Abstract
Previous studies have observed an association between maternal circadian rhythm disruption and preterm birth (PTB). However, the underlying molecular mechanisms and the potential of circadian clock genes to serve as predictors of PTB remain unexplored. We examined the association of 10 core circadian transcripts in maternal blood with spontaneous PTB (sPTB) vs term births using a nested case-control study design. We used a public gene expression dataset (GSE59491), which was nested within the All Our Babies (AOB) study cohort in Canada. Maternal blood was sampled in Trimesters 2–3 from women with sPTB (n = 51) and term births (n = 106), matched for five demographic variables. In 2nd trimester maternal blood, only CLOCK and CRY2 transcripts were significantly lower in sPTB vs term (P = 0.02–0.03, false discovery rate (FDR) < 0.20). A change of PER3 mRNA from trimesters 2–3 was significantly associated with sPTB (decline in sPTB, P = 0.02, FDR < 0.20). When CLOCK and CRY2 were modeled together in 2nd trimester blood, the odds of being in the low level of both circadian gene transcripts was greater in sPTB vs term (OR = 4.86, 95%CI = (1.75,13.51), P < 0.01). Using GSVA and Pearson correlation, we identified 98 common pathways that were negatively or positively correlated with CLOCK and CRY2 expression (all P < 0.05, FDR < 0.10). The top three identified pathways were amyotrophic lateral sclerosis, degradation of extracellular matrix, and inwardly rectifying potassium channels. These three processes have previously been shown to be involved in neuron death, parturition, and uterine excitability during pregnancy, respectively.
Keywords: spontaneous preterm birth, circadian clock genes, CLOCK, CRY2, logistic regression, gene set variation analysis, pregnancy, human
Low transcript levels of the circadian clock genes CLOCK and CRY2 in 2nd trimester maternal blood are associated with an increased risk of spontaneous preterm birth.
Introduction
Preterm birth (PTB) is the leading cause of perinatal morbidity and mortality in the USA [1, 2], accounting for more than 50% of long-term morbidity and 60–80% of perinatal mortality [3]. PTB is defined as birth occurring between Weeks 20 and 37 of gestation. PTB is associated with an increased risk of severe developmental delays and lifelong medical problems [4]. About 15 million babies are born preterm each year in the world [1, 2]. The extensive medical cost associated with PTB puts a tremendous financial burden on families and healthcare systems, with an estimated annual cost of approximately $26 billion in 2005 in the USA [5]. Additionally, from 2005 to 2016, the average cost of a PTB increased by 25% [6]. Spontaneous PTB (sPTB), including spontaneous preterm labor (sPTL) and preterm premature rupture of membranes (PPROM), account for two-thirds of all PTB in the USA [7, 8]. About 95% of sPTB cases are intractable to current interventions, and few predictors exist to identify women at risk for PTB [5, 9]. However, recent findings suggest that circadian rhythms play a role in sPTB etiologies.
Circadian rhythms are 24-hour oscillations in behavior and physiology. Circadian rhythms exist across all types of organisms from bacteria and plants to mammals, including primates and humans [10–13]. Within cells, circadian rhythms are driven by endogenous biological “clocks”. The cellular clock is formed of a complex set of transcription factors and transcriptional regulators, which to a great extent have been conserved across species [14]. The mammalian core molecular clock consists of the transcription factors Brain and Muscle ARNT-like protein 1 (ARNTL, BMAL1, or MOP3), Cryptochromes 1 and 2 (CRY1, CRY2), Circadian Locomotor Output Cycles Kaput (CLOCK), and Period genes (PER1, PER2, and PER3) [15]. BMAL1 and CLOCK dimerize and initiate the transcription of CRY1/CRY2 and PER1/PER2/PER3, which in turn dimerize and inhibit their own transcription in a ~24-hour oscillation [13]. To regulate the 24-hour transcription–translation feedback loop, a large number of additional transcription factors, kinases, and DNA regulatory enzymes participate in this large regulatory network to fine tune the transcriptional activity of the molecular clock [11, 13]. Studies in both animal models and humans have demonstrated that circadian rhythms are involved in maintaining female reproductive health [16–21] and pregnancy success [19, 22–24]. In pregnant women, numerous observational studies have shown an association between maternal circadian rhythm disruption (e.g., shift work-related chrono disruption) and spontaneous abortion, miscarriage, and PTB [19, 25–30]. However, to date, the underlying molecular mechanisms and the potential of clock genes as biomarkers to predict/classify these adverse reproductive outcomes in humans are still unclear.
In this study, we examined the association of 10 core circadian transcripts (ARNTL, ARNTL2, CLOCK, CRY1, CRY2, NPAS2, PER1, PER2, PER3, and TIMELESS) in maternal blood with sPTB vs term birth using a microarray gene expression dataset from a Canadian cohort (GSE59491) in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database. These core clock genes were selected based on their critical roles in circadian rhythms [31] and the detectability of their mRNAs in the available microarray data. We also analyzed the circadian gene-correlated and sPTB-associated biological pathways using the Gene Set Variation Analysis (GSVA), which has greater noise/dimension reduction and biological interpretability [32].
Materials and methods
Human research approval
The secondary use of publicly available, deidentified human data does not constitute human subjects research as defined by 45 CFR 46.102. The original study [33] was approved by the Conjoint Health Research Ethics Board, University of Calgary, Canada (Ethics #20821 and #22128).
Selection of pregnant women and maternal blood processing
The samples were nested within the All Our Babies (AOB) study cohort, a community-based longitudinal pregnancy cohort (N = 1878, May 2008–December 2010) in Calgary, Alberta, Canada. The inclusion criteria included women 18 years of age, gestation age <18 weeks at time of recruitment, and singleton pregnancy [33]. The pregnant women with multifetal pregnancy and pre-existing medical conditions including diabetes, high blood pressure, autoimmune disorders, kidney disease, cardiovascular disease or chronic infection were excluded [33]. Figure S1 summarizes the selection of the participants in the study cohort and the nested cased-control study.
Spontaneous PTB was defined as a delivery that occurred ≥20 and <37 weeks of gestation, including sPTL or PPROM [33]. Term birth was ≥37 weeks-gestation [33]. Women who had sPTB were confirmed by a manual review of the medical charts [33].
Maternal blood total RNA from each pregnant woman in both Trimesters 2 and 3 was extracted, respectively and then hybridized to Affymetrix Human Gene 2.1 ST (Affymetrix, Santa Clara, CA, USA) for microarray measurement [33]. The generated raw gene expression values were normalized using the Robust MultiArray Average (RMA) method with a log base 2 (log2) transformation (GSE59491) [33].
