Abstract
Objective: This study was undertaken to investigate eukaryotic translation initiation factor 3 subunit B (EIF3B) expression and its clinical value for indicating disease progression and prognosis in adult Philadelphia chromosome negative acute lymphoblastic leukemia (Ph− ALL) patients. Methods: Totally, 76 adult Ph− ALL patients and 30 healthy donors (HDs) were included. Bone marrow (BM) samples before therapy (baseline), after 4-week therapy of Ph− ALL patients and the BM samples of HDs were collected. Then, EIF3B expression in BM was detected by reverse transcription quantitative polymerase chain reaction. Results: EIF3B expression was increased in Ph− ALL patients compared with HDs, which distinguished Ph− ALL patients from HDs (area under the curve [AUC]: 0.928; 95% confidence interval [CI]: 0.882−0.974) by receiver operating characteristic curve. Furthermore, higher baseline EIF3B expression was associated with elevated white blood cell and bone marrow blasts, while it was associated with lower complete remission (CR) within 4 weeks and less allogeneic hematopoietic stem cell transplant achievements in Ph− ALL patients. Additionally, higher baseline EIF3B expression was associated with decreased disease-free survival but not overall survival. However, it was associated with raised 1-year mortality and 3-year mortality in Ph− ALL patients. After 4-week therapy, EIF3B expression was reduced in total Ph− ALL patients. Notably, the reduction of EIF3B expression was more obvious in Ph− ALL patients who achieved CR within 4 weeks compared with Ph− ALL patients who did not achieve CR within 4 weeks. Conclusion: EIF3B overexpression is related to worsened clinical features, poor treatment response and survival in adult Ph− ALL patients.
Keywords: adult Philadelphia chromosome negative acute lymphoblastic leukemia, eukaryotic translation initiation factor 3 subunit B, clinical feature, treatment response, survival profiles
Introduction
Acute lymphoblastic leukemia (ALL) is an aggressive hematological malignant disorder marked by a malignant proliferation and expansion of immature lymphoid progenitor cells that can invade bone marrow, blood, and extramedullary sites, affecting both the pediatric population and the adult population. 1 Philadelphia chromosome (a byproduct of reciprocal translocation t [9; 22] [q34; q11]) negative ALL (Ph− ALL) is a subtype of ALL.2,3 Although the remission rate is achieved in more than 80% of adult Ph− ALL patients with mainstay multidrug chemotherapy regimens, a considerable number of these patients eventually become refractory to initial therapy or/and experience a relapse despite the aggressive consolidation and maintenance chemotherapy regimens, even after allogeneic stem cell transplantation.4,5 In addition, limited effective targeted therapies and lack of effective biomarkers remain as major obstacles to the improvement of prognosis in adult Ph− ALL patients. 6 Therefore, efforts are needed to identify more potential and effective biomarkers for guiding treatment strategies and improving prognosis in adult Ph− ALL patients.
Eukaryotic translation initiation factor 3 (EIF3), the most complex translation initiation factor consisting of 13 non-identical subunits (EIF3A to M), is vital in the entire process of translational initiation processes. 7 As a major scaffold protein, EIF3 subunit B (EIF3B) contains two domains (the N-terminal domain and the WD40β domain), which is related to multiple cellular processes, including cell cycle progression, apoptosis, transcription, signal transduction and others. 7 A series of recent findings has unraveled that EIF3B participates in the pathogenesis of multiple cancers including hematological malignancies.8–16 As an example, the downregulation of EIF3B represses the proliferation and promotes the apoptosis of cells in chronic myeloid leukemia. 16 Another study illustrates that knockdown of EIF3B impedes cell proliferation, cell migration, and enhances cell apoptosis in acute myeloid leukemia. 14
Considering that EIF3B is involved in the enhancement of malignant cells’ activities in above-mentioned hematological malignancies, we speculated that EIF3B might participate in the development and progression of Ph− ALL patients, while little information was found yet.
Herein, the focus of this current study was to investigate the EIF3B expression in adult Ph− ALL patients and its potentially predictive value for disease progression, treatment response, and survival profiles.
Methods
Patients and Controls
A total of 76 adult Ph− ALL patients who were admitted to our hospital from January 2015 to October 2019 were screened out and analyzed in this retrospective cohort study. The screening criteria included: (i) patient was diagnosed as primary Ph- ALL confirmed by bone marrow morphology, immunology, cytogenetics and molecular biology (MICM) examinations; (ii) patient had fresh-frozen and well-preserved bone marrow sample that was collected prior to the initiation of therapy; (iii) patient had age within 18−75 years; (iv) patient's clinical data were available for analysis. However, patients were not included in the analysis if they had the following conditions: (i) patients had received treatment for ALL in another hospital before being admitted to our hospital; (ii) patients were proven to be concomitant with other malignant diseases; (iii) patients had no treatment response data; and (iv) patients had incomplete follow-up records. Furthermore, this study also included 30 healthy donors (HDs) who volunteered to undergo bone marrow donation and provide bone marrow samples for the study use, during the same period.
