Abstract
SARS-COV-2 infection causes severe respiratory tract illness leading to asphyxia and death. The onset of infection is associated with loss of smell, blurred vision, headache with bronchopulmonary symptoms. The clinical observations of neurological abnormalities lead us to address the question, does the virus enter into brain and what is the underlying mechanism of brain infection? The working hypothesis is, SARS-COV-2 Spike epitopes modify blood brain barrier and infect glial cells to induce brain inflammation in genetically diverse human population. The hypothesis is tested by determining binding or interacting ability of virus Spike epitope peptides M1Lys60 and Ala240Glu300 with human toll-like receptor 8 (TLR 8), brain targeted Vascular Cell adhesion Molecules (VCAM1) proteins, Zonula Occludens (ZO), glial cell specific protein NDRG2 and Apo- S100B. The molecular dynamic experiments are performed, and root mean square deviation (RMSD) values are determined for interactions between the Spike peptides and selected proteins. The observations demonstrate formation of heterodimeric complex between the epitope peptides and selected protein structures. The viral epitopes have ability to bind with HLA-DRB1 15:01, 07:01 or 03.01 alleles thus found immunogenic in nature. The observations altogether suggest entry of these Spike protein epitopes into human brain causes inflammation.
Keywords: Spike epitopes, SARS-COV-2, Glia, Blood brain barrier, Brain
Introduction/background
Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-COV-2) causes recent pandemic with heightened mortality rate all over the world. Acute respiratory distress (ARD) with uncontrolled surge of cytokines is found to worsen the disease condition. The viral infection causes lung collapse with pneumonia [1], [2] and cardiovascular failure [3]. The genetic mark up with susceptibility of infection in population was not known until recent genome wide association study (GWAS) which suggests 3p21.31 gene cluster is critical for SARS-COV-2 infection with respiratory failure [4]. Several investigations [5], [6], [7] also indicated possible genetic factors for determining hosts’ susceptibility to SARS-COV-2. Progressive infection causes kidney malfunction [8] and multi-organ failure [9]. The meta-analysis of COVID-19 patients in pandemic demonstrates close association with neuropsychiatric disorders [10]. The presence of virus particles in cerebrospinal fluid has been demonstrated from infected patients [11], [12] which indicates presence of the virus into central nervous system. It is reported that SARS-COV-2 infection activates astrocytes and microglia in patients [13]. Though mechanism is still not clear. We identified unique Spike epitopes which have binding affinity to HLA-DRB1 alleles 15:01; 07:01 and 03:01 with variable immunogenicity to T helper cells. The brain receptor binding efficacy of these viral peptides are tested by demonstration of heterodimer formation with selected human brain targeted proteins. The mapping of viral receptors in human brain and immune therapy approach to block these virus epitopes are currently underway.
The hypothesis/theory
Our working hypotheses are two folds; we propose (a) SARS-COV-2 spike epitope peptides modify blood brain barrier proteins and enter into brain cause inflammation; and (b) Viral spike epitope peptides induce activated T helper cells recognized by HLA-DRB1 alleles in genetically diverse human population leading to chronic brain inflammation and neurodegeneration.
Evaluation of hypothesis/idea
The specific interaction patterns between virus spike epitope sequences and human brain targeted proteins are still not clear. It has been reported that, seven members of human Corona Virus family (hCOV) : SARS-COV, SARS-COV-2, MERS-COV, HCoV-OC43, HCoV-229E, HCoV-HKU1 and HCoV-NL63 are related to each other and possibly infect human central nervous system (CNS) [12]. Virus infection causes alteration in blood brain barrier integrity [14], [15]. The postmortem analysis of COVID-19 patients demonstrates presence of activated astrocytes, microglia and cytotoxic T cells in olfactory bulb, brain stem and cerebellum [13], [16], [17].
