Abstract
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor that represents <5% of all thyroid malignancies and is generally more aggressive than differentiated thyroid cancer. The aim of this study is to provide an update, through review of clinical studies of patients with MTC published between January 1, 2016, and June 1, 2021, on recent advances in the diagnosis and treatment of MTC. This review focuses on updates in biochemical testing, imaging, hereditary disease, surgical management, adjuvant therapies, and prognosis. Recent advances reviewed herein have sought to diagnose MTC at earlier stages of disease, predict when patients with a hereditary syndrome may develop MTC, use functional imaging to assess for distant metastases, perform optimal initial surgery with appropriate lymphadenectomy, employ targeted systemic therapies for patients with progressive metastatic disease, and better predict patient-specific outcomes.
Keywords: medullary thyroid cancer, hereditary, thyroidectomy, calcitonin, neuroendocrine
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor that arises from the calcitonin-producing parafollicular C-cells and represents <5% of all thyroid malignancies. 1 While rare, the age-adjusted incidence of MTC has increased significantly over the last 30 years from 0.14 to 0.21 per 100,000 people (p < 0.001). The greatest increase in MTC incidence has been observed in patients with localized disease. 2 Approximately 25% of MTC is associated with a hereditary syndrome.
Multiple guidelines, including from the American Thyroid Association (ATA) and the European Society for Medical Oncology, provide extensive reviews of the best available evidence on MTC management recommendations.3,4 The aim of this study is to provide an update on recent advances in the diagnosis and treatment of MTC with a focus on biochemical markers, imaging, hereditary disease, surgical management, adjuvant therapies, and prognosis.
Methods
A PubMed search was conducted to evaluate for clinical studies of patients with MTC published between January 1, 2016, and June 1, 2021, using the following terms, “carcinoma, medullary,” “thyroid cancer,” “medullary thyroid carcinoma,” or “medullary thyroid cancer.” Systematic reviews, clinical practice guidelines, and clinical studies were included. Studies were required to be published in English. The articles were screened and categorized by two authors (MBK and LMW). The articles were then critically reviewed by three authors (MBK, LMW, and OEO). None of the authors have any conflicts of interest to report. There were no specific funding sources supporting this work.
Results
Diagnostic laboratory studies
Screening serum calcitonin in nodular thyroid disease
Calcitonin is a sensitive tumor marker for MTC and correlates with C-cell mass and burden of disease. Preoperative diagnosis of MTC, which may not be evident on preoperative fine needle aspiration (FNA) biopsy, is essential to guiding appropriate initial surgical management. In one tertiary care center, one-third of patients who had MTC diagnosed on final pathology in an index nodule did not have MTC diagnosed on preoperative FNA of that index nodule. 5 Therefore, additional methods to identify MTC preoperatively in patients with nodular thyroid disease have been proposed, including calcitonin screening. In a series where more than 10,000 patients with nodular thyroid disease underwent calcitonin screening, 0.4% had an elevated basal calcitonin level and confirmed with a pentagastrin-stimulated calcitonin level. Preoperative FNA was suspicious for malignancy in only two-thirds of the patients, and all had MTC on final pathology. Thus, calcitonin was more specific and sensitive than FNA for diagnosing MTC. When compared to a historical cohort, MTC patients identified through calcitonin screening were diagnosed at an earlier stage, more likely to achieve complete remission (p < 0.0001) and had prolonged survival (p = 0.0005). 6 More recently, a 2020 Cochrane review assessed screening calcitonin levels in more than 72,000 patients with thyroid nodules. With a median prevalence of MTC of 0.32%, calcitonin detected MTC with a sensitivity of 83–100% and specificity 94–100%. Using a threshold of 10 pg/mL for serum calcitonin increased the sensitivity to 100% and specificity to 97.2%. 7 ATA guidelines recommend physician choice on calcitonin screening in patients with nodular thyroid disease. 3
Stimulated calcitonin
Measurement of calcitonin after pharmacologic stimulation has been used to differentiate whether calcitonin elevation is derived from thyroid C-cells versus from a non-thyroid origin, to identify patients with normal basal calcitonin and MTC, and to determine timing of thyroidectomy in patients with rearranged during transfection (RET) mutations detected on genetic testing.8,9 Pentagastrin stimulation, now no longer available worldwide, has largely been replaced with calcium stimulation with equivalent results. 10 Multiple studies have attempted to set parameters for detecting MTC using basal calcitonin, stimulated calcitonin, or combined measurements.11,12 In a group of consecutive patients with thyroid nodules and elevated basal calcitonin, Niederle et al. found that basal calcitonin ⩾100 pg/mL for males and ⩾23 pg/mL for females had a 100% sensitivity for diagnosing lateral neck lymph node metastasis. Diagnostic cutoffs for detecting any MTC was identified with basal calcitonin >43 pg/mL in men (sensitivity 53% and specificity 100%) and >23 pg/mL in women (sensitivity 81%, specificity 100%) with no improvement in detection of MTC with calcium-stimulated calcitonin levels alone or added to basal calcitonin. 11 This study suggests that with modern immunochemiluminometric calcitonin assays, calcium stimulation may not be necessary. 11 These findings were supported with results from a recent meta-regression analysis. 13 However, Fugazzola et al. 14 found that with using basal calcitonin cutoffs of >34 pg/mL for men and >30 pg/mL for women (area under curve 0.97 for men and 0.91 for women), the addition of calcium stimulation increased the sensitivity of MTC detection, particularly for patients with small MTC. In patients with thyroid nodular disease and basal calcitonin 10–100 pg/mL, stimulated calcitonin levels may be a useful adjunct to detect MTC and to facilitate appropriate initial surgery, although risks of potential overtreatment must be considered.
