Abstract
Background:
Venous thromboembolism is a significant source of morbidity and mortality following total hip replacement and total knee replacement. Apixaban has been proven to be efficacious without increased risk of bleeding in phase-III trials in patients undergoing total knee replacement and total hip replacement. Due to paucity of data on safety of apixaban in Indian patients, this phase-IV study was conducted to evaluate safety of apixaban in patients undergoing total knee replacement and total hip replacement.
Methods:
In this non-comparative phase-IV clinical trial, patients undergoing elective total knee replacement or total hip replacement surgery, or a revision of at least one component of total knee replacement or total hip replacement, were enrolled. The eligible patients were given the approved dosage of apixaban 12 to 24 h after completing the skin wound closure. The primary safety outcome was the composite of the International Society on Thrombosis and Haemostasis–defined major bleeding and clinically relevant non-major bleeding events at the end of the treatment. The secondary efficacy endpoint was the composite of venous thromboembolism/all-cause death at the end of the treatment.
Results:
A total of 498 patients received apixaban prophylaxis therapy. Six (1.2%) bleeding adverse events were observed during the treatment period. Only one bleeding event was adjudicated as an International Society on Thrombosis and Haemostasis–defined clinically relevant non-major bleeding event (moderate severity). There were no fatal bleeding events and no deaths following the treatment. One venous thromboembolism event, that is, symptomatic distal left leg DVT, was reported in a total knee replacement patient and was adjudicated during the treatment period.
Conclusion:
Apixaban demonstrated a favorable safety profile for venous thromboembolism prevention in Indian patients undergoing total knee replacement or total hip replacement.
Keywords: Total knee replacement, total hip replacement, thromboembolism, venous, anticoagulant
Introduction
Venous thromboembolism (VTE) comprises pulmonary embolism (PE) and deep-vein thrombosis (DVT). 1 These complications are associated with significant morbidity and mortality subsequent to total hip replacement (THR) or total knee replacement (TKR). 2 The American College of Chest Physicians (ACCP) and the American Academy of Orthopedic Surgeons have computed an estimate of symptomatic VTE rate for the immediate postoperative period to be 1.5%, 1% for DVT, and 0.5% for PE. For major orthopedic surgeries like TKR, THR, and hip fracture surgery (HFS), a combined 35-day untreated baseline risk for symptomatic VTE was estimated to be 4.3%. 3 The occurrence rate of VTE in anticoagulated patients who have undergone TKR and THR at 90 days can go up to 5% for DVT and up to 2% for PE. 4 A 5-year Korean study reported an incidence of postoperative DVT for hip replacement arthroplasty and knee replacement arthroplasty as 0.15% and 0.22%, respectively. 5 The incidence of DVT in Indian patients undergoing orthopedic surgery without any prophylaxis ranges from 0.98% to 23.3%. 6
The ACCP clinical practice guidelines for VTE prophylaxis post THR or TKR procedures have recommended either pharmacological agents or mechanical compressive devices, or both as the standard of care for thromboprophylaxis. 3 ACCP endorses the routine use of traditional anticoagulants; for example, low-molecular-weight heparins (LMWHs), vitamin K antagonists (VKAs) like warfarin, fondaparinux, aspirin, and newer oral anticoagulants—apixaban, rivaroxaban, or dabigatran for thromboprophylaxis post THR or TKR surgery. 3
Apixaban directly inhibits factor Xa 3 and has been approved in India for “prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery.” 7 Apixaban has been evaluated in phase-III orthopedic studies like ADVANCE-2 and ADVANCE-3. ADVANCE-2 and ADVANCE-3 clinical studies recruited patients who had undergone elective knee- or hip-replacement surgeries, respectively. Both the studies concluded apixaban to be superior to enoxaparin without increasing the risk of bleeding.8,9
There is a paucity of data on safety of apixaban in Indian patients undergoing major orthopedic surgeries. Hence, we conducted this study to evaluate the safety of apixaban 2.5 mg orally twice daily for 2 weeks in patients undergoing TKR or for 5 weeks in patients undergoing THR.
Methods
Patients
Inclusion and exclusion criteria
Patients aged ⩾ 18 years undergoing elective TKR or THR surgery, or a revision of at least one component of TKR or THR, were eligible to participate in this study. Patients were included if they were willing to provide informed consent to participate in the study and willing to take precautions to avoid pregnancy.
