Abstract
Study objective
Chest computed tomography (chest CT) is routinely obtained to assess disease severity in COVID-19. While pulmonary findings are well-described in COVID-19, the implications of cardiovascular findings are less well understood. We evaluated the impact of cardiovascular findings on chest CT on the adverse composite outcome (ACO) of hospitalized COVID-19 patients.
Setting/participants
245 COVID-19 patients who underwent chest CT at Rush University Health System were included.
Design
Cardiovascular findings, including coronary artery calcification (CAC), aortic calcification, signs of right ventricular strain [right ventricular to left ventricular diameter ratio, pulmonary artery to aorta diameter ratio, interventricular septal position, and inferior vena cava (IVC) reflux], were measured by trained physicians.
Interventions/main outcome measures
These findings, along with pulmonary findings, were analyzed using univariable logistic analysis to determine the risk of ACO defined as intensive care admission, need for non-invasive positive pressure ventilation, intubation, in-hospital and 60-day mortality. Secondary endpoints included individual components of the ACO.
Results
Aortic calcification was independently associated with an increased risk of the ACO (odds ratio 1.86, 95% confidence interval (1.11–3.17) p < 0.05). Aortic calcification, CAC, abnormal septal position, or IVC reflux of contrast were all significantly associated with 60-day mortality and major adverse cardiovascular events. IVC reflux was associated with in-hospital mortality (p = 0.005).
Conclusion
Incidental cardiovascular findings on chest CT are clinically important imaging markers in COVID-19. It is important to ascertain and routinely report cardiovascular findings on CT imaging of COVID-19 patients as they have potential to identify high risk patients.
Keywords: Chest computed tomography, COVID-19, Aortic calcification, Coronary artery calcification, Right ventricular strain
Graphical abstract
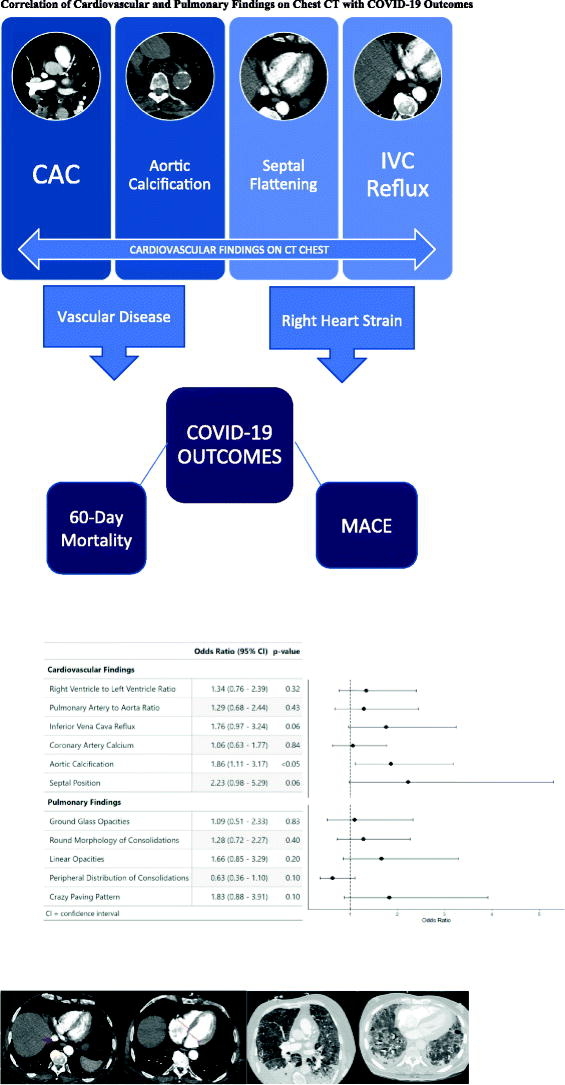
The correlation of cardiovascular and pulmonary findings on CT Chest with composite outcome consisting of ICU admission, need for invasive ventilation via endotracheal intubation or non-invasive positive pressure ventilation, in-hospital mortality, and 60-day mortality is illustrated above with the respective ORs. Only aortic calcification was independently associated with higher risk of composite outcome (p < 0.05). Coronary artery calcification, aortic calcification, septal flattening, and IVC reflux were all independently associated with higher risk of 60-day mortality and MACE (p < 0.05). CT: Computed Tomography, IVC: inferior vena cava, MACE: Major adverse cardiovascular event, OR: odds ratio.
1. Introduction
Computed tomography (CT) of the chest (chest CT) is routinely used for the diagnosis and management of patients with COVID-19. There is growing evidence for the use of chest CT in COVID-19 for determination of disease severity, risk stratification, guidance of treatment, and assessment of treatment response [1], [2], [3]. CT allows for detection of early parenchymal lung disease, pulmonary embolism, myocardial injury and acute heart failure [2]. According to the 2020 Multinational Consensus Statement from the Fleischner Society, chest CT is indicated in COVID-19 patients with moderate to severe clinical features in the presence of clinical worsening to determine secondary cardiopulmonary abnormalities, or in the setting of continued functional impairment/hypoxemia after recovery [2]. Characteristic pulmonary findings in COVID-19 patients have been well described and standardized CT reporting language have been proposed by multiple professional societies, including the Radiological Society of North America [4], [5], [6].
