Abstract
Oil pollution is of increasing concern for environmental safety and the use of microbial surfactants in oil remediation has become inevitable for their efficacy and ecofriendly nature. In this work, biosurfactants of bacteria isolated from oil-contaminated soil have been characterized. Four potent biosurfactant-producing strains (SD4, SD11, SD12 and SD13) were selected from 27 isolates based on drop collapse assay and emulsification index, and identified as species belonging to Bacillus, Burkholderia, Providencia and Klebsiella, revealed from their 16S rRNA gene-based analysis. Detailed morphological and biochemical characteristics of each selected isolate were determined. Their growth conditions for maximum biosurfactant production were optimized and found quite similar among the four isolates with a pH of 3.0 and temperature 37°C after 6 or 7 days of growth on kerosene. The biosurfactants of SD4, SD11 and SD12 appeared to be glycolipids and that of SD13 a lipopeptide. Emulsification activity of most of the biosurfactants was stable at low and high temperatures (4–100°C), a wide range of pH (2–10) and salt concentrations (2–7% NaCl). Each biosurfactant showed antimicrobial activity against two or more pathogenic bacteria. The biosurfactants were well-capable of emulsifying kerosene, diesel and soya bean, and could efficiently degrade diesel.
Keywords: microbial surfactants, biosurfactant-producing bacteria, petroleum hydrocarbon bioremediation, crude oil biodegradation, emulsification index, production optimization
1. Introduction
Petroleum-based fuels have been a major concern for life and environment especially in industrialized and developing countries [1]. Over the years, numerous natural and anthropogenic incidents have led to an enormous release of petroleum oil into nature thus posing a serious threat to the quality and sustainability of ecosystems [2,3]. Petroleum oil contains many aromatic toxic compounds such as benzene, ethylbenzene, toluene, xylene, etc. that are harmful for most life forms [4–7]. In addition to causing physical damage to habitats, the toxic ingredients of petroleum make mutagenic and carcinogenic changes to people [8]. Exposure to benzene and benzopyrene, for example, was found associated with an increased risk of leukaemia and lung cancer, respectively [9,10]. However, microbial populations particularly some bacteria and fungi manage to thrive on these rather harmful aromatic pollutants [11]. Regardless of the pollutants' high toxicity and hydrophobicity, bacterial species including those of the genera Pseudomonas, Bacillus, Streptomyces, Stenotrophomonas, etc. have been found inhabiting such petroleum-rich niches [12,13]. One of the key properties that allow these microbes to endure polluted environments is their ability to uptake petroleum hydrocarbons and facilitate their degradation by the production of a group of surface-active agents known as biosurfactants [14]. The biosurfactants are excreted from microbial cells or produced at the cell surface and include a broad range of chemical structures with diverse surface properties. They can be low molecular weight biomolecules that are generally glycolipids such as trehalose lipids, sophorolipids and rhamnolipids or lipopeptides such as surfactin, gramicidin S and polymyxin, or high molecular weight compounds such as polysaccharides, proteins, lipopolysaccharides, lipoproteins or complex mixtures of these biopolymers [15]. Biochemically, the microbial surfactants consist of both hydrophilic and hydrophobic moieties [16]. This amphipathic nature allows biosurfactants to partition at the interface between aqueous and hydrophobic phases, e.g. oil and water, or oil and rock interfaces, thus reducing the surface and interfacial tensions [17]. The biosurfactants, therefore, appear very effective in mobilization, increase of bioavailability and degradation of residual oil at a contaminated area [18]. Other important functions of biosurfactants include antimicrobial and antiviral activities, immunomodulation, enzyme inhibition, regulation of cell surface properties facilitating attachment to or detachment from surfaces, etc. [19,20].
A variety of synthetic or chemical surfactants are also available and used for the environmental bioremediation of petroleum hydrocarbons [18]. But microbial surfactants are of particular importance in this regard since they offer several advantages over their synthetic counterparts. For example, biosurfactants show better foaming capacity, selectivity and specific activity as compared to the synthetic surfactants [21]. Moreover, biosurfactants, due to their higher biodegradability, are less toxic than the chemical surfactants [22]. In addition, microbial surfactants are more stable and efficient over a wide range of environmental conditions, e.g. temperatures, pH and salinity [23]. Hence, biosurfactants are considered better candidates for environmental oil recovery processes and supposed to replace the synthetic surfactants.
Due to the importance of microbial surfactants, the present study has been carried out to isolate efficient biosurfactant-producing bacteria and characterize their secreted surfactants for potential application in hydrocarbon bioremediation. Hence, bacterial species isolated from oil-contaminated sites were screened for biosurfactant production and selected isolates were examined for optimum yields at various culture conditions. Morphological, biochemical and taxonomic characteristics of the isolates and preliminary characteristics of their surfactants have also been studied. Additionally, the degradation of diesel oil by the biosurfactants under laboratory conditions was evaluated.
2. Material and methods
2.1. Soil samples
The soil was collected from three different locations of Chittagong (see electronic supplementary material, figure S1) in April 2018. The top layer (0–15 cm) of the surface soil was collected using sterile spatula into sterile zip-locked bags and kept in an icebox during transportation to the laboratory. The physico-chemical properties of the soil, e.g. pH and the temperature, were measured at the collection sites (table 1).
Table 1.
Location and physico-chemical properties of the samples.
| location | GPS coordinates | soil temperature | soil pH | strains isolated |
|---|---|---|---|---|
| Shoraipara fuel station, Pahartali | 22.35586828 N, 91.78846921 E | 30°C | 8 | SD1, SD2, SD3, SD4 |
| engine filling station, Chittagong Railway Academy | 22.32053532 N, 91.78405199 E | 31°C | 8.5 | SD5, SD6, SD7 |
| engine washing station, Chittagong Railway Academy | 22.32338341 N, 91.78185608 E | 29°C | 9 | SD8, SD9, SD10 |
| main station, Chittagong Railway Academy | 22.32121629 N, 91.78552304 E | 32°C | 8 | SD11, SD12, SD13 |
2.2. Enrichment and isolation
One gram of each soil sample was dissolved in 99 ml of Mckeen medium containing 25 g glucose, 2.5 g monosodium glutamate, 3.0 g yeast extract, 1.0 g MgSO4·7H2O, 1.0 g K2HPO4, 0.5 g KCl and 1.0 ml trace element solution (0.64 g MnSO4 · 7H2O, 0.16 g CuSO4·5H2O and 0.015 g FeSO4·7H2O in 100 ml of distilled water) per 1 l distilled water [24]. After incubation at 37°C for 3 days at 150 r.p.m., 100 µl of the suspension was spread over Mckeen agar plates and incubated at 37°C. Single colonies from the plate were picked and repeatedly streaked on fresh plates until pure cultures appeared that were preserved as slant cultures.
2.3. Hydrocarbon overlay assay
Initial screening of the isolates for biosurfactant production was performed by hydrocarbon overlay assay as described by Hanano et al. [25]. One microliter of culture was spread over a McKeen agar plate coated with 100 µl of kerosene and incubated at 37°C for 7 days. Colony surrounded by an emulsified halo was considered positive for biosurfactant production.
