Abstract
Purpose
We describe the spectrum of acute neurological disorders among hospitalized patients who recently had COVID-19 mRNA vaccination.
Method
We performed a prospective study at 7 acute hospitals in Singapore. Hospitalized patients who were referred for neurological complaints and had COVID-19 mRNA vaccines, BNT162b2 and mRNA-1273, in the last 6 weeks were classified into central nervous system (CNS) syndromes, cerebrovascular disorders, peripheral nervous system (PNS) disorders, autonomic nervous system (ANS) disorders and immunization stress-related responses (ISRR).
Results
From 30 December 2020 to 20 April 2021, 1,398,074 persons (median age 59 years, 54.5% males) received COVID-19 mRNA vaccine (86.7% BNT162b2, 13.3% mRNA-1273); 915,344(65.5%) completed 2 doses. Four hundred and fifty-seven(0.03%) patients were referred for neurological complaints [median age 67(20–97) years, 281(61.5%) males; 95.8% received BNT162b2 and 4.2% mRNA-1273], classified into 73(16.0%) CNS syndromes, 286(62.6%) cerebrovascular disorders, 59(12.9%) PNS disorders, 0 ANS disorders and 39(8.5%) ISRRs. Eleven of 27 patients with cranial mononeuropathy had Bell's palsy. Of 33 patients with seizures, only 4 were unprovoked and occurred within 2 weeks of vaccination. All strokes occurred among individuals with pre-existing cardiovascular risk factors. We recorded 2 cases of cerebral venous thrombosis; none were vaccine-induced thrombotic thrombocytopenia. Five had mild flares of immune-mediated diseases.
Conclusion
Our observational study does not establish causality of the described disorders to vaccines. Though limited by the lack of baseline incidence data of several conditions, we observed no obvious signal of serious neurological morbidity associated with mRNA vaccination. The benefits of COVID-19 vaccination outweigh concerns over neurological adverse events.
Keywords: SARS-CoV-2, Vaccination, Clinical study, Adverse events, Safety
1. Introduction
Singapore began her vaccination program with the BNT162b2 (Pfizer-BioNTech) vaccine on 30 December 2020, followed by the mRNA-1273 (Moderna) vaccine on 12 March 2021 [1,2], focusing on healthcare workers and elderly individuals [3]. The unprecedented pace of COVID-19 vaccine development and testing [4]; use of novel mRNA technology and large-scale vaccination programs have engendered concerns of adverse events following immunization (AEFI) [5,6], including neurological disorders. The potential for exacerbating pre-existing immune-mediated diseases and development of vaccine-enhanced disease [7], as well as novel adverse events such as vaccine-induced thrombotic thrombocytopenia (VITT) syndrome have contributed to fears of unexpected AEFI from mRNA vaccines [8,9]. Data from the Centres for Disease Control (CDC) Vaccine Adverse Event Reporting System (VAERS), with 51,755,447 vaccine doses administered, did not show an increased rate of neurological disorders [10]. While non-specific symptoms such as headache were common (13.2%), no neurological disorders were identified from a large app-based survey of patients who had received BNT162b2 in the UK [11]. The data from the European Union was also similarly reassuring [12]. This in the context of very high efficacy of these vaccines to protect against COVID-19 infection, hospitalization and severe illness. In a recent study from Mexico, recording 6,536 AEFI in 704,003 patients who had received the first dose of BNT162b2, only 17 patients had neurological disorders deemed serious [13]. To address the safety concerns of serious neurological AEFI, we extended our ongoing study of COVID-19-associated neurological disorders in Singapore public hospitals [14] to describe neurological disorders occurring in patients recently vaccinated with mRNA vaccines.
2. Materials and method
2.1. Study design, setting and patient population
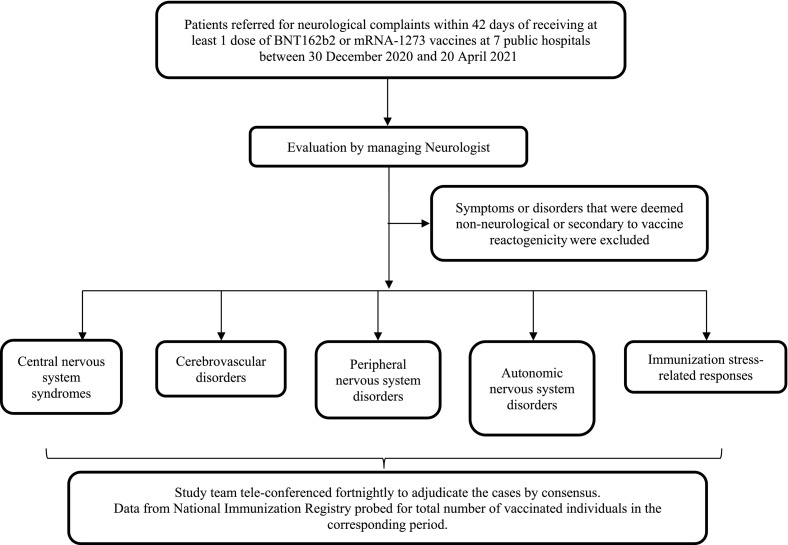
We performed a multi-centre prospective observational cohort study of patients in 7 Singapore public acute hospitals (Fig. 1 ). We included all sequential hospitalized patients who were referred for neurological complaints and had received at least 1 dose of BNT162b2 or mRNA-1273 vaccines in the last 42 days. We examined a hospital-based cohort to focus on serious neurological AEFI as defined by World Health Organization (WHO) [7]. Immediate and short term AEFIs are conventionally studied over 42 days [15]. The study covered 30 December 2020 to 20 April 2021, when selected high risk population groups (healthcare, frontline workers and elderly individuals) were vaccinated [3]. To contextualize our findings, data from the National Immunization Registry was probed for the total number of vaccinated individuals in the corresponding period. The protocol was approved by the Institutional Review Board (CIRB 2020/2410).
Fig. 1.
Study methodology and flowchart.
2.2. Data collection and case ascertainment
-
1.
Patients were evaluated at the discretion of the managing neurologist and relevant clinical, radiological and laboratory data collated. Interval between the last vaccine dose and symptom onset (latency) was calculated.
-
2.
Symptoms or disorders that were deemed non-neurological (e.g., musculoskeletal pain, syncope) or secondary to vaccine reactogenicity [16], namely unspecified headache, malaise and myalgia occurring within 24h of vaccination, were excluded.
-
3.