Bone Marrow Sample and Clinical Data Collection
In the current study, 76 bone marrow samples before therapy (baseline) of adult Ph− ALL patients and 30 bone marrow samples of HDs were collected from hospital sample bank. Among 76 adult Ph− ALL patients, 33 patients also had well-preserved bone marrow samples that were obtained after 4-week therapy, which were also collected from the hospital sample bank. In summary, the bone marrow samples at baseline, after 4-week therapy of Ph− ALL patients, and the bone marrow samples of HDs were collected. Then, EIF3B expression in bone marrow samples was detected by reverse transcription quantitative polymerase chain reaction (RT-qPCR). After separation of monocyte cells from bone marrow samples by density gradient centrifugation, the EIF3B expression in the monocyte cells (including the lymphocytes) was determined by RT-qPCR. Besides, adult Ph− ALL patients’ clinical data including demographics (age, gender), immunophenotype (T-cell acute lymphoblastic leukemia [T-ALL], B-cell acute lymphoblastic leukemia [B-ALL]), central nervous system leukemia (CNSL), laboratory index (white blood cell [WBC] count, hemoglobin [HGB], blood platelet [PLT], bone marrow blasts), outcome (complete remission [CR] within 4 weeks, total CR, allogeneic hematopoietic stem cell transplant [allo-HSCT]),s and follow-up information were collected from medical records.
RT-qPCR Assay
Total RNA was extracted by TRIzol™ Reagent (Invitrogen™), and then RNA was reverse-transcribed with Quanti Nova Reverse Transcription Kit (Qiagen, Duesseldorf, Nordrhein-Westfalen,Germany). After that, the qPCR procedure was carried out using the QuantiNova SYBR Green PCR Kit (Qiagen, Duesseldorf,Nordrhein-Westfalen, Germany). The genomic DNA amplified by primers were removed by DNase treatment prior to qPCR procedures. Lastly, the relative expression of EIF3B was calculated using the 2−ΔΔCt method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal reference. The following is the primers applied in qPCR: EIF3B, Forward Primer (5′-3′): CGTATGTGCGTTGGTCTCCTAA; Reverse Primer (5′-3′): CCTTGGTGGCTGAATCTCTGAA; GAPDH, Forward Primer (5′-3′): TGACCACAGTCCATGCCATCAC, Reverse Primer (5′-3′): GCCTGCTTCACCACCTTCTTGA). Additionally, we used another internal reference 18 s RNA with re-designed EIF3B primer to validate the results, which indicated that that the EIF3B Ct values by two different primers were highly consistent with each other, and so was the calculated EIF3B expression using the 2−ΔΔCt method (Supplemental Figure 1). The EIF3B primer that cover an exon–exon boundary: EIF3B forward: 5′-GGAGTATGAACGGTGCCTTAGC-3′; reverse: 5′-AGCGGTCCTTGTTGTTCTTCTG-3′; 18 s rRNA forward: 5′-GTGGAGCGATTTGTCTGGTT-3′; reverse: 5′-CGCTGAGCCAGTCAGTGTAG-3′.
Treatment and Remission Evaluation
As recommended by Chinese expert panel consensus on diagnosis and treatment of adult ALL, 17 all adult Ph− ALL patients received the Chinese Acute Lymphoblastic Leukemia Cooperative Group 2008 (CALLG2008) protocol, which included a prophase treatment chemotherapy regimen, induction chemotherapy regimen, consolidation chemotherapy regimen, maintenance chemotherapy therapy, as well as central nervous system prophylaxis regimen. Bone marrow morphologic analysis was conducted for remission status evaluation on the 28 ± 7 days after initiation of induction therapy with a 4-week VDCLP regimen (Vincristine, Daunorubicin, Cyclophosphamide, L-asparaginase, Prednisone). CR was defined as no circulating blasts or extramedullary disease, trilineage hematopoiesis and <5% blasts, absolute neutrophil count> 1.0 × 109/mL, peripheral blood PLT> 100 × 109/mL, and no recurrence for 4 weeks. Non-CR patients after the induction therapy were given salvage therapy. There were 52 patients who achieved CR within 4 weeks after initiation of induction therapy, and totally 65 patients achieved CR after induction therapy and salvage therapy. As for patients who were candidates for allo-HSCT, the allo-HSCT (human leukocyte antigen [HLA]-matched donor or haploidentical related donor) was administered for them after 3 to 5 courses of consolidation therapy. Other patients continued to undergo the consolidation and maintenance therapy.
Survival Evaluation
Follow-up information was extracted from medical records, which were used to calculate disease-free survival (DFS) and overall survival (OS) with a last visit date up to 2019/12/31. The median follow-up duration was 15 months, a range of 0.0−54.0 months. DFS was defined as the duration from the date of CR to the date of relapse or death in CR status, where the relapse was defined as reappearance of blasts in the blood or bone marrow (>5%) or in any extramedullary site after a CR. Patients not known to have relapsed or died at last follow-up were censored on the date they were last examined. OS was defined as the duration from the date of diagnosis to the date of death or last follow-up.
