Abstract
Purpose
One of the essential goals regarding the successful control of rabies infection is the development of a safe, effective, and inexpensive vaccine. the current study aimed to evaluate the inactivation potential of β-propiolactone (βPL), binary ethyleneimine (BEI), and hydrogen peroxide (H2O2).
Materials and Methods
Estimating the inactivation kinetics of βPL, BEI, and H2O2 revealed that the tested inactivants could completely and irreversibly inactivate rabies virus within 2, 12, and 4 hours, respectively while maintaining its viral immunogenicity. The potency of βPL, BEI, and H2O2 inactivated vaccines was higher than the World Health Organization acceptance limit and were in the order of 3.75, 4.21, and 3.64 IU/mL, respectively. Monitoring the humoral and cellular immunity elicited post-immunization using Staphylococcus aureus derived hyaluronic acid (HA) and bacillus Calmette-Guérin purified protein derivative (PPD) adjuvanted rabies vaccine candidates were carried out using enzyme-linked immunosorbent assay.
Results
Results demonstrated that both adjuvants could progressively enhance the release of anti-rabies total immunoglobulin G as well as the pro-inflammatory mediators (interferon-gamma and interleukin-5) relative to time. However, a higher immune response was developed in the case of HA adjuvanted rabies vaccine compared to PPD adjuvanted one. The harmful consequences of the tested adjuvants were considered via investigating the histopathological changes in the tissues of the immunized rats using hematoxylin and eosin stain. Lower adverse effects were observed post-vaccination with HA and PPD adjuvanted vaccines compared to that detected following administration of the currently used alum as standard adjuvant.
Conclusion
Our findings suggested that HA and PPD could serve as a promising platform for the development of newly adjuvanted rabies vaccines with elevated immune enhancing potentials and lower risk of health hazards.
Keywords: β-Propiolactone, Binary ethyleneimine, Immune enhancers, Hyaluronic acid, Rabies vaccines
Introduction
Rabies is a global zoonotic disease of major public health and economic significance. It remains one of the most serious neglected viral diseases with a case fatality rate of about 100% [1]. Despite the presence of post-exposure prophylaxis, rabies infection resulted in approximately 59,000 deaths worldwide each year with the majority of cases in Africa and Asia. That’s one person dies every 9 minutes due to rabies infection every day, 40% of whom are mostly children [2]. It causes severe economic loss in many developing countries approaching 5.5 billion dollars per year. Rabies infection is accompanied by acute encephalomyelitis that progresses rapidly to coma or death within days after the onset of symptoms [3]. Even though the international efforts to produce an effective rabies vaccine, the disease has not been eliminated as it has many animal reservoirs [4]. There is always an attempt to formulate an affordable and safe rabies vaccine via the use of inactivated rabies virus [5]. β-propiolactone (βPL) is the currently recommended inactivating agent for rabies virus, where it maintains the viral antigenicity compared to other chemicals, such as formaldehyde and phenol [6]. However, βPL is a potentially carcinogenic and expensive inactivant, thus it is important to find other inactivating agents which are safe, cheap, and available [7].
Although the mass production of cell culture based inactivated rabies vaccines is costly, but it is mostly used worldwide [8]. Additionally, there is always a need to improve the immune-enhancing potentials of the prepared cell culture-based rabies vaccines in developing countries via the use of adjuvants such as alum [9]. In alum adjuvanted vaccines the antigen becomes readily absorbed by the subject and more immunogenic [10]. On the other side, searching for an alternative adjuvant to alum is an essential issue in order to overcome its well-known side effects [9]. Attention has now been directed to the enhancement of immunogenicity of viral vaccines via the development of bio-adhesive delivery systems using natural constituents, such as lectins and fimbrial proteins. These bio-adhesives attach to mucosal cell surfaces through receptor-mediated mechanisms. Hyaluronic acid (HA) is an example of these natural bio-adhesives which has the potential in developing an effective immune response. It was found that the combination of the antigen with either esterified HA polymers or auto-cross-linked HA polymers resulted in significant improvement in the immune response [11]. Among other immunostimulants, it was also demonstrated that bacillus Calmette-Guérin (BCG) is a live attenuated tuberculosis vaccine that has the potential to stimulate non-specific cross-protection against different pathogens as it improves the antigen recognition and induces rapid inflammatory response [12]. Consequently, the current study aimed to evaluate the inactivation potential of βPL, binary ethyleneimine (BEI), and H2O2. Monitoring the humoral and cellular immune response elicited post-vaccination using Staphylococcus aureus derived HA and BCG purified protein derivative (PPD) adjuvanted rabies vaccines. In addition to assessment of histopathological changes associated with the administration of these vaccines in laboratory animals.
Materials and Methods
Ethical approval
All procedures involving animals followed the regulations approved by the Egyptian Network of Research Ethics Committee which was created in 2008. An ethical approval for the present study protocol was granted by the Holding Company for Biological Products and Vaccines, Sera, and Drugs (VACSERA) ethical committee, Cairo, Egypt (approval no., 0728019157). The scarified and dead animals were decanted according to the safety and occupational health guidelines. The study sticked to the National Institute of Health (NIH) regulations regarding the animal care and the use program of laboratory animals. There are no human subjects in this study and informed consent is unapplicable.
