Abstract
Biflavonoids, composed of two monoflavonoid residues, occur naturally in angiosperms, bryophytes, ferns, and gymnosperms. More than 592 biflavonoids have been structurally elucidated, and they can be classified into two groups of C-C and C-linear fragments-C, based on whether the linker between the two residues contains an atom. As the linker can be established on two arbitrary rings from different residues, the C-C type contains various subtypes, as does the C-linear fragment-C type. Biflavonoids have a wide range of pharmacological activities, including anti-inflammatory, antioxidant, antibacterial, antiviral, antidiabetic, antitumor, and cytotoxic properties, and they can be applied in Alzheimer’s disease and Parkinson’s disease. This review mainly summarizes the distribution and chemistry of biflavonoids; additionally, their bioactivities, pharmacokinetics, and synthesis are discussed.
Keywords: biflavonoids, chemistry, pharmacology, pharmacokinetics, synthesis
1. Introduction
Flavonoids, one of the main classes of secondary metabolites in plants, and have representative scaffolds as flavones, chalcones, isoflavones, aurones, and xanthones. Biflavonoids, as members of the flavonoid family, are comprised of two monoflavonoids by a direct connection, or a linear linker. In 2017, Gontijo et al., summarized 139 biflavonoids and their medical applications [1]. In the same year, Sheng Yu et al. [2] also reviewed the phytochemistry, pharmacology, and pharmaceutics of amentoflavone in biflavonoids, including a comprehensive description and summary of the source and current situation of amentoflavone derivatives. It is known that amentoflavone can be obtained from different parts of 127 plants, 45 kinds of derivatives that belong to the same type of connection with amentoflavone. The pharmacological effects of amentoflavone are summarized, including its anti-inflammatory, antioxidation, antitumor, antiaging, antidiabetes, antiviral, central nervous, cardiovascular system, antifungal, and other pharmacological effects. The amentoflavone family is recorded in detail.
In this report, 592 biflavonoids, as well as their distribution, structural scaffolds, and chemical subtype are reviewed. In addition, the pharmacology and synthesis of biflavonoids are summarized.
2. Distribution of Biflavonoids
A total of 592 biflavonoids are widely distributed in angiosperms, ferns, gymnosperms, and bryophytes, but most of them are found in angiosperms, including: Anacardiaceae, Apiaceae, Aristolochiaceae, Asteraceae, Balsaminaceae, Berberidaceae, Caprifoliaceae, Chloranthaceae, Clusiaceae (especially Garcinia), Daphniphyllaceae, Ephedraceae, Ericaceae, Euphorbiaceae, Gentianaceae, Juglandaceae, Lanariaceae, Leguminosae, Liliaceae, Lythraceae, Menispermaceae, Moraceae, Myrtaceae, Ochnaceae, Polygonaceae, Rosaceae, Rubiaceae, Theaceae, Thymelaeaceae, Velloziaceae, and Vitaceae. The vast majority of biflavonoids are come from Clusiaceae, Thymelaeaceae, Ochnaceae, and Selaginellaceae, which account for approximately 50% of the biflavonoids in all families. The standard names of the plant families are from The Plant List (2013), which was published in http://www.theplantlist.org/ (accessed on 21 October 2020).
3. The Scaffold of Biflavonoids
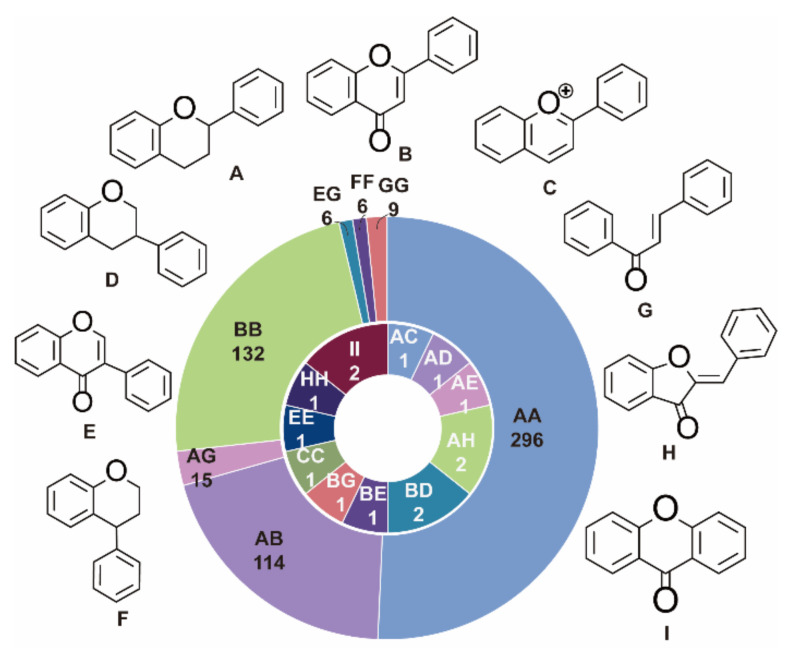
In these 592 biflavonoids, according to the C6-C3-C6 combination pattern, flavan (A), flavone (B), anthocyanidin (C), isoflavan (D), isoflavone (E), neoflavan (F), chalcone (G), aurone (H), and xanthone (I) were the main monoflavonoid scaffolds. According to the different monomer combination types, 592 biflavonoids were divided into 17 kinds, including: AA (flavan-flavan), AB (flavan-flavone), AC (flavan-anthocyanidin), AD (flavan-isofalvan), AE (flavan-isoflavone), AG (flavan-chalcone), AH (flavan-aurone), BB (flavone-flavone), BD (flavone-isoflavan), BE (flavone-isoflavone), BG (flavone-chalcone), CC (anthocyanidin-anthocyanidin), EE (isoflavone-isoflavone), EG (isoflavone-chalcone), FF (neoflavan-neoflavan), GG (chalcone-chalcone), HH (aurone-aurone), and II (xanthone-xanthone) (Figure 1). Among them, AA type biflavonoids are abundant in natural plants, and have good development prospects.
