Abstract
Frailty is a clinical syndrome caused by homeostasis imbalance. It is characterized by marked vulnerability to endogenous or exogenous stressors, reduced self-care ability, and increased mortality risk. This aging-related syndrome is common in individuals older than 65 years and carries an increased risk for poor health outcomes. These include falls, incident disability, incapacity, and mortality. In addition, it can result in a poor prognosis for other comorbidities. With the aging population, frailty increases the burden of adverse health outcomes. Studies on frailty are at their infancy. In addition, there is a lack of thorough understanding of its pathogenesis. Several studies have suggested that frailty is caused by chronic inflammation due to enhanced intestinal permeability following gut microbiota imbalance as well as pathogen-related antibodies entering the circulation system. These result in musculoskeletal system disorders and neurodegenerative diseases. However, this assumption has not been validated in large cohort-based studies. Several studies have suggested that inflammation is not the only cause of frailty. Hence, further studies are necessary to extend our understanding of its pathogenesis. This review summarizes the research findings in the field and expands on the possible role of the gut microbiota in frailty syndrome.
Keywords: frailty, gut microbiota, chronic inflammation, neurodegenerative diseases, inflammatory factors
1. Frailty syndrome
Frailty syndrome is an aging-related syndrome and is characterized by morphological and physiological changes in multiple organs and systems. This leads to homeostasis imbalance and marked vulnerability to endogenous and/or exogenous stressors [1,2]. At present, frailty is clinically defined with frailty phenotype (FP) and frailty index (FI) (Table 1).
Table 1.
Common frailty assessment models [4]
| Frailty assessment models | Type | Contents | Author |
|---|---|---|---|
| Frailty phenotype (FP) | Criteria | Criteria: weight loss, weakness, slowness, low activity levels, poor endurance | Fried, 2001 |
| ≥3 met: frail | |||
| 1–2 met: pro-frail | |||
| 0 met: non-frail | |||
| Frailty index (FI) | Index | Calculating the proportion of abnormal items to the total items (FI value) | Mitnitski, 2001 |
| FI ≥ 25%: frail | |||
| 8 < FI < 25%: pro-frail | |||
| FI ≤ 8%: non-frail |
Frailty syndrome has been highly associated with age. A meta-analysis [3] based on 21 studies demonstrated that the incidence rate of frailty syndrome ranges between 4 and 59.1% and is positively correlated with age. The incidence peak occurs in populations aged 85 years. Along with increased longevity and the higher proportion of the elderly in the population, the number of individuals with frailty syndrome keeps increasing [4]. Studies have demonstrated that frailty is closely related to chronic diseases, such as cardiovascular diseases, Alzheimer’s Disease (AD), and Parkinson’s Disease (PD) [4,5,6]. The prevention and treatment of frailty may reduce the risk of other chronic diseases and improve prognosis. Hence, understanding the pathogenesis and prevention of frailty may lower the burden of adverse health outcomes in the elderly. It is an important public health concern to understand the cause and pathogenesis of frailty.
2. Gut microbiota
Higher organisms contain diversified microflora, which includes, bacteria, archaebacteria, viruses, fungi, and protozoa [7]. The intestinal tract of mammals is rich in nutrition and has a constant temperature, making it ideal for the survival of microorganisms. Microorganisms reside in all mucosal surfaces of the host but are mainly distributed in the gastrointestinal tract. Most microorganisms are anaerobes outnumbering aerobe and facultative anaerobes by 100 to 1,000 times [8]. A sequencing analysis on the gut microbiota of a cohort of 386 Chinese individuals indicated that 95% of the gut microbiota were Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, of which, 90% fell into 15 genera, such as Bacteroidetes, Clostridia, Clostridium leptum, and Eubacteria [9].
There are approximately 1014 [10] bacterial cells in the intestinal tract of an adult. This is 10 times as much as the number of human cells [11]. Their combined genome (also known as the microbiome) tops over 5 million genes, two orders of magnitude higher than the genetic potentiality of the host [7,12]. The consequent huge gene product library (e.g., RNA and protein) exerts an influence on the physiological activities of the host [13]. Studies have shown that approximately 400,000 of the 4,026,600 messenger RNAs in the human transcriptome are from gut microbiota [14]. This lays a material basis for the gut microbiota to participate in regulating the physiological activities of the host: (1) Gut microbiota can stimulate the metabolism of polysaccharides [14], synthesize essential vitamins, and regulate fat absorption and distribution [10]. (2) The microbiota is essential for the development and differentiation of intestinal epithelial cells of the host. In addition, they can facilitate the maturation of gut-associated lymphoid tissues (GALTs), tissue regeneration (especially intestinal villi), and intestinal tract movement. (3) The microbiota plays a prominent role in shaping the immune microenvironment. This is done by promoting the development of lymphatics and the differentiation of immunocytes and regulating the generation of immune mediators [14]. In addition, they can stabilize the immune system of the host. (4) Gut microbiota has also been shown to regulate tissue homeostasis (e.g., induces cell proliferation and stem cell differentiation) and bone mineral density (BMD) of the host [14] and (5) the microbiota can affect the nervous system of the host through three pathways of the gut–brain axis, i.e., immune system, neuroendocrine system, and vagus nerve. This is important for modifying and controlling cognitive activities such as anxiety, pain, and depression [15].
The gut microbiota is influenced by the host and under dynamic fluctuations. Studies on the gut microbiota in children and adults suggest that the gut microbiota keeps changing from birth to old age [16,17]. After 65 years of age, the gut microbiota enters a degeneration period with decreased bacterial diversity and is usually dominated by Bacteroidetes [18]. Furthermore, changes in diet, lifestyle, sanitary conditions, or antibiotic use of the host could also affect the gut microbiome composition [14,19,20,21]. For instance, Firmicutes/Bacteroidetes ratio has been generally accepted as an index for obesity [22]. Unlike the genome of the host, the microbiome changes rapidly along with changes in gut microbiome composition or a single microbial gene. This leads to changes in the transcriptome, proteome, and metabolic profiling [14], and influences the host. Thus, alterations in the gut microbiota may exert a profound impact on the health of the elderly [15,23].
3. Direct evidence of correlation between gut microbiota and frailty
An imbalance in gut microbiota may trigger a chronic inflammatory status [22] and increase the risks of cardiovascular diseases, Type II diabetes, and cancer [24,25,26]. As studies on frailty syndrome increase, researchers have identified that gut microbiota imbalance in the elderly may be associated with frailty [27]. Nevertheless, there are only limited studies on the gut microbiota and frailty. A small number of exploratory studies have revealed a possible correlation [15,24].
