Abstract
Background:
In the current literature, deposits in calcific tendinitis are described as amorphous masses of hydroxyapatite with a size in the range of 5 to 20 μm. Theoretically, these are too big to be phagocytized by macrophages and induce an inflammatory reaction.
Purpose:
To better characterize the deposits seen in calcific tendinitis.
Study Design:
Case series; Level of evidence, 4.
Methods:
Included in the study were 6 patients with a history of at least 1 year of shoulder pain (range, 1-14 years). Shoulder arthroscopy was performed under general anesthesia, and calcium deposits from the supraspinatus tendon and biopsies from the adjacent subacromial bursa were taken. Samples were analyzed by light microscopy and immunostained for macrophages. Scanning electron microscopy and energy-dispersive x-ray (EDX) analysis were used to assess the morphology and chemical composition of the calcific deposits.
Results:
Light microscopy showed round and bulky calcium deposits partially surrounded by activated CD68-positive macrophages within inflammatory tissue. Some hemosiderin positive mononuclear cells, indicative for (micro-) hemorrhage, were seen. Scanning electron microscopy revealed that the large calcific deposits (1-20 μm) were composed of rod-like structures. These highly crystalline rods had a size of approximately 100 nm in length and 20 nm in width. Chemical composition by EDX analysis showed that crystals were composed of mainly calcium, oxygen, and phosphorus, equaling the chemical composition of hydroxyapatite.
Conclusion:
Deposits in calcific tendinitis of the rotator cuff are not amorphous but composed of highly crystalline structures. Fragmentation of these aggregates and subsequent release of the needle-like nanocrystals might initiate the strong inflammatory reaction often seen in patients with calcifying tendinitis of the rotator cuff.
Keywords: calcific tendinitis, calcifying tendinosis, crystalline, rotator cuff, shoulder
Calcific deposits are found in 3% of asymptomatic shoulders 14 and in 10% to 42% of painful shoulders. 4,5,9,12 It is believed that the disease starts with a fibrocartilaginous metaplasia of the tenocytes and that the process evolves in 3 stages: the precalcific, the calcific, and the postcalcific. 19 At the end of the calcific stage, a resorptive phase is observed. To date, it is not known why, after years of quiescence, resorption of the calcific deposits in the rotator cuff takes place and, in particular, why this process is accompanied by a fulminant and often painful inflammation. 14,16 Microtrauma 2 and change in the bonding capacity of the organic molecules 8 have been suggested as trigging factors for resorption. The process of inflammation in the tendon tissue is heralded by the appearance of thin-walled vascular channels at the periphery of the calcific deposits. 18 Macrophages and multinucleated cells around calcium deposits are detected. These cells have phagocytic capacities and might be responsible for the initiation of the resorption. Macrophages located within mineralized regions of tissue have been seen to contain mineral. 1,18
Until recently, the calcific deposits have been described as amorphous and round-shaped with a size in the range of 5 to 20 μm, which is theoretically too large to be absorbed by histiocytes. 3,6,8,17,18 The mechanism by which resorption of these bulky round masses proceeds has not yet been explained.
In this study, we investigated the chemical composition and nano- and microstructure of the calcific deposits at the symptomatic stage of calcific tendinitis.
Methods
The study protocol was approved by the responsible ethical committee, and all included patients provided written informed consent. Included were 6 patients (5 women, 1 man; mean age, 49 years; range, 44-54 years) with symptomatic calcific tendinitis of the supraspinatus tendon from November 2019 to November 2020. Before surgery, patients had undergone at least 1 year of nonoperative treatment (range, 1-14 years), including physiotherapy and a period of nonsteroidal anti-inflammatory drug intake. None had undergone shock-wave therapy. Of the 6 patients, 5 had corticosteroid infiltrations on average 4.2 months (range, 2-8 months) before surgery.
All patients underwent surgery under general anesthetic. After diagnostic arthroscopy of the glenohumeral joint, a limited subacromial bursectomy was performed, and the calcium deposits were localized with a spinal needle. The calcific deposits were taken with a closed rongeur from the bulky white masses, while arthroscopic flow was stopped for a few seconds. The overlying bursal tissue, together with the tendon adjacent to the calcific deposits, were harvested with a small arthroscopic rongeur from the lateral arthroscopic portal for histological analyses.