2.4. Drop collapse assay
Drop collapse assay was carried out according to the description of [26] using cell-free supernatant prepared from the centrifugation of a 48 h culture at 5000 r.p.m. for 20 min at 4°C. A single drop of diesel oil was placed on a glass slide upon which one drop of the supernatant was dropped. After 1–2 min, the flattening property was recorded. If the drop collapsed the result was scored as positive while if it remained beaded the result was considered negative.
2.5. Blood agar assay
To perform blood agar assay, fresh cultures were streaked on blood agar plates (Himedia, India) containing 5–7% sheep blood. After incubation at 37°C for 48–72 h, the formation of a clear halo surrounding the colonies was scored as a positive result [27].
2.6. Determination of emulsification index
The emulsification index (E24) was determined as previously reported [28]. Three microlitres of kerosene was added to the same amount of cell-free supernatant and vortexed for 2 min. After 24 h, the height of the stable emulsion layer was measured. Water was used as negative control. E24 was defined as the percentage of the height of the emulsified layer divided by the total height of the liquid column:
2.7. PCR and sequencing of 16S rRNA gene
To amplify 16S rRNA gene sequences, cells from the stock culture were inoculated in nutrient broth containing 5.0 g peptone, 3.0 g yeast extract, 5.0 g NaCl in 1 l distilled water and incubated overnight at 37°C. The activated cultures were further grown in nutrient broth at 37°C overnight and their genomic DNA was extracted using a Maxwell 16 Blood DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. PCR was carried out with the genomic DNA using the primers 27F (5′-AGAGTTTGATCNTGGCTCAG-3′) and 1492R (5′-GCTTACCTTGTTACGACTT-3′). Sequencing of the purified PCR products was performed as previously described [29]. The sequences were submitted to GenBank under the accession nos. MZ254917–MZ254920.
2.8. Sequence analysis
Taxonomic affiliation of the isolates was determined based on the identity of their 16S rRNA gene sequences with those in the GenBank database and with the nearest type strains in EZBioCloud database as described in [29].
2.9. Phylogenetic tree construction
To construct a phylogenetic tree, sequences were aligned using ClustalW algorithms in the Geneious application (Geneious Prime 2021.1; https://www.geneious.com) [30]. Sequences of the type strains (T) were obtained from EZBioCloud with the accession numbers AE016877 (Bacillus cereus ATCC 14579), LASD01000006 (Burkholderia contaminans LMG 23361), CP022823 (Klebsiella quasivariicola KPN1705) and HQ888847 (Providencia thailandensis C1112). Phylogenetic tree of the aligned sequences was constructed using the maximum-likelihood method with Tamura–Nei distance algorithm in molecular evolutionary genetics analysis (MEGA) application according to a previous report [31].
2.10. Morphological and biochemical characterization
Characterization of the selected isolates by determination of colony morphology, biochemical and growth characteristics and fermentation of various carbohydrates were carried out as described previously [32,33].
2.11. Optimization of culture conditions
To determine optimum culture condition and hydrocarbon for biosurfactant production, the strains were grown for different incubation times (3–11 days), temperatures (25–50°C), pH (3–9) and hydrocarbon sources (kerosene, diesel, octane and soya bean) in Mckeen medium and the E24 value at each was determined.
2.12. Extraction of biosurfactant
Extraction of biosurfactant was carried out as previously described [34]. Briefly, activated cultures were incubated at 37°C for 7 days at 150 r.p.m. Culture supernatant was collected by centrifugation at 5000 r.p.m. for 20 min at 4°C and pH was adjusted to 2 with 1 M H2SO4. Equal volume of chloroform–methanol mixture (2 : 1) was then added and shaken vigorously for 5 min and allowed to stand until phase separation. The bottom solvent phase was then removed by a separating funnel and the upper aqueous phase was collected. The partially purified biosurfactant was concentrated by evaporation and preserved at −20°C until analysed.
2.13. Characterization of biosurfactants
The chemical nature of the partially purified biosurfactants was determined by various biochemical examinations. Ninhydrin test was performed as reported by Feignier et al. [35], biuret test was performed according to Patowary et al. [36], Molisch's test was performed according to the method of Vanavil et al. [37] and thin layer chromatography according to Lamilla et al. [38].
2.14. Determination of antimicrobial activity
Antimicrobial activity of selected isolates was determined using partially purified biosurfactant against clinical and environmental bacteria of both Gram-positive and Gram-negative strains including Bacillus cereus (ATCC 14574), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 9027), Salmonella typhi (ATCC 14028), Vibrio cholera (ATCC 14035) and Escherichia coli (ATCC 25922) by disc diffusion method as described previously [39].
2.15. Determination of stability
The stability of biosurfactant was assessed from the determination of E24 under various conditions such as temperature, pH and salinity. Thermal stability was estimated by placing at 4–121°C for 30 min followed by cooling to room temperature. pH stability was evaluated in the range of pH 1–10 adjusted with 1 N HCl or 1 N NaOH. Salinity was assessed using NaCl of 2–7% w/v.
2.16. Degradation of diesel oil by the selected strains
Degradation of diesel oil by the selected strains was measured by the gravimetric method described by Ganesh & Lin [40] in 100 ml minimal salt medium (MSM) containing 1.8 g K2HPO4, 4.0 g NH4Cl, 0.2 g MgSO4 . 7H2O, 0.1 g NaCl, 0.01 g FeSO4. 7H2O per litre enriched with 2% (v/v) filter-sterilized crude oil as the carbon source cultured at 37°C for 7 days at 150 r.p.m. The residual oil was recovered using the solvent extraction method by adding dichloromethane to the media. The media/solvent mixture was decanted into a separating funnel, shaken well and the organic phase was drained into a previously weighed beaker. After evaporation of dichloromethane, the beaker was again weighed until a consistent weight was obtained. The difference between the two weights provided the weight of the residual oil. The same procedure was used for oil extraction from the negative control media maintained under the same conditions without any inoculation. Degradation of oil was calculated by the following formula:
3. Results
3.1. Selection and characterization of biosurfactant producing bacteria
Following enrichment in Mckeen media supplemented with 0.1% kerosene, 13 colonies that produced emulsified halos on hydrocarbon overlay agar were initially selected (table 2). Further screening based on the drop collapse assay and emulsification index (E24; table 2) sorted out four isolates as efficient producers of biosurfactants (SD4, SD11, SD12, SD13). Although the hemolytic test, a method traditionally used in the screening, was performed, the technique has been reported not very reliable to detect biosurfactant production [41]. The selection criteria, therefore, relied principally upon the results of the drop collapse method and E24. All the four selected isolates showed a vigorous collapse in the drop collapse test and an E24 > 50%. The four isolates were taxonomically identified from their 16S rRNA gene-based analysis (figure 1a), and morphological and biochemical characteristics (table 3). The 16S rRNA gene sequence of the four strains showed maximum similarity to species of Bacillus, Burkholderia, Providencia and Klebsiella, respectively. This taxonomic affiliation was further supported by the phylogenetic relationship of the isolates with their closest type strains (figure 1b). Morphological and biochemical analysis (table 3) suggested that the isolates were non-motile, indole-negative and catalase-positive strains and, except the Bacillus strain (SD4), all were Gram-negative. All the four isolates could ferment glucose, fructose and sucrose. While the Burkholderia (SD11) and Klebsiella (SD13) strains also fermented raffinose, rhamnose, mannitol and lactose, Bacillus (SD4) and Providencia (SD12) isolates did not. Other biochemical properties such as cellular arrangement; citrate, nitrate, urease, methyl red, Voges–Proskauer, starch hydrolysis, deep glucose agar tests; and oxygen relationship were also determined (table 3) and found consistent with the taxonomic annotation according to Bergey's manual [42].