CT-guided staging of COVID-19 severity based on the typical temporal pattern of subpleural ground glass opacities, crazy paving, consolidation at disease peak, and absorption of consolidation has been shown to be an accurate predictor of clinical severity (p < 0.001) [5]. In a recent study of COVID-19 where a CT-based lobar involvement severity score was utilized, a higher score was independently associated with death on multivariate analysis [hazard ratio (HR) 3.74; 95% confidence interval (CI) 1.1–12.77/p = 0.03] [6].
While the importance of pulmonary findings in COVID-19 is well described, the significance of incidental cardiovascular findings on CT imaging on adverse outcomes is less well studied, especially with regards to long-term clinical outcomes following hospitalization [7]. In the setting of pulmonary embolism, CT findings of right ventricular (RV) strain such as RV to left ventricular (LV) diameter ratio > 0.9, pulmonary artery (PA) to aorta (Ao) diameter ratio > 1.0, and reflux of contrast into the inferior vena cava (IVC), are established as poor prognostic factors and predictors of short term mortality [8], [9], [10]. These imaging findings are a result of increasing impedance and resistance on the pulmonary vasculature, resulting in RV strain [11]. Coronary artery calcification (CAC) is a readily identifiable finding on chest CT that is a potent and well-established marker of cardiovascular risk [12]. Moreover, there is mounting evidence that cardiovascular disease (CVD) such as coronary artery disease (CAD) and cerebrovascular disease, hypertension, and diabetes are all associated with increased mortality in COVID-19 [13], [14], [15], [16]. The risk of mortality in COVID-19 patients with underlying CVD is higher than the general population. The risk has been reported as high as 10.5% in patients with CVD, 7.3% in patients with hypertension, and 6% in patients with diabetes in a report from 44,672 confirmed cases from the Chinese Center for Disease Control and Prevention, in contrast to the 2.3% case fatality rate in those without these underlying conditions [17], [18]. As the morbidity and mortality of COVID-19 continue to rise, especially in those with underlying CVD, early risk stratification is critical.
Given the lack of standardization of reporting cardiovascular findings on chest CT in COVID-19, the prognostic implications of incidental cardiovascular findings are largely unknown. The aim of our study was to investigate the correlation of incidental cardiovascular findings on chest CT with both cardiopulmonary disease severity and mortality in COVID-19 patients.
2. Materials and methods
2.1. Study population
We conducted a retrospective cohort study on 245 adult patients hospitalized at Rush University System for Health (RUSH) between March to June 2020 who were diagnosed with COVID-19 and underwent clinically indicated chest CT imaging. The study was reviewed and approved by the Institutional Review Board at Rush University Medical Center. Inclusion criteria were ≥ 18 years of age, hospitalization with positive COVID test, and performance of a chest CT. Baseline demographic information and comorbidities were extracted from our electronic medical records (EPIC). Patient outcomes and CT data were collected through manual chart review.
2.2. CT image acquisition and analysis
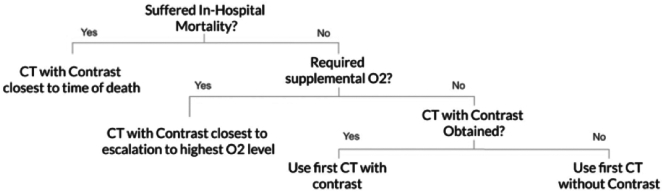
Only one chest CT scan was analyzed per patient. Chest CTs performed after occurrence of one of the composite endpoints were excluded from analysis. If multiple CT scans were performed, preference was given to contrast enhanced CTs that were performed closest to the patient's time of death or escalation to the highest level of oxygen therapy (Fig. 1). If the patient survived and did not require oxygen supplementation, the first CT scan performed during the hospitalization was included, with preference given to contrast-enhanced CT studies.
Fig. 1.
Choice of chest CT per patient.
Pathway of Chest CT selection is shown above. If multiple CT scans were performed, preference was given to contrast-enhanced CTs that were performed closest to the patient's time of death or escalation to the highest level of oxygen therapy.
CT: computed tomography. O2: oxygen.
CT scans were manually reviewed by two physicians (MIP and MR) who were blinded to the patients' clinical history. Each of these physicians underwent standardized training from a cardiovascular imaging specialist (AR) to measure and identify RV/LV diameter and ratio, PA to Ao diameter and ratio, and presence or absence of the following: IVC reflux of contrast, CAC, aortic calcification, and abnormal interventricular septal position (septal flattening or bowing of the septum into the LV). If contrast-enhanced CT was not performed, the first non-contrast CT was chosen per protocol (Fig. 1) and CAC and aortic calcification were measured. RV/LV diameter and ratio, PA to Ao diameter and ratio, IVC reflux, and septal position were not measured on non-contrast CT.
