Abstract
Background: Clinical studies have reported improved neurological outcomes in patients who were taking vitamin D supplements. This study investigates the effect of intramuscular (IM) vitamin D supplementation in patients with acute ischemic stroke (AIS) on neurological outcomes and inflammatory marker levels.
Methods: This study included patients diagnosed with AIS (n = 60) from the Neurology Unit of Loghman Hakim Hospital, Tehran, Iran, during the year 2019. Patients with AIS were allocated randomly into two groups who received a single dose of 300000 IU IM vitamin D and a control group that did not receive vitamin D supplementation. Serum vitamin D concentration, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) levels, as primary outcomes, and the Modified Rankin Scale (MRS), the National Institute of Health Stroke Scale (NIHSS), and the Mini-Mental State Examination (MMSE), as secondary outcomes, were measured at the baseline and the end of the study (6 weeks).
Results: Eventually, 59 patients with AIS completed the intervention study. A single dose of 300000 IU increased vitamin D level; moreover, vitamin D supplementation improved MRS and IL-6 levels significantly (P = 0.01, P = 0.02, respectively). There were reverse correlations between serum vitamin D and NIHSS and TNF-α after vitamin D administration. However, no statistically significant effect of vitamin D on the TNF-α or NIHSS and MMSE was seen compared to the control group.
Conclusion: Vitamin D probably due to a single dose and short duration of administration, as well as a short follow-up period, had no favorable effects on TNF-α level and NIHSS score.
Key Words: Vitamin D, Ischemic Stroke, Neurologic Manifestations, Inflammation, Cognitive Function
Introduction
Vitamin D deficiency is prevalent in patients with stroke, and as shown in previous studies, it preceded stroke. In previous meta-analyses, vitamin D deficiency was associated with an increased risk of stroke1 and poor functional outcome, as well as a rise in all-cause mortality of 1.86-fold and 3.56-fold, respectively.2 Recent studies have linked the 25-hydroxyvitamin D [25(OH)D] deficiency to functional impairments and poor survival outcomes.3,4 Vitamin D deficiency also increases the severity of stroke associated with an increase in the National Institute of Health Stroke Scale (NIHSS) index.5
Inflammation is considered to be a risk factor for ischemic strokes. Various literature proposes that vitamin D may exert anti-inflammatory markers, so that its deficiency is correlated with poorer outcomes in patients with acute stroke.6 The neuroprotective ability of pretreatment with 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] has been demonstrated in several in vivo and in vitro studies. In a rat model of cerebral ischemia caused by middle cerebral artery (MCA) ligation, pretreatment with 1,25(OH)2D for eight days was correlated with a significant decrease in ischemic brain infarction volume.7 A low serum level of vitamin D has been linked with cognitive decline;8 also, vitamin D deficiency in the short-term phase of ischemic stroke was associated with a higher incidence of 1-month cognitive impairment.9 Also, there is increasing evidence showing that vitamin D deficiency is linked to an increased risk of stroke “evolution”.10 A recent study showed a significant improvement in stroke patients’ outcome after administering vitamin D for three months. They concluded that screening for vitamin D deficiency in patients with stroke was essential, and vitamin D replenishment would improve the stroke outcome.11 In this study, vitamin D level was not measured at the time of assessing the outcome of stroke after three months and alteration in the inflammatory markers and cognitive impairment was not paid attention.
Randomized controlled trials (RCTs) are limited and have not been sufficiently driven to detect the association between vitamin D supplementation level and severity of the stroke; also, its inflammatory aspects are still unclear.11,12 To the best of our knowledge, there is no clinical trial study that has investigated the association between inflammatory markers, cognitive impairment, and vitamin D level. Hence, in this study, we attempted to define whether a single dose of vitamin D supplementation affected the tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) levels as the serum markers of inflammation as well as clinical status of patients with ischemic stroke.
Materials and Methods
Study design and ethics statements: The study has been approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethics committee number: IR.SBMU.RETECH.REC.1397.642). Furthermore, this double-blind randomized controlled clinical trial was registered in the Iranian Registry of Clinical Trials (number: IRCT20170104031773N2).
Sample size: G*Power software version 3.1 (Erdfelder, Faul, and Buchner, 1996) was employed to estimate the sample size. The below values were assumed to estimate the sample size. Effect size was equal to 1.65, obtained from previous studies,11 α error probability was equal to 0.05, and β error probability equal to 0.95. Finally, the sample size was estimated as 11 patients for each group. Regarding dropout (30%), the sample size was considered 30 patients per group.