3.1. Potential correlation between frailty and intestinal microorganism diversity
It is reasonable to believe that there is a correlation between frailty and intestinal microorganism diversity. The gut microbiota in the elderly is characterized by reduced bacterial community diversity and an increase in some microorganism species [5,28,29,30,31]. A small-scale exploratory study compared the gut microbiotas of individuals with and without frailty. The study discovered that Lactobacilli is decreased in frail people compared with non-frail ones, and the same thing happens in Faecalibacterium prausnitzii (with anti-inflammatory and immunoregulation effects); while Enterobacteriaceae, which could increase opportunistic infections [32], was found to be higher in frail people. Another study performed on 728 female twins suggested a negative correlation between frailty and intestinal microorganism diversity [29]. Eubacterium dolichum (related to a high-fat diet [33]) and Eggerthella lenta (a pathogen [34]) are the most abundant gut microbiota in individuals with frailty. Like that of the elderly, F. prausnitzii in the gut microbiota of individuals with frailty decreased [35].
Studies using larger cohorts are necessary to determine whether the gut microbiota is associated with frailty. Additionally, it is necessary to perform high-quality clinical trials on intervention treatment and assess the effects of probiotics supplements and/or prebiotics on frailty syndrome. A randomized double-blind placebo-controlled clinical trial investigated interventional treatments [36]: 60 volunteers received a 13-week treatment with probiotic preparations (inulin and fructo-oligosaccharide). Subsequent frailty assessments demonstrated that frailty was not improved. Nevertheless, compared to the placebo group, individuals receiving probiotic treatment had significant improvements in two indicators (i.e., fatigue and grip).
3.2. The gut microbiota and frailty may influence each other
In addition to the potential correlation, the gut microbiota and frailty may influence each other. The microbiota may be possibly affected by drugs taken by patients with frailty, especially proton pump inhibitors (PPIs), which are prescription drugs administered to older individuals with frailty [37,38,39]. A large population-based study on frailty demonstrated a decrease in symbiotic bacteria abundance and bacterial diversity and increased levels of pathogenic streptococci in the intestinal tract of patients taking PPIs. These results were validated in a study on fraternal twins taking PPIs [40]. These findings are of clinical significance because it proves that the use of PPIs and alterations in the gut microbiota were related to Clostridium difficile infection (CDI). CDI may give rise to a poor prognosis and frailty in elderly patients with multiple complications [41]. In addition, non-steroid anti-inflammatory drugs (NSAIDs) that have been extensively administered in the elderly may lead to alterations in the gut microbiota [42].
4. Role of gut microbiota in the pathogenesis of frailty
The above studies revealed a direct correlation between the gut microbiota and frailty. Adverse outcomes brought by aging may include alterations in the gut microbiota [43,44] and an inflammatory reaction (immunosenescence) [45], followed by frailty. Studies have asserted that the gut microbiota may play a part in the pathogenesis of frailty through chronic inflammation [5]. However, the relationship with regards to the alterations in the gut microbiota, chronic inflammation, and frailty is not unilateral but complicated and interrelated [46,47]. The mutual influences among alterations in the gut microbiota, chronic inflammation, and frailty will be discussed in the following sections.
4.1. Frailty syndrome and immune-related disease in the context of aging
As mentioned above, frailty syndrome leads to homeostasis imbalance and marked vulnerability to endogenous and/or exogenous stressors [1,2]. For example, it is well studied that frailty syndrome is always with a chronic low-grade inflammation, which is a contributing factor of immune related disease [48]. So, it is reasonable to believe that the elder population with frailty syndrome is highly like with immune-related diseases.
The clinical manifestations of frailty syndrome are usually loss of muscle and bone tissue, which may partially be explained by an impaired immune system as studies by Cornish et al. who revealed that there is substantial “cross-talk” between muscle and bone and the immune system [49].
Stavropoulou and Bezirtzoglou found that the elder population with frailty is always the one facing immune-related disease. Usually, fragile elder population is facing problems like changes in hormonal, increase in the pro-inflammatory cytokines release, abnormality of the telomere, and these problems can cause the dysfunction of the immune system [50].
4.2. Gut microbiota, chronic inflammation, musculoskeletal system disorders (MSDs), and frailty
A possible cause may be aging-triggered alterations in the gut microbiota which results in chronic inflammation. Increased inflammatory factors could directly or indirectly lead to typical symptoms of frailty like reduced grip and muscle and bone loss [51,52].
Studies have demonstrated that changes in gut microbiome composition and intestinal permeability due to aging may give rise to an inflammatory reaction. Studies have revealed that aging is associated with decreased probiotics (e.g., Enterococcus faecalis, Bacillus faecalis, and Lactobacilli) and a lower Firmicutes/Bacteroidetes ratio, despite the tremendous differences in the gut microbiota of populations of different races and in different regions and environments [53].
Some beneficial microorganisms are vital to human health because they can inhibit the expansion of pathogenic bacterial communities, and generate mucus and products of lipid metabolism, for example, short-chain fatty acid (SCFA), bacterial polysaccharide (PSA), and Serum Amyloid A (SAA), through fermenting starch and dietary fiber to maintain a complete intestinal tract barrier [54]. It has been reported that SCFA can act on internal regulatory T cells (cTreg) through the G protein-coupled receptor GPR43 to improve the number of cTregs which is reduced by vancomycin, and up-regulate the genes of Foxp3 and IL-10 in cTreg cells in sterile mouse, so as to alleviate colitis [55]. Another research shows that SCFA can increase the immunoglobulin A (IgA) level in rat saliva, thus revealing a possible mechanism of how cellulose enhances rats’ immunity [56].
Intestinal beneficial bacteria decrease with age, while the relative abundance of other bacteria increases, including symbiotic bacteria that are pathogenic and inflammatory [57]. Such microorganisms are mainly facultative anaerobes (e.g., Clostridia and Staphylococci). In addition, studies have demonstrated that an increase in pathogenic bacteria is associated with an increase in inflammatory cytokines [58]. Enhanced intestinal permeability enables bacteria and their products (including pathogen-associated molecular pattern (PAMP), damage-associated molecular pattern (DAMP), and microorganism-associated molecular pattern (MAMP)) to enter the circulatory system. This consequently results in a chronic pro-inflammatory status [59]. This hypothesis is supported in animal models, however, no explicit evidence has been identified in the elderly who have no obvious inflammatory disease [60].
An inflammatory reaction manifests with an increase in inflammatory factors (e.g., IL-6, C-reactive protein, tumor necrosis factor-α (TNF-α), and neopterin) [61]. Several studies have demonstrated that high levels of inflammatory molecules in the blood have been correlated with frailty [62]. Hence, chronic inflammation attributable to alterations in the gut microbiota may be a key cause of frailty.