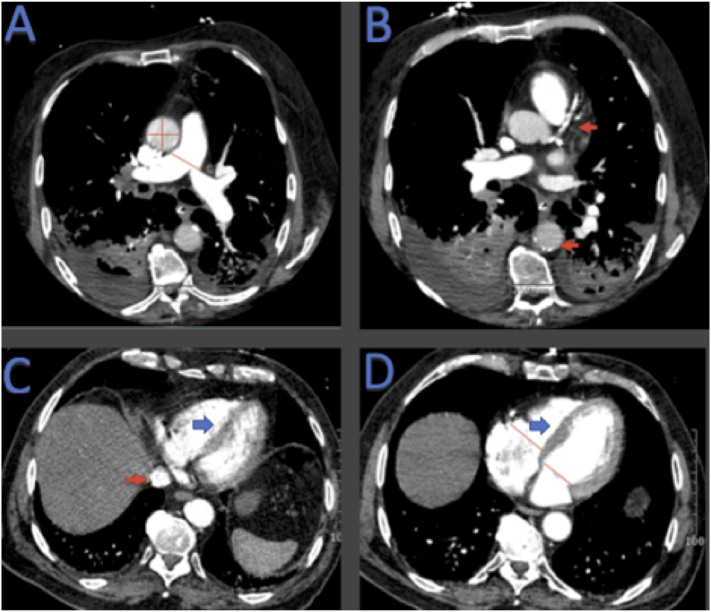
Cardiovascular CT findings are shown in Fig. 2. PA diameter was measured just proximal to the bifurcation of the pulmonary trunk on axial slices. The diameter of the ascending aorta was performed on the same axial slice at the level of the right PA (Fig. 2A). Presence or absence of CAC and aortic calcification were recorded as binary variables (Fig. 2B). The threshold for defining CAC and aortic calcification was set to ≥130 Hounsfield units. Presence or absence of IVC reflux was recorded (Fig. 2C). RV and LV diameters were measured on a single axial CT slice at the point of maximal distance between the interventricular septum and the ventricular free wall perpendicular to the long axis of the heart on axial sections (Fig. 2D). In addition, the interventricular septum was evaluated for the presence of right to left bowing or flattening, labeled as abnormal septal position (Fig. 2C) Normal septal position was defined as bowing towards the right ventricle (Fig. 2D). Septal position was recorded as presence or absence of abnormal septal position.
Fig. 2.
Cardiovascular findings measured on Chest CT.
PA diameter was measured just proximal to the bifurcation of the pulmonary trunk ascending aortic length and width were measured on the same axial CT slice at the level of the right pulmonary artery (red lines) (A). Presence or absence of coronary calcium and aortic calcification was measured in binary fashion (red arrow) (B). Presence or absence of IVC reflux was measured (red arrow) (C). RV and LV diameters were measured on a single axial CT slice as described in text (red lines) (D). Interventricular septum was evaluated in binary fashion for normal (D) versus abnormal (C) position. CT: computed tomography, IVC: inferior vena cava, LV: left ventricle, PA: pulmonary artery, RV: right ventricle.
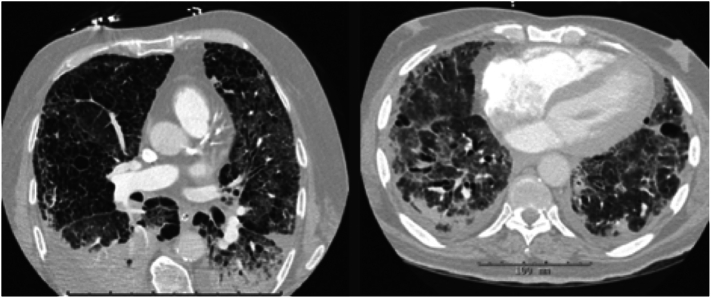
Pulmonary findings were extracted from the clinically recorded radiology report, interpreted by an attending radiologist. The typical findings related to COVID-19 infection recorded were the following: presence of ground glass opacities, interlobular and intralobular septal thickening overlying an area of ground glass opacity (also referred to as “crazy-paving pattern”), consolidation, peripheral distribution of consolidation, rounded or nodular consolidation, and linear opacities (Fig. 3). Presence of pulmonary embolism was also documented per the clinical radiology report.
Fig. 3.
Pulmonary findings on chest CT.
The pulmonary findings from the clinically recorded report included the typical COVID-19 findings of ground glass opacities, interlobular and intralobular septal thickening referred to as “crazy-paving” and peripheral distribution of consolidations, all of which are demonstrated above.
The primary endpoint was adverse composite outcome for severe COVID-19 infection, which consisted of need for intensive care unit (ICU) admission, need for invasive ventilation via endotracheal intubation or non-invasive positive pressure ventilation, in-hospital mortality, and 60-day mortality.