Perioperative calcitonin in MTC
In patients with previously untreated sporadic MTC, preoperative basal calcitonin measurements have been used to predict the extent of disease and plan surgery. Patients with disease in the ipsilateral (central and lateral) neck had basal calcitonin of at least 20 pg/mL, contralateral central neck at least 50 pg/mL, contralateral lateral neck at least 200 pg/mL, and upper mediastinum at least 500 pg/mL. 15 A recent follow-up study of 1026 patients with MTC showed that when calcitonin levels are ⩾500 pg/mL, only 50% of patients achieved biochemical cure; all distant metastases were seen in patients with basal calcitonin at this level or higher. No patients were cured at preoperative calcitonin level >10,000 pg/mL. 16
While calcitonin elevation post-operatively indicates persistent disease, a recent study demonstrated that time to post-operative calcitonin normalization is dependent on both nodal disease burden and preoperative calcitonin levels. Calcitonin normalizes in a consistent pattern in node-negative patients with preoperative calcitonin levels <1000 pg/mL (3.5–6.6 days). 17 However, patients with node-positive disease with a preoperative serum calcitonin level >1000 pg/mL took on average 57.7 days to normalize compared to 6.6 days in those without nodal metastases. Length of time to calcitonin normalization was proportional to nodal burden of disease. Those with more than 10 positive nodes demonstrated a mean time to calcitonin normalization of 57.1 days compared to 5.2 days in patients with 1–5 positive nodes and 7 days in patients with 6–10 positive nodes. 17 Thus, patients with calcitonin >1000 pg/mL and those with >10 positive lymph nodes have prolonged time to calcitonin normalization which must be considered when planning timing of first post-operative calcitonin level.
Calcitonin doubling time in MTC
In patients with persistent hypercalcitonemia after surgery, biochemical markers with calculated doubling times have been used to monitor MTC recurrence, progression, and survival. Barbet et al. found in multivariate analysis that calcitonin and carcinoembryonic antigen (CEA) doubling times are independent risk factors for survival. When calcitonin doubling time was <0.5 years, 5-year survival was 25%. However, when calcitonin doubling time was >2 years, 100% of patients were alive at 10 years. 18 A meta-analysis found that CEA doubling time had a higher predictive value compared to calcitonin doubling time, 19 and measurement of both is recommended in follow-up of MTC. 3 One small study that evaluated calcitonin doubling time in patients after detection of distant metastases found short survival with doubling time of ⩽1.5 years compared to relatively long-term survival with patients with a longer doubling time. 20
Some limitations of calcitonin include inter-assay variability, a concentration-dependent half-life, and rapid degradation at room temperature or refrigeration. With development of increasingly reliable assays, 21 investigation into other biomarkers continues.
Serum carbohydrate antigen 19.9 (Ca 19.9) in MTC
Recently, elevated serum carbohydrate antigen 19.9 (Ca 19.9) has been recognized as a biomarker associated with poor prognosis in MTC. Elisei et al. 22 found Ca 19.9 to be elevated in 16% of patients with advanced structural recurrent/persistent MTC and none who were biochemically cured. Elevated Ca 19.9 was a predictor of mortality (odds ratio (OR): 3.78, p = 0.04). Another study found that Ca 19.9 doubling times of <6 months and <1 year were associated with mortality but not progressive structural disease according to response evaluation criteria in solid tumors (RECIST). These authors urge consideration Ca 19.9 secretion and doubling time as criteria for initiating systemic therapy independent of progressive structural disease. 23
Procalcitonin
Procalcitonin is the peptide precursor molecule of calcitonin, produced in response to inflammation, and is used in clinical practice to diagnose sepsis. However, procalcitonin is a more stable protein with a more predictable half-life and less inter-assay variability than calcitonin, promoting study of its potential as a marker in the initial diagnosis of MTC and as a tumor marker for follow-up of disease course. 24 Despite heterogeneity in the primary research, two recent systematic reviews found procalcitonin to have good correlation to calcitonin levels and burden of disease in patients diagnosed with MTC.25,26 A 2016 systematic review of 15 studies observed strong correlation between procalcitonin and calcitonin levels (r = 0.60–0.95), primary tumor diameter, lymph node metastases, and distant metastases. For a procalcitonin >0.1 ng/mL, the specificity of detecting MTC was 98–100% while sensitivity was 91–100%. 26 A 2019 meta-analysis evaluating the use of procalcitonin in detecting MTC recurrence found a pooled sensitivity of 96% (95% CI: 92–99%) and pooled specificity of 96% (95% CI: 87–100%). 27 Procalcitonin may also be a useful marker in the small population of calcitonin-negative MTC patients. While most prior studies have been confined to outpatient settings and excluded patients with signs or symptoms of systemic infection, potential exists for the use of procalcitonin as a more accessible tumor marker for MTC.
Micro-RNAs
Circulating cell-free micro-RNAs are frequently detected in the bloodstream of patients with cancer. Romeo et al. 28 conducted a prospective study to identify micro-RNAs overexpressed in MTC compared to healthy controls and reported several important findings. First, miR-375 is the micro-RNA most commonly overexpressed in MTC. In addition, miR-375 was able to differentiate significantly between MTC patients in remission from those with persistent/recurrent disease and patients with distant metastases compared to locoregional disease. Finally, patients with higher levels of miR-375 demonstrated a worse overall survival (hazard ratio (HR) 10.6, 95% CI: 3.8–29.5). On multivariate analysis, elevated levels of miR-375 was the only significant prognostic factor (HR: 5.52, 95% CI: 1.98–15.41, p = 0.001). miR-375 is a promising biomarker and prognostic marker for patients with MTC. 28
While basal calcitonin and CEA remain the standard biomarkers of MTC, there are multiple avenues of investigation for markers that are specific to MTC and can better predict disease prognosis in addition to disease burden.
Imaging
Evaluation of primary tumor
Ultrasound (US) is first-line imaging for patients with suspected MTC and is associated with 75.3% sensitivity, 93.1% specificity, and overall accuracy 80.4%. 29 US features associated with MTC include irregular shape, height/width ratio <1, lack of a peripheral halo, hypoechogenecity, calcification (typically macrocalcification), and hypervascularity.29–31 Two studies that compared US features of papillary thyroid cancer (PTC) versus MTC both found larger lesion size, heterogeneous echotexture, and hypervascularity as significantly more characteristic of MTC than PTC.30,31
US classification systems can be applied for both diagnosis and prognosis in MTC. Valderrabano et al. 32 found that the 2015 ATA thyroid nodule guidelines’ sonographic criteria to predict malignancy perform well in MTC. Specific US features in MTC can also have prognostic implications. In a study that used modified Thyroid Imaging and Reporting Data Systems (TI-RADS) to separate retrospectively MTC nodules into groups based on “high-risk” versus “low-risk” features, the “high-risk” group had significantly higher nodal staging, preoperative calcitonin level, and rates of disease recurrence. The ultrasonographically “low-risk” group had significantly more biochemical cures. 33 On multivariate analysis, extrathyroidal extension was positively associated with disease recurrence. US is superior to axial or functional imaging for detecting initial intrathyroidal tumor and initial or recurrent neck lymph node metastases.29,34
However, in contrast to these findings, a recent study from Matrone et al. evaluated the performance of five societal US risk stratification systems on 152 known MTC nodules. Fewer than half of the MTC nodules were classified by any of the systems as having features suggestive of high risk of malignancy (45.4–47.4%), and FNA was only recommended for 48.7–63.8% of the MTC nodules. 35 This study concluded that MTC may have a different sonographic appearance than differentiated thyroid cancer and that US risk stratification systems currently in use may fail to identify MTC.
