Abstract
Subacute thyroiditis (SAT) is a thyroid disease of viral or post-viral origin. Whether SAT represents a complication of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still unclear. Our aim was to systematically review the literature to 1) explore the size of the literature about SAT in COVID-19 and 2) evaluate the clinical characteristics of SAT. PubMed/MEDLINE, Embase, and Scopus were searched until April 20, 2021. Original papers, case reports, and case series reporting SAT in COVID-19 patients were included. Authors and their country, journal, year of publication, COVID-19 and SAT clinical presentation, thyroid function, therapy, and follow-up data were extracted. Nineteen papers (17 case reports and 2 case series) were included, describing 27 patients, 74.1% females, aged 18 to 69 years. COVID-19 was diagnosed by nasopharyngeal swab in 66.7% cases and required hospitalization in 11.1%. In 83.3% cases, SAT occurred after COVID-19. Neck pain was present in 92.6% cases and fever in 74.1%. Median TSH, fT3, and fT4 were 0.01 mU/l, 10.79 pmol/l, and 27.2 pmol/l, respectively. C-reactive-protein and erythrocyte sedimentation rate were elevated in 96% of cases. Typical ultrasonographic characteristics of SAT were observed in 83.3% of cases. Steroids were the most frequent SAT therapy. Complete remission of SAT was recorded in most cases. In conclusion, the size and quality of published data of SAT in COVID-19 patients are poor, with only case reports and case series being available. SAT clinical presentation in COVID-19 patients seems to be similar to what is generally expected.
Keywords: subacute thyroiditis (SAT), subacute thyroiditis de Quervain, SARS-CoV-2, COVID-19, thyroid
Introduction
Subacute thyroiditis (SAT) is a self-limited thyroid disease of viral or post-viral origin. SAT, also known as de Quervain thyroiditis, is typically characterized by a triphasic clinical course of thyrotoxicosis, hypothyroidism, and return to normal thyroid function. From a clinical point of view, SAT presents with neck pain typically with radiation to the ears and a wide spectrum of systemic symptoms, which include fever, asthenia, and malaise. In the initial phase, many patients also present clinical and/or biochemical manifestation of mild-moderate thyrotoxicosis, such as tremor and palpitations (1). Thyroid follicles are infiltrated, resulting in disrupted basement membrane and rupture of the follicles. This injury is thought to be the result of cytolytic T-cell recognition of viral and cell antigens (2).
While persistent hypothyroidism is a rare event, high circulating levels of inflammatory markers, such as C-reactive protein (CRP), and, more specifically, erythrocyte sedimentation rate (ESR) represent the most frequent biochemical finding at presentation (3, 4).
Several respiratory viruses, including coxsackievirus (5), mumps (6), Epstein–Barr virus (7), cytomegalovirus (8), and influenza virus (9), were reported to be associated with SAT development (10). However, owing to the fact that specific antiviral treatment is not required in most cases, diagnostic evaluations aimed at identifying the etiological viruses are not routinely performed in these patients. Thus, the viral or post-viral origin of SAT is supported by both direct and indirect evidences. Epidemiological data showed an overlap of seasonal outbreaks of infectious diseases and SAT outbreaks (6, 11). Indeed, high titers of virus-specific antibodies or positive virus swabs were found in patients harboring SAT and an association between presence of antibodies to specific viruses and SAT was observed (5, 6, 12). On the other hand, virus culture from thyroid tissue as well as viral RNA identification from thyroid cytological samples yielded conflicting results (13–15). At present, it is unclear whether follicle damage in SAT is caused by direct viral infection of the gland or by the host’s immunological response to the viral infection.
With the beginning of the coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a thyroid impact was considered due to the potential of SARS-CoV-2 to cause multiorgan effects. Among the thyroid SARS-CoV-2 complications, SAT was early reported by some case report articles (16), and the question whether SAT might be an underestimated SARS-CoV-2 manifestation was raised (17). Subsequently, several original articles, editorials, and reviews were published on the thyroid sequelae experienced by patients with COVID-19 (18–22). More recently, a systematic review found that COVID-19 patients can develop thyroid dysfunction, frequently non-thyroidal illness syndrome, when hospitalized in an intensive care unit. Furthermore, several data supported the notion that having a thyroid disease would not increase the risk for SARS-CoV-2 infection, and thyroid patients do not need a COVID-19-adapted follow-up (23). According to the available data summarized in review articles, whether SAT can represent a COVID-19 complication remains an open question. Specifically, the prevalence of COVID-19-related SAT and the similarity of its clinical presentation to usual SAT remain unknown.
Therefore, the present study was conceived to summarize the published data about the association between COVID-19 and SAT. The literature was systematically reviewed to retrieve the largest number of original papers, case reports, and case series articles reporting SAT in patients diagnosed with SARS-CoV-2. The aims of the present study were to 1) explore the size and quality of the literature about SAT in COVID-19 and 2) evaluate the clinical characteristics of SAT in these patients.
Materials and Methods
Review Conduction
The systematic review was conducted according to the PRISMA statement (24), and the checklist is reported as supplemental file ( Supplemental File 1 ).
Search Strategy
A comprehensive computer literature search of the PubMed/MEDLINE, Embase, and Scopus databases was conducted to find published articles on the topic of our review. The search algorithm was created based on combinations of specific terms: (“De Quervain thyroiditis” OR “subacute thyroiditis”) AND (“SARS-CoV-2” OR COVID OR COVID-19 OR coronavirus). A beginning date limit was not used, and the search was updated until April 20, 2021, without language restrictions. To identify additional studies and expand our search, the references of the retrieved articles were also screened.
The authors declare that the study selection was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Study Selection
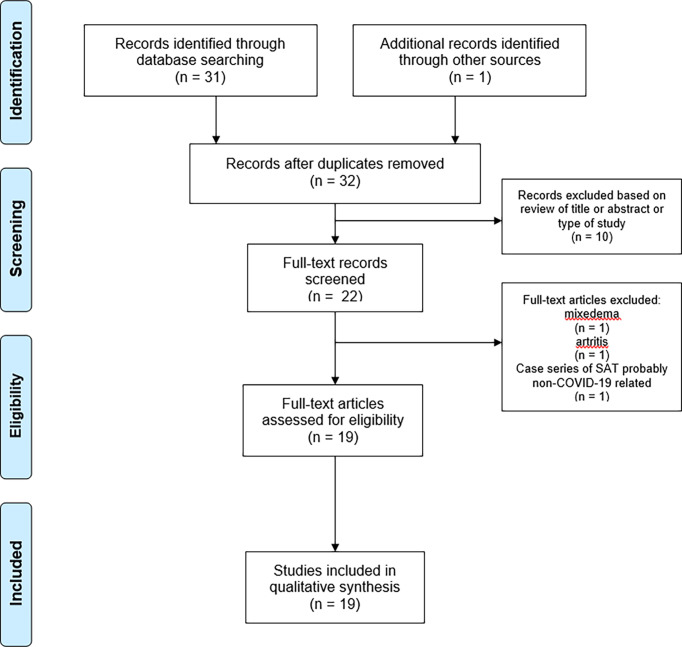
Studies or subsets of studies that report data on the detection of SAT in patients with previous or concurrent occurrence of COVID-19 were eligible for inclusion. The main exclusion criteria were a) articles not within the field of interest of this review; b) review articles, editorials, or comments; c) articles that did not provide clear study characteristics or reports that had overlapping patient data; and d) cases in which the SAT diagnosis was not clearly rendered. Two authors (LC, LS) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and abovementioned exclusion criteria. Then, the same two researchers independently reviewed the full text of the articles to determine their final inclusion. Disagreements were solved in a final mutual meeting involving also other two authors (PT, MR). Figure 1 illustrates the research strategy and flow of articles.