Figure 1.
The scaffold of biflavonoids.
4. Subtypes of Biflavonoids
4.1. C-C Type
According to the connection mode of biflavonoids, they are divided into three major groups.
Group A is about C-C linkages (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8); C-C type biflavonoids have a large number, so they can according the positions of their combinations, divide into: 2-3′′, 2′-2′′′, 2′-6′′, 2′-8′′, 3-3′′, 3-3′′′, 3′-3′′′, 3′-4′′′,3′-5′′, 3-6′′, 3′-6′′, 3-7′′, 3′-7′′, 3-8′′, 3′-8′′ , 4-6′′, 4-8′′, 4′-8′′, 5-5′′, 6-6′′, 6-γ, 6-8′′, 7-7′′, and 8-8′′.
Table 1.
The 2-3′′, 2′-2′′′, 2′-6′′, 2′-8′′, and 3-3′′ subtypes of biflavonoids.
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| 2-3′′ | 1 | Linobiflavonoid | AH | Thym | [1] |
| 2′-2′′′ | 2 | I-3,II-3,I-5,II-5,I-7,II-7,I-4′,II-4′-octahydroxy[I-2′,II-2′]biflavone | BB | Clus | [3] |
| 3 | I-3,II-3,I-5,II-5,I-7,II-7,I-4′,II-4′-octamethoxy[I-2′,II-2′]biflavone | BB | Clus | [3] | |
| 4 | 3,3′′-di-O-α-L-rhamnopyranoside,2′,2′′′-bimyricetin | BB | Myrt | [4] | |
| 5 | Acuminatanol | AA | Anac | [5] | |
| 6 | Theasinensin A | AA | Thea | [6] | |
| 7 | Theasinensin B | AA | Thea | [6] | |
| 2′-6′′ | 8 | 2′,6′′-Biapignin | BB | Sela | [7] |
| 2′-8′′ | 9 | 2′,8′′-Biapignin | BB | Sela | [7] |
| 3-3′′ | 10 | Chamaejasmine | AA | Thym, Legu | [8] |
| 11 | 7-methoxychamaejasmin | AA | Thym | [8] | |
| 12 | Ruixianglangdu B | AA | Thym | [8] | |
| 13 | Isosikokianin | AA | Thym | [8] | |
| 14 | 7,4′,7′′,4′′-tetramethylisochamaejasmin | AA | Ochn | [9] | |
| 15 | 7,7′′-di-O-methylchamaejasmin | AA | Legu | [10] | |
| 16 | Campylospermone A | AA | Ochn | [10,11] | |
| 17 | Campylospermone B | AA | Ochn | [11] | |
| 18 | Isochamaejasmine | AA | Thym | [12] | |
| 19 | 7,7′′-di-O-glucosylisochamaejasmin | AA | Thym | [13] | |
| 20 | Neochamaejasmin A | AA | Thym | [8] | |
| 21 | Chamaejasmenin B | AA | Thym | [8] | |
| 22 | Chamaejasmenin C | AA | Thym | [8] | |
| 23 | Sikokianin A | AA | Thym | [8] | |
| 24 | 7-methoxyneochamaejasmin A | AA | Thym | [8] | |
| 25 | Chamaejasmenin D | AA | Thym | [8] | |
| 26 | Isoneochamaejasmin A | AA | Thym | [8] | |
| 27 | Isochamaejasmine B | AA | Thym | [8] | |
| 28 | Neochamaejasmin B | AA | Thym | [8] | |
| 29 | Sikokianin B | AA | Thym | [12] | |
| 30 | Chamaejasmenin A | AA | Thym | [8] | |
| 31 | Sikokianin C | AA | Thym | [8,12] | |
| 32 | Ruixianglangdu A | AA | Thym | [8] | |
| 33 | Isosikokianin A | AA | Thym | [8] | |
| 34 | Asteryomenin | AA | Aste | [14] | |
| 35 | Wikstaiwanone C | AA | Thym | [12] | |
| 36 | Sikokianin D | AA | Thym | [15] | |
| 37 | 2′′′-dehydroxy-2,2′′-bisteppogenin | AA | Thym | [1] | |
| 38 | 2,2′′-bisteppogenin 7-O-β-glucopyranoside | AA | Thym | [1] | |
| 39 | 2,2′′-bisteppogenin | AA | Thym | [1] | |
| 40 | Apigenil-(I-3,II-3)-naringenin | AA | Legu | [1] | |
| 41 | 6-aminoacryloylchamaejasmin | AA | Legu, Thym | [1,16] | |
| 42 | Ormocarpin | AA | Legu | [1,13,17] | |
| 43 | (−)-7,7′′-di-O-glucosylchamaejasmin | AA | Legu | [1,13] | |