Functional imaging for detection of recurrent or metastatic disease
ATA guidelines recommend contrast-enhanced computed tomography (CT) of the neck and chest for initial detection of lung and mediastinal metastases in MTC. Contrast-enhanced magnetic resonance imaging (MRI) or three-phase contrast-enhanced liver CT is recommended for further evaluation of liver metastases. Bone metastases are best evaluated with MRI and bone scintigraphy. 3
Multiple recent studies have confirmed that of the radiopharmaceuticals available, 18F-DOPA PET/CT has the highest diagnostic performance in detecting persistent or recurrent MTC in both patient-based and lesion-based analyses.36–38 The utility of detecting MTC with 18F-DOPA PET/CT increases proportionally to increases in basal calcitonin in the context of both initial and recurrent disease.39,40 One study evaluating the utility of F-DOPA PET/CT in patients with persistent or recurrent disease found the sensitivity of the study was markedly improved with a serum calcitonin level greater than 150 pg/mL (sensitivity 91% versus 29%) as the cutoff for imaging for metastatic disease according to 2015 ATA guidelines.3,38
Despite 2009 guidelines that recommended use of 18F-DOPA PET/CT to detect persistent/recurrent MTC in patients with calcitonin >150 pg/mL, functional imaging is not recommended in these cases in the 2015 guidelines.3,41 The 2020 European Association of Nuclear Medicine (EANM) guidelines recommended the use of 18F-DOPA PET/CT in the context of persistent/recurrent MTC.42,43 As discussed in a recent editorial in Thyroid, reconsideration of the recommendation for 18F-DOPA PET/CT in patients with calcitonin >150 pg/mL after initial therapy for MTC is warranted. 43
Hereditary versus sporadic MTC
One-fourth of MTC is hereditary in the setting of three different syndromes: multiple endocrine neoplasia (MEN) 2A, MEN2B, and familial MTC. Activating point mutations in the RET proto-oncogene, located on chromosome 10q11, is responsible for these diseases, whereas somatic RET mutations occur in approximately 60% of patients with sporadic MTC. 44 An additional group of patients with sporadic MTC may have somatic mutations of HRAS, KRAS, or nRAS. RAS mutations have been identified in 10–15% of sporadic MTC cases.
Recent research has helped define prognosis for MTC in patients with hereditary RET mutations. Genotype-phenotype correlations in MTC associated with RET mutations has evolved since these mutations were identified. In 2015, the updated ATA MTC guidelines replaced the A, B, C, and D risk categories with moderate-, high-, and highest-risk categories.3,41 Highest risk patients (codon 918 mutation) have an MEN2B phenotype. These risk categories are based on “aggressiveness” of MTC, “based on development of MTC at an early age.” 3
There are several key differences in the presentation of hereditary compared to sporadic MTC. Hereditary MTC presents at a significantly younger age, even after excluding patients identified via genetic screening. However, in a large cohort of patients with MTC, Saltiki et al. found that compared to sporadic MTC, hereditary MTC patients had significantly more microscopic disease (⩽1 cm), multifocality, C-cell hyperplasia, and smaller tumor size.45,46 Sporadic MTC patients had significantly more capsular invasion, soft tissue invasion, and distant metastases than hereditary MTC patients. Sporadic MTC patients with Stage-IV disease had significantly more progression. 46
A recent study comparing patients with moderate- to high-risk RET codon mutations found that patients with high-risk mutations were identified earlier (age 23.0 vs 42.3, p < 0.0001) and moderate-risk patients had more T3/T4 tumors at diagnosis. However, there was no significant difference in N or M stage at diagnosis. 47 Multivariable analysis demonstrated that increasing age at diagnosis (HR: 1.05 per year; 95% CI: 1.03 to 1.08), T3/T4 tumor (HR: 2.73; 95% CI: 1.22–6.11), and M1 status at diagnosis (HR: 3.93; 95% CI: 1.61–9.59)—but not high-risk mutation—were significantly associated with an increased risk for death. There were no differences in time to development of distant metastatic disease or overall survival between moderate-risk and high-risk groups. 47
Similarly, a recent study of 387 RET germline mutation carriers with MTC found that age at thyroidectomy increased significantly from highest to moderate risk. 45 Interestingly, despite younger age at diagnosis in highest-risk groups, primary tumor size and time to lymph node metastases were similar between groups. Taken together, these studies demonstrate that the biological behavior of hereditary MTC is similar among risk categories. Perhaps, as posited by Voss et al., 47 high-risk mutations might be better described by their presentation with MTC at an earlier age rather than by “aggressiveness.”
Surgery (neck management)
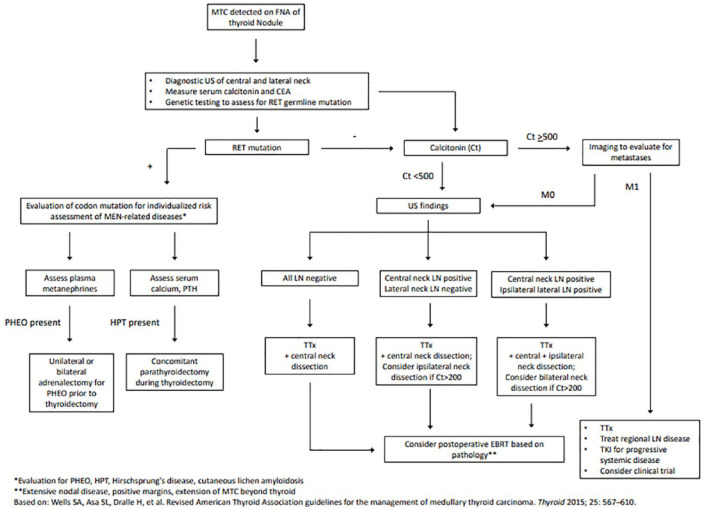
Surgical treatment of intrathyroidal MTC involves total thyroidectomy and bilateral central neck lymph node dissection (LND). Compartment-oriented LND of other involved cervical lymph node basins should be undertaken at the time of initial surgery. 3 However, controversy remains in the approach to management of potentially uninvolved lateral neck lymph node basins, particularly in patients with markedly elevated calcitonin levels (Figure 1 contains an algorithm for this approach). For patients with basal serum calcitonin >200 pg/mL, contralateral lateral neck dissection should be considered if ipsilateral lateral neck lymph nodes are involved. However, no consensus was reached on dissection of lateral neck lymph node compartments (II–V) in patients with MTC and no evidence of neck lymphadenopathy or distant metastases. 3 Surgical treatment of patients with MTC changed over time (1983–2012) with significantly more patients undergoing total thyroidectomy and LND and an increase in the number of lymph nodes harvested per dissection. 2 However, important questions remain regarding the optimal extent of LND.