Figure 1.
Search strategy and flow of articles.
Data Extraction
For each included study, information was extracted concerning reference data (authors, journal, year of publication, and country of origin). Number of patients evaluated, clinical data regarding COVID-19 presentation and SAT presentation, biochemical evaluation of thyroid function parameters, therapy for subacute thyroiditis, and long-term follow-up data were also extracted.
Each case report and case series were carefully evaluated to verify that no patient was included in more than one study.
Results
Retrieved Articles
Using the above search strategy, 32 records were initially found. Among these, 14 were excluded because they did not fit with the study aim while the remaining 18 (16, 17, 25–41) were included in the systematic review. Another paper (40), not included in the initial pool of retrieved records, was added to the systematic review because it was known by the authors. Finally, 19 articles, consisting of 17 case reports and 2 case series (with four and six SAT cases), were included. Remarkably, no original articles including large sample size were found. Figure 1 illustrates the flow of articles.
General Features of Included Articles
The 19 articles included in the systematic review included a total number of 27 SAT in patients diagnosed with COVID-19. The 19 studies were published from May 21, 2020 (16), to April 14, 2021 (41), by authors from 10 countries: seven cases from Italy, seven from Iran, four from the United States of America, two from Spain, two form India, one from Mexico, one from the Philippines, one from Singapore, one from Turkey, and one from the United Kingdom. The main demographic and clinical characteristics (regarding COVID-19 and SAT presentation) of each included patient are summarized in Table 1 .
Table 1.
Description of the anagraphic characteristics and clinical presentation of COVID-19 and SAT of the 27 included patients.
| Year | N. | Reference | Country | Age | Gender | Preexisting Thyroid Disease | COVID-19 Diagnosis | Clinical presentation of COVID-19 | Onset after COVID-19 (days) | General | Neck pain | Fever | Palpitations | Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 1 | (32) | Spain | 46 | F | NO | Positive IgG | Asymptomatic | YES | YES | YES | NO | Prednisone (40 mg/day as the starting dose, gradually tapered) | |
| 2020 | 2 | (25) | Turkey | 41 | F | NO | RT-PCR | Asymptomatic | 0 | NO | YES | YES | NO | Prednisolone 16 mg daily |
| 2020 | 3 | (16) | Italy | 18 | F | NO | RT-PCR | Mild upper respiratory symptoms (rhinorrhea and cough) | 19 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2020 | 4 | (17) | Italy | 38 | F | NO | RT-PCR | Mild upper respiratory symptoms | 16 | YES | YES | YES | YES c | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2020 | 5 | (17) | Italy | 29 | F | NO | Positive IgG | Rhinorrhea | 30 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered), propranolol 40 mg/day |
| 2020 | 6 | (17) | Italy | 29 | F | NO | RT-PCR | Fever, cough, rhinorrhea, anosmia | 36 | YES | YES | NO | YES | Ibuprofen 600 mg/day |
| 2020 | 7 | (17) | Italy | 46 | F | NO | RT-PR | Fever, cough, rhinorrhea, anosmia, asthenia | 20 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2020 | 8 | (28) | Mexico | 37 | F | NO | RT-PCR | Odynophagia and anosmia | 30 | YES | YES | NO | NO | No treatment (still thyrotoxic after 1 month) |
| 2020 | 9 | (39) | India | 58 | M | NO | RT-PCR | Fever | 0 | NO | YES | YES | YES | Prednisolone (30 mg/day as the starting dose, gradually tapered), propranolol 40 mg/day |
| 2021 | 10 | (29) | USA | 37 | M | NO | RT-PCR | Productive cough, fever, chills, dyspnea | 30 | YES | YES | NO | YES | Aspirin, propranolol |
| 2021 | 11 | (37) | Iran | 33 | M | NO | RT-PCR | Interstitial pneumonia | 8 | YES | YES | YES | YES | Dexamethasone 4 mg every 8 h for 5 days, then oral prednisone 25 mg daily with tapering |
| 2021 | 12 | (34) | UK | 57 | F | NO | Likely diagnosis a | Mild upper respiratory symptoms, anosmia | 60 | YES | YES | NO | YES | Ibuprofen 200 mg three times per day and paracetamol 1 g three times per day |
| 2020 | 13 | (38) | Italy | 69 | F | NO | RT-PCR | Interstitial pneumonia | 5 | YES | NO b | NO | YES | First methimazole, then shifted to methylprednisolone 40 mg/die for 3 days, then prednisone 25 mg/die progressively tapered |
| 2021 | 14 | (36) | USA | 41 | F | NO | RT-PCR | Fever, cough, and coryza | 14 | YES | YES | YES | YES | Ibuprofen 600 mg every 6 h and prednisone 40 mg daily. |
| 2021 | 15 | (41) | USA | 67 | M | NO | RT-PCR | Heart failure and interstitial pneumonia | 0 | YES | NO | YES | YES | Methimazole for 1 month, after no recovery shifted to prednisone |
| 2020 | 16 | (27) | Singapore | 34 | M | NO | RT-PCR | Fever, mild upper respiratory symptoms, anosmia | 3 | NO | YES | NO | YES | Prednisolone (20 mg/day as starting dose, gradually tapered) |
| 2020 | 17 | (33) | USA | 29 | F | NO | RT-PCR | Mild upper respiratory symptoms | 49 | YES | YES | YES | YES | Prednisone (40 mg/day as the starting dose, gradually tapered), atenolol 50 mg/die |
| 2020 | 18 | (30) | Spain | 28 | F | NO | RT-PCR | Diarrhea, abdominal pain | 14 | YES | YES | YES | YES | Aspirin 500 mg and propranolol 40 mg every 6 hours |
| 2020 | 19 | (26) | Italy | 43 | F | NO | RT-PCR | Fever, mild upper respiratory symptoms | 45 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2021 | 20 | (40) | India | 29 | F | NO | RT-PCR | Fever, cough, and other flu-like symptoms | 45 | NO | YES | YES | YES | Indomethacin 25 mg and propanol 40 mg thrice daily |
| 2020 | 21 | (31) | Philippines | 47 | F | NO | RT-PCR | Lobar pneumonia | -47 | YES | YES | NO | NO | Mefenamic acid, later shifted to celecoxib |
| 2021 | 22 | (35) | Iran | 37 | F | NO | Positive IgG | Myalgia for a few days | 30 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2021 | 23 | (35) | Iran | 35 | M | NO | Positive IgG | Asymptomatic | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) | |
| 2021 | 24 | (35) | Iran | 41 | F | NO | Positive IgG | Low-grade fever and mild myalgia for few days | 30 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2021 | 25 | (35) | Iran | 52 | M | NO | Positive IgG | Low-grade fever, dry cough, and mild myalgia for few days | 30 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
| 2021 | 26 | (35) | Iran | 34 | F | NO | Positive IgG | Asymptomatic | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) | |
| 2021 | 27 | (35) | Iran | 26 | F | NO | Positive IgG | Self-limited dry cough for 1 week | 30 | YES | YES | YES | YES | Prednisone (25 mg/day as the starting dose, gradually tapered) |
RT-PCR, real-time polymerase chain reaction; COVID-19, coronavirus disease 2019; SAT, subacute thyroiditis.
