality, and extract data. ZND, YFY, LY, HL analyzed the data. ZND, ZRD, ZC, JH, and TL wrote, reviewed, and/or revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Taishan Scholars Program for Young Expert of Shandong Province (tsqn20161064), the National Natural Science Foundation of China (81874178 and 82073200), and funds for Independent Cultivation of Innovative Team from Universities in Jinan (Grant No. 2020GXRC023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.733530/full#supplementary-material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and Development of Sorafenib: A Multikinase Inhibitor for Treating Cancer. Nat Rev Drug Discov (2006) 5(10):835–44. doi: 10.1038/nrd2130 [DOI] [PubMed] [Google Scholar]
- 3. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol (2020) 38(36):4317–45. doi: 10.1200/JCO.20.02672 [DOI] [PubMed] [Google Scholar]
- 4. Rizzo A, Dadduzio V, Ricci AD, Massari F, Di Federico A, Gadaleta-Caldarola G, et al. Lenvatinib Plus Pembrolizumab: The Next Frontier for the Treatment of Hepatocellular Carcinoma? Expert Opin Investig Drugs (2021) 1–8. doi: 10.1080/13543784.2021.1948532 [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 7. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob Health (2020) 8(2):e180–90. doi: 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 8. Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol (2016) 17(7):956–65. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 10. Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, et al. Comprehensive Analyses of Tumor Immunity: Implications for Cancer Immunotherapy. Genome Biol (2016) 17(1):174. doi: 10.1186/s13059-016-1028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global Patterns of Hepatocellular Carcinoma Management From Diagnosis to Death: The BRIDGE Study. Liver Int (2015) 35(9):2155–66. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A, Zakaria MK, Fatima K, et al. Hepatitis B Virus, HBx Mutants and Their Role in Hepatocellular Carcinoma. World J Gastroenterol (2014) 20(30):10238–48. doi: 10.3748/wjg.v20.i30.10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 Study of Pembrolizumab and Circulating Biomarkers to Predict Anticancer Response in Advanced, Unresectable Hepatocellular Carcinoma. Cancer (2019) 125(20):3603–14. doi: 10.1002/cncr.32339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Liu R, Su X, Pan Y, Han X, Shao C, et al. Harnessing Tumor-Associated Macrophages as Aids for Cancer Immunotherapy. Mol Cancer (2019) 18(1):177. doi: 10.1186/s12943-019-1102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol (2021) 39(27):2991–3001. doi: 10.1200/JCO.20.03555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rizzo A, Ricci AD, Brandi G. Atezolizumab in Advanced Hepatocellular Carcinoma: Good Things Come to Those Who Wait. Immunotherapy (2021) 13(8):637–44. doi: 10.2217/imt-2021-0026 [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo B, Moga C, Harstall C, Schopflocher D. A Principal Component Analysis Is Conducted for a Case Series Quality Appraisal Checklist. J Clin Epidemiol (2016) 69:199–207.e2. doi: 10.1016/j.jclinepi.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 20. Cancer Genome Atlas Research Network . Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell (2017) 169(7):1327–41.e23. doi: 10.1016/j.cell.2017.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner GP, Kin K, Lynch VJ. Measurement of mRNA Abundance Using RNA-Seq Data: RPKM Measure Is Inconsistent Among Samples. Theory Biosci (2012) 131(4):281–5. doi: 10.1007/s12064-012-0162-3 [DOI] [PubMed] [Google Scholar]
- 22. Hänzelmann S, Castelo R, Guinney J. GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC Bioinf (2013) 14:7. doi: 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity (2013) 39(4):782–95. doi: 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 24. Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture From Expression Data. Nat Commun (2013) 4:2612. doi: 10.1038/ncomms3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tahmasebi Birgani M, Carloni V. Tumor Microenvironment, A Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci (2017) 18(2):405. doi: 10.3390/ijms18020405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in Patients With Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab With or Without Bevacizumab in Unresectable Hepatocellular Carcinoma (GO30140): An Open-Label, Multicentre, Phase 1b Study. Lancet Oncol (2020) 21(6):808–20. doi: 10.1016/S1470-2045(20)30156-X [DOI] [PubMed] [Google Scholar]
- 28. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 29. Wainberg ZA, Segal NH, Jaeger D, Lee K-H, Marshall J, Antonia SJ, et al. Safety and Clinical Activity of Durvalumab Monotherapy in Patients With Hepatocellular Carcinoma (HCC). J Clin Oncol (2017) 35(15_suppl):4071. doi: 10.1200/JCO.2017.35.15_suppl.4071 [DOI] [Google Scholar]
