Key Points
Question
Is fractional carbon dioxide laser an effective treatment for vaginal symptoms associated with menopause?
Findings
This sham-controlled, double-blinded, randomized clinical trial included 90 women with postmenopausal vaginal symptoms. After 12 months, treatment with fractional carbon dioxide laser compared with sham treatment resulted in a change in visual analog scale score for overall symptom severity of –17.2 vs –26.6 (range, 0-100; lower scores indicate less symptom severity) and Vulvovaginal Symptom Questionnaire score of –3.1 vs –1.6 (range 0-20; lower scores indicate less symptoms). Neither comparison was statistically significant.
Meaning
Among women with postmenopausal vaginal symptoms, treatment with fractional carbon dioxide laser vs sham treatment did not improve vaginal symptoms after 12 months.
Abstract
Importance
Postmenopausal vaginal symptoms are common and frequently detrimental to a woman’s quality of life. Fractional carbon dioxide vaginal laser is increasingly offered as a treatment, but the efficacy remains unproven.
Objective
To determine the efficacy of fractional carbon dioxide laser for treatment of vaginal symptoms associated with menopause.
Design, Setting, and Participants
A double-blind, randomized, sham-controlled trial with 12-month follow-up was undertaken at a single tertiary referral hospital in Sydney, Australia. Enrollment commenced on September 19, 2016, with final follow-up on June 30, 2020. Participants were postmenopausal women with vaginal symptoms substantive enough to seek medical treatment. Of 232 participants approached, 85 were randomized.
Interventions
Three treatments using a fractional microablative carbon dioxide laser system performed 4 to 8 weeks apart, with 43 women randomized to the laser group and 42 to the sham group.
Main Outcomes and Measures
The co–primary outcomes were symptom severity assessed using a visual analog scale (VAS; range, 0-100; 0 indicates no symptoms and 100 indicates the most severe symptoms) and the Vulvovaginal Symptom Questionnaire (VSQ; range, 0-20; 0 indicates no symptoms and 20 indicates the most severe symptoms) at 12 months. The minimal clinically important difference was specified as a 50% decrease in both VAS and VSQ severity scores. There were 5 prespecified secondary outcomes, including quality of life (range, 0-100; higher scores indicate better quality of life), the Vaginal Health Index Score (range, 5-25; higher scores indicate better health), and vaginal histology (premenopausal or postmenopausal status).
Results
Of 85 randomized participants (mean [SD] age, 57 [8] years), 78 (91.7%) completed the 12-month follow-up. From baseline to 12 months, there was no significant difference between the carbon dioxide laser group and the sham group in change in symptom severity (VAS score for overall vaginal symptoms: –17.2 vs –26.6; difference, 9.4 [95% CI, –28.6 to 47.5]; VAS score for the most severe symptom: –24.5 vs –20.4; difference –4.1 [95% CI, –32.5 to 24.3]; VSQ score: –3.1 vs –1.6; difference, –1.5 [95% CI, –5.9 to 3.0]). There were no significant differences between the laser and sham group in the mean quality of life score (6.3 vs 1.4; difference, 4.8 [95% CI, –3.9 to 13.5]) and Vaginal Health Index Score (0.9 vs 1.3; difference, –0.4 [95% CI, –4.3 to 3.6]) or in histological comparisons between laser and sham treatment groups. There were 16 adverse events in the laser group and 17 in the sham group, including vaginal pain/discomfort (44% vs 68%), spotting, discharge, and lower urinary tract symptoms. No severe adverse events were reported in either group.
Conclusions and Relevance
Among women with postmenopausal vaginal symptoms, treatment with fractional carbon dioxide laser vs sham treatment did not significantly improve vaginal symptoms after 12 months.
Trial Registration
Australian and New Zealand Clinical Trials Registry: ACTRN12616001403426
This randomized clinical trial examines the efficacy of fractional carbon dioxide laser for the management of vaginal symptoms associated with menopause.
Introduction
Vaginal symptoms associated with menopause are common, with an estimated prevalence of 40% to 60% among postmenopausal women.1 Menopause may have been natural or iatrogenic from treatment of hormone-sensitive tumors, such as breast cancer.2 Vaginal symptoms can cause physical discomfort and may negatively affect sexual function, relationships, and quality of life. Current treatments, such as nonhormonal vaginal lubricants and moisturizers and topical or systemic estrogen therapies, are often ineffective, relatively contraindicated in women with breast cancer, or declined by women avoiding exposure to exogenous hormones.1,2
Fractional laser treatments are used as a nonhormonal alternative for postmenopausal vaginal symptoms. Preliminary data showed histological changes consistent with improvement in vaginal atrophy after treatment,3 with observational studies suggesting a 75% to 100% reduction in symptoms for women treated with this technology.4,5
Medical devices are often adopted into clinical practice with a lower level of evidence than pharmaceutical products, with rapid dissemination before stringent evaluation.6 In 2018, the US Food and Drug Administration published a warning regarding the efficacy and safety profile of laser and thermal vaginal treatments following widespread global uptake in use of these treatments.7 Since that statement, 3 randomized studies that compared vaginal laser treatment with vaginal estrogen reported no significant differences between these treatments, although none used a sham control.8,9,10 This double-blind, randomized, sham-controlled trial was conducted to evaluate fractional laser treatment for postmenopausal vaginal symptoms.