aNo direct demonstration of SARS-CoV-2.
bWhile on morphine for back surgery.
cAtrial fibrillation.
Demographic Features of Patients
Twenty (74.1%) patients were females and seven (25.9%) males. Patients’ age at SAT occurrence ranged from 18 to 69 years (median 37.5, IQR 33–46). No patient was previously diagnosed with thyroid disease.
COVID-19 Diagnosis and Presentation
COVID-19 diagnosis was rendered through real-time polymerase chain reaction (RT-PCR) on nasopharyngeal swab in 18 cases (66.7%), and by positivity of specific IgG in eight cases (29.6%). In one case, COVID-19 was suspected based on the presence of its typical symptoms (anosmia, dysgeusia) with no direct evidence of SARS-CoV-2 infection. COVID-19 was asymptomatic in 3 cases (11.1%), with mild upper respiratory symptoms in 21 cases (77.7%), and with pneumonia requiring hospitalization in 3 cases (11.1%). SARS-CoV-2-related peculiar symptoms (i.e., anosmia or dysgeusia) were reported in 5 (18.5%) out of 27 cases.
Time Interval Between SAT Occurrence and COVID-19 Diagnosis
The timing of SAT diagnosis with respect to COVID-19 was described in 24 cases:
In 20 (83.3%) cases, SAT occurred after COVID-19 onset, after a median of 30 (IQR 16–32) days. In three (12.5%) cases, SAT and COVID-19 were synchronous. In one (4.2%) patient (N # 21), the onset of SAT preceded by 47 days the molecular confirmation of COVID-19. This latter case should be briefly overviewed. At SAT diagnosis, the patient did not have fever or respiratory symptoms but a right lower lobe pneumonia was found on a chest radiography. Unfortunately, the reverse transcription-polymerase chain reaction for SARS-CoV-2 using nasopharyngeal and oropharyngeal swabs was performed only after several weeks after the onset of SAT symptoms and found positive. Although the possibility that SARS-CoV-2 infection was already present at the onset of SAT cannot be ascertained, the authors suggested that SAT might occur also in patients with confirmed COVID-19 without respiratory manifestations.
SAT Clinical Presentation
Neck pain was present in 25 (92.6%) cases; notably, one of the two patients without neck pain was taking opioid drugs since a recent surgical intervention (N. #13). General symptomatology (i.e., asthenia and malaise) was described in 23 (85.2%) patients. Fever was recorded in 20 (74.1%) patients. Palpitations were experienced by 22 (81.5%) patients, and one presented a new-onset episode of atrial fibrillation.
Thyroid Laboratory Tests and Inflammation Markers
Serum assessment was quite heterogeneous among the 19 studies. Available data are summarized in Table 2 .
Table 2.
Biochemical evaluation of thyroid function parameters, thyroglobulin, thyroid autoantibodies, and inflammatory markers in the 27 included patients.
| Year | N. | Ref | TSH | TSH ref range | FT3 | FT3 ref range | FT4 | FT4 ref range | Tg | TgAb | TPOAb | TRAb | CRP/ESR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 1 | (32) | 0.11 | 0.5–4.78 mIU/ml | N/A | N/A | 2.18 | 0.89–1.76 ng/dl | N/A | N/A | Slightly positive | N/A | High |
| 2020 | 2 | (25) | <0.008 | N/A | 7.7 | 3.1–6.8 pmol/l | 25.7 | 12–21 pmol/l | N/A | 0 | 0 | 0 | High |
| 2020 | 3 | (16) | <0.04 | 0.5–4.1 mIU/l | 8.7 | 4.6–8.4 pmol/l | 27.2 | 11–23 nmol/l | 5.6 | 120 UI/ml (positive) | 0 | 0 | High |
| 2020 | 4 | (17) | 0.1 | 0.4–4.5 mIU/ml | 8.0 | 2.3 to 4.2 pmol/l | 29.3 | 6–16 pmol/l | 75.3 | 0 | 0 | 0 | High |
| 2020 | 5 | (17) | < 0.01 | 0.4–4.5 mIU/ml | 8.9 | 2.3 to 4.2 pmol/l | 31.8 | 6–16 pmol/l | 80 | 38 (positive) | 0 | 0 | High |
| 2020 | 6 | (17) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 2020 | 7 | (17) | <0.01 | 0.4–4.5 mIU/ml | 6.9 | 2.3 to 4.2 pmol/l | 27.8 | 6–16 pmol/l | N/A | N/A | N/A | 0 | Normal |
| 2020 | 8 | (28) | <0.001 | N/A | 211a | 80–150 ng/dla | 1.6 | 0.7–1.48 ng/dl | N/A | 0 | 0 | N/A | High |
| 2020 | 9 | (39) | <0.005 | 0.27–4.2 mIU/l | 2.88a | 0.80–2.0 ng/mla | 20.11b | 5.10–14.1 μg/dlb | N/A | N/A | N/A | N/A | High |
| 2021 | 10 | (29) | 0.01 | 0.4–4.5 μIU/ml | 202a | 80–150 ng/dla | 2.3 | 0.6–1.3 ng/dl | N/A | N/A | 0 | 0 | High |
| 2021 | 11 | (37) | <0.001 | N/A | 236 a | 75–195 ng/dl a | 23.1b | 4–11 μg/dlb | N/A | N/A | 0 | 0 | High |
| 2021 | 12 | (34) | 0.1 | 0.27–4.2 mU/l | N/A | N/A | 21.2 | 12–22 pmol/l | N/A | 6.61 U/ml (positive) | 71.8 UI/ml (positive) | 0 | High |