We classified the cases into 4 groups, similar to our previous study[14] where we described 39 patients with neurological disorders from a cohort of 47,572 COVID-19 patients: i) Central nervous system (CNS) syndromes, ii) Cerebrovascular disorders, iii) Peripheral nervous system (PNS) disorders (including cranial neuropathies) and iv) Autonomic nervous system (ANS) disorders. We added a group for immunization stress-related responses (ISRR), as defined by the WHO manual on causality assessment of an AEFI [7,17]. This also includes functional neurological disorders (FND) temporally related to vaccination.
-
4.
The study team tele-conferenced fortnightly to adjudicate the cases by consensus.
2.3. Statistical analysis
Categorical variables are summarized as numbers (n) and percentages (%). Continuous variables are expressed as median (interquartile range), and, based on their distribution, compared using t-test and Mann-Whitney U tests as appropriate.
3. Results
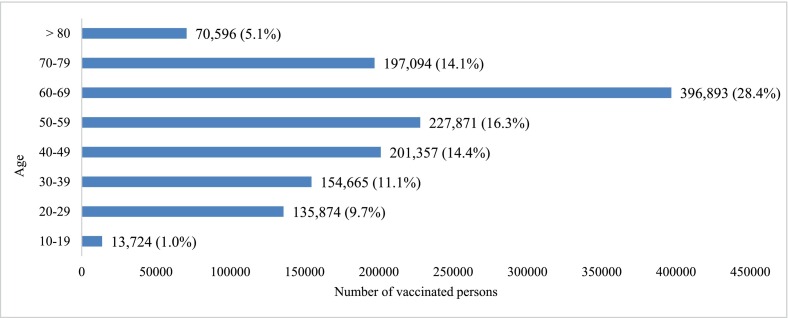
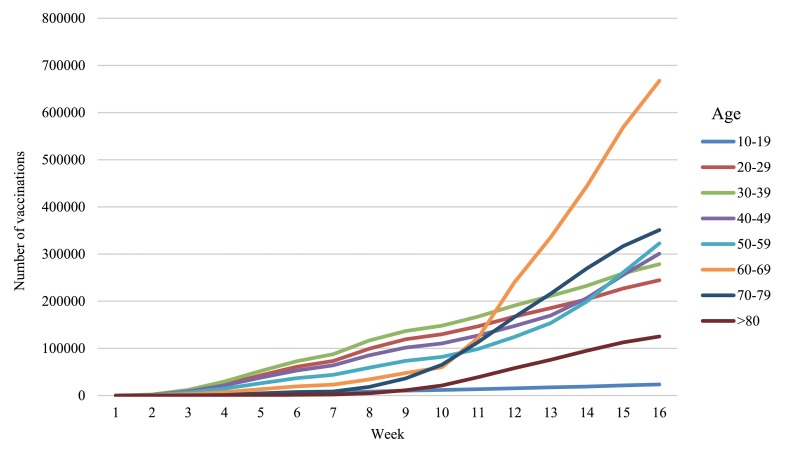
From 30 December 2020 to 20 April 2021, 1,398,074 individuals in Singapore received COVID-19 mRNA vaccines (86.7% BNT162b2, 13.3% mRNA-1273), of whom 915,344 (65.5%) completed 2 doses. Median age was 59(15–121) years; 54.5% males. The demographic profile of the vaccinated group and pace of vaccination according to age groups are illustrated in Appendix Figs. A.1 and A.2.
During this period, 457 (0.03%) patients were referred for neurological complaints, median age 67(20–97) years, 281 (61.5%) males; none had recent symptomatic COVID-19, as the national policy was to delay vaccination by 6 months post-COVID-19 infection. Four hundred and thirty-eight patients (95.8%) received BNT162b2 and 19 (4.2%) had the mRNA-1273 vaccine. Two hundred and seventy-three (59.7%) cases occurred after the first dose. Seventy-three (16.0%) were grouped into CNS syndromes, 286 (62.6%) cerebrovascular disorders, 59 (12.9%) PNS disorders, none (0%) ANS disorders and 39 (8.5%) ISRR. Median latency was 9(0–40) days. Table 1 summarizes the key neurological disorders and their temporal relationship to vaccines.
Table 1.
Summary of key neurological disorders in disease groups and temporal relationship to vaccine.
| Category | Key neurological disorders in each category⁎ | Vaccine type |
Patients with symptom onset after first dose, n | Median latency, days (range) | |
|---|---|---|---|---|---|
| BNT162b2 | mRNA-1273 | ||||
| Central Nervous System syndromes (n = 73) |
Seizures (n = 33) |
31 |
2 |
22 |
10 (0–38) |
| Encephalopathy (n = 4) |
4 | 0 | 4 | 16 (3–26) | |
| Demyelinating diseases (multiple sclerosis and optic neuritis) (n = 4) |
4 | 0 | 3 | 12.5 (1−33) | |
| Cerebrovascular disorders (n = 286) |
All clinical/imaging proven acute ischemic stroke (n = 246) |
234 |
12 |
143 |
9 (0–40) |
| Transient ischemic attack (n = 37) |
35 | 2 | 22 | 11 (0−33) | |
| Cerebral venous thrombosis (n = 2) |
2 | 0 | 0 | 8.5 (8–9) | |
| Peripheral Nervous System disorders (n = 59) |
Bell's palsy (n = 11) |
11 |
0 |
6 |
7 (1–29) |
| Cranial nerve III and VI palsies (n = 11) |
10 | 1 | 8 | 8 (1–14) | |
| Guillain-Barré syndrome (n = 2) |
2 | 0 | 2 | 4 (0–8) | |
| Autonomic Nervous System disorders (n = 0) |
- | 0 | 0 | 0 | - |
| Immunization Stress Related Response (n = 39) |
- | 39 | 0 | 25 | 2 (0–35) |
Cases not presented here include: primary headaches (n = 19), Parkinson's disease fluctuations (n = 5), dystonia (n = 1), transient global amnesia (n = 4), spondylotic and ischemic myelopathies (n = 2), acute psychosis (n = 1), central retinal artery occlusion (n = 1), cranial nerve V mononeuropathy (n = 4), vestibular neuronitis (n = 1), non-spondylotic radiculopathy (n = 3), spondylotic radiculopathy (n = 5), chronic inflammatory demyelinating polyradiculoneuropathy (n = 1), diabetic polyneuropathy (n = 1), myasthenia gravis (n = 2), myopathies (n = 2) and other peripheral vestibulopathies (n = 16).