Studies have demonstrated the direct correlation between increased inflammatory factor levels and frailty [45,46,47,51,52,53,54,57,58]. The direct correlation between frailty and elevated IL-6 levels (a pro-inflammatory cytokine) was demonstrated in a study that involved community-dwelling elderly individuals [63]. The IL-6 level in the serum of individuals with frailty was higher compared to individuals without frailty. This exploratory study enrolled 11 senior citizens with frailty and 19 without frailty. This finding was validated subsequently in a large-scale study in elderly individuals under different nursing conditions, as well as in cell culture and mouse model-based studies. This suggested a direct correlation between chronic inflammation and immune activation, characterized by elevated IL-6 levels and frailty syndrome [64]. Additional inflammatory molecules (e.g., C-reactive protein and TNF-α) were also demonstrated to be related to frailty syndrome [65]. In addition, increased neopterin level (a marker of immune activation), independent of IL-6 levels, were confirmed to be associated with frailty in a cohort of community-dwelling elderly individuals [66]. These indicated that immune activation may induce frailty relative to chronic inflammation [67]. An increase in total white blood cell counts (TWCC) (higher than typical ranges) is often regarded as part of the complete blood counts for routine measurement in clinical practice. This is a laboratory index secondary to systemic inflammation induced by acute bacterial infections. Several studies have demonstrated a direct correlation between increased TWCC (even within the normal range) and frailty. Specific subsets, including neutrophils and monocytes, have been confirmed to be associated with frailty [68]. In addition, an increase in other cell subsets, such as differentiated CD8+/CD28− T cells and CCR5+ T cells, has also been associated with frailty [69,70,71].
As previously mentioned, the direct correlation between frailty and inflammatory molecules has been demonstrated. Higher expression levels of inflammatory molecules activate the inflammatory pathway, and it is a molecular mechanism of chronic inflammation in individuals with frailty [72]. How does chronic inflammation play a role in the pathogenesis of frailty? It was discovered that several inflammatory molecules (e.g. IL-6) may directly induce frailty or reduce key assessment indicators (e.g., muscle mass, strength, and exercise performance) [73,74]. A study on 3,075 elderly individuals demonstrated that high levels of IL-6 were linked to reduced muscle mass and strength. The relationship between the IL-6 level and grip has been the most consistent. The increase in IL-6 level by each standard deviation (SD) results in a decrease in grip by 1.1–2.4 kg [61]. Studies have demonstrated that higher levels of inflammatory molecules were negatively related to hemoglobin levels and levels of insulin-like growth factor-1 (IGF-1), albumin, micronutrients, and vitamins [63,75,76]. IGF1, in particular, is an indispensable growth factor for muscle regeneration and muscle structural integrity and protects the body from unstable carotid atheromatous plaques [77,78]. In vitro studies showed that IL-1, IL-6, and TNF-α could inhibit IGF1-mediated anabolism. In addition, IL-6 reduces the generation of IGF1 and IGF-binding protein 3 (IGFBP-3) [79]. An observational study suggested that high levels of IL-6 and low levels of IGF1 could exert a synergistic effect to decrease muscle strength and effectively predict progressive disabilities and death [80,81]. Furthermore, inflammation interferes with long-chain peptide synthesis which is indispensable for muscle energy and protein anabolism [82].
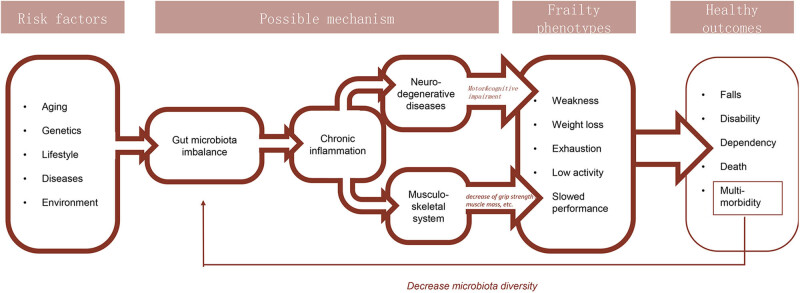
Chronic inflammation directly or indirectly plays a key role in the pathogenesis of frailty (as shown in Figure 1). Chronic inflammation causes typical symptoms of frailty, such as reduced grip and muscle mass by damaging the musculoskeletal system. In addition, other factors besides chronic inflammation may play a vital role in the pathogenesis of frailty. Some studies found no correlation between elevated IL-6 levels and frailty [83,84], while other studies found that administration of statins that had an anti-inflammatory effect did not alleviate frailty [85]. Given this, the pathogenesis of frailty is extremely complicated. Chronic inflammation is probably one of the causes. Furthermore, the gut microbiota may influence the host through anabolic resistance [86] and reduced bioavailability of nutrients [87]. Additional studies are necessary to identify causal factors for frailty.
Figure 1.
Current understanding of the pathogenesis of frailty syndrome.
5. Gut microbiota, neuroinflammation, neurodegenerative diseases, and frailty
Another possible cause of frailty may be related to gut microbiota. The mechanism may be through the gut–brain axis, which affects the nervous system to induce neurodegenerative diseases. This affects cognitive function to trigger or deteriorate frailty-related symptoms, including difficulty in moving and incapacity [37,88,89]. Such cognitive impairment-related frailty is known as “cognitive frailty” [90,91].
The concept of the gut–brain axis has been fully demonstrated and widely accepted. A study by Muller et al. [92] further verified this concept by discovering the direct modulation of gut-extrinsic sympathetic neurons by the gut microbiota. It is reported that the depletion of rat microbiota can induce an increase in the expression level of cFos, a neuronal transcription factor. The implantation of SCFA-producing bacteria rescues the increase of cFos expression, which further verifies that gut microbiota can directly regulate the development of these sympathetic neurons.
A large cohort study suggested a significant correlation between bacterial diversity and cognitive function [93]. This study sequenced fecal samples from 1,551 subjects and analyzed diversity to correlate with multiple cognitive functions, which included verbal fluency and response time. The authors observed a correlation between higher language diversity and shorter response time and higher bacterial diversity. There are only a limited number of studies on how gut microbiota imbalance impairs cognitive function and ultimately lead to frailty. However, several animal models and some studies on humans suggested a correlation between the gut microbiota and cognition [94,95,96,97].
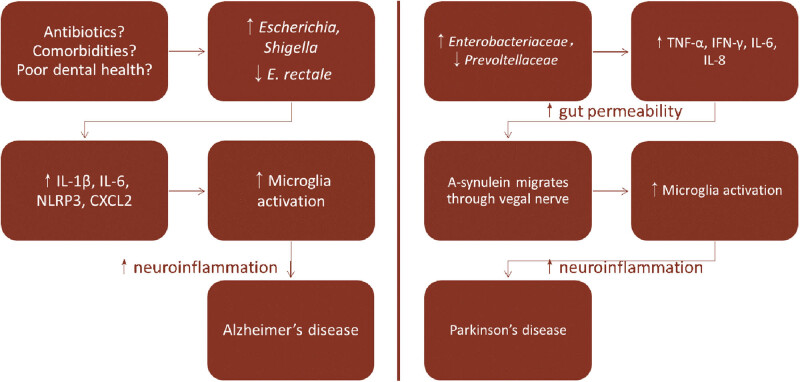
Patients with Parkinson’s Disease (PD) suffer from shaking, stiffness, and difficulty in walking, balance, and coordination [98]. The primary pathogenesis lies in the unusual folding and aggregation of the protein α-synuclein, which forms protein clumps (i.e., Lewy bodies and Lewy neuritis) and affects the nervous system. Neuroinflammation induces gut–brain axis damage and is considered as a possible cause of abnormal folding of α-synuclein [99]. A study performed on 19 PD patients demonstrated that neuroinflammation may be triggered by chronic inflammation in the colonic mucosa [100]. Compared to healthy individuals, PD patients had higher mRNA expression levels of pro-inflammatory cytokines (e.g., TNF-α, IFN-γ, IL-6, and IL-1β) and neurogliocyte activation markers in colonic biopsies. It was observed that the levels of pro-inflammatory cytokines and the duration of the disease were negatively correlated. Another retrospective study indicated that vagotomy reduced the risk of PD. This suggested an interaction between the intestinal tract and the central nervous system [101]. Furthermore, it was observed that abnormally folded α-synuclein appears in the nerve plexuses of intestinal submucosa and myenteron before it aggregates in the brain. Hence, abnormal proteins may migrate to the brain from the intestinal tract in a “prion-like” form [102]. This assumption is supported by the cephalo-caudal gradient of the distribution of α-synuclein in the enteric nervous system (ENS) of PD patients during the early stages [103]. Hence, this could be the reason why PD patients suffer from gastrointestinal symptoms like constipation and dysporia several years before dyskinesia [104].