| 2020 | 13 | (38) | 0.08 | 0.27–4.2 mU/l | 5.5 | 2–4.4 pg/ml | 24.6 | 0.3–17 pg/ml | 187 | N/A | N/A | N/A | N/A |
| 2021 | 14 | (36) | <0.008 | 0.7–4.20 mIU/l | 3.39a | 1.232–3.08 nmol/la | 60.63 | 11.61–23.22 pmol/l | N/A | N/A | 96.71 (positive) | 0 | High |
| 2021 | 15 | (41) | 0.029 | 0.27–4.2 μIU/ml | 1.2a | 0.80–2.0 ng/mla | 2.1 | 0.8–1.7 ng/dl | N/A | 0 | 0 | 0 | High |
| 2020 | 16 | (27) | <0.01 | 0.65–3.70 mU/l | 13.4 | 3.2–5.3 pmol/l | 41.8 | 8.8–14.4 pmol/l | N/A | N/A | 0 | 0 | High |
| 2020 | 17 | (33) | 0.01 | mU/l | 374a | 80–150 ng/la | 4.4 | 0.6–1.3 ng/l | N/A | N/A | 0 | 0 | High |
| 2020 | 18 | (30) | <0.001 | 0.38–5.33 mU/l | N/A | N/A | 37.5 | 7.0–16.0 pmol/l | N/A | 0 | 0 | 0 | High |
| 2020 | 19 | (26) | 0.006 | 0.27–4.2 mU/l | 7.03 | 1.71–3.71 pg/ml | 2.69 | 0.7–1.48 ng/dl | 188 | 0 | 0 | 0 | High |
| 2021 | 20 | (40) | 0.007 | N/A | 5.05a | ng/mla | 7.77 | ng/dl | N/A | 0 | 0 | 0 | High |
| 2020 | 21 | (31) | 0.05 | 0.47–4.68 μIU/ml | 1.4a | 0.97–1.69 ng/ml a | 1.68 | 0.78–2.19 pg/ml | N/A | 0 | 0 | 0 | High |
| 2021 | 22 | (35) | <0.01 | 0.4–4.0 mU/l | 25.4 | 3.1–6.8 pmol/l | 2.3 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
| 2021 | 23 | (35) | 0.12 | 0.4–4.0 mU/l | 19.3 | 3.1–6.8 pmol/l | 24.7 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
| 2021 | 24 | (35) | <0.01 | 0.4–4.0 mU/l | 23.7 | 3.1–6.8 pmol/l | 21.9 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
| 2021 | 25 | (35) | 0.17 | 0.4–4.0 mU/l | 21.6 | 3.1–6.8 pmol/l | 26.7 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
| 2021 | 26 | (35) | 0.23 | 0.4–4.0 mU/l | 18.1 | 3.1–6.8 pmol/l | 18.4 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
| 2021 | 27 | (35) | 0.07 | 0.4–4.0 mU/l | 18.9 | 3.1–6.8 pmol/l | 19.5 | 12–21 pmol/l | N/A | N/A | N/A | N/A | High |
N/A, not available; TgAb, anti-thyroglobulin antibodies; TPOAb, anti-thyroperoxydase antibodies; TRAb, anti-TSH-receptor antibodies; CRP/ESR, C-reactive protein/erythrocyte sedimentation rate.
aTotal T3.
bTotal T4.
Thyroid function tests were performed in all patients but one and showed overt thyrotoxicosis in all cases. Particularly, median TSH (available in 26 out of 27 cases) was 0.01 mU/l (IQR 0.008–0.07), median free-T4 (23 cases) was 27.2 pmol/l (IQR 22.4-31.1) with a median increase of 1.27 times (IQR 1.17–1.83) the upper limit of the reference range, and median-free T3 (15 cases) was 10.79 pmol/l (IQR 8.5–19.1) with a median increase of 2.11 times (IQR 1.67–2.80) of the upper limit of the reference range. Total T4 was measured in three (11.1%) patients ranging from 13.5 to 23.1 μg/dl. Total T3 was measured in eight (29.6%) patients with a median value of 2.15 ng/ml (IQR 1.865–2.49).
Thyroid autoantibodies were measured in 18 (66.6%) of cases. Indeed, anti-thyroglobulin antibodies (TgAb) and anti-thyroperoxydase antibodies (TPOAb) can be detected in some non-autoimmune thyroid diseases, such as SAT (42). Although some studies reported the de novo appearance of TgAb in up to 25%–50% of SAT patients, and, to a lesser extent, TPOAb, this positivity is usually transient and most patients do not develop autoimmune sequelae (43). Moreover, anti TSH-receptor antibody (TRAb) testing is performed in some patients in the thyrotoxic phase of SAT to exclude the presence of Graves’ disease.
Positive TgAb tests were found in 3 out of 11 patients in whom they were measured. Positive TPOAb tests were found in 3 out of 16 patients. TRAb measurement was performed in 15 patients with negative results in all cases. Thyroglobulin was measured in five (18.5%) patients and was always elevated.
SAT-specific inflammation markers, such as CRP and ESR, were performed in 25 patients and were high in 24 (96%).
Thyroid-Specific Imaging
The results of thyroid-specific imaging performed for the included patients are summarized in Table 3 . Thyroid ultrasound was performed in 24 cases (88.9%). The typical ultrasonographic characteristics of SAT (patchy hypoechogenic areas with reduced vascularization) were observed in 20 cases, while in the remaining cases non-specific patterns were described. Thyroid 99mTc-pertechnetate scintigraphy was performed in nine (33.3%) patients, with the uptake being either reduced (four cases) or absent (five cases).
Table 3.