Methods
Study Design
This study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (HREC/15/POWH/585). The study protocol and statistical analysis plan are available in Supplement 1. Participants were recruited from primary care physicians, gynecologists, and social media support groups for breast cancer and/or menopause. Eligible women were assessed and treated at a university teaching hospital in Sydney, Australia, after providing written informed consent.
Individuals eligible for inclusion were women 18 years and older who were fluent in English and had not previously received vaginal energy-based treatment for menopausal symptoms. Participants must have been amenorrheic for at least 12 months, either naturally or iatrogenically, and experiencing 1 or more of the following vaginal symptoms: dyspareunia, burning, itching, or dryness severe enough to prompt presentation to seek further treatment. Medical treatments (personal lubricants, vaginal moisturizers, or estrogen) may have been previously used and considered ineffective, offered but not used, or contraindicated. Participants were required to discontinue vaginal estrogen for 6 months before inclusion in the trial.
Exclusion criteria included pelvic organ prolapse protruding more than 1 cm beyond the hymen (greater than stage 2), active genital or urinary tract infections, previous vaginal mesh surgery, or ongoing chronic medical conditions that may have interfered with study completion.
Randomization
Participants were randomized prior to the first treatment, using a third-party telephone service, in a 1:1 ratio in balanced blocks of 8, following a computer-generated randomization sequence.
Procedure
Specialized equipment required for the procedure was purchased from the manufacturer’s local distributor and fitted to a preexisting fractional microablative carbon dioxide laser system (SmartXide2 V2LR, MonaLisa Touch, DEKA Laser). Treating physicians were all independently laser certified and underwent standard procedural training.
Women underwent 3 treatments 1 month apart (minimum, 4 weeks; maximum, 8 weeks) as per recommended practice and allowing for participant flexibility. The laser treatment was performed at standard settings (40 W power, 1000 μs dwell time, 1000 μm DOT spacing, SmartStack 2 on DP emission mode), delivering fluence of 5.37 J/cm2. The sham treatment was performed at minimal energy settings with no tissue effect (0.5 W power, 100 μs dwell time, 2000 μm DOT spacing, SmartStack 1 on SmartPulse emission mode), delivering no energy (fluence, 0 J/cm2). Visual and auditory effects from the laser were identical between the groups.
Lignocaine, 4%, topical anesthetic cream was applied to the vaginal introitus for 20 minutes to reduce tactile differences or pain perception. The same procedural maneuvers were performed in both groups. A plume evacuator (ULT JUMBO Filtertrolley 2.0, ULT AG) operated to remove visual and olfactory effects from smoke plume.
Data Collection
Data were collected electronically and stored on an approved secure online database (REDCap, Vanderbilt University). Demographic data were collected at enrollment. Symptom data were collected at enrollment, each treatment visit, and 6 and 12 months after initial treatment. Cytology and biopsy were collected at enrollment and at 6 months after initial treatment.
Outcomes
To maintain blinding, separate clinicians performed either the assessment or the treatment, with clinicians continuing those roles for the duration of the study for that participant. The treating clinician accessed the participants’ group assignment prior to each treatment, entered energy parameters, performed the randomized treatment, and had no other participant interaction, with all other study personnel blinded.
Change in symptom severity at 12 months was assessed by the co–primary outcomes. Severity of overall vaginal symptoms and individual symptoms of dyspareunia, dysuria, vaginal dryness, burning, and itching were assessed on a visual analog scale (VAS) ranging from 0 to 100, in which 0 indicated no symptoms and 100 indicated the greatest possible symptom severity. Overall VAS symptom score and the highest scoring symptom at baseline for a participant (denoted as the most severe symptom) were compared throughout the study. The Vulvovaginal Symptom Questionnaire (VSQ) is a 21-item validated instrument to measure vulvovaginal symptoms in postmenopausal women. This questionnaire assesses 4 domains (symptoms, emotions, life-impact, and sexual-impact) with scores ranging from of 0 to 20, in which 0 indicates no symptoms and 20 indicates severe symptoms.11 The minimal clinically important difference in symptom severity was determined to be a 50% reduction in severity for both the VAS and VSQ.12 Both outcomes had to be statistically significant to declare the study results to be “positive.”
Validated instruments were used to assess quality of life (Assessment of Quality of Life–6D scale13; score range, 0-100; 0 represents the worst quality of life and 100 represents the best) and sexual satisfaction (Monash University Women’s Health Program Female Sexuality Satisfaction Questionnaire; score range, 5-54; 5 represents the lowest sexual satisfaction and 54 represents the highest).14 Additional secondary outcomes, including treatment discomfort, acceptability, and satisfaction, as well as vaginal lubricant use, were collected but are not reported in this article.