Statistical Analysis
A Kolmogorov–Smirnov test was carried out to determine the normality. Normally or approximatively normally distributed data were described as mean with standard deviation (SD); skewed distributed data were described as median with interquartile range (IQR); categorical data were expressed as number and percentage (No. [%]). Comparison of EIF3B expression between Ph−ALL patients and HDs was determined by a Wilcoxon rank sum test. Receiver-operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to assess the performance of EIF3B expression in distinguishing Ph−ALL patients from HDs. Paired comparison of EIF3B expression at baseline and after 4-week therapy was determined by a Wilcoxon signed rank test. Ph−ALL patients were divided into four quartiles according to their baseline EIF3B expression as follows: Quartile 1 (Q1, EIF3B expression ranked in 0%–25% among all patients; median [range] EIF3B expression: 1.292 [0.855−2.042]), Quartile 2 (Q2, EIF3B expression ranked in 25%−50% among all patients; median [range] EIF3B expression: 2.838 [2.068−3.238]), Quartile 3 (Q3, EIF3B expression ranked in 50%−75% among all patients); median (range) EIF3B expression: 3.708 (3.316−4.284)), and Quartile 4 (Q4, EIF3B expression ranked in 75%−100% among all patients; median [range] EIF3B expression: 4.974 [4.335−8.347]). Correlation of baseline EIF3B expression with patients’ clinical features and outcomes was determined by the Spearman's rank correlation test or χ2 test for trend. DFS and OS were demonstrated by a Kaplan–Meier curve, and the correlation of DFS and OS with baseline EIF3B expression was determined by a log-rank test. Correlation of relapse rate and mortality with baseline EIF3B expression was determined by the χ2 test for trend. Independent factors related to DFS or OS were analyzed using forward stepwise multivariate Cox’s proportional hazard regression model. All statistical analyses were performed using SPSS 22.0 statistical software (IBM), and all figures were plotted using GraphPad Prism 8.01 software (GraphPad Software Inc.). P value < .05 was considered as statistically significance.
Results
Characteristics of Adult Ph− ALL Patients
As for demographics, the mean age was 32.2 ± 10.0 years, and 53 (69.7%) adult Ph− ALL patients were <35 years and 23 (30.3%) adult Ph− ALL patients were ≥35 years; there were 33 (43.4%) females and 43 (56.6%) males (Table 1). In terms of immunophenotype, 10 (13.2%) adult Ph− ALL patients had T-ALL immunophenotype and 66 (86.8%) adult Ph− ALL patients had B-ALL immunophenotype. As to CNSL, 5 (6.6%) adult Ph− ALL patients presented with CNSL. Regarding outcome, 52 (68.4%) adult Ph− ALL patients achieved CR within 4 weeks; 65 (85.5%) adult Ph− ALL patients achieved total CR; 27 (35.5%) adult Ph− ALL patients received allo-HSCT. Other information about laboratory indices in adult Ph− ALL patients is displayed in Table 1.
Table 1.
Clinical characteristics of Ph− ALL patients.
| Items | Ph− ALL patients (N = 76) |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 32.2 ± 10.0 |
| <35, No. (%) | 53 (69.7) |
| ≥35, No. (%) | 23 (30.3) |
| Gender, No. (%) | |
| Female | 33 (43.4) |
| Male | 43 (56.6) |
| Immunophenotype, No. (%) | |
| T-ALL | 10 (13.2) |
| B-ALL | 66 (86.8) |
| CNSL, No. (%) | |
| No | 71 (93.4) |
| Yes | 5 (6.6) |
| Laboratory index | |
| WBC ( × 109/L), median (IQR) | 22.0 (11.9−44.8) |
| Increased WBC at diagnosis # , No. (%) | |
| No | 51 (67.1) |
| Yes | 25 (32.9) |
| HGB (g/L), median (IQR) | 90.6 (67.5−108.3) |
| <100, No. (%) | 53 (69.7) |
| ≥100, No. (%) | 23 (30.3) |
| PLT ( × 109/L), median (IQR) | 47.5 (25.8−81.0) |
| <100, No. (%) | 67 (88.2) |
| ≥100, No. (%) | 9 (11.8) |
| Bone marrow blasts (%), median (IQR) | 78.8 (68.1−88.2) |
| <78%, No. (%) | 38 (50.0) |
| ≥78%, No. (%) | 38 (50.0) |
| Outcome | |
| CR within 4 weeks, No. (%) | |
| No | 24 (31.6) |
| Yes | 52 (68.4) |
| Total CR, No. (%) | |
| No | 11 (14.5) |
| Yes | 65 (85.5) |
| Allo-HSCT, No. (%) | |
| No | 49 (64.5) |
| Yes | 27 (35.5) |
# increased WBC was defined as B-ALL patients >30 × 109/L at diagnosis, and T-ALL patients >100 × 109/L at diagnosis. Ph−, Philadelphia chromosome negative; ALL, acute lymphoblastic leukemia; SD, standard deviation; CNSL, central nervous system leukemia; WBC, white blood cell; IQR, interquartile range; HGB, hemoglobin; PLT, platelet; CR, complete remission; Allo-HSCT, allogeneic hematopoietic stem cell transplantation.