Laboratory animals
Five suckling mice families and 20 adult mice were used for evaluation of the safety profiles of the prepared vaccines. Five groups of 16 adult Swiss albino male mice of 4–6 weeks old (14–16 g) were used for testing the potency of each vaccine. Three groups of 120–150 g Sprague Dawley rats (10 rats/group) were immunized according to a predetermined schedule to estimate the level of the developed immune response.
Rabies virus
Fixed rabies virus (FRV/K2) was kindly supplied by el-Karamany [13] (former general manager of research and development sector), the VACSERA, Egypt. Rabies virus was adapted to Vero cells (ATCC [American Type Culture Collection] CCL-81) by alternating 37 passages in mice and Vero cells (20 cycles in mice and 17 passages in Vero cells). The adapted viral strain was used to prepare the test vaccine and recorded a viral titer of 7.5 log10 MICLD50 (mouse intracerebral lethal dose 50%)/mL.
Inactivants
Binary ethylene amine and hydrogen peroxide were kindly supplied from Sigma-Aldrich (Burlington, MA, USA). Binary ethylene amine was converted into BEI using 0.2 N NaOH and finally used as 0.001 M. H2O2 was used as 0.3 M, whereas βPL was used at a final concentration of 0.0035 M.
Reference rat anti-rabies immunoglobulin G
Rat anti-rabies immunoglobulin G (IgG) was kindly supplied from VACSERA and it was used as 6.5 IU/mL.
Vaccine preparation
Rabies vaccine was prepared using Vero cells, where exhausted growth medium was decanted from tissue culture flasks and rabies virus was inoculated at one multiplicity of infection/mL. Flasks were incubated at 37°C (Jouan, Saint-Herblain, France) for 1–1.5 hours and shaken at 15-minute intervals to ensure the even distribution of the virus on the cultured area. Maintenance medium was added to the infected flasks and cells were examined daily for detection of morphological changes using inverted microscope (Helmut Hund GmbH, Wetzlar, Germany). On the third day, the infection medium was harvested and replaced with fresh medium. Medium containing cell free virus was harvested at 4–5 days interval. On the 15th–20th day, the cell associated virus was also collected through 3 cycles of freezing and thawing and cold centrifuged (Jouan). Cell pellets were decanted. The infectivity titer of the pooled viral harvests (cell free and cell associated virus) was evaluated using mice inoculation assay. Viral suspension was concentrated by the aid of hollow fiber cartridge ultra-filtration system [9].
Virus inactivation
The previously prepared bulk virus was divided into three aliquots. Chemical inactivants such as βPL, BEI, and H2O2 were added to each bulk at a final concentration of 0.0035 M for βPL, 0.001M for BEI, and 0.3 M for H2O2. Treated viral bulks were incubated at 37°C for 24 hours and samples were collected for evaluation of the inactivation kinetics according to the designed protocol. Samples were collected at predetermined time interval and were 10-fold serially diluted in Hanks’ balanced salt solution. Each dilution (0.03 mL/mouse) was inoculated intracerebrally in Swiss albino male mice of 15–17 g body weight (6 mice/dilution). Mice mortality was recorded for 14 days post-injection keeping in consideration that the number of deaths was recorded starting from the 5th day post-inoculation. The viral infectivity titer was determined according to Reed and Muench [14] in 1938. The presence of residual living virus was traced via intracerebrally injecting the inactivated virus in 5 suckling mice families and 20 adult mice. The vaccine was considered safe on condition that all inoculated mice remained alive for a period of 28 days post-intracerebral inoculation [6].
Adjuvants
S. aureus derived HA and PPD of BCG were kindly supplied from VACSERA. PPD was routinely produced in BCG department (Tuberculin PPD RT23 “SSI”), batch no. A25/2020 and its release was based on certified quality control procedures. While HA was not a routine production line, but it was prepared as the research and development sector’s self-activity and its purity was examined using gel electrophoresis to detected clear sole bands with an average molecular weight of about 700 kDa. Both adjuvants were mixed with the prepared vaccines at a final concentration of 10 µg/mL and incubated over night at 4°C.
Vaccine potency
Prepared vaccine candidates were 5-fold serially diluted in phosphate buffer saline. Each dilution (0.3 mL) was intraperitoneally (IP) injected into 16 adult Swiss albino male mice of 4–6 weeks old and weighing 14–16 g. Injection was carried out at zero time and a second dose was administered on the 7th day post-prime vaccination. On the 14th day, vaccinated mice were challenged intracerebrally using 50 LD50 (lethal dose 50%) of challenge virus standard kindly supplied from rabies vaccine research unit, VACSERA. Mortality was recorded starting from the 5th to the 15th day post-challenge. ED50 (the effective dose that protects 50% of challenged mice) of test vaccines was determined according to the NIH protocol [15].