The physician-reported outcome of Vaginal Health Index Score (VHI; range, 5-25; score <15 considered vaginal atrophy)15 was collected at each visit using the following 5 parameters: vaginal elasticity, vaginal fluid amount, pH, epithelial integrity, and hydration.
Vaginal cytological samples assessed the vaginal maturation index (the ratio of parabasal to intermediate to superficial cells), in which a higher score indicates superior estrogenization.16 However, within the first 10 participants enrolled, it was determined that these samples were unreliable, with poor cellular content or preservation.8 An amendment approved by the ethics committee was enacted with specific consent for a full-thickness vaginal skin biopsy, taken 3 cm above the hymenal ring on the right at baseline and on the left 6 months after the first treatment, using Tischler biopsy forceps. A minimum of 3 weeks between vaginal biopsy and first trial treatment was required to ensure skin healing.
Histological specimens were assessed by specialist gynecological pathologists blinded to randomization group and sample sequence (before or after treatment). Samples were categorized as premenopausal or postmenopausal samples based on the following criteria: changes to collagen (reduced type I to type III fibril ratio, loss of trabecular disposition, absent fibrillogenesis), lamina propria (absence of activated fibroblasts, loss of mucopolysaccharides), epithelium (thinning of superficial layer; superficial keratinization; loss of rugae, elastic fibers, and subepithelial papillae; increase in subepithelial connective tissue), and decreased vascularization.3,17,18
Sample Size Calculation
At trial registration, prospective observational studies reported 90% reduction in symptoms after treatment.19,20 The clinical trial steering committee considered a smaller effect would be sufficient to judge the intervention as clinically effective and sample size calculation was based on a more moderate 50% reduction of symptom severity at 12 months. An estimated placebo response of 30% improvement in symptom severity was made based on previous sham-controlled procedural studies.21 The calculated sample size based on these parameters was 48 individuals. Following independent scientific and statistical review by the ethics committee, the sample size was recommended to be increased to 78 to reduce the probability of a type II error, given the positive outcomes from previous observational studies. An allowance of 10% was made to account for dropout and loss to follow-up.
Statistical Analysis
Mixed-effects model analyses were used for outcomes gathered from repeated measures of symptom change throughout the study, including intergroup and intragroup comparison. Study participants were considered as random effects, with treatment group and visit number as fixed effects. The estimated difference in mean change from baseline and the corresponding 95% CIs were calculated using mixed analysis of variance. Nonparametric continuous variables were assessed using Wilcoxon signed-rank tests or Mann-Whitney U tests and parametric variables were assessed with t tests as appropriate. Analyses of multiple time points were adjusted using the Holm-Bonferroni method and both unadjusted and adjusted P values are reported. Statistical significance was defined as P < .05. Categorical data were analyzed using the Pearson χ2 test (or Fisher exact test if expected values were <5 in any cell of the contingency table).
Missing data were assessed prior to analysis and, if determined to be missing at random, managed by pairwise deletion and mixed model analyses.22 Subgroup analyses were decided a priori (natural vs iatrogenic menopause) and performed using mixed-effects model analyses, as above. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All analyses were performed using SPSS software, version 26.0 (IBM Corp) and independently verified, without awareness of the initial results, using SAS software, version 9.4 (SAS Institute, Inc).
Results
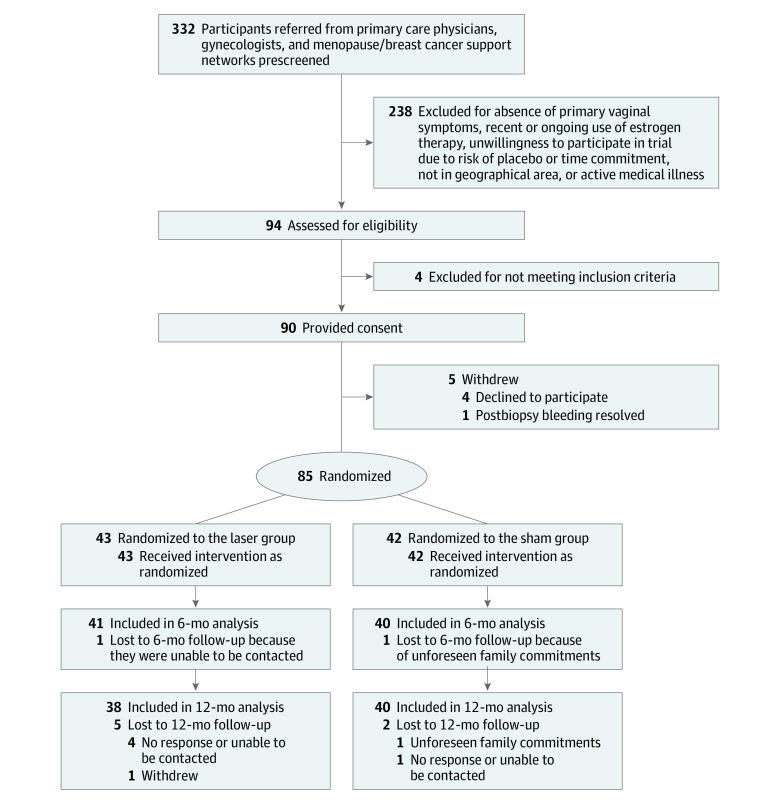
Of 232 women initially contacted, 90 participants were recruited and provided consent and 85 participants were treated between September 2016 and June 2019 (Figure 1). Patient demographics are presented in the Table, with no statistically significant differences between the laser (n = 43) and sham (n = 42) groups. No participant discontinued treatment after randomization, and 12-month follow-up was completed by 38 participants (88.4%) in the laser group and 40 participants (95.2%) in the sham group. For the primary outcomes, missing data at any time point occurred in 2.8% of measurements.