3.1. CNS syndromes
Median age of the 73 patients was 62(22–94) years, 35 males. Of 33 (7.2%) patients [median age 63(25–85) years, 17 males] who developed seizures after vaccination, 17 (51.5%) were first-onset seizures; the rest had pre-existing epilepsy. Twenty-two (66.7%) occurred after the first dose. Median latency was 10(0–38) days; 21 (63.6%) occurred in the first 14 days (18 with identified triggers) and 12 (36.4%) between 15 and 42 days (11 with identified triggers). Four (0.9%) patients had first-onset unprovoked seizures, defined as convulsive episodes occurring in the absence of a potentially responsible clinical condition, normal electroencephalogram and unremarkable MRI brain. They did not require anti-epileptic drugs. Median latency for these 4 patients was 8(1–15) days.
Four patients (0.9%) [median age 60.5(46–94) years, 2 males] presented with encephalopathy at median 16(3–26) days after the first dose. Three were attributed to presumed infections (tuberculosis (TB), herpes simplex virus (HSV), bacterial translocation from cerebrospinal fluid leak) and one to diabetic hyperglycaemia. All improved with treatment.
Two (0.4%) female patients developed optic neuritis (48 and 62 years). One was idiopathic, occurring 33 days after the second dose; the other had newly-diagnosed aquaporin-4 (AQP-4) antibody-positive optic neuritis developing 1 day after the first dose. Two (0.4%) female patients (22 and 38 years) presented with mild, multiple sclerosis (MS) relapses 17 and 8 days after the first dose. Both were not on disease-modifying drugs and have had yearly exacerbations at baseline. All 4 patients improved with corticosteroids.
We recorded 19 (4.2%) primary headaches, 5 (1.1%) Parkinson's disease fluctuations, 1 (0.2%) dystonia, 4 (0.9%) transient global amnesia, 2 (0.4%) spondylotic and ischemic myelopathies, and 1 (0.2%) acute psychosis.
3.2. Cerebrovascular disorders
Of 286 (62.6%) patients [median age 70(38–97) years, 197 males], 246 (86.0%) had acute ischemic stroke (AIS), 37 (12.9%) transient ischemic attack (TIA), 2 (0.7%) cerebral venous thrombosis (CVT) and 1 (0.4%) isolated central retinal artery occlusion. AIS was proven on neuroimaging in 243 (98.8%), median age 70 (38–97) years, 169 males; of these, 143 (58.8%) occurred after the first dose. Median latency was 9(0–40) days. All had at least 1 cardiovascular risk factor (age > 50 years, hypertension, hyperlipidaemia, diabetes mellitus, cardiac disease, BMI ≥ 25.0 kg/m 2, smoking, atrial fibrillation). The stroke subtypes (TOAST classification) were 71 (29.2%) small-vessel, 73 (30.0%) large vessel, 61 (25.1%) cardioembolic, 36 (14.8%) undetermined and 2 (0.8%) other aetiologies (dissection and hypercoagulable state from malignancy). Eleven (4.5%) were ≤ 50 years of age (Appendix Table A.1). Their median latency of 6(0–40) days was similar to the rest of the cohort (p = 0.42). Forty-one (17.3%) patients received revascularization treatments (thrombolysis or endovascular therapy); the rest received standard of care medical therapy. One hundred and forty-four (59.3%) had a good functional outcome [modified Rankin Scale (mRS) 0–2] upon discharge.
Eleven (4.5%) AIS patients [median age 80(64–97) years, 8 males] had thrombocytopenia (Appendix Table A.2), none akin to VITT. Compared to the rest of imaging-proven AIS patients, they were older (median age 80 vs 70 years, p = 0.007), but their median latency was similar (6 vs 9 days, p = 0.29).
Two (0.4%) patients had extensive CVT (60 and 62 years, both females), 9 and 8 days after second dose respectively. Evaluation for prothrombotic factors (Appendix Table A.3) and malignancy for patient 1 was unremarkable. Despite decompressive hemicraniotomy and anticoagulation with heparin followed by warfarin, she remained neurologically debilitated (mRS 5) 6 weeks later. Patient 2 had a family history of thrombosis (her son had unprovoked pulmonary embolism). Her prothrombotic evaluation (Appendix Table A.3) showed low antithrombin III levels (55%, normal range: 80–120%), attributed to acute thrombosis. She improved with anticoagulation, heparin then warfarin, and recovered to mRS 2 upon discharge. None had thrombocytopenia. Anti-Platelet Factor 4 antibodies were not tested in both patients.
3.3. PNS disorders
We encountered 59 (12.9%) patients with PNS disorders [median age 69(28–92) years, 33 males]. All except 3 received BNT162b2. Twenty-seven (5.9%) [median age 69(28–92) years, 13 males] had cranial mononeuropathies. Eleven (2.4%) [median age 66(28–92) years, 5 males] had Bell’'s palsy, 6 (54.5%) of whom occurred after the first dose. Median latency was 7(1–29) days. Six (54.5%) patients made partial recovery within 1–2 months, 1 (9.1%) fully recovered at 3 months, while 4 (36.4%) patients are pending reviews.
We recorded 8 (1.8%) isolated cranial nerve (CN) III and 3 (0.7%) CN VI palsies [median age 70(59–83) years, 7 males]. Eight (72.7%) occurred after the first dose. Median latency from the last vaccine dose was 8(1–14) days. Clinical and laboratory data suggested an ischemic mechanism (7 had diabetes mellitus and 9 had hypertension). Seven (63.6%) patients made partial recovery within 1–2 months and 1 (9.1%) fully recovered at 2 months; reviews are pending for the rest.
Four (0.9%) patients had unilateral CN V mononeuropathy; 2 had exacerbation of trigeminal neuralgia and 2 transient unilateral face numbness [median age 67.5(59–71) years, all females, median latency 14(1–19) days]. One (0.2%) patient had vestibular neuronitis on the same day as his second dose. All improved with symptomatic treatment.
An elderly man developed multiple (left partial III, IV and VI) cranial neuropathies on the same day as his first dose. The rest of his neurological examination, contrast-enhanced MRI brain, spinal tap and nerve conduction studies were normal. However, serum GM1 IgG was markedly raised, suggesting a forme-fruste of Guillain-Barré syndrome (GBS). He improved within a week without immunotherapy. Another elderly man had acute inflammatory demyelinating polyradiculoneuropathy variant of GBS 8 days after the first dose. He improved with intravenous immunoglobulin.
Three (0.7%) patients [median age 70(52–85) years, 2 males] developed unilateral non-spondylotic radiculopathy at median 1(0–14) day. Two (66.7%) occurred after first dose. One was associated with zoster reactivation in the same segments and foot drop that remained at 2 months. Another had a wrist drop that recovered after 2 months.