Bioinformatics and statistical analyses
Figure 1 summarizes the pipeline of our bioinformatics and statistical analyses. We retrieved 10 core circadian genes (ARNTL, ARNTL2, CLOCK, CRY1, CRY2, NPAS2, PER1, PER2, PER3, and TIMELESS) from the dataset GSE59491. The mRNA levels (log2-transformed) of all 10 core circadian genes in 2nd and 3rd trimester maternal blood were summarized as means with standard deviations (SDs). We examined the mean differences in gene mRNA levels between the sPTB and term groups by using a two-sample, two-tailed t-test. Probability values were adjusted for multiple comparisons using a false discovery rate of 20% (FDR = 0.20) [34]. To test if there were threshold associations between circadian gene mRNA levels and sPTB, we categorized mRNA values into two levels: ≤median and >median. The level of >median for each circadian gene was set as reference. Odds ratios for the threshold associations of sPTB with the categorized circadian gene mRNA levels were calculated in binary logistic regression models.
Figure 1.
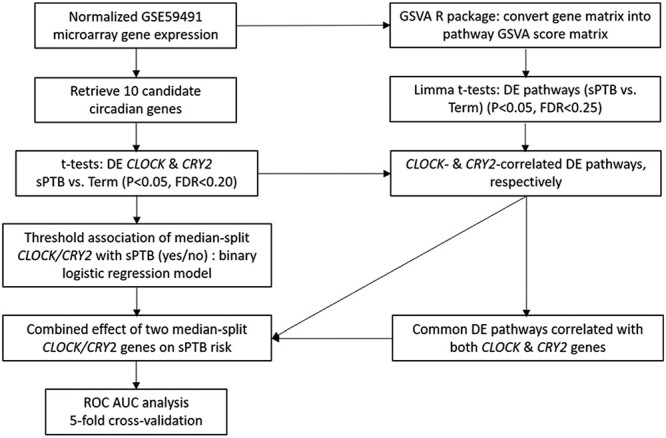
Flowchart summarizing the methodological steps of data analysis used in this study. Gene Set Variation Analysis (GSVA) was used to explore gene expression relationships between sPTB-clock gene changes and gene expression pathway changes. For details on statistical analysis see Bioinformatics and Statistical Analyses section. Abbreviations: DE, differentially expressed; ROC AUC, receiver operating characteristic area under the curve.
Next, we compared the differences of the changes of 10 core clock genes’ mRNA levels from the 2nd to 3rd trimester between sPTB and term birth using t-tests. The corresponding FDR q values were calculated with the SAS proc multtest procedure to correct multiple comparisons. The cut-off values for statistical significance were set as P < 0.05 and FDR q < 0.20 [34].
To examine if there was a combined effect of two significant circadian genes on the risk of sPTB, we combined the categorized two circadian gene mRNA levels into one three-level variable: Level 1—both gene mRNA levels <median; Level 3—both gene mRNA levels ≥median; and Level 2—all other combinations. The association between the combined two circadian genes variable (three levels) and sPTB (yes/no) was assessed with a binary logistic regression model. The cv.glm function in the R boot package was used for conducting a 5-fold cross-validation to examine if the final model had a possible overfitting problem (Figure 1). Based on our previous method [35], the levels of the identified CLOCK and CRY2 transcripts were linearly combined to generate a continuous risk score. We assessed the performance of this risk score to classify sPTB vs term births with the receiver operating characteristic (ROC) analysis. We applied leave-one-out validation to validate the final area under curve (AUC) curve of the risk score by using the SAS “predprobs = crossvalidate” option in the proc logistic procedure. Finally, we calculated the sensitivity, specificity, and the corresponding maximum Youden J index for the final AUC curve [35].
In order to explore the biological pathways that are involved in sPTB-clock gene relationship, the GSVA, an unsupervised method to estimate the variation of pathway activity over a sample population [32], was applied. The GSVA algorithm includes: the nonparametric kernel estimation of the cumulative density function for each gene expression profile; the rank-ordered expression-level statistic for each sample; and the calculation of the Kolmogorov–Smirnov-like rank statistic (i.e., GSVA enrichment score) for each gene set and each sample [32]. Compared to the classical gene set enrichment analysis [36], the GSVA method increases the statistical power to detect subtle changes of pathway activity over a sample population [32]. As shown in Figure 1, the cleaned and normalized microarray gene expression matrix (GSE59491) was converted into the sample-wise GSVA score matrix by using the pathway subcollection—c2.cp.v7.1.symbols.gmt in the Molecular Signatures Database (MSigDB). A moderated t-statistic in limma R package [37] was applied to identify differentially expressed pathways between sPTB and term births (i.e., sPTB-associated pathways) with the cutoffs of P < 0.05 and Benjamini and Hochberg adjusted P < 0.25 [38] (Figure 1). The Pearson Correlation statistic was used to examine the correlations of the identified DE clock gene mRNA levels with the sPTB-associated pathways (P < 0.05 and Benjamini and Hochberg adjusted P < 0.10) [39] (Figure 1). The subset of the pathways associated with both sPTB and clock genes were referred to as circadian gene-correlated sPTB-associated pathways. Finally, we overlapped the pools of sPTB-associated pathways correlated with individual circadian genes to get common up- or down-regulated pathways in sPTB that were shared by circadian genes (Figure 1).
All data management and statistical analyses were performed with SAS v9.4 (SAS Institute, Cary, NC, USA) and R (R Development Core Team).
Results
Demographics of the participants in our studied groups and description of nested case-control data
In the sPTB group, the included pregnant women consisted of 76.5% Caucasian with a mean maternal age of 31 years, a mean pre-pregnancy body mass index (BMI) of 25, 19.6% smoked during pregnancy, and 52.9% were nulliparous [33].
We had 51 sPTB samples at 2nd trimester (17–23 weeks’ gestation) and 47 sPTB samples at 3rd trimester (27–33 weeks’ gestation) as well as 114 term samples from both trimesters. Spontaneous PTBs were matched to term births at ratios of 1:1 (n = 2), 1:2 (n = 135), and 1:3 (n = 20) on five characteristics, i.e., maternal age (<35 years vs ≥35 years), pre-pregnancy BMI (<18.5, 18.5–24.9, 25–29.9, and ≥ 30 kg/m2), race/ethnicity (Caucasian vs non-Caucasian), smoking during pregnancy (yes vs no), and parity (no previous birth vs at least one previous birth) (Figure S1). Eight women delivered at term without matched cases and were excluded. The final dataset was composed of 51 sPTBs for 2nd trimester (15 sPTLs and 36 PPROM), 47 sPTB for 3rd trimester (14 sPTLs and 33 PPROM) and matched 106 term births for both trimesters (Figure S1).