Empirical data
We selected two Spike peptides with amino acid sequences 1–60 (M1Lys60) and 241–300 (Ala240Glu300) (NH2-terminal sequences) to determine their binding efficacy with brain receptor proteins. The viral peptides are found to bind with HLA-DRB1 alleles 03:01, 07:01 and 15:01 to a variable extent (Table 1 ) (IEDB epitope analysis tools). The observations presented in Table1 suggested that viral peptides are immunogenic in nature. We performed molecular dynamics and docking experiments (md) using AutoDock Tools (Script, La Jolla), PyMol software systems (US), to determine interaction patterns between membrane bound alpha helical SARS-COV-2 Spike epitopes (PDB: Spike Chain_A_1-60 and Spike Chain_A_241-300) (Identified epitope peptides, Subhajit Dasgupta) and selected protein sequences : human tight junction protein Zo (PDB: 3SHU), integrin VCAM1 (PDB: 1VSC) [18], innate immune responder TLR8 (PDB: 3W3N) [19], glial protein NDRG2 (2XMR) [20] and rat Apo S100B (PDB: 1B4C) [21]. The md experiments were performed by using protein database (PDB) format of Spike peptides and selected proteins. We aligned the PDB formatted Spike peptides with each of selected brain derived peptides considering outlier rejection in 10 cycles and cut off value 2 Angstrom unit. The md run was set for 2 min (120 × 109 nsec), 30 Frames per second with ray tracing parameter and each aligned Spike peptide as mobile selection for each selected host protein (target selection). We determined root mean square deviation (RMSD) value (Angstrom) for each molecular dynamics run. The frame per second versus rendering time plot for each molecular dynamic interaction has been presented with docking results on blood brain barrier tight junction protein Zonula Occluden-1 (ZO-1) (Fig. 1 A), Vascular Cell Adhesion Factor (VCAM) (Fig. 1B), human Toll-like receptor 8 (TLR8) (Fig. 2 ). The molecular docking experiments also demonstrates specificity of Spike peptide epitopes to form complex with human astrocyte specific protein N-myc downstream-regulated gene 2 (NDRG2) (Fig. 3 A) and glial specific calcium regulator protein Apo- S100B (Fig. 3B).
Table 1.
SARS-COV2 N-terminal Spike peptide epitopes efficiently bind with HLA-DRB1 alleles and demonstrate CD4 cell immunogenicity.
| N-terminal Spike peptides | Immunogenic CD4 epitope(s) | IEDB combined score | Epitope binding efficacy with HLA-DRB1 alleles (IC50 nM) ‡ 03:01 07:01 15:01 | ||
|---|---|---|---|---|---|
| Peptide A: 1-60 |
LVSLLSVLL LLSVLLMGC |
52.20664 56.00644 |
– – |
42 | 90 |
| Peptide C: 241-300 |
ITRFQTLLA |
49.766 |
– |
INITRFQTL (351/242.9) |
INITRFQTL (452/100.3) |
The IEDB epitope analysis tool is used to determine IC(50) values; IC (50) value less than 50 nM is considered as high affinity binding of peptide with HLADRB1 allele; IC (50) greater than 50 less than 500 nM is intermediate affinity. IC (50) greater than 500 nM less than 5000 nM is considered as low to poor affinity or no affinity. The core peptides are assessed for their affinity profile with alleles.
Fig. 1.
SARS-COV-2 Spike epitope peptides (amino acid sequences 1–60 and 241–300) form complex with blood brain barrier protein Zonula Occludens and integrin vascular cell adhesion molecule (VCAM). The molecular docking experiments were performed to determine interaction between alpha helical SARS-COV-2 Spike epitope peptides (PDB id: Spike Chain_A_1-60 and Spike Chain_A_ 241–300) with (A) Zonula Occludens (Zo ;PDB id: 3SHU). RMSD for md run with Spike Chain_A_241-300 is 2.907 Angstrom. Inset figure shows secondary protein structures interacting with each other in docking experiment. (B) VCAM (PDB id: 1VSC) to form heterodimeric complex. RMSD for md run with Spike Chain_A-1–60 is 5.605 Angstrom and Spike Chain_A_241-300 is 5.065 Angstrom. Inset figure shows secondary protein structures interacting with each other in docking experiment. The md plot shows Frame/sec value @30 frames per sec run for 120x 109 nsec Rendering time. The interacting protein sequences are in highlighted box and provided in both the figures.
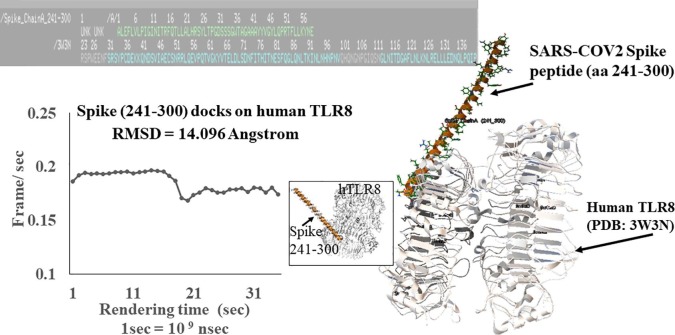
Fig. 2.
SARS-COV-2 Spike peptide (amino acid sequence 241–300) form heterodimeric complex with human toll-like receptor 8 (hTLR8). The molecular docking experiments were performed to determine interaction between alpha helical SARS-COV-2 Spike epitope peptide PDB id: Spike Chain_A_ 241–300) with human toll-like receptor 8 (TLR8). RMSD for md run with Spike Chain_A_241-300 is 14.096 Angstrom. Inset figure shows secondary protein structures interacting with each other in docking experiment. The md plot shows Frame/sec value @30 frames per sec run for 120x 10(9) nsec Rendering time. The interacting protein sequences are shown in the figure.