| 44 | (−)-(2S, 3S, 2′′S, 3′′R)-7-O-glucosylchamaejasmin | AA | Legu | [1] | |
| 45 | (2S, 3R, 2′′S, 3′′R)-7-O-glucosylchamaejasmin | AA | Legu | [1,17] | |
| 46 | Campilospermone B | AA | Ochn | [1] | |
| 47 | Neochamaejasmin C | AA | Thym | [1] | |
| 48 | 7-methoxyneochamaejasmin B | AA | Thym | [1] | |
| 49 | 2′′′-dehydroxy-2,2′′-bisteppogenin 7-O-β-glucopyranoside | AA | Thym | [1] | |
| 50 | 3′′-epidiphysin | FF | Legu | [1] | |
| 51 | 5,5′′-di-O-methyldiphysin | FF | Legu | [1,17] | |
| 52 | Diphysin | FF | Legu | [1] | |
| 53 | 2,3-didehydro-(+)-chamaejasmin | AA | Thym | [16] | |
| 54 | 3,3′-biliquiritigenin | AA | Ochn, Legu | [17] | |
| 55 | Euchamaejasmin A | AA | Thym | [18] | |
| 56 | 7-O-β-D-glucopyranoside-diphysin | FF | Legu | [17] | |
| 57 | 3-Epimer, 5,5′-dideoxy-diphysin | FF | Anac | [19] | |
| 58 | 3,3′-diepimer, 5,5′-dideoxy-diphysin | FF | Anac | [19] | |
| 59 | 6′′′-hydroxylophirone | AG | Ochn | [20] |
* Anac: Anacardiaceae; Aste: Asteraceae; Clus: Clusiaceae; Legu: Leguminosae; Myrt: Myrtaceae; Ochn: Ochnaceae; Sela: Selaginellaceae; Thea: Theaceae; and Thym: Thymelaeaceae.
Table 2.
The 3-3′′′, 3′-3′′′, 3′-4′′′, 3′-5′′, 3-6′′, 3′-6′′, 3-7′′, and 3′-7′′ subtypes of biflavonoids.
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| 3-3′′′ | 60 | Taiwaniaflavone | BB | Cupr, Sela, Taxo | [21,22,23] |
| 61 | 7-O-methyltaiwaniaflavone | BB | Taxo | [23] | |
| 62 | 4′′′,7-di-O-methyltaiwaniaflavone | BB | Taxo | [23] | |
| 63 | Lupinabisone A | AB | Legu | [24] | |
| 3′-3′′′ | 64 | 2,3-dihydro-3′,3′′′-biapigenin | BB | Sela | [7] |
| 65 | 3′,3′′-binaringenin | BB | Sela | [7,25] | |
| 66 | Thuidinin | BB | Thui | [25] | |
| 67 | Kudzuisoflavone A | EE | Faba | [26] | |
| 3′-4′′′ | 68 | Chrysocauloflavone III | AB | Sela | [7] |
| 69 | Japoflavone D | BB | Capr | [27] | |
| 3′-5′′ | 70 | Aulacomniumbiaureusidin | HH | Aula | [28] |
| 3-6′′ | 71 | Daphnodorin K1 | AB | Thym | [29] |
| 72 | Daphnodorin K2 | AB | Thym | [29] | |
| 73 | Wikstaiwanone A | AB | Thym | [12] | |
| 74 | Wikstaiwanone B | AB | Thym | [12] | |
| 75 | Stephaflavone A | BB | Meni | [30] | |
| 76 | Stephaflavone B | BB | Meni | [30] | |
| 77 | Isomanniflavone | AA | Clus | [1] | |
| 78 | Ridiculuflavone | BB | Aris | [1] | |
| 79 | Afzelone B | AA | Ochn | [31] | |
| 80 | Ridiculuflavone A | BB | Aris | [32,33] | |
| 81 | Ridiculuflavone B | BB | Aris | [32,33] | |
| 82 | Ridiculuflavone D | BB | Aris | [32] | |
| 83 | Ridiculuflavone C | BB | Aris | [32] | |
| 84 | 4′′′,5,5′′,7′′-tetrahydroxy-3′′′,4′,7-trimethoxy-3,6′′-biflavone | BB | Aris | [32,34] | |
| 3′-6′′ | 85 | Robustaflavone | BB | Anac, Arau, Clus, Sela | [35,36] |
| 86 | 7′′-O-methylrobustaflavone | BB | Sela | [7] | |
| 87 | 4′-O-methylrobustaflavone | BB | Sela | [7,35,37] | |
| 88 | 7,4′-di-O-methylrobustaflavone | BB | Sela | [7,38] | |
| 89 | 4′,4′′′-di-O-methylrobustaflvone | BB | Sela | [7,39] | |
| 90 | 4′,7′′-di-O-methylrobustaflavone | BB | Sela | [7,37] | |
| 91 | 7,4′,4′′′-tri-O-methylrobustaflvone | BB | Sela | [7,39] | |
| 92 | Imbricataflavone A | AB | Podo, Sela | [7,35,40] | |