Table 2.
Screening of the isolates for biosurfactant production.
| isolates | hydrocarbon overlay agara | drop collapseb | blood hemolysisa | emulsification index (E24) (%) |
|---|---|---|---|---|
| SD1 | + | − | − | 0 |
| SD2 | + | − | − | 0 |
| SD3 | + | − | − | 5 |
| SD4 | + | +++ | + | 62.5 |
| SD5 | + | − | − | 6 |
| SD6 | + | ++ | − | 50 |
| SD7 | + | − | − | 50 |
| SD8 | + | ++ | + | 47.8 |
| SD9 | + | − | − | 25 |
| SD10 | + | − | − | 0 |
| SD11 | + | +++ | − | 55 |
| SD12 | + | +++ | + | 70 |
| SD13 | + | +++ | + | 74 |
a+ = positive result; − = negative result.
b+++ = vigorous collapse; ++ = moderate collapse; + = scanty collapse.
Figure 1.
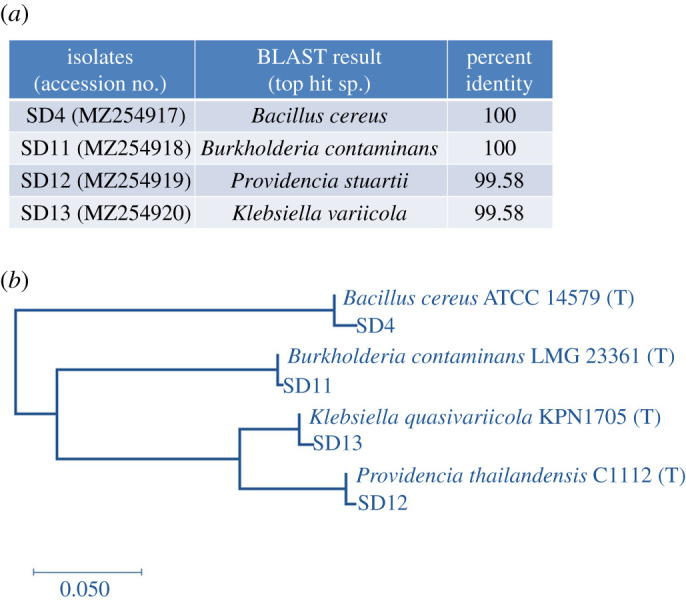
Phylotypes of the selected isolates. (a) Taxonomic affiliations of the four isolates based on sequence identity of their 16S rRNA genes. Accession numbers of the strains are provided in parentheses. (b) Phylogenetic tree of the isolates and their closest type strains (T).
Table 3.
Morphological, cultural and biochemical characteristics of the selected strains. + = positive result; – = negative result.
| features | Bacillus SD4 | Burkholderia SD11 | Providencia SD12 | Klebsiella SD13 |
|---|---|---|---|---|
| colony morphology | circular, raised, entire, smooth, off-white colour | circular, raised, entire, smooth, off-white colour | circular, raised, entire, smooth, off-white colour | circular, raised, entire, smooth, off-white colour |
| slant characteristics | effuse | filiform | arborescent | arborescent |
| Gram staining | + | − | − | − |
| motility test | − | − | − | − |
| cell arrangement | single | single | single | single |
| indole test | − | − | − | − |
| catalase test | + | + | + | + |
| citrate test | + | + | − | − |
| nitrate test | + | + | − | − |
| urease test | − | − | − | + |
| methyl red test | + | − | − | + |
| Voges–Proskauer test | + | − | − | + |
| deep glucose agar test | grow on the surface of medium | grow on the surface of medium | grow throughout medium | grow throughout medium |
| oxygen relationship | strictly aerobic | strictly aerobic | facultative anaerobic | facultative anaerobic |
| starch hydrolysis | + | − | + | − |
| fermentation of carbohydrates | ||||
| glucose | + | + | + | + |
| fructose | + | + | + | + |
| dextrose | + | + | + | + |
| sucrose | + | + | + | + |
| maltose | + | + | − | + |
| raffinose | − | + | − | + |
| rhamnose | − | + | − | + |
| mannitol | − | + | − | + |
| lactose | − | + | − | + |
| starch | + | − | + | + |
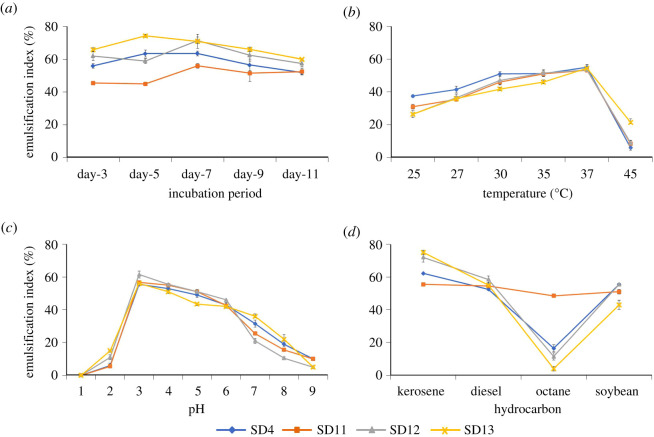
3.2. Optimum growth conditions for biosurfactant production
The influence of various culture conditions on the production of biosurfactants was analysed and presented as a function of the emulsification index (E24, %) (figure 2). While the production continued as long as day 11, the highest E24 was obtained on day 5 in the Bacillus (SD4) and Klebsiella (SD13) strains, and on day 7 in Burkholderia (SD11) and Providencia (SD12) strains (figure 2a). For all isolates, the optimum production temperature was found at 37°C (figure 2b). Although considerable biosurfactant production was also observed below this temperature, the production sharply dropped at 45°C in all isolates. Acidic conditions, on the other hand, appeared to favour biosurfactant production in the isolates with the highest yield taking place at pH 3 (figure 2c). In fact, the E24 was considerably high in most of the acidic range from pH 3 to 6 and decreased below or above this range although small emulsification was still observed at pH 2 and 9. With regards to the use of hydrocarbons in the media, kerosene was generally found most suitable for biosurfactant production followed by diesel and soya bean (figure 2d). By contrast, when octane was used as the carbon source, the emulsification capacity was very poor except for SD11 which showed a relatively better emulsification with octane. It appears that SD11 was the only isolate that could use all the hydrocarbon sources equally well for emulsification.
Figure 2.
Effect of growth conditions on emulsification activity of culture filtrates. The isolates were grown at different sets of culture conditions such as incubation period (a), temperature (b), pH (c) and carbon source (d), and the emulsification index of culture supernatant was recorded. Error bars represent one standard deviation of the mean of three experiments.