The Value of EIF3B for Distinguishing Adult Ph− ALL Patients From HDs
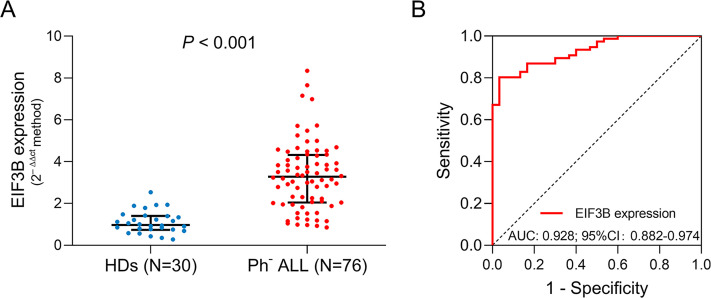
EIF3B expression was increased in adult Ph− ALL patients (median: 3.277 [IQR: 2.048−4.322]) compared with HDs (median: 0.973 [IQR: 0.737−1.403]) (P < .001) (Figure 1A). The following ROC curve analysis manifested that EIF3B expression could distinguish adult Ph− ALL patients from HDs with an AUC of 0.928 (95% confidence interval [CI]: 0.882−0.974) (Figure 1B).
Figure 1.
EIF3B expression discriminated adult Ph− ALL patients from HDs. Comparison of EIF3B between HDs and adult Ph− ALL patients (A). The performance of EIF3B expression in discriminating adult Ph− ALL patients from HDs (B).
Abbreviations: EIF3B, eukaryotic translation initiation factor 3 subunit B; Ph− ALL, Philadelphia chromosome− negative acute lymphoblastic leukemia; HDs, healthy donors.
Association of EIF3B with Adult Ph− ALL Patients’ Characteristics
Based on the baseline EIF3B expression, adult Ph−ALL patients were divided into four quartiles: Quartile 1 (Q1, EIF3B expression ranked in 0%–25% among all patients), Quartile 2 (Q2, EIF3B expression ranked in 25%–50% among all patients), Quartile 3 (Q3, EIF3B expression ranked in 50%−75% among all patients), and Quartile 4 (Q4, EIF3B expression ranked in 75%−100% among all patients). Higher baseline EIF3B expression was associated with increased WBC (P = .002) and bone marrow blasts (P = 0.007), while it was associated with lower CR within 4 weeks (P = .028) and less allo-HSCT achievements (P = .043) (Table 2). In addition, no association of baseline EIF3B expression with age (P = .777), gender (P = .607), immunophenotype (P = .763), CNSL (P = .064), HGB (P = .212), PLT (P = .284) or total CR (P = .060) was observed in adult Ph− ALL patients (Table 2).
Table 2.
Correlation of baseline EIF3B with Ph− ALL patients’ clinical characteristics and outcomes.
| Items | Baseline EIF3B expression* | P value | |||
|---|---|---|---|---|---|
| Q1 (0-25%, n = 19) | Q2 (25-50%, n = 19) | Q3 (50-75%, n = 19) | Q4 (75-100%, n = 19) | ||
| Demographics | |||||
| Age (years), mean ± SD | 32.6 ± 7.4 | 31.3 ± 9.4 | 30.2 ± 8.3 | 34.8 ± 13.9 | .777 |
| Gender, No. (%) | .607 | ||||
| Female | 9 (47.4) | 8 (42.1) | 9 (47.4) | 7 (36.8) | |
| Male | 10 (52.6) | 11 (57.9) | 10 (52.6) | 12 (63.2) | |
| Immunophenotype, No. (%) | .763 | ||||
| T-ALL | 2 (10.5) | 2 (10.5) | 4 (21.1) | 2 (10.5) | |
| B-ALL | 17 (89.5) | 17 (89.5) | 15 (78.9) | 17 (89.5) | |
| CNSL, No. (%) | .064 | ||||
| No | 19 (100.0) | 18 (94.7) | 18 (94.7) | 16 (84.2) | |
| Yes | 0 (0.0) | 1 (5.3) | 1 (5.3) | 3 (15.8) | |
| Laboratory index | |||||
| WBC ( × 109/L), median (IQR) | 14.6 (10.5-24.7) | 13.0 (9.5-29.5) | 26.2 (11.3-56.3) | 38.4 (21.1-62.9) | .002 |
| HGB (g/L), median (IQR) | 92.8 (72.2-108.0) | 94.1 (77.9-126.8) | 89.7 (68.1-98.5) | 67.3 (60.7-108.5) | .212 |
| PLT ( × 109/L), median (IQR) | 63.7 (31.5-82.3) | 49.2 (17.4-89.4) | 48.2 (27.6-81.9) | 33.9 (18.9-70.6) | .284 |
| Bone marrow blasts (%), median (IQR) | 72.8 (61.9-82.3) | 78.2 (59.6-85.4) | 81.6 (69.4-88.6) | 88.2 (71.3-93.4) | .007 |
| Outcome | |||||
| CR within 4 weeks, No. (%) | .028 | ||||
| No | 3 (15.8) | 5 (26.3) | 7 (36.8) | 9 (47.4) | |
| Yes | 16 (84.2) | 14 (73.7) | 12 (63.2) | 10 (52.6) | |
| Total CR, No. (%) | .060 | ||||