Individual components of the adverse composite outcome were selected as secondary endpoints. Major adverse cardiovascular events (MACE) that occurred within 60 days of admission, defined as occurrence of acute coronary syndrome (ACS), stroke, life-threatening arrhythmia, deep venous thrombosis, acute heart failure, need for renal replacement therapy, and pulmonary embolism were also included as a secondary endpoint. Acute coronary syndrome was defined per the 2018 joint task force of the European Society of Cardiology, American Heart Association, American College of Cardiology Foundation, and World Health Federation definition: documented abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia. This included documented ACS, NSTEMI, STEMI, and MI. Life-threatening arrhythmia was defined as asystole, ventricular tachycardia >30 s, new grade 3 atrioventricular block/complete heart block, or any arrhythmia requiring defibrillation or cardiopulmonary resuscitation. Acute heart failure was defined as admission for symptoms of acute heart failure.
2.3. Statistical analysis
Normally distributed continuous variables were compared with Student t-tests and are displayed with means and standard deviation. Categorical variables were compared with the Pearson chi-square test and are presented as counts and proportions.
Univariable logistic regression was performed with each individual CT finding as a risk for the adverse composite outcome for severe COVID-19 infection. Odds ratio (OR)s with 95% confidence intervals (CI) are presented.
From the results of these models along with prior studied models and clinical experience, particular variables were chosen for further examination by analyzing correlation with the separate components of the adverse composite outcome in addition to correlation MACE [19].
Lastly, both a cardiac and pulmonary multivariable model was created to assess which would better correlate with the adverse composite outcome. The sensitivity and specificity of each model was then plotted on a receiver operation curve where the area under the curve of each model was calculated, which were then compared using Delong's test.
The threshold for p-value significance was set to 0.05. All statistical analysis was performed using RStudio version 1.3 (Boston, Massachusetts). Receiver operating characteristic (ROC) plotting and comparisons were performed using the pROC package in RStudio.
3. Results
3.1. Patient characteristics
Our study cohort consisted of 245 COVID-19 positive patients evaluated with chest CT imaging, 189 of which were performed with iodinated contrast.
The clinical characteristics, comorbidities, and drug therapies are reported in Table 1. Median age of those who experienced the adverse composite outcome was 58 (46.75–73.25) years, those without 55 (43.75–65.00) years. The cohort was 58% male and with 28% Caucasian and 27% African American.
Table 1.
Clinical characteristics.
| Yes (N %) |
No (N %) |
p-Value | |
|---|---|---|---|
| (Total n = 124) | (Total n = 121) | ||
| Female (%) | 44 (35.5) | 60 (49.6) | 0.035 |
| Age (median [IQR]) | 58.00 [46.75, 73.25] | 55.00 [43.75, 65.00] | 0.037 |
| BMI (median [IQR]) | 27.40 [23.50, 31.85] | 27.75 [24.05, 33.60] | 0.433 |
| Systolic BP (median [IQR]) | 129.50 [115.00, 143.50] | 131.00 [117.75, 147.00] | 0.659 |
| Diastolic BP (median [IQR]) | 72.50 [61.00, 86.00] | 74.00 [64.75, 84.00] | 0.493 |
| Heart rate (mean (SD)) | 102.76 (21.13) | 102.90 (20.54) | 0.958 |
| Current smoker (%) | 4 (4.0) | 4 (3.6) | 1.000 |
| Race (%) | 0.067 | ||
| White | 40 (36.0) | 29 (26.1) | |
| Other | 46 (41.4) | 42 (37.8) | |
| Black or African American | 25 (22.5) | 40 (36.0) | |
| Comorbidities | |||
| CAD (%) | 48 (38.7) | 18 (15.0) | <0.001 |
| Hypertension (%) | 82 (66.1) | 66 (55.0) | 0.099 |
| Atrial Fibrillation (%) | 35 (28.2) | 6 (5.0) | <0.001 |
| COPD (%) | 11 (8.9) | 3 (2.5) | 0.062 |
| Asthma (%) | 12 (9.7) | 13 (10.8) | 0.931 |
| Diabetes mellitus (%) | 61 (49.2) | 47 (39.2) | 0.148 |
| Cancer (%) | 13 (10.5) | 12 (10.0) | 1.000 |
| Stroke (%) | 22 (17.7) | 7 (5.8) | 0.007 |
| Prior DVT or PE (%) | 48 (38.7) | 8 (6.7) | <0.001 |
| In-hospital treatments | |||
| Intravenous steroids (%) | 52 (41.9) | 29 (24.2) | 0.005 |
| Remdesivir (%) | 18 (14.5) | 10 (8.3) | 0.189 |
| Intravenous antibiotics (%) | 97 (78.2) | 57 (47.5) | <0.001 |
| Imaging characteristics | |||
| Contrast-enhanced (%) | 92 (74.2) | 101 (83.5) | 0.105 |
| PE diagnosed on imaging (%) | 17 (14.0) | 7 (5.8) | 0.053 |
Those with the adverse composite outcome were more likely to be male versus female (64.5% vs. 50.4%; p < 0.05); have a history of cardiovascular disease [CAD (38.7% vs. 15.0%; p < 0.001), atrial fibrillation (28.2% vs. 5.0%; p < 0.001), and stroke (17.7% vs. 5.8%; p < 0.01)]; and have a history of prior deep vein thrombosis or pulmonary embolism (38.7% vs. 6.7%; p < 0.001) compared with those without the specified composite endpoint.