A PD mouse model (overexpressing α-synuclein) further revealed an important correlation between neuroinflammation and the gut microbiota. The comparison between germ-free and normal mice in this model indicated that the gut microbiota was indispensable to trigger dyskinesia, neurogliocyte activation (neuroinflammation), and aggregation of α-synuclein [105]. Additionally, the transfer of gut microbiota from PD mice (overexpressing α-synuclein) into germ-free mice resulted in sports injury, while transferring gut microbiota from healthy mice did not.
Human studies have demonstrated the correlation between the gut microbiota and PD. A study assessed the gut microbiome composition of 72 PD patients: [106] Compared to healthy subjects, Prevotellaceae in the feces of PD patients were significantly lower, while the relative abundance of Enterobacteriaceae increased. This was positively correlated with the severity of postural instability and gait disturbance (PIGD) of patients. The authors speculated that Prevotellaceae could significantly increase the synthesis of thiamine and folic acid to generate mucoprotein. Hence, a decrease in Prevotellaceae may contribute to reduced vitamin content and enhanced intestinal permeability observed in PD patients.
Alzheimer’s Disease (AD) is the most common type of dementia. The disease process is associated with amyloid plaques and neurofibrillary tangles in the brain [107]. The correlation between the intestinal tract and AD was first demonstrated in a mouse model. Compared to conventionally fed mice with a complete gut microbiota, germ-free mice had memory dysfunction (a typical symptom of AD) [108]. Moreover, the administration of endotoxin could increase β-amyloid protein levels in the hippocampus of mice and induce cognitive defects [109]. An interventional study demonstrated that higher numbers of Actinomycetes and Bacteroidetes [110] decreased neurogliocyte activation markers and enhanced brain-derived neurotrophic factor (BDNF) in the gut microbiota of older rats treated with VSL#3 (a probiotic mixture of eight Gram-positive bacterial strains).
Animal models have suggested the potential relationship between AD and gut microbiota [102,103,104], however, this relationship has only been supported by a limited number of research studies on humans with AD. Microbiota in the feces of AD patients was first analyzed in a study conducted in 2017 [111]. In this study, compared to the control group and patients negative for amyloid protein, patients who were positive for amyloid proteins had more pro-inflammatory cytokines (e.g., IL-6, CXCL2, NLRP3, and IL-1α) and lower levels of IL-10, an anti-inflammatory cytokine. In terms of the gut microbiota, the level of Eubacterium rectale in patients who were positive for amyloid protein was lower, while the levels of Escherichia and Shigella that cause infections were increased. In addition, it is found that pro-inflammatory cytokines (e.g., IL-1α, NLRP3, and CXCL2) were positively correlated with the abundance of Escherichia and Shigella, and negatively correlated with Bacillus stearothermophilus level in the rectum.
Animal models and some human studies have demonstrated the relationship between chronic inflammatory status triggered by gut microbiota and the marked upregulation of immune factors and neuroinflammation characterized by neurogliocyte activation. Hence, some investigators speculated that the upregulation of inflammatory factors, due to gut microbiota imbalance, leads to neuroinflammation through the gut–brain axis. Subsequently, neuroinflammation takes part in the onset/progression of PD and AD [5] (as shown in Figure 2). Dyskinesia and cognitive disorders brought about by PD and AD will cause/deteriorate symptoms of frailty, which include slower movement and reduced physical activity, lower the self-care ability and increase the risk of death [37]. Nevertheless, the assumption has not been fully demonstrated in human studies. How neuroinflammation participates in the pathogenesis of PD and AD remains to be deciphered.
Figure 2.
Possible pathogenic mechanism of PD and AD due to gut microbiota imbalance [5].
Apart from the impact on central nervous system, the enteric neurons are under the regulation of the gut flora as well. Take the association between IL-33 and enteric neurons for an example. Malik et al. [112] observed a higher level of pro-inflammatory microbiota in mice lacking IL-33, which indicated a direct association between IL-33 level and gut microbiome. On the other hand, the IL-33 level is associated with the release of 5-HT. IL-33, as an alarmin cytokine, could be sensed by Enterochromaffin (EC) cells [113], resulting in the release of serotonin (5-HT) [114], a neurotransmitter that activates enteric neurons and promotes gut motility, which is essentially compromised in frail people [115]. Therefore, it is reasonable to relate gut microbiota-related IL-33 increase with 5-HT decrease-related frailty symptoms.
6. Discussion and conclusion
As the population ages, frailty syndrome will bring a huge medical burden to society. Previous studies have suggested that gut microbiota imbalance may be a cause of frailty. Animal models and a few human studies have demonstrated that individuals with frailty tend to have increased levels of inflammatory factors (e.g. IL-6, C-reactive protein, and TNF-α) and a chronic inflammatory status. Inflammatory factors have been demonstrated to directly or indirectly reduce key indicators of frailty, such as muscle mass and grip. In addition, gut microbiota imbalance has been demonstrated to be associated with the higher expression of inflammatory factors. Studies have suggested that gut microbiota imbalance leads to enhanced intestinal permeability. This in turn triggers the entry of pathogen-related antibodies like PAMP and DAMP to the circulatory system to subsequently trigger an inflammatory reaction. As a result, investigators believe that the chronic inflammatory status due to gut microbiota imbalance could directly or indirectly give rise to the typical symptoms of frailty (by causing cardiovascular diseases or damaging the musculoskeletal system). In addition, higher levels of inflammatory factors due to gut microbiota may further influence the nervous system of the host via the gut–brain axis to induce neuroinflammation (neurogliocyte activation) leading to neurodegenerative diseases, i.e., dyskinesia and/or cognitive disorders in patients with frailty (as shown in Figure 1).
