Abstract
A 65-year-old female with newly diagnosed cholangiocarcinoma was referred for a FDG PET/CT for initial staging. There was a region of moderate FDG avidity localizing to the hepatic hilum representing the primary site of malignancy. An unexpected moderately FDG avid focus was demonstrated in the spinous process of the T11 vertebra with no corresponding mass lesion seen on low dose CT and no evidence of distant metastatic disease elsewhere. A percutaneous CT guided needle bone biopsy was performed which confirmed a solitary T11 spinous process metastasis on histopathology.
Keywords: PET/CT, Cholangiocarcinoma, Metastasis, Cancer, Bone biopsy
Introduction
Cholangiocarcinoma is an aggressive malignancy which arises from the bile duct epithelium. Metastatic spread is typically via regional lymph nodes, liver, lungs and peritoneum. Bone metastases are not uncommon but typically present in later stages of disease and at multiple sites. We present a case of metastatic cholangiocarcinoma which demonstrated a solitary, FDG avid spinous process metastasis which was confirmed via CT guided percutaneous needle biopsy and histopathological analysis.
Case presentation
A 65-year-old female presented to the hospital with epigastric abdominal pain, pruritis and weight loss. Liver function tests showed obstructive jaundice with a bilirubin of 103 umol/L (normal range <19). Hepatitis screen showed past infection with Hepatitis B virus (Hep B core antibody positive). Initial CT of the abdomen/pelvis showed irregular, nodular, enhancing tissue at the hepatic hilum with marked intra-hepatic dilatation, suspicious for a cholangiocarcinoma (Fig. 1A). MRI demonstrated increased delayed enhancement of the common bile duct consistent with an infiltrating neoplastic process (Fig. 1B). The patient underwent an endoscopic retrograde cholangiopancreatogram with placement of a biliary stent. The patient underwent FDG-PET for initial staging, which demonstrated an irregular, moderately avid region localizing to the hepatic hilum on CT, representing the primary site of malignancy. There was also an unexpected focus of moderate avidity localizing to the T11 vertebra at the junction of the laminae and the spinous process with no corresponding mass lesion on CT (Fig. 2B-D). This was thought to be reactive/inflammatory with solitary bony metastasis not excluded. There was no FDG avid metastatic disease elsewhere. CT guided percutaneous needle biopsy of the bone was performed several weeks later. A bone biopsy needle was introduced into the site of FDG avidity in the T11 vertebral body using a left posterolateral approach (Fig. 3). Immunohistopathological analysis of the sample showed scattered superficial strips of mucinous epithelium lined by hyperchromatic atypical nuclei with gland formation with positive staining for CAM 5.2, CK 7 and CK 19, consistent with metastatic biliary adenocarcinoma. Given the presence of distant metastasis, the patient was referred to the medical oncology team and was treated with palliative intent carboplatin/gemcitabine chemotherapy.
Fig. 1.