The major exclusion criteria were bleeding or coagulation disorder in the patients or first-degree relative, active bleeding or at a risk of bleeding, history of major surgery or trauma within the past 90 days, and active hepatobiliary disease. The concomitant use of any antiplatelet agents (including clopidogrel but other than aspirin), oral anticoagulants (VKAs and direct factor Xa or II inhibitors), unfractionated heparin, LMWH, and fondaparinux was prohibited.
Study design and medications
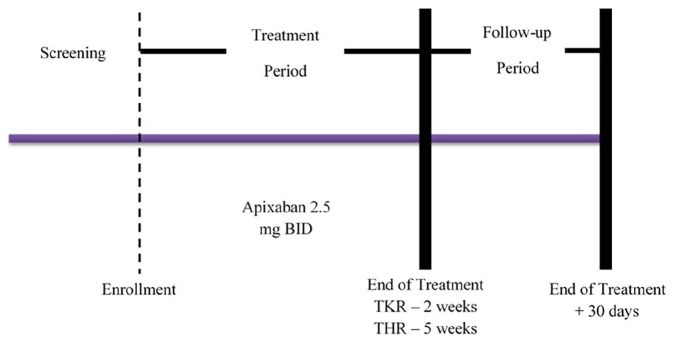
The present study was a multi-center, phase-IV, prospective, non-comparative clinical trial with endpoint adjudication. The study was funded by Bristol-Myers Squibb and Pfizer. The study was compliant with the norms set in the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the respective institutional ethics committees of the participating centers prior patient enrollment. The participating patients provided written informed consent prior their enrollment. The patients were screened until 14 days before the surgery and potentially eligible patients were assigned a unique sequential number by the interactive voice response system (IVRS). The eligible patients were assigned to apixaban 2.5 mg by the IVRS between 4 days prior to surgery to 24 h after surgery (refer Figure 1).
Figure 1.
Study design.
The first oral dose of apixaban 2.5 mg was given 12 to 24 h after completing skin wound closure, typically on the morning of day 2, followed by twice a day dosing for the next 12 ± 2 days in patients who underwent TKR and for 35 ± 2 days in patients who underwent THR. The follow-up visit was scheduled 30 days after the last dose of the study drug. The study drugs were supplied by Bristol-Myers Squibb. The total duration of the study for TKR was 42 days and for THR was 65 days.
Procedures
Patients from both the surgery groups were evaluated on day 1, day 2, and on the day of discharge from the hospital. The subsequent follow-ups were conducted on day 7 ± 2, and day 12 ± 2 for patients who underwent TKR. The patients who underwent THR, an additional follow-up was scheduled on day 35 ± 2. During the follow-up, the patients were checked for suspected symptomatic VTE, bleeding events, or any adverse events (AEs) or serious AEs (SAEs). Patients with signs and/or symptoms of VTE underwent appropriate diagnostic evaluations such as ultrasound, computer tomography (CT), and magnetic resonance imaging (MRI). If study medication was discontinued for a suspected DVT and/or PE, alternative antithrombotic prophylaxis was initiated per the investigator’s discretion and standard-of-care. Follow-up assessments were conducted on day 42 ± 2 for patients who underwent TKR and on day 65 ± 2 for patients who underwent THR.
Outcome measures
The primary safety outcome was to assess the composite of the International Society on Thrombosis and Haemostasis (ISTH) for major bleeding and clinically relevant non-major (CRNM) bleeding in patients undergoing elective TKR or THR at the end of treatment + 2 days. The ISTH major bleeding was defined as
a fatal bleeding, or bleeding in critical organs such as brain, spine, eye, retroperitoneum, pericardium, and skeletal muscle, or resulting in reduction of hemoglobin by 2 g/dL or more, or transfusion of 2 units or more of packed red cells or whole blood within 24 hours. 10
The CRNM bleeding was defined as the bleeding that was clinically acute and overt, and did not qualify as a major bleed, but required medical intervention. 11 An Independent Central Adjudication Committee (ICAC) evaluated the bleeding AEs and SAEs.
The secondary efficacy endpoint was the composite of VTE/all-cause death at the end of the treatment + 2 days, where VTE was the combination of DVT and non-fatal PE.