| No | 1 (5.3) | 2 (10.5) | 3 (15.8) | 5 (26.3) | |
| Yes | 18 (94.7) | 17 (89.5) | 16 (84.2) | 14 (73.7) | |
| Allo-HSCT, No. (%) | 0.043 | ||||
| No | 9 (47.4) | 12 (63.2) | 13 (68.4) | 15 (78.9) | |
| Yes | 10 (52.6) | 7 (36.8) | 6 (31.6) | 4 (21.1) | |
Correlation was determined by Spearman's rank correlation test or χ2 test for trend. * Q1: quartile 1, Q2: quartile 2, Q3: quartile 3, Q4: quartile 4. EIF3B, eukaryotic translation initiation factor 3 subunit B; Ph−, Philadelphia chromosome negative; ALL, acute lymphoblastic leukemia; SD, standard deviation; CNSL, central nervous system leukemia; WBC, white blood cell; IQR, interquartile range; HGB, hemoglobin; PLT, platelet; CR, complete remission; Allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Association of Baseline EIF3B with Survivals in Adult Ph− ALL Patients
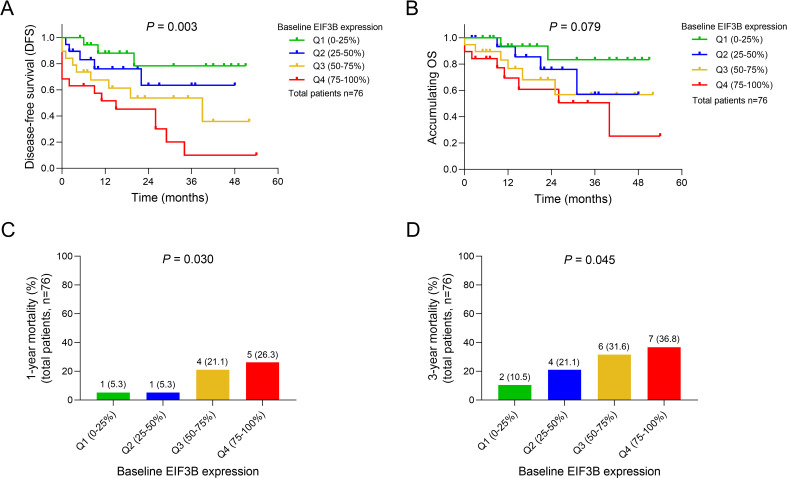
Higher baseline EIF3B expression was associated with shorter DFS (P = .003) (Figure 2A), while there was no association of baseline EIF3B expression with accumulating OS (P = .079) (Figure 2B) in adult Ph− ALL patients. However, higher baseline EIF3B expression was associated with increased 1-year mortality (P = .030) (Figure 2C) and 3-year mortality (P = .045) (Figure 2D) in adult Ph− ALL patients.
Figure 2.
Difference in survival profiles among adult Ph− ALL patients with different EIF3B expression tier. Comparisons of DFS (A), accumulating OS (B), 1-year mortality (C) and 3-year mortality (D) among adult Ph− ALL patients with different baseline EIF3B expression tier.
Abbreviations: Ph− ALL, Philadelphia chromosome− negative acute lymphoblastic leukemia; DFS, disease-free survival; OS, overall survival; EIF3B, eukaryotic translation initiation factor 3 subunit B.
Independent Factors of DFS and OS in Adult Ph− ALL Patients
Forward stepwise Cox's proportional hazard regression analyses revealed that higher baseline EIF3B (P = .012; hazard ratio (HR) [95% confidence interval, 95%CI] = 1.571 [1.104−2.236]), increased WBC at diagnosis (P = .008; HR [95%CI] = 2.966 [1.321−6.659]) were independent factors for decreased DFS, while CR within 4 weeks (P < .001; HR [95%CI] = 0.095 [0.037−0.248]) and allo-HSCT (P = .043; HR [95%CI] = 0.259 [0.070−0.957]) were independent factors for prolonged DFS. Furthermore, higher baseline EIF3B (P = .009; HR [95%CI] = 1.890 [1.170−3.052]), increased WBC at diagnosis (P = .009; HR [95%CI] = 3.644 [1.387−9.576]) were independent factors for shorter OS, while CR within 4 weeks (P < .001; HR [95%CI] = 0.087 [0.030−0.254]) was an independent factor for longer OS (Table 3).
Table 3.
Analysis of factors independently related to DFS and OS.
| Items | Forward stepwise Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| DFS | ||||
| Higher baseline EIF3Ba | 0.012 | 1.571 | 1.104 | 2.236 |
| Increased WBC at diagnosisb | 0.008 | 2.966 | 1.321 | 6.659 |
| CR within 4 weeks | <0.001 | 0.095 | 0.037 | 0.248 |
| Allo-HSCT | 0.043 | 0.259 | 0.070 | 0.957 |
| OS | ||||
| Higher baseline EIF3Ba | 0.009 | 1.890 | 1.170 | 3.052 |
| Increased WBC at diagnosisb | 0.009 | 3.644 | 1.387 | 9.576 |
| CR within 4 weeks | <0.001 | 0.087 | 0.030 | 0.254 |
* Baseline EIF3B expression in Ph− ALL patients was categorized as quartile 1 (Q1, 0-25%) = 1, quartile 2 (Q2, 25-50%) = 2, quartile 3 (Q3, 50-75%) = 3 and quartile 4 (Q4, 75-100%) = 4.