Figure 1.
Algorithm for management of newly diagnosed MTC.
CEA, carcinoembryonic antigen; Ct, Calcitonin; EBRT, external beam radiation therapy; FNA, fine needle aspiration; HPT, hyperparathyroidism; LN, lymph nodes; MTC, medullary thyroid cancer; PHEO, pheochromocytoma; RET, rearranged during transfection; TKI, tyrosine kinase inhibitors; Ttx, total thyroidectomy.
One retrospective study of 316 patients who had undergone resection with curative intent for MTC (1986–2017) found that LND without evidence of structural disease does not improve recurrence or survival. In patients who had a preoperative calcitonin of ⩾200 pg/mL and no structural disease in the neck lymph nodes, they compared 45 patients who underwent “prophylactic” ipsilateral LND with the 44 who did not. There was no difference at 10 years in incidence of neck recurrence (20.9% LND vs 30.4% no LND, p = 0.46), incidence of distant recurrence (18.3% vs 18.4%, p = 0.97), disease-specific survival (86% vs 93%, p = 0.53), or overall survival (82% vs 90%, p = 0.6). 48
To predict involvement of lymph nodes based on intrathyroidal tumor characteristics of MTC, Niederle et al. identified that lymph node metastases are only found in patients who have a desmoplastic stromal reaction (DSR) in their intrathyroidal tumor. DSR is defined as cancer-associated and newly formed stroma surrounding invasive epithelial cells and can be identified intraoperatively on frozen section during initial MTC resection to guide surgical treatment.49,50 Of the 360 patients included over 25 years, 17.8% of tumors were DSR-negative and no patient in that group had lateral lymph node or distant metastases at diagnosis or during follow-up. In the DSR-positive group (82.2%), lymph node and distant metastases were present in 31.4% and 6.4% of patients, respectively. Using these data, if the patient’s intrathyroidal MTC is DSR-negative on frozen section, a prophylactic lateral neck LND is avoided. 50
Machens et al. highlighted that tumor size predicts lymph node and distant metastasis and lymph node metastasis predicts distant metastasis. In a recent study of 1115 patients with MTC (hereditary 307 and sporadic 808), increasing primary tumor size was correlated with lymph node and distant metastases. 51 In both hereditary and sporadic MTC, distant metastases almost never occur in the absence of lymph node metastases. In fact, in patients with node-positive, sporadic MTC, distant metastasis was 13.8 times more frequent (23.5% vs 1.7%) than in patients with node-negative sporadic MTC. In multivariable logistic regression analysis for sporadic MTC, lymph node metastasis contributed to distant metastasis (odds ratio: 12.4) more than primary tumor size. 51 Taken together, these findings provide some additional guidance based on tumor size, tumor characteristics, and preoperative calcitonin levels on which patients benefit from lateral neck dissection in the absence of structural lateral neck disease.
Adjuvant therapies
Targeted therapies
Patients presenting with MTC detected on physical exam have cervical lymph node metastases in 70% and distant metastases in 10%. 3 Cytotoxic chemotherapy has had disappointing results in halting disease progression. Therefore, extensive research in the last decade to identify targeted therapies for MTC has resulted in multiple United States Food and Drug Administration (FDA)-approved medications. However, systemic therapies in MTC are not curative and their impact is time-limited by frequent tumor escape. Their use is also limited by toxic side effects. Weitzman and Cabanillas reviewed the landscape of thyroid cancer treatment with multitargeted tyrosine kinase inhibitors (MKIs) and provide a compelling approach to the use of systemic therapy in MTC—systemic therapies should be used to treat clinically significant, progressive disease that has not responded appropriately to alternative therapies in patients with good performance status. 52 Specifically, appropriate uses of systemic therapy in MTC are in patients with symptomatic disease or disease that threatens vital structures or is rapidly progressive. 52 More recent reports suggest that rapidly progressive disease may be defined either structurally with RECIST criteria or with biochemical markers.20,23
Cabozantinib and vandetanib were the first two FDA-approved MKIs for treatment of progressive or symptomatic MTC with locally advanced or metastatic disease. Both are small molecule inhibitors that target RET and vascular endothelial growth factor-2 (VEGF-2) by blocking both angiogenic and proliferative pathways. Even though both drugs are effective in producing a partial response or stable disease, limitations of these drugs include frequent tumor escape and intolerable side effects.53,54
The Efficacy of XL184 in Advanced Medullary Thyroid Cancer (EXAM) trial and the ZETA trial evaluated the efficacy and safety of cabozantinib and vandetanib in patients with unresectable locally advanced or metastatic MTC.53,55,56 Tappenden et al. 57 performed a systematic review of data through 2016 and an economic analysis to evaluate the cost and benefit of use of these drugs in England. The analysis summarized that with similar effects, both drugs significantly improved progression-free survival (PFS) more than the placebo (p < 0.001). There has been no significant survival benefit for either drug compared to placebo. 57 The greatest clinical benefit of cabozantinib appears to be in patients with RET M918T mutations, a common somatic mutation in MTC.57–59
Two additional MKIs, sorafenib and lenvatinib, are approved for use in radioiodine-refractory differentiated thyroid cancer and have been studied for use in MTC. With disease control rate of 75% or greater in MTC, sorafenib continues to be investigated to provide an alternative treatment for patients who do not have access to or have progressive disease on other therapies.60,61 Similarly, a phase-II trial of lenvatinib demonstrated median PFS of 9 months in patients with unresectable MTC with 67% of patients exhibiting PFS at 6 months. In addition, the time-to-response for lenvatinib was short at a median of 3.5 months (95% CI: 1.9–3.7). Its side effect profile is similar to the VEGF-inhibitors with patients commonly experiencing diarrhea, proteinuria, fatigue, hypertension, poor appetite, and weight loss. However, skin toxicities may be less severe than similar MKIs. 62 A phase-II study from Japan demonstrated median PFS of 9.2 months and response rate of 22% in patients with metastatic MTC. 63 A recent, small study showed potential clinical benefit (disease stabilization) of lenvatinib as salvage therapy in patients who lost clinical benefit with other tyrosine kinase inhibitors (TKIs). 64 The results of the phase-III trial of lenvatinib for the treatment of iodine-refractory differentiated thyroid cancer and MTC are pending (NCT00784303). Neoadjuvant use of TKIs (selpercatinib, vandetinib, levantinib, and sunitinib) for unresectable MTC has also been described in several case reports whereby dramatic local tumor response allowed resection and long-term survival.65–68
Despite their effect in improvement of PFS, clinical utility of MKIs is limited by their toxicities. There has been no significant improvement in overall survival, and their duration of effectiveness is limited by intrinsic tumor resistance mechanisms. Two specific RET-kinase inhibitors, selpercatinib and pralsetinib, were FDA-approved for treatment of RET-mutant MTC in 2020.59,69 While there have been no trials directly comparing the MKIs to these RET-kinase inhibitors, these agents seem to be better tolerated than MKIs possibly due to the lack of VEGF inhibition. 70
