Abstract
Sudden cardiac arrest from anomalous coronary artery from the opposite sinus of Valsalva is rarely observed in children under 10 years of age. We report a 7-week-old infant with a brief resolved unexplained event from left anomalous aortic origin of a coronary artery who underwent unroofing and again developed syncope at 8 years of age. Ischemia was detected by stress echocardiography both times. (Level of Difficulty: Advanced.)
Key Words: anomalous coronary artery, congenital heart disease, dobutamine, exercise, stress echocardiography, sudden cardiac arrest
Abbreviations and Acronyms: AAOCA, anomalous aortic origin of a coronary artery; ALCA-R, anomalous left coronary artery originating from the right sinus of Valsalva; AoV, aortic valve; BRUE, brief resolved unexplained event; DSE, dobutamine stress echocardiography; ESE, exercise stress echocardiography; LV, left ventricle/ventricular; SCA, sudden cardiac arrest; WMA, wall motion abnormality
Central Illustration
History of Presentation
A 7-week-old male infant was admitted following a brief resolved unexplained event (BRUE) with limpness, cyanosis, and apnea with feeding. There was resolution of symptoms with minimal intervention by the mother. Past medical history and physical exam were unremarkable. Electrocardiography was normal.
Learning Objectives
-
•
To understand the risk of SCA in infants and children <10 years of age with ALCA-R.
-
•
To underscore importance of ischemia testing for infants and children with anatomic coronary lesions, especially those with long intramural or interarterial course.
-
•
To recognize pediatric stress echocardiography (dobutamine and exercise) as a potentially useful modality requiring no radiation exposure for diagnosing ischemia in children with AAOCA.
Differential Diagnosis
The differential diagnosis for a BRUE in an infant includes respiratory, cardiac, neurologic, infectious, or metabolic etiologies.
Investigations
Secondary to the presentation and lack of apparent etiology, an echocardiogram was performed, revealing an anomalous left coronary artery originating from the right sinus of Valsalva (ALCA-R) (Figure 1) in this child with normal left ventricular (LV) size and function, and no resting wall motion abnormalities (WMAs). To investigate the functional importance of ALCA-R, dobutamine stress echocardiography (DSE) was performed to assess for ischemia. ECG during DSE showed nonspecific ST-T-wave abnormalities. At low-dose dobutamine (2.5-20 μg/kg/min), LV function augmented well without WMAs. However, at higher dobutamine doses (30-40 μg/kg/min), the child developed global LV dilatation and new anteroseptal and posterior wall hypokinesis at a blood pressure of 119/58 mm Hg and heart rate of 185 beats/min. These findings are indicative of ischemia of the left coronary artery (LCA) territory (Videos 1A and 1B). There was complete reversal of WMAs 20 minutes into recovery. The biphasic effect, characterized by augmentation of LV function at low doses and deterioration of function at higher doses of dobutamine, was present, indicating ischemic but viable myocardium (1).
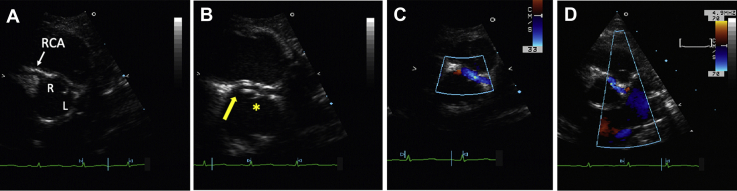
Figure 1.
Origin and Course of the Anomalous Artery
Two-dimensional and color Doppler echocardiogram showing (A, B) origin and (C, D) course of the left main coronary artery from the right sinus of Valsalva. The asterisk indicates the intercoronary commissure. The arrow indicates the ostium. L = left; R = right; RCA = right coronary artery.
Management
The patient underwent unroofing of the ALCA-R at 2 months of age. The ALCA-R ostium emanated from the right coronary sinus just lateral to the right coronary artery. The most proximal portion of the ALCA-R was intramural, and then exited at an acute angle from the right coronary sinus to run between the great arteries. The ALCA-R passed behind the commissure of the left-right sinuses of the aortic valve (AoV). Given that the child weighed only 3 kg, unroofing was performed to the level of but not past the intercoronary commissure to avoid destabilizing the AoV. The surgery was uncomplicated. Resting post-surgical echocardiography showed no WMAs.
The infant was discharged home with resolution of feeding issues and on beta-blockers.