Statistical analyses
Sample size calculation
The sample size of the study was determined as per the requirement of the regulatory agency and not statistical considerations. With 500 patients in the apixaban arm and an event rate of 4.36% for ISTH major or CRNM bleeding, the 95% confidence interval (CI) for the event rate would be (2.6%, 6.2%) and width of the 95% CI would be 3.6%.
Frequency distribution and summary statistics for demographic and baseline variables were presented by the type of surgery. The event rate with 95% CI of the adjudicated major bleeding or CRNM bleeding events and the composite of VTE and all-cause death during the treatment period were summarized. Sub-group analyses by surgery type were also conducted.
Results
Patients
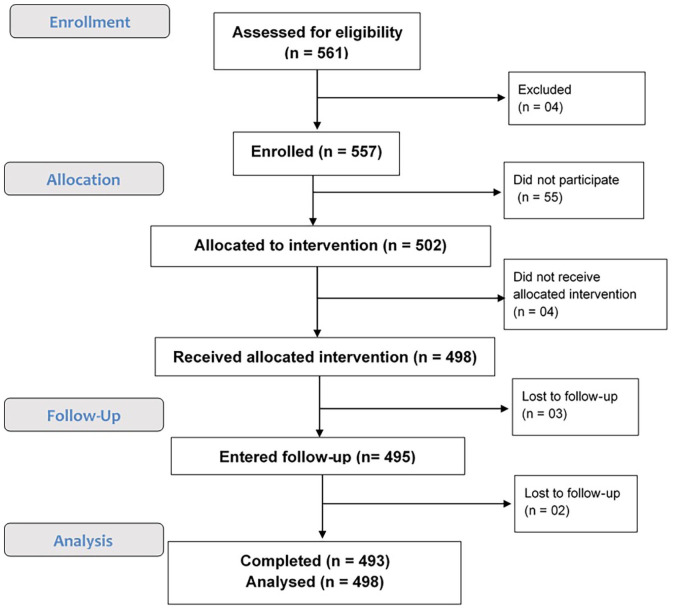
Across 17 centers in India, 561 patients were screened, and 557 patients were enrolled between April 2015 and June 2018. Among the eligible patients, 498 received the prophylaxis treatment with apixaban 2.5 mg BID. The study was completed by 493 patients (refer Figure 2). The baseline characteristics and demographics of the patients are highlighted in Table 1.
Figure 2.
Patient disposition.
Table 1.
Baseline demographic characteristics of treated patients.
| No. of TKR patients (n = 326) | No. of THR patients (n = 172) | |
|---|---|---|
| Race | ||
| Asian | 326 (100%) | 172 (100%) |
| Gender | ||
| Male | 89 (27.3%) | 119 (69.2%) |
| Female | 237 (72.7%) | 53 (30.8%) |
| Age range (years) | 23–82 | 18–74 |
| < 65 | 208 (63.8%) | 163 (94.8%) |
| 65 to < 75 | 96 (29.4%) | 9 (5.2%) |
| ⩾ 75 | 22 (6.7%) | 0 |
| Weight, mean (kg) | 67.7 | 60.2 |
| Level of renal impairment, measured by CrCL, n (%) | ||
| Severe: < 30 mL/min | 1 (0.3) | 1 (0.6) |
| Moderate: ⩽ 30 to < 60 mL/min | 45 (13.8) | 12 (7.0) |
| Mild: ⩽ 60 to < 90 mL/min | 137 (42.0) | 49 (28.5) |
| Normal: ⩾ 90 mL/min | 142 (43.6) | 108 (62.8) |
| Not available | 1 (0.3) | 2 (1.2) |
| No. of blood units transferred (n ± SD) | 48 ± 0.25 | 29 ± 0.41 |
TKR: total knee replacement; THR: total hip replacement; SD: standard deviation.
The mean duration of exposure for the TKR group was 12 ± 1.37 days and for the THR group was 34.7 ± 2.81 days. Majority of the patients were noted to have treatment compliance between 80% and 120%; 99.1% patients from the TKR group and 99.4% patients from the THR group.
Safety results
Only one event, an incision-site hemorrhage of moderate intensity, was adjudicated as an ISTH-defined CRNM bleeding event in the THR surgery group.
There were no fatal bleeding events following treatment with apixaban in the study population, regardless of the type of orthopedic surgery and the associated different lengths of VTE prophylaxis.
As shown in Table 2, there were a total of six (1.2%) bleeding AEs reported in 498 patients (three from each surgery type). The onset of these AEs occurred during the treatment period. Of these, four were considered not related to apixaban and two were considered related to apixaban.