Selpercatinib was tested in the Phase 1/2 LIBRETTO-001 international multicenter clinical trial. This prospective, open-label study for patients with any solid tumor exhibiting an activating RET mutation enrolled 531 patients, 55 of whom had MTC. In patients with MTC who were previously treated with vandetanib, cabozantinib, or both, 69% had an objective response (95% CI, 55–81%); 5 patients (9%) had a complete response and 33 (60%) had a partial response. Responses were observed in patients with all qualifying RET mutations. 69 Similar responses were observed in patients without prior treatment (73%; 95% CI, 62–82%); 10 patients (11%) had a complete response, and 54 (61%) had a partial response. Responses were quite durable with a 1-year PFS of 82% (95% CI, 69–90%) in patients with prior systemic therapy and 92% (95% CI, 82–97%) in patients without prior systemic therapy. Thirty percent of patients experienced a treatment-related adverse event that resulted in a dose reduction, and the overall dropout rate due to adverse events was 2%. Hypertension was the most common grade III or IV adverse event (21%). 69 The Phase I/II ARROW trial is currently testing pralsetinib (formerly known as BLU-667) in people who have RET-altered MTC and other RET-mutated tumors. Preliminary reports presented at the European Society for Medical Oncology on 9/20/20 demonstrated durable responses and a well-tolerated safety profile in systemic treatment-naïve and previously treated patients regardless of RET mutation genotype, including a high response rate in patients with gatekeeper mutations resistant to MKIs. While preliminary, these results suggest a potential pathway to overcome some of the limitations of MKIs.
External beam radiation therapy
Due to lack of improvement in overall survival with targeted systemic therapies, research into additional adjunctive therapies to target recurrent or metastatic MTC continues. The goal of post-operative external beam radiation therapy (EBRT) in treatment of patients with MTC has been to achieve local control in those at high risk of regional recurrence. Given the lack of randomized control trials (RCTs) evaluating patients who received adjuvant EBRT with those who did not, there remain no clear recommendations on this adjuvant therapy. 3 A 2019 systematic review highlights the heterogeneity of studies and subsequent difficulty in providing any recommendations on which patients may benefit from adjuvant EBRT. 71 A meta-analysis found that patients with MTC who underwent adjuvant EBRT had a 38% reduction in risk of locoregional recurrence (95% CI: 26–50%, p < 0.0001) with a trend toward better local control with doses greater than 60 Gy. However, given the selection bias inherent in many of these studies (i.e. radiotherapy reserved for patients with substantially more aggressive disease), it is possible that the impact of EBRT is higher. These findings do not change ATA recommendations to consider adjuvant EBRT to the neck and mediastinum in patients at high risk for local recurrence (residual macroscopic or microscopic disease, extrathyroidal extension, or extensive lymph node metastases) and those at risk of airway obstruction. 3
Peptide receptor radionuclide therapy
Since Lutathera (177Lu-DOTATATE) became the first FDA-approved peptide receptor radionuclide therapy (PRRT) in 2018 for the treatment of gastropancreatic neuroendocrine tumors, there has been increasing interest in somatostatin receptor-targeting PRRT for treatment of advanced, progressive, or metastatic MTC. As MTC cells have been shown to express somatostatin receptors in vitro and in vivo, and express cholecystokinin 2 receptor (CCK2R), both of these have been targets for PRRT. A 2020 review summarized the 19 published studies and 4 clinical trials. Most cases of MTC were treated with PRRT with somatostatin analogues radiolabeled with Yttrium-90 or Lutetium-177. There was a radiographic disease control rate of 62.4% (most with stable disease) with treatment discontinuation for toxicity in 1.3% of cases. 72 Lee and Kim published a 2020 meta-analysis evaluating PRRT in patients with thyroid cancer. Five articles assessed the radiological response of PRRT specifically in MTC. The pooled proportion of patients with disease control in MTC was nearly 60% with serious adverse events occurring in 2.79%. 73 These early data suggest that PRRT may be considered in selected patients with advanced, progressive, or metastatic MTC in the context of a clinical trial.72,73
Prognosis
In 2018, the AJCC 8th Edition staging manual first included MTC as a stand-alone chapter; it provided guidelines for anatomic staging at the time of diagnosis with a goal of predicting overall survival. 74 Disease characteristics at the time of diagnosis, including tumor size, presence and extent of gross invasion into surrounding tissues, cervical nodal metastases, and distant metastatic disease are factors that combine to predict survival. Dynamic risk stratification (DRS) has been proposed as a method to enhance prognosis by factoring in response to initial therapy to staging at diagnosis. 75 This is particularly relevant for patients with Stage-III or -IV disease who have an excellent response to initial therapy. The DRS system uses biochemically and structurally complete response after initial therapy as categories to predict mortality and may be better at predicting disease-free recurrence than tumor node metastasis (TNM) staging. 75
Multiple recent studies have defined contemporary factors that predict the evolution of presentation or prognosis in MTC. As may be predicted by the increase in MKI development, disease-specific survival has increased for those with regional and metastatic disease but not for those with localized disease; overall survival has not changed over time. 2 MTC survival is impacted by age at diagnosis. A recent SEER-18 study of 1457 patients with MTC found that older age was an independent prognostic factor for worse disease-specific mortality, controlling for disease stage and disease management. 76 Matrone et al. evaluated clinical data of 432 patients with sporadic MTC and compared groups by age (< or ⩾65 years). The clinical presentation and TNM stage of MTC was the same between groups and each had undergone equivalent surgical and therapeutic management. While there was no significant difference in cancer-related deaths between groups, there was a lower survival rate in the older group (HR: 2.5 (CI 95%: 1.27–4.94), p < 0.01). 77 Raue et al. 78 also found that increasing age at diagnosis was an independent predictor of disease-specific survival in MTC. Micro-MTC, defined as MTC measuring ⩽1 cm, has significantly increased over time as the proportion of newly diagnosed MTC cases (25.1% of cases 2003–2012). 2 A recent meta-analysis showed that compared to macro-MTC, micro-MTC had significantly lower rate of extrathyroidal extension and cervical lymph node metastases, but no difference in multifocality or rate of distant metastases. Disease-free survival (DFS) was significantly longer in micro-MTC (HR: 0.406, 95% CI: 0.288–0.575). 79
As discussed earlier, calcitonin and CEA doubling time have been shown to be important predictors of disease progression and mortality in MTC. Yeh et al. demonstrated that in patients with metastatic or recurrent MTC, tumor volume doubling time is also predictive of overall survival. Patients with an average tumor volume doubling time of ⩽1 year (median overall survival: 11.1 years) had a significantly worse prognosis than patients with higher average tumor volume doubling time. 80 These data, combined with calcitonin and CEA doubling time, can add valuable information to patient prognosis.