3.3. Chemical nature of the biosurfactants
The preliminary chemical structure of the biosurfactants was assessed from a series of biochemical reactions (table 4). Biosurfactants of Bacillus (SD4), Burkholderia (SD11) and Providencia (SD12) strains were found negative in the ninhydrin and biuret tests and in TLC sprayed with ninhydrin suggesting the absence of amino acids, but positive in Molisch test and TLC exposed to iodine vapour indicating the presence of carbohydrates and lipids. Taken together, biosurfactants of the three isolates seem glycolipid in nature. The Klebsiella (SD13) biosurfactant, however, showed quite a contrasting result in the biochemical tests with negative reaction in the Molisch test and positive reactions in both protein and lipid detection tests suggesting it to be a lipopeptide.
Table 4.
Results of biochemical tests for chemical characterization of the biosurfactants. + = positive result; − = negative result.
| isolates | protein detection |
carbohydrate detection | lipid detection | interpretation | ||
|---|---|---|---|---|---|---|
| ninhydrin | biuret | TLC (ninhydrin) | Molisch | TLC (iodine vapour) | ||
| SD4 | − | − | − | + | + | glycolipid |
| SD11 | − | − | − | + | + | glycolipid |
| SD12 | − | − | − | + | + | glycolipid |
| SD13 | + | + | + | – | + | lipopeptide |
3.4. Stability of the biosurfactants
The biosurfactant of each isolate was found to display a stable emulsification activity over a wide range of abiotic conditions (figure 3). Temperature sensitivity was assessed in a limit of 4–121°C. The Klebsiella (SD13) biosurfactant appeared most thermostable with relatively high emulsifying activity all along this temperature range (figure 3a). Biosurfactants of Bacillus (SD4) and Burkholderia (SD11) strains also showed similar stability with a slim decline over a temperature of 100°C. The Providencia (SD12) biosurfactant was, however, relatively less stable at temperatures below 25°C and above 70°C. High pH did not have much effect on the emulsifying capacity as the biosurfactants remained stable in both highly acidic and highly alkaline conditions (figure 3b). The biosurfactants of all four isolates maintained nearly constant values of E24 over pH 2–9. The tolerance of the biosurfactants to ionic stress was also examined at 2–10% NaCl (figure 3c). The biosurfactants of Burkholderia, Providencia and Klebsiella strains showed similar emulsification activity forming stable emulsions at all these saline concentrations, whereas that of the Bacillus strain greatly diminished below 3% NaCl and gradually increased at higher ionic strengths.
Figure 3.
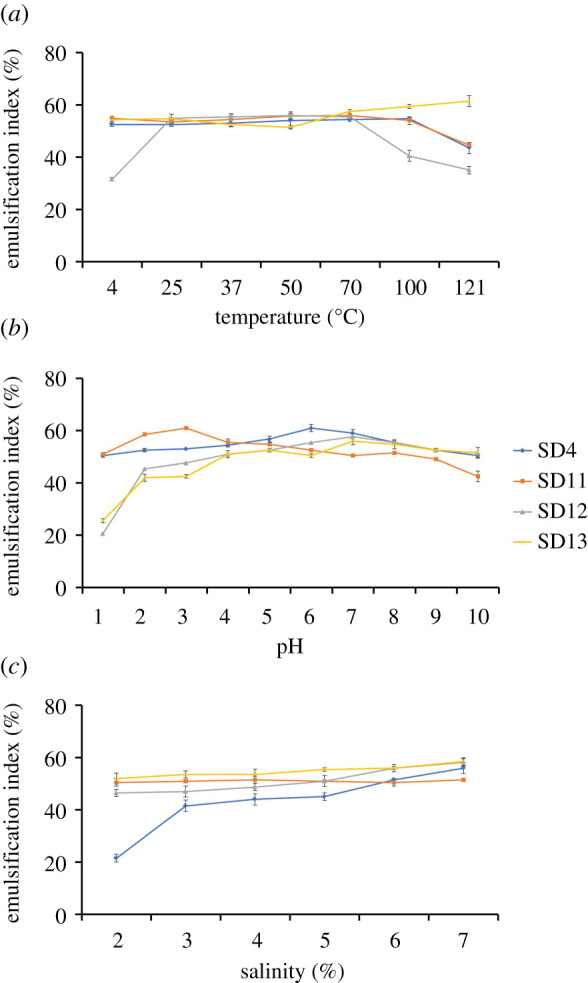
Stability of the biosurfactants at various (a) temperature, (b) pH and (c) salinity. The error bars represent one standard deviation of the mean, n = 3.
3.5. Antimicrobial activity
The biosurfactants were tested as antimicrobial agents against six pathogenic or indicator organisms including B. cereus, P. aeruginosa, S. aureus, S. typhi, V. cholera and E. coli (table 5). The biosurfactant obtained from the Bacillus (SD4) strain was found most effective among the four isolates showing activity against a maximum of five test organisms having no effects against only B. cereus. Bacillus cereus was, in fact, the most unaffected organisms of the six test strains resisting biosurfactants of most isolates. Only the Burkholderia (SD11) strain exhibited antagonistic activity against it. Vibrio cholera, in contrast, was inhibited by biosurfactants of all four isolates. Biosurfactant from the Providencia (SD12) strain appeared the least effective with only two of the six test strains, V. cholera and E. coli, being inhibited. In general, the biosurfactants were more effective against the Gram-negative strains (69%) in comparison to the Gram-positive bacteria (38%).
Table 5.
Antimicrobial activity of the biosurfactants against pathogenic or indicator bacterial strains. + = presence of activity; – = no activity.
| test strains | biosurfactant from |
|||
|---|---|---|---|---|
| SD4 | SD11 | SD12 | SD13 | |
| Gram-positive strains | ||||
| Bacillus cereus | − | + | − | − |
| Staphylococcus aureus | + | − | − | + |
| Gram-negative strains | ||||
| Pseudomonas aeruginosa | + | − | − | + |
| Salmonella typhi | + | + | − | − |
| Vibrio cholera | + | + | + | + |
| Escherichia coli | + | − | + | + |
3.6. Oil emulsification potential
The emulsifying capacity of the biosurfactants was measured using four different oil hydrocarbons, i.e. kerosene, diesel, octane and soya bean (figure 4a). Kerosene was found the most suitable substrate for emulsification followed by diesel and soya bean. Biosurfactants from all four isolates, especially Klebsiella (E24 = 75%), demonstrated better emulsification with kerosene as compared to the other hydrocarbons. Considerable emulsion (approx. 50%) was also formed with diesel and soya bean. With octane, however, the emulsification activity was generally very poor with the exception of the Burkholderia (SD11) biosurfactant which showed relatively higher emulsification with octane. Although E24 with octane was found to be 48.5% for the biosurfactant from Burkholderia, it was less than 20% for biosurfactants obtained from the other three isolates. Hence, the Burkholderia biosurfactant appears to have comparably broader substrate specificity. The ability of the isolates to degrade diesel oil was also studied by the determination of the amount of diesel oil left in the culture media after 7 days of growth (figure 4b); 40–52% degradation of diesel was achieved by the isolates which indicate their potential application in oil bioremediation.
Figure 4.