However, the above assumptions have not been validated in large cohort-based studies. The relationship of gut microbiota imbalance, chronic inflammation, and frailty is not unilateral but complicated and interrelated. Several studies have suggested that chronic inflammation due to gut microbiota imbalance may not be the only cause of frailty. It is worth noting that individuals with frailty are on long-term medication due to preexisting chronic diseases (complications). It has been demonstrated that medications could alter gut microbiome composition. Hence, future studies are necessary to determine whether gut microbiota is a cause of frailty or a result of long-term medication in people with frailty. In addition, factors that may affect the gut microbiota, such as lifestyle, diet, and other health complications, need to be considered comprehensively. Lastly, studies on the pathogenesis of frailty should emphasize the prevention and treatment of frailty to improve the health and ease the medical burden of the elderly.
Acknowledgments
Not applicable
Abbreviations
- MSDs
musculoskeletal system disorders
- FP
frailty phenotype
- FI
frailty index
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- GALTs
gut-associated lymphoid tissues
- BMD
bone mineral density
- PPIs
proton pump inhibitors
- CDI
Clostridium difficile infection
- NSAIDs
non-steroid anti-inflammatory drugs
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- MAMP
microorganism-associated molecular pattern
- TNF-α
tumor necrosis factor-α
- TWCC
total white blood cell counts
- SD
standard deviation
- IGF-1
insulin-like growth factor-1
- ENS
enteric nervous system
- PIGD
postural instability and gait disturbance
- BDNF
brain-derived neurotrophic factor
Footnotes
Funding information: This study was supported by the Health Commission of ZheJiang Province (No. 2020KY393).
Conflict of interest: The author(s) declare(s) that there is no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. 10.1093/gerona/56.3.m146. [DOI] [PubMed]; Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- [2].Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–7. 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed]; Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S. et al. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed]; Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- [4].Rohrmann S. Epidemiology of frailty in older people. NV, editor. Springer; 2020. [DOI] [PubMed]; Rohrmann S. Epidemiology of frailty in older people. Springer; 2020. . NV, editor. [DOI] [PubMed] [Google Scholar]
- [5].Di Sabatino A, Lenti MV, Cammalleri L, Corazza GR, Pilotto A. Frailty and the gut. Dig Liver Dis. 2018;50(6):533–41. 10.1016/j.dld.2018.03.010. [DOI] [PubMed]; Di Sabatino A, Lenti MV, Cammalleri L, Corazza GR, Pilotto A. Frailty and the gut. Dig Liver Dis. 2018;50(6):533–41. doi: 10.1016/j.dld.2018.03.010. [DOI] [PubMed] [Google Scholar]
- [6].Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020;1216:55–64. 10.1007/978-3-030-33330-0_7. [DOI] [PubMed]; Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020;1216:55–64. doi: 10.1007/978-3-030-33330-0_7. [DOI] [PubMed] [Google Scholar]
- [7].Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, et al. The human microbiome project: a community resource for the healthy human microbiome. Plos Biol. 2012;10(8):e1001377. [DOI] [PMC free article] [PubMed]; Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW. et al. The human microbiome project: a community resource for the healthy human microbiome. Plos Biol. 2012;10(8):e1001377. doi: 10.1371/journal.pbio.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70. 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed]; Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sommer F, Bäckhed F. The gut microbiota – masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. 10.1038/nrmicro2974. [DOI] [PubMed]; Sommer F, Bäckhed F. The gut microbiota – masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- [10].Luo J, Jin F. Recent advances in understanding the impact of intestinal microbiota on host behavior. Chin Sci Bull. 2014;59(22):2169.; Luo J, Jin F. Recent advances in understanding the impact of intestinal microbiota on host behavior. Chin Sci Bull. 2014;59(22):2169. [Google Scholar]
- [11].Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100(18):10452–9. 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed]; Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100(18):10452–9. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Methé B, Nelson K, Pop M, Creasy H, Giglio M, Huttenhower C, et al. A framework for human microbiome research. Nature. 2012;486(7402):215–21. 10.1038/nature11209. [DOI] [PMC free article] [PubMed]; Methé B, Nelson K, Pop M, Creasy H, Giglio M, Huttenhower C. et al. A framework for human microbiome research. Nature. 2012;486(7402):215–21. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lynch DB, Jeffery IB, Cusack S, O’Connor EM, O’Toole PW. Diet-microbiota-health interactions in older subjects: implications for healthy aging. Interdiscip Top Gerontol. 2015;40:141–54. 10.1159/000364976. [DOI] [PubMed]; Lynch DB, Jeffery IB, Cusack S, O’Connor EM, O’Toole PW. Diet-microbiota-health interactions in older subjects: implications for healthy aging. Interdiscip Top Gerontol. 2015;40:141–54. doi: 10.1159/000364976. [DOI] [PubMed] [Google Scholar]
- [14].Smith PM, Garrett WS. The gut microbiota and mucosal T cells. Front Microbiol. 2011;26(2):111. [DOI] [PMC free article] [PubMed]; Smith PM, Garrett WS. The gut microbiota and mucosal T cells. Front Microbiol. 2011;26(2):111. doi: 10.3389/fmicb.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–5. 10.1126/science.aac8469. [DOI] [PubMed]; O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–5. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- [16].Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G327–33. 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed]; Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO. et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G327–33. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adriansjach J, Baum ST, Lefkowitz EJ, Van Der Pol WJ, Buford TW, Colman RJ. Age-related differences in the gut microbiome of rhesus macaques. J Gerontol A Biol Sci Med Sci. 2020;75(7):1293–8. 10.1093/gerona/glaa048. [DOI] [PMC free article] [PubMed]; Adriansjach J, Baum ST, Lefkowitz EJ, Van Der Pol WJ, Buford TW, Colman RJ. Age-related differences in the gut microbiome of rhesus macaques. J Gerontol A Biol Sci Med Sci. 2020;75(7):1293–8. doi: 10.1093/gerona/glaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bi Y, Yang R. Human gut microbiota, nutrition and health. Chin Sci Bull. 2019;64(3):260–71.; Bi Y, Yang R. Human gut microbiota, nutrition and health. Chin Sci Bull. 2019;64(3):260–71. [Google Scholar]
- [19].Shanahan F, van Sinderen D, O’Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66(9):1709–17. 10.1136/gutjnl-2017-313872. [DOI] [PubMed]; Shanahan F, van Sinderen D, O’Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66(9):1709–17. doi: 10.1136/gutjnl-2017-313872. [DOI] [PubMed] [Google Scholar]
- [20].Tran TTT, Cousin FJ, Lynch DB, Menon R, Brulc J, Brown JR, et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7(1):39. 10.1186/s40168-019-0654-1. [DOI] [PMC free article] [PubMed]; Tran TTT, Cousin FJ, Lynch DB, Menon R, Brulc J, Brown JR. et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7(1):39. doi: 10.1186/s40168-019-0654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67(1):40–51. 10.1099/jmm.0.000640. [DOI] [PMC free article] [PubMed]; Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67(1):40–51. doi: 10.1099/jmm.0.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, et al. Age-related inflammation: the contribution of different organs, tissues and systems. how to face it for therapeutic approaches. Curr Pharm Des. 2010;16(6):609–18. 10.2174/138161210790883840. [DOI] [PubMed]; Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G. et al. Age-related inflammation: the contribution of different organs, tissues and systems. how to face it for therapeutic approaches. Curr Pharm Des. 2010;16(6):609–18. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- [23].Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–20. 10.1159/000490615. [DOI] [PMC free article] [PubMed]; Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–20. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Palmer S, Albergante L, Blackburn CC, Newman TJ. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci USA. 2018;115(8):1883–8. 10.1073/pnas.1714478115. [DOI] [PMC free article] [PubMed]; Palmer S, Albergante L, Blackburn CC, Newman TJ. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci USA. 2018;115(8):1883–8. doi: 10.1073/pnas.1714478115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19(1):26–30. 10.1097/mco.0000000000000242. [DOI] [PubMed]; Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19(1):26–30. doi: 10.1097/mco.0000000000000242. [DOI] [PubMed] [Google Scholar]