Fig. 3.
SARS-COV-2 Spike epitope peptides form complex with astrocyte activation protein NDRG2 and calcium modulator protein Apo-S100B. The molecular docking experiments demonstrate SARS-COV-2 alpha helix peptides with amino acid sequence 1–60 and 241–300 form dimeric complex with (A) NDRG2. The RMSD value for md run with Spike Chain_A_1-60 and NDRG2 is 5.495 Angstrom and md run with Spike Chain_A_241-300 and NDRG2 is 7.857 Angstrom. and (B) Apo-S100B. The molecular dynamic run between Spike Chain_A_1-60 and Apo- S100B shows RMSD value 0.539 Angstrom. The md run between Spike Chain_A_241-300 and Apo S100B shows RMSD value 0.777 Angstrom unit. Inset figure shows secondary protein structures interacting with each other in docking experiment (Apo- S100B one isomer out of 20 is taken for md run experiment). The md plot for each individual interaction shows Frame/sec value @30 frames per sec run for 120x 10(9) nsec Rendering time. The highlighted interacting protein sequences are shown in the figure.
Consequences of the hypothesis and discussion
The molecular dynamics (md) and docking experiments provide clear demonstrations on mechanism of interaction between Spike epitopes (1-60 and 241-300) and selected blood brain barrier protein Zo, innate immune responder TLR8 and brain derived glial cell proteins NDRG2 and Apo-S100B. The interaction profile indicates of SARS-COV-2 in blood brain barrier endothelium and cause inflammation in brain. The use of md method is a new way to identify interaction between homologous as well as non-homologues proteins on the basis of atom-to-atom alignment profile between two interacting proteins. The computational approach with md has been used to demonstrate mode of action of natural compound as possible drug and therapeutics in cancer [22], [23]. Recently, therapeutic importance of human antimicrobial peptide LL-37 for COVID has been analyzed by md methods [24]. In the aspect, our observations provide new findings on Spike protein action on selected proteins in human brain.
The blood brain barrier (BBB) composed of endothelial cells, astrocyte-foot process and axons - all play vital role in maintaining CNS integrity. The junctions between the BBB cells selectively pass nutrients and small molecules from periphery to CNS. Our findings (Fig. 1A and B) that, viral spike epitopes [1–60: M1Lys60 and 241–300: Ala240Glu300)] form complex with tight junction protein Zo-1 and integrin VCAM-1, suggest a mechanism of Spike peptides on viral entry to CNS through BBB. The Spike peptide interaction pattern with human TLR8 (Fig. 2) is another critical observation showing involvement of TLR8. The elevated expression of VCAM1 is demonstrated in microglia in brain lesions of multiple sclerosis patients [25], [26]. The possibility that, chronic inflammation in brain through interaction between Spike epitope and VCAM1 integrin leading to autoimmune onset of multiple sclerosis cannot be ignored in aging population. We found Spike peptides bind with astrocyte activation protein NDRG2 (tumor suppressor protein and stress response gene product). The elevated production of this protein shows activation of astrocyte and presence of abnormal synaptic glutamate during neurodegenerative diseases and neuropathic pain [27], [28]. We pursue these findings to determine specific binding region of SARS-COV-2 Spike peptides for the receptor(s) present in human brain and develop immunotherapy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
I (S.D.G) acknowledge NeuroDrug Research LLC. and Biology division, Trident Technical College, Charleston for resources.
Consent statement/ethical approval
There is no animal model or human subject involved in the manuscript. The consent statement/ ethical approval is not required.
Funding statement
The research is supported by internal funding from NeuroDrug Research LLC. There is no Gov’t or other fund associated with the project.