Table 2.
Summary of adverse events—by type of surgery.
| TKR (N = 326) | THR (N = 172) | |
|---|---|---|
| Primary endpoint: ISTH-defined major and CRNM bleeding | 0 | 1 (Moderate incision-site hemorrhage a ) |
| Bleeding AEs | ||
| Not related | 3 (2 mild decreased hemoglobin; 1 mild hematuria) | 1 (Moderate incision-site hemorrhage a ) |
| Related to study drug | 0 | 2 (1 Mild serosanguinous wound discharge; 1 moderate incision-site hemorrhage a ) |
| AEs leading to discontinuation | ||
| Not related | 1 (Mild pyrexia) | 0 |
| Related to study drug | 1 (Mild DVT—popliteal) | 2 (1 Mild serosanguinous wound discharge; 1 moderate incision-site hemorrhage a ) |
| Serious adverse events | ||
| Not related | 2 (1 mild anemia; 1 mild vertigo) | 0 |
| Related to study drug | 1 (Mild DVT—popliteal) | 0 |
| AEs | ||
| Not related | 132 (125 mild events; 6 moderate events; 1 severe event) | 34 (31 mild events; 3 moderate events) |
| Related to study drug | 4 (3 mild: DVT, incision site pain, urinary retention; 1 moderate: hypertension) | 2 (1 mild: serosanguinous wound discharge; 1 moderate: incision-site hemorrhage a ) |
TKR: total knee replacement; THR: total hip replacement; CRNM bleeding: clinically relevant nonmajor bleeding; DVT: deep vein thrombosis; AE: adverse event; ISTH: International Society on Thrombosis and Haemostasis.
This event was adjudicated as an ISTH-defined CRNM bleeding event.
In the TKR group, 132 AEs occurred in 82 patients which were considered to be not related to apixaban. In the THR group, 34 such AEs occurred in 19 patients which were determined to be not related to apixaban. A total of four drug-related AEs were reported in TKR group and 2 in THR group (Table 2).
There were three (0.01%) SAEs reported in the TKR surgery group onset during the treatment period. Of these three SAEs, two events (anemia and vertigo) were reported in the same subject and were considered not related to study treatment. The third SAE was a mild DVT that was adjudicated and considered as a secondary efficacy event which led to discontinuation. No SAEs were reported in the THR group.
The most common AE (> 5%) was incision site pain, reported in 23 out of 326 (7.1%) TKR patients.
Efficacy results
Only one VTE event (symptomatic distal left leg DVT) was reported in a patient from TKR group and adjudicated during the treatment period, resulting in an overall event rate (95% CI) of the composite of all-cause deaths + adjudicated VTE (DVT/PE) of 0.20% (0, 1.25).
Discussion
In this study, apixaban 2.5 mg b.i.d was given to patients undergoing TKR or THR surgery, as an antithrombotic prophylaxis. Six (1.2%) bleeding adverse events were observed in 498 patients during the treatment period and only two of these events were considered to be related to apixaban. The two related bleeding adverse events occurred in the THR surgery group. One of the events was mild serosanguinous wound discharge. The other event was moderate incision-site hemorrhage and was adjudicated as an ISTH-defined CRNM bleeding event of moderate severity. There were no fatal bleeding events reported in the study population. One VTE event, that is, symptomatic distal left leg DVT was reported in a TKR subject and was adjudicated during the treatment period.
In ADVANCE-2, efficacy and safety of apixaban 2.5 mg (twice daily) was assessed in comparison with subcutaneous enoxaparin 40 mg (once daily) given prophylactically to patients undergoing elective TKR. In the study, 4% of the patients treated prophylactically with apixaban experienced a major or CRNM bleeding was reported. In ADVANCE-3, apixaban 2.5 mg (twice daily) and subcutaneous enoxaparin 40 mg (every 24 h) were assessed for thromboprophylaxis after hip replacement surgery. As per the results of ADVANCE-3, 4.8% patients receiving apixaban were reported to have major and clinically relevant nonmajor bleeding events.8,9 Raskob et al. conducted a pooled analysis to compare VTE and bleeding events of apixaban with enoxaparin in 8464 patients from ADVANCE-2 and ADVANCE-3 trials. In the apixaban group, 0.7% patients experienced major bleeding events. Bleeding events at the surgical site which would necessitate reoperation or intervention were very uncommon in both groups (< 1 in 1000 patients). The analysis indicated that apixaban has a favorable balance of antithrombotic benefit and bleeding risk as compared with enoxaparin. In the apixaban group, a proportion of bleeding events occurred prior to the first dose of apixaban. Hence, the absolute incidence of major bleeding at the surgical site associated with apixaban was 0.2%. 12 Yu et al., 13 in their meta-analysis, reported that bleeding events occurred in 4.09% patients of apixaban group from the 16 studies included. The safety analysis of the present study revealed that the bleeding events occurred in 0.01% patients treated with apixaban.