- [26].Chapelet G, Boureau AS, Montassier E, Le Bastard Q, Batard E, Lepelletier D, et al. Cancer and microbiota in elderly patients: challenges and management. Geriatr Psychol Neuropsychiatr Vieil. 2019;17(1):20–30. 10.1684/pnv.2019.0783. [DOI] [PubMed]; Chapelet G, Boureau AS, Montassier E, Le Bastard Q, Batard E, Lepelletier D. et al. Cancer and microbiota in elderly patients: challenges and management. Geriatr Psychol Neuropsychiatr Vieil. 2019;17(1):20–30. doi: 10.1684/pnv.2019.0783. [DOI] [PubMed] [Google Scholar]
- [27].Mello AM, Paroni G, Daragjati J, Pilotto A. Gastrointestinal microbiota and their contribution to healthy aging. Dig Dis. 2016;34(3):194–201. 10.1159/000443350. [DOI] [PubMed]; Mello AM, Paroni G, Daragjati J, Pilotto A. Gastrointestinal microbiota and their contribution to healthy aging. Dig Dis. 2016;34(3):194–201. doi: 10.1159/000443350. [DOI] [PubMed] [Google Scholar]
- [28].Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8(2):82–97. 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed]; Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8(2):82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut. 2018;67(12):2213–22. 10.1136/gutjnl-2017-315542. [DOI] [PubMed]; An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D. Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut. 2018;67(12):2213–22. doi: 10.1136/gutjnl-2017-315542. [DOI] [PubMed] [Google Scholar]
- [30].Maffei VJ, Kim S, Blanchard ET, Luo M, Jazwinski SM, Taylor CM, et al. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci. 2017;72(11):1474–82. 10.1093/gerona/glx042. [DOI] [PMC free article] [PubMed]; Maffei VJ, Kim S, Blanchard ET, Luo M, Jazwinski SM, Taylor CM. et al. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci. 2017;72(11):1474–82. doi: 10.1093/gerona/glx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang L, Liao J, Chen Q, Chen M, Kuang Y, Chen L, et al. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res. 2020;32:2001–11. 10.1007/s40520-019-01385-2. [DOI] [PubMed]; Zhang L, Liao J, Chen Q, Chen M, Kuang Y, Chen L. et al. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res. 2020;32:2001–11. doi: 10.1007/s40520-019-01385-2. [DOI] [PubMed] [Google Scholar]
- [32].van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71(10):6438–42. 10.1128/aem.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed]; van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71(10):6438–42. doi: 10.1128/aem.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pallister T, Jackson MA, Martin TC, Glastonbury CA, Jennings A, Beaumont M, et al. Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. Int J Obes (Lond). 2017;41(7):1106–13. 10.1038/ijo.2017.70. [DOI] [PMC free article] [PubMed]; Pallister T, Jackson MA, Martin TC, Glastonbury CA, Jennings A, Beaumont M. et al. Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. Int J Obes (Lond) 2017;41(7):1106–13. doi: 10.1038/ijo.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gardiner BJ, Tai AY, Kotsanas D, Francis MJ, Roberts SA, Ballard SA, et al. Clinical and microbiological characteristics of Eggerthella lenta bacteremia. J Clin Microbiol. 2015;53(2):626–35. 10.1128/jcm.02926-14. [DOI] [PMC free article] [PubMed]; Gardiner BJ, Tai AY, Kotsanas D, Francis MJ, Roberts SA, Ballard SA. et al. Clinical and microbiological characteristics of Eggerthella lenta bacteremia. J Clin Microbiol. 2015;53(2):626–35. doi: 10.1128/jcm.02926-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed]; Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE. et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016;17(6):932. 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed]; Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M. et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016;17(6):932. doi: 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mannucci PM, Nobili A. Multimorbidity and polypharmacy in the elderly: lessons from REPOSI. Intern Emerg Med. 2014;9(7):723–34. 10.1007/s11739-014-1124-1. [DOI] [PubMed]; Mannucci PM, Nobili A. Multimorbidity and polypharmacy in the elderly: lessons from REPOSI. Intern Emerg Med. 2014;9(7):723–34. doi: 10.1007/s11739-014-1124-1. [DOI] [PubMed] [Google Scholar]
- [38].Pasina L, Nobili A, Tettamanti M, Salerno F, Corrao S, Marengoni A, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med. 2011;22(2):205–10. 10.1016/j.ejim.2010.11.009. [DOI] [PubMed]; Pasina L, Nobili A, Tettamanti M, Salerno F, Corrao S, Marengoni A. et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med. 2011;22(2):205–10. doi: 10.1016/j.ejim.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [39].Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7(1):11102. 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed]; Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA. et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7(1):11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56. 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed]; Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC. et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. 10.1056/NEJMra1403772. [DOI] [PubMed]; Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- [42].Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103(2):227–34. 10.1017/s0007114509991553. [DOI] [PubMed]; Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103(2):227–34. doi: 10.1017/s0007114509991553. [DOI] [PubMed] [Google Scholar]
- [43].Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. 10.1038/nature11319. [DOI] [PubMed]; Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- [44].García-Peña C, Álvarez-Cisneros T, Quiroz-Baez R, Friedland RP. Microbiota and aging. a review and commentary. Arch Med Res. 2017;48(8):681–9. 10.1016/j.arcmed.2017.11.005. [DOI] [PubMed]; García-Peña C, Álvarez-Cisneros T, Quiroz-Baez R, Friedland RP. Microbiota and aging. a review and commentary. Arch Med Res. 2017;48(8):681–9. doi: 10.1016/j.arcmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- [45].Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed]; Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- [46].Rehman T. Role of the gut microbiota in age-related chronic inflammation. Endocr Metab Immun Disord Drug Targets. 2012;12(4):361–7. 10.2174/187153012803832620. [DOI] [PubMed]; Rehman T. Role of the gut microbiota in age-related chronic inflammation. Endocr Metab Immun Disord Drug Targets. 2012;12(4):361–7. doi: 10.2174/187153012803832620. [DOI] [PubMed] [Google Scholar]
- [47].Gemikonakli G, Mach J, Hilmer SN. Interactions between the aging gut microbiome and common geriatric giants: polypharmacy, frailty and dementia. J Gerontol A Biol Sci Med Sci. 2021;76(6):1019–28. 10.1093/gerona/glaa047. [DOI] [PubMed]; Gemikonakli G, Mach J, Hilmer SN. Interactions between the aging gut microbiome and common geriatric giants: polypharmacy, frailty and dementia. J Gerontol A Biol Sci Med Sci. 2021;76(6):1019–28. doi: 10.1093/gerona/glaa047. [DOI] [PubMed] [Google Scholar]
- [48].Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41:400–9. [DOI] [PubMed]; Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41:400–9. doi: 10.1016/j.clinthera.2019.02.001. [DOI] [PubMed] [Google Scholar]
- [49].Cornish SM, Chilibeck PD, Candow DG. Potential importance of immune system response to exercise on aging muscle and bone. Curr Osteoporos Rep. 2020;18(4):350–6. 10.1007/s11914-020-00596-1. [DOI] [PubMed]; Cornish SM, Chilibeck PD, Candow DG. Potential importance of immune system response to exercise on aging muscle and bone. Curr Osteoporos Rep. 2020;18(4):350–6. doi: 10.1007/s11914-020-00596-1. [DOI] [PubMed] [Google Scholar]
- [50].Stavropoulou E, Bezirtzoglou E. Human microbiota in aging and infection: a review. Crit Rev Food Sci Nutr. 2017;59(4):537–45. 10.1080/10408398.2017.1379469. [DOI] [PubMed]; Stavropoulou E, Bezirtzoglou E. Human microbiota in aging and infection: a review. Crit Rev Food Sci Nutr. 2017;59(4):537–45. doi: 10.1080/10408398.2017.1379469. [DOI] [PubMed] [Google Scholar]
- [51].Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633. 10.3390/nu11071633. [DOI] [PMC free article] [PubMed]; Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C. et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633. doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Casati M, Ferri E, Azzolino D, Cesari M, Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124:110639. 10.1016/j.exger.2019.110639. [DOI] [PubMed]; Casati M, Ferri E, Azzolino D, Cesari M, Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124:110639. doi: 10.1016/j.exger.2019.110639. [DOI] [PubMed] [Google Scholar]