Participants : A total of 60 patients with first-ever stroke were recruited from the Neurology Unit of Loghman Hakim Hospital, Tehran, from November 2018 to September 2019. Patients aged 40-70 years hospitalized within 24 hours after the onset of the stroke symptoms who had a deficiency of vitamin D were eligible for the study. Diagnosis of ischemic stroke was made on the basis of clinical presentation and neurologic examination and was confirmed by a brain computed tomography (CT) scan without contrast. Patients with a previous history of any types of stroke [intracerebral hemorrhage (ICH), hemorrhagic and ischemic infarct, or transient ischemic attack (TIA)], neurodegenerative diseases [Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS)], current mental or psychiatric disorders, malignancy, renal or liver diseases, a current history of alcohol, tobacco, and drug dependency, receiving tissue plasminogen activator (tPA), or thrombectomy were excluded from the study. The aphasic patients, patients with loss of consciousness, patients with severe stroke, and those with high NIHSS who could not participate in the Mini-Mental State Examination (MMSE) were also excluded. Each subject agreed and signed the written informed consent prior to participation in the study.
Intervention: Patients with serum 25(OH)D ≤ 20 ng/ml were categorized as deficient in vitamin D, 21-29 ng/ml as vitamin D insufficient, and ≥ 30 ng/ml as usual vitamin D level. The research included only patients with vitamin D deficiency. Participants were randomized into two groups using computer-generated block randomization. Group A received a single dose of intramuscular (IM) vitamin D (300000 IU). According to the Endocrine Society guidelines, a common approach for patients with severe vitamin D deficiency is to treat with 50000 IU of vitamin D2 or D3 orally once per week for six to eight weeks. The single IM dose of 300000 IU vitamin D equals to an oral dose of 50000 IU per week for six weeks.13 Group B did not receive a vitamin D supplement. Routine medical treatment of acute ischemic stroke (AIS) and physiotherapy sessions were continued in both groups. The final follow-up visit was carried out six weeks after discharge.
Primary and secondary outcomes : A detailed clinical examination and medical history were taken on the first visit. The quantitative analysis of serum vitamin-D was determined by high-performance liquid chromatography (HPLC) (Agilent-1100 series, Germany). The time it took to obtain a response was 24 hours. Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and TNF-α were used from Diaclone Company, France. Whole-blood samples were collected in the early morning after overnight fasting. The samples were stored for 15 hours at room temperature and then centrifuged at 1000 × g for 20 minutes. The supernatant was then separated and stored at -70 °C until testing. Repeated freezing and thawing were avoided for all samples. The procedures for the measurement of IL-6 and TNF-α level followed the instructions provided by the kit manufacturers. The clinical consequences were measured through NIHSS, Modified Rankin Scale (MRS), and MMSE scales as secondary outcomes. All measurements were performed at the initiation and termination of the follow-up by trained neurologists blinded to the subject in a neurology clinic (6 weeks after patient discharge).
The SPSS software (version 22, IBM Corporation, Armonk, NY, USA) was used to analyze data. We used the Kolmogorov-Smirnov test (K-S test) to assess the normal distribution of variables. Numeric and categorical variables were expressed as mean and standard deviation (SD) and frequency and percentage, respectively. Independent samples t-test and chi-square test were performed to explore the differences between the treatment and placebo groups in quantitative and categorical data. Furthermore, within-group differences before and after the intervention were assessed by the paired t-test. The results were expressed as 95% confidence intervals (CIs), and P-values of < 0.05 were considered statistically significant in all tests.
Results
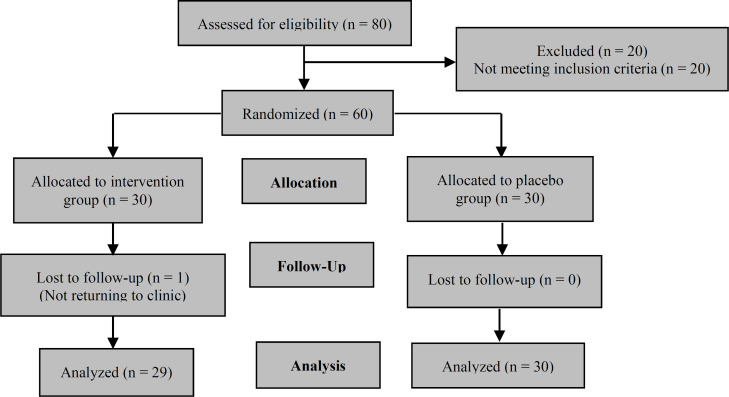
In this randomized double-blinded clinical trial study, 59 patients were enrolled (65% women and 35% men, age: 62.33 ± 11.23 years). One participant did not complete the research protocol, and ultimately, the study was performed by 29 patients in the A group and 30 patients in the B group. Figure 1 demonstrates the flowchart of the study.
