Abstract
Molecular mechanisms of malignant transformation in spinal cord gliomas are not well-understood. Our objective was to investigate genetic causes of malignant transformation in a primary spinal cord glioma. A 32-year-old female patient presented with bilateral lower extremity weakness and was diagnosed with a primary spinal cord glioma from T9 to T12, with a syrinx extending from the craniocervical junction to the conus. She underwent resection in 2006. Pathology showed an abundance of Rosenthal fibers, calcification and degenerative features consistent with a low-grade pilocytic astrocytoma. She presented in 2020 with tumor recurrence and underwent re-resection. Whole exome sequencing, DNA methylation profiling and immunohistochemistry were performed on her initial and recurrent tumor samples. Immunohistochemical profiling of her recurrent tumor showed pleomorphic cells with extensive necrosis consistent with a high-grade glioma. DNA methylation profiling showed that the initial tumor clustered with pilocytic astrocytomas, whereas the recurrent lesion clustered with anaplastic astrocytomas, confirming malignant transformation. Whole-exome sequencing showed interim acquisition of a rare fibroblast growth factor receptor-transforming acidic coiled-coil (FGFR1-TACC1) gene fusion. We report an FGFR1-TACC1 fusion associated with malignant transformation in a primary spinal cord glioma. Our study adds to growing reports of FGFR-TACC fusions, which are amenable to receptor tyrosine kinase inhibition.
Keywords: Fibroblast growth factor receptor fusion (FGFR fusion), spinal cord glioma, malignant transformation, case report
Introduction
Primary spinal cord gliomas constitute roughly 6–8% of all spinal cord tumors (1). Of these, 25% are high grade (WHO grade III and above) (2). Primary high-grade gliomas of the spine are very rare and carry a dismal prognosis with median survival duration ranging from 12 to 14 months (3). Very little is known about their pathogenesis. Malignant transformation occurs in up to 20% of intracranial low-grade gliomas (4), however its underlying mechanisms are poorly understood.
In this paper we present a patient with primary low-grade spinal cord glioma resected in 2006 with recurrence in 2020 as a high-grade glioma. Sequencing of her tumor from 2006 compared to 2020 showed interim acquisition of an FGFR1-TACC1 fusion. This finding has therapeutic implications for this subset of very aggressive tumors.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/jss-21-24).
Case presentation
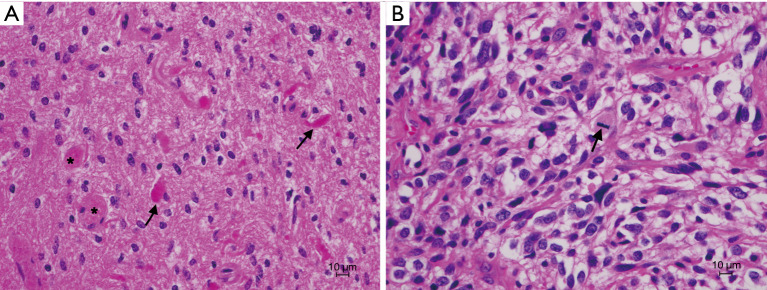
A 32-year-old female underwent tumor resection for an intramedullary thoracic mass in 2006. Her presenting symptom was bilateral lower extremity weakness. Magnetic resonance imaging (MRI) imaging showed a hyperintense lesion from T9 to T12, and a syrinx extending from the craniocervical junction to the conus. Pathology showed an abundance of Rosenthal fibers, calcification and frank degenerative features. Immunohistochemistry detected plasmacytic cells and infiltrating macrophages with occasional accumulation of fibrillary astrocytes, consistent with a diagnosis of pilocytic astrocytoma, WHO grade I (Figure 1A).
Figure 1.
H&E stain of patient’s tumor from 2006 (A) compared to 2020 (B). (A) Pilocytic astrocytoma with low cellularity and scattered piloid cells. Rosenthal fibers (arrows) and eosinophilic granular bodies (stars) are present. (B) Recurrent tumor showing increased cellularity and larger pleomorphic elements. Mitotic figures are present (arrow). H&E, hematoxylin and eosin.
She presented in 2020 with exophytic tumor recurrence and underwent partial re-resection for tumor debulking. Pathology (Figure 1B) showed a pleomorphic neoplasm admixed with extensive necrosis, hemorrhage and hyalinized vessels. The neoplastic cells had marked nuclear atypia and exhibited elongated and rounded shape, including some giant bizarre and rhabdoid cells. Microvascular proliferation was noted. Tumor cells were strongly positive for glial fibrillary acidic protein (GFAP), and negative for mutant isocitrate dehydrogenase 1 (IDH1), R132H, BRAF V600E and H3K37M, consistent with a high-grade glioma.