- [53].Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed]; Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J. et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol. 2014;30:54–62. 10.1016/j.coi.2014.07.003. [DOI] [PubMed]; Shapiro H, Thaiss CA, Levy M, Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr Opin Immunol. 2014;30:54–62. doi: 10.1016/j.coi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- [55].Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M, et al. The microbial metabolites,short chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed]; Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M. et al. The microbial metabolites,short chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamamoto Y, Takahahi T, To M, Nakagawa Y, Hayashi T, Shimizu T, et al. The salivary IgA flow rate is increased by high concentrations of short -chain fatty acids in the cecum of rats ingesting fructooligosaccharides. Nutrients. 2016;8(8):1–13. [DOI] [PMC free article] [PubMed]; Yamamoto Y, Takahahi T, To M, Nakagawa Y, Hayashi T, Shimizu T. et al. The salivary IgA flow rate is increased by high concentrations of short -chain fatty acids in the cecum of rats ingesting fructooligosaccharides. Nutrients. 2016;8(8):1–13. doi: 10.3390/nu8080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, et al. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY). 2013;5(12):902–12. 10.18632/aging.100623. [DOI] [PMC free article] [PubMed]; Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C. et al. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 2013;5(12):902–12. doi: 10.18632/aging.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed]; Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E. et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63(4):776–81. 10.1111/jgs.13310. [DOI] [PMC free article] [PubMed]; Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63(4):776–81. doi: 10.1111/jgs.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–66.e4. 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed]; Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–66.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41(3):400–9. 10.1016/j.clinthera.2019.02.001. [DOI] [PubMed]; Chen Y, Liu S, Leng SX. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019;41(3):400–9. doi: 10.1016/j.clinthera.2019.02.001. [DOI] [PubMed] [Google Scholar]
- [62].Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34(2):275–80. 10.1177/0363546505279912. [DOI] [PubMed]; Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34(2):275–80. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- [63].Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–71. 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed]; Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50(7):1268–71. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- [64].Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85 + Study. Mech Ageing Dev. 2012;133(6):456–66. 10.1016/j.mad.2012.05.005. [DOI] [PubMed]; Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C. et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85 + Study. Mech Ageing Dev. 2012;133(6):456–66. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- [65].Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–41. 10.1001/archinte.162.20.2333. [DOI] [PubMed]; Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH. et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- [66].Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40(4):475–81. 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed]; Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A. et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40(4):475–81. doi: 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–41. 10.2147/cia.s45300. [DOI] [PMC free article] [PubMed]; Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–41. doi: 10.2147/cia.s45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the women’s health and aging studies I. Exp Gerontol. 2009;44(8):511–6. 10.1016/j.exger.2009.05.005. [DOI] [PubMed]; Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the women’s health and aging studies I. Exp Gerontol. 2009;44(8):511–6. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- [69].Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005;40(1–2):81–7. 10.1016/j.exger.2004.09.006. [DOI] [PubMed]; Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005;40(1–2):81–7. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [70].De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56(5):904–8. 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed]; De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56(5):904–8. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391(6665):344–5. 10.1038/34814. [DOI] [PubMed]; Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C. et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391(6665):344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- [72].Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–6. 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed]; Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA. et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130(3):161–6. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed]; Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- [74].Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. 10.1093/gerona/57.5.m326. [DOI] [PubMed]; Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB. et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- [75].Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9b):3103–9. 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed]; Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9b):3103–9. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. 10.1007/bf03324545. [DOI] [PubMed]; Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M. et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–7. doi: 10.1007/bf03324545. [DOI] [PubMed] [Google Scholar]
- [77].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. 10.1056/nejm200003233421202. [DOI] [PubMed]; Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/nejm200003233421202. [DOI] [PubMed] [Google Scholar]
- [78].Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, et al. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. 2016;133(23):2263–78. 10.1161/circulationaha.116.021805. [DOI] [PMC free article] [PubMed]; Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P. et al. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. 2016;133(23):2263–78. doi: 10.1161/circulationaha.116.021805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lazarus DD, Moldawer LL, Lowry SF. Insulin-like growth factor-1 activity is inhibited by interleukin-1 alpha, tumor necrosis factor-alpha, and interleukin-6. Lymphokine Cytokine Res. 1993;12(4):219–23. [PubMed]; Lazarus DD, Moldawer LL, Lowry SF. Insulin-like growth factor-1 activity is inhibited by interleukin-1 alpha, tumor necrosis factor-alpha, and interleukin-6. Lymphokine Cytokine Res. 1993;12(4):219–23. [PubMed] [Google Scholar]
- [80].Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafè M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–7. 10.1152/ajpendo.00319.2002. [DOI] [PubMed]; Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafè M. et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–7. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- [81].Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–25. 10.1210/jc.2002-021694. [DOI] [PubMed]; Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- [82].Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–71. 10.2337/db10-0415. [DOI] [PMC free article] [PubMed]; Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS. et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–71. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Arai Y, Takayama M, Gondo Y, Inagaki H, Yamamura K, Nakazawa S, et al. Adipose endocrine function, insulin-like growth factor-1 axis, and exceptional survival beyond 100 years of age. J Gerontol A Biol Sci Med Sci. 2008;63(11):1209–18. 10.1093/gerona/63.11.1209. [DOI] [PubMed]; Arai Y, Takayama M, Gondo Y, Inagaki H, Yamamura K, Nakazawa S. et al. Adipose endocrine function, insulin-like growth factor-1 axis, and exceptional survival beyond 100 years of age. J Gerontol A Biol Sci Med Sci. 2008;63(11):1209–18. doi: 10.1093/gerona/63.11.1209. [DOI] [PubMed] [Google Scholar]