Histopathology
Tissue samples were routinely fixed in 4% buffered formalin, and the tissue was completely embedded in paraffin. Tissue sections were routinely stained with hematoxylin-eosin and Von Kossa stain. In addition, immunohistochemistry for CD68 was performed using standard procedure. CD68 is particularly useful as an immunohistochemical marker of the macrophage lineage, including tissue histiocytes. It allows for the documentation of the interaction between calcifications and tissue macrophages.
Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectrometry Analysis
Samples were gently spread with a spatula on a 7 × 7–mm silicon wafer and were air-dried without ethanol or acetone. Unlike procedures in other studies, we did not treat the samples with any metals and imaged them directly. The wafer was attached to an aluminum stub with carbon tape and brought into a scanning electron microscope (Gemini 450; Zeiss) for imaging without coating. Low-voltage scanning electron microscopy (<1 KeV) was used to identify the hydroxyapatite crystals. Specifically, an acceleration voltage of 0.8 KeV and a beam current of 200 pA were applied. Images were acquired with a secondary electron (Everhart-Thornley type SE) detector. The samples were imaged at a working distance between 4 and 5 mm.
For energy-dispersive x-ray spectroscopy (EDX) analysis, an x-ray detector (X-MAX80, AZTec Advanced; Oxford Instruments) was used. The acceleration voltage for EDX was 10 keV. Briefly, with the EDX analysis, electrons interact with the sample upon electron irradiation and produce x-rays with element-characteristic energies, which can be measured with a dedicated detector. Based on the energy spectrum of a desired region of interest of the specimen, the elemental, stoichiometric composition can be deduced. 7
Results
Histopathology
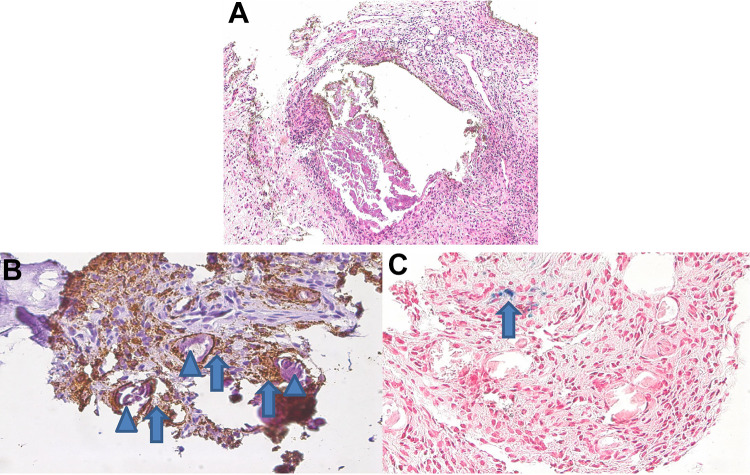
On histopathological examination, deposits of large, bulky, pleomorphic, and fragmented nonbirefringent calcifications were demarked by fibroblastic proliferations with scarring fibrosis, lymphocytes, scattered granulocytes, and macrophages (Figure 1A). Most of the calcified fragments (85%) were surrounded by CD68-positive macrophages (Figure 1, B and C), independent of the fragment size or shape. Remarkably, adjacent to the areas with inflammation, there were focal aggregates of hemosiderin-laden macrophages, indicative for recurrent hemorrhage.
Figure 1.
(A) Bulky calcifications demarked by scarring fibrosis and marked inflammation (hematoxylin and eosin, 200×). (B) Calcified fragments (arrowheads) were surrounded by CD68-positive macrophages (arrows); CD68 immunostaining (brown), 400×. (C) Adjacent to the areas with inflammation, there were focal aggregates of hemosiderin (blue)-laden macrophages (arrow).
Electron Microscopy
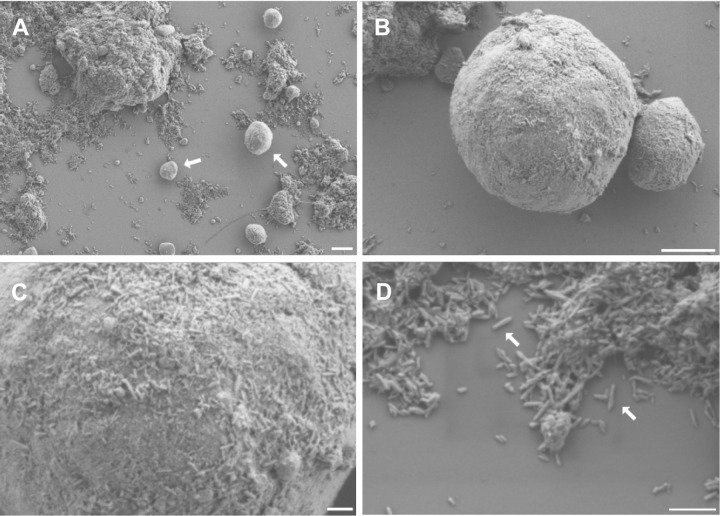
Scanning electron microscopy images showed round (size, 1-10 µm) and bulky aggregates (Figure 2). These bulky structures, as well as the round elements, appeared to be composed of tiny, rod-like structures. All fragments of the bulky aggregates were covered by these rods. The rods had a size (mean ± SD) of approximately 96.9 ± 26.1 nm in length (4 samples; 375 measurements) and 21.4 ± 4.5 nm in width (4 samples, 258 measurements). The EDX analysis demonstrated that these rods were composed of mainly calcium, oxygen, and phosphorus, resembling the chemical composition of hydroxyapatite.