| 93 | Caesalflavone | AB | Legu, Sela | [7,41,42] | |
| 94 | Uncinatabiflavone D | AB | Sela | [7,43,44] | |
| 95 | 7,4′,7′′-tri-O-methyl-2,3-dihydrorobustaflavone | AB | Sela | [7,39] | |
| 96 | 5-O-methyl-2,3-dihydrorobustaflavone | AB | Sela | [7,42] | |
| 97 | Macrophylloflavone | AB | Sela | [45] | |
| 98 | 2,3-dihydrorobustaflavone 7,7′′-dimethyl ether | AB | Sela, Thym | [1] | |
| 99 | Imbricataflavone B | AB | Podo | [40] | |
| 100 | 4′-O-methyl-2′′,3′′-dihydrorobustaflavone | AB | Sela, Thym | [7,43] | |
| 101 | 7,4′-di-O-methyl-2′′,3′′-dihydrorobustaflvone | AB | Arau, Sela, Thym | [7,37] | |
| 102 | 4′,7′′-di-O-methyl-2′′,3′′-dihydrorobustaflavone | AB | Arau, Sela, Thym | [36] | |
| 103 | 7,4′,7′′-tri-O-methyl-2′′,3′′-dihydrorobustaflavone | AB | Sela, Thym | [7,37] | |
| 104 | Robustaflavanone | AA | Sela, Thym | [7,38] | |
| 105 | Uncinatabiflavone A | AA | Sela | [7,43,44] | |
| 106 | Uncinatabiflavone B | AA | Sela | [7,43,44] | |
| 107 | Uncinatabiflavone C | AA | Sela | [7,43,44] | |
| 108 | 7,4′,7′′-tri-O-methyl-2,3,2′′,3′′-tetrahydrorobustaflavone | AA | Sela | [7,35] | |
| 109 | Abiesin | BB | Pina | [46] | |
| 110 | 5′-hydroxyrobustaflavone | BB | Hylo | [47] | |
| 111 | 2′′,3′′-dihydro-5′′′-hydroxyrobustaflavone | AB | Mnia | [48] | |
| 112 | 5′,6′′-biluteolin | BB | Hylo, Dicr | [49] | |
| 113 | 2,3-dihydro-5′,6′′-biluteolin | AB | Dicr | [49] | |
| 114 | 2′′,3′′-dihydro-5′,6′′-biluteolin | AB | Mnia | [50] | |
| 3-7′′ | 115 | 5,5′′,6′′,7,8-pentahydroxy-2,2′′-bis(p-hydroxyphenyl)-4H,4′′H(3,7′′-bichromene)-4,4′′-dione | BB | Anac | [51] |
| 3′-7′′ | 116 | Lophirone M | AB | Ochn | [52] |
| 117 | Lophirone M hexa-acetate | AB | Ochn | [52] |
* Aula: Aulacomniaceae; Arau: Araucariaceae; Aris: Aristolochiaceae; Capr: Caprifoliaceae; Cupr: Cupressaceae; Faba: Fabaceae; Meni: Menispermaceae; Podo: Podocarpaceae; Taxa: Taxaceae; Taxo: Taxodiaceae; and Thui: Thuidiaceae.
Table 3.
The 3-8′′ subtype of biflavonoids.
| No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|
| 118 | Garciniaflavone E | AB | Clus | [1] |
| 119 | Garciniaflavone F | AB | Clus | [1] |
| 120 | Morelloflavone-7′′-O-β-D-glucosíde | BB | Clus | [1] |
| 121 | (+)-4′′′-O-methylmorelloflavone | AB | Clus | [1] |
| 122 | Biapigenin | BB | Clus | [1] |
| 123 | 4′′′-O-methyl-I3,II8-binaringenin | AA | Clus | [1,53] |
| 124 | Volkensiflavone | AB | Clus | [54] |
| 125 | Morelloflavone | AB | Clus | [55] |
| 126 | Spicataside | AB | Clus | [55] |
| 127 | Fukugiside | AB | Clus | [55] |
| 128 | 3′′′-O-methylfukugetin | AB | Clus | [1] |
| 129 | Garcinianin | AB | Clus | [55,56,57] |
| 130 | Madrunoudeaside | AB | Clus | [58] |
| 131 | Morelloflavone-7′-sulfate | AB | Clus | [59] |
| 132 | (2R,3S)-morelloflavone | AB | Mora | [60] |
| 133 | 7,4′,7′′,3′′′,4′′′-penta-O-acetylmorelloflavone | BB | Mora | [60] |
| 134 | 7,4′,7′′,3′′′,4′′′-penta-O-methylmorelloflavone | BB | Mora | [60] |
| 135 | 7,4′,7′′,3′′′,4′′′-penta-O-butanoylmorelloflavone | BB | Mora | [60] |