3.2. CT association with the primary adverse composite outcome
Primary analysis results are summarized in Table 2 and in the Graphical Abstract. Among cardiovascular findings, aortic calcification showed a statistically significant correlation with the adverse composite outcome (OR 1.86 [95% CI: 1.11–3.17]; p < 0.05). Though not significant, septal position (OR 2.23 [95% CI: 0.98–5.29]; p = 0.06) and IVC reflux (OR 1.76 [95% CI: 0.97–3.24]; p = 0.06) revealed a trend towards association with the adverse composite outcome. RV to LV ratio, PA to Aorta Ratio, and CAC were not significantly associated with the adverse composite outcome.
Table 2.
CT association with composite outcome.
| Cardiac CT variables | Odds ratio (95% CI) | p-Value |
|---|---|---|
| RV to LV ratio | 1.34 (0.76–2.39) | 0.32 |
| PA to Aorta ratio | 1.29 (0.68–2.44) | 0.43 |
| IVC Reflux | 1.76 (0.97–3.24) | 0.06 |
| Coronary artery calcification | 1.06 (0.63–1.77) | 0.84 |
| Aortic calcification | 1.86 (1.11–3.17) | <0.05 |
| Septal position | 2.23 (0.98–5.29) | 0.06 |
| Pulmonary variables | Odds ratio (95% CI) | p-Value |
|---|---|---|
| Ground glass opacities | 1.09 (0.51–2.33) | 0.83 |
| Round morphology | 1.28 (0.72–2.27) | 0.40 |
| Linear opacities | 1.66 (0.85–3.29) | 0.20 |
| Peripheral distribution | 0.63 (0.36–1.10) | 0.10 |
| Crazy paving | 1.83 (0.88–3.91) | 0.10 |
Bold indicate that aortic calcification was independently associated with an increased risk of the ACO (odds ratio 1.86, 95% confidence interval (1.11–3.17) p < 0.05).
None of the pulmonary findings were associated with the adverse composite outcome: ground glass opacities (OR 1.09 [95% CI: 0.51–2.33]; p = 0.83), round morphology of consolidations (OR 1.28 [95% CI: 0.72–2.27]; p = 0.40), linear opacities (OR 1.66 [95% CI: 0.85–3.29]; p = 0.20), peripheral distribution of consolidations (OR 0.63 [CI: 0.36–1.10]; p = 0.10), and crazy paving (OR 1.83 [CI: 0.88–3.91]; p = 0.10) (Table 2).
3.3. CT association with secondary endpoints
In our patient cohort, the most common MACE outcomes observed were pulmonary embolism (11.8%), life-threatening arrhythmia (11.4%), need for renal replacement therapy (8.6%), and deep venous thrombosis (6.5%). The presence of CAC, aortic calcification, and flattening or right to left bowing of the interventricular septum were all independently associated with 60-day mortality and one or more MACE. These results are summarized in Table 3. Those who had IVC reflux on their CT scan were more likely to be intubated (40.3% vs. 18.9%; p < 0.01), experienced in-hospital mortality (22.7% vs. 7.4%; p < 0.01) and 60-day mortality (26.9% vs. 10.7%; p < 0.01), and have one or more MACE (41.8% vs. 24.6%; p < 0.05), all of which are components of the secondary endpoint.
Table 3.
CT findings correlation with composite outcome components.
| Cardiac findings | Coronary artery calcium |
Aortic calcification |
||||
|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-Value | |
| n | 98 | 145 | 92 | 151 | ||
| Intubated (%) | 32 (32.7) | 41 (28.3) | 0.557 | 31 (33.7) | 42 (27.8) | 0.409 |
| In-hospital mortality (%) | 17 (17.3) | 14 (9.7) | 0.122 | 17 (18.5) | 14 (9.3) | 0.062 |
| 60-day mortality (%) | 25 (25.5) | 15 (10.3) | 0.003 | 24 (26.1) | 16 (10.6) | 0.003 |
| ICU requirement (%) | 46 (46.9) | 70 (48.3) | 0.941 | 51 (55.4) | 65 (43.0) | 0.081 |
| ≥1 MACE (%) | 40 (40.8) | 37 (25.5) | 0.018 | 40 (43.5) | 37 (24.5) | 0.003 |
| Cardiac findings | Septal position |
IVC reflux |
||||
|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-Value | |
| n | 161 | 28 | 67 | 122 | ||
| Intubated (%) | 40 (24.8) | 10 (35.7) | 0.331 | 27 (40.3) | 23 (18.9) | 0.002 |
| In-hospital mortality (%) | 17 (10.6) | 7 (25.0) | 0.073 | 15 (22.7) | 9 (7.4) | 0.005 |
| 60-day mortality (%) | 21 (13.0) | 10 (35.7) | 0.007 | 18 (26.9) | 13 (10.7) | 0.008 |
| ICU requirement (%) | 68 (42.2) | 17 (60.7) | 0.108 | 37 (55.2) | 48 (39.3) | 0.052 |
| ≥1 MACE (%) | 44 (27.3) | 14 (50.0) | 0.029 | 28 (41.8) | 30 (24.6) | 0.022 |
| Lung findings | Linear opacities |
Peripheral distribution |
||||
|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-Value | |
| n | 43 | 200 | 169 | 74 | ||
| Intubated (%) | 15 (34.9) | 58 (29.0) | 0.562 | 46 (27.2) | 27 (36.5) | 0.194 |
| In-hospital mortality (%) | 13 (30.2) | 18 (9.0) | <0.001 | 20 (11.9) | 11 (14.9) | 0.670 |
| 60-day mortality (%) | 16 (37.2) | 24 (12.0) | <0.001 | 24 (14.2) | 16 (21.6) | 0.212 |
| ICU requirement (%) | 24 (55.8) | 92 (46.0) | 0.317 | 74 (43.8) | 42 (56.8) | 0.085 |
| ≥1 MACE (%) | 17 (39.5) | 60 (30.0) | 0.299 | 47 (27.8) | 30 (40.5) | 0.070 |
Patients with linear opacities were more likely to experience in-hospital mortality (30.2% vs. 9.0%; p < 0.001) and 60-day mortality (37.2% vs. 12.0%; p < 0.001). There were no significant differences when the individual outcomes were analyzed in patients with the remainder of the pulmonary findings.