CLOCK and CRY2 are differentially expressed in maternal blood between sPTB and term birth
Disrupted molecular clock function in transgenic mouse models has consistently been associated with poor pregnancy outcomes [40–45]. Further, disrupted circadian rhythms, through mistimed daily light exposure, such as during shift work, increases the risk of mistimed birth [19], indicating that molecular clock function is important in pregnancy. To explore if changes in clock gene mRNA levels in maternal blood were associated with sPTB, we analyzed transcript levels of the core circadian clock genes ARNTL, ARNTL2, CLOCK, CRY1, CRY2, NPAS2, PER1, PER2, PER3, and TIMELESS in maternal blood in women with sPTB and term birth. As shown in Tables 1 and 2, out of 10 core circadian gene transcripts measured in 2nd trimester maternal blood, CLOCK and CRY2 were differentially expressed between sPTB and term births, where the means of CLOCK and CRY2 transcripts were lower in sPTB than in term (term birth mRNA expression mean ± SD: CLOCK [7.58 ± 0.17] and CRY2 [7.58 ± 0.12] vs mRNA levels in sPTB: CLOCK [7.51 ± 0.17] and CRY2 [7.53 ± 0.13]; t-statistic: P = 0.02–0.03 and FDR = 0.15). In contrast, the mean differences of all other studied genes were not statistically significant between the two groups (Table 1, P > 0.05 and FDR > 0.20). None of the circadian gene transcripts in 3rd trimester maternal blood were significantly associated with sPTB (P > 0.05 and FDR > 0.20) (Table 1).
Table 1.
Descriptive statistics of 10 candidate circadian genes’ expression levels in the 2nd and 3rd trimester maternal blood (sPTB vs term).
| Term | sPTB | |||||
|---|---|---|---|---|---|---|
| Gene | n | Meana (SD) | n | Mean (SD) | P b | FDRc |
| Trimester 2: | ||||||
| ARNTL | 106 | 9.25 (0.20) | 51 | 9.26 (0.22) | 0.6959 | 0.8699 |
| ARNTL2 | 106 | 3.39 (0.20) | 51 | 3.42 (0.20) | 0.3417 | 0.5695 |
| CLOCK | 106 | 7.58 (0.17) | 51 | 7.51 (0.17) | 0.0230 | 0.1500 |
| CRY1 | 106 | 5.98 (0.23) | 51 | 5.97 (0.22) | 0.8375 | 0.9306 |
| CRY2 | 106 | 7.58 (0.12) | 51 | 7.53 (0.13) | 0.0300 | 0.1500 |
| NPAS2 | 106 | 5.78 (0.41) | 51 | 5.66 (0.35) | 0.0789 | 0.2630 |
| PER1 | 106 | 6.60 (0.25) | 51 | 6.60 (0.28) | 0.9457 | 0.9457 |
| PER2 | 106 | 6.33 (0.17) | 51 | 6.32 (0.20) | 0.6333 | 0.8699 |
| PER3 | 106 | 5.54 (0.27) | 51 | 5.47 (0.26) | 0.1343 | 0.3358 |
| TIMELESS | 106 | 4.61 (0.37) | 51 | 4.54 (0.35) | 0.2321 | 0.4642 |
| Trimester 3: | ||||||
| ARNTL | 106 | 9.24 (0.18) | 47 | 9.21 (0.22) | 0.2689 | 0.3830 |
| ARNTL2 | 106 | 3.42 (0.18) | 47 | 3.45 (0.19) | 0.3064 | 0.3830 |
| CLOCK | 106 | 7.53 (0.17) | 47 | 7.46 (0.19) | 0.0467 | 0.2335 |
| CRY1 | 106 | 5.98 (0.19) | 47 | 5.93 (0.20) | 0.2275 | 0.3830 |
| CRY2 | 106 | 7.55 (0.16) | 47 | 7.51 (0.17) | 0.1006 | 0.3353 |
| NPAS2 | 106 | 5.79 (0.46) | 47 | 5.61 (0.41) | 0.0288 | 0.2335 |
| PER1 | 106 | 6.62 (0.27) | 47 | 6.68 (0.31) | 0.2307 | 0.3830 |
| PER2 | 106 | 6.30 (0.19) | 47 | 6.34 (0.23) | 0.2241 | 0.3830 |
| PER3 | 106 | 5.52 (0.25) | 47 | 5.55 (0.24) | 0.6015 | 0.6683 |
| TIMELESS | 106 | 4.62 (0.33) | 47 | 4.62 (0.34) | 0.9394 | 0.9394 |
aMean of gene expression values, expressed as normalized log2 (RMA signal intensity), where RMA robust multiarray average.
bTwo-sample t test, α < 0.05 as significant, two-tailed.
cFDR values were calculated with the fdrtool package in R to correct comparisons.
Table 2.
Associations of sPTB with the categorized mRNA levels of circadian genes (median split) in 2nd trimester maternal blood using logistic regressions.
| N (%) | Term, n (%) | sPTB, n (%) | ORsPTB vs term (95% CI) | P | FDR | |
|---|---|---|---|---|---|---|
| ARNTL | ||||||
| ≤median | 78 (100.0) | 53 (68.0) | 25 (32.0) | 0.96 (0.49, 1.88) | 0.9084 | 0.9084 |
| >median | 79 (100.0) | 53 (67.1) | 26 (32.9) | Ref. | ||
| ARNTL2 | ||||||
| ≤median | 78 (100.0) | 54 (69.2) | 24 (30.8) | 0.86 (0.44, 1.67) | 0.6486 | 0.8108 |
| >median | 79 (100.0) | 52 (65.8) | 27 (34.2) | Ref. | ||
| CLOCK | ||||||
| ≤median | 78 (100.0) | 46 (59.0) | 32 (41.0) | 2.20 (1.11, 4.36) | 0.0244 | 0.1220 |
| >median | 79 (100.0) | 60 (76.0) | 19 (24.0) | Ref. | ||
| CRY1 | ||||||
| ≤median | 78 (100.0) | 53 (68.0) | 25 (32.0) | 1.04 (0.53, 2.03) | 0.9084 | 0.9084 |
| >median | 79 (100.0) | 53 (67.1) | 26 (32.9) | Ref. | ||
| CRY2 | ||||||
| ≤median | 78 (100.0) | 46 (59.0) | 32 (41.0) | 2.20 (1.11, 4.36) | 0.0244 | 0.1220 |
| >median | 79 (100.0) | 60 (76.0) | 19 (24.0) | Ref. | ||
| NPAS2 | ||||||
| ≤median | 78 (100.0) | 50 (64.1) | 28 (36.9) | 1.36 (0.70, 2.67) | 0.3648 | 0.7296 |
| >median | 79 (100.0) | 56 (70.9) | 23 (29.1) | Ref. | ||
| PER1 | ||||||
| ≤median | 78 (100.0) | 51 (65.4) | 27 (34.6) | 1.21 (0.62, 2.37) | 0.5712 | 0.8108 |
| >median | 79 (100.0) | 55 (69.6) | 24 (30.4) | Ref. | ||
| PER2 | ||||||
| ≤median | 78 (100.0) | 51 (65.4) | 27 (34.6) | 1.21 (0.62, 2.37) | 0.5712 | 0.8108 |
| >median | 79 (100.0) | 55 (69.6) | 24 (30.4) | Ref. | ||
| PER3 | ||||||
| ≤median | 78 (100.0) | 48 (61.5) | 30 (38.5) | 1.73 (0.88, 3.39) | 0.1136 | 0.2840 |
| >median | 79 (100.0) | 58 (73.1) | 21 (26.6) | Ref. | ||
| TIMELESS | ||||||
| ≤median | 78 (100.0) | 48 (61.5) | 30 (38.5) | 1.73 (0.88, 3.39) | 0.1136 | 0.2840 |
| >median | 79 (100.0) | 58 (73.1) | 21 (26.6) | Ref. | ||
“Ref.” = “reference”.