| 136 | Talbotaflavone | BB | Clus | [1,61] |
| 137 | Balsamiside A | AB | Bals | [62] |
| 138 | Balsamiside B | AB | Bals | [62] |
| 139 | Balsamiside C | AB | Bals | [62] |
| 140 | Balsamiside D | AB | Bals | [62] |
| 141 | Daphnodorin D1 | AB | Thym | [29] |
| 142 | Daphnodorin D2 | AB | Thym | [29] |
| 143 | Wikstrol A | AB | Thym | [63] |
| 144 | Wikstrol B | AB | Thym | [63] |
| 145 | II-3,I-5, II-5,II-7,I-4′,II-4′-hexahydroxy-(I-3,II-8)-flavonylflavanonol | BB | Clus | [1] |
| 146 | GB-1a | AA | Clus | [54,64] |
| 147 | GB-2a | AB | Clus | [65] |
| 148 | GB-1a-7′′-O-glycoside | AA | Clus | [55] |
| 149 | Xanthochymuside | AA | Clus | [55,66] |
| 150 | Kolaflavanone | AA | Clus | [55,64] |
| 151 | GB-1 | AA | Clus | [55,64] |
| 152 | GB-2 | AA | Clus | [55,64] |
| 153 | Manniflavanone | AA | Clus | [54,64] |
| 154 | GB-2a-II-4′-OMe | BB | Clus | [65] |
| 155 | Buchananiflavone | AA | Clus | [1,67] |
| 156 | Manniflavone-7′′-O-β-D-glucopyranoside | AB | Clus | [1] |
| 157 | (2R,3S,2′′R)3,8′′-binaringenin-7′′-O-β-glucoside | AA | Clus | [1,68] |
| 158 | (2R, 3S, 2′′R, 3′′R)GB1-7′′-O-β-glucoside | AA | Clus | [1,68] |
| 159 | Ent-naringenil-(I-3α,II-8)-4′-O-metilnaringenin | BB | Clus | [1,53] |
| 160 | 3,8′′-biapigenin | BB | Poly, Clus | [69,70] |
| 161 | Sumaflavone | BB | Anac | [7,71] |
| 162 | 4′-methoxydaphnodorin D1 | AB | Thym | [72] |
| 163 | 4′-methoxydaphnodorin D2 | AB | Thym | [72] |
| 164 | Pancibiflavonol | AB | Clus | [73] |
| 165 | Volkensiflavone 7-sulfate | AB | Clus | [74] |
| 166 | 8-(3′,4′,5,7-tetrahydroxyflavanon-3-yl)-4′,5,7-trihydroxyflavone | AB | Clus | [75] |
| 167 | GB-3 | AA | Clus | [64] |
| 168 | GB-4 | AA | Clus | [76] |
| 169 | GB-2b | AA | Clus | [64] |
| 170 | GB-4a | AA | Clus | [76] |
| 171 | 4′′′-O-methylfukugetin | AB | Clus | [77] |
| 172 | Lupinalbisone B | AB | Legu | [24] |
* Bals: Balsaminaceae; Dicr: Dicranaceae; Hylo: Hylocomiaceae Mnia: Mniaceae; Mora: Moraceae; Poly: Polygonaceae; and Pina: Pinaceae.
Table 4.
The 3′-8′′ subtype of biflavonoids.
| No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|
| 173 | Amentoflavone | BB | Anac, Capr, Clus, Cupr, Gink, Pina, Podo, Pter, Sela, Taxa, Taxo | [2] |
| 174 | Isoginkgetin | BB | Gink, Sela | [2,7] |
| 175 | 3′,8′′-biisokaempferide | BB | Pter, Vell | [1,2] |
| 176 | 7,7′′-di-O-methylamentoflavone | BB | Arau, Cupr, Podo, Sela, Taxo | [2,7] |
| 177 | 4′,7′′-di-O-methylamentoflavone | BB | Arau, Sela, Taxo | [2,7] |
| 178 | 5′-methoxybilobetin | BB | Gink, Mnia | [2] |
| 179 | 7,4,7′′,4′′′-tetra-O-methylamentoflavone | BB | Sela | [2,7] |
| 180 | 7,4′,7′′-tri-O-methylamentoflavone | BB | Arau, Ceph, Cupr, Taxa | [2] |
| 181 | Acetyl ginkgetin | BB | Gink | [78] |
| 182 | Acetyl isogenkgetin | BB | Gink | [78] |
| 183 | Acetyl sciadopitysin | BB | Gink | [78] |
| 184 | 6-C-methyl-7-O-methylamentoflavone | BB | Ceph | [79] |
| 185 | 3′′′-hydroxy-4′′′,7-dimethylamentoflavone | BB | Aris, Taxa | [80] |
| 186 | Anacarduflavone | BB | Anac | [81] |
| 187 | Bilcarobetin | BB | Gink, Sela | [2,7] |