Conclusion
In conclusion, multiple recent updates in MTC include advancements in biochemical testing, imaging, hereditary disease, surgical management, adjuvant therapies, and prognosis. Use of basal and stimulated calcitonin to screen patients with thyroid nodular disease for MTC and to predict outcome of MTC continues to be explored. Alternate biochemical markers including Ca 19.9, procalcitonin, and micro-RNAs may help identify and predict the extent of disease and prognosis for MTC. While imaging of the primary tumor and cervical lymph nodes is initially undertaken with US, there is some debate as to whether current US risk systems are able to identify MTC with good sensitivity and specificity. Recent research reaffirms 18F-DOPA PET/CT as the best radiopharmaceutical to identify recurrent or metastatic disease in patients with calcitonin >150 pg/mL. Hereditary MTC presents at a younger age in higher risk mutations; prognosis appears similar regardless of risk category. Neck management in patients with elevated calcitonin but clinically negative lateral neck lymph nodes remains debated. MKIs have shown promise in terms of prolonging DFS in patients with progressive, metastatic disease but have substantial toxicities and do not improve overall survival. Two new highly selective RET inhibitors—selpercatinib and pralsetinib—have shown promising results in MTC patients with a RET mutation, regardless of mutation or the number or type of prior systemic therapies received. Finally, predicting prognosis may be improved by more dynamic staging systems.
Footnotes
Author contributions: MAB-K: conceptualization; formal analysis; investigation; methodology; resources; supervision; and writing—original draft. OEO: formal analysis; resources; and writing—original draft. LFM-W: conceptualization; formal analysis; methodology; resources; supervision; writing—original draft; and writing—review and editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lilah F. Morris-Wiseman  https://orcid.org/0000-0002-9751-8687
https://orcid.org/0000-0002-9751-8687
Contributor Information
Marisa A. Bartz-Kurycki, Department of Surgery, University of Arizona Tucson College of Medicine, Tucson, AZ, USA
Omowunmi E. Oluwo, Department of Surgery, University of Arizona Tucson College of Medicine, Tucson, AZ, USA
Lilah F. Morris-Wiseman, MD Chief of Endocrine Surgery, Assistant Professor of Surgery, Johns Hopkins University School of Medicine, 600 N. Wolfe Avenue, Blalock 606, Baltimore, MD 21287, USA.
References
- 1. Pereira M, Williams VL, Hallanger Johnson J, et al. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid 2020; 30: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 2. Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017; 161: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25: 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1856–1883. [DOI] [PubMed] [Google Scholar]
- 5. Workman AD, Soylu S, Kamani D, et al. Limitations of preoperative cytology for medullary thyroid cancer: proposal for improved preoperative diagnosis for optimal initial medullary thyroid carcinoma specific surgery. Head Neck 2021; 43: 920–927. [DOI] [PubMed] [Google Scholar]
- 6. Elisei R, Bottici V, Luchetti F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab 2004; 89: 163–168. [DOI] [PubMed] [Google Scholar]
- 7. Verbeek HHG, de Groot JWB, Sluiter WJ, et al. Calcitonin testing for detection of medullary thyroid cancer in people with thyroid nodules. Cochrane Database Syst Rev 2020; 3: CD010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allelein S, Ehlers M, Morneau C, et al. Measurement of basal serum calcitonin for the diagnosis of medullary thyroid cancer. Horm Metab Res 2018; 50: 23–28. [DOI] [PubMed] [Google Scholar]
- 9. Baetu M, Olariu CA, Moldoveanu G, et al. Calcitonin stimulation tests: rationale, technical issues and side effects: a review. Horm Metab Res 2021; 53: 355–363. [DOI] [PubMed] [Google Scholar]
- 10. Niederle MB, Scheuba C, Gessl A, et al. Calcium-stimulated calcitonin—the “new standard” in the diagnosis of thyroid C-cell disease-clinically relevant gender-specific cut-off levels for an “old test.” Biochem Med 2018; 28: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niederle MB, Scheuba C, Riss P, et al. Early diagnosis of medullary thyroid cancer: are calcitonin stimulation tests still indicated in the era of highly sensitive calcitonin immunoassays? Thyroid 2020; 30: 974–984. [DOI] [PubMed] [Google Scholar]
- 12. Pina G, Dubois S, Murat A, et al. Is basal ultrasensitive measurement of calcitonin capable of substituting for the pentagastrin-stimulation test. Clin Endocrinol 2013; 78: 358–364. [DOI] [PubMed] [Google Scholar]
- 13. Vardarli I, Weber M, Weidemann F, et al. Diagnostic accuracy of routine calcitonin measurement for the detection of medullary thyroid carcinoma in the management of patients with nodular thyroid disease: a meta-analysis. Endocr Connect 2021; 10: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fugazzola L, Di Stefano M, Censi S, et al. Basal and stimulated calcitonin for the diagnosis of medullary thyroid cancer: updated thresholds and safety assessment. J Endocrinol Invest 2021; 44: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010; 95: 2655–2663. [DOI] [PubMed] [Google Scholar]
- 16. Machens A, Lorenz K, Dralle H. Prediction of biochemical cure in patients with medullary thyroid cancer. Br J Surg 2020; 107: 695–704. [DOI] [PubMed] [Google Scholar]
- 17. Machens A, Lorenz K, Dralle H. Time to calcitonin normalization after surgery for node-negative and node-positive medullary thyroid cancer. Br J Surg 2019; 106: 412–418. [DOI] [PubMed] [Google Scholar]
- 18. Barbet J, Campion L, Kraeber-Bodéré F, et al. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 2005; 90: 6077–6084. [DOI] [PubMed] [Google Scholar]
- 19. Meijer JAA, le Cessie S, van den Hout WB, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol 2010; 72: 534–542. [DOI] [PubMed] [Google Scholar]