Five (1.1%) patients with spondylotic radiculopathy, 1 (0.2%) chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), 1 (0.2%) diabetic polyneuropathy, 1 (0.2%) myasthenia gravis (MG) and 2 (0.4%) myopathies had symptom exacerbation after vaccination. Another patient developed new-onset, seropositive, non-thymomatous, generalised MG 17 days after BNT162b2 second dose. He improved with corticosteroids and pyridostigmine. There were 16 (3.5%) other cases of peripheral vestibulopathy, including benign paroxysmal positional vertigo.
3.4. ISRR
Thirty-nine (8.5%) patients developed ISRR [median age 51(20–73) years, 16 males]. Of these, 25 (64.1%) occurred after the first dose. Median latency was 2(0–35) days. Seven (17.9%) patients had previous psychiatric illness or functional disorder. Six (15.4%) had previous migraine. Sixteen (41.0%) patients had a single neurological symptom: sensory complaints (n = 9), dizziness (n = 2), headache (n = 2), focal twitching (n = 2), and forgetfulness (n = 1); 23 (59.0%) were polysymptomatic: sensory (n = 17), motor (10 weakness and 2 cramps), headache (n = 7), giddiness (n = 5), unsteadiness (n = 3), abnormal movement/twitching (n = 2), dysarthria (n = 2), and visual blurring (n = 2). All except one patient improved prior to discharge.
4. Discussion
Our hospital-based study covered a 4-month period during which 1,398,074 people received at least 1 dose of COVID-19 mRNA vaccine. We recorded only 457 patients with a spectrum of neurological disorders encompassing cranial neuropathies, seizures, AIS, CVT and ISRR. Our study adds value to ongoing pharmacovigilance by describing clinical features and outcomes of the disorders seen after mRNA vaccination in a real-world setting. Unlike the pivotal studies, our cohort was predominantly Asian.
Without attempting to establish or refute causality of these disorders to the mRNA vaccines due to the observational nature of our study, we discuss the possible association of neurological disorders to mRNA vaccines by considering:
-
a)
Temporal association and time window of increased risk: This is based on published literature to elucidate a reasonable time relationship to vaccination [15,18].
-
b)
Biological plausibility of the disorders' association to vaccination, guided by contemporaneous literature, and
-
c)
For individual disorders, we correlate with available baseline incidence data
We then categorized the likelihood of vaccination or a concurrent and coincidental illness explaining the disorder using the WHO AEFI causality assessment, Brighton criteria framework [19]; Appendix Table A.4).
4.1. CNS syndromes - Seizures
Vaccines can trigger seizures, typically within 24 h for inactivated vaccines and 5–12 days after live-attenuated vaccines [15,20]. The risk period is unknown for novel mRNA vaccines and no cases have been reported at the time of writing. Four patients had first-onset unprovoked seizures within the first 15 days following vaccination without underlying predisposition or concurrent disease. However, unprovoked seizures without a cause are not uncommon. The 4 cases out of 1,398,074 vaccinated persons occurring in 4 months fall within the expected baseline incidence in the general population of 1.9–5.1 per 100,000 person-month [21], and could be categorized at best, “probable” vaccine-related. Another 18 patients who had seizures within the first 2 weeks but had an alternative explanation, such as poorly controlled epilepsy, were categorized only as “possible”. The seizures in the remaining 11 patients were deemed as “unlikely” or “unrelated” as they occurred more than 2 weeks after vaccination and had an alternative explanation.
4.2. CNS syndromes – Encephalopathy
The described risk period for meningoencephalitis after vaccination is 21 days [15]. There have been no reported cases of encephalopathy following mRNA vaccine at the time of writing. The 2 patients who presented with meningoencephalitis within this interval would only fall into the “possible” category because their illness could be better-explained by the presumed clinical diagnosis (supported by MR imaging features of TB and HSV infection, but not confirmed microbiologically). The other 2 patients with encephalopathy, who developed illness outside the risk period and had alternative etiologies (CSF leak and hyperglycaemia), were labelled “unlikely” or “unrelated”.
4.3. CNS syndromes – Immune-mediated disorders
Dysimmune processes tend to occur 5–28 days after inactivated vaccines; with additional days added for the incubation period of live attenuated vaccines [18]. One case of new-onset MS 7 days after the first dose of BNT162b2 was reported [22]. However, an observational study of MS patients who received the BNT162b2 vaccine showed relapse rates (2.1% and 1.6% after first and second dose respectively) similar to non-vaccinated patients [23]. Therefore, we categorized the mild MS flares in 2 patients within this time-frame as only “possible” vaccine-related. The association between vaccination and optic neuritis is less clear [24]. However, the AQP-4 antibody positive optic neuritis that occurred only 1 day after BNT162b2 first dose and the idiopathic optic neuritis that developed 33 days after BNT162b2 second dose were likely coincidental and classified as “unlikely”. Likewise, the other CNS syndromes recorded were deemed “unlikely” or “unrelated”.
4.4. Cerebrovascular diseases
Common disorders such as stroke may occur coincidentally after vaccination. No relationship was found in a recent study based on a national database in Scotland that recorded 0.82 million people who received BNT162b2 [25]. The 246 AIS cases out of 1,398,074 vaccinated persons we observed over 4 months lies within the expected local baseline incidence of 16.6 per 100,000 person-month [26]. The median age (70 vs 69 years) and stroke subtypes of our imaging-proven AIS patients were similar to that reported previously, including the Singapore Stroke Registry [26,27]. Nevertheless, to address concerns of possible links to mRNA vaccines, we analysed patients who were deemed least likely to develop AIS (age ≤ 50 years), although this would not account for potential interaction between age and vaccination. No pattern with regards to stroke etiology is discerned; nine of 11 patients had at least 2 cardiovascular risk factors (Appendix Table A.1). They also did not have signs of dysimmunity, prothrombotic state or thrombocytopenia. Compared to other AIS patients, we did not find clustering of latency in this group. Overall, the AIS/TIA cases could be explained by pre-existing cardiovascular risk factors and likely occurred independent of the vaccine. We also did not observe any resemblance to VITT; neither did we find any specific stroke pattern nor clustering of latency (6 vs 9 days, p = 0.29) in those with thrombocytopenia (Appendix Table A.2).
Majority of CVTs reported post-vaccination have occurred following the adenovirus vector vaccines [28,29]. Recent reports of possible association between mRNA vaccine and CVT [30,31], have been balanced by reassuring data from the Scotland national database [25]. Our 2 CVT cases, 1 idiopathic, the other with family history, could be categorized as “probable” and “possible” vaccine-related respectively. Similar to AIS [32], the challenge to discerning a causal link between CVT and mRNA vaccine is the uncertainty of the risk period.