Figure 1.
Study flowchart
Regarding major risk factors of atherosclerosis, 44 (70.0%) and 28 (46.7%) patients had cardiovascular disease (CVD) history and diabetes mellitus (DM), respectively. No significant differences were shown in terms of demographic parameters between the two groups (P > 0.050). The clinical features of the patients are presented in table 1.
Table 1.
General characteristics of patients with ischemic stroke
| Variable | Vitamin D group (n = 29) | Placebo group (n = 30) | P |
|---|---|---|---|
| Sex [n (%)] | 0.787 | ||
| Men | 10 (36.7) | 10 (33.3) | |
| Women | 19 (63.3) | 20 (66.7) | |
| Age (year) (mean ± SD) | 62.07 ± 12.08 | 62.60 ± 10.71 | 0.855 |
| CVD [n (%)] | 0.573 | ||
| Yes | 22 (73.3) | 20 (66.7) | |
| No | 8 (26.7) | 10 (33.3) | |
| Diabetes [n (%)] | |||
| Yes | 14 (46.7) | 14 (46.7) | |
| No | 16 (53.3) | 16 (53.3) | |
| Diabetes [n (%)] | > 0.999 | ||
| Yes | 10 (36.7) | 10 (33.3) | |
| No | 19 (63.3) | 20 (66.7) |
CVD: Cardiovascular disease; SD: Standard deviation
Primary outcomes: The baseline level of vitamin D in both groups was not statistically different (P = 0.700). The serum concentration of vitamin D differed from 18.86 ± 6.61 to 37.80 ± 21.73 ng/ml and from 18.99 ± 7.13 to 16.53 ± 14.98 in A and B groups, respectively (P = 0.001, P = 0.378). Vitamin D levels were significantly different between the two groups after six weeks (P = 0.001) (Table 2). The findings showed no significant differences for any measured serum inflammatory markers at baseline between the two groups (P > 0.050). In this interventional trial, vitamin D supplementation revealed a statistically notable effect on the serum IL-6 but no effect on TNF-α, although after six weeks in the intervention group, there was a negative correlation between serum level of vitamin D and TNF-α (r = -0.490, P = 0.021).
Table 2.
Serum levels of patients with ischemic stroke before and after the intervention
| Variable | Vitamin D group (n = 29) | Placebo group (n = 30) | P |
|---|---|---|---|
| Vitamin D (ng/ml) | |||
| Baseline | 18.86 ± 7.01 | 18.99 ± 7.13 | 0.939 |
| After | 37.80 ± 21.73 | 16.53 ± 14.98 | 0.001* |
| P | 0.001 | 0.378 | |
| IL-6 (pg/ml) | |||
| Baseline | 115.88 ± 43.39 | 128.97 ± 38.57 | 0.214 |
| After | 55.72 ± 22.51 | 68.91 ± 23.81 | 0.028 |
| P | 0.001 | 0.001 | |
| TNF-α (pg/ml) | |||
| Baseline | 274.54 ± 123.50 | 309.52 ± 154.24 | 0.328 |
| After | 81.70 ± 43.64 | 87.52 ± 42.33 | 0.596 |
| P | 0.001 | 0.001 |
Data are presented as mean ± standard deviation (SD);
IL-6: Interleukin 6; TNF-α: Tumor necrosis factor alpha
P < 0.050
Secondary outcomes: As indicated in table 3, there is no statistical difference between the two groups based on the evaluation of NIHSS, MRS, and MMSE in all patients on admission day (P > 0.050). During the study period, our results showed a decrease in scores of NIHSS and MRS (P = 0.001) and an improvement in MMSE (P = 0.050) in both groups.
Table 3.