Results of thyroid-specific imaging in the 27 included patients.
| Year | N. | Ref | Ultrasound | US typical for SAT | Scintigraphy |
|---|---|---|---|---|---|
| 2020 | 1 | (32) | Enlarged thyroid with heterogeneous echotexture | NO | Reduced uptake |
| 2020 | 2 | (25) | Increased vascularity, heterogeneous parenchyma | NO | N/A |
| 2020 | 3 | (16) | Multiple, diffuse hypoechoic areas | YES | N/A |
| 2020 | 4 | (17) | Enlarged thyroid gland with multiple hypoechoic areas and absent vascularization at color Doppler | YES | N/A |
| 2020 | 5 | (17) | Increased thyroid volume with bilateral diffuse hypoechoic areas and absent vascularization at color Doppler ultrasonography | YES | No uptake |
| 2020 | 6 | (17) | Increased thyroid volume (25 ml) with bilateral diffuse hypoechoic areas | YES | N/A |
| 2020 | 7 | (17) | Increased thyroid volume (18 ml) with bilateral diffuse hypoechoic areas and absent to mild vascularization at color Doppler ultrasonography | YES | N/A |
| 2020 | 8 | (28) | N/A | No uptake | |
| 2020 | 9 | (39) | Diffuse bilateral enlargement of thyroid with hypoechogenicity and increased vascularity on color Doppler | YES | Reduced uptake |
| 2021 | 10 | (29) | Diffusely heterogeneous echotexture | NO | N/A |
| 2021 | 11 | (37) | Bilateral ill-defined hypoechoic areas | YES | N/A |
| 2021 | 12 | (34) | Patchy areas of variably-reduced parenchymal echogenicity bilaterally | YES | Reduced uptake |
| 2020 | 13 | (38) | Enlarged hypoechoic thyroid, decreased vascularity | YES | No uptake |
| 2021 | 14 | (36) | Heterogeneous thyroid gland with bilateral patchy ill-defined hypoechoic areas | YES | N/A |
| 2021 | 15 | (41) | Mildly enlarged thyroid gland with no increased vascularity and 5-mm bilateral cysts | NO | N/A |
| 2020 | 16 | (27) | Enlarged thyroid gland with heterogeneous echotexture; hypoechoic areas with ill-defined margins corresponding to the hard regions palpable. Reduced blood flow in both lobes | YES | N/A |
| 2020 | 17 | (33) | N/A | N/A | |
| 2020 | 18 | (30) | N/A | No uptake | |
| 2020 | 19 | (26) | Diffusely enlarged and hypoechogenic thyroid gland. | YES | Reduced uptake |
| 2021 | 20 | (40) | Enlarged heterogeneously hypoechoic left lobe of thyroid and isthmus with normal vascularity, and bulky right lobe of thyroid with few ill-defined hypoechoic areas which suggestive of thyroiditis. | YES | No uptake |
| 2020 | 21 | (31) | Slightly enlarged right thyroid lobe, with ill-defined hypoechogenicity and normal vascularity in both lobes | YES | N/A |
| 2021 | 22 | (35) | Hypoechoic areas | YES | N/A |
| 2021 | 23 | (35) | Hypoechoic areas | YES | N/A |
| 2021 | 24 | (35) | Hypoechoic areas | YES | N/A |
| 2021 | 25 | (35) | Hypoechoic areas | YES | N/A |
| 2021 | 26 | (35) | Hypoechoic areas | YES | N/A |
| 2021 | 27 | (35) | Hypoechoic areas | YES | N/A |
N/A, not available; US, ultrasound.
SAT Treatment
Steroidal therapy was the most frequently administered therapy for SAT: the most used agent was prednisone (in 13 cases), but less frequently dexamethasone (in one case), prednisolone (in two cases), and methylprednisolone (in one case) were used. The median of prednisone equivalents administered per day was 25 mg (IQR 25–35). Seven patients received non-steroidal anti-inflammatory agents (including aspirin, indomethacin, and mefenamic acid). These therapies were often paired with beta-blockers (most frequently propranolol). Two patients with thyrotoxicosis were initially treated with anti-thyroid drugs and subsequently switched to steroid therapy.
SAT Outcome
A complete resolution of symptoms and thyrotoxicosis was recorded in most cases, with the exception of four patients (14.8%) in whom an evolution toward subclinical hypothyroidism was witnessed and another one who developed overt hypothyroidism.
Risk of Bias
Data extracted from the articles were almost complete in all the above outcomes except that regarding the laboratory tests. In fact, unfortunately, the antibody profile was reported in less than 50% of cases with a significant risk of bias due to missing results. On the contrary, the risk of bias was negligible in all the other outcomes.
Summary of Findings
The synthesis of results was made according to PRISMA statement (24). As reported in Table 4 , based on the data found in the literature, the certainty of evidence was moderate in all outcomes except that of laboratory tests, which was lower.
Table 4.
Summary of findings about COVID-19-associated SAT.
| Outcome | Participants (n) | Certainty of the evidence | Comments |
|---|---|---|---|
| Gender | 27 | Moderate | SAT more often recurs in women |
| Presentation of COVID-19 | 27 | Moderate | In patients with SAT COVID-19 usually presents with mild upper respiratory symptoms |
| Time interval between SAT and COVID-19 | 24 | Moderate | SAT typically manifests 3–60 days after COVID-19 |
| SAT clinical presentation | 27 | Moderate | COVID-19-associated SAT usually manifests as classical SAT (i.e., neck pain, asthenia and malaise, palpitations, fever) |
| Thyroid laboratory tests and inflammation markers | Variable | Low to moderate | Overt thyrotoxicosis is present in all cases of SAT; CRP and ESR, and thyroglobulin are typically elevated |
| Thyroid US | 24 | Moderate | The ultrasonographic characteristics of classical SAT (patchy hypoechogenic areas with reduced vascularization) are observed in COVID-19-associated SAT |
| SAT treatment | 27 | Moderate | Steroidal therapy was the most frequently administered therapy |
| SAT outcome | 27 | Moderate | A complete resolution of symptoms and euthyroidism is reached in most cases |
SAT, subacute thyroiditis; US, ultrasound; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Discussion
SAT is generally secondary to upper respiratory tract infections by several viruses (3, 10); thus, SAT might represent a potential complication of SARS-CoV-2 infection. The fact that SARS-CoV-2 recognizes angiotensin-converting enzyme 2 (ACE-2) as its cellular entry receptor (44) and the recent demonstration of ACE-2 expression in follicular thyroid cells would further support this possibility (45–47).
Here we conceived a systematic review to achieve more solid evidence about the relationship between SAT and COVID-19. Particularly, we aimed to evaluate the size and quality of the published literature on this topic, and the clinical characteristics of SAT in this setting of patients.
Regarding the first objective, we found 19 studies reporting a total of 27 patients with SAT. Surprisingly, we did not find original articles with large sample size although the first case report was published 1 year ago (16). The 17 case reports (16, 25–34, 36–41) and the 2 case series (17, 35) described carefully the history of patients and their SAT. In this context, it should be highlighted that two recent systematic reviews on a similar topic included 21 (48) and 17 (49) SAT cases, respectively. Our systematic review, conducted 3 months later and conceived with two specific aims, found 27 cases. The fact that the number of reported SAT cases in literature did not exponentially increase in a time frame during which COVID-19 cases significantly increased worldwide indirectly confirms that SAT is a rare complication of COVID-19. Furthermore, because of the lack of large-sample studies, both size and quality of the literature about SAT in COVID-19 patients have to be considered poor. From this point of view, taking up the pertinent question raised by Brancatella et al. (17) of whether SAT is an underestimated manifestation of SARS-CoV-2, the issue remains unsolved. A most reasonable answer would be that SAT represents, at best, a rare complication of COVID-19.