4.5. PNS disorders
We recorded 11 cases of Bell’'s palsy. It is still unclear if the incidence after mRNA vaccines is above the background rate or disproportionate to that of other viral vaccines [2,[33], [34], [35], [36]]. One report estimated a higher incidence of 106 per 100,000 person-years, against the background rate of 15–30 per 100,000 person-years [36]. The EudraVigilance data suggests a greater than 3-fold increase in Bell's palsy with mRNA vaccines compared to other vaccines [36]. In Singapore, Bell's palsy incidence after mRNA vaccination is 3.45 per 100,000 person-month [37], at the higher end of the range of background incidence of 1.1–4.4 per 100,000 person-month.
CN III and VI mononeuropathy occurred in 8 and 3 patients respectively. Post-vaccination cranial neuropathies other than Bell's palsy is rare. There were 68 reports to the US VAERS over a period of more than 20 years. The commonest cranial neuropathies (excluding VII) were III (n = 22), IV (n = 9) and VI (n = 32), occurring largely in isolation at median 9 days. No clustering according to age group or vaccine types was observed [38]. At the time of writing, there was one report of a patient who developed CN VI palsy 2 days after BNT162b2 [39]. Although our patients developed cranial III and VI neuropathies at median 8(1–14) days post vaccination, the link to vaccination is uncertain as these patients also had risk factors for microvascular ischemia. These cases would therefore fall into “possible” category.
The low occurrence of GBS (n = 2) is perhaps surprising given the slightly elevated risk of GBS after influenza vaccine [40]. The national incidence of GBS has not increased since vaccination started compared to a baseline of 9 GBS cases per month pre-COVID (personal communications). We recorded 1 patient with new-onset MG, similar to a recent report of 2 patients who developed MG 1 and 7 days after BNT162b2 second dose [22]. Our 3 patients who had exacerbation of MG, CIDP and myositis (2 already had frequent relapses), together with the 2 MS cases, suggest that mRNA vaccines may only occasionally contribute to flares of immune-mediated disease.
4.6. ISRR
We recorded 39 patients (8.5%) with ISRR, of whom only a small proportion had previous FND or psychiatric illness. Consistent with previous reports [17,41], these patients were younger, a slightly higher proportion were female and majority were polysymptomatic with sensory symptoms being the commonest complaint. There were no clusters of ISRR.
4.7. Comparison with COVID-19 neurology case-mix
WHO had suggested vigilance for potential vaccine-enhanced disease, an AEFI following some live-attenuated vaccines. This refers to a potential increased risk of COVID-19 like disease or its complications. At present, there is no evidence that either of these risks exist for COVID-19 vaccines [7]. We categorized the patients similar to that used to describe COVID-19 neurological complications to facilitate detecting such AEFI. In the Singapore study of COVID-19 associated neurological complications, as well as a subsequent nation-wide review [14,42], we did not see a significant association of GBS with COVID-19. Likewise, we recorded only 2 patients with GBS after vaccination. However, Bell's palsy predominated the PNS case-mix in both cohorts (5 of 7 post-COVID-19 PNS complications, and 11 of 59 PNS disorders post-vaccination) [14]. Whilst CVT is a significant complication of COVID-19 [14,43], whether this condition is related to COVID-19 mRNA vaccines remains unclear [32]. AIS is associated with COVID-19, often in young patients without cardiovascular risk factors but with signs of dysimmunity and prothrombotic tendency [14,44]. On the other hand, AIS in our post-vaccine cohort is likely coincidental. We encountered 4 cases each of limited dysautonomia [45] and severe encephalitis [14,46] associated with COVID-19 but none after vaccination.
Our study has several limitations. Investigations were performed at the discretion of the managing neurologist. Not all patients were tested for COVID-19, although none had COVID-19 symptoms and local transmission was less than 5 community cases per day during the study period. As our study was based in public acute hospitals, we would have missed patients consulting outpatient clinics, private hospitals and non-neurologists. The greater number of Bell's palsy, 25 recorded by Singapore's drug regulatory authority, Health Sciences Authority (HSA), versus 11 in our study, during the same period illustrates this point [37]. The first 4 months of the vaccine drive was focused on frontline workers and elderly patients (Appendix Fig. A.2), hence our study findings may not be generalizable to the general population. A large number of individuals were vaccinated over a short period of time, engendering overestimation of normally infrequent AEFI [35]. Our study findings therefore do not reflect that of a steady-state vaccination programme. In addition, not all the background incidence rates of the disorders described were available. Data from a control group comprising unvaccinated patients hospitalized in the same period was also unavailable. The published risk periods that guided us might be inapplicable to novel vaccines and regimens (2 doses over a few weeks). The discussion on biological plausibility, although guided by contemporaneous literature, is also inevitably quite arbitrary. The cases described in our observational study, which cannot establish causality to vaccination, could have been purely coincidental and occurred independent of the vaccine. Despite these limitations, we present systematically collected data in a multi-centre study and contextualised them against the total number of vaccinated individuals, instead of reporting individual cases with uncertain link to vaccine, in order to identify possible signals of serious AEFIs.
5. Conclusion
Over a 4-month period during which approximately 1.4 million people received the COVID-19 mRNA vaccines, we recorded a spectrum of neurological disorders in only 457 hospitalized patients - including 11 Bell's palsy. We did not observe VITT and recorded 2 patients with CVT. Largely mild exacerbation of immune-mediated diseases was seen in only 5 patients; 8.5% had ISRR. Notwithstanding the prominence of Bell's palsy, the vaccine did not recapitulate the neurological complications of COVID-19. Our study does not establish causality between vaccination and any of the described disorders. Nonetheless, we observed no obvious signal of serious neurological morbidity, suggesting that benefits of COVID-19 vaccination outweigh concerns over neurological adverse events.
Contributors
Study concept and design: JSK, TMT, DWSC, RCSS, KT, TU.
Acquisition of data, analysis and interpretation of data: JSK, RHMH, MHY, HJC, YG, KPY, BYQT, LLLY, AMLQ, IS, TMT, MS, JA, SR, GJC, ACH, AA, MPS, MC, SMK, LLF, TU.
Drafting of manuscript: JSK, HJC, YKP, DWSC, MS, TU.
Critical revision of manuscript for important intellectual content: All.
Study supervision: DWSC, RCSS, KT, TU.
All authors approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent for publication
Waiver of consent was granted by the Singapore Health Services institutional review board for collection of anonymized clinical data.