Figure 2.
(A, B) The samples appear as round (size, 1-10 µm; arrows in A) and bulky aggregates. (C, D) The surface of the round elements, as well as the bulky aggregates, are composed of rod-like structures of approximately 100 nm in length and 20 nm in width (arrows in D). They are clumped in an irregular manner. Scale bars: A = 2 µm, B = 1 µm, C = 200 nm, D = 300 nm.
In addition, some samples contained almost rectangular crystals (not shown) that resembled the birefringent crystals seen by light microscopy. However, chemical composition by EDX analysis demonstrated that these crystals consisted mainly of sodium and chloride, indicating that they originate from the rinsing solution during arthroscopy. Similar observations of such crystals have been described in other publications. 6
Discussion
To our knowledge, we demonstrated for the first time that deposits in calcific tendinitis are composed of highly crystalline rod-like structures of approximately 100 nm length and 20 nm width. This is in contrast to the current literature, where these deposits have been described as amorphous masses with a particle size in the range of 5 to 20 μm. 3,6,8,17 –19 An explanation for our diverse depiction of these “amorphous” masses may be that we used low-voltage scanning electron microscopy and analyzed uncoated material. In previous studies, electron microscopy was performed on processed, dehydrated, and embedded tissues. In addition, they were stained either with uranyl acetate and lead citrate 1,19 or with gold. 6 In other electron microscopy studies, resolution was too low to detect structures on a nanometer scale. 8 The finding that calcific deposits are composed of very small (in a nanoscale range) and sharp crystals is of clinical relevance. The proinflammatory effects of hydroxyapatite have been well described in the literature. 10,13 In addition, it is known that the size and shape of the crystals largely affect the scale of inflammatory reaction to these particles. 11,15 For instance, Lebre et al 11 demonstrated that hydroxyapatite particles of 100 nm in size with a “needle-like” shape evoked the strongest inflammatory response as opposed to larger and smooth spherical particles. Taking these findings together, it is understandable why resorption of the fragmented calcium deposits is usually accompanied by a fulminant inflammatory reaction. Conversely, it may also explain why the large rounded aggregates do not evoke any reaction as long as the bulky masses do not disintegrate.
Calcium phosphate crystals form in body solutions with a sufficiently high supersaturation and a specific pH. 8 Various additives such as proteins, electrolytes, and metals affect stability of the crystals through ionic bonding. We can assume that the same forces hold the needle-like crystals together. Disintegration might therefore occur through a change of the pH or the chemical composition in the fluid around the aggregates. Another explanation may be a mechanical fragmentation by microtrauma.
On light microscopy, we have demonstrated that CD68-activated macrophages are in close vicinity to fragments of the calcific deposits. Furthermore, we observed hemosiderin-laden macrophages around the clusters of the inflammatory cells, which are indicative for a recent hemorrhage. These hemosiderin-laden macrophages may originate from prior injections or neovascularization, but they may also be caused by microtrauma, as some of the patients recall a minor distortion or vigorous movement with the shoulder before the symptomatic episode started.
The study has some limitations. First, the number of patients was low. However, as the nanostructure of the calcific deposits was identical in all samples and calcific deposits, we doubt that a larger number of patients would have led to a different conclusion. Second, the preoperative symptomatic phase of the patients varied by a wide range (1-14 years). However, patients’ recall of pain is sometimes vague and symptoms are often not continuous. In addition, the asymptomatic phase may have taken years, with some reaction in the surrounding tissue. Therefore, we assumed that all patients had a chronic disease with an acute inflammatory deterioration. Third, it can only be speculated whether the entire aggregate is composed of the needle-like-crystals. Further characterizations would involve decomposition of aggregates and identification of the components, but appropriate methods would need to be identified, evaluated, and validated and are currently out of the scope of this study. However, as the aggregates and all fragments thereof were covered by the crystals, it seems conceivable that the entire mass consists of these nanostructures seen on the surface.