Figure 1. Flow of Participants in a Study of the Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Symptom Severity in Women With Postmenopausal Vaginal Symptoms.
Table. Population Characteristics in a Study of the Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Women With Postmenopausal Vaginal Symptoms.
| Characteristic | No. (%) | |
|---|---|---|
| Laser group (n = 43) | Sham group (n = 42) | |
| Age, mean (SD), y | 55 (7) | 58 (8) |
| Race and ethnicity | ||
| White | 42 (98) | 40 (95) |
| Othera | 1 (2) | 2 (5) |
| Tertiary education | 35 (81) | 32 (76) |
| Age at menarche, mean (SD), y | 13 (2) | 13 (2) |
| Age at menopause, mean (SD), y | 48 (5) | 49 (6) |
| Time after menopause, median (IQR), y | 8 (4-14) | 6 (3-9) |
| Iatrogenic menopause | 20 (47) | 21 (50) |
| Parity | (n = 40) | (n = 37) |
| 0 | 4 (10) | 6 (16) |
| 1 | 10 (25) | 7 (19) |
| 2 | 17 (43) | 16 (43) |
| ≥3 | 9 (22) | 8 (22) |
| Sexually active | 23 (54) | 21 (50) |
| Regular alcohol consumptionb | 33 (77) | 26 (62) |
| Current nonsmoker | 41 (95) | 42 (100) |
| Regular exercise | 33 (77) | 34 (81) |
| Other medical comorbiditiesc | 17 (40) | 19 (45) |
| Previous hysterectomy | 2 (5) | 4 (10) |
Two participants were Asian (1 in the laser group and 1 in the sham group) and 1 participant (in the sham group) was Aboriginal Australian.
Reported routine consumption of at least 1 unit of alcohol per week.
Current chronic condition, excluding history of breast cancer.
Primary Outcomes
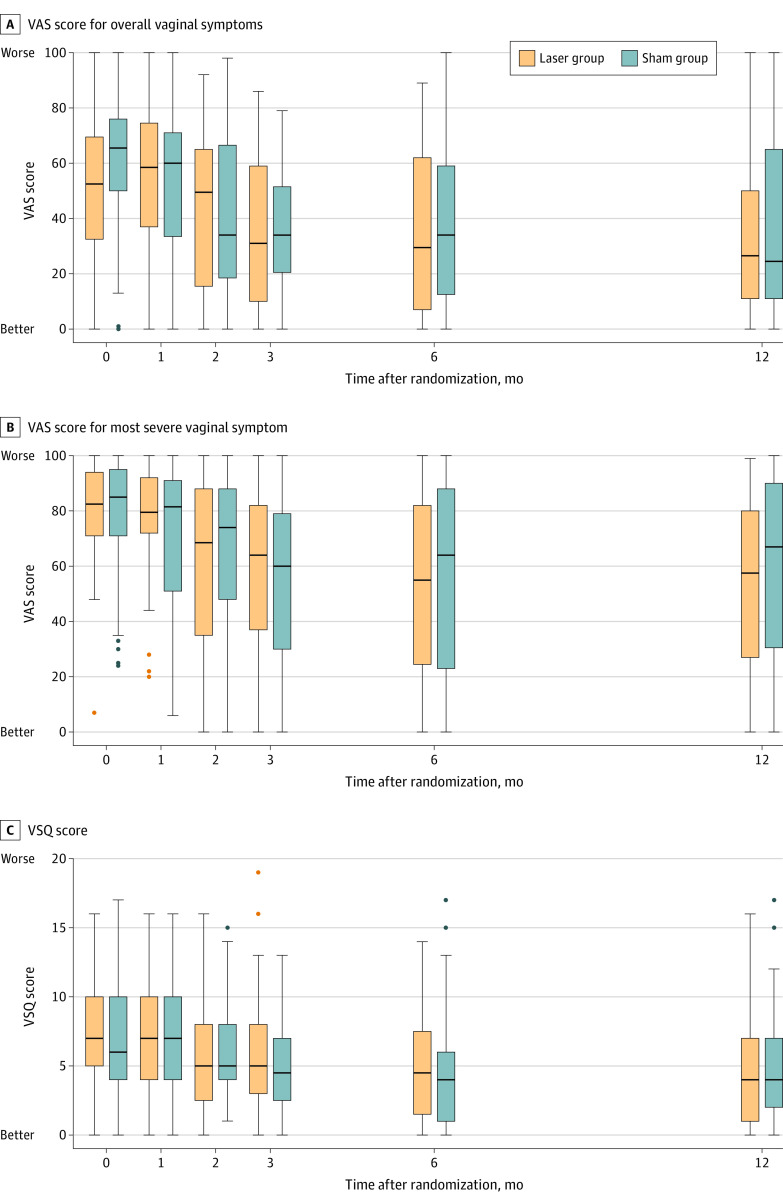
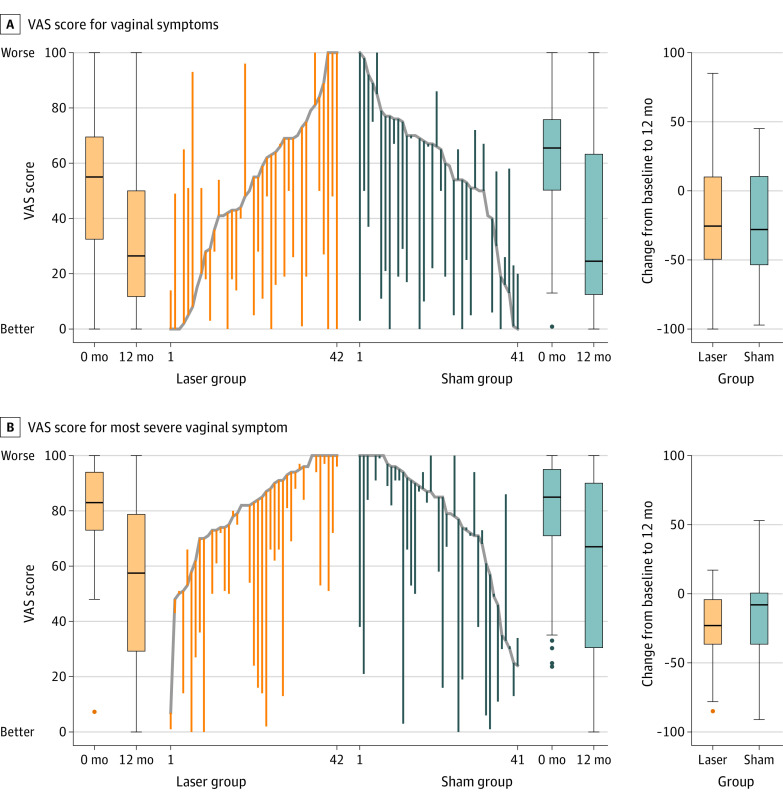
There was no significant difference between the laser and sham groups from baseline to 12 months in the change in VAS score for overall symptoms (–17.2 vs –26.6; difference, 9.4 [95% CI, –28.7 to 47.5]) or for the most severe symptom (–24.5 vs –20.4; difference, –4.1 [95% CI, –32.5 to 24.3]) (Figure 2). There was no significant difference between the laser and sham groups from baseline to 12 months in total VSQ score (3.1 vs –1.6; difference, –1.5 [95% CI, –6.0 to 3.0]) (Figure 2) or any individual domain (eTable 1 in Supplement 2). Subgroup analyses comparing natural and iatrogenic menopause groups demonstrated no significant difference in change in VAS score for the most severe symptom and overall vaginal symptoms over time (eFigure 1 in Supplement 2). Parallel line plots for individual paired data and associated boxplots are provided in Figure 3.