Axial contrast enhanced CT (A) in portal-venous phase showed irregular, nodular, enhancing tissue at the hepatic hilum with marked intra-hepatic dilatation, suspicious for a cholangiocarcinoma. T1 weighted axial delayed gadolinium enhanced MRCP (B) demonstrates increased delayed enhancement of the common bile duct consistent with an infiltrating neoplastic process (Fig B, white arrow)
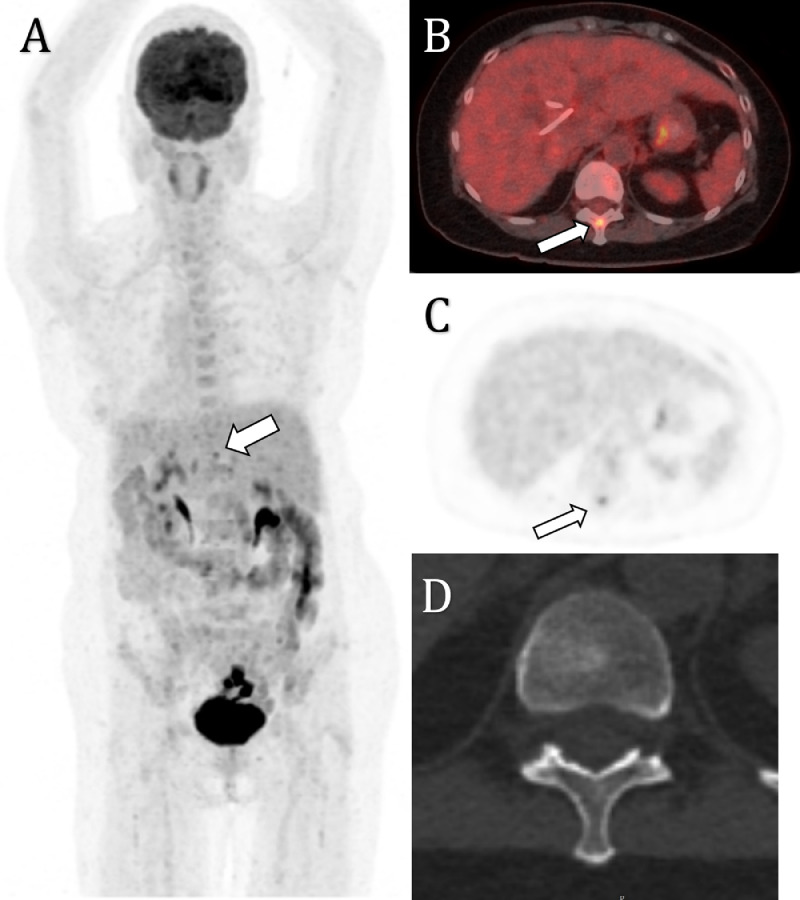
Fig. 2.
Maximal intensity projection (A), axial PET (B), and fused PET/CT (C) demonstrates an irregular, moderately avid region (SUVmax 6.2) localising to the hepatic hilum on CT, representing the primary site of malignancy. There is a focus of moderate avidity (SUVmax 6.2, white arrow) localising to the T11 vertebra at the junction of the laminae and the spinous process with no corresponding lesion on 1.25mm slice-thickness CT (D)
Fig. 3.
Axial CT acquired during image guided percutaneous bone biopsy. A bone biopsy needle was introduced into the site of FDG avidity in the T11 vertebral body using a left posterolateral approach
Discussion
Cholangiocarcinoma is an aggressive malignancy which presents late, is difficult to diagnose and is associated with high mortality [1], [2], [3], [4]. It has an incidence of 1-2 per 100,000 in western countries, with a slightly higher incidence in Asia due to the presence of endemic parasites such as Opisthorcus viverrine and Clonorchis sinenis [2,4]. Cholangiocarcinoma arises from the epithelium of the hepatic bile ducts. Metastatic spread of disease typically occurs first via the lymphatics to regional lymph nodes, then consequently via haematogenous spread to liver, lungs and peritoneum [1]. Bone metastases have been reported in the literature, however, these are often found in later stages of disease, at multiple vertebral levels and concurrently with other sites of metastases [5], [6], [7]. Bony metastases from cholangiocarcinoma typically show a mixed osteolytic/osteoblastic pattern on CT and predominantly involve the anterior spinal elements [8]. A solitary FDG-avid spinous process metastasis on presentation of cholangiocarcinoma is extremely rare. Surgical resection is the only chance for cure in patients with localized disease, and distant metastases impedes curative intent resections [9].
Furthermore, incidental, isolated FDG uptake in the spinous process without mass lesion on CT can be mistakenly attributed to benign processes such as Bastruup's disease [10], [11].