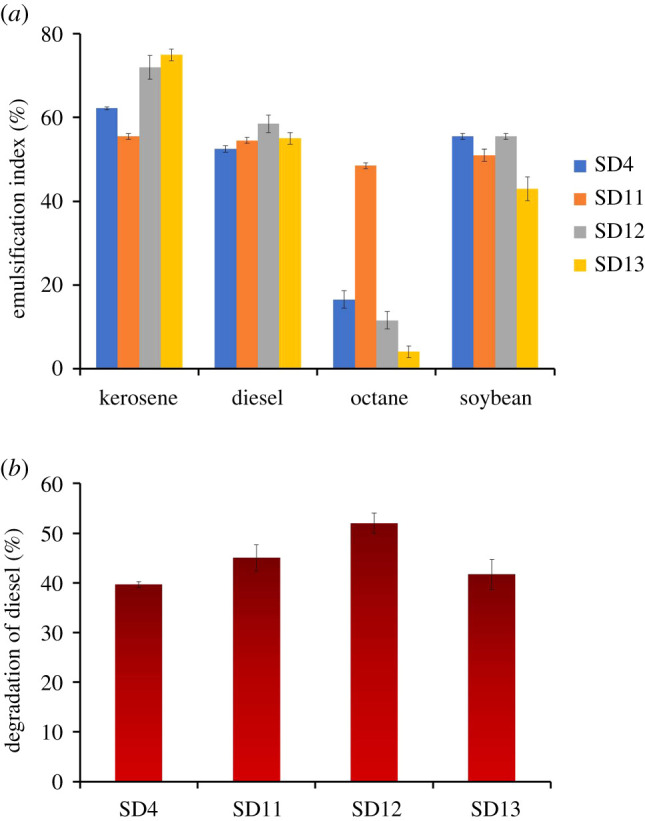
Emulsification activity of the biosurfactants on different types of oil (a), and degradation of diesel by the isolates (b). The error bars represent one standard deviation of the mean, n = 3.
4. Discussion
Bacterial surfactants play a major role in the emulsification of petroleum hydrocarbons; hence they are regarded as alternatives to chemical surfactants for superior properties like biodegradability, less toxicity, eco-friendliness and high specificity [43]. The biosurfactant-producing bacteria are found in diverse environments but mostly isolated from places rich in organic hydrophobic contaminants [44]. In the present work, therefore, biosurfactant producers were searched in oil-contaminated sites. The isolates were examined by various screening methods including hydrocarbon overlay assay, blood agar assay, drop collapse method and emulsification indices since previous reports have recommended use of multiple techniques to screen for efficient biosurfactant-producing strains [44,45]. The initial screening was based on the hydrocarbon overlay assay sorting out 13 isolates for further selection. Blood agar assay, although widely used for screening of biosurfactant production [41], has been reported to give false positive and negative results [41]. Hence, the emulsification activity which is regarded to be a very reliable and accurate method to screen for biosurfactant production [43], together with the drop collapse assay, was basically considered [41] in this study in the final selection of four potent strains from the 13 isolates (46.15%). The four isolates were identified based on their 16S rRNA gene sequences. About 500 bp of the approximately 1500 bp sequence that has been found to be of high quality was used for the taxonomic identification. Limitations of identification by relatively short sequences were, however, described [46]. A nearly full-length sequence is said to be helpful for making a confident species or strain level identification [47], although several reports argued that a shorter sequence such as approximately 500 bp can also provide necessary divergence for the purpose [48,49]. In fact, both 500 and 1500 bp are common lengths to be sequenced and compared for phylotype determinations, and sequences of various lengths are found in databases and the literature [49–57]. Nevertheless, analysis of a nearly full-length sequence of the 16S rRNA gene is usually recommended, especially when reporting a new species or when it is necessary to differentiate between specific strains in a genus. Indeed, full-length sequences are supposed to provide relatively better resolution than short reads particularly for strains having high sequence similarity since it is indeterminate which segment of the 16S rRNA gene would provide the differentiation. On the other hand, for clinical isolates, the initial 500 bp has been reported sufficient for taxonomic differentiation [49]. Recently, Farrance and Hong examined 208 diverse bacterial sequences of 131 randomly selected genera by both the initial 500 bp and the 1500 bp sequences [48]. They found that 93.7% of the samples did not show any difference in the species level identification between the two approaches, whereas in only 5.3% of the samples the full-length sequences showed better resolution. Bacterial identification in the MicroSeq system is also based on 500 bp sequences, and identification using sequences shorter than 500 bp has been reported as well [58–62]. In the present study, analysis of the approximately 500 bp sequence of the four isolates exhibited greater than 99% identity to the closest GenBank sequences. Each was found to be affiliated with a different genus: Bacillus, Burkholderia, Providencia and Klebsiella. Bacterial strains from these four genera are well known as being capable of producing biosurfactants and degradation of petroleum hydrocarbons [43,44,63–65]. Most particularly, the members of the genus Bacillus have been very frequently isolated from the soil of oil-polluted sites and reported as an effective bioresource for biosurfactants [44,66]. The isolates, except the Bacillus strain, were Gram-negative. The dominance of Gram-negative species seems common in soil with a history of contamination by oil or its byproducts, a characteristic that has been suggested to contribute in the survival of these populations in such harsh environments [67].
In any strain, however, culture conditions play a major role in the growth of the strain itself and also in its production of a particular metabolite. It is, therefore, important to find out the optimum culture condition and suitable hydrocarbon source to achieve the maximum yield [68]. In the present work, the highest production of biosurfactant was found at day 5 or day 7 depending on the strain. A similar incubation time of maximum production was also reported in several other analyses [69–71]. Among the other factors, a temperature of 37°C and pH in the range of 3–6 appeared to be most suitable for the selected isolates to produce biosurfactants. The optimal temperature was close to the soil temperature (approx. 30°C) during isolation which indicates a direct correlation of biosurfactant production to the growth of the microbes under suitable temperature, i.e. higher production as the cell density increases. The optimal pH, in contrast, was found lower than that of the soil (approx. 8) from which the bacteria were obtained. While similar growth conditions were observed in several other bacteria, many species also showed optimum yield for different temperatures and pH, either higher or lower [45,68].
Preliminary characterization of the chemical nature of the partially purified biosurfactants indicated that the biosurfactants had glycolipid structures with the exception of the Klebsiella biosurfactant that was a lipopeptide. The glycolipid biosurfactants have recently gained special attention for their ecofriendly nature, high efficiency in biodegradation as well as other special activities such as pesticidal, antifungal and antibacterial activities [72,73]. Accordingly, the glycolipid biosurfactants obtained in this work also showed antagonistic activity against several of the Gram-positive and Gram-negative bacteria. The most potent of these biosurfactants was that produced by the Bacillus strain (SD4) which demonstrated inhibitory effects against five of the six test organisms. Although previous research has shown that glycolipid biosurfactants such as mannosylerythritol have significant antimicrobial activity against Gram-positive bacteria [73], those extracted in the present study, in contrast, were usually more effective against the Gram-negative strains.