| 188 | CGY-1 | BB | Lili | [82] |
| 189 | Ginkgetin | BB | Arau, Gink, Sela, Taxa, Taxo | [2,7] |
| 190 | Ginkgetin 7-O-D-glucopyranoside | BB | Gink | [83] |
| 191 | Heveaflavone | BB | Euph, Sela | [2,7] |
| 192 | 7-O-gluamentoflavone | BB | Cupr, Gink | [84] |
| 193 | Isoginkgetin 7-O-D-glucopyranoside | BB | Gink | [83] |
| 194 | Amentoflavone 7′′-O-β-D-glucopyranoside | BB | Cupr, Gink | [84] |
| 195 | Kayaflavone | BB | Podo, Sela, Taxa, Taxo | [2,7] |
| 196 | Oliveriflavone | BB | Taxa | [79] |
| 197 | Oliveriflavone B | BB | Taxa | [85] |
| 198 | Oliveriflavone C | BB | Taxa | [85] |
| 199 | Podocarduflavone B | BB | Podo | [2] |
| 200 | Podocarpusflavone A | BB | Podo, Sela | [2,7] |
| 201 | Sciadopitysin | BB | Cupr, Gink, Podo, Taxa, Taxo | [2] |
| 202 | Sequoiaflavone | BB | Sela, Taxo | [2,7] |
| 203 | Sotetsuflavone | BB | Cyca, Sela, Taxo | [2,7] |
| 204 | Taiwaniaflavone A | BB | Ceph | [2] |
| 205 | Dulcisbiflavonoid A | BB | Clus | [59] |
| 206 | Putraflavone | BB | Euph, Podo | [1] |
| 207 | (2S,2′′S)-2,3-di-hydroisoginkgetin | AB | Cyca | [86] |
| 208 | 2,3-dihydro-6-methylginkgetin | AB | Ceph | [1] |
| 209 | 2,3-dihydrosciadopitysin | AB | Gink, Taxo | [83] |
| 210 | 4′,7′′-di-O-methyl-2,3-dihydroamentoflavone | AB | Sela | [7] |
| 211 | (2S)-2,3-dihydroamentoflavone | AB | Sela | [7] |
| 212 | 7,4′-di-O-methyl-2,3-dihydroamentoflavone | AB | Cupr, Sela | [7] |
| 213 | 7,4′,7′′-tri-O-methyl-2,3-dihydroamentoflavone | AB | Arau, Sela, Taxa | [7] |
| 214 | 2,3-dihydro-4-O-methylamentoflavone | AB | Cyca, Sela | [87] |
| 215 | (2S)-4′-O-methyl-2,3-tetrahydroamentoflavone | AB | Cyca, Sela | [7] |
| 216 | Garciniaflavone A | AB | Clus | [1,2] |
| 217 | Garciniaflavone B | AB | Clus | [1,2] |
| 218 | Garciniaflavone C | AB | Clus | [1,2] |
| 219 | Garciniaflavone D | AB | Clus | [1,2] |
| 220 | 6′′-hydroxy-2,3-dihydroamentoflavone | AB | Sela | [7] |
| 221 | Selamariscina A | AB | Sela | [36] |
| 222 | 2′′,3′′-dihydroamentoflavone | AB | Anac, Cyca, Sela, Taxo | [7] |
| 223 | 4′-O-methyl-2′′,3′′-dihydroamentoflavone | AB | Cyca, Sela | [1,7] |
| 224 | (2S,2′′S)-2,3,2′′,3′′-tetrahydroisoginkgetin | AA | Arau, Cyca, Podo | [86] |
| 225 | (2S,2′′S)-2,3,2′′,3′′-tetrahydroamentoflavone | AA | Anac, Cyca, Sela | [7,86] |
| 226 | (2S,2′′S)-4′-O-methyl-2,3,2′′,3′′-tetrahydroamentoflavone | AA | Sela | [7] |
| 227 | Taxusbiflavone A | BB | Capr | [88] |
| 228 | 3′′′-methoxyamentoflavone | BB | Anac | [89] |
| 229 | 3′′′,5′-dihydroxyamentoflavone | BB | Dicr | [49] |
| 230 | (2S,2′′S)-3′,4′,4′′′,5,5′′,7′′-hexahydroxy-8,3′′′-biflavanone | AA | Anac | [81] |
| 231 | 3′,5,5′′-trihydroxy-4′,4′′′,7′′-trimethoxy-8,3′′′-biflavanone | AA | Anac | [90] |
| 232 | 5,5′′-dihydroxy-3′,4′,4′′′,7′′-tetramethoxy-8,3′′′-biflavanone | AA | Anac | [91] |
| 233 | Anacarduflavanone | AA | Anac | [92] |
* Capr: Caprifoliaceae; Ceph: Cephalotaxaceae; Cyca: Cycadaceae; Euph: Euphorbiaceae; Gink: Ginkgoaceae; Lili: Liliaceae; Pter: Pteridiaceae; and Vell: Velloziaceae.
Table 5.
The 4-6′′ subtype of biflavonoids.