As such, histopathological clarification is paramount to diagnostic workup in these cases to prognosticate and plan future treatment. Surgical biopsy of these vertebral sites is typically the gold standard to provide adequate volume of material for immunological and histopathological analysis. CT guided percutaneous needle biopsy provides a cost-effective, less invasive, and faster alternative to open surgical biopsy [12]. Given the spinous process’ small anatomical volume and proximity to the spinal cord, needle biopsies of these sites are technically challenging and should be performed by experienced interventional radiologists.
Conclusion
Isolated metastasis of cholangiocarcinoma to the spinous process is uncommon, but nevertheless a possibility in patients with biliary cancer. Percutaneous biopsy of these sites is often technically challenging but should be considered to confirm metastatic disease to guide patient management.
Footnotes
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient consent: Personal patient information was removed from the presented radiology images and appropriate patient consent was acquired.
References
- 1.Khan S, Thomas HC, Davidson BR, Cholangiocarcinoma Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;336(9493):1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Landis S.H., Murray T., Bolden S., Wingo P.A. Cancer statistics. CA Cancer J. Clin. 1998;48(1):6–29. doi: 10.3322/canjclin.48.1.6. 1998. [DOI] [PubMed] [Google Scholar]
- 3.Sangsin A, Saiudom D, Pongmanee S, Saengsin J, Leerapun T, Murakami H. Natural history and prognostic factors of cholangiocarcinoma with spinal metastasis: a 10-year single center study. Clin Spine Surg. 2018;31(3):E160–E165. doi: 10.1097/BSD.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ketpueak T, Thiennimitr P, Apaijai N, Chattipakorn S, Chattipakorn N. Association of chronic opisthorchis infestation and microbiota alteration on tumorigenesis in cholangiocarcinoma. Clin Trans Gastroenterol. 2021;12:1. doi: 10.14309/ctg.0000000000000292. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowsiriroj P, Paholpak P, Sirichativapee W. Cholangiocarcinoma with spinal metastasis: single center survival analysis. J Clin Neurosci. 2017;38:43–48. doi: 10.1016/j.jocn.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Katayose Y, Nakagawa K, Yamamoto K, Yoshida H, Hayashi H, Mizuma M. Lymph nodes metastasis is a risk factor for bone metastasis from extrahepatic cholangiocarcinoma. Hepatogastroenterology. 2012;59:1758–1760. doi: 10.5754/hge11806. [DOI] [PubMed] [Google Scholar]
- 7.Hahn F, Müller L, Mähringer-Kunz A, Tanyildizi Y, Dos Santos DP, Düber C. Distant metastases in patients with intrahepatic cholangiocarcinoma: does location matter? A retrospective analysis of 370 patients. J Oncol. 2020;2020 doi: 10.1155/2020/7195373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thammaroj P, Chimcherd A, Chowchuen P, Panitchote A, Sumananont C, Wongsurawat N. Imaging features of bone metastases from cholangiocarcinoma. Eur J Radiol. 2020 doi: 10.1016/j.ejrad.2020.109118. [DOI] [PubMed] [Google Scholar]
- 9.Rahnemai-Azar A.A., Weisbrod A.B., Dillhoff M., Schmidt C., Pawlik T.M. Intrahepatic cholangiocarcinoma: current management and emerging therapies. Expert Rev Gastroenterol Hepatol. 2017;11(5):439–449. doi: 10.1080/17474124.2017.1309290. [DOI] [PubMed] [Google Scholar]
- 10.Lin E. Baastrup's disease (kissing spine) demonstrated by FDG PET/CT. Skeletal Radiol. 2008;37(2):173–175. doi: 10.1007/s00256-007-0379-2. [DOI] [PubMed] [Google Scholar]
- 11.Rosen RS, Fayad L, Wahl RL. Increased 18F-FDG uptake in degenerative disease of the spine: Characterization with 18F-FDG PET/CT. J. Nucl. Med. 2006;47(8):1274–1280. [PubMed] [Google Scholar]
- 12.Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17(7):975–981. doi: 10.1007/s00586-008-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]