Considering the second aim of the present systematic review, SAT occurred generally after COVID-19. Its clinical presentation appears to be similar to “classic” forms of SAT, encompassing neck pain, asthenia/malaise, fever, and palpitations. Under a biochemical point of view, the reported patients always presented with thyrotoxicosis, usually with elevated serum inflammation markers. When available, the ultrasonographic/scintigraphic features of these patients were generally typical of virus-related thyroiditis. In addition, most patients were treated with steroids with complete resolution of symptoms. It should be noted that the median initial dose of prednisone employed in the reported patients (25 mg/day) is lower than the one recommended by the most recent guidelines (40 mg daily) (50). In general, patients with post-COVID-19 SAT presented a moderate-mild form of the disease, in terms of both clinical and biochemical presentation, with no peculiar clinical features.
The COVID-19 presentation and severity in the 27 patients with SAT deserve to be discussed. First, some specific and peculiar symptoms of COVID-19, such as anosmia and/or dysgeusia, were reported in a minority of SAT patients (19%). It is worth underlining that the above symptoms received great informative emphasis, making it unlikely that they were left unrecognized. Second, most patients experienced a pauci- or asymptomatic COVID-19 disease, with only three patients requiring hospitalization, and that their median age was 37 years, much younger than the typical COVID-19 hospitalized patients. Moreover, the female-to-male ratio was high, this being the opposite of what happens in a case series of hospitalized COVID-19 patients (51). In this context, it should be highlighted that in a recent study by Trimboli et al. (52) aimed at searching for an association between SAT and SARS-CoV-2 in COVID-19-specific symptoms and contact tracing data, it was found that among the 10 included SAT patients, none had positive SARS-CoV-2 diagnostic tests, and only one case had a contact with people who were diagnosed with SARS-CoV-2.
To date, several large series studies as well as several reviews have evaluated the thyroid impact in COVID-19 hospitalized/discharged patients (18–22). Surprisingly, even if these large case series (18–20, 23) were specifically aimed at describing the thyroidal repercussions of COVID-19, no occurrence of SAT was reported, allowing the following speculation. It could be that, among the hospitalized COVID-19 patients, the typical symptoms of SAT were misdiagnosed in severe COVID-19 disease or masked by the routine use of high-dose corticosteroids currently employed in critical COVID-19 patients (53). This hypothesis would hold particularly true only for the so-called “second wave” of the pandemic, since corticosteroids were contraindicated by most national guidelines in the early phase of the pandemic (54). It could be thus hypothesized that SAT would be a typical complication of less severe forms of COVID-19 occurring in community-dwelling, young female subjects that are less frequently included in clinical studies, while hospitalized patients would more often experience non-thyroidal illness syndrome due to the hyperinflammatory state typical of severe COVID-19 (55–57). In this context, a systematic review by Ruggeri et al. (58) provided a comprehensive review of all COVID-19-related inflammatory disorders. This paper highlighted, from a slightly different point of view, that thyroid dysfunction is frequently observed in COVID-19 patients, regardless of the underlying thyroid disease, and physicians should be aware of its possible occurrence. The fact that no extreme increases in the number of SAT cases occurred in the last months, even in areas greatly affected by COVID-19 (59), could be due to the fact that social distancing measures and mask use reduced the diffusion of other SAT-causative viruses, similarly to what happened with the H1N1 virus (60).
The here reviewed evidence has several limitations: even if the clues in favor of a SARS-CoV-2-induced SAT exist, the size of published cases is poor, with only case reports and case series being available. Also, our review process was greatly limited by the nature of the available literature and by the lack of uniformity in part of the data, mainly the laboratory tests performed.
These results can give evidence-based information to help clinicians who encounter cases of COVID-19-related SAT in everyday practice. In particular, available literature suggests that these forms of SAT are rather mild and do not require any specific treatment when compared with “classic” SAT forms. Nevertheless, this complication does not appear to be particularly frequent in COVID-19 patients, especially in those who require hospitalization. More data regarding larger series of patients with a more uniform evaluation of thyroid function parameters will be required to draw more firm conclusions on the real incidence of SAT in COVID-19 patients. Moreover, long-term follow-up data will indicate if patients who experience COVID-19-related SAT are at risk for long-term thyroid sequelae.
In conclusion, the size of published data of SAT in COVID-19 patients is poor and the SAT clinical presentation in COVID-19 patients appears overall similar from that generally expected. According to these evidence-based data, SAT cannot be considered as a direct or frequent complication of SARS-CoV-2. However, since the rapid worldwide diffusion of SARS-CoV-2 and its variants, the present findings might change in the next future.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
PT, LCr, and MR designed and conceptualized the study, analyzed the data, and drafted the manuscript for intellectual content; all the co-authors interpreted the data and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.707726/full#supplementary-material
Abbreviations
COVID-19, Coronavirus Disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAT, subacute thyroiditis; T3, triiodothyronine; T4, thyroxine; TgAb, anti-thyroglobulin antibodies; TPOAb, anti-thyroperoxidase antibodies; TSH, thyroid-stimulating hormone.