Figure 2. Severity of Symptoms in a Study of the Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Women With Postmenopausal Vaginal Symptoms.
A and B, Visual analog scale (VAS) scores range from 0 to 100, with 0 indicating no symptoms and 100 indicating maximal symptom severity. C, The Vulvovaginal Symptom Questionnaire (VSQ) is a is a 21-item validated instrument used to measure vulvovaginal symptoms in postmenopausal women. It assesses 4 domains (symptoms, emotion, life-impact, and sex-impact) with possible scores ranging from 0 to 20, where 0 indicates no symptoms and 20 indicates severe symptoms. The line represents the median, boxes represent the IQR, whiskers represent the range (excluding outliers), and dots represent outliers greater than 1.5 box lengths (ie, IQR) from the median.
Figure 3. Overall and Most Severe Vaginal Symptoms in a Study of the Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Women With Postmenopausal Vaginal Symptoms.
Visual analog scale (VAS) scores ranged from 0 to 100, with 0 indicating no symptoms and 100 indicating maximal symptom severity. The highest scoring vaginal symptom at baseline was taken to be the “most severe” symptom for the participant throughout the trial. The line represents the median, the boxes represent the IQR, the whiskers represent the range (excluding outliers), and dots represent outliers greater than 1.5 box lengths (ie, IQR) from the median. The trend lines indicate the baseline score. Increased scores indicate increased severity of symptom. Decreased scores indicate symptom improvement.
There was no significant difference in the number of participants who reported a reduction greater than 50% in the severity of the most severe symptom between laser and sham groups at 12 months (8 [22%] vs 8 [21%]; P = .43).