3.4. Cardiac and pulmonary multivariable models of the primary adverse composite outcome
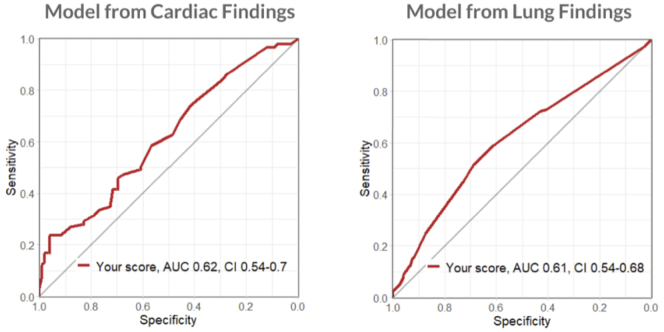
The multivariable models were poorly predictive in both the cardiac and the pulmonary models (AUC 0.62 [CI 0.54–0.70] and 0.61 [0.54–0.68], respectively) (Fig. 4). There was no significant difference between the predictive ability between the two models, p = 0.95.
Fig. 4.
Cardiac versus pulmonary multivariable models.
As plotted on a receiver operation curve, the cardiac and pulmonary multivariable models were poorly predictive (AUC 0.62 [CI 0.54–0.70] and 0.61 [0.54–0.68], respectively), with no significant difference between the predictive ability between the two models, p = 0.95
AUC: area under the curve.
4. Discussion
In our cohort study of 245 adult patients admitted to a tertiary care facility with laboratory-confirmed diagnosis of COVID-19 who underwent chest CT, aortic calcification showed the strongest independent association with the primary composite outcome consisting of intensive care unit admission, need for non-invasive positive pressure ventilation or endotracheal intubation, in-hospital mortality, and 60-day mortality (Graphical Abstract). Furthermore, individual imaging findings including aortic calcification, CAC, abnormal septal position, and IVC reflux were independently associated with both 60-day mortality and MACE. Our data support the theory that findings of RV strain as well as concomitant cardiovascular disease are associated with worse clinical outcomes in patients with COVID-19, and may serve as significant imaging markers conferring a worse prognosis (Graphical Abstract GRa).
The results of our study are suggestive that large vessel atherosclerosis can be a significant and strong marker of poor outcomes in COVID-19. Our findings bolster a recent smaller multi-center study of patients with COVID-19 who underwent low-dose chest CT, which showed that aortic wall calcification volume was the only significant regressor for severe disease within multivariate regression analysis of aortic calcification, age, sex, and the authors' cardiovascular comorbidity score (p = 0.004). Authors suggest that aortic wall calcification may thus be an independent biomarker for severe COVID-19, which was defined similarly to our study: ICU admission, intubation, and death [20].
Secondly, our findings of CAC correlation with increased 60-day mortality are supported by others. Our findings are corroborated by a recent cross-sectional study of 209 COVID-19 hospitalized patients with no prior cardiovascular disease who underwent chest CT. Adjusting for age, sex, hypertension, smoking, and diabetes, the presence of CAC was significantly associated with first occurrence of mechanical noninvasive or invasive ventilation, extracorporeal membrane oxygenation (ECMO), or death within 30 days of hospitalization (HR 4.4 [95% CI: 2.4–8.0]; p < 0.0001) [21]. Our results demonstrate that the presence of aortic calcification is associated with a similar composite outcomeIn another study, CAC score was not significantly associated with myocardial injury by multivariable logistic regression. In contrast to our study, CAC was adjusted by age, which was surmised to prevent its predictive value from being significant [22].