Ethics approval
The study was approved by the Singapore Health Services institutional review board (CIRB 2020/2410).
Data availability statement
Study protocol, statistical code and dataset are available from the corresponding author upon reasonable request. All data relevant to the study are included in the article and supplemental material. Patient-related dataset will be shared upon request from any qualified investigator, maintaining anonymization of the individual patients.
Declaration of Competing Interest
None.
Appendix A
Appendix Fig. A.1. Demographic profile of the national COVID-19 mRNA vaccination program during study period - 30 December 2020 to 20 April 2021. (National Immunization Registry).
Appendix Fig. A.2. Weekly cumulative number of vaccinations by age groups (30 December 2020 to 20 April 2021. (National Immunization Registry).
Appendix Table A.1. Demographics, stroke characteristics and temporal relationship to vaccine of young (≤ 50 years) patients with imaging proven AIS.
| Patient | Age, y/Sex | Cardiovascular risk factorsb | Location of infarcts/TOAST classification | Temporal relationship to vaccine, days (after 1st/2nd dose) |
|---|---|---|---|---|
| 1 | 43/female | Hypertension BMI 30.1 kg/m2 |
Left cerebellar infarct/undetermined (cryptogenic) | 40 (after BNT162b2 2nd dose) |
| 2 | 43/female | Hypertension BMI 28.1 kg/m2 |
Left middle cerebral artery infarct/undetermined (cryptogenic) | 15 (after BNT162b2 1st dose) |
| 3 | 45/female | Hypertension BMI 27.3 kg/m2 |
Left middle cerebral artery infarct/undetermineda | 6 (after BNT162b2 1st dose) |
| 4 | 41/female | Smoking (unknown pack years) | Right frontal infarct/large vessel | 25 (after BNT162b2 1st dose) 1 (after BNT162b2 2nd dose) |
| 5 | 38/female | Smoking (10 pack-years) |
Right parietal infarct / undetermined (cryptogenic) |
28 (after BNT162b2 1st dose) 17 (after BNT162b2 2nd dose) |
| 6 | 42/male | Hyperlipidemia Diabetes mellitus | Right thalamus infarct/small vessel | 13 (after BNT162b2 1st dose) |
| 7 | 47/male | Hyperlipidemia Diabetes mellitus BMI 26.7 kg/m2 |
Left putamen and corona radiata infarct/small vessel | 2 (after mRNA-1273 1st dose) |
| 8 | 48/male | Hypertension Hyperlipidemia Ischemic heart disease (ejection fraction 20%) BMI 31.4 kg/m2 |
Right temporal lobe, right hippocampus, bilateral occipital lobes, right cerebellum, right thalamus infarcts/cardioembolic | 0 (After mRNA-1273 1st dose) |
| 9 | 45/male | Hypertension Hyperlipidemia Ischemic heart disease Diabetes mellitus Peripheral vascular disease Left ventricular thrombus |
Left middle cerebral artery infarct/cardioembolic | 22 (after BNT162b2 1st dose) 1 (after BNT162b2 2nd dose) |
| 10 | 50/male | Ischemic heart disease Old stroke Smoking |
Left thalamus infarct/undetermined (cryptogenic) | 22 (after BNT162b2 1st dose) 1 (after BNT162b2 2nd dose) |
| 11 | 50/male | Hypertension Hyperlipidemia BMI 28.4 kg/m2 | Left corona radiata, left lentiform nucleus and right high frontal subcortical infarcts/undetermineda | 28 (after BNT162b2 2nd dose) |
AIS = acute ischemic stroke; BMI = body mass index; TOAST = Trial of Org 10172 in Acute Stroke Treatment.
Incomplete stroke evaluation as patient declined cardioembolic work-up.
Cardiovascular risk factors include – Age > 50 years, hypertension, hyperlipidemia, diabetes mellitus, cardiac disease, BMI ≥ 25.0 kg/m2, smoking, atrial fibrillation.
Appendix Table A.2. Demographics, stroke characteristics and temporal relationship to vaccine of imaging proven AIS patients with thrombocytopenia.a
| Patient (Age, y/Sex) | Cardiovascular risk factorsb | Location of infarcts/TOAST classification | Temporal relationship to vaccine, days (after 1st/2nd dose) | Etiology for thrombocytopenia | Initial platelet level, x 109/l | Platelet trend |
|---|---|---|---|---|---|---|
| 1 (81/male) |
Hypertension Hyperlipidemia Diabetes mellitus Ischemic heart disease Atrial fibrillation BMI 27.6 kg/m2 |
Right middle and anterior cerebral artery infarcts/cardioembolic | 23 (after BNT162b2 1st dose) | Chest infection | 119 | Normalized after about 2 weeks |
| 2 (83/male) |
Hypertension Hyperlipidemia Diabetes mellitus Ischemic heart disease Atrial fibrillation Smoking |
Left middle and anterior cerebral artery infarcts/cardioembolic | 42 (after BNT162b2 1st dose) 21 (after BNT162b2 2nd dose) |
None | 117 | Stable when checked 2 days later, background chronic thrombocy-topenia |
| 3 (80/female) |
Hypertension | Left corona radiata and lentiform nucleus infarcts/undetermined (cryptogenic) | 28 (after BNT162b2 1st dose) 2 (after BNT162b2 2nd dose) |
None | 120 | Improved when checked 2 days later |
| 4 (74/male) |
Hypertension Hyperlipidemia Old stroke Smoking |
Left pons infarct/small vessel | 30 (after BNT162b2 1st dose) 2 (after BNT162b2 2nd dose) |
None | 115 | Stable when checked 2 days later |
| 5 (73/male) |
Hypertension Hyperlipidemia Ischemic heart disease Valvular atrial fibrillation BMI 25.4 kg/m2 |
Left cerebellar infarct/cardioembolic | 28 (after BNT162b2 1st dose) 7 (after BNT162b2 2nd dose) |
None | 118 | Normalized after 5 days |
| 6 (86/female) |
Hypertension Atrial fibrillation BMI 25.1 kg/m2 |
Right middle cerebral artery infarct/cardioembolic | 25 (after BNT162b2 1st dose) 4 (after BNT162b2 2nd dose) |
None | 139 | Not repeated |
| 7 (64/male) |
– | Right putamen, corona radiata and posterior limb internal capsule infarcts/undetermined (cryptogenic) | 10 (after BNT162b2 1st dose) | Alcoholic pancreatitis | 65 | Normalized after 8 days |
| 8 (68/female) |
Hypertension Hyperlipidemia Ischemic heart disease Diabetes mellitus Old stroke Smoking BMI 25.2 kg/m2 |
Left middle cerebral artery infarct/large vessel | 2 (after BNT162b2 1st dose) | Possible urinary tract infection | 123 | Not repeated |
| 9 (76/male) |
Hypertension Hyperlipidemia Ischemic heart disease Diabetes mellitus |
Right thalamic infarct/large vessel | 6 (after BNT162b2 1st dose) | None | 126 | Normalized when checked about 2 weeks later |
| 10 (86/male) |
Hypertension Atrial fibrillation | Right middle cerebral artery infarct/cardioembolic | 24 (after BNT162b2 1st dose) 2 (after BNT162b2 2nd dose) |
None | 139 | Normalized after 6 days |
| 11 (97/male) |
Hypertension Hyperlipidemia Ischemic heart disease Diabetes mellitus Atrial fibrillation Old stroke BMI 26.8 kg/m2 |
Right middle and anterior cerebral artery infarcts/cardioembolic | 15 (after BNT162b2 1st dose) | None | 130 | Not repeated, background chronic thrombocy-teopenia |
AIS = acute ischemic stroke; BMI = body mass index; TOAST: Trial of Org 10172 in Acute Stroke Treatment.