Secondary Outcomes
Quality of life did not significantly differ between the groups at either baseline or 12 months, with mean difference in the Assessment of Quality of Life–6D scale of 6.3 in the laser group vs 1.4 in the sham group (difference, 4.9 [95% CI, –3.9 to 13.5]). Sexual activity rates and quality of sex were not significantly different between the groups at baseline or 12 months (eTable 2 in Supplement 2).
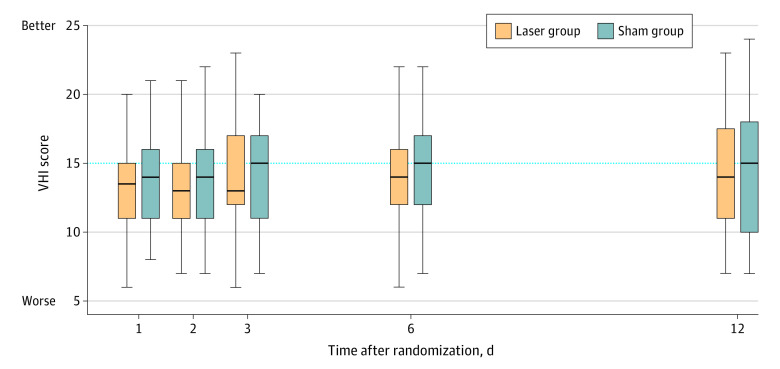
There was no statistically significant difference between total scores and individual domains of the VHI between laser and sham groups at any time point, with mean difference from baseline to 12 months of 0.9 in the laser group vs 1.3 in the sham group (difference, –0.4 [95% CI, –4.3 to 3.6]) (Figure 4).
Figure 4. Vaginal Health Index Scores in a Study of the Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Women With Postmenopausal Vaginal Symptoms.
The Vaginal Health Index Score (VHI) ranges from 5 to 25, with a score less than 15 considered to indicate vaginal atrophy. The median is represented by the line; the interquartile range is represented by the bar (75th percentile by maximal edge; 25th percentile by minimal edge); and the range is represented by the whiskers. Values under the dotted line indicate vaginal atrophy.
Forty-six paired vaginal wall biopsies were analyzed, and a shift from postmenopausal to premenopausal histology was reported in 2 of 22 participants (9%) after laser treatment and 3 of 24 (12.5%) after sham treatment. Comparing laser vs sham treatment groups, a shift after treatment from premenopausal to postmenopausal histology was reported in 0 of 22 participants in the laser group vs 3 of 24 (12%) in the sham group. There was no significant difference between laser and sham groups at baseline or 12 months.
Adverse Events
Overall, 16 participants in the laser group and 17 participants in the sham group reported an adverse event at least once during treatment. These included self-resolving vaginal pain or discomfort (44% vs 68%), spotting (30% vs 5%), lower urinary tract symptoms or confirmed urinary tract infection (15% vs 5%), and vaginal discharge (11% vs 11%). One participant in the laser group reported an upper urinary tract infection after treatment that was managed with oral antibiotics.
There were 3 adverse events after vaginal biopsy with bleeding treated by pressure and application of silver nitrate. Persistent bleeding in 2 participants required repeat silver nitrate, with 1 participant withdrawing from the trial before randomization and 1 continuing with the trial.
Discussion
In this randomized clinical trial involving women with postmenopausal vaginal symptoms, treatment with fractional carbon dioxide laser vs sham treatment did not result in improvement in symptom severity, quality of life, or vaginal histology.
The demand for effective alternatives to hormonal treatments for vaginal menopausal symptoms, particularly when hormonal treatments are contraindicated (eg, hormone-sensitive breast cancer), has contributed to a rapid dissemination and uptake of commercially available fractional carbon dioxide laser, despite no data from sham-controlled trials to date.4,5 Introduction of devices and treatments in women’s health prior to evidence of efficacy and safety have had consequential effects on both individual health and health care policy, with synthetic mesh procedures for vaginal prolapse being a notable example.23 The physical, psychosocial, and economic effects of vaginal symptoms of menopause are substantive, although not life-threatening. Clinical outcomes and cost-effectiveness of treatments, whether devices or drugs, should be evaluated rigorously prior to integration into routine treatment. The annual cost of laser treatment to the individual for management of vaginal menopausal symptoms was reported to be $2733,24 and because there is no demonstratable difference vs sham treatment, it cannot be considered to be cost-effective.
Strengths of this study include double-blinding and use of a full sham protocol to quantify placebo effect. These methods are underutilized to evaluate medical devices because it is a substantial undertaking for both the participants and clinicians.6 The broad inclusion criteria support external validity of the results because participants represented a clinically realistic target population for laser treatments. This study included women who presented to a clinician with troublesome symptoms rather than using an arbitrary questionnaire score cutoff because this more closely reflects clinical practice. Use of a range of assessment tools (patient, clinician, and laboratory) enables comparability between previous studies, as does the use of the same assessment tools as in those studies. For example, the VHI has been used in 23 studies of vaginal laser treatment but never validated for this intervention.4,5,16 Results from the current study demonstrated no significant difference in VHI in the laser group compared with the sham group.