Another important feature of the glycolipid biosurfactants is their stability over an extreme range of pH, salinity and temperature [73] which is in line with the findings of the present research. Both glycolipid and lipopeptide biosurfactants of the present work exhibited good stability in maintaining emulsification at a wide range of pH, temperatures and salt concentrations thus indicating their suitability for application in extreme environmental or industrial conditions. The synthetic surfactants, on the other hand, are highly susceptible to such conditions. For example, salt concentrations over 2% NaCl were reported enough to inactivate a synthetic surfactant [74], whereas the emulsifying activity of the biosurfactants of this study remained unchanged from 2% to as high as 7% of NaCl.
To summarize, four bacterial strains and their secreted surfactants were characterized in this work. The partially purified biosurfactants had relatively high activity, formed stable emulsions with different hydrocarbons and showed good antimicrobial activity. Moreover, the biosurfactants also exhibited high levels of pH, salinity and thermal stability, and potential to degrade diesel oil, all which indicate their prospects for application in bioremediation and oil recovery processes under harsh conditions.
Supplementary Material
Acknowledgement
The authors would like to thank Dr Zobaidul Alam, Department of Microbiology, University of Chittagong for providing laboratory facilities and some chemicals. The authors also thank members of the Biochemistry and Pathogenesis of Microbes—BPM Research Group, Department of Biochemistry and Molecular Biology, University of Chittagong for their help.
Contributor Information
Ferdausi Ali, Email: seema@cu.ac.bd.
Tanim Jabid Hossain, Email: tanim.bmb@gmail.com.
Data accessibility
Sequence data presented in this study can be found in NCBIData Bank with the accession nos. MZ254917–MZ254920.
Authors' contributions
F.A. and T.J.H. contributed to conception and design, and supervised the study; S.D. and F.A. carried out laboratory experiments and generated data; F.A., T.J.H. and T.D. analysed the data; S.I.C. and T.J.H. performed sequence and phylogenetic analysis; T.J.H. wrote and prepared the manuscript; T.D. prepared the electronic supplementary material, figure S1; S.A.Z., M.N.A.C., F.A. and T.D. helped in finding information regarding methods and discussion; M.S.U. reviewed the manuscript; all authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was partially funded by the University of Chittagong.
References
- 1.Ahuja D, Tatsutani M.. 2009. Sustainable energy for developing countries. S.A.P.I.EN.S. Surveys and Perspectives Integrating Environment and Society.
- 2.Saadoun IMK. 2015. Impact of oil spills on marine life. London, UK: IntechOpen. [Google Scholar]
- 3.Bashir I, Lone FA, Bhat RA, Mir SA, Dar ZA, Dar SA. 2020. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and biotechnology: sustainable approaches to pollution degradation (eds Hakeem KR, Bhat RA, Qadri H), pp. 1-26. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 4.Strømgren T, Sørstrøm SE, Schou L, Kaarstad I, Aunaas T, Brakstad OG, Johansen Ø. 1995. Acute toxic effects of produced water in relation to chemical composition and dispersion. Mar. Environ. Res. 40, 147-169. ( 10.1016/0141-1136(94)00143-D) [DOI] [Google Scholar]
- 5.Henderson SB, Grigson SJW, Johnson P, Roddie BD. 1999. Potential impact of production chemicals on the toxicity of produced water discharges from North Sea oil platforms. Mar. Pollut. Bull. 38, 1141-1151. ( 10.1016/S0025-326X(99)00144-7) [DOI] [Google Scholar]
- 6.Washburn L, Stone S, MacIntyre S. 1999. Dispersion of produced water in a coastal environment and its biological implications. Cont. Shelf Res. 19, 57-78. ( 10.1016/S0278-4343(98)00068-5) [DOI] [Google Scholar]
- 7.Zhang X, Xu D, Zhu C, Lundaa T, Scherr KE. 2012. Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa strains. Chem. Eng. J. 209, 138-146. ( 10.1016/j.cej.2012.07.110) [DOI] [Google Scholar]
- 8.Pashin YUV, Bakhitova LM. 1979. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Perspect. 30, 185-189. ( 10.2307/3429123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denissenko MF, Pao A, Tang M, Pfeifer GP. 1996. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 274, 430-432. ( 10.1126/science.274.5286.430) [DOI] [PubMed] [Google Scholar]
- 10.Khalade A, Jaakkola MS, Pukkala E, Jaakkola JJ. 2010. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environ. Health 9, 31. ( 10.1186/1476-069X-9-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joutey NT, Bahafid W, Sayel H, ElGhachtouli N. 2013. Biodegradation: involved microorganisms and genetically engineered microorganisms. London, UK: IntechOpen. [Google Scholar]
- 12.Yoshida N, et al. 2005. Bacterial communities in petroleum oil in stockpiles. J. Biosci. Bioeng. 99, 143-149. ( 10.1263/jbb.99.143) [DOI] [PubMed] [Google Scholar]
- 13.Cai M, et al. 2015. Crude oil as a microbial seed bank with unexpected functional potentials. Sci. Rep. 5, 16057. ( 10.1038/srep16057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varjani SJ, Gnansounou E. 2017. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 245, 1258-1265. ( 10.1016/j.biortech.2017.08.028) [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg E, Ron EZ. 1999. High- and low-molecular-mass microbial surfactants. Appl. Microbiol. Biotechnol. 52, 154-162. ( 10.1007/s002530051502) [DOI] [PubMed] [Google Scholar]
- 16.Adetunji AI, Olaniran AO. 2021. Production and potential biotechnological applications of microbial surfactants: an overview. Saudi J. Biol. Sci. 28, 669-679. ( 10.1016/j.sjbs.2020.10.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. 2016. Biosurfactants: multifunctional biomolecules of the 21st Century. Int. J. Mol. Sci. 17, 401. ( 10.3390/ijms17030401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, Dirisala VR, Kodali VP. 2018. Role of biosurfactants in bioremediation of oil pollution: a review. Petroleum 4, 241-249. ( 10.1016/j.petlm.2018.03.007) [DOI] [Google Scholar]
- 19.Floris R, Rizzo C, Giudice AL. 2018. Biosurfactants from marine microorganisms. In Metabolomics: new insights into biology and medicine. London, UK: IntechOpen. ( 10.5772/intechopen.80493) [DOI] [Google Scholar]
- 20.Wang W, Cai B, Shao Z. 2014. Oil degradation and biosurfactant production by the deep sea bacterium Dietzia maris As-13–3. Front. Microbiol. 5, 711. ( 10.3389/fmicb.2014.00711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulugeta K, Kamaraj M, Tafesse M, Aravind J. 2021. A review on production, properties, and applications of microbial surfactants as a promising biomolecule for environmental applications. In Strategies and tools for pollutant mitigation: avenues to a cleaner environment (eds Aravind J, Kamaraj M, Prashanthi Devi M, Rajakumar S), pp. 3-28. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 22.Akbari S, Abdurahman NH, Yunus RM, Fayaz F, Alara OR. 2018. Biosurfactants—a new frontier for social and environmental safety: a mini review. Biotechnol. Res. Innov. 2, 81-90. ( 10.1016/j.biori.2018.09.001) [DOI] [Google Scholar]