Thrombocytopenia is defined as platelet count <140 × 109/l.
Cardiovascular risk factors include – Age > 50 years, hypertension, hyperlipidemia, diabetes mellitus, cardiac disease, BMI ≥ 25.0 kg/m2, smoking, atrial fibrillation.
Appendix Table A.3. Pertinent clinical, radiological and laboratory evaluation of patients with cerebral venous thrombosis.
| Patient | Neurological complications | Site and extent of venous thrombosis on imaging | Prothrombotic evaluation performed | Platelet level on admission, x 109/l |
|---|---|---|---|---|
| 1 | Intraparenchymal hemorrhage | Thrombosis of the transverse, sigmoid and internal jugular sinuses | Erythrocyte sedimentation rate Antinuclear antibody Anti-double stranded DNA Anti-cardiolipin IgM/IgG Lupus anticoagulant Anti-ß2 glycoprotein 1 Factors V and VIII Antithrombin III Protein C and S Factor V Leiden gene Heparin-induced platelet aggregation study |
383 |
| 2 | Seizures Intraparenchymal hemorrhage |
Thrombosis of the superior sagittal, transverse and sigmoid sinuses, internal jugular and cortical veins | Anti-cardiolipin IgM/IgG Lupus anticoagulant Antithrombin III Protein C and S |
250 |
.
Appendix Table A.4. World Health Organization (WHO) adverse event following immunization (AEFI) causality assessment, Brighton criteria [1].
| Likelihood of disease association with vaccine | Definition |
|---|---|
| Certain | Clinical event with a plausible time to vaccine administration, and which cannot be explained by concurrent disease or other drugs. |
| Probable | Clinical event with a reasonable time relationship to vaccine administration, and is unlikely to be attributed to concurrent disease or other drugs. |
| Possible | Clinical event with a reasonable time relationship to vaccine administration, but which could also be explained by concurrent disease or other drugs. |
| Unlikely | Clinical event whose time relationship to vaccine administration makes a causal connection improbable, but which could plausibly be explained by underlying disease or other drugs. |
| Unrelated | Clinical event with an incompatible time relationship to vaccine administration and which could be explained by underlying disease or other drugs. |
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. (Epub 2020 Dec 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health Government Accepts Recommendations of Expert Committee on COVID-19 Vaccination [Internet] Ministry of Health; Singapore (SG): 2020 Dec 27. https://www.moh.gov.sg/news-highlights/details/government-accepts-recommendations-of-expert-committee-on-covid-19-vaccination [cited 2021 Jun 14]. Available from: [Google Scholar]
- 4.Calina D., Hartung T., Docea A.O., et al. COVID-19 vaccines: ethical framework concerning human challenge studies. Daru. 2020;28(2):807–812. doi: 10.1007/s40199-020-00371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calina D., Hernández A.F., Hartung T., et al. Challenges and scientific prospects of the newest generation of mRNA-based vaccines against SARS-CoV-2. Life. 2021;11(9):907. doi: 10.3390/life11090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronald N.K., Darja K., Alan L.P., et al. Vaccine- and natural infection-induced mechanisms that could modulate vaccine safety. Toxicol. Rep. 2020;7:1448–1458. doi: 10.1016/j.toxrep.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Covid-19 vaccines: safety surveillance manual - monitoring and responding to adverse events following immunization [Internet] World Health Organization; Geneva (CH): 2020. https://www.who.int/publications/m/item/covid-19-vaccines-safety-surveillance-manual---monitoring-and-responding-to-adverse-events-following-immunization [cited 2021 Jun 14]. 26 p. Available from: [Google Scholar]
- 8.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. (Epub 2021 Apr 9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greinacher A., Thiele T., Warkentin T.E., et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goss A.L., Samudralwar R.D., Das R.R., et al. ANA investigates: neurological complications of COVID -19 vaccines. Ann. Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. (Epub 2021 Mar 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menni C., Klaser K., May A., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021 Apr 27;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonio F.H., Daniela C., Konstantinos P., et al. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol. Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Grimshaw M., Ceballos-Liceaga S.E., Hernández-Vanegas L.E., et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin. Immunol. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh J.S., De Silva D.A., Quek A.M.L., et al. Neurology of COVID-19 in Singapore. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117118. https://pubmed.ncbi.nlm.nih.gov/32977228/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene S.K., Kulldorff M., Lewis E.M., et al. Near real-time surveillance for influenza vaccine safety: proof-of-concept in the vaccine safety datalink project. Epidemiol. Rev. 2010;171(2):177–188. doi: 10.1093/aje/kwp345. (Epub 2009 Dec 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hervé C., Laupèze B., Del Giudice G., et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4 doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Immunization Stress-related Response: A Manual for Program Managers and Health Professionals to Prevent, Identify and Respond to Stress-related Responses Following Immunization [Internet] World Health Organization; Geneva (CH): 2019. https://www.who.int/publications/i/item/10665330277 [cited 2021 Jun 14]. 62 p. Available from: [Google Scholar]
- 18.Law B. Coalition for Epidemic Preparedness Innovations; Davos (CH): 2021. SO2- D2.5.2.1 – AESI Case Definition Companion Guide for 1st tier AESI Anaphylaxis [Internet]https://brightoncollaboration.us/wp-content/uploads/2020/11/SPEAC_D2.5.2.1_AESI-Case-Definition-Companion-Guide.pdf [cited 2021 Jun 17]. 39 p. Available from: [Google Scholar]