# increased WBC was defined as B-ALL patients >30 × 109/L at diagnosis, and T-ALL patients >100 × 109/L at diagnosis. DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI: confidence interval; EIF3B, eukaryotic translation initiation factor 3 subunit B; CR, complete remission; Allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Change of EIF3B Expression After 4-Week Therapy in Adult Ph− ALL Patients
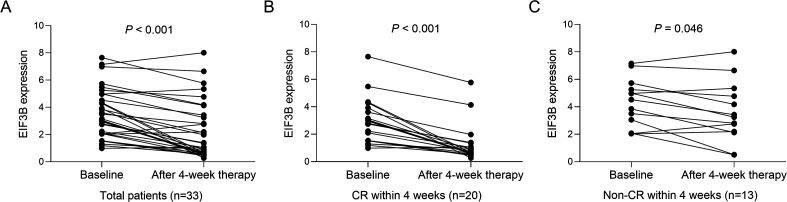
In all adult Ph− ALL patients (patients who achieved CR within 4 weeks and patients who did not achieve CR within 4 weeks), EIF3B expression was attenuated after 4-week therapy compared with that at baseline (P < .001) (Figure 3A). In patients who achieved CR within 4 weeks, EIF3B expression was dramatically attenuated after 4-week therapy compared with that at baseline (P < .001) (Figure 3B). For patients who did not achieve CR within 4 weeks, EIF3B expression was only slightly reduced after 4-week therapy compared with that at baseline (P = .046) (Figure 3C).
Figure 3.
EIF3B expression at baseline and after 4-week therapy in adult Ph− ALL patients. Comparison of EIF3B expression between baseline and after 4-week therapy in total adult Ph− ALL patients (A). Comparison of EIF3B expression between baseline and after 4-week therapy in adult Ph− ALL patients who received CR within 4 weeks (B). Comparison of EIF3B expression between baseline and after 4-week therapy in adult Ph− ALL patients who did not receive CR within 4 weeks (C).
Abbreviations: EIF3B, eukaryotic translation initiation factor 3 subunit B; Ph− ALL, Philadelphia chromosome− negative acute lymphoblastic leukemia; CR, complete remission.
Discussion
Preceding studies have elucidated that EIF3B is overexpressed in multiple solider cancers such as non-small cell lung cancer and gastric cancer, and EIF3B overexpression is closely linked to exacerbated clinical features and worse prognosis.8–13 In addition to the role of EIF3B in hematological malignancy, EIF3B is functionally linked to the pathogenesis of acute myeloid leukemia and chronic myeloid leukemia.14–16 In acute myeloid leukemia, knockdown of EIF3B inhibits cell proliferation and migration while it enhances apoptosis by downregulating the WNT signaling. 14 In chronic myeloid leukemia, EIF3B inhibition suppresses cell proliferation but facilitates cell apoptosis by arresting cell cycle progression and regulating the expression of Rap guanine nucleotide exchange factor 1 (C3G/RAPGEF1). 15 On the basis of aforementioned mechanisms, it is likely that EIF3B might present with clinical implication in the development and progression of hematological malignancy, including Ph− ALL. However, no evidence about the clinical relevance of EIF3B in Ph− ALL patients has been published yet.
In this current study, we detected baseline EIF3B expression in adult Ph− ALL patients and HDs. We discovered that baseline EIF3 expression was increased in adult Ph− ALL patients compared with HDs, and it could distinguish adult Ph− ALL patients from HDs. This finding could be explained by that: EIF3B might promote cell proliferation and repress cell apoptosis via downregulating the apoptosis inhibitory protein such as B cell leukemia/lymphoma 2 (BCL2) expression and activating the Akt pathway, which contributed to the malignant proliferation of immature lymphoid progenitor cells and the development of Ph− ALL; therefore, EIF3B could distinguish adult Ph− ALL patients from HDs.11,12,18 Furthermore, we analyzed the association of EIF3B with clinical characteristics before therapy in adult Ph− ALL patients. We observed that higher EIF3B expression was associated with increased WBC level and bone marrow blasts. The following are possible explanations: (i) EIF3B might enhance the proliferation and migration and inhibit the apoptosis of immature WBC by interfering with cell cycle progression in the bone marrow, which resulted in the overproduction of abnormal WBCs and bone marrow blasts, therefore, higher EIF3B expression was associated with elevated WBC level and bone marrow blasts in adult Ph− ALL patients.7,14 (ii) EIF3B might maintain malignant cell survival via promoting cell proliferation enhancement factor such as C3G (inhibited proapoptotic pathway such as the p38 mitogen activated kinase-like protein [MAPK] pathway and the extracellular regulated MAP kinase/proteinkinase B [ERK/AKT] cascade), which led to exacerbated clinical features in adult Ph− ALL patients. 15 However, the inferential explanations needed further validation.