References
- 1. Stasiak M, Lewiński A. New Aspects in the Pathogenesis and Management of Subacute Thyroiditis. Rev Endocr Metab Disord (2021) 5:1–13. doi: 10.1007/s11154-021-09648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kojima M, Nakamura S, Oyama T, Sugihara S, Sakata N, Masawa N. Cellular Composition of Subacute Thyroiditis. An Immunohistochemical Study of Six Cases. Pathol Res Pract (2002) 198(12):833–7. doi: 10.1078/0344-0338-00344 [DOI] [PubMed] [Google Scholar]
- 3. Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical Characteristics of 852 Patients With Subacute Thyroiditis Before Treatment. Intern Med (2008) 47(8):725–9. doi: 10.2169/internalmedicine.47.0740 [DOI] [PubMed] [Google Scholar]
- 4. Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute Thyroiditis: Clinical Characteristics and Treatment Outcome in Fifty-Six Consecutive Patients Diagnosed Between 1999 and 2005. J Endocrinol Invest (2007) 30(8):631–5. doi: 10.1007/BF03347442 [DOI] [PubMed] [Google Scholar]
- 5. Volpé R, Row VV, Ezrin C. Circulating Viral and Thyroid Antibodies in Subacute Thyroiditis. J Clin Endocrinol Metab (1967) 27(9):1275–84. doi: 10.1210/jcem-27-9-1275 [DOI] [PubMed] [Google Scholar]
- 6. Eylan E, Zmucky R, Sheba C. Mumps Virus and Subacute Thyroiditis; Evidence of a Causal Association. Lancet (1957) 272(6978):1062–3. doi: 10.1016/S0140-6736(57)91438-1 [DOI] [PubMed] [Google Scholar]
- 7. Volta C, Carano N, Street ME, Bernasconi S. Atypical Subacute Thyroiditis Caused by Epstein-Barr Virus Infection in a Three-Year-Old Girl. Thyroid (2005) 15(10):1189–91. doi: 10.1089/thy.2005.15.1189 [DOI] [PubMed] [Google Scholar]
- 8. Al Maawali A, Al Yaarubi S, Al Futaisi A. An Infant With Cytomegalovirus-Induced Subacute Thyroiditis. J Pediatr Endocrinol Metab (2008) 21(2):191–3. doi: 10.1515/JPEM.2008.21.2.191 [DOI] [PubMed] [Google Scholar]
- 9. Dimos G, Pappas G, Akritidis N. Subacute Thyroiditis in the Course of Novel H1N1 Influenza Infection. Endocrine (2010) 37(3):440–1. doi: 10.1007/s12020-010-9327-3 [DOI] [PubMed] [Google Scholar]
- 10. Desailloud R, Hober D. Viruses and Thyroiditis: An Update. Virol J (2009) 6:5. doi: 10.1186/1743-422X-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volpé R. Thyroiditis: Current Views of Pathogenesis. Med Clin North Am (1975) 59(5):1163–75. doi: 10.1016/S0025-7125(16)31965-4 [DOI] [PubMed] [Google Scholar]
- 12. Parmar RC, Bavdekar SB, Sahu DR, Warke S, Kamat JR. Thyroiditis as a Presenting Feature of Mumps. Pediatr Infect Dis J (2001) 20(6):637–8. doi: 10.1097/00006454-200106000-00023 [DOI] [PubMed] [Google Scholar]
- 13. Mori K, Yoshida K, Funato T, Ishii T, Nomura T, Fukuzawa H, et al. Failure in Detection of Epstein-Barr Virus and Cytomegalovirus in Specimen Obtained by Fine Needle Aspiration Biopsy of Thyroid in Patients With Subacute Thyroiditis. Tohoku J Exp Med (1998) 186(1):13–7. doi: 10.1620/tjem.186.13 [DOI] [PubMed] [Google Scholar]
- 14. Debons-Guillemin MC, Valla J, Gazeau J, Wybier-Franqui J, Giron ML, Toubert ME, et al. No Evidence of Spumaretrovirus Infection Markers in 19 Cases of De Quervain's Thyroiditis. AIDS Res Hum Retroviruses (1992) 8(9):1547. doi: 10.1089/aid.1992.8.1547 [DOI] [PubMed] [Google Scholar]
- 15. Luotola K, Hyöty H, Salmi J, Miettinen A, Helin H, Pasternack A. Evaluation of Infectious Etiology in Subacute Thyroiditis–Lack of Association With Coxsackievirus Infection. APMIS (1998) 106(4):500–4. doi: 10.1111/j.1699-0463.1998.tb01378.x [DOI] [PubMed] [Google Scholar]
- 16. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute Thyroiditis After Sars-COV-2 Infection. J Clin Endocrinol Metab (2020) 105(7):2367–70. doi: 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brancatella A, Ricci D, Cappellani D, Viola N, Sgrò D, Santini F, et al. Is Subacute Thyroiditis an Underestimated Manifestation of SARS-CoV-2 Infection? Insights From a Case Series. J Clin Endocrinol Metab (2020) 105(10):e3742–6. doi: 10.1210/clinem/dgaa537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status and Outcome in 191 Patients With COVID-19. J Clin Endocrinol Metab (2020) 106(2):e926–35. doi: 10.1210/clinem/dgaa813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M, et al. Thyroid Function Before, During and After COVID-19. J Clin Endocrinol Metab (2020) 106(2):03–e811. doi: 10.1210/clinem/dgaa830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen M, Zhou W, Xu W. Thyroid Function Analysis in 50 Patients With COVID-19: A Retrospective Study. Thyroid (2020) 31(1):8–11. doi: 10.1089/thy.2020.0363 [DOI] [PubMed] [Google Scholar]
- 21. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in Patients With COVID-19: The THYRCOV Study. Eur J Endocrinol (2020) 183(4):381–7. doi: 10.1530/EJE-20-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Croce L, Gangemi D, Ancona G, Liboà F, Bendotti G, Minelli L, et al. The Cytokine Storm and Thyroid Hormone Changes in COVID-19. J Endocrinol Invest (2021) 44(5):891–904. doi: 10.1007/s40618-021-01506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trimboli P, Camponovo C, Scappaticcio L, Bellastella G, Piccardo A, Rotondi M. Thyroid Sequelae of COVID-19: A Systematic Review of Reviews. Rev Endocr Metab Disord (2021) 22(2):485–91. doi: 10.1007/s11154-021-09653-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asfuroglu Kalkan E, Ates I. A Case of Subacute Thyroiditis Associated With Covid-19 Infection. J Endocrinol Invest (2020) 43(8):1173–4. doi: 10.1007/s40618-020-01316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute Thyroiditis in a Patient Infected With SARS-COV-2: An Endocrine Complication Linked to the COVID-19 Pandemic. Hormones (Athens) (2020) 20:219–21. doi: 10.1007/s42000-020-00230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute Thyroiditis Associated With COVID-19. BMJ Case Rep (2020) 13(8):e237336. doi: 10.1136/bcr-2020-237336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute Thyroiditis Associated With COVID-19. Case Rep Endocrinol (2020) 2020:8891539. doi: 10.1155/2020/8891539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute Thyroiditis in the Setting of Coronavirus Disease 2019. Am J Med Sci (2021) 361(3):400–2. doi: 10.1016/j.amjms.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruano R, Zorzano-Martinez M, Campos A, Rius F, Hernández M. Subacute Thyroiditis Might be a Complication Triggered by SARS-CoV-2. Endocrinol Diabetes Nutr (2020). doi: 10.1016/j.endinu.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. San Juan MDJ, Florencio MQV, Joven MH. Subacute Thyroiditis In a Patient With Coronavirus Disease 2019. AACE Clin Case Rep (2020) 6(6):e361–e4. doi: 10.4158/ACCR-2020-0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Álvarez Martín MC, Del Peso Gilsanz C, Hernández López A. Subacute De Quervain Thyroiditis After SARS-CoV-2 Infection. Endocrinol Diabetes Nutr (2020) S2530–0164(20):30244–5. doi: 10.1016/j.endinu.