Immune response
Three groups of 120–150 g body weight Sprague Dawley strain of albino rats (10 rats/each) were immunized via IP injection of five doses of adjuvanted test vaccines at days 0, 3, 7, 14, and 28. Blood samples were collected from the retro orbital plexus of the vaccinated rats at predetermined time intervals and incubated at 37°C till complete coagulation. Clotted blood samples were cold centrifuged (Jouan) to separate immune sera and stored at −80°C until use. Evaluation of humoral immunity was carried out via assessment of rabies antibody level in the collected sera using home reference enzyme-linked immunosorbent assay (ELISA) kit. Whereas, cellular immunity was traced based on evaluation of interfer on gamma (IFN-γ) and interleukin type 5 (IL-5) levels according to the manufacturer’s protocols (BD Biosciences Pharmingen, San Jose, CA, USA) [9].
Histopathological changes
Hematoxylin and eosin staining of tissue sections was carried out to detect histopathological changes occurring in adult male rats immunized with HA, PPD, and alum adjuvanted rabies vaccines compared to unadjuvanted vaccine. At the end of the immunization schedule (10 days after last dose), rats were sacrificed. The kidney and the liver were obtained from dissected animals and washed in normal saline followed by 10% neutral buffered formalin. Specimens were obtained from each of the dissected organs and washed with tap water followed by dehydration for 30 minutes suing serially diluted ethanol (50%, 60%, 70%, 85%, and 95%). Specimens were incubated in 1:1 mixture of ethanol and xylene for 30 minutes, washed twice using xylene for 1 hour and transferred to xylene and paraffin mixture for 30 minutes. Transverse sections (5 mm) were dewaxed at 60°C, immersed in xylene for 1 hour, rehydrated using a series of ethanol (95%, 80%, 70%, and 60%) for 2 minutes, and washed using tap water. Sections were stained using hematoxylin and eosin. Slides were mounted using Entellan embedding agent, covered with coverslips, and examined using a light microscope connected to a digital camera [16].
Statistical analysis
All experiments were performed in three independent tests. Data were presented as the mean±standard deviation and analyzed using one-way analysis of variance and Student t-test. The results were considered statistically significant at probability less than 0.05.
Results
Inactivation kinetics
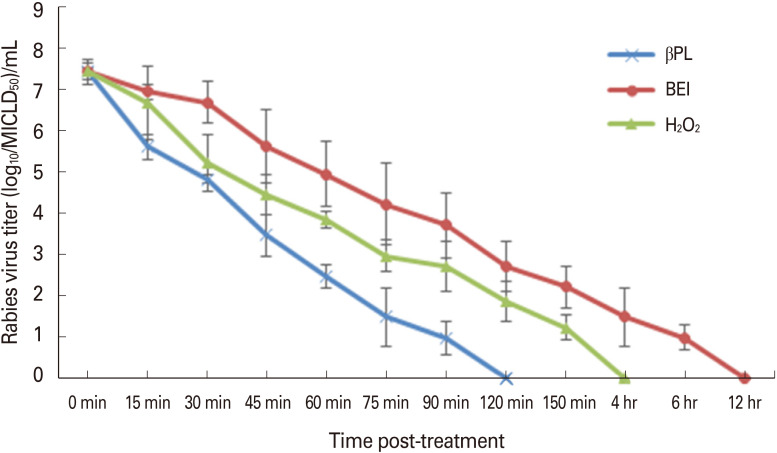
Data recorded revealed that βPL and H2O2 showed faster inactivation potentials compared with that of BEI. The recorded mean depletion rate of rabies virus infectivity titer was in the order of 4.3, 3.2, and 2.5 log10/hr post-treatment with βPL, H2O2, and BEI, respectively. It was clear that the inactivation potential of βPL was significantly elevated (p<0.05) compared to that of H2O2 and BEI, where the virus was completely inactivated within 2 hours. Whereas, H2O2 and BEI completely inactivated the virus within 4 and 12 hours, respectively. In the meantime, no residual living virus was detected during the observation period (28 days) post-inoculation of the inactivated virus using the tested inactivants (Fig. 1).
Fig. 1. Inactivation kinetics of rabies virus stocks using β-propiolactone (βPL), binary ethyleneimine (BEI), and hydrogen peroxide (H2O2) relative to time post-treatment using mice inoculation assay. MICLD50, mouse intracerebral lethal dose 50%.
Vaccine potency
Regarding the inactivation efficacy and related vaccine potency, it was noticed that the prepared rabies vaccines using the tested inactivants were potent according to the World Health Organization (WHO) recommendations; ED50 >2.5 IU/mL. There was non-significant difference (p>0.05) between the recorded ED50 (4.21±0.03 IU/mL) in case of BEI inactivated vaccine compared to that of βPL (3.75±0.06 IU/mL) and H2O2 (3.64±0.15 IU/mL) inactivated vaccine candidates.
Immune response
Evaluation of vaccine immunogenicity was based on the determination of the humoral immune response (total anti-rabies IgG) using direct ELISA compared with home reference serum (6.5 IU/mL). It was noticed that anti-rabies IgG was elevated relative to time and there was non-significant difference between the antibody levels in case of immunization with un adjuvanted BEI, βPL, and H2O2 inactivated vaccines (p>0.05) during the first 14 days. While on the 21st day post-vaccination, BEI and H2O2 inactivated vaccines showed significantly arising antibody levels (p<0.05) compared to that detected post-vaccination with βPL inactivated vaccine (Fig. 2A). Regarding the immunogenicity of adjuvanted vaccines, it was noticed that the HA adjuvanted βPL and H2O2 inactivated vaccines showed a significantly (p<0.01) elevated anti-rabies IgG as compared to that detected post-vaccination with PPD adjuvanted and unadjuvanted vaccine candidates (Fig. 2B, C). Similarly, HA adjuvanted BEI inactivated vaccine exhibited higher antibody level compared to PPD adjuvanted BEI inactivated vaccine (Fig. 2D).