| No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|
| 234 | Sarcandrone D | AA | Chlo | [1] |
| 235 | Procyanidin B5 | AA | Dava, Malv, Pina, Rosa, Sapi | [93,94] |
| 236 | Epicatechin 3-O-gallate-(4β→6)-epicatechin 3-O-(4-hydroxybenzoate) | AA | Myro | [95] |
| 237 | 3′-O-galloylprocyanidin B5 | AA | Vita | [95,96] |
| 238 | 3,3′-di-O-galloylprocyanidin B5 | AA | Poly | [97] |
| 239 | Epigallocatechin 3-O-gallate-(4β→6)-epicatechin 3-O-gallate | AA | Thea | [98] |
| 240 | Epicatechin 3-O-gallate-(4β→6)-epigallocatechin 3-O-gallate | AA | Thea | [98] |
| 241 | Epigallocatechin-(4β→6)-epigallocatechin 3-O-gallate | AA | Cist | [99] |
| 242 | 3,3′-di-O-galloylprodelphinidin B5 | AA | Myri | [100] |
| 243 | Epiafzelechin 3-O-gallate-(4β→6)-epigallocatechin 3-O-gallate | AA | Thea | [98] |
| 244 | Procyanidin B6 | AA | Cupr, Eric, Rosa | [93] |
| 245 | Procyanidin B7 | AA | Cupr, Eric | [93] |
| 246 | Procyanidin B8 | AA | Eric, Rosa, Sali | [93] |
| 247 | Ent-epicatechin-(4α→6)-ent-epicatechin | AA | Malp | [101] |
| 248 | Fisetinidol-(4β→6)-fisetinidol-4β-ol | AA | Legu | [102] |
| 249 | Fisetinidol-(4β→6)-fisetinidol | AA | Legu | [103] |
| 250 | Fisetinidol-(4β→6)-fisetinidol-4α-ol | AA | Legu | [102] |
| 251 | Fisetinidol-(4β→6)-ent-epifisetinidol | AA | Legu | [103] |
| 252 | Fisetinidol-(4α→6)-fisetinidol-4β-ol | AA | Legu | [102] |
| 253 | Fisetinidol-(4α→6)-fisetinidol | AA | Legu | [103] |
| 254 | Fisetinidol-(4α→6)-fisetinidol-4α-ol | AA | Legu | [102] |
| 255 | Fisetinidol-(4α→6)-ent-epifisetinidol | AA | Legu | [103] |
| 256 | Globiflorin 3B1 | AA | Legu | [104] |
| 257 | Globiflorin 3B2 | AA | Legu | [104] |
| 258 | Guibourtinidol-(4α→6)-afzelechin | AA | Legu | [105] |
| 259 | ent-Guibourtinidol-(4β→6)-catechin | AA | Rosa | [106] |
| 260 | Epicatechin-(4β→6)-epicatechin-(4β→2)-phloroglucinol | AA | Legu, Pina, Rosa | [107,108,109] |
| 261 | Guibourtinidol-(4α→6)-epicatechin-8-carboxylic acid | AA | Legu | [104] |
| 262 | Guibourtinidol-(4α→6)-catechin-8-carboxylic acid | AA | Legu | [104] |
| 263 | Epioritin-(4β→6)-epioritin-4α-ol | AA | Legu | [110] |
| 264 | Epioritin-(4β→6)-epioritin-4β-ol | AA | Legu | [110] |
| 265 | Epioritin-(4β→6)-oritin-4α-ol | AA | Legu | [111] |
| 266 | Epioritin-(4β→6)-ent-oritin-4α-ol | AA | Legu | [110] |
| 267 | Ent-Oritin-(4β→6)-epioritin-4α-ol | AA | Legu | [110] |
| 268 | Ent-Oritin-(4α→6)-epioritin-4α-ol | AA | Legu | [111] |
| 269 | Ent-Oritin-(4α→6)-epioritin-4β-ol | AA | Legu | [111] |
| 270 | Ent-Oritin-(4β→6)-oritin-4α-ol | AA | Legu | [110] |
| 271 | Ent-Oritin-(4α→6)-oritin-4α-ol | AA | Legu | [111] |
* Chlo: Chloranthaceae; Cist: Cistaceae; Dava: Davalliaceae; Eric: Ericaceae; Malv: Malvaceae; Malp: Malpighiaceae; Myri: Myricaceae; Myro: Myrothamnaceae; Rosa: Rosaceae; Sali: Salicaceae; and Sapi: Sapindaceae.
Table 6.
The 4-8′′ subtype of biflavonoids.
| No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|
| 272 | Juglbiflavone A | AB | Jugl | [112] |
| 273 | Sarcandrone | AA | Chlo | [1] |
| 274 | Procyanidin B2 | AA | Aizo, Rosa, Sapi | [94,113,114] |
| 275 | Procyanidin B2 7′-xyloside | AA | Legu | [115] |
| 276 | 3′-galloylprocyanidin B2 | AA | Poly,Vita | [6] |
| 277 | 3,3′-digalloylprocyanidin B2 | AA | Poly, Thea, Rosa | [6,114] |
| 278 | 3′-O-(3,4-di-O-methylgalloyl)procyanidin B2 | AA | Poly | [94] |
| 279 | Epicatechin-(4α→8)-epicatechin | AA | Rosa | [116] |
| 280 | Procyanidin B1 | AA | Legu | [114] |
| 281 | 3′-(4-hydroxybenzoyl)procyanidin B1 | AA | Hama | [117] |
| 282 | 3-galloylprocyanidin B1 | AA | Poly | [114,118] |
| 283 | 3′-O-(1-hydroxy-6-oxo-2-cyclohexene-1-carboxylate)procyanidin B1 | AA | Sali | [119] |
| 284 | Procyanidin B4 | AA | Rosa | [113] |
| 285 | 3-O-β-D-glucopyranoside, 3′-O-(6-O-E-cinnamoyl-β-D-glucopyranoside)Procyanidin B4 | AA | Legu | [120] |
| 286 | 3′-galloylprocyanidin B4 | AA | Euph | [6] |