Group comparisons of neurological scores of patients with ischemic stroke before and after the intervention
| Variable | Vitamin D group (n = 29) | Placebo group (n = 30) | P |
|---|---|---|---|
| NIHSS | |||
| Baseline | 10.16 ± 4.70 | 12.08 ± 4.14 | 0.125 |
| After | 5.60 ± 4.16 | 6.60 ± 3.30 | 0.313 |
| P | 0.001 | 0.001 | |
| MRS | |||
| Baseline | 3.10 ± 0.84 | 3.53 ± 1.10 | 0.121 |
| After | 1.60 ± 1.16 | 2.33 ± 1.15 | 0.014 |
| P | 0.001 | 0.001 | |
| MMSE | |||
| Baseline | 19.73 ± 5.65 | 20.26 ± 6.18 | 0.807 |
| After | 24.40 ± 3.64 | 23.26 ± 4.69 | 0.466 |
| P | 0.001 | 0.013 |
Data are presented as mean ± standard deviation (SD)
NIHSS: National Institute of Health Stroke Scale; MRS: Modified Rankin Scale; MMSE: Mini-Mental State Examination
Comparative analysis revealed substantial differences for MRS, six weeks following the stroke, between the intervention and control groups; however, these differences did not happen for NIHSS and MMSE scores. Based on the Pearson correlation test, there was a reverse correlation between serum level of vitamin D and NIHSS (r = -0.519, P = 0.003).
Discussion
The present study showed that a single dose of 300000 IU supplementary vitamin D raised the serum vitamin D level. Following vitamin D administration, there were reverse correlations between the serum vitamin D, NIHSS, and TNF-α levels. Vitamin D supplementation in patients with stroke significantly decreased serum IL-6 level and MRS score. However, no statistically significant effect of vitamin D on the TNF-α levels could be detected in the current study compared to the control group.
According to the literature, low levels of vitamin D, especially in the elderly, were associated with an increased risk of poor functional performance, all-cause mortality, and stroke recurrence in patients with ischemic stroke.2 Additionally, it was shown that in vitamin D-deficient patients, cognitive functions significantly were impaired after one month of AIS.9 Low vitamin D levels may increase cerebral susceptibility for larger infarctions as well as endothelial dysfunction.14 However, in a study, it was found that vitamin D toxicity or hypervitaminosis D could promote larger infarct sizes. In addition, it can also induce hemorrhagic stroke through mechanisms, such as endothelial shear stress and inflammation.15 Our study showed no significant increase in the hemorrhagic transformation in the studied patients after vitamin D administration, which might be due to the fact that our intervention did not cause hypervitaminosis in vitamin D-deficient patients.
Inflammation plays a significant role in stroke pathogenesis. The anti-inflammatory effects of vitamin D are documented in other diseases such as MS, epilepsy, and acute stroke.16,17 The suggested mechanisms of neuroprotection induced by vitamin D are complex. Vitamin D has a strong anti-inflammatory impact through several pathways, including inhibiting prostaglandin (PG) and cyclooxygenase-2 (COX-2) pathways, matrix metalloproteinase-9 (MMP-9) reduction, and anti-inflammatory cytokine upregulation.5,6 Deficiency in 25(OH)D can increase the ongoing immune-inflammatory responses and attenuate neuroprotection, leading to adverse prognosis in patients with stroke.18 Therefore, a sufficient vitamin D level could have a protective role against ischemic reperfusion in brain ischemia.
In vivo and in vitro studies indicated that acute and subchronic use of calcitriol in the immature rats with hypoxic-ischemic brain damage and co-application of this compound together with glutamate to primary neuronal cultures provided significant neuroprotection. In contrast, post-treatment with calcitriol at the end of the excitotoxic insult is not appropriate.19 Wang et al. demonstrated that calcitriol pretreatment was necessary to improve neuronal survival following hypoxia-ischemia. Following 8-day pretreatment of animals with calcitriol, they observed neuroprotection using a rat model of stroke, whereas 4-day treatment was ineffective.7 Considering calcitriol administration during the post-ischemic phase, Oermann et al.20 and Losem-Heinrichs et al.21 using a rat model of focal cerebral ischemia showed that this treatment did not affect the size of the lesions. However, the authors found indicators of neuroprotection in secondarily-injured remote cortical regions.