In addition, the prognostic value of EIF3B in adult Ph− ALL patients has not been studied yet. In this current study, we observed that higher EIF3B expression (before therapy) was associated with lower CR within 4 weeks, allo-HSCT, shorter DFS, while it was not associated with OS in adult Ph− ALL patients. However, higher EIF3B expression (before therapy) was associated with increased 1-year mortality and 3-year mortality in adult Ph− ALL patients. Subsequent forward stepwise Cox’s proportional hazard regression analysis manifested that higher EIF3B expression (before therapy) could independently predict shorter DFS and OS in adult Ph− ALL patients. These could be explained by that: (i) EIF3B probably induced drug resistance and subsequent poor treatment outcomes, which in turn resulted in worse prognosis in adult Ph− ALL patients. 11 (ii) Ph− ALL patients were eligible for post-consolidation therapy allo-HSCT once they achieved CR; furthermore, based on our finding, adult Ph− ALL patients with higher EIF3B expression had lower CR within 4 weeks; thereby, adult Ph− ALL patients with higher EIF3B expression presented less cases received allo-HSCT. 19 (iii) EIF3B might attenuate the apoptosis and facilitate the proliferation and migration of malignant cells by interfering with the cell cycle, which enhanced the aggressiveness of malignant cells.14–16 As observed in this current study, higher EIF3B expression was associated with increased WBC level and bone marrow blasts, which accelerated the disease progression and aggravated the malignancy of disease, thereby, leading to worse prognosis in adult Ph− ALL patients. (iv) EIF3B expression was not associated with OS in Ph− ALL patients, which might be explained by that few adult Ph− ALL patients died during the follow-up period that greatly reduced the statistic power. Other than EIF3B there have been a number of genes that are prognostic markers for ALL, such as BCR/ABL1. 20 Compared with those, EIF3B was also proven to be an independent predictive factor, which was also of clinical significance.
Besides, in this current study, the EIF3B expression after 4-week therapy was detected in adult Ph− ALL patients, which found that EIF3B expression (after 4-week therapy) was reduced in adult Ph− ALL patients. Of note, the reduction of EIF3B expression was more obvious in adult Ph− ALL patients who achieved CR within 4 weeks compared with adult Ph− ALL patients who did not achieve CR within 4 weeks. The possible reasons were that: (i) With 4-week therapy, the aggressiveness and number of malignant cells were probably reduced; meanwhile, EIF3B expression indicated the aggressiveness of malignant cells; therefore, EIF3B expression after 4-week therapy was reduced compared to pre-treatment EIF3B expression in adult Ph− ALL patients. (ii) As EIF3B expression reflected the aggressiveness of malignant cells, the rapid reduction of EIF3B expression indicated the attenuated aggressiveness of malignant cells and reduced number of malignant cells; therefore, adult Ph− ALL patients with more reduction of EIF3B expression had a higher probability of achieving CR.
Nevertheless, several shortcomings existed in this current study. First, as it was difficult to recruit HDs (very limited healthy volunteers for bone marrow donation), the sample size of HDs was relatively small, which might reduce the statistic power. Second, EIF3B expression did not correlate with OS in adult Ph− ALL patients, which might be explained by that few adult Ph− ALL patients died during the follow-up duration that greatly reduced the statistic power; thereby, further studies with extended follow-up duration would be desirable for exploring the impact of EIF3B on OS more objectively. Third, only EIF3B expression before therapy and after 4-week induction therapy of Ph− ALL patients were collected, while EIF3B expression after a certain time point of consolidation treatment was not assessed; thus, further explorations should be warranted. Fourth, included patients were all primary adult Ph− ALL patients, while relapsed/refractory adult Ph− ALL patients were not included; thus, further studies with both primary and relapsed/refractory adult Ph− ALL patients were needed for investigating the longitudinal change of EIF3B expression during treatment. Lastly, the experiment about the detailed mechanism of EIF3B in the pathogenies of adult Ph− ALL was not performed; thereby, further experiments were needed.
To conclude, EIF3B serves a possible biomarker for reflecting exacerbated clinical features, poor treatment response, and worse survivals, which might help with disease management, treatment optimization, and prognosis improvement in adult Ph− ALL patients.
Supplemental Material
Supplemental material, sj-tif-1-tct-10.1177_15330338211041464 for EIF3B Associates with Exacerbated Clinical Features, Poor Treatment Response and Survival in Adult Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia Patients by Feiyue Zhu, Yesong Fu and Xiaojuan He in Technology in Cancer Research & Treatment
Supplemental material, sj-xlsx-3-tct-10.1177_15330338211041464 for EIF3B Associates with Exacerbated Clinical Features, Poor Treatment Response and Survival in Adult Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia Patients by Feiyue Zhu, Yesong Fu and Xiaojuan He in Technology in Cancer Research & Treatment
Acknowledgement
We thank the MedSci company (www.medsci.cn/) for statistics, language editing, and formatting of our manuscript.
Glossary
Abbreviations
- EIF3B
eukaryotic translation initiation factor 3 subunit B
- Ph-
Philadelphia chromosome negative
- ALL
acute lymphoblastic leukemia
- HDs
healthy donors
- BM
bone marrow
- AUC
area under the curve
- CR
complete remission
- EIF3
eukaryotic translation initiation factor 3
- MICM
morphology, immunology, cytogenetics and molecular biology
- T-ALL
T-cell acute lymphoblastic leukemia
- B-ALL
B-cell acute lymphoblastic leukemia
- CNSL
central nervous system leukemia
- WBC
white blood cell
- HGB
hemoglobin
- PLT
platelet
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- CALLG2008
Chinese Acute Lymphoblastic Leukemia Cooperative Group 2008
- VDCLP
Vincristine, Daunorubicin, Cyclophosphamide, L-asparaginase, Prednisone
- HSCT
hematopoietic stem cell transplantation
- HLA
human leukocyte antigen
- DFS
disease free survival
- OS
overall survival
- IQR
interquartile range
- SD
standard deviation
- ROC
receiver-operating characteristic
- C3G/RAPGEF1
Rapguanine nucleotide exchange factor 1
- BCL2
B cell leukemia/lymphoma 2
- MAPK
mitogen activated kinase-like protein
- ERK/AKT
extracellular regulated MAP kinase/protein kinase B
Footnotes
Ethical Statement: This study was approved by Ethics Committee of Loudi Central Hospital, 2019-ethics (scientific research)-025. The adult Ph− ALL patients, HDs, or their families provided the written informed consents.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Feiyue Zhu https://orcid.org/0000-0003-0773-9329
Supplemental material: Supplemental material for this article is available online.