Our findings are supportive of the value of qualitative measurement of CAC and aortic calcification as prognostic markers for COVID-19 disease severity. In a retrospective analysis of 248 patients admitted with COVID-19 who underwent chest CT, qualitative visual assessment of a CAC score > 1 was associated with shorter overall survival (HR 2.76, 95% CI 1.4–5.45, p < 0.01) [23]. Given the association of 60-day mortality with CAC in our study, the binary assessment of CAC should not be overlooked since these patients can be appropriately treated for secondary prevention of CAD.
Our data also highlight that individual variables of RV strain may serve as high-risk imaging markers for 60-day mortality. A single center study of 332 patients with COVID-19 showed that increased PA diameter was significantly associated with myocardial injury defined as high-sensitivity troponin >20 ng/L, (OR adjusted 1.10, 95% CI 1.02–1.19 p = 0.01). It was also significantly associated with death (HR adjusted 1.09, 95% CI 1.02–1.17, p = 0.01) [7]. Our study contributes to the implications of these findings, demonstratingthat in addition to increased PA diameter, other markers of RV strain, i.e. abnormal septal position and IVC reflux, are significantly associated with increased 60-day mortality and MACE.
Neither combined cardiovascular or pulmonary findings used in our single multivariable model had predictive ability for our adverse composite outcome. A recent prospective study of 106 hospitalized COVID 19 patients analyzed a radiology-based chest CT scoring system in addition to biomarkers to determine if CAC is an accurate predictor of short-term clinical outcomes. Their adverse outcomes were defined as requirement of mechanical ventilation and death within 10 days of chest CT [24]. The authors found that the “volume of disease” as measured by pulmonary findings along with age, C-reactive protein, and lymphocyte percentage were better predictors for adverse outcomes compared to CAC and aortic calcification. These results are similar to our findings, as we found that aortic calcification and CAC were not associated with short-term mortality, but rather significantly associated with 60-day mortality.
Lastly, our findings emphasize the importance of recognizing and reporting both pulmonary and cardiovascular findings detected on chest CTs obtained on patients with COVID-19. Recognition of high-risk cardiovascular findings on clinically indicated CTs is of paramount importance, potentially predicting a more severe clinical course.
We therefore stress that cardiovascular findings should be recognized and reported when imaging patients with COVID-19. In particular, the presence of vascular disease and markers of RV strain may be helpful in identifying patients who have higher risk of adverse outcomes in COVID-19.
4.1. Study limitations
The results may be limited by selective inclusion of patients undergoing chest CT, with an even smaller cohort of contrast CTs. This population may have weakened the association with adverse composite outcomes. The observation of strong trends but not statistically significant, towards in-hospital mortality in patients with IVC reflux and abnormal septal position could be a Type II error. Also, RV strain is ideally measured in the four-chamber plane aligned on the short axis plane through anterior mitral valve papillary muscle and apex of right ventricle. In this study, the performance of the measurements in the axial section may have introduced underestimation of the severity of the condition. This may have weakened the predictive value of RV to LV diameter ratio.
Of note, IVC reflux, abnormal septal position, PA/Ao ratio and RV/LV ratio were analyzed in 189 contrast-enhanced CTs compared to the 243 total CTs including those without contrast used to analyze CAC and aortic calcification. The difference in CT number may have reduced power of analysis of RV strain compared to analysis of aortic calcification: the strongest predictor of composite outcome. Secondly, the 30-day mortality rate of our cohort was 10.54%, compared to the 60-day mortality rate of 18.2%. Though the lower short-term mortality rate may have affected composite outcome, secondary analysis highlighted the correlation of CAC, aortic calcification, IVC reflux and septal position with long-term mortality rate (p = 0.003, p = 0.003, p = 0.008, p = 0.007, respectively). Further studies are needed to elucidate the prognostic potential of these cardiovascular markers.
5. Conclusions
Our study demonstrated that cardiovascular findings on chest CT are important imaging markers in symptomatic COVID-19 patients. Findings of aortic calcification, CAC, abnormal septal position, and IVC reflux of contrast are independently associated with mortality. We believe that it is important to ascertain and routinely report cardiovascular findings on CT imaging of patients with COVID-19 with the potential to identify high risk patients. Larger studies are needed to validate these findings.
Funding
The authors received no financial support for the research, authorship, or publication of this manuscript.
Author's contributions
All authors contributed to the study conception and design. Maria Isabel Planek, Anupama Rao, and Stella Kyung have led the study from its inception to final revisions. Material preparation, data collection, and analysis were performed by Max Ruge, Jeanne Du Fay de Lavallaz, Joanne Gomez, Stella Kyung, J Alan Simmons, and Maria Isabel Planek. Data collection also performed by those mentioned in acknowledgements. The original draft of the manuscript was written by Maria Isabel Planek, and all authors have contributed to previous versions of the manuscript. Revision of the manuscript was led and written by Maria Isabel Planek. Supervision of the manuscript has been under Tisha M. Suboc, Kim A. Williams, Annabelle Santos Volgman, and Anupama Rao. All authors read and approved the final manuscript.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used during the current study are available from the corresponding author on reasonable request.
Ethic's approval
The study was reviewed and approved by the Institutional Review Board at Rush University Medical Center. This retrospective chart review study involving human participants is in accordance of the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Rush University Medical Center approved this study.