Limitations
This study has several limitations. First, at the time of protocol development and registration, data arising from prospective studies reported very high response rates with almost complete elimination of symptoms.19,20 This is a common occurrence with new technologies. The original power calculation necessarily included these data, and the sample size was increased by an independent committee to account for a type II error. The estimated clinically important difference of 50% may be considered high, although this is in keeping with other randomized trials of menopausal symptoms12,25 and appropriate when the indication for intervention is not life-threatening and the trial intervention is costly, as in this study.
Second, a change in laboratory-based assessment was required during the study due to technical challenges. Vaginal cytology was initially used as an indicator of local response in vaginal epithelium, but poor cellular content and preservation was noted in early samples. Vaginal biopsy was added to the protocol, which provided fewer but more reliable paired samples for blinded histological assessment and determination of premenopausal or postmenopausal vaginal epithelium. The lack of significant differences between laser and sham groups contrast with data from nonblinded and nonrandomized studies that suggested laser restoration of vaginal epithelium to a premenopausal state.3,17,18 Histological outcomes from this study were consistent with a randomized, blinded trial of the erbium YAG laser, estrogen, and sham treatments in 24 sheep, which showed no significant difference of laser over sham treatment in vaginal histology in the animal model.26 Although histological or cytological change was important to objectively assess the intervention, any change needs to be accompanied by symptom improvement to be clinically meaningful, and this study did not find any benefit in any end point.
Third, although this study did not determine any statistical or clinical differences in symptoms for women after breast cancer treatment or natural menopause, the study was not powered to detect such differences. Approximately 50% of participants in this study had previously treated breast cancer, with impairment of sexual function commonly reported. In a survey study of 194 women conducted in 2015-2016, sexual dysfunction was reported by 69% of women with breast cancer compared with 20% without breast cancer.2 For such women, treatments are limited to nonhormonal options and, although laser treatment is frequently offered as a choice among this group, results from this study demonstrated no significant response compared with a sham procedure.
Fourth, although this study did not identify any safety issues, it was not powered to determine the risk of uncommon, but clinically relevant, complications, such as postcoital vaginal wall lacerations, worsening dyspareunia, increased scarring, and rigidity of vaginal tissues.27,28 Minor complications were noted, including spotting and discomfort, likely secondary to trauma from probe maneuvers. It was also possible in the sham group that even in the absence of laser energy, this trauma contributed to symptomatic improvement, given the theory of tissue injury and repair as a possible mechanism of action of laser treatments.29,30
Conclusions
Among women with postmenopausal vaginal symptoms, treatment with fractional carbon dioxide laser vs sham treatment did not significantly improve vaginal symptoms after 12 months.
Trial protocol and statistical analysis plan
eTable 1. Primary and secondary outcomes from baseline to 12 months
eFigure 1. Analyses for natural vs. iatrogenic menopause subgroups, from baseline to 12 months
eTable 2. Sexual activity and quality of sexual experience at baseline, 6 and 12 months
Data sharing statement
References
- 1.Mili N, Paschou SA, Armeni A, Georgopoulos N, Goulis DG, Lambrinoudaki I. Genitourinary syndrome of menopause: a systematic review on prevalence and treatment. Menopause. 2021;28(6):706-716. doi: 10.1097/GME.0000000000001752 [DOI] [PubMed] [Google Scholar]
- 2.Gambardella A, Esposito D, Accardo G, et al. Sexual function and sex hormones in breast cancer patients. Endocrine. 2018;60(3):510-515. doi: 10.1007/s12020-017-1470-7 [DOI] [PubMed] [Google Scholar]
- 3.Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429-436. doi: 10.1007/s10103-014-1677-2 [DOI] [PubMed] [Google Scholar]
- 4.Li F, Picard-Fortin V, Maheux-Lacroix S, et al. The efficacy of vaginal laser and other energy-based treatments on genital symptoms in postmenopausal women: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(3):668-683. doi: 10.1016/j.jmig.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Song S, Budden A, Short A, Nesbitt-Hawes E, Deans R, Abbott J. The evidence for laser treatments to the vulvo-vagina: making sure we do not repeat past mistakes. Aust N Z J Obstet Gynaecol. 2018;58(2):148-162. doi: 10.1111/ajo.12735 [DOI] [PubMed] [Google Scholar]