The patient experienced normal growth without cardiac symptoms. No stress imaging was performed until 7 years of age, when routine treadmill exercise stress echocardiography (ESE) showed decreased LV wall augmentation without ischemia. Over the next year, his exercise capacity diminished at home. Repeat ESE at 8 years of age revealed new antero- and inferoseptal WMAs during exercise—findings almost identical to the abnormal WMAs prior to unroofing—and consistent with ischemia in the LCA territory (Videos 1C and 1D). Subsequent coronary computed tomography angiography demonstrated a slit-like orifice of the dominant ALCA-R, with a long 8-mm intramural course and narrowing of the interarterial portion of the vessel (Figure 2).
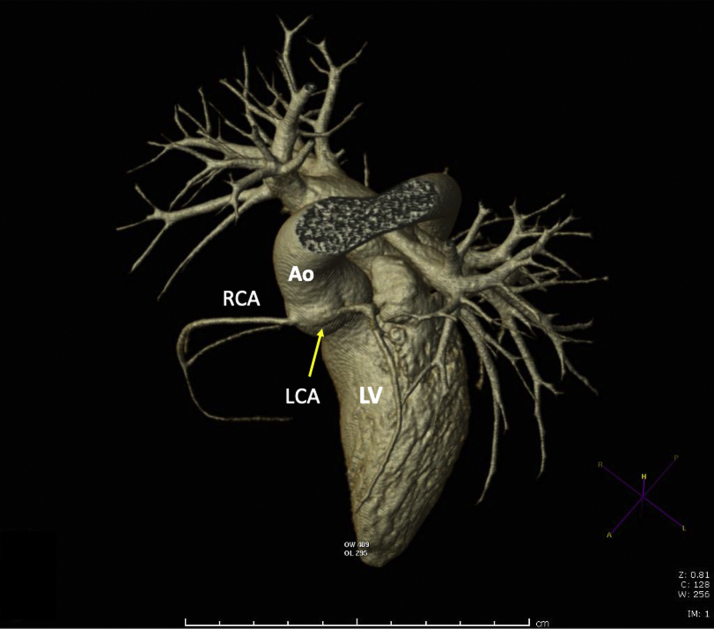
Figure 2.
Coronary Computed Tomography Angiogram of ALCA-R
Three-dimensional coronary computed tomography angiogram showing narrowing of the anomalous left coronary artery originating from the right sinus of Valsalva (ALCA-R) as it emerges from the aortic wall. The arrow indicates the LCA which has both an intramural and an interarterial course. Ao = aorta; LCA = left coronary artery; LV = left ventricle; RCA = right coronary artery.
While awaiting cardiac surgery as an outpatient, the child had a sudden syncopal episode following physical activity, becoming pale and cold without documented arrhythmia, and then recovered spontaneously. He was admitted for emergent cardiac surgery in which takedown of the left-right AoV commissure exposed the ALCA-R, coursing behind the commissure with a long intramural course before exiting the aortic wall. Button reimplantation of the coronary ostium in the left sinus of Valsalva was performed, followed by patch augmentation of the aortic wall and resuspension of the commissure. Surgery was again well tolerated. Pre-discharge transesophageal echocardiography and echocardiography showed laminar flow and no WMAs or aortic regurgitation.
Discussion
This unusual case documents a child with an ALCA-R who experienced recurrent sudden cardiac arrest (SCA), one during infancy, and again 8 years status post-coronary unroofing. Although anomalous aortic origin of a coronary artery (AAOCA) is the second leading cause of SCA in older children and young adults (2), the risk of SCA owing to AAOCA is exceedingly low in young children, with only 1 reported case of sudden cardiac death in a patient <10 years of age (3). Many SCAs associated with AAOCA are during or following intense physical exercise (4). Ischemia likely results from multiple etiologies, resulting in diminished coronary flow including decreased diastolic coronary fill time with increased heart rates during exercise; kinking or torsion of any interarterial segments; and acute angle of coronary takeoff and a resulting slit-like orifice compromising flow (5,6). Compression of the AAOCA by the dilation of the great arteries during exercise may also contribute to ischemia. Recently intramural segments of the coronary artery has been recognized as a significant cause of SCA in children (7). Our patient, who had both a long intramural segment in addition to interarterial compression as the coronary exited the aortic wall, may have suffered the cumulative effects of several causes of ischemia. Despite this, because ischemia can be intermittent, rarely does a case have both the anatomic and physiologic correlates of ischemia.