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehmood MA, Bapna M, Arshad M. A Case of Post-COVID-19 Subacute Thyroiditis. Cureus (2020) 12(12):e12301. doi: 10.7759/cureus.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dworakowska D, Morley S, Mulholland N, Grossman AB. Covid-19 Related Thyroiditis: A Novel Disease Entity? Clin Endocrinol (Oxf) (2021) 95(3):369–77. doi: 10.1111/cen.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sohrabpour S, Heidari F, Karimi E, Ansari R, Tajdini A. Subacute Thyroiditis in COVID-19 Patients. Eur Thyroid J (2021) 9(6):321–3. doi: 10.1159/000511707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khatri A, Charlap E, Kim A. Subacute Thyroiditis From COVID-19 Infection: A Case Report and Review of Literature. Eur Thyroid J (2021) 9(6):324–8. doi: 10.1159/000511872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davoodi L, Oladi Z, Jafarpour H, Zakariaei Z, Soleymani E, Razavi A. A 33-Year-Old Man With COVID-19 Presented With Subacute Thyroiditis: A Rare Case Report and Literature Review. New Microbes New Infect (2021) 41:100871. doi: 10.1016/j.nmni.2021.100871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: A Potential Trigger for Subacute Thyroiditis? Insights From a Case Report. J Endocrinol Invest (2020) 43(8):1171–2. doi: 10.1007/s40618-020-01312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chakraborty U, Ghosh S, Chandra A, Ray AK. Subacute Thyroiditis as a Presenting Manifestation of COVID-19: A Report of an Exceedingly Rare Clinical Entity. BMJ Case Rep (2020) 13(12):e239953. doi: 10.1136/bcr-2020-239953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Safwan M, Vijayan KN, Najeeb, TG J. Post-Coronavirus Disease 2019 De Quervain’s Thyroiditis – A Case Report and Literature Review. innovare Journals Med Sci (2021) 9(2):4–6. doi: 10.22159/ijms.2021.v9i2.40956 [DOI] [Google Scholar]
- 41. Mathews SE, Castellanos-Diaz J, Srihari A, Kadiyala S, Leey-Casella J, Ghayee HK, et al. Subacute Thyroiditis and Heart Failure in a Patient Presenting With COVID-19. J Investig Med High Impact Case Rep (2021) 9:23247096211009412. doi: 10.1177/23247096211009412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ricci D, Brancatella A, Marinò M, Rotondi M, Chiovato L, Vitti P, et al. The Detection of Serum IgMs to Thyroglobulin in Subacute Thyroiditis Suggests a Protective Role of IgMs in Thyroid Autoimmunity. J Clin Endocrinol Metab (2020) 105(6):e2261–70. doi: 10.1210/clinem/dgaa038 [DOI] [PubMed] [Google Scholar]
- 43. Nishihara E, Amino N, Kudo T, Kohsaka K, Ito M, Fukata S, et al. Moderate Frequency of Anti-Thyroglobulin Antibodies in the Early Phase of Subacute Thyroiditis. Eur Thyroid J (2019) 8(5):268–72. doi: 10.1159/000501033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-Converting Enzyme 2 is a Functional Receptor for the SARS Coronavirus. Nature (2003) 426(6965):450–4. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect Dis Poverty (2020) 9(1):45. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of SARS-COV-2 Receptor ACE-2 mRNA in Thyroid Cells: A Clue for COVID-19-Related Subacute Thyroiditis. J Endocrinol Invest (2020) 44:1085–90. doi: 10.1007/s40618-020-01436-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coperchini F, Ricci G, Croce L, Denegri M, Ruggiero R, Villani L, et al. Modulation of ACE-2 mRNA by Inflammatory Cytokines in Human Thyroid Cells: A Pilot Study. Endocrine (2021). doi: 10.1007/s12020-021-02807-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aemaz Ur Rehman M, Farooq H, Ali MM, Ebaad Ur Rehman M, Dar QA, Hussain A. The Association of Subacute Thyroiditis With COVID-19: A Systematic Review. SN Compr Clin Med (2021), 1–13. doi: 10.1007/s42399-021-00912-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christensen J, O'Callaghan K, Sinclair H, Hawke K, Love A, Hajkowicz K, et al. Risk Factors, Treatment and Outcomes of Subacute Thyroiditis Secondary to COVID-19: A Systematic Review. Intern Med J (2021). doi: 10.1111/imj.15432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid (2016) 26(10):1343–421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 51. McPadden J, Warner F, Young HP, Hurley NC, Pulk RA, Singh A, et al. Clinical Characteristics and Outcomes for 7,995 Patients With SARS-CoV-2 Infection. PloS One (2021) 16(3):e0243291. doi: 10.1371/journal.pone.0243291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trimboli P, Camponovo C, Franscella S, Bernasconi E, Buetti N. Subacute Thyroiditis During the COVID-19 Pandemic: Searching for a Clinical Association With SARS-CoV-2. Int J Endocrinol (2021) 2021:5588592. doi: 10.1155/2021/5588592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients With Covid-19 - Preliminary Report. N Engl J Med (2020) 394:693–704. doi: 10.1101/2020.06.22.20137273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, Harris E, et al. Scope, Quality, and Inclusivity of Clinical Guidelines Produced Early in the Covid-19 Pandemic: Rapid Review. BMJ (2020) 369:m1936. doi: 10.1136/bmj.m1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coperchini F, Chiovato L, Ricci G, Croce L, Magri F, Rotondi M. The Cytokine Storm in COVID-19: Further Advances in Our Understanding the Role of Specific Chemokines Involved. Cytokine Growth Factor Rev (2021) 58:82–91. doi: 10.1016/j.cytogfr.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coperchini F, Chiovato L, Rotondi M. Interleukin-6, CXCL10 and Infiltrating Macrophages in COVID-19-Related Cytokine Storm: Not One for All But All for One! Front Immunol (2021) 12:668507. doi: 10.3389/fimmu.2021.668507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev (2020) 53:25–32. doi: 10.1016/j.cytogfr.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruggeri RM, Campennì A, Deandreis D, Siracusa M, Tozzoli R, Petranović Ovčariček P, et al. SARS-COV-2-Related Immune-Inflammatory Thyroid Disorders: Facts and Perspectives. Expert Rev Clin Immunol (2021) 17(7):737–59. doi: 10.1080/1744666X.2021.1932467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pirola I, Gandossi E, Rotondi M, Marini F, Cristiano A, Chiovato L, et al. Incidence of De Quervain's Thyroiditis During the COVID-19 Pandemic in an Area Heavily Affected by Sars-CoV-2 Infection. Endocrine (2021). doi: 10.1007/s12020-021-02841-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones N. How COVID-19 is Changing the Cold and Flu Season. Nature (2020) 588(7838):388–90. doi: 10.1038/d41586-020-03519-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.