Conclusion
In the present study, we demonstrated that the amorphous masses of calcific deposits are aggregates composed of rod-like crystals at a nanometer scale. Particles of this size and shape were able to induce an abundant inflammatory reaction.
Acknowledgment
The authors thank Hans-Rudi Schmid, Head of the Laboratory Department, Kantonsspital Baden, for his support.
Footnotes
Final revision submitted May 28, 2021; accepted June 9, 2021.
The authors have declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the regional ethics committee (Ethikkommission Nordwest- und Zentralschweiz; ref No. EKNZ-2018-01266).
References
- 1. Archer RS, Bayley JI, Archer CW, Ali SY. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J Anat. 1993;182:1–11. [PMC free article] [PubMed] [Google Scholar]
- 2. Bishop W. Calcification of the supraspinatus tendon: cause, pathologic picture and relation to the scalenus anticus syndrome. Arch Surg. 1939;39:231–246. [Google Scholar]
- 3. Darrieutort-Laffite C, Arnolfo P, Garraud T, et al. Rotator cuff tenocytes differentiate into hypertrophic chondrocyte-like cells to produce calcium deposits in an alkaline phosphatase-dependent manner. J Clin Med. 2019;8(10):1544–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farin PU, Jaroma H. Sonographic findings of rotator cuff calcifications. J Ultrasound Med. 1995;14(1):7–14. [DOI] [PubMed] [Google Scholar]
- 5. Friedman MS. Calcified tendinitis of the shoulder. Am J Surg. 1957;94(1):56–61. [DOI] [PubMed] [Google Scholar]
- 6. Gartner J, Simons B. Analysis of calcific deposits in calcifying tendinitis. Clin Orthop Relat Res. 1990;(254):111–120. [PubMed] [Google Scholar]
- 7. Goldstein J, Newbury D, Michael J, Ritchie N, Scott J. Scanning Electron Microscopy and X-ray Microanalysis. 3rd ed. Springer; 2003. [Google Scholar]
- 8. Grases F, Muntaner-Gimbernat L, Vilchez-Mira M, et al. Characterization of deposits in patients with calcific tendinopathy of the supraspinatus. Role of phytate and osteopontin. J Orthop Res. 2015;33(4):475–482. [DOI] [PubMed] [Google Scholar]
- 9. Harmon PH. Methods and results in the treatment of 2,580 painful shoulders, with special reference to calcific tendinitis and the frozen shoulder. Am J Surg. 1958;95(4):527–544. [DOI] [PubMed] [Google Scholar]
- 10. Jin C, Frayssinet P, Pelker R, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci USA. 2011;108(36):14867–14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebre F, Sridharan R, Sawkins MJ, Kelly DJ, O’Brien FJ, Lavelle EC. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci Rep. 2017;7(1):2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louwerens JK, Sierevelt IN, van Hove RP, van den Bekerom MP, van Noort A. Prevalence of calcific deposits within the rotator cuff tendons in adults with and without subacromial pain syndrome: clinical and radiologic analysis of 1219 patients. J Shoulder Elbow Surg. 2015;24(10):1588–1593. [DOI] [PubMed] [Google Scholar]
- 13. Maurer KH, Schumacher HR. Hydroxyapatite phagocytosis by human polymorphonuclear leucocytes. Ann Rheum Dis. 1979;38(1):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLaughlin HL. Lesions of the musculotendinous cuff of the shoulder; observations on the pathology, course and treatment of calcific deposits. Ann Surg. 1946;124:354–362. [PubMed] [Google Scholar]
- 15. Mestres G, Espanol M, Xia W, Persson C, Ginebra MP, Ott MK. Inflammatory response to nano- and microstructured hydroxyapatite. PLoS One. 2015;10(3):e0120381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinals RS, Short CL. Calcific periarthritis involving multiple sites. Arth Rheum. 1966;9(4):566–574. [Google Scholar]
- 17. Riley GP, Harrall RL, Constant CR, Cawston TE, Hazleman BL. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis. 1996;55(2):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5(4):183–191. [DOI] [PubMed] [Google Scholar]
- 19. Uhthoff HK, Sarkar K, Maynard JA. Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res. 1976;118:164–168. [PubMed] [Google Scholar]