- 6.Van Norman GA. Drugs, devices, and the FDA: part 2: an overview of approval processes: FDA approval of medical devices. JACC Basic Transl Sci. 2016;1(4):277-287. ,doi: 10.1016/j.jacbts.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statement from FDA Commissioner Scott Gottlieb, MD, on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” News release. US Food and Drug Administration . Updated August 2, 2018. Accessed September 19, 2021. https://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm615013.htm
- 8.Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1):21-28. doi: 10.1097/GME.0000000000000955 [DOI] [PubMed] [Google Scholar]
- 9.Paraiso MFR, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27(1):50-56. doi: 10.1097/GME.0000000000001416 [DOI] [PubMed] [Google Scholar]
- 10.Politano CA, Costa-Paiva L, Aguiar LB, Machado HC, Baccaro LF. Fractional CO2 laser versus promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause. 2019;26(8):833-840. doi: 10.1097/GME.0000000000001333 [DOI] [PubMed] [Google Scholar]
- 11.Erekson EA, Yip SO, Wedderburn TS, et al. The Vulvovaginal Symptoms Questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause. 2013;20(9):973-979. doi: 10.1097/GME.0b013e318282600b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms: a randomized clinical trial. JAMA Intern Med. 2018;178(5):681-690. doi: 10.1001/jamainternmed.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen J, Inder KJ, Lewin TJ, Attia JR, Kelly BJ. Construct validity of the Assessment of Quality of Life - 6D (AQoL-6D) in community samples. Health Qual Life Outcomes. 2013;11:61. doi: 10.1186/1477-7525-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison SL, Bell RJ, La China M, Holden SL, Davis SR. Assessing sexual function in well women: validity and reliability of the Monash Women’s Health Program Female Sexual Satisfaction Questionnaire. J Sex Med. 2008;5(11):2575-2586. doi: 10.1111/j.1743-6109.2008.00967.x [DOI] [PubMed] [Google Scholar]
- 15.Bachmann G. Urogenital ageing: an old problem newly recognized. Maturitas. 1995;22(suppl):S1-S5. doi: 10.1016/0378-5122(95)00956-6 [DOI] [PubMed] [Google Scholar]
- 16.Weber MA, Limpens J, Roovers JPWR. Assessment of vaginal atrophy: a review. Int Urogynecol J. 2015;26(1):15-28. doi: 10.1007/s00192-014-2464-0 [DOI] [PubMed] [Google Scholar]
- 17.Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22(8):845-849. doi: 10.1097/GME.0000000000000401 [DOI] [PubMed] [Google Scholar]
- 18.Samuels JB, Garcia MA. Treatment to external labia and vaginal canal with CO2 laser for symptoms of vulvovaginal atrophy in postmenopausal women. Aesthet Surg J. 2019;39(1):83-93. doi: 10.1093/asj/sjy087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvatore S, Maggiore ULR, Origoni M, et al. Microablative fractional CO2 laser improves dyspareunia related to vulvovaginal atrophy: a pilot study. J Endometr Pelvic Pain Disord. 2014;6(3):150-6. doi: 10.5301/je.5000184 [DOI] [Google Scholar]
- 20.Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17(4):363-369. doi: 10.3109/13697137.2014.899347 [DOI] [PubMed] [Google Scholar]
- 21.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82(4):878-884. doi: 10.1016/j.fertnstert.2004.03.046 [DOI] [PubMed] [Google Scholar]
- 22.Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022-1028. doi: 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 23.Urogynecologic Surgical Mesh Implants. US Food and Drug Administration . Updated October 7, 2019. Accessed September 20, 2020. https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants
- 24.Wallace SL, St Martin B, Lee K, Sokol ER. A cost-effectiveness analysis of vaginal carbon dioxide laser therapy compared with standard medical therapies for genitourinary syndrome of menopause-associated dyspareunia. Am J Obstet Gynecol. 2020;223(6):890.e1-890.e12. doi: 10.1016/j.ajog.2020.06.032 [DOI] [PubMed] [Google Scholar]
- 25.Constantine GD, Revicki DA, Kagan R, et al. Evaluation of clinical meaningfulness of estrogen plus progesterone oral capsule (TX-001HR) on moderate to severe vasomotor symptoms. Menopause. 2019;26(5):513-519. doi: 10.1097/GME.0000000000001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackova K, Mazzer AM, Mori Da Cunha M, et al. Vaginal Er:YAG laser application in the menopausal ewe model: a randomised estrogen and sham-controlled trial. BJOG. 2021;128(6):1087-1096. doi: 10.1111/1471-0528.16558 [DOI] [PubMed] [Google Scholar]
- 27.Al-Badr A, Alkhamis WH. Laser vaginal tightening complications: report of three cases. Lasers Surg Med. 2019;51(9):757-759. doi: 10.1002/lsm.23110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26(4):423-427. doi: 10.1097/GME.0000000000001293 [DOI] [PubMed] [Google Scholar]
- 29.Capon A, Mordon S. Can thermal lasers promote skin wound healing? Am J Clin Dermatol. 2003;4(1):1-12. doi: 10.2165/00128071-200304010-00001 [DOI] [PubMed] [Google Scholar]
- 30.Hantash BM, Bedi VP, Kapadia B, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39(2):96-107. doi: 10.1002/lsm.20468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eTable 1. Primary and secondary outcomes from baseline to 12 months
eFigure 1. Analyses for natural vs. iatrogenic menopause subgroups, from baseline to 12 months
eTable 2. Sexual activity and quality of sexual experience at baseline, 6 and 12 months
Data sharing statement