Fig. 2. (A-D) Humoral immune response (total anti-rabies immunoglobulin G [IgG]) developed post-immunization with β-propiolactone (βPL), binary ethyleneimine (BEI), and hydrogen peroxide (H2O2) inactivated rabies virus and in combination with hyaluronic acid (HA) and purified protein derivative (PPD) adjuvanted vaccines. Statistically significant difference (*p<0.05). Statistically significant difference between HA adjuvanted vaccines compared to PPD and unadjuvanted vaccines (**p<0.01).
Concerning the cellular immune response detected post-vaccination with adjuvanted and unadjuvanted vaccine candidates, it was noticed that IFN-γ levels showed progressive elevated values relative to time. However, there was insignificant difference (p>0.05) in IFN-γ levels in the sera of the tested groups immunized using different adjuvanted inactivated vaccine candidates until the 14th day post-immunization. Further detection of IFN-γ levels revealed significant elevated IFN-γ levels in case of immunization with BEI inactivated vaccine compared to βPL and H2O2 inactivated vaccines, where they showed almost plateau shape followed by a declining phase during the 28th–35th day post-vaccination (Fig. 3A). On the other hand, the use of HA as an adjuvant in case of BEI and H2O2 inactivated vaccines resulted in a significant (p<0.01) elevation in IFN-γ levels compared to PPD adjuvanted one followed by unadjuvanted vaccines (Fig. 3C, D).
Fig. 3. (A-D) Evaluation of interferon gamma (IFN-γ) level in rat serum post-vaccination with hyaluronic acid (HA) and purified protein derivative (PPD) adjuvanted as well as unadjuvanted vaccines relative to time using enzyme-linked immunosorbent assay. Statistically significant difference (*p<0.05). Statistically significant difference between HA adjuvanted vaccines compared to PPD and unadjuvanted vaccines (**p<0.01).
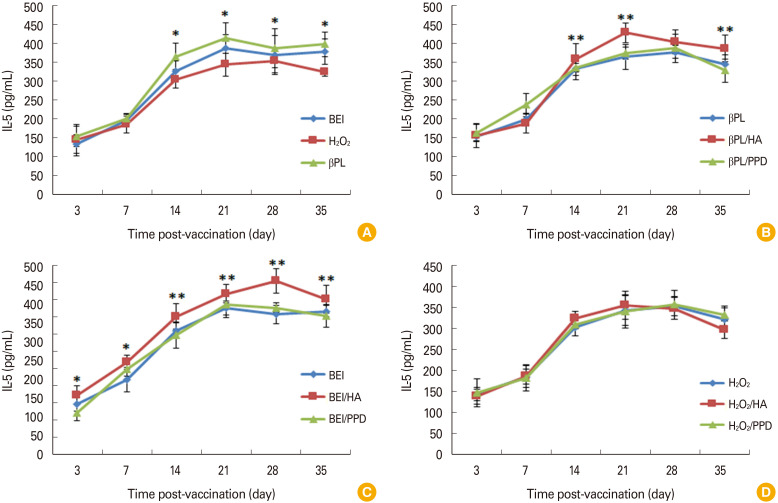
IL-5 showed a successive elevation post-immunization with unadjuvanted vaccines till the peak was detected between the 14th and 21st day post-vaccination with βPL, BEI, and H2O2 inactivated vaccines followed by a declining phase dur ing the 28th and 35th day in case of immunization with H2O2 inactivated vaccine. Higher IL-5 levels were detected post-immunization with unadjuvanted βPL and BEI inactivated vaccines compared to H2O2 inactivated one (Fig. 4A). It was also observed that IL-5 levels in HA adjuvanted vaccines either βPL or BEI inactivated were significantly (p<0.01) higher than that in case of PPD adjuvanted as well as in unadjuvanted vaccines (Fig. 4B, C).
Fig. 4. (A-D) Evaluation of interleukin type 5 (IL-5) level in sera samples of rats immunized with hyaluronic acid (HA) and purified protein derivative (PPD) adjuvanted and unadjuvanted vaccines relative to time using enzyme-linked immunosorbent assay. Statistically significant difference (*p<0.05). Statistically significant difference between HA adjuvanted vaccines compared to PPD and unadjuvanted vaccines (**p<0.01).