- 30. Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The Head and Neck Cancer Immune Landscape and Its Immunotherapeutic Implications. JCI Insight (2016) 1(17):e89829. doi: 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galvis MM, Borges GA, Oliveira TB, Toledo IP, Castilho RM, Guerra ENS, et al. Immunotherapy Improves Efficacy and Safety of Patients With HPV Positive and Negative Head and Neck Cancer: A Systematic Review and Meta-Analysis. Crit Rev Oncol Hematol (2020) 150:102966. doi: 10.1016/j.critrevonc.2020.102966 [DOI] [PubMed] [Google Scholar]
- 32. Welters MJP, Ma W, Santegoets S, Goedemans R, Ehsan I, Jordanova ES, et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin Cancer Res (2018) 24(3):634–47. doi: 10.1158/1078-0432.CCR-17-2140 [DOI] [PubMed] [Google Scholar]
- 33. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and Genetic Properties of Tumors Associated With Local Immune Cytolytic Activity. Cell (2015) 160(1-2):48–61. doi: 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis B Virus Infection. Nat Rev Dis Primers (2018) 4:18035. doi: 10.1038/nrdp.2018.35 [DOI] [PubMed] [Google Scholar]
- 35. Villanueva A. Hepatocellular Carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 36. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: Current Concepts and Future Challenges. Nat Rev Gastroenterol Hepatol (2019) 16(7):411–28. doi: 10.1038/s41575-019-0145-7 [DOI] [PubMed] [Google Scholar]
- 37. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a More Accurate Estimate of the Prevalence of Hepatitis C in the United States. Hepatology (2015) 62(5):1353–63. doi: 10.1002/hep.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob Health (2016) 4(9):e609–16. doi: 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- 39. Riley RS, June CH, Langer R, Mitchell MJ. Delivery Technologies for Cancer Immunotherapy. Nat Rev Drug Discovery (2019) 18(3):175–96. doi: 10.1038/s41573-018-0006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and Safety of Sorafenib in Patients With Advanced Hepatocellular Carcinoma: Subanalyses of a Phase III Trial. J Hepatol (2012) 57(4):821–9. doi: 10.1016/j.jhep.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 41. Ho WJ, Danilova L, Lim SJ, Verma R, Xavier S, Leatherman JM, et al. Viral Status, Immune Microenvironment and Immunological Response to Checkpoint Inhibitors in Hepatocellular Carcinoma. J Immunother Cancer (2020) 8(1):e000394. doi: 10.1136/jitc-2019-000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akbar SK, Horiike N, Onji M. Prognostic Importance of Antigen-Presenting Dendritic Cells During Vaccine Therapy in a Murine Hepatitis B Virus Carrier. Immunology (1999) 96(1):98–108. doi: 10.1046/j.1365-2567.1999.00668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The Prevalence of Hepatitis C Virus Infection in the United States, 1988 Through 1994. N Engl J Med (1999) 341(8):556–62. doi: 10.1056/NEJM199908193410802 [DOI] [PubMed] [Google Scholar]
- 44. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bian J, Lin J, Long J, Yang X, Yang X, Lu X, et al. T Lymphocytes in Hepatocellular Carcinoma Immune Microenvironment: Insights Into Human Immunology and Immunotherapy. Am J Cancer Res (2020) 10(12):4585–606. [PMC free article] [PubMed] [Google Scholar]
- 46. Pei Y, Yeo Y. Drug Delivery to Macrophages: Challenges and Opportunities. J Control Release (2016) 240:202–11. doi: 10.1016/j.jconrel.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 47. Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased Regulatory T Cells Correlate With CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology (2007) 132(7):2328–39. doi: 10.1053/j.gastro.2007.03.102 [DOI] [PubMed] [Google Scholar]
- 48. Peltanova B, Raudenska M, Masarik M. Effect of Tumor Microenvironment on Pathogenesis of the Head and Neck Squamous Cell Carcinoma: A Systematic Review. Mol Cancer (2019) 18(1):63. doi: 10.1186/s12943-019-0983-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoshizawa K, Abe H, Kubo Y, Kitahara T, Aizawa R, Matsuoka M, et al. Expansion of CD4(+)CD25(+)FoxP3(+) Regulatory T Cells in Hepatitis C Virus-Related Chronic Hepatitis, Cirrhosis and Hepatocellular Carcinoma. Hepatol Res (2010) 40(2):179–87. doi: 10.1111/j.1872-034X.2009.00587.x [DOI] [PubMed] [Google Scholar]
- 50. Roderburg C, Wree A, Demir M, Schmelzle M, Tacke F. The Role of the Innate Immune System in the Development and Treatment of Hepatocellular Carcinoma. Hepatic Oncol (2020) 7(1):Hep17. doi: 10.2217/hep-2019-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust Enumeration of Cell Subsets From Tissue Expression Profiles. Nat Methods (2015) 12(5):453–7. doi: 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kelley RK, WO J, Hazra S, Benzaghou F, Yau T, Cheng AL, et al. Cabozantinib in Combination With Atezolizumab Versus Sorafenib in Treatment-Naive Advanced Hepatocellular Carcinoma: COSMIC-312 Phase III Study Design. Future Oncol (2020) 16(21):1525–36. doi: 10.2217/fon-2020-0283 [DOI] [PubMed] [Google Scholar]
- 53. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab Plus a Bevacizumab Biosimilar (IBI305) Versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): A Randomised, Open-Label, Phase 2-3 Study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.