VTE and death from all-cause was reported in 1.4% and 15.06% patients from ADVANCE-2 and ADVANCE-3 trials, respectively, who had received apixaban. Major thromboembolism was reported in 0.5% patients from the apixaban group in ADVANCE-2 and in 1.09% patients from the apixaban group in ADVANCE-3.8,9 In the pooled analysis by Raskob et al., 12 major VTE was reported in 0.7% patients from apixaban group and in 1.5% patients from the enoxaparin group. Total efficacy events (“composite of DVT, non-fatal PE, and all-cause mortality”) occurred in 6.81% patients treated with apixaban. 13 In this study, 1 VTE event (distal left leg DVT) was reported in a TKR patient and was adjudicated during the treatment period.
There is a correlation between increased risk of excessive hemorrhage in patients during an invasive procedure and surgery and genetics. Though the evidence on association between bleeding symptoms and race and ethnicity is sparse, Mauer et al. 14 reported less bleeding symptoms in Asian healthy volunteers as compared to Caucasians. The presence of “prothrombin gene mutation of factor V Leiden” is responsible for a 3 to 4 times increased risk of VTE. Since this trait is less prevalent in Asian population as compared to Caucasians, the prevalence of VTE is implied to be lower. The Asian population is also reported to have a 3 to 5 times lower incidence of symptomatic first-time idiopathic and secondary VTE. 15 The results of the present study concur with the lower bleeding risk associated with the race and reports a lower rate of bleeding events as well as lower rate of VTE events in Indian patients.
This study was however limited in terms of the sample size and inadequate efficacy data since it was a single-arm, non-comparative study. A comparative study will help to ascertain the benefit-to-risk ratio of apixaban as compared to other available therapeutic options in Indian population.
Conclusion
Overall, apixaban was concluded to have a favorable safety profile for VTE prevention in Indian patients undergoing TKR or THR.
Acknowledgments
The authors would like to acknowledge the investigators who were a part of the study and helped in conducting the study and reported unbiased results: Dr. Bharath Loganathan and Dr. Chinmaya Sharma (Shalby Hospital Ltd., Ahmedabad); Dr. Naresh Shetty (M.S. Ramaiah College and Hospital, Bangalore); Dr. Ashish Kumar (King George Medical University, Lucknow); Dr. Rajesh Malhotra (All India Institute Of Medical Sciences, New Delhi); Dr. Navaladi Shankar (Apollo Hospital, Chennai); Dr. Bhagvatula Narayan Prasad (CARE Hospital, Hyderabad); Dr. Sajal Mitra (Government Medical College, Nagpur); Dr. Ravindran Mecheryvalappil Krishnankutty (Government Medical College, Kozhikode); Dr. Arvind Goregaonkar (Lokmanya Tilak Municipal General Hospital, Sion, Mumbai); Dr. Girish Gadekar (Mahatma Gandhi Mission Medical College and Hospital, Aurangabad); Dr. Binoy Sridharadhan (Government Medical College, Thiruvananthanapuram); Dr. Sachin Karmarkar (Noble Hospital, Pune); Dr. Sanjeev Mahajan (Fortis Hospital, Ludhiana); Dr. Keyur Buch (CIMS Hospital, Ahmedabad). The authors would also like to thank Ms. Vaidehi Wadhwa (Medical Excellence, Pfizer) for her medical writing and editorial support in preparing this manuscript.
Footnotes
Author contributions: Study conduct: P.H., A.A., and S.P.K. Manuscript drafting: P.H., A.A., S.P.K., K.N.B., and T.S.R. Manuscript editing and review: P.H., A.A., S.P.K., K.N.B., and T.S.R. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author disclosures: Panchal H, Agashe A and Sancheti PK were the investigators of the study. Kulkarni NB and Taur SR are employees of Pfizer Ltd. Additionally, Taur SR holds stock in Pfizer Ltd.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Pfizer Inc. and Bristol Myers Squibb.