Histopathological changes
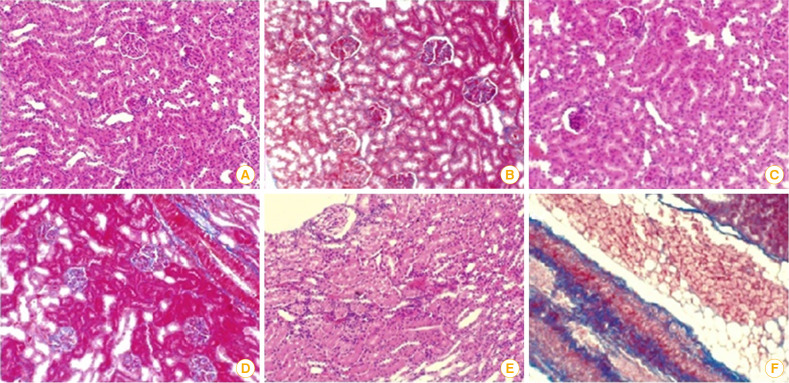
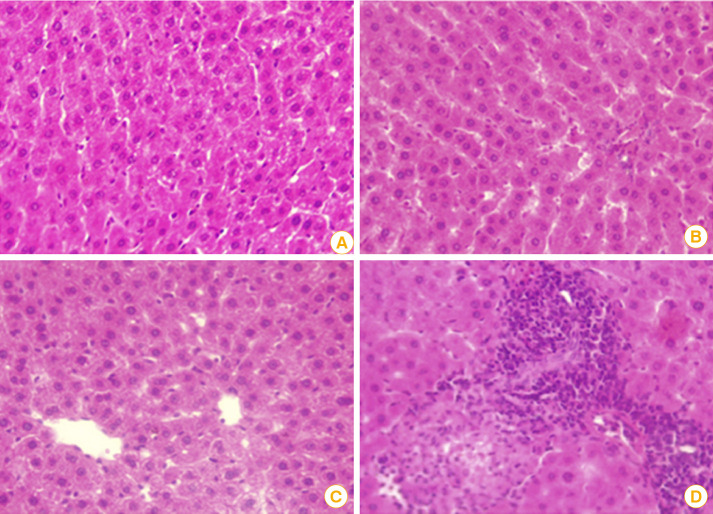
Hematoxylin and eosin-stained kidney sections of male rats post-immunization with HA and PPD adjuvanted rabies inactivated vaccine candidates showed that both HA and PPD adjuvanted vaccines showed almost normal kidney architecture as indicated by the normal appearance of glomerular space and renal tubules (Fig. 5C, D) in addition to normal liver cells and nuclei (Fig. 6B, C). However, kidney sections in rats administered alum as standard adjuvant showed infiltrated glomerular space and renal tubules as well as increase of connective tissue (Fig. 5E, F). Moreover, portal fibrosis, vacuolated cytoplasm, and cellular infiltration were apparent in hepatic sections of rats immunized with alum adjuvanted vaccine (Fig. 6D).
Fig. 5. (A) A photomicrograph of kidney section in adult male rat post-immunization with unadjuvanted vaccine showing normal histological architecture of kidney and normal renal corpuscles with normal tubules. (B) Kidney section following unadjuvanted vaccine administration showing normal renal tubular distribution of connective tissue. (C) Kidney section of hyaluronic acid adjuvanted vaccine post-administration in rat revealing a glomerular space with almost normal architecture. (D) Kidney section of male rat vaccinated with purified protein derivative adjuvanted vaccine demonstrating somewhat normal kidney with an obliterated glomerular space and renal tubules. (E) Kidney section post-immunization with alum adjuvanted rabies vaccine as standard adjuvant showing an obliterated and infiltrated glomerular space and renal tubules. (F) Kidney section post-alum adjuvanted vaccine administration demonstrating obliterated glomerular space and renal tubules as well as increase in connective tissue (H&E, ×400).
Fig. 6. (A) A photomicrograph of liver section of adult rat following administration of unadjuvanted vaccine showing histological architecture of normal liver lobular structure with central vein hepatocytes. (B) Liver section post-immunization with hyaluronic acid adjuvanted rabies vaccine illustrating a slight hepatic sinusoidal with almost normal liver architecture. (C) Liver section post-administration of purified protein derivative adjuvanted rabies vaccine showing somewhat normal liver cells and nuclei. (D) Hepatic section post-administration of alum adjuvanted vaccine revealing portal fibrosis, vacuolated cytoplasm, and cellular infiltration (H&E, ×400).