Consent to participate
As this is a large retrospective cohort study, formal written consent was deemed able to be waived per our institution IRB/Ethics Committee. Research HIPAA Authorization and HIPAA Waiver of Informed Consent were obtained. This is available on request. It was deemed that all protected health information involved no more than minimal risk to the privacy of individuals involved. As above, all aspects of the study were reviewed and approved by the Institutional Review Board at Rush University Medical Center.
Consent for publication
Not applicable, as above. There is no identifying information in this manuscript.
Declaration of competing interest
The authors have no conflicts of interest to disclose. The authors have no competing interests. The Conflict of Interest Form is attached.
Acknowledgments
Athina Bouroukas, Alexander Hlepas, Annas Rahman, Alexandra Sarau, Anna Zemke, Allison Zimmerman, Benjamin Schwartz, Clay Hoster, Gianna Bosco, Mary Potkonjak, Priya Patel, Tai Tri Nguyen, Vishal Birk. We are grateful for our colleagues across all specialties and fields at Rush University Medical Center who work tirelessly to implement the best possible care for our patients with COVID-19.
Footnotes
Presented at the American Heart Association: Scientific Sessions, Virtual Conference, November 2020.
References
- 1.Agricola E., Beneduce A., Esposito A., Ingallina G., Palumbo D., Palmisano A., et al. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc. Imaging. 2020;13(8):1792–1808. doi: 10.1016/j.jcmg.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RC Rubin G.D., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., Schluger N.W., Volpi A., Yim J.J., IBK Martin, Anderson D.J., Kong C., Altes T., Bush A., Desai S.R., Goldin O., Goo J.M., Humbert M., Inoue Y., Kauczor H.U., Luo F., Mazzone P.J., Prokop M., Remy-Jardin M., Richeldi L., Schaefer-Prokop C.M., Tomiyama N., Wells A.U., Leung A.N. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology. 2020;296(1):172–180. doi: 10.1148/radiol.2020201365. Epub 2020 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson S.K.F., Abbara S., Bhalla S., Chung J.H., Chung M., et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol. Cardiothorac. Imaging. 2020;2(2) doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L., et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020;12:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante G., Fazzari F., Cozzi O., Maurina M., Bragato R., D'Orazio F., et al. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc. Res. 2020;116(14):2239–2246. doi: 10.1093/cvr/cvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutte H., Mortensen J., Mork M.L., Kristoffersen U.S., Jensen C.V., Petersen C.L., et al. Non-ECG-gated CT pulmonary angiography and the prediction of right ventricular dysfunction in patients suspected of pulmonary embolism. Clin. Physiol. Funct. Imaging. 2017;37(6):575–581. doi: 10.1111/cpf.12325. [DOI] [PubMed] [Google Scholar]
- 9.He H.S.M., Zalta B., Haramati L.B. Computed tomography evaluation of right heart dysfunction in patients with acute pulmonary embolism. J. Comput. Assist. Tomogr. 2006;30(2):262–266. doi: 10.1097/00004728-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Contractor S.M.P., Sharma V.K., Gor D.M. Role of helical CT in detecting right ventricular dysfunction secondary to acute pulmonary embolism. J. Comput. Assist. Tomogr. 2002;26:587–591. doi: 10.1097/00004728-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Ghuysen A., Ghaye B., Willems V., Lambermont B., Gerard P., Dondelinger R.F., et al. Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax. 2005;60(11):956–961. doi: 10.1136/thx.2005.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 2018;72(4):434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan,China. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakkar A.N., Tea I., Al-Mallah M.H. Cardiovascular implications of COVID-19 infections. Methodist Debakey Cardiovasc. J. 2020;16(2):146–154. doi: 10.14797/mdcj-16-2-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19.Kang D.K., Thilo C., Schoepf U.J., Barraza J.M., Jr., Nance J.W., Jr., Bastarrika G., et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc. Imaging. 2011;4(8):841–849. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Fervers P., Kottlors J., Zopfs D., Bremm J., Maintz D., Safarov O., et al. Calcification of the thoracic aorta on low-dose chest CT predicts severe COVID-19. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc. Imaging. 2020;13(11):2468–2470. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosyns B., Motoc A., Luchian M.L., Lochy S., Belsack D. Coronary calcium score in COVID-19 hospitalized patients. JACC Cardiovasc. Imaging. 2020;13(12):2698. doi: 10.1016/j.jcmg.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombi D., Villani G.D., Maffi G., Risoli C., Bodini F.C., Petrini M., et al. Qualitative and quantitative chest CT parameters as predictors of specific mortality in COVID-19 patients. Emerg. Radiol. 2020;27(6):701–710. doi: 10.1007/s10140-020-01867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matos J., Paparo F., Mussetto I., Bacigalupo L., Veneziano A., Perugin Bernardi S., et al. Evaluation of novel coronavirus disease (COVID-19) using quantitative lung CT and clinical data: prediction of short-term outcome. Eur. Radiol. Exp. 2020;4(1):39. doi: 10.1186/s41747-020-00167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.