- 20. Ito Y, Miyauchi A, Kihara M, et al. Calcitonin doubling time in medullary thyroid carcinoma after the detection of distant metastases keenly predicts patients’ carcinoma death. Endocr J 2016; 63: 663–667. [DOI] [PubMed] [Google Scholar]
- 21. Kahaly GJ, Algeciras-Schimnich A, Davis TE, et al. United States and European multicenter prospective study for the analytical performance and clinical validation of a novel sensitive fully automated immunoassay for calcitonin. Clin Chem 2017; 63: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 22. Elisei R, Lorusso L, Piaggi P, et al. Elevated level of serum carbohydrate antigen 19.9 as predictor of mortality in patients with advanced medullary thyroid cancer. Eur J Endocrinol 2015; 173: 297–304. [DOI] [PubMed] [Google Scholar]
- 23. Lorusso L, Romei C, Piaggi P, et al. Ca19.9 positivity and doubling time are prognostic factors of mortality in patients with advanced medullary thyroid cancer with no evidence of structural disease progression according to response evaluation criteria in solid tumors. Thyroid 2021; 31: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 24. Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab 2014; 99: 2986–2994. [DOI] [PubMed] [Google Scholar]
- 25. Trimboli P, Seregni E, Treglia G, et al. Procalcitonin for detecting medullary thyroid carcinoma: a systematic review. Endocr Relat Cancer 2015; 22: R157–R164. [DOI] [PubMed] [Google Scholar]
- 26. Karagiannis AKA, Girio-Fragkoulakis C, Nakouti T. Procalcitonin: a new biomarker for medullary thyroid cancer? A systematic review. Anticancer Res 2016; 36: 3803–3810. [PubMed] [Google Scholar]
- 27. Trimboli P, Giovanella L. Procalcitonin as marker of recurrent medullary thyroid carcinoma: a systematic review and meta-analysis. Endocrinol Metab 2018; 33: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romeo P, Colombo C, Granata R, et al. Circulating miR-375 as a novel prognostic marker for metastatic medullary thyroid cancer patients. Endocr Relat Cancer 2018; 25: 217–231. [DOI] [PubMed] [Google Scholar]
- 29. Wang L, Kou H, Chen W, et al. The diagnostic value of ultrasound in medullary thyroid carcinoma: a comparison with computed tomography. Technol Cancer Res Treat 2020; 19: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai X, Liu M, Xia Y, et al. Hypervascularity is more frequent in medullary thyroid carcinoma compared with papillary thyroid carcinoma. Medicine 2016; 95: e5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu M-J, Liu Z-F, Hou Y-Y, et al. Ultrasonographic characteristics of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Oncotarget 2017; 8: 27520–27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valderrabano P, Klippenstein DL, Tourtelot JB, et al. New American Thyroid Association sonographic patterns for thyroid nodules perform well in medullary thyroid carcinoma: institutional experience, systematic review, and meta-analysis. Thyroid 2016; 26: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao J, Zheng X, Gao M, et al. Ultrasound features of medullary thyroid cancer as predictors of biological behavior. Cancer Imaging 2021; 21: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castroneves LA, Filho GC, de Freitas RMC, et al. Comparison of 68Ga PET/CT to other imaging studies in medullary thyroid cancer: superiority in detecting bone metastases. J Clin Endocrinol Metab 2018; 103: 3250–3259. [DOI] [PubMed] [Google Scholar]
- 35. Matrone A, Gambale C, Biagini M, et al. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Euro J Endocrinol 2021; 185: 193–200. [DOI] [PubMed] [Google Scholar]
- 36. Lee SW, Shim SR, Jeong SY, et al. Comparison of 5 different PET radiopharmaceuticals for the detection of recurrent medullary thyroid carcinoma: a network meta-analysis. Clin Nucl Med 2020; 45: 341–348. [DOI] [PubMed] [Google Scholar]
- 37. Treglia G, Tamburello A, Giovanella L. Detection rate of somatostatin receptor PET in patients with recurrent medullary thyroid carcinoma: a systematic review and a meta-analysis. Hormones 2017; 16: 362–372. [DOI] [PubMed] [Google Scholar]
- 38. Romero-Lluch AR, Cuenca-Cuenca JI, Guerrero-Vázquez R, et al. Diagnostic utility of PET/CT with 18F-DOPA and 18F-FDG in persistent or recurrent medullary thyroid carcinoma: the importance of calcitonin and carcinoembryonic antigen cutoff. Eur J Nucl Med Mol Imaging 2017; 44: 2004–2013. [DOI] [PubMed] [Google Scholar]
- 39. Brammen L, Niederle MB, Riss P, et al. Medullary thyroid carcinoma: do ultrasonography and F-DOPA-PET/CT influence the initial surgical strategy? Ann Surg Oncol 2018; 25: 3919–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golubić AT, Pasini Nemir E, Žuvić M, et al. The value of 18F-DOPA PET/CT in patients with medullary thyroid carcinoma and increased calcitonin values. Nucl Med Commun 2017; 38: 636–641. [DOI] [PubMed] [Google Scholar]
- 41. Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 2009; 19: 565–612. [DOI] [PubMed] [Google Scholar]
- 42. Giovanella L, Treglia G, Iakovou I, et al. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2020; 47: 61–77. [DOI] [PubMed] [Google Scholar]
- 43. Castinetti F, Taïeb D. Positron emission tomography imaging in medullary thyroid carcinoma: time for reappraisal. Thyroid 2021; 31: 151–155. [DOI] [PubMed] [Google Scholar]
- 44. Ciampi R, Romei C, Ramone T, et al. Genetic landscape of somatic mutations in a large cohort of sporadic medullary thyroid carcinomas studied by next-generation targeted sequencing. iScience 2019; 20: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Machens A, Lorenz K, Weber F, et al. Lymph node metastasis in hereditary medullary thyroid cancer is independent of the underlying RET germline mutation. Eur J Surg Oncol 2021; 47: 920–923. [DOI] [PubMed] [Google Scholar]
- 46. Saltiki K, Simeakis G, Anagnostou E, et al. Different outcomes in sporadic versus familial medullary thyroid cancer. Head Neck 2019; 41: 154–161. [DOI] [PubMed] [Google Scholar]