Lower transcript levels of CLOCK or CRY2 in the 2nd trimester maternal blood increased the risk of sPTB
To assess the threshold association between mRNA levels of each core circadian gene with sPTB, we divided each core circadian gene mRNA into two levels (median split) (2-quantile) and determined the odds ratio of sPTB vs term birth in each quantile. In the 2nd trimester maternal blood, the odds of being in the lower quantile of both CLOCK and CRY2 genes was greater in sPTB vs term birth than that of being in the higher quantile (odds ratio (OR) (95% CI) = 2.20 (1.11, 4.36), P = 0.02, FDR = 0.12) (Table 2). All other studied genes had no threshold associations with the risk of sPTB (P > 0.05 and FDR > 0.20) (Table 2). In the 3rd trimester maternal blood, none of the 10 circadian genes studied had threshold associations with sPTB (P > 0.05 and FDR > 0.02) (Table S1).
Specific decline of PER3 transcript from 2nd to 3rd trimester in sPTB
Table 3 demonstrated that the change in PER3 mRNA levels from the 2nd to 3rd trimester was significantly different between sPTB and term birth (P = 0.0153 and FDR q = 0.1530). The PER3 mRNA levels had a slight increase over time (Delta(T2−T3) = 0.0182, SD = 0.2803, Table 3) without significance in term (P = 0.5063, data not shown), but a significant decline in sPTB from Trimesters 2 to 3 (Delta(T2−T3)= − 0.0979, SD=0.2446, Table 3) (P=0.0086, data not shown).
Table 3.
Comparisons of the changes of 10 clock genes’ mRNA levels across two different trimesters between sPTB and term.
| Mean Difference(T2−T3)a in Term | Mean Difference(T2–T3) in sPTB | ||||||
|---|---|---|---|---|---|---|---|
| Gene Name | N | Delta(T2−T3) (SD) | N | Delta(T2−T3) (SD) | tterm-sPTB | P * | FDR* |
| ARNTL | 106 | 0.0013 (0.1551) | 47 | 0.0565 (0.1764) | −1.95 | 0.0536 | 0.2680 |
| ARNTL2 | 106 | −0.0307 (0.2521) | 47 | −0.0323 (0.2586) | 0.04 | 0.9717 | 0.9717 |
| CLOCK | 106 | 0.0497 (0.1851) | 47 | 0.0372 (0.1968) | 1.13 | 0.5994 | 0.7493 |
| CRY1 | 106 | −0.0001 (0.2504) | 47 | 0.0262 (0.2504) | −0.60 | 0.5495 | 0.7493 |
| CRY2 | 106 | 0.0274 (0.1736) | 47 | 0.0209 (0.956) | 0.20 | 0.8391 | 0.9323 |
| NPAS2 | 106 | −0.0049 (0.3946) | 47 | 0.0804 (0.3083) | −1.31 | 0.1910 | 0.5372 |
| PER1 | 106 | −0.0272 (0.3104) | 47 | −0.0914 (0.3816) | 1.10 | 0.2738 | 0.5372 |
| PER2 | 106 | 0.0348 (0.2197) | 47 | −0.0152 (0.2508) | 1.24 | 0.2159 | 0.5372 |
| PER3 | 106 | 0.0182 (0.2803) | 47 | −0.0979 (0.2446) | 2.45 | 0.0153 | 0.1530 |
| TIMELESS | 106 | −0.0129 (0.4546) | 47 | −0.0922 (0.4573) | 0.99 | 0.3223 | 0.5372 |
aMean Difference(T2−T3) represents the difference of the candidate gene mRNA levels between Trimesters 2 and 3.
*Cut-off values for statistical significance: P < 0.05, q < 0.20.
N, number of samples.
Increased risk of sPTB in 2nd trimester maternal blood samples with low mRNA levels of both CLOCK and CRY2
To evaluate a combined effect of the two significant circadian genes (median split) on the risk of sPTB, we modeled the two categorized CLOCK and CRY2 genes together. The results demonstrated that the odds of being in the high risk level of two circadian genes (i.e., lower quantile for both CLOCK and CRY2) was greater in sPTB vs term (reference: low risk level, i.e., higher quantile for both CLOCK and CRY2) (OR=4.86 (1.75, 13.51), P=0.0025) whereas the medium risk level (i.e., all other combinations of two genes) had a nonsignificant OR (OR=2.24 (0.87, 5.78), P=0.0946) (Table 4). Five-fold cross-validation analysis indicated that the above model (combining two clock gene transcripts into a single variable) had no significant overfitting; the 5-fold cross-validation estimation error, delta1, was 0.2097, which was very close to the bias-corrected estimation error (delta2=0.2092) (data not shown). The performance of the linearly combined transcripts to classify sPTB vs term births was assessed with the ROC analysis (Figure 2). The resulted area under the ROC curve (AUC) was 0.66 (95% CI: 0.57–0.75, P = 0.0005, reference: by chance) with a sensitivity for sPTB of 76% and a specificity of 51% (data not shown). The leave-one-out validation demonstrated that the cross-validated AUC was 0.64 (95% CI: 0.54–0.73), which was very close to the nonvalidated AUC (Figure 2).
Table 4.
Combined effect of CLOCK and CRY2 (median splits) on sPTB in 2nd trimester maternal blood (n = 51 sPTB, n = 106 term) with logistic regression model.
| N (%) | Term, n (%) | sPTB, n (%) | ORsPTB vs term (95% CI) | P | |
|---|---|---|---|---|---|
| High risk level* | 40 (100.0) | 20 (50.0) | 20 (50.0) | 4.86 (1.75, 13.51) | 0.0025 |
| Middle risk level*** | 76 (100.0) | 52 (68.4) | 24 (31.6) | 2.24 (0.87, 5.78) | 0.0946 |
| Low risk level** | 41 (100.0) | 34 (32.1) | 7 (13.7) | Ref. |
*Both CLOCK and CRY2 transcripts ≤ median.