We observed a reverse correlation among TNF-α and serum level of vitamin D in the intervention group. In a randomized placebo-controlled trial, Pilz et al.22 and Schleithoff et al.23 reported that vitamin D supplementation raised the cytokine IL-10 and decreased the inflammatory marker TNF-α in patients with CVDs. Matthews et al. prescribed vitamin D 50000-100000 IU immediately after admission and supplemented with vitamin D for up to five days in surgical intensive care unit (SICU) patients, showing significant improvement in functional outcome in three months, fewer disabilities, and fewer deaths.24 In addition, a study showed that single-dose vitamin D replacement improved the outcome of ischemic stroke considerably. The authors affirmed that there was no consideration of confounding factors concerning the stroke, and the findings could not be generalized. The level of vitamin D was not assessed after three months while assessing stroke outcomes.11 However, in a RCT conducted by Momosaki et al., there were no considerable improvements in the rehabilitation after administering 2000 IU of vitamin D3 compared to the placebo-treated group. This study's major limitation was that serum 25(OH)D levels were not measured, so patients without vitamin D deficiency might have been included.12 Witham et al. published a RCT versus placebo where they observed no change in blood pressure or improvement in other vascular health markers related to vitamin D supplementation.25 There was no effect of vitamin D supplementation on myocardial infarction (MI) risk in an analysis of six RCTs.26,27
These findings were consistent with our results showing that mortality had no significant difference between groups. Both animal and clinical studies have indicated that the volume of infarction is related to low levels of vitamin D. It has been well-founded that the amount of infarction is closely linked to initial neurological defects and is a powerful prognostic indicator of stroke outcome. The initial neurological deficit was also indicated to be more severe in patients with lower 25(OH)D levels.15,28 The current study showed no correlation between NIHSS and MRS scores at the stroke onset and 25(OH)D levels. Hence, the severity of the initial infarction may not explain all the correlations between 25(OH)D and the stroke outcome. However, there was a strong association between the second NIHSS score and 25(OH)D in our intervention group.
Conclusion
Vitamin D deficiency is a potential risk factor for stroke, and vitamin D supplementation showed a favorable outcome in patients with ischemic stroke with vitamin D deficiency. Single-dose replacement of IM vitamin D has significantly improved the outcome of ischemic stroke. However, due to a single dose, short duration of supplementation, and short-term follow-up, there were no favorable effects on the TNF-α level and NIHSS score. Screening for vitamin D status is essential in patients with ischemic stroke, and supplementation should be administered to maintain vitamin D at a normal level. Nonetheless, more RCTs are necessary to demonstrate whether vitamin D supplementation enhances post-stroke outcomes and to clarify the relationship between the length of treatment with vitamin D and neuroprotection.
Limitations: This study has several limitations. The most important shortcoming of our study was the small sample size; hence, the findings should be interpreted with caution. Second, we could not have assessed the severity of stroke by radiologic measures like the Alberta Stroke Program Early CT Score (ASPECTS). Third, unfortunately, we had dropout rate of over 50% in a 3-month follow-up. Forth, confounding factors affecting the patients were not considered, and the results cannot be generalized.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes:
How to cite this article: Rezaei O, Ramezani M, Roozbeh M, Fazeli B, Hajiesmaeili M, Pakdaman H, et al. Does vitamin D administration play a role in outcome of patients with acute ischemic stroke? A randomized controlled trial. Curr J Neurol 2021; 20(1): 8-14.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Qiu H, Wang M, Mi D, Zhao J, Tu W, Liu Q. Vitamin D status and the risk of recurrent stroke and mortality in ischemic stroke patients: Data from a 24-month follow-up study in China. J Nutr Health Aging. 2017;21(7):766–71. doi: 10.1007/s12603-016-0821-z. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Wang J, Xu Z. Prognostic utility of serum 25-hydroxyvitamin D in patients with stroke: A meta-analysis. J Neurol. 2020;267(11):3177–86. doi: 10.1007/s00415-019-09599-0. [DOI] [PubMed] [Google Scholar]