- [84].Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB, et al. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med. 2009;122(10):947–54. 10.1016/j.amjmed.2009.04.016. [DOI] [PMC free article] [PubMed]; Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB. et al. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med. 2009;122(10):947–54. doi: 10.1016/j.amjmed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Newman AB, Kooperberg CL, et al. Statin use and incident frailty in women aged 65 years or older: prospective findings from the women’s health initiative observational study. J Gerontol A Biol Sci Med Sci. 2008;63(4):369–75. 10.1093/gerona/63.4.369. [DOI] [PubMed]; LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Newman AB, Kooperberg CL. et al. Statin use and incident frailty in women aged 65 years or older: prospective findings from the women’s health initiative observational study. J Gerontol A Biol Sci Med Sci. 2008;63(4):369–75. doi: 10.1093/gerona/63.4.369. [DOI] [PubMed] [Google Scholar]
- [86].Ni Lochlainn M, Bowyer RCE, Steves CJ. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10(7):929. 10.3390/nu10070929. [DOI] [PMC free article] [PubMed]; Ni Lochlainn M, Bowyer RCE, Steves CJ. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10(7):929. doi: 10.3390/nu10070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ticinesi A, Tana C, Nouvenne A. The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care. 2019;22(1):4–12. 10.1097/mco.0000000000000521. [DOI] [PubMed]; Ticinesi A, Tana C, Nouvenne A. The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care. 2019;22(1):4–12. doi: 10.1097/mco.0000000000000521. [DOI] [PubMed] [Google Scholar]
- [88].Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–511. 10.2147/cia.s139163. [DOI] [PMC free article] [PubMed]; Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–511. doi: 10.2147/cia.s139163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cell Mol Life Sci. 2018;75(1):119–28. 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed]; O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cell Mol Life Sci. 2018;75(1):119–28. doi: 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Baptista LC, Sun Y, Carter CS, Buford TW. Crosstalk between the gut microbiome and bioactive lipids: therapeutic targets in cognitive frailty. Front Nutr. 2020;7:17. 10.3389/fnut.2020.00017. [DOI] [PMC free article] [PubMed]; Baptista LC, Sun Y, Carter CS, Buford TW. Crosstalk between the gut microbiome and bioactive lipids: therapeutic targets in cognitive frailty. Front Nutr. 2020;7:17. doi: 10.3389/fnut.2020.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Scassellati C, Ciani M, Galoforo AC, Zanardini R, Bonvicini C, Geroldi C. Molecular mechanisms in cognitive frailty: potential therapeutic targets for oxygen-ozone treatment. Mech Ageing Dev. 2020;186:111210. 10.1016/j.mad.2020.111210. [DOI] [PubMed]; Scassellati C, Ciani M, Galoforo AC, Zanardini R, Bonvicini C, Geroldi C. Molecular mechanisms in cognitive frailty: potential therapeutic targets for oxygen-ozone treatment. Mech Ageing Dev. 2020;186:111210. doi: 10.1016/j.mad.2020.111210. [DOI] [PubMed] [Google Scholar]
- [92].Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583(7816):441–6. 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed]; Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A. et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583(7816):441–6. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD, et al. An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front Aging Neurosci. 2018;10:398. 10.3389/fnagi.2018.00398. [DOI] [PMC free article] [PubMed]; Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD. et al. An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front Aging Neurosci. 2018;10:398. doi: 10.3389/fnagi.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75(1):149–60. 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed]; Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75(1):149–60. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):e76993. 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed]; De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI. et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed]; Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB. et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed]; Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. 10.1136/jnnp.2007.131045. [DOI] [PubMed]; Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- [99].Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37):10609–20. 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed]; Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37):10609–20. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–8. 10.1016/j.nbd.2012.09.007. [DOI] [PubMed]; Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H. et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–8. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- [101].Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9. 10.1002/ana.24448. [DOI] [PubMed]; Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P. et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- [102].Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM. Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Parkinsonism Relat Disord. 2016;27:1–8. 10.1016/j.parkreldis.2016.03.012. [DOI] [PubMed]; Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM. Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Parkinsonism Relat Disord. 2016;27:1–8. doi: 10.1016/j.parkreldis.2016.03.012. [DOI] [PubMed] [Google Scholar]
- [103].Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed]; Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL. et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260(5):1332–8. 10.1007/s00415-012-6801-2. [DOI] [PubMed]; Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C. et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260(5):1332–8. doi: 10.1007/s00415-012-6801-2. [DOI] [PubMed] [Google Scholar]
- [105].Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167(6):1469–80.e12. 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed]; Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE. et al. Gut Microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167(6):1469–80.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–8. 10.1002/mds.26069. [DOI] [PubMed]; Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–8. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- [107].Association As. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–44. 10.1016/j.jalz.2011.02.004. [DOI] [PubMed]; Association As. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–44. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [108].Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17. 10.1136/gut.2009.202515. [DOI] [PubMed]; Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ. et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- [109].Kahn MS, Kranjac D, Alonzo CA, Haase JH, Cedillos RO, McLinden KA, et al. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229(1):176–84. 10.1016/j.bbr.2012.01.010. [DOI] [PubMed]; Kahn MS, Kranjac D, Alonzo CA, Haase JH, Cedillos RO, McLinden KA. et al. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229(1):176–84. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- [110].Distrutti E, O’Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA, et al. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9(9):e106503. 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed]; Distrutti E, O’Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA. et al. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9(9):e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–8. 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed]; Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–8. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- [112].Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, et al. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126(12):4469–81. 10.1172/JCI88625. [DOI] [PMC free article] [PubMed]; Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P. et al. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126(12):4469–81. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Veiga-Fernandes H, Mucida D. Neuro-immune interactions at barrier surfaces. Cell. 2016;165(4):801–11. [DOI] [PMC free article] [PubMed]; Veiga-Fernandes H, Mucida D. Neuro-immune interactions at barrier surfaces. Cell. 2016;165(4):801–11. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF, et al. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity. 2021;54(1):151–63. [DOI] [PMC free article] [PubMed]; Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF. et al. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity. 2021;54(1):151–63. doi: 10.1016/j.immuni.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Serra‐Prat M, Mans E, Palomera E, Clave P. Gastrointestinal peptides, gastrointestinal motility, and anorexia of aging in frail elderly persons. Neurogastroenterol Motil. 2013;25(4):291-e245. [DOI] [PubMed]; Serra‐Prat M, Mans E, Palomera E, Clave P. Gastrointestinal peptides, gastrointestinal motility, and anorexia of aging in frail elderly persons. Neurogastroenterol Motil. 2013;25(4):291-e245. doi: 10.1111/nmo.12055. [DOI] [PubMed] [Google Scholar]