- 23.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. 2010. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 87, 427-444. ( 10.1007/s00253-010-2589-0) [DOI] [PubMed] [Google Scholar]
- 24.Sudarmono P, Wibisana A, Listriyani LW, Sungkar S. 2019. Characterization and synergistic antimicrobial evaluation of lipopeptides from Bacillus amyloliquefaciens isolated from oil-contaminated soil. Int. J. Microbiol. 2019, e3704198. ( 10.1155/2019/3704198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanano A, Shaban M, Almousally I. 2017. Biochemical, molecular, and transcriptional highlights of the biosynthesis of an effective biosurfactant produced by Bacillus safensis PHA3, a petroleum-dwelling bacteria. Front. Microbiol. 8, 77. ( 10.3389/fmicb.2017.00077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson A, Molin G. 1987. Capacity for biosurfactant production of environmental Pseudomonas and Vibrionaceae growing on carbohydrates. Appl. Microbiol. Biotechnol. 26, 439-442. ( 10.1007/BF00253528) [DOI] [Google Scholar]
- 27.Carrillo PG, Mardaraz C, Pitta-Alvarez SI, Giulietti AM. 1996. Isolation and selection of biosurfactant-producing bacteria. World J. Microbiol. Biotechnol. 12, 82-84. ( 10.1007/BF00327807) [DOI] [PubMed] [Google Scholar]
- 28.Nitschke M, Pastore GM. 2004. Biosurfactant production by Bacillus subtilis using cassava-processing effluent. Appl. Biochem. Biotechnol. 112, 163-172. ( 10.1385/ABAB:112:3:163) [DOI] [PubMed] [Google Scholar]
- 29.Hossain TJ, Chowdhury SI, Mozumder HA, Chowdhury MNA, Ali F, Rahman N, Dey S. 2020. Hydrolytic exoenzymes produced by bacteria isolated and identified from the gastrointestinal tract of bombay duck. Front. Microbiol. 11, 2097. ( 10.3389/fmicb.2020.02097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearse M, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647-1649. ( 10.1093/bioinformatics/bts199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hossain TJ, Manabe S, Ito Y, Iida T, Kosono S, Ueda K, Hosomi A, Inoue D, Suzuki T. 2018. Enrichment and characterization of a bacterial mixture capable of utilizing C-mannosyl tryptophan as a carbon source. Glycoconj. J. 35, 165-176. ( 10.1007/s10719-017-9807-2) [DOI] [PubMed] [Google Scholar]
- 32.Carter GR. 1990. Isolation and identification of bacteria from clinical specimens. In Diagnostic procedure in veterinary bacteriology and mycology (eds Carter GR, Cole JR), pp. 19-39, 5th edn. San Diego, CA: Academic Press. [Google Scholar]
- 33.Hossain TJ, Alam MK, Sikdar D. 2011. Chemical and microbiological quality assessment of raw and processed liquid market milks of Bangladesh. Cont. J. Food Sci. Technol. 5, 6-17. ( 10.3923/rjdsci.2010.28.34) [DOI] [Google Scholar]
- 34.Sarin S, Khamsri B, Sarin C. 2011. Isolation of biosurfactant producing bacteria with antimicrobial activity against bacterial pathogens. EnvironmentAsia 4, 1-5. ( 10.14456/ea.2011.1) [DOI] [Google Scholar]
- 35.Feignier C, Besson F, Michel G. 1995. Studies on lipopeptide biosynthesis by Bacillus subtilis: isolation and characterization of iturin−, surfactin+ mutants. FEMS Microbiol. Lett. 127, 11-15. ( 10.1111/j.1574-6968.1995.tb07442.x) [DOI] [PubMed] [Google Scholar]
- 36.Patowary K, Saikia RR, Kalita MC, Deka S. 2015. Degradation of polyaromatic hydrocarbons employing biosurfactant-producing Bacillus pumilus KS2. Ann. Microbiol. 65, 225-234. ( 10.1007/s13213-014-0854-7) [DOI] [Google Scholar]
- 37.Vanavil B, Perumalsamy M, Rao AS. 2013. Biosurfactant production from novel air isolate NITT6 L: screening, characterization and optimization of media. J. Microbiol. Biotechnol. 23, 1229-1243. ( 10.4014/jmb.1212.12031) [DOI] [PubMed] [Google Scholar]
- 38.Lamilla C, Schalchli H, Briceño G, Leiva B, Donoso-Piñol P, Barrientos L, Rocha VAL, Freire DMG, Diez MC. 2021. A pesticide biopurification system: a source of biosurfactant-producing bacteria with environmental biotechnology applications. Agronomy 11, 624. ( 10.3390/agronomy11040624) [DOI] [Google Scholar]
- 39.Ndlovu T, Rautenbach M, Vosloo JA, Khan S, Khan W. 2017. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Expr. 7, 108. ( 10.1186/s13568-017-0363-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganesh A, Lin J. 2009. Diesel degradation and biosurfactant production by Gram-positive isolates. Afr. J. Biotechnol. 8, 5847-5854. ( 10.5897/AJB09.811) [DOI] [Google Scholar]
- 41.Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. 2004. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods 56, 339-347. ( 10.1016/j.mimet.2003.11.001) [DOI] [PubMed] [Google Scholar]
- 42.Garrity GM, Bell JA, Lilburn TG. 2004. Taxonomic outline of the prokaryotes. In Bergey's manual of systematic bacteriology, 2nd edn. New York, NY: Springer-Verlag. [Google Scholar]
- 43.Ahmad Z, Arshad M, Asghar HN, Sheikh MA, Crowley DE. 2016. Isolation, screening and functional characterization of biosurfactant producing bacteria isolated from crude oil contaminated site. Int. J. Agric. Biol. 18, 542-548. ( 10.17957/IJAB/15.0126) [DOI] [Google Scholar]
- 44.Soltanighias T, Singh AEA, Satpute SK, Banpurkar AG, Koolivand A, Rahi P. 2019. Assessment of biosurfactant-producing bacteria from oil contaminated soils and their hydrocarbon degradation potential. Environ. Sustain. 2, 285-296. ( 10.1007/s42398-019-00074-0) [DOI] [Google Scholar]
- 45.Nwaguma IV, Chikere CB, Okpokwasili GC. 2016. Isolation, characterization, and application of biosurfactant by Klebsiella pneumoniae strain IVN51 isolated from hydrocarbon-polluted soil in Ogoniland, Nigeria. Bioresour. Bioprocess. 3, 40. ( 10.1186/s40643-016-0118-4) [DOI] [Google Scholar]
- 46.Srinivasan R, Karaoz U, Volegova M, MacKichan J, Kato-Maeda M, Miller S, Nadarajan R, Brodie EL, Lynch SV. 2015. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. PLoS ONE 10, e0117617. ( 10.1371/journal.pone.0117617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JS, et al. 2019. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 10, 5029. ( 10.1038/s41467-019-13036-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrance CE, Hong S. 2015 Is it essential to sequence the entire 16S rRNA gene for bacterial identification? See http://www.americanpharmaceuticalreview.com/Featured-Articles/181783-Is-it-Essential-to-Sequence-the-Entire-16S-rRNA-Gene-for-Bacterial-Identification/ (accessed 4 September 2021).