Discussion
Rabies is a fatal viral infection transmitted through the bites of rabid animals. If not prevented, rabies virus attacks the cells of the central nervous system leading to death [17]. Rabies infection is considered one of the important causes of human mortality despite the presence of effective prophylactic measures. This may be due to that the achievement of successful post-exposure prophylaxis against rabies is encountered by some problems such as the cost as well as other factors related to vaccine potency during its formulation [18]. Thus, the current study aimed to estimate the inactivation potentials of βPL, BEI, and H2O2 as the inactivation of rabies virus is considered a critical factor in the production of rabies vaccine. It was observed that the inactivation kinetics of βPL was comparable to that of H2O2, while BEI showed somewhat a delayed inactivation potential (12 hours). βPL, H2O2, and BEI recorded a mean depletion rate of rabies virus infectivity titer in the order of 4.3, 3.2, and 2.5 log10/hr, respectively till complete inactivation. Also, the potency of test vaccines was in the accepted range according to WHO recommendations (>2.5 IU/mL) and nearly similar (WHO, 1996). That was in agreement with another study, where it reported that H2O2 showed promising inactivation kinetics of rabies virus and the potency of H2O2 inactivated rabies vaccine was acceptable compared to that of βPL inactivated one. It was also reported that H2O2 is considered safe to the environment as it is decomposed to oxygen and water thus there is no need for complicated purification processes for its removal from the vaccine [19]. Contrary to our findings, another study pointed out that although βPL is a common inactivating agent but sometimes it failed to inactivate the virus completely. On the other hand, H2O2 retains its viral inactivation potential without causing destruction of the antigenic epitopes [10]. The present study also highlighted the effectiveness of BEI to inactivate rabies virus, where it completely inactivated the virus within 12 hours and the potency of the BEI inactivated vaccine was 4.2 IU/mL. In the same context, it was reported that BEI is an effective rabies virus inactivating agent that has an advantage over βPL, where BEI is more stable, inexpensive and could be easily prepared with less handling hazards. It was also found that although both βPL and BEI inactivate the virus through alkylation of the viral genome, BEI preserved the virus structure as it passes through the viral capsid and alkylates the viral genome. On the other side, modification of the viral proteins should be kept in mind when βPL is used as inactivating agent. Not only the ability of the inactivating agent to maintain the viral antigenicity is an essential goal during the preparation of viral inactivated vaccines, but also ensuring the absence of the residual living virus is another important point that should be taken into consideration [20]. Therefore, the current study proved the ability of the tested inactivants to completely inactivate rabies virus.
The immune response developed following vaccination goes hand in hand with the inactivation kinetics and safety concerns of viral vaccines. The immunogenic potential of cell culture derived rabies vaccines is commonly evaluated via determining the humoral response as indicated by measuring rabies virus neutralizing antibody titer developed post-immunization. Additionally, the detection of cytokines such as IFN-γ and interleukin-5 levels following vaccination is a respectable marker for investigating the cell mediated immune response, where during viral infection the CD4 T cells differentiate into either type 1 cytokine producing Th1 cells or type 2 cytokine producing Th2 cells. Th1 cells play an essential role in anti-viral immunity, where they secrete type 1 panel of cytokines including IFN-γ, IL-2, and tumor necrosis factor-α. These cells also stimulate the interaction between CD8 T cells and dendritic cells and aid B cells to release high affinity neutralizing antibodies. Whereas, Th2 cells secrete type 2 cytokines such as IL-4, IL-5, and IL-13 and promote the humoral immune response of B cells as well as the immunopathology during viral infection [21]. Concerning the effectiveness of different inactivants to enhance eliciting significant immune response, the current findings demonstrated that elevated anti-rabies IgG and IFN-γ levels were recorded post-immunization with BEI-inactivated vaccines compared to βPL and H2O2 inactivated vaccines. That was in accordance with a study which reported that most of BEI-inactivated viruses could elicit a protective immune response due to the induction of significant levels of neutralizing antibodies [20]. Another study related the level of the developed immune response to the denaturation potential of the tested inactivant on the viral epitopes, where they examined the effect of different inactivants on the epitopes of Rift Valley Fever (RVF) virus glycoproteins. It was demonstrated that BEI exhibited very low adverse effect on RVF epitopes, whereas βPL significantly altered seven epitopes and the least number of intact epitopes was accompanied by the highest immune response [22]. Induction of remarkable level of immune response is also critically influenced by the formulation of the vaccine, where higher amount of the released antibodies as well as longer-lasting protection could be achieved via the incorporation of an adjuvant during vaccine preparation [23]. Despite that alum is the first used adjuvant in licensed human vaccines, it has some disadvantages due to its carcinogenicity, exaggerated stimulatory effect in localized area and its inability to elicit an immune response of a weak antigen [24]. In the present study we explored the application of new adjuvants of different bacterial origins to compare their potentials to reach optimum and safe immune response against rabies infection. Regarding the recorded effectiveness of S. aureus derived adjuvants as an immunostimulant in rabies vaccine, it was reported that staphylococcus derived enterotoxin C2 could be applied as a successful adjuvant for rabies vaccine. This study demonstrated that mice immunized with staphylococcal enterotoxin C2 adjuvanted rabies vaccine exhibited elevated levels of anti-rabies IgG as well as IFN-γ and IL-4 producing cells as compared to unadjuvanted vaccine [25]. HA is a type of carbohydrate-based vehicle that attracted the attention of researchers to be studied as adjuvant and delivery system due to its immune modulating properties, biocompatibility, biodegradability as well as its low toxicity [26]. A study demonstrated that HA-coated nanoparticles could enhance the activation and maturation of dendritic cells. In addition to promoting the release of co-stimulatory molecules, they enhance antigen-specific CD4+ and CD8+ T-cell responses, cytokines, antigen-specific IgG antibody, and the generation of memory T-cells. The use of cationic liposome–HA hybrid nanoparticles as potential vaccine delivery system for intranasal vaccination with subunit antigens was also demonstrated [27] in addition to the successful application of HA as nanocarriers in non-invasive transdermal vaccination [28]. The immune enhancing potential of HA is attributed to its ability to deliver the antigens to desired body sites [27]. It was also reported that the HA polymers reduce the antigen clearance rate which subsequently extended the contact time between the antigen and the absorbing membrane. Moreover, an effective antigen transport occurs via HA induced widening at the tight cellular junctions [11]. Similarly, it was found that the co-delivery of BCG as an adjuvant in DNA vaccines resulted in an enhancement of the immune response in terms of the released immunoglobulins as well as the cell-mediated immunity [29].