**Both CLOCK and CRY2 transcripts > median.
***All other combinations.
Figure 2.
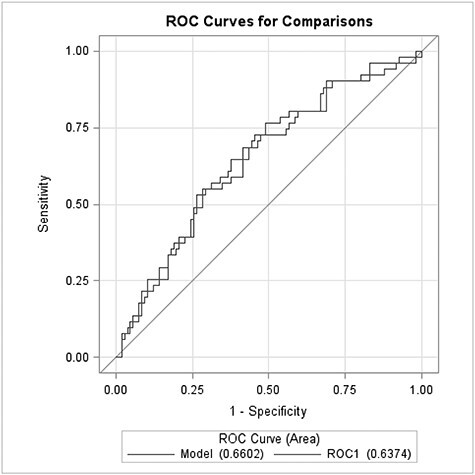
Visualization of the ROC analysis with the linearly combined CLOCK and CRY2 transcripts. The blue line represents the nonvalidated ROC curve (AUC=0.66, 95% CI=0.57–0.75, P = 0.0005, ref.: by chance) for the combined transcripts. The red line represents the cross-validated ROC curve (AUC=0.64, 95% CI=0.54–0.73) for the combined transcripts.
sPTB-associated biological pathways commonly correlated with both CLOCK and CRY2 gene transcripts
Both CLOCK and CRY2 are transcriptional regulators [14, 15], and changes in their expression would be expected to impact target gene expression patterns as well as molecular clock function. To determine if CLOCK and CRY2 associated pathways were impacted in our term and sPTB groups, we used the GSVA and limma R packages. We identified 315 out of 1707 pathways significantly different between sPTB and term birth, among which 199 and 116 pathways were down- and up-regulated, respectively (P < 0.05 and adjusted P < 0.25) (Table S2). To further explore the sPTB-associated pathways that were commonly correlated with both circadian genes (CLOCK and CRY2), we used Pearson Correlation statistic to examine CLOCK/CRY2 correlated sPTB-associated pathways. We found that 296 of 315 associated sPTB-pathways were significantly correlated with CLOCK, whereas 100 out of 315 were significantly correlated with CRY2 (absolute correlation coefficient r = (0.1789–0.6470), P ≤ 0.02, and FDR ≤ 0.03 for CLOCK gene; r = (0.1730–0.2952), P ≤ 0.03, and FDR < 0.10 for CRY2 gene) (Table S3). The combination of both CLOCK and CRY2 correlated sPTB-associated pathways resulted in 98 common pathways (30 up- and 68 down-regulated in sPTB) negatively or positively correlated with the two circadian genes (all P < 0.05 and FDR < 0.10) (Tables S4 and S5). Based on the absolute correlation coefficient values of CLOCK in sPTB, the top three correlated and up-regulated pathways are AMYOTROPHIC LATERAL SCLEROSIS ALS, DEGRADATION OF THE EXTRACELLULAR MATRIX, and INWARDLY RECTIFYING K CHANNELS (r = −0.56 to −0.53) (Table 5) and the top three correlated and down-regulated pathways include TRNA PROCESSING, TRNA PROCESSING IN THE NUCLEUS, and TRANSPORT OF MATURE TRANSCRIPT TO CYTOPLASM (r = 0.58–0.65) (Table 5).
Table 5.
Top three increased and decreased pathways in sPTB which were negatively and positively correlated with both CLOCK and CRY2 gene mRNA levels in 2nd trimester maternal blood, respectively (all P < 0.05 and FDR < 0.10).
| Pathway Name | Correlation Coefficient | |
|---|---|---|
| CLOCK | CRY2 | |
| Increased Pathways in sPTB: | ||
| KEGG_AMYOTROPHIC_LATERAL_SCLEROSIS_ALS | −0.56 | −0.17 |
| REACTOME_DEGRADATION_OF_THE_EXTRACELLULAR_MATRIX | −0.54 | −0.21 |
| REACTOME_INWARDLY_RECTIFYING_K_CHANNELS | −0.53 | −0.22 |
| Decreased Pathways in sPTB: | ||
| REACTOME_TRNA_PROCESSING | 0.65 | 0.18 |
| REACTOME_TRNA_PROCESSING_IN_THE_NUCLEUS | 0.62 | 0.20 |
| REACTOME_TRANSPORT_OF_MATURE_TRANSCRIPT_TO_CYTOPLASM | 0.58 | 0.18 |
Discussion
Numerous studies in rodents have demonstrated the role of circadian rhythms through the action of clock genes in pregnancy success [40–45]. In humans, although observational studies have documented an association between maternal circadian rhythm disruption and PTB [27, 30, 46] and polymorphisms of CLOCK and PER3 have been associated with adverse pregnancy outcome [47, 48], to date, the systematic evaluation of core circadian gene transcripts in maternal blood in relation to sPTB has not yet been reported. Using a publicly available data set, we show that reduced levels of both CLOCK and CRY2 mRNAs in 2nd trimester maternal blood increased the odds ratio of sPTB about 5-fold, indicating that low mRNA levels of CLOCK and CRY2 genes in maternal blood may be useful in detecting increased risk of sPTB as early as the 2nd trimester.
Disrupted molecular clock function is associated with increased risk of sPTB
Over the last decade, numerous studies have found an association between changed molecular clock gene expression and adverse pregnancy outcomes. This is particularly true for CLOCK. Lower CLOCK gene expression at both mRNA and protein levels was detected in fetal tissue and placental chorionic villi of spontaneous abortion (miscarriage), whose etiologies may overlap with that of PTB [49–51], as compared to those of induced abortion in a Chinese population [52]. Using a publicly available maternal blood mRNA data set, we found that the odds of either low CLOCK or CRY2 transcript levels (≤median) in the 2nd trimester maternal blood had a 2-fold increase in the sPTB group as compared to the term birth group. When both CLOCK and CRY2 levels were low, the risk of sPTB was ~5-fold higher. To our knowledge, our results show for the first time that low mRNA levels of CLOCK and CRY2 transcripts in maternal blood in the 2nd trimester of pregnancy is a potential new biomarker allowing to classify the risk of sPTB in midpregnancy. Importantly, the additional eight core circadian genes we analyzed were not differentially expressed in maternal blood between sPTB and term births in the 2nd or 3rd trimester of pregnancy, making CLOCK and CRY2 promising biomarkers allowing to identify women at increased risk of sPTB as early as the 2nd trimester of pregnancy. We are not the first to report that circadian rhythm changes in midpregnancy are a potential predictor of pregnancy outcomes. A small cohort study in pregnant women found that women who had preterm labor did not have increased uterine contractions during the night period in the late 2nd trimester/early 3rd trimester of pregnancy [46], whereas another study revealed that uterine contraction frequency was significantly greater in women who had PTB [53]. Such controversy may be related to the use of the different definitions for the outcome and/or the different measuring time of the participants. More work is required to further our understanding and classification of what is considered normal circadian rhythm function in pregnancy, and how changes in circadian function adapt throughout pregnancy.