Ethical approval: 1. Shalby Hospital Ltd Ethics Committee—Protocol 02, 16 August 2014.
2. Ethical Review Board M.S. Ramaiah Medical College & Hospital—Protocol 02, 20 September 2014.
3. King George Medical University Institutional Ethics Committee—Protocol 02, 28 September 2015.
4. All India Institute Of Medical Sciences Institutional Ethics Committee—Protocol 02, 30 August 2014.
5. Apollo Hospital Educational & Research Foundation Ethics Committee—Apollo Hospital, Protocol 02, 17 January 2017.
6. Care Hospital IEC CARE Convergence Center, Protocol 02, 13 June 2016.
7. Government Medical College, IEC, Protocol 02, 26 December 2016.
8. Institutional Review Board- Sancheti Institute for Orthopedics & Rehabilitation Sancheti Research Center, Protocol 02, 8 August 2014.
9. Government Medical College IEC Inst of Maternal and Child Health, Protocol 02, 19 May 2016.
10. Lokmanya Tilak Municipal Medical College Staff and Research Society EC, Protocol 02, 8 August 2016.
11. IEC B. J. Medical College & Civil Hospital, Protocol 02, 22 September 2016.
12. Mahatma Gandhi Mission ECRHS; Pharmacology Department, Protocol 02, 15 October 2016.
13. Government Medical College Dept of Pharmacology Human Ethics Committee, Protocol 02, 17 April 2017.
14. IEC, Noble Hospital, Protocol 02, 30 December 2016.
15. Sahyadri Hospitals Ltd Ethics Committee Sahyadri Clinical Research & Development Center, Protocol 02, 30 August 2016.
16. IEC, Fortis Hospital, Protocol 02, 07 December 2017.
Informed consent: Written informed consent was obtained from all subjects before the study
Trial registration: The trial was registered with the Clinical Trial Registry of India; Reg. No: CTRI/2015/03/005598; Available at: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid = 7433&EncHid =&userName = apixaban
ORCID iD: Santosh R Taur  https://orcid.org/0000-0002-6865-230X
https://orcid.org/0000-0002-6865-230X
References
- 1. Agnelli G, Buller HA, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368: 699–708. [DOI] [PubMed] [Google Scholar]
- 2. Zhang J, Chen Z, Zheng J, et al. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg 2015; 135(6): 759–772. [DOI] [PubMed] [Google Scholar]
- 3. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141(2Suppl): e278S–e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bala A, Huddleston JI, 3rd, Goodman SB, et al. Venous Thromboembolism prophylaxis After TKA: Aspirin, Warfarin, Enoxaparin, or Factor Xa inhibitors? Clin Orthop Relat Res 2017; 475: 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SY, Ro du H, Chung CY, et al. Incidence of deep vein thrombosis after major lower limb orthopedic surgery: analysis of a nationwide claim registry. Yonsei Med J 2015; 56(1): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parameswaran A, Krishnamoorthy VP, Oommen AT, et al. Is pre-operative assessment of coagulation profile with Thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery. J Clin Orthop Trauma 2016; 7(Suppl. 2): 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drugs@CDSCO, https://cdscoonline.gov.in/CDSCO/Drugs (accessed 23rd January 2020).
- 8. Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010; 375: 807–815. [DOI] [PubMed] [Google Scholar]
- 9. Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010; 363: 2487–2498. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 11. Hellenbart EL, Faulkenberg KD, Finks SW. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag 2017; 13: 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raskob GE, Gallus AS, Pineo GF, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement. J Bone Joint Surg Br 2012; 94-B: 257–264. [DOI] [PubMed] [Google Scholar]
- 13. Yu Z, Shan P, Yang X, et al. Comparison of efficiency and safety of rivaroxaban, apixaban and enoxaparin for thromboprophylaxis after arthroplastic surgery: a meta-analysis. Biosci Rep 2018; 38: BSR20180423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mauer A, Khazanov N, Levenkova N, et al. Impact of sex, age, race, ethnicity and aspirin use on bleeding symptoms in healthy adults. J Thromb Haemost 2011; 9(1): 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White R, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res 2009; 123: S11–S17. [DOI] [PubMed] [Google Scholar]