References
- 1.Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395(10230):1146-1162. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu H, Doki N, Kanamori H, et al. Prognostic impact of cytogenetic abnormalities in adult patients with Philadelphia chromosome-negative ALL who underwent an allogeneic transplant. Bone Marrow Transplant. 2019;54(12):2020-2026. [DOI] [PubMed] [Google Scholar]
- 3.Carroll WL, Bhojwani D, Min DJ, et al. Pediatric acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2003;2003(1):102-131. [DOI] [PubMed] [Google Scholar]
- 4.Frey NV, Luger SM. How I treat adults with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood. 2015;126(5):589-596. [DOI] [PubMed] [Google Scholar]
- 5.Richard-Carpentier G, Kantarjian H, Jabbour E. Recent advances in adult acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2019;14(2):106-118. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Shi P, Deng M, et al. Low dose triptolide reverses chemoresistance in adult acute lymphoblastic leukemia cells via reactive oxygen species generation and DNA damage response disruption. Oncotarget. 2016;7(51):85515-85528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng X, Li J, Liu P. The biological roles of translation initiation factor 3b. Int J Biol Sci. 2018;14(12):1630-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang Y, Zhang X, Yan L, et al. Eukaryotic translation initiation factor 3b is both a promising prognostic biomarker and a potential therapeutic target for patients with clear cell renal cell carcinoma. J Cancer. 2017;8(15):3049-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu F, Xu CZ, Gu J, et al. Eukaryotic translation initiation factor 3B accelerates the progression of esophageal squamous cell carcinoma by activating beta-catenin signaling pathway. Oncotarget. 2016;7(28):43401-43411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue Q, Meng L, Jia B, Han W. Expression of eukaryotic translation initiation factor 3 subunit B in liver cancer and its prognostic significance. Exp Ther Med. 2020;20(1):436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Wen X, Luan F, et al. EIF3B Is associated with poor outcomes in gastric cancer patients and promotes cancer progression via the PI3 K/AKT/mTOR signaling pathway. Cancer Manag Res. 2019;2019(11):7877-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian Y, Zhao K, Yuan L, et al. EIF3B Correlates with advanced disease stages and poor prognosis, and it promotes proliferation and inhibits apoptosis in non-small cell lung cancer. Cancer Biomark. 2018;23(2):291-300. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Ru Y, Sanchez-Carbayo M, Wang X, Kieft JS, Theodorescu D. Translation initiation factor eIF3b expression in human cancer and its role in tumor growth and lung colonization. Clin Cancer Res. 2013;19(11):2850-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Wu L. Knockdown of eukaryotic translation initiation factor 3 subunit B inhibits cell proliferation and migration and promotes apoptosis by downregulating WNT signaling pathway in acute myeloid leukemia. Int J Clin Exp Pathol. 2020;13(1):99-106. [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Wei Z, Chang X, et al. eIF3b regulates the cell proliferation and apoptosis processes in chronic myelogenous leukemia cell lines via regulating the expression of C3G. Biotechnol Lett. 2020;42(7):1275-1286. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, He K, Wang J, et al. Inhibition of eukaryotic initiation factor 3B suppresses proliferation and promotes apoptosis of chronic myeloid leukemia cells. Adv Clin Exp Med. 2019;28(12):1639-1645. [DOI] [PubMed] [Google Scholar]
- 17.Chinese Society of Hematology CMA, Society of Hematological Malignancies Chinese Anti-Cancer A. [A Chinese expert panel consensus on diagnosis and treatment of adult acute lymphoblastic leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2012;33(9):789-792. [DOI] [PubMed] [Google Scholar]
- 18.Xu F, Zhang S, Liu Z, et al. TEX9 And eIF3b functionally synergize to promote the progression of esophageal squamous cell carcinoma. BMC Cancer. 2019;19(1):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giebel S, Marks DI, Boissel N, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the acute leukemia working party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019;54(6):798-809. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Cho B. Prognostic factors and treatment of pediatric acute lymphoblastic leukemia. Korean J Pediatr. 2017;60(5):129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tct-10.1177_15330338211041464 for EIF3B Associates with Exacerbated Clinical Features, Poor Treatment Response and Survival in Adult Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia Patients by Feiyue Zhu, Yesong Fu and Xiaojuan He in Technology in Cancer Research & Treatment
Supplemental material, sj-xlsx-3-tct-10.1177_15330338211041464 for EIF3B Associates with Exacerbated Clinical Features, Poor Treatment Response and Survival in Adult Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia Patients by Feiyue Zhu, Yesong Fu and Xiaojuan He in Technology in Cancer Research & Treatment