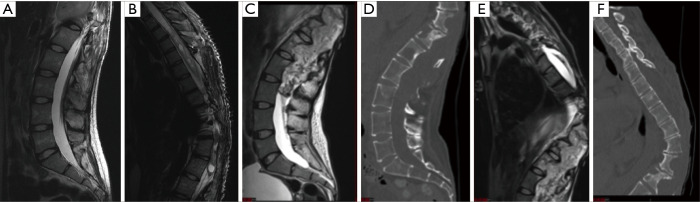
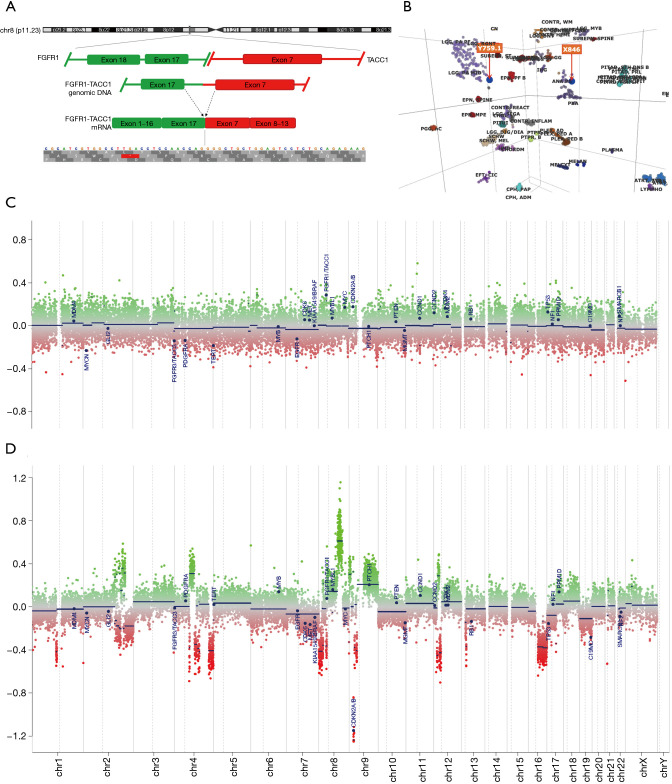
Radiological evaluation from 2006 (Figure 2A,2B) compared to 2020 (Figure 2C-2F) showed progression to a fungating lesion with extensive soft-tissue invasion and bony remodeling. Next-generation sequencing was performed on her tumor from 2020 compared to 2006. Genomic DNA and RNA were extracted from 5-µm sections of formalin fixed paraffin embedded tissue sections using the AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany). Next-generation sequencing was performed using a custom amplicon-based brain tumor specific panel (PBTP) using an Ion S5™ XL Sequencing System (Thermo Fisher Scientific) at minimum depth of 100× (5). She was found to have acquired a fusion of FGFR1-TACC1 (Figure 3A). DNA methylation assays from the 2006 and 2020 specimens were performed using the Infinium MethylationEPIC platform (Illumina, USA) and showed that her initial tumor clustered with pilocytic astrocytomas, whereas the recurrent tumor clustered with anaplastic astrocytomas (Figure 3B). Several interim copy number alterations were also observed including loss of TP53 and CDKN2a (Figure 3C,3D). Final pathology was consistent with WHO grade III high-grade glioma. Post-operatively she continued radiation treatment and made a satisfactory post-operative recovery. However, she was transitioned to hospice care and succumbed to her disease 5 months after surgery.
Figure 2.
Imaging findings from 2006 (A,B) compared to 2020 (C-F). (A,B) T2-weighted MRI imaging of the lumbar (A) and thoracic spine (B) from 2006. (C-F) T2-weighted MRI imaging of the lumbar (C) and thoracic spine (E) and CT imaging of the lumbar (D) and thoracic spine (F) from 2020. Images show interim growth of a fungating exophytic mass from T10 to L4 involving the thecal sac, posterior spinal elements and soft tissues of the back and skin (C,E) associated with a syrinx (E). CT imaging from 2020 (D,F) shows bony erosion of posterior as well as anterior spinal elements. CT, computed tomography; MRI, magnetic resonance imaging.
Figure 3.
Genomic and epigenomic tumor characterization. (A) Next-generation sequencing showing fusion of FGFR1-TACC1 on chr8. (B) DNA methylation profiling shows that tumor from 2006 (Y759.1) clustered with pilocytic astrocytomas whereas tumor from 2020 (X846) clustered with anaplastic astrocytomas. (C,D) Interim acquisition of hotspots for DNA copy number gain or loss between 2006 (C) and 2020 (D).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Virginia Institutional Review Board and with the Declaration of Helsinki (as revised in 2013). Written informed consent was not required for retrospective analysis of de-identified patient data. The patient was deceased at the time of the writeup therefore it was not possible to seek her consent.