Although the immune response developed post-immunization with adjuvanted vaccine is greater than that obtained in case of unadjuvanted one, the absolute safety of adjuvanted vaccines can’t be guaranteed. The major problems caused by administration of adjuvanted vaccines are the occurrence of inflammation, abscess or nodules at the injection site as well as fever and hypersensitivity in addition to other consequences due to the adjuvant’s toxicity to tissues or organs [30]. Thus, the present study investigated the histopathological changes developed post-immunization with the newly adjuvanted rabies vaccines (namely HA and PPD) compared to the currently used alum as a standard adjuvant as well as the unadjuvanted vaccine. The currently tested adjuvants were used at the lowest concentration (10 µg/mL) that could be applied to avoid any exaggerated inflammatory reactions induced in response to the administration of these adjuvants [31]. In coincidence with our findings, it was demonstrated that alum showed necrotic muscle fibers and granulomas at the injection site [32]. In addition, severe histological changes were observed post-administration of alum adjuvanted tetanus toxoid vaccine, where the kidney cells of the vaccinated mice showed mononuclear cellular infiltration, renal tubule vacuolation as well as congestion of peritubular capillaries [33]. On the contrary, the observed histopathological changes encountered with HA and PPD adjuvanted rabies vaccines were much lower than that in case of alum adjuvanted one. That may suggest lower adverse effects associated with the administration of these types of newly promising adjuvanted vaccines. To the best of our knowledge, this is the first report comparing the inactivation potentials of βPL, BEI, and H2O2 on rabies virus and demonstrating the immune potentials of HA and PPD as adjuvants in the preparation of rabies vaccine. The vaccines developed during the recent coronavirus disease 2019 (COVID-19) pandemic (especially the Chinese βPL inactivated-alum adjuvanted vaccine against COVID-19) is a strong reminder for the evolving need for developing an efficient and safe whole-virus-inactivated vaccine to target several viral antigens using different inactivating agents and adjuvants [34]. Despite that the present study was carried out on rabies virus not on coronavirus, the current findings might draw attention to BEI and H2O2 as potential immunogenic viral inactivating agents alternative to βPL. Simultaneously, exploring the efficiencies of new adjuvants (such as HA and BCG derived PPD) either for enhanced immune response or for lower adverse effects should be addressed.
In conclusion, finally, it is worth to point out that hydrogen peroxide could be applied as rabies virus inactivating agent without adversely affecting its immunogenic potentials. Additionally, the application of HA and BCG derived PPD as adjuvants could enhance the elicited humoral as well as the cell mediated immune response with lower adverse effects. However, more intensified studies regarding the application of these bacterial derived proteins as adjuvants should be conducted in comparison to the standard adjuvants such as alum with an investigation of the cellular immune reactivity to draw a whole picture for their role as adjuvants and related pathways affecting the immune system.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Masiira B, Makumbi I, Matovu JK, et al. Long term trends and spatial distribution of animal bite injuries and deaths due to human rabies infection in Uganda, 2001-2015. PLoS One. 2018;13:e0198568. doi: 10.1371/journal.pone.0198568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan B, Burke R, Bassaline D. Notes from the field: rabies exposures from fox bites and challenges to completing postexposure prophylaxis after Hurricane Irma: Palm Beach County, Florida, August-September 2017. MMWR Morb Mortal Wkly Rep. 2019;68:795–797. doi: 10.15585/mmwr.mm6836a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrell MJ. Developments in human rabies prophylaxis. Rev Sci Tech. 2018;37:629–647. doi: 10.20506/rst.37.2.2829. [DOI] [PubMed] [Google Scholar]
- 4.Fu ZF. Rabies and rabies research: past, present and future. Vaccine. 1997;15 Suppl:S20–S24. doi: 10.1016/s0264-410x(96)00312-x. [DOI] [PubMed] [Google Scholar]
- 5.Fisher CR, Schnell MJ. New developments in rabies vaccination. Rev Sci Tech. 2018;37:657–672. doi: 10.20506/rst.37.2.2831. [DOI] [PubMed] [Google Scholar]
- 6.Morgeaux S, Tordo N, Gontier C, Perrin P. Beta-propiolactone treatment impairs the biological activity of residual DNA from BHK-21 cells infected with rabies virus. Vaccine. 1993;11:82–90. doi: 10.1016/0264-410x(93)90343-v. [DOI] [PubMed] [Google Scholar]