- 3.Nie Z, Ji XC, Wang J, Zhang HX. Serum levels of 25-hydroxyvitamin D predicts infarct volume and mortality in ischemic stroke patients. J Neuroimmunol. 2017;313:41–5. doi: 10.1016/j.jneuroim.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Wajda J, Swiat M, Owczarek AJ, Brzozowska A, Olszanecka-Glinianowicz M, Chudek J. Severity of vitamin D deficiency predicts mortality in ischemic stroke patients. Dis Markers. 2019;2019:3652894. doi: 10.1155/2019/3652894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KY, Chung PW, Kim YB, Moon HS, Suh BC, Won YS, et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc Dis. 2015;40(1-2):73–80. doi: 10.1159/000434691. [DOI] [PubMed] [Google Scholar]
- 6.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS One. 2015;10(11):e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, et al. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39(5):873–80. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 8.Grant WB. Does vitamin D reduce the risk of dementia? J Alzheimers Dis. 2009;17(1):151–9. doi: 10.3233/JAD-2009-1024. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Liu Y, Huang G, Zhu J, Feng W, He J. Association between vitamin D status and cognitive impairment in acute ischemic stroke patients: A prospective cohort study. Clin Interv Aging. 2018;13:2503–9. doi: 10.2147/CIA.S187142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T. Lower vitamin D status is associated with an increased risk of ischemic stroke: A systematic review and meta-analysis. Nutrients. 2018;10(3):277. doi: 10.3390/nu10030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasimhan S, Balasubramanian P. Role of vitamin D in the outcome of ischemic stroke- a randomized controlled trial. J Clin Diagn Res. 2017;11(2):CC06–CC10. doi: 10.7860/JCDR/2017/24299.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momosaki R, Abo M, Urashima M. Vitamin D Supplementation and post-stroke rehabilitation: A randomized, double-blind, placebo-controlled trial. Nutrients. 2019;11(6):1295. doi: 10.3390/nu11061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson-Hughes B. Vitamin D deficiency in adults: Definition, clinical manifestations, and treatment. UpToDate [Online] [cited Feb 2021]. Available from: URL: https://www.uptodate.com/contents/vitamin-d-deficiency-in-adults-definition-clinical-manifestations-and-treatment.
- 14.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15(3):188–97. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Daubail B, Jacquin A, Guilland JC, Hervieu M, Osseby GV, Rouaud O, et al. Serum 25-hydroxyvitamin D predicts severity and prognosis in stroke patients. Eur J Neurol. 2013;20(1):57–61. doi: 10.1111/j.1468-1331.2012.03758.x. [DOI] [PubMed] [Google Scholar]
- 16.Mpandzou G, Ait Ben Haddou E, Regragui W, Benomar A, Yahyaoui M. Vitamin D deficiency and its role in neurological conditions: A review. Rev Neurol (Paris) 2016;172(2):109–22. doi: 10.1016/j.neurol.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Zhu Z, Liu Y, Tu X, He J. Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. 2018;8(2):e00885. doi: 10.1002/brb3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Cho KH, Im SH, Choi J, Yu J, Kim M. Decrement of serum vitamin D level after stroke. Ann Rehabil Med. 2017;41(6):944–50. doi: 10.5535/arm.2017.41.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajta M, Makarewicz D, Zieminska E, Jantas D, Domin H, Lason W, et al. Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem Int. 2009;55(5):265–74. doi: 10.1016/j.neuint.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Oermann E, Bidmon HJ, Witte OW, Zilles K. Effects of 1alpha,25 dihydroxyvitamin D3 on the expression of HO-1 and GFAP in glial cells of the photothrombotically lesioned cerebral cortex. J Chem Neuroanat. 2004;28(4):225–38. doi: 10.1016/j.jchemneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Losem-Heinrichs E, Gorg B, Redecker C, Schleicher A, Witte OW, Zilles K, et al. 1alpha,25-dihydroxy-vitamin D3 in combination with 17beta-estradiol lowers the cortical expression of heat shock protein-27 following experimentally induced focal cortical ischemia in rats. Arch Biochem Biophys. 2005;439(1):70–9. doi: 10.1016/j.abb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, Marz W. Vitamin D supplementation: A promising approach for the prevention and treatment of strokes. Curr Drug Targets. 2011;12(1):88–96. doi: 10.2174/138945011793591563. [DOI] [PubMed] [Google Scholar]
- 23.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 24.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204(1):37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22(10):864–70. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Thomas T, Briot K. Vitamin D: Skeletal and muscular effects. Presse Med. 2013;42(10):1351–7. doi: 10.1016/j.lpm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Ji H, Tong Y, Zhang ZB. Prognostic value of serum 25-hydroxyvitamin D in patients with stroke. Neurochem Res. 2014;39(7):1332–7. doi: 10.1007/s11064-014-1316-0. [DOI] [PubMed] [Google Scholar]