Discussion
Targeted therapy against receptor tyrosine kinases has been successfully used for BCR-ABL fusions in chronic myelogenous leukemia, PML-RARA fusions in pro-myelocytic leukemia and EML4-ALK fusions in non-small cell lung cancer (6). FGFR-TACC fusions are seen in ~3% of human glioblastoma multiforme (7) but also in IDH wild-type gliomas (WHO grades II and III), urothelial carcinoma, papillary renal carcinoma, non-small cell lung cancer, cholangiocarcinoma and nasopharyngeal carcinoma. FGFR-TACC results from an in-frame duplication with inversion leading to fusion of the FGFR N-terminus with the TACC C-terminus (7). An FGFR1-TACC1 gene fusion was recently reported in association with malignant transformation in a spinal cord pilocytic astrocytoma while this manuscript was under review (8). Another recent series also identified FGFR1-TACC1 fusions in 3 out of 26 patients (2 pilocytic astrocytomas and 1 rosette-forming glioneuronal tumor) (9). Our findings corroborate these recent studies and suggest that FGFR-TACC fusions in spinal cord gliomas are likely under-reported.
Binding of FGF to its receptor leads to activation of downstream RAS/mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling, ultimately resulting in proliferation, differentiation and survival via incompletely understood mechanisms (10). Similarly to other fusion proteins, FGFR-TACC fusions contain a constitutively active receptor tyrosine kinase which leads to unchecked signaling. Furthermore, the TACC coiled-coil domain promotes dimerization of the fusion protein leading to autophosphorylation and further receptor activation. Patients with FGFR-TACC fusion-driven high-grade gliomas are amenable to receptor tyrosine kinase inhibitor treatment.
Following early success in pre-clinical and phase I trials, several advanced-stage trials are ongoing for receptor tyrosine kinase inhibitors targeting FGFR such as Infigratinib for patients with FGFR-TACC fusions. Phase II results of Infigratinib in patients with recurrent high-grade gliomas showed partial response or stable disease in roughly one-third of patients with FGFR alterations (11). Unfortunately, our patient transitioned to hospice care and expired before her final diagnosis and trial eligibility were determined. Of note, we also observed copy number variations in her recurrent tumor including loss of TP53 and CDKN2a which have been implicated in several tumor types. Further studies are needed to directly establish a causal role for FGFR1-TACC1 fusions in malignant transformation.
Summary and conclusions
In this paper we describe a patient with malignant transformation of a primary spinal cord glioma associated with a rare FGFR1-TACC1 fusion. Patients with this fusion may be amenable to personalized chemotherapy using small molecule receptor tyrosine kinase inhibitors targeting FGFR. This study is limited by retrospective analysis of single patient data.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Virginia Institutional Review Board and with the Declaration of Helsinki (as revised in 2013). Written informed consent was not required for retrospective analysis of de-identified patient data. The patient was deceased at the time of the writeup therefore it was not possible to seek her consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/jss-21-24
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jss-21-24). The authors have no conflicts of interest to declare.
References
- 1.Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus 2015;39:E14. 10.3171/2015.5.FOCUS15158 [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep 2011;11:320-8. 10.1007/s11910-011-0190-2 [DOI] [PubMed] [Google Scholar]
- 3.Bowers DC, Weprin BE. Intramedullary Spinal Cord Tumors. Curr Treat Options Neurol 2003;5:207-12. 10.1007/s11940-003-0011-2 [DOI] [PubMed] [Google Scholar]
- 4.Murphy ES, Leyrer CM, Parsons M, et al. Risk Factors for Malignant Transformation of Low-Grade Glioma. Int J Radiat Oncol Biol Phys 2018;100:965-71. 10.1016/j.ijrobp.2017.12.258 [DOI] [PubMed] [Google Scholar]
- 5.Raffeld M, Abdullaev Z, Pack SD, et al. High level MYCN amplification and distinct methylation signature define an aggressive subtype of spinal cord ependymoma. Acta Neuropathol Commun 2020;8:101. 10.1186/s40478-020-00973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambacorti-Passerini C., Part I: Milestones in personalised medicine--imatinib. Lancet Oncol 2008;9:600. 10.1016/S1470-2045(08)70152-9 [DOI] [PubMed] [Google Scholar]
- 7.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231-5. 10.1126/science.1220834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daoud EV, Patel A, Gagan J, et al. Spinal Cord Pilocytic Astrocytoma With FGFR1-TACC1 Fusion and Anaplastic Transformation. J Neuropathol Exp Neurol 2021;80:283-5. 10.1093/jnen/nlaa122 [DOI] [PubMed] [Google Scholar]
- 9.Perwein T, Benesch M, Kandels D, et al. High frequency of disease progression in pediatric spinal cord low-grade glioma (LGG): management strategies and results from the German LGG study group. Neuro Oncol 2021;23:1148-62. 10.1093/neuonc/noaa296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasorella A, Sanson M, Iavarone A. FGFR-TACC gene fusions in human glioma. Neuro Oncol 2017;19:475-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassman AB, Sepúlveda-Sánchez JM, Cloughesy T, et al. OS10.6 Infigratinib (BGJ398) in patients with recurrent gliomas with fibroblast growth factor receptor (FGFR) alterations: a multicenter phase II study. Neuro Oncol 2019;21:iii21-2. 10.1093/neuonc/noz126.072 [DOI] [Google Scholar]