- 7.Spaninger E, Bren U. Carcinogenesis of β-propiolactone: a computational study. Chem Res Toxicol. 2020;33:769–781. doi: 10.1021/acs.chemrestox.9b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandi S, Kumar M. Development in immunoprophylaxis against rabies for animals and humans. Avicenna J Med Biotechnol. 2010;2:3–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Dedloff MR, Effler CS, Holban AM, Gestal MC. Use of biopolymers in mucosally-administered vaccinations for respiratory disease. Materials (Basel) 2019;12:2445. doi: 10.3390/ma12152445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Chaphalkar SR. Vaccine adjuvants: the current necessity of life. Shiraz E-Med J. 2015;16:e28061 [Google Scholar]
- 11.Moon SH, Shin EC, Noh YW, Lim YT. Evaluation of hyaluronic acid-based combination adjuvant containing monophosphoryl lipid A and aluminum salt for hepatitis B vaccine. Vaccine. 2015;33:4762–4769. doi: 10.1016/j.vaccine.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Covian C, Fernandez-Fierro A, Retamal-Diaz A, et al. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.el-Karamany RM. Production in Vero cells of an inactivated rabies vaccine from strain FRV/K for animal and human use. Acta Virol. 1987;31:321–328. [PubMed] [Google Scholar]
- 14.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 15.Rooijakkers E, Groen J, Uittenbogarrd J, van Herwijnen J, Osterhaus A. Development and evaluation of alternative testing methods for the in vivo NIH potency test used for the quality control of inactivated rabies vaccines. Dev Biol Stand. 1996;86:137–145. [PubMed] [Google Scholar]
- 16.Eid RA, Ahmed Zaki MS, Alghamd MA, et al. Ameliorative effect of vitamin E on biochemical and ultrastructural changes in artemether-induced renal toxicity in rats. Int J Morphol. 2020;38:461–471. [Google Scholar]
- 17.Fooks AR, Cliquet F, Finke S, et al. Rabies. Nat Rev Dis Primers. 2017;3:17091. doi: 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 18.Ullas PT, Desai A, Madhusudana SN. Rabies DNA vaccines: current status and future. World J Vaccines. 2012;2:36–45. [Google Scholar]
- 19.Abd-Elghaffar AA, Ali AE, Boseila AA, Amin MA. Inactivation of rabies virus by hydrogen peroxide. Vaccine. 2016;34:798–802. doi: 10.1016/j.vaccine.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Delrue I, Verzele D, Madder A, Nauwynck HJ. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges. Expert Rev Vaccines. 2012;11:695–719. doi: 10.1586/erv.12.38. [DOI] [PubMed] [Google Scholar]
- 21.Venkataswamy MM, Madhusudana SN, Sanyal SS, et al. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin Exp Vaccine Res. 2015;4:68–74. doi: 10.7774/cevr.2015.4.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn NK, Besselaar TG. A study of the effect of chemical inactivants on the epitopes of Rift Valley fever virus glycoproteins using monoclonal antibodies. J Virol Methods. 1991;33:367–374. doi: 10.1016/0166-0934(91)90036-y. [DOI] [PubMed] [Google Scholar]
- 23.Clapp T, Siebert P, Chen D, Jones Braun L. Vaccines with aluminum-containing adjuvants: optimizing vaccine efficacy and thermal stability. J Pharm Sci. 2011;100:388–401. doi: 10.1002/jps.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao S, Li Y, Zhang Q, et al. Staphylococcal enterotoxin C2 as an adjuvant for rabies vaccine induces specific immune responses in mice. Pathog Dis. 2018;76:fty049. doi: 10.1093/femspd/fty049. [DOI] [PubMed] [Google Scholar]
- 26.Bashiri S, Koirala P, Toth I, Skwarczynski M. Carbohydrate immune adjuvants in subunit vaccines. Pharmaceutics. 2020;12:965. doi: 10.3390/pharmaceutics12100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Sahdev P, Ochyl LJ, Akerberg J, Moon JJ. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–129. doi: 10.1016/j.jconrel.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KS, Kim H, Park Y, et al. Noninvasive transdermal vaccination using hyaluronan nanocarriers and laser adjuvant. Adv Funct Mater. 2016;26:2512–2522. doi: 10.1002/adfm.201504879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Guo YJ, Sun SH, et al. DNA vaccination using bacillus Calmette-Guerin-DNA as an adjuvant to enhance immune response to three kinds of swine diseases. Scand J Immunol. 2005;62:371–377. doi: 10.1111/j.1365-3083.2005.01674.x. [DOI] [PubMed] [Google Scholar]
- 30.Principi N, Esposito S. Aluminum in vaccines: does it create a safety problem? Vaccine. 2018;36:5825–5831. doi: 10.1016/j.vaccine.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 31.Nies I, Hidalgo K, Bondy SC, Campbell A. Distinctive cellular response to aluminum based adjuvants. Environ Toxicol Pharmacol. 2020;78:103404. doi: 10.1016/j.etap.2020.103404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter D, Reed SG. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010;5:409–413. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issa AM, Salim MS, Zidan H, Mohamed AF, Farrag AR. Evaluation of the effects of aluminum phosphate and calcium phosphate nanoparticles as adjuvants in vaccinated mice. Int J Chem Eng Appl. 2014;5:367–373. [Google Scholar]
- 34.Sundaram AK, Ewing D, Liang Z, et al. Immunogenicity of adjuvanted psoralen-inactivated SARS-CoV-2 vaccines and SARS-CoV-2 spike protein DNA vaccines in BALB/c mice. Pathogens. 2021;10:626. doi: 10.3390/pathogens10050626. [DOI] [PMC free article] [PubMed] [Google Scholar]