- 49.Clarridge JE. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17, 840-862. ( 10.1128/CMR.17.4.840-862.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sibley CD, Grinwis ME, Field TR, Eshaghurshan CS, Faria MM, Dowd SE, Parkins MD, Rabin HR, Surette MG. 2011. Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS ONE 6, e22702. ( 10.1371/journal.pone.0022702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murata T, Iida T, Shiomi Y, Tagomori K, Akeda Y, Yanagihara I, Mushiake S, Ishiguro F, Honda T. 2001. A large outbreak of foodborne infection attributed to Providencia alcalifaciens. J. Infect. Dis. 184, 1050-1055. ( 10.1086/323458) [DOI] [PubMed] [Google Scholar]
- 52.Gichuhi J, Khamis F, Van den Berg J, Mohamed S, Ekesi S, Herren JK. 2020. Influence of inoculated gut bacteria on the development of Bactrocera dorsalis and on its susceptibility to the entomopathogenic fungus, Metarhizium anisopliae. BMC Microbiol. 20, 321. ( 10.1186/s12866-020-02015-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu N, Tu Q, Tung H, Hieu D, Romero-Jovel S. 2014. Detection of tetrodotoxin-producing Providencia rettgeri T892 in Lagocephalus pufferfish. World J. Microbiol. Biotechnol. 30, 1829-1835. ( 10.1007/s11274-014-1601-8) [DOI] [PubMed] [Google Scholar]
- 54.Ventorino V, et al. 2017. Bio-based succinate production from Arundo donax hydrolysate with the new natural succinic acid-producing strain Basfia succiniciproducens BPP7. Bioenerg. Res. 10, 488-498. ( 10.1007/s12155-017-9814-y) [DOI] [Google Scholar]
- 55.Vinoj G, Pati R, Sonawane A, Vaseeharan B. 2015. In vitro cytotoxic effects of gold nanoparticles coated with functional acyl homoserine lactone lactonase protein from Bacillus licheniformis and their antibiofilm activity against Proteus species. Antimicrob. Agents Chemother. 59, 763-771. ( 10.1128/AAC.03047-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SH, Vaidya YH, Joshi CG, Kunjadia AP. 2016. Culture-dependent assessment of bacterial diversity from human milk with lactational mastitis. Comp. Clin. Pathol. 25, 437-443. ( 10.1007/s00580-015-2205-x) [DOI] [Google Scholar]
- 57.Abdelshafy Mohamad OA, Ma J-B, Liu Y-H, Zhang D, Hua S, Bhute S, Hedlund BP, Li W-J, Li L. 2020. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front. Plant Sci. 11, 47. ( 10.3389/fpls.2020.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosshard PP, Abels S, Zbinden R, Böttger EC, Altwegg M. 2003. Ribosomal DNA sequencing for identification of aerobic Gram-positive rods in the clinical laboratory (an 18-month evaluation). J. Clin. Microbiol. 41, 4134-4140. ( 10.1128/JCM.41.9.4134-4140.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel JB, Leonard DGB, Pan X, Musser JM, Berman RE, Nachamkin I. 2000. Sequence-based identification of Mycobacterium species using the MicroSeq 500 16S rDNA bacterial identification system. J. Clin. Microbiol. 38, 246-251. ( 10.1128/JCM.38.1.246-251.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall L, Doerr KA, Wohlfiel SL, Roberts GD. 2003. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J. Clin. Microbiol. 41, 1447-1453. ( 10.1128/JCM.41.4.1447-1453.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilck MB, Wu Y, Howe JG, Crouch JY, Edberg SC. 2001. Endocarditis caused by culture-negative organisms visible by Brown and Brenn staining: utility of PCR and DNA sequencing for diagnosis. J. Clin. Microbiol. 39, 2025-2027. ( 10.1128/JCM.39.5.2025-2027.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amaresan N, Jayakumar V, Thajuddin N. 2014. Isolation and characterization of endophytic bacteria associated with chilli (Capsicum annuum) grown in coastal agricultural ecosystem. Indian J. Biotechnol. 13, 247-255. [Google Scholar]
- 63.Wanjohi L, Mwamburi L, Too E, Aloo B, Kosgei J. 2015. Isolation and identification of bacteria with bioremediation potential of oil spills in lake Nakuru, Kenya. Asian J. Microbiol. Biotechnol. Environ. Sci. 17, 831-838. [Google Scholar]
- 64.AlKaabi N, Al-Ghouti MA, Jaoua S, Zouari N. 2020. Potential for native hydrocarbon-degrading bacteria to remediate highly weathered oil-polluted soils in Qatar through self-purification and bioaugmentation in biopiles. Biotechnol. Rep. 28, e00543. ( 10.1016/j.btre.2020.e00543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhagobaty RK. 2020. Hydrocarbon-utilizing bacteria of natural crude oil seepages, Digboi oilfield, Northeastern region of India. J. Sediment. Environ. 5, 177-185. ( 10.1007/s43217-020-00013-8) [DOI] [Google Scholar]
- 66.Singh P, Patil Y, Rale V. 2019. Biosurfactant production: emerging trends and promising strategies. J. Appl. Microbiol. 126, 2-13. ( 10.1111/jam.14057) [DOI] [PubMed] [Google Scholar]
- 67.Bicca FC, Fleck LC, Ayub MAZ. 1999. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev. Microbiol. 30, 231-236. ( 10.1590/S0001-37141999000300008) [DOI] [Google Scholar]
- 68.El-Sersy NA. 2012. Plackett-Burman design to optimize biosurfactant production by marine Bacillus subtilis N10. Rom. Biotechnol. Lett. 17, 16. ( 10.21608/ejm.2011.260) [DOI] [Google Scholar]
- 69.Ramadan EM, Kheiralla ZM, Fouad MA, El Tayeb TS, Gomaa EZ. 2011. Optimization of biosurfactant production by Bacillus licheniformis isolated from oil-contaminated Egyptian soil. Egypt. J. Microbiol. 45, 1-20. [Google Scholar]
- 70.Padmapriya B, Suganthi S, Anishya RS. 2013. Screening, optimization and production of biosurfactants by Candida species isolated from oil polluted soils. American-Eurasian J. Agric. Environ 13, 227-233. ( 10.5829/idosi.aejaes.2013.13.02.2744) [DOI] [Google Scholar]
- 71.Yaraguppi DA, Bagewadi ZK, Muddapur UM, Mulla SI. 2020. Response surface methodology-based optimization of biosurfactant production from isolated Bacillus aryabhattai strain ZDY2. J. Pet. Explor. Prod. Technol. 10, 2483-2498. ( 10.1007/s13202-020-00866-9) [DOI] [Google Scholar]
- 72.Mnif I, Ghribi D. 2016. Glycolipid biosurfactants: main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 96, 4310-4320. ( 10.1002/jsfa.7759) [DOI] [PubMed] [Google Scholar]
- 73.Shu Q, Lou H, Wei T, Liu X, Chen Q. 2021. Contributions of glycolipid biosurfactants and glycolipid-modified materials to antimicrobial strategy: a review. Pharmaceutics 13, 227. ( 10.3390/pharmaceutics13020227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desai JD, Banat IM. 1997. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 61, 47-64. ( 10.1128/mmbr.61.1.47-64.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data presented in this study can be found in NCBIData Bank with the accession nos. MZ254917–MZ254920.