Transthoracic echocardiography with color-flow Doppler is the primary tool for screening for AAOCA (4). With the advent of coronary computed tomography angiography, excellent visualization of AAOCA can be obtained with limited radiation exposure. Here, we demonstrate that in children with AAOCA, stress echocardiography (DSE in infants and young children and ESE in older children) can be used for ischemia assessment. Exercise is the preferred and most physiologic stressor for stress imaging, as it most closely replicates how a child may develop ischemia.
To our knowledge, this is the youngest reported infant to have a documented SCA from AAOCA and to have undergone DSE to assess for ischemia. Prior to this, DSE has not been validated in children <1 year of age. DSE has been used in other pediatric cohorts (ie, cardiac transplants) to rule out ischemia (1). Here, DSE determined the physiological impact of AAOCA. In adults and older children, DSE is used to assess viability of myocardium. We observed the same pattern in this infant, in which the biphasic response pre-intervention was followed by resolution of BRUE symptoms post-unroofing. This case report demonstrates that pediatric stress echocardiography, both pharmacologic and exercise, which examines myocardial wall thickening with stress, can be an effective noninvasive diagnostic tool for identifying ischemia in children with AAOCA. Of note, electrocardiograms performed during DSE are not a sensitive means of identifying ischemia.
Following ALCA-R diagnosis, patients are typically referred for surgical treatment owing to high risk of SCD. Unroofing the intramural segment of the ALCA-R and reimplantation with ostial translocation are 2 commonly recommended procedures when the child is sufficiently large to undergo surgery (4). At experienced centers, these procedures pose a relatively low risk (4). Our case highlights that as the child grows, cardiovascular anatomy and spatial relationships between structures may evolve. Therefore, close follow-up and stress imaging even after successful unroofing should be performed in children to rule out ischemia with exertion.
Follow-Up
The patient was discharged without further cardiac symptoms, now 4 years after definitive therapy with LCA reimplantation.
Conclusions
ALCA-R can pose a risk of SCA in infants and children under 10 years of age. Pediatric stress echocardiography can help detect ischemia in infants and young children.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
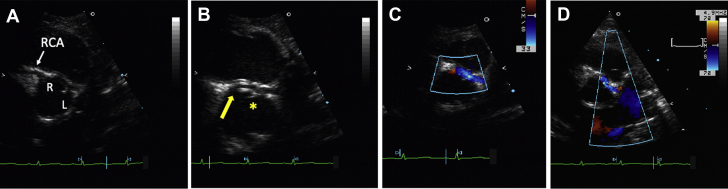
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
References
- 1.Pellikka P.A., Arruda-Olson A., Chaudhry F.A. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: From the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:1–41.e8. doi: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Molossi S., Martínez-Bravo L.E., Mery C.M. Anomalous aortic origin of a coronary artery. Methodist Debakey Cardiovasc J. 2019;15:111–121. doi: 10.14797/mdcj-15-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishnoi R.N., McMillan K.N., Thompson W.R. Unusual sudden cardiac death from an anomalous left coronary artery from the right sinus of Valsalva. Cardiol Young. 2014;24:732–734. doi: 10.1017/S1047951113001005. [DOI] [PubMed] [Google Scholar]
- 4.Brothers J.A., Frommelt M.A., Jaquiss R.D.B. Expert consensus guidelines: Anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153:1440–1457. doi: 10.1016/j.jtcvs.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 5.Khalighi K., Sharma M., Toor A. Anomalous left main coronary artery arising from the right sinus of Valsalva in a young man presenting with recurrent syncope and myocardial infarction. Case Rep Cardiol. 2018;2018:9805061. doi: 10.1155/2018/9805061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J.A., Cecchin F., Jones T.K., Portman M.A. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593–597. doi: 10.1016/s0735-1097(00)01136-0. [DOI] [PubMed] [Google Scholar]
- 7.Jegatheeswaran A., Devlin P.J., McCrindle B.W. Features associated with myocardial ischemia in anomalous aortic origin of a coronary artery: A Congenital Heart Surgeons’ Society study. J Thorac Cardiovasc Surg. 2019;158:822–834.e3. doi: 10.1016/j.jtcvs.2019.02.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.
(A, B) Dobutamine stress echocardiography at 7 weeks of age. Parasternal long-axis views. Global left ventricular dilation with new anteroseptal wall motion abnormalities (WMAs) in left coronary artery territory (B) on post–stress imaging as compared (A) with pre-stress. (C, D) Exercise stress echocardiography at 8 years of age. Four-chamber view. (D) Development of anteroseptal WMAs with exercise (C) from baseline. Arrows indicate WMA.