PER3 mRNA change from Trimesters 2 to 3 and sPTB risk
We also found that the change of PER3 mRNA from Trimesters 2 to 3 was significantly different between sPTB and term (decline in sPTB but no change in term). In humans, a PER3 single-nucleotide polymorphism has been documented to be associated with PTB [48]. In mice, the variation of Per3 transcript was evidenced to be causally associated with and also responsive to stress and alcohol [54]. Maternal stress during pregnancy has been evidenced to increase the risk of PTB in a case-control study [55]. In addition, studies also indicated that maternal stress may be involved in the regulation of parturition in different domestic animal species [56]. However, whether the dynamic change of PER3 transcript across Trimesters 2 and 3 in sPTB is related to progressive maternal stress during pregnancy and/or parturition is unknown. More rigorous studies are needed for further clarification.
CLOCK and CRY2 associated pathways and their potential role in sPTB
To further explore the molecular mechanism(s) underlying the relationship between the mRNA levels of CLOCK and CRY2 genes with sPTB, we examined the pathways enriched by both circadian genes and sPTB. The results revealed 30 up- and 68 down-regulated pathways in sPTB. The top three CLOCK/CRY2 correlated and up-regulated pathways in sPTB are Amyotrophic Lateral Sclerosis (ALS), Degradation of the Extracellular Matrix (ECM), and Inwardly Rectifying K Channels.
ALS is a pathway related to neuron death [57]. Circadian rhythm dysfunction has been documented to induce neuron death via neuroinflammation and oxidative stress [58]. Evidence also showed that the neuronal activity of cervix-related sensory neurons increases during pregnancy in mice and plays a role in cervical ripening and parturition [59]. It will be of interest for future studies to determine if there is a causative link between lower clock gene expression and increased ALS pathway activity in maternal blood in sPTB, in particular with regard to cervix-related sensory neuron death and premature cervical ripening.
A second pathway we found to be associated with CLOCK and CRY2 involved the ECM, the noncellular component in tissues that constantly undergoes remodeling [60, 61]. ECM remodeling is essential for tissue morphogenesis and cell differentiation [60, 61]. Studies have demonstrated that the protein levels of amniotic fluid matrix metalloproteinase-2 (MMP-2) and MMP-9, two enzymes regulating ECM remodeling and degradation [62], were increased in PPRM [63, 64]. Particularly, studies indicate that ECM degradation and remodeling is required for parturition and its abnormal alteration may result in PTB [65]. However, the exact mechanism underlying the relationship between an increase in ECM pathways and lower clock gene expression and sPTB is unclear.
Inwardly rectifying K channels (Kir channels) are integral membrane proteins responsible for transporting potassium (K+) with a greater tendency for K+ uptake than K+ export [66, 67]. The Kir channels exist in a variety of cell types (e.g., cardiac myocytes, neurons, blood cells, epithelial cells, etc.) [68]. In rat glial cells, an increase in Kir channels was associated with the arrest of the cell cycle [69, 70]. In myometrial cells, the Kir channel 7.1 (Kir7.1) plays an important role in myometrium excitability and allows to maintain uterine quiescence throughout pregnancy in mice [71]. However, in vitro results in animal cells appear to be controversial with our findings, where we found that lower clock gene expression correlated with increased Kir7.1 pathway activity. This could be related to different species, tissues, sampling time, or pathway interaction that might cause a functional downregulation of Kir7.1, relieving Kir7.1-promoted relaxation of the myometrium. More rigorous studies are needed to further clarify these associations.
In contrast, the top three CLOCK/CRY2 correlated and down-regulated pathways in sPTB that we found in the present study include tRNA Processing, tRNA Processing in the Nucleus, and Transport of Mature Transcript to Cytoplasm. These down-regulated pathways in sPTB are consistent with the findings in our previous study, in which several tRNA-related pathways (e.g., cytosolic tRNA aminoacylation, tRNA charging, tRNA aminoacylation, and aminoacyl-tRNA biosynthesis) or RNA metabolism pathways (including RNA intracellular transport) correlated with two lncRNAs were significantly decreased in sPTB [39, 72]. This consistency suggests that these circadian clock genes-correlated and down-regulated pathways in sPTB may also be related to epigenetic regulation.
Strengths and limitations of the present study
Our study presented several strengths. We used 10 core clock gene candidates and conducted the integrated pathway analysis. These analyses improved the biological plausibility of the gene-disease relationship as well as the biological understanding and interpretation of the final model. In addition, the application of leave-one-out cross-validation increased the internal validation of the final ROC model. However, it is important to note the limitations of the current study. The dataset analyzed is publicly available (NCBI GEO GSE59491), and we do not have time-of-day information or time-of-year information about sample collection. In addition, our findings could not be validated in a second cohort as only one microarray maternal blood gene expression profiling dataset regarding sPTB (GSE59491) in the NCBI GEO database is currently available. Further, the origins of clock gene transcripts in maternal blood were unknown. Finally, the performance of the combined CLOCK/CRY2 transcripts to classify sPTB vs term births was modest, probably due to the relatively small number of sPTB cases (n = 51) and the heterogeneity of sPTB. Other biomarkers or clinical predictors may need to be included in the model for the improvement. These are all important caveats, as the body’s circadian time keeping system adapts to the time-of-day and the time-of-year, and the key characteristic of molecular clock genes is their circadian expression. That said, we believe our findings remain relevant, and that low levels of CLOCK and CRY2 mRNAs in maternal blood are novel biomarkers that can easily be screened for in 2nd trimester maternal blood. Indeed, we specifically found that a reduction in CLOCK and CRY2 mRNA levels is associated with sPTB, whereas none of the other eight molecular clock gene mRNA levels were associated with sPTB in 2nd trimester maternal blood. This indicates that independent of time-of-day, reduced CLOCK and CRY2 transcript levels are associated with an increased risk for sPTB during the 2nd trimester of pregnancy. To validate these genes as biomarkers for sPTB it will be important to repeat the study controlling for time-of-day of sample collection and time-of-year.
Conclusions
Here we describe that low transcript levels of both CLOCK and CRY2 genes in 2nd trimester maternal blood may be informative in identifying women at increased risk of sPTB. The underlying mechanism may be partially linked to the abnormal circadian clock regulation of the pathways such as increased neuron death, abnormal tissue/organ morphogenesis, and cell cycle arrest/altered uterine excitability, as well as decreased RNA processing and RNA transport. Additional pregnancy cohort studies are needed to examine the robustness and generalizability of our findings.