- 47. Voss RK, Feng L, Lee JE, et al. Medullary thyroid carcinoma in MEN2A: ATA moderate- or high-risk RET mutations do not predict disease aggressiveness. J Clin Endocrinol Metab 2017; 102: 2807–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spanheimer PM, Ganly I, Chou JF, et al. Prophylactic lateral neck dissection for medullary thyroid carcinoma is not associated with improved survival. Ann Surg Oncol. Epub ahead of print 21 March 2021. DOI: 10.1245/s10434-021-09683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaserer K, Scheuba C, Neuhold N, et al. Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol 2001; 25: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 50. Niederle MB, Riss P, Selberherr A, et al. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br J Surg 2021; 108: 174–181. [DOI] [PubMed] [Google Scholar]
- 51. Machens A, Lorenz K, Weber F, et al. Exceptionality of distant metastases in node-negative hereditary and sporadic medullary thyroid cancer: lessons learned. J Clin Endocrinol Metab 2021; 106: e2968–e2979. [DOI] [PubMed] [Google Scholar]
- 52. Weitzman SP, Cabanillas ME. The treatment landscape in thyroid cancer: a focus on cabozantinib. Cancer Manag Res 2015; 7: 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012; 30: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013; 31: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol 2017; 28: 2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kreissl MC, Bastholt L, Elisei R, et al. Efficacy and safety of vandetanib in progressive and symptomatic medullary thyroid cancer: post hoc analysis from the ZETA trial. J Clin Oncol 2020; 38: 2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tappenden P, Carroll C, Hamilton J, et al. Cabozantinib and vandetanib for unresectable locally advanced or metastatic medullary thyroid cancer: a systematic review and economic model. Health Technol Assess 2019; 23: 1–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sherman SI, Clary DO, Elisei R, et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 2016; 122: 3856–3864. [DOI] [PubMed] [Google Scholar]
- 59. Subbiah V, Yang D, Velcheti V, et al. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol 2020; 38: 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ito Y, Onoda N, Ito KI, et al. Sorafenib in Japanese patients with locally advanced or metastatic medullary thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid 2017; 27: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Castroneves LA, Negrão MV, de Freitas RM, et al. Sorafenib for the treatment of progressive metastatic medullary thyroid cancer: efficacy and safety analysis. Thyroid 2016; 26: 414–419. [DOI] [PubMed] [Google Scholar]
- 62. Schlumberger M, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res 2016; 22: 44–53. [DOI] [PubMed] [Google Scholar]
- 63. Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019; 15: 717–726. [DOI] [PubMed] [Google Scholar]
- 64. Matrone A, Prete A, Nervo A, et al. Lenvatinib as a salvage therapy for advanced metastatic medullary thyroid cancer. J Endocrinol Invest 2021; 44: 2139–2151. [DOI] [PubMed] [Google Scholar]
- 65. Jozaghi Y, Zafereo M, Williams MD, et al. Neoadjuvant selpercatinib for advanced medullary thyroid cancer. Head Neck 2021; 43: E7–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cleary JM, Sadow PM, Randolph GW, et al. Neoadjuvant treatment of unresectable medullary thyroid cancer with sunitinib. J Clin Oncol 2010; 28: e390–e392. [DOI] [PubMed] [Google Scholar]
- 67. Milner TD, Ronghe M, Shaikh MG, et al. Vandetanib tumor shrinkage in metastatic medullary thyroid cancer allowing surgical resection of the primary site: a case report. J Pediatr Hematol Oncol 2019; 41: e329–e332. [DOI] [PubMed] [Google Scholar]
- 68. Golingan H, Hunis B, Golding AC, et al. Neoadjuvant lenvatinib in advanced unresectable medullary thyroid carcinoma: a case report. AACE Clin Case Rep 2020; 6: e73–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020; 383: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gild ML, Tsang VHM, Clifton-Bligh RJ, et al. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol 2021; 17: 225–234. [DOI] [PubMed] [Google Scholar]
- 71. Rowell NP. The role of external beam radiotherapy in the management of medullary carcinoma of the thyroid: a systematic review. Radiother Oncol 2019; 136: 113–120. [DOI] [PubMed] [Google Scholar]
- 72. Grossrubatscher E, Fanciulli G, Pes L, et al. Advances in the management of medullary thyroid carcinoma: focus on peptide receptor radionuclide therapy. J Clin Med 2020; 9: 3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee DY, Kim Y. Peptide receptor radionuclide therapy in patients with differentiated thyroid cancer: a meta-analysis. Clin Nucl Med 2020; 45: 604–610. [DOI] [PubMed] [Google Scholar]
- 74. Rosen JE, Lloyd R v, Brierley JD, et al. Thyroid—medullary. In: Amin MB. (ed.) AJCC cancer staging manual. Chicago, IL: Springer Nature, 2018, pp. 891–901. [Google Scholar]
- 75. Choi JB, Lee SG, Kim MJ, et al. Dynamic risk stratification in medullary thyroid carcinoma: single institution experiences. Medicine 2018; 97: e9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sahli ZT, Canner JK, Zeiger MA, et al. Association between age and disease specific mortality in medullary thyroid cancer. Am J Surg 2021; 221: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Matrone A, Gambale C, Prete A, et al. Impact of advanced age on the clinical presentation and outcome of sporadic medullary thyroid carcinoma. Cancers 2020; 13: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Raue F, Bruckner T, Frank-Raue K. Similar stage-dependent survival and outcome in sporadic and hereditary medullary thyroid carcinoma. J Clin Endocrinol Metab 2021; 106: e3582–e3591. [DOI] [PubMed] [Google Scholar]
- 79. Kim JH, Pyo JS, Cho WJ. Clinicopathological significance and prognosis of medullary thyroid microcarcinoma: a meta-analysis. World J Surg 2017; 41: 2551–2558. [DOI] [PubMed] [Google Scholar]
- 80. Yeh T, Yeung M, Sherman EJ, et al. Structural doubling time predicts overall survival in patients with medullary thyroid cancer in patients with rapidly progressive metastatic medullary thyroid cancer treated with molecular targeted therapies. Thyroid 2020; 30: 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]