References
- 1.Kniep I., Heinemann A., Edler C., Sperhake J.P., Püschel K., Ondruschka B., et al. COVID-19 lungs in post-mortem computed tomography COVID-19-lungen in der post-mortem-computertomographie. Rechtsmedizin (Berl) 2021;31(2):145–147. doi: 10.1007/s00194-021-00462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojha V., Mani A., Pandey N.N., Sharma S., Kumar S. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020;30(11):6129–6138. doi: 10.1007/s00330-020-06975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severe Covid G.G., Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., et al. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenti L., Griffini S., Lamorte G., Grovetti E., Uceda Renteria S.C., Malvestiti F., et al. Chromosome 3 cluster rs11385942 variant links complement activation with severe COVID-19. J Autoimmun. 2021;117:102595. doi: 10.1016/j.jaut.2021.102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou Y., Zhao J., Martin W., Kallianpur A., Chung M.K., Jehi L., et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littera R., Campagna M., Deidda S., Angioni G., Cipri S., Melis M., et al. Human leukocyte antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. The Sardinian Experience. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.605688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng W.H., Tipih T., Makoah N.A., Vermeulen J.-G., Goedhals D., Sempa J.B., et al. Comorbidities in SARS-CoV-2 patients a systematic review and meta-analysis. mBio. 2021;12(1) doi: 10.1128/mBio.03647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulchandani R., Lyngdoh T., Kakkar A.K. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51(1) doi: 10.1111/eci.v51.110.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perchetti G.A., Nalla A.K., Huang M.-L., Zhu H., Wei Y., Stensland L., et al. Validation of SARS-CoV-2 detection across multiple specimen types. J Clin Virol. 2020;128:104438. doi: 10.1016/j.jcv.2020.104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgello S. Coronaviruses and the central nervous system. J Neurovirol. 2020;26(4):459–473. doi: 10.1007/s13365-020-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Dalahmah O., Thakur K.T., Nordvig A.S., Prust M.L., Roth W., Lignelli A., et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol Commun. 2020;8(1) doi: 10.1186/s40478-020-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds J.L., Mahajan S.D. SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation. J Neuroimmune Pharmacol. 2021;16(1):4–6. doi: 10.1007/s11481-020-09975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas G., Medeiros Geraldo L.H., Gedeão Salomão N., Viana Paes M., Regina Souza Lima F., Carvalho Alcantara Gomes F. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: insights and perspectives. Brain Behav Immun Health. 2020;7:100127. doi: 10.1016/j.bbih.2020.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J.H., Pepinsky R.B., Stehle T., Liu J.H., Karpusas M., Browning B., et al. The crystal structure of an N-terminal two-domain fragment of vascular cell adhesion molecule 1 (VCAM-1): a cyclic peptide based on the domain 1 C-D loop can inhibit VCAM-1-alpha 4 integrin interaction. Proc Natl Acad Sci U S A. 1995;92(12):5714–5718. doi: 10.1073/pnas.92.12.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanji H., Ohto U., Shibata T., Miyake K., Shimizu T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science. 2013;339(6126):1426–1429. doi: 10.1126/science.1229159. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J., Kim Y., Kang H.B., Jaroszewski L., Deacon A.M., Lee H., et al. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J Biol Chem. 2011;286(14):12450–12460. doi: 10.1074/jbc.M110.170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drohat A.C., Tjandra N., Baldisseri D.M., Weber D.J. The use of dipolar couplings for determining the solution structure of rat apo-S100B(betabeta) Protein Sci. 1999;8(4):800–809. doi: 10.1110/ps.8.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alpsoy A., Utturkar S.M., Carter B.C., Dhiman A., Torregrosa-Allen S.E., Currie M.P., et al. BRD9 is a critical regulator of androgen receptor signaling and prostate cancer progression. Cancer Res. 2021;81(4):820–833. doi: 10.1158/0008-5472.CAN-20-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokhande K.B., Nagar S., Swamy K.V. Molecular interaction studies of Deguelin and its derivatives with Cyclin D1 and Cyclin E in cancer cell signaling pathway: the computational approach. Sci Rep. 2019;9(1):1778. doi: 10.1038/s41598-018-38332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lokhande K.B., Banerjee T., Swamy K.V., Ghosh P., Deshpande M. An in silico scientific basis for LL-37 as a therapeutic for Covid-19. Proteins. 2021 doi: 10.1002/prot.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson J.W., Bö L., Mörk S., Chang A., Ransohoff R.M., Trapp B.D. VCAM-1-positive microglia target oligodendrocytes at the border of multiple sclerosis lesions. J Neuropathol Exp Neurol. 2002;61(6):539–546. doi: 10.1093/jnen/61.6.539. [DOI] [PubMed] [Google Scholar]
- 26.Haarmann A., Nowak E., Deiss A., van der Pol S., Monoranu C.M., Kooij G., et al. Soluble VCAM-1 impairs human brain endothelial barrier integrity via integrin alpha-4-transduced outside-in signalling. Acta Neuropathol. 2015;129(5):639–652. doi: 10.1007/s00401-015-1417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flügge G., Araya-Callis C., Garea-Rodriguez E., Stadelmann-Nessler C., Fuchs E. NDRG2 as a marker protein for brain astrocytes. Cell Tissue Res. 2014;357(1):31–41. doi: 10.1007/s00441-014-1837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonkeren S.L., Massen M., van der Horst R., Koch A., Vaes N., Melotte V. Nervous NDRGs: the N-myc downstream-regulated gene family in the central and peripheral nervous system. Neurogenetics. 2019;20(4):173–186. doi: 10.1007/s10048-019-00587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]