Supplementary Material
Grant Support: This research was funded by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R00 HD084759 (H.M.H.), the USDA National Institute of Food and Agriculture Hatch project MICL1018024 (H.M.H.) and by the March of Dimes Grant no 5-FY19-111 (H.M.H.).
Contributor Information
Guoli Zhou, Clinical & Translational Sciences Institute, Michigan State University, East Lansing, USA.
Thu V Duong, Department of Animal Science, The Reproductive and Developmental Sciences Program, College of Agriculture and Natural Resources, Michigan State University, East Lansing, USA.
Eric P Kasten, Clinical & Translational Sciences Institute, Michigan State University, East Lansing, USA; Department of Radiology, Michigan State University, East Lansing, USA.
Hanne M Hoffmann, Department of Animal Science, The Reproductive and Developmental Sciences Program, College of Agriculture and Natural Resources, Michigan State University, East Lansing, USA.
Authors’ contributions
G.Z. developed research ideas, conducted data analyses including statistical analysis and bioinformatics analysis, interpreted the data, and drafted manuscript. H.M.H. developed research ideas, interpreted the data, and drafted manuscript. T.V.D. and E.P.K. assisted with manuscript reviewing/revising. All authors gave final approval of the version to be published.
Data availability
Data are publicity available at NCBI GEO GSE59491.
Conflict of interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1]. Kassebaum NJ, Barber RM, Dandona L, Hay SI, Larson HJ, Lim SS, Lopez AD, Mokdad AH, Naghavi M, Pinho C, Steiner C, Vos T et al. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016; 388:1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012; 379:2162–2172. [DOI] [PubMed] [Google Scholar]
- [3]. Wang W, Yen H, Chen CH, Jasani N, Soni R, Koscica K, Reznik SE. Prevention of inflammation-associated preterm birth by knockdown of the endothelin-1-matrix metalloproteinase-1 pathway. Mol Med 2010; 16:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Mullan C. Management of preterm labour. Obstet Gynaecol Reprod Med 2018; 28:208–214. [Google Scholar]
- [5]. Birth P, Behrman PRE, Butler AS, Birth UP, Healthy A, Isbn O, Pdf T, Press NA, Press NA, Academy N, Academy N, Press NA . Institute of medicine (US) committee on understanding premature birth and assuring healthy outcomes. Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Butler AS, editors. Washington (DC): National Academies Press (US); 2007. PMID: 20669423. [PubMed]
- [6]. Dimes M of. March of Dimes 2020 Report Card 2020.
- [7]. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Berkowitz GS, Blackmore-Prince C, Lapinski RH, Savitz DA. Risk factors for preterm birth subtypes. Epidemiology. 1998; 9:279–285. [PubMed] [Google Scholar]
- [9]. di Renzo GC, Roura LC, European Association of Perinatal Medicine-Study Group on Preterm Birth . Guidelines for the management of spontaneous preterm labor. J Perinat Med 2006; 34:359–366. [DOI] [PubMed] [Google Scholar]
- [10]. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 2006; 15:R271–R277. [DOI] [PubMed] [Google Scholar]
- [11]. Leung JM, Martinez ME. Circadian rhythms in environmental health sciences. Curr Environ Heal Reports 2020; 7:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Zee PC, Attarian H, Videnovic A. Circadian rhythm abnormalities. Contin Lifelong Learn Neurol 2013; 19:132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 2014; 24:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lowrey PL, Takahashi JS. MAMMALIAN CIRCADIAN BIOLOGY: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 2004; 5:407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet 2011; 74:175–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update 2014; 20:293–307. [DOI] [PubMed] [Google Scholar]
- [17]. Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 2014; 5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. McCarthy RT, Jungheim ES, Fay JC, Bates K, Herzog ED, England SK. Riding the rhythm of melatonin through pregnancy to deliver on time. Front Endocrinol (Lausanne) 2019; 10:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Yaw A, McLane-Svoboda A, Hoffmann H. Shiftwork and light at night negatively impact molecular and endocrine timekeeping in the female reproductive axis in humans and rodents. Int J Mol Sci 2020; 22:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Sen A, Hoffmann HM. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol Cell Endocrinol 2020; 501:110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Hoffmann HM, Meadows JD, Breuer JA, Yaw AM, Nguyen D, Tonsfeldt KJ, Chin AY, Devries BM, Trang C, Oosterhouse HJ, Lee JS, Doser JW et al. The transcription factors SIX3 and VAX1 are required for suprachiasmatic nucleus circadian output and fertility in female mice. J Neurosci Res 2021. doi: 10.1002/jnr.24864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Valenzuela FJ, Vera J, Venegas C, Pino F, Lagunas C. Circadian system and melatonin hormone: Risk factors for complications during pregnancy. Obstet Gynecol Int 2015; 2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Wharfe MD, Mark PJ, Wyrwoll CS, Smith JT, Yap C, Clarke MW, Waddell BJ. Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J Endocrinol 2016; 228:135–147. [DOI] [PubMed] [Google Scholar]
- [24]. Martin-Fairey CA, Zhao P, Wan L, Roenneberg T, Fay J, Ma X, McCarthy R, Jungheim ES, England SK, Herzog ED. Pregnancy induces an earlier chronotype in both mice and women. J Biol Rhythms 2019; 34(3):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Zhu JL, Hjollund NH, Andersen A-MN, Olsen J. Shift work, job stress, and late fetal loss: The National Birth Cohort in Denmark. J Occup Environ Med 2004; 46:1144–1149. [DOI] [PubMed] [Google Scholar]
- [26]. Whelan EA, Lawson CC, Grajewski B, Hibert EN, Spiegelman D, Rich-Edwards JW. Work schedule during pregnancy and spontaneous abortion. Epidemiology 2007; 18:350–355. [DOI] [PubMed] [Google Scholar]
- [27]. Lawson CC, Rocheleau CM, Whelan EA, Hibert ENL, Grajewski B, Spiegelman D, Rich-Edwards JW. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol 2012; 206:327–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Grajewski B, Whelan EA, Lawson CC, Hein MJ, Waters MA, Anderson JL, MacDonald LA, Mertens CJ, Tseng C-Y, Cassinelli RT. Miscarriage among flight attendants. Epidemiology 2015; 26:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Begtrup LM, Specht IO, Hammer PEC, Flachs EM, Garde AH, Hansen J, Hansen ÅM, Kolstad HA, Larsen AD, Bonde JP. Night work and miscarriage: A Danish nationwide register-based cohort study. Occup Environ Med 2019; 76:302–308. [DOI] [PubMed] [Google Scholar]
- [30]. Suzumori N, Ebara T, Matsuki T, Yamada Y, Kato S, Omori T, Saitoh S, Kamijima M, Sugiura-Ogasawara M. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: A large prospective cohort study—Japan environment and children’s study. Birth 2020; 47:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Albrecht U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012; 74:246–260. [DOI] [PubMed] [Google Scholar]