- 19.Puliyel J., Naik P. Revised World Health Organization (WHO)’s causality assessment of adverse events following immunization- a critique. F1000Res. 2018;7:243. doi: 10.12688/f1000research.13694.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheffer I.E. Vaccination triggers, rather than causes, seizures. Epilepsy Curr. 2015;15(6):335–337. doi: 10.5698/1535-7511-15.6.335. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4657773/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser W.A., Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49(Suppl. 1):8–12. doi: 10.1111/j.1528-1167.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 22.Watad A., De Marco G., Mahajna H., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mrna/dna sars-cov-2 vaccination. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. (Epub 2021 Apr 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J.Y., Margo C.E. Ocular adverse events following vaccination: overview and update. Surv. Ophthalmol. 2021;S0039–6257(21) doi: 10.1016/j.survophthal.2021.04.001. 00099–0. [DOI] [PubMed] [Google Scholar]
- 25.Simpson C.R., Shi T., Vasileiou E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021 Jun 9;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. https://www.nature.com/articles/s41591-021-01408-4.pdf Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeng M., Koke W., Ms J.C., et al. National Registry of Diseases Office; Singapore (SG): 2020 Jun. Singapore Stroke Registry Annual Report 2018 [Internet]https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/ssr-web-report-2018.pdf?sfvrsn=58eb7c4c_0 [cited 2021 Jun 14]. 49 p. Available from: [Google Scholar]
- 27.Hart R.G., Diener H.-C., Coutts S.B., et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 28.See I., Su J.R., Lale A., et al. US Case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Mayhani T., Saber S., Stubbs M.J., et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J. Neurol. Neurosurg. Psychiatry. 2021;92:1247–1248. doi: 10.1136/jnnp-2021-326984. https://jnnp-bmj-com.libproxy1.nus.edu.sg/content/jnnp/early/2021/06/03/jnnp-2021-326984.full.pdf 326984. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Dias L., Soares-Dos-Reis R., Meira J., et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. 2021;30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taquet M., Husain M., Geddes J.R., et al. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. medRxiv. 2021 doi: 10.1101/2021.04.27.21256153v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smadja D.M., Yue Q.-Y., Chocron R., et al. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021 doi: 10.1183/13993003.00956-2021. https://erj.ersjournals.com/content/early/2021/04/08/13993003.00956-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledford H. US authorization of first COVID vaccine marks new phase in safety monitoring. Nature. 2020;588(7838):377–378. doi: 10.1038/d41586-020-03542-4. [DOI] [PubMed] [Google Scholar]
- 34.Renoud L., Khouri C., Revol B., et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the world health organization pharmacovigilance database. JAMA Intern. Med. 2021;181(9):1243–1245. doi: 10.1001/jamainternmed.2021.2219. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2779389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozonoff A., Nanishi E., Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect. Dis. 2021;21(4):450–452. doi: 10.1016/S1473-3099(21)00076-1. (Epub 2021 Feb 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozonoff A., Nanishi E., Levy O. Bell’s palsy and SARS-CoV-2 vaccines—an unfolding story – authors’ reply. Lancet Infect. Dis. 2021;21(9):1211–1212. doi: 10.1016/S1473-3099(21)00323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Sciences Authority . Health Sciences Authority; Singapore (SG): 2021. HSA’s safety update #1 Pfizer-BioNTech and Moderna COVID-19 vaccines (30 December 2020–18 April 2021) [Internet]https://www.hsa.gov.sg/docs/default-source/hprg-vcb/safety-update-on-covid19-vaccines/hsa-safety-update-no-2-on-covid-19-vaccines-(23-may-2021).pdf [cited 2021 Jun 14]. 6 p. Available from: [Google Scholar]
- 38.Woo E.J., Winiecki S.K., Ou A.C. Motor palsies of cranial nerves (excluding VII) after vaccination reports to the US vaccine adverse event reporting system. Hum Vaccin Immunother. 2014;10(2):301–305. doi: 10.4161/hv.27032. (Epub 2013 Nov 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Capo D.P., Stevens S.M., Cavuoto K.M. Acute abducens nerve palsy following COVID-19 vaccination. J. AAPOS. 2021;S1091–8531(21) doi: 10.1016/j.jaapos.2021.05.003. 00109–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stowe J., Andrews N., Wise L., et al. Investigation of the temporal association of guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom general practice research database. Epidemiol. Rev. 2008 Feb 1;169(3):382–388. doi: 10.1093/aje/kwn310. (Epub 2008 Nov 24) [DOI] [PubMed] [Google Scholar]
- 41.Loharikar A., Suragh T.A., MacDonald N.E., et al. Anxiety-related adverse events following immunization (AEFI): a systematic review of published clusters of illness. Vaccine. 2018;36(2):299–305. doi: 10.1016/j.vaccine.2017.11.017. (Epub 2017 Nov 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umapathi T., Er B., Koh J.S., et al. Guillain-Barré syndrome decreases in Singapore during the COVID-19 pandemic. J. Peripher. Nerv. Syst. 2021 Jun;26(2):235–236. doi: 10.1111/jns.12439. (Epub 2021 Mar 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu T.M., Goh C., Tan Y.K., et al. Cerebral venous thrombosis in patients with COVID-19 infection: a case series and systematic review. J. Stroke Cerebrovasc. Dis. 2020 Dec;29(12):105379. doi: 10.1016/j.jstrokecerebrovasdis.2020.105379. (Epub 2020 Oct 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu T.M., Seet C.Y.H., Koh J.S., et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw. Open. 2021;4(4):e217498. doi: 10.1001/jamanetworkopen.2021.7498. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2779040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umapathi T., Poh M.Q., Fan B.E., et al. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin. Auton. Res. 2020;30(6):571–573. doi: 10.1007/s10286-020-00733-x. (Epub 2020 Sep 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umapathi T., Quek W.M.J., Yen J.M., et al. Encephalopathy in COVID-19 patients; viral, parainfectious, or both? eNeurologicalSci. 2020;21:100275. doi: 10.1016/j.ensci.2020.100275. https://www.sciencedirect.com/science/article/pii/S240565022030054X?via%3Dihub Erratum in: eNeurologicalSci. 2021 Jun;23:100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study protocol, statistical code and dataset are available from the corresponding author upon reasonable request. All data relevant to the study are included in the article and supplemental material. Patient-related dataset will be shared upon request from any qualified investigator, maintaining anonymization of the individual patients.