- [32]. Hänzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 2013; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Heng YJ, Pennell CE, McDonald SW, Vinturache AE, Xu J, Lee MWF, Briollais L, Lyon AW, Slater DM, Bocking AD, De Koning L, Olson DM et al. Maternal whole blood gene expression at 18 and 28 weeks of gestation associated with spontaneous preterm birth in asymptomatic women. PLoS One 2016; 11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Feng Y, Wang Y, Liu H, Liu Z, Mills C, Owzar K, Xie J, Han Y, Qiang DC, Brhane Y, Mclaughlin J, Brennan P et al. Novel genetic variants in the P38MAPK pathway gene ZAK and susceptibility to lung cancer. Mol Carcinog 2018; 57:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Zhou G, Holzman C, Heng YJ, Kibschull M, Lye SJ. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. J Matern Neonatal Med 2020; 0:1–9. [DOI] [PubMed] [Google Scholar]
- [36]. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Lomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci 2005; 102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Zhou G, Holzman C, Chen B, Wang P, Heng YJ, Kibschull M, Lye SJ, Kasten EP. EBF1-correlated long non-coding RNA transcript levels in 3rd trimester maternal blood and risk of spontaneous preterm birth. Reprod Sci 2021; 28:541–549. [DOI] [PubMed] [Google Scholar]
- [39]. Zhou G, Holzman C, Heng YJ, Kibschull M, Lye SJ, Vazquez A. EBF1 gene mRNA levels in maternal blood and spontaneous preterm birth. Reprod Sci 2020; 27:316–324. [DOI] [PubMed] [Google Scholar]
- [40]. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 2004; 14:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 2003; 23:11202–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female ClockΔ19 mutant mice. Reprod Fertil Dev 2004; 16:801–810. [DOI] [PubMed] [Google Scholar]
- [43]. Dolatshad H, Campbell EA, O’hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod 2005; 21:68–79. [DOI] [PubMed] [Google Scholar]
- [44]. Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction 2008; 135:559–568. [DOI] [PubMed] [Google Scholar]
- [45]. Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction 2010; 139:1077–1090. [DOI] [PubMed] [Google Scholar]
- [46]. Germain AM, Valenzuela GJ, Ivankovic M, Ducsay CA, Gabella C, Serón-Ferré M. Relationship of circadian rhythms of uterine activity with term and preterm delivery. Am J Obstet Gynecol 1993; 168:1271–1277. [DOI] [PubMed] [Google Scholar]
- [47]. Hodžic A, Lavtar P, Ristanovic M, Novakovic I, Dotlic J, Peterlin B. Genetic variation in the clock gene is associated with idiopathic recurrent spontaneous abortion. PLoS One 2018; 13:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Kovac U, Jasper EA, Smith CJ, Baer RJ, Bedell B, Donovan BM, Weathers N, Zmrzljak UP, Jelliffe-Pawlowski LL, Rozman D, Ryckman KK. The association of polymorphisms in circadian clock and lipid metabolism genes with 2nd trimester lipid levels and preterm birth. Front Genet 2019; 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Hammoud E, Bujold E, Krapp M, Diamond M, Baumann P. Recurrent miscarriages and risks of PTB. Fertil Steril 2004; 82:S18.15363686 [Google Scholar]
- [50]. Oliver-Williams C, Fleming M, Wood AM, Smith GCS. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980-2008: A historical cohort study. BJOG An Int J Obstet Gynaecol 2015; 122:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Fukuta K, Yoneda S, Yoneda N, Shiozaki A, Nakashima A, Minamisaka T, Imura J, Saito S. Risk factors for spontaneous miscarriage above 12 weeks or premature delivery in patients undergoing cervical polypectomy during pregnancy. BMC Pregnancy Childbirth 2020; 20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Li R, Cheng S, Wang Z. Circadian clock gene plays a key role on ovarian cycle and spontaneous abortion. Cell Physiol Biochem 2015; 37:911–920. [DOI] [PubMed] [Google Scholar]
- [53]. Iams JD. What have we learned about uterine contractions and preterm birth? The HUAM prediction study. Semin Perinatol 2003; 27:204–211. [DOI] [PubMed] [Google Scholar]
- [54]. Wang X, Mozhui K, Li Z, Mulligan MK, Ingels JF, Zhou X, Hori RT, Chen H, Cook MN, Williams RW, Lu L. A promoter polymorphism in the Per3 gene is associated with alcohol and stress response. Transl Psychiatry 2012; 2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Lilliecreutz C, Larén J, Sydsjö G, Josefsson A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC Pregnancy Childbirth 2016; 16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Nagel C, Aurich C, Aurich J. Stress effects on the regulation of parturition in different domestic animal species. Anim Reprod Sci 2019; 207:153–161. [DOI] [PubMed] [Google Scholar]
- [57]. Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia or Genes and Genomes. Nucleic Acids Res 2000; 28:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Circadian rhythms, Neuroinflammation and oxidative stress in the story of Parkinson’s disease. Cell 2020; 9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Mowa CN, Papka RE. The role of sensory neurons in cervical ripening: Effects of estogen and neuropeptides. J Histochem Cytochem 2004; 52:1249–1258. [DOI] [PubMed] [Google Scholar]
- [60]. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123:4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res 2003; 92:827–839. [DOI] [PubMed] [Google Scholar]
- [63]. Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998; 179:1248–1253. [DOI] [PubMed] [Google Scholar]
- [64]. Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: An indirect support for endogenous pathway to membrane rupture. J Perinat Med 1999; 27:362–368. [DOI] [PubMed] [Google Scholar]
- [65]. Geng J, Huang C, Jiang S. Roles and regulation of the matrix metalloproteinase system in parturition. Mol Reprod Dev 2016; 83:276–286. [DOI] [PubMed] [Google Scholar]
- [66]. Minor DL, Masseling SJ, Yuh NJ, Lily YJ. Transmembrane structure of an inwardly rectifying potassium channel. Cell 1999; 96:879–891. [DOI] [PubMed] [Google Scholar]
- [67]. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev 2010; 90:291–366. [DOI] [PubMed] [Google Scholar]
- [68]. Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, Jassal B, Korninger F, May B, Milacic M, Roca CD et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res 2018; 46:D649–D655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia 2000; 30:39–48. [DOI] [PubMed] [Google Scholar]
- [70]. Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: From K + buffering to cell differentiation. J Neurochem 2008; 107:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. McCloskey C, Rada C, Bailey E, McCavera S, Berg HA, Atia J, Rand DA, Shmygol A, Chan Y, Quenby S, Brosens JJ, Vatish M et al. The inwardly rectifying K + channel KIR 7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med 2014; 6:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, Miller DE, Litterman N, Jiang P-P, Russell L, Hinds DA, Hu Y et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med 2017; 377:1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicity available at NCBI GEO GSE59491.