| 287 | Procyanidin B3 | AA | Sali | [113,121] |
| 288 | 3-rhamnoside-procyanidin B3 | AA | Faga | [122] |
| 289 | 3-glucoside-procyanidin B3 | AA | Faga | [122] |
| 290 | 3-O-β-D-glucopyranoside, 3′-O-(2-O-E-cinnamoyl-β-D-glucopyranoside)procyanidin B3 | AA | Legu | [120] |
| 291 | Procyanidin B3 3′-rhamnoside | AA | Faga | [122] |
| 292 | Procyanidin B3 3′-O-glucoside | AA | Rosa | [123] |
| 293 | Procyanidin B3 7-glucoside | AA | Poly | [124] |
| 294 | Catechin-(4α→8)-catechin 7-xyloside | AA | Betu | [125] |
| 295 | 3-galloylprocyanidin B3 | AA | Rosa | [126] |
| 296 | 3,3′-di-Ac-3′′′-O-β-D-glucopyranoside procyanidin B3 | AA | Poly | [127] |
| 297 | 3′-O-(1-Hydroxy-6-oxo-2-cyclohexene-1-carboxylate)procyanidin B3 | AA | Sali | [119] |
| 298 | Epicatechin-(4β→8)-ent-epicatechin | AA | Arec | [128] |
| 299 | Ent-epicatechin-(4α→8)-epicatechin | AA | Arec | [128] |
| 300 | Ent-epicatechin-(4α→8)-catechin | AA | Arec | [128] |
| 301 | Ent-epicatechin-(4α→8)-ent-epicatechin | AA | Arec | [128] |
| 302 | 3-O-(3,4,5-trihydroxybenzoyl)ent-epicatechin-(4α→8)-ent-epicatechin | AA | Malp | [101] |
| 303 | 3′-O-(3,4,5-trihydroxybenzoyl)ent-epicatechin-(4α→8)-ent-epicatechin | AA | Malp | [101] |
| 304 | 3,3′-bis-O-(3,4,5-trihydroxybenzoyl)ent-epicatechin-(4α→8)-ent-epicatechin | AA | Malp | [101] |
| 305 | Auricassidin | AA | Legu | [129] |
| 306 | 3,3′,4′,5,7-pentahydroxyflavan-(4→8)-3,4′,5,7-tetrahydroxyflavan | AA | Legu | [130] |
| 307 | Epicatechin-(4β→8)-epiafzelechin | AA | Legu | [131,132] |
| 308 | Catechin-(4α→8)-epiafzelechin | AA | Legu | [133] |
| 309 | Epicatechin-(4β→8)-ent-epiafzelechin | AA | Legu | [134] |
| 310 | Ent-epicatechin-(4α→8)-epiafzelechin | AA | Legu | [134] |
| 311 | Ent-epicatechin-(4α→8)-ent-epiafzelechin | AA | Legu | [134] |
| 312 | Epiguibourtinidol-(4β→8)-epicatechin | AA | Legu | [135] |
| 313 | Guibourtinidol-(4β→8)-epicatechin | AA | Legu | [105] |
| 314 | Guibourtinidol-(4β→8)-epiafzelechin | AA | Legu | [105] |
| 315 | Guibourtinidol-(4α→8)-epicatechin | AA | Legu | [104] |
| 316 | Guibourtinidol-(4α→8)-epiafzelechin | AA | Legu | [105] |
| 317 | Guibourtinidol-(4β→8)-catechin | AA | Legu | [104] |
| 318 | Guibourtinidol-(4α→8)-catechin | AA | Legu | [104] |
| 319 | Calodenin C | AA | Legu | [136] |
| 320 | Ent-guibourtinidol-(4β→8)-epicatechin | AA | Legu | [105] |
| 321 | Epiafzelechin-(4β→8)-epicatechin | AA | Legu, Poly | [134,137] |
| 322 | 3′-O-(3,4,5-trihydroxybenzoyl)epiafzelechin-(4β→8)-epicatechin | AA | Poly | [137] |
| 323 | 3,3′-bis-O-(3,4,5-trihydroxybenzoyl)epiafzelechin-(4β→8)-epicatechin | AA | Poly | [137] |
| 324 | Ouratea proanthocyanidin A | AA | Cela, Ochn | [138] |
| 325 | Ouratea proanthocyanidin B | AA | Cela, Ochn | [138] |
| 326 | Epiafzelechin-(4ξ→8)-epicatechin | AA | Poly | [94] |
| 327 | 3′-O-(4-hydroxybenzoyl)epiafzelechin-(4ξ→8)-epicatechin | AA | Poly | [94] |
| 328 | 3′-O-(3-hydroxy-4,5-dimethoxybenzoyl)epiafzelechin-(4ξ→8)-epicatechin | AA | Poly | [94] |
| 329 | Gambiriin C | AA | Rubi | [139] |
| 330 | Afzelechin-(4α→8)-epicatechin | AA | Rhiz | [140] |
| 331 | 3′-O-(4-hydroxy-3-methoxybenzoyl), 3-O-α-L-rhamnopyranoside-afzelechin-(4α→8)-epicatechin | AA | Euph | [141] |
* Arec: Arecaceae; Aizo: Aizoaceae; Betu: Betulaceae; Faga: Fagaceae; Hama: Hamamelidaceae; Jugl: Juglandaceae; and Vita: Vitaceae.
Table 7.
The 4-8′′ subtype of biflavonoids.
| No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|
| 332 | 3′-O-(4-hydroxy-3,5-dimethoxybenzoyl), 3-O-α-L-rhamnopyranoside-afzelechin-(4α→8)-epicatechin | AA | Euph | [141] |
| 333 | afzelechin-(4α→8)-catechin | AA | Rhiz, Rosa | [121,140] |
| 334 | 3-O-α-L-rhamnopyranoside-afzelechin-(4α→8)-catechin | AA | Faga | [142] |
| 335 | 3-O-β-D-glucopyranoside-afzelechin-(4α→8)-catechin | AA | Faga | [142] |
| 336 | Epiafzelechin-(4β→8)-ent-epicatechin | AA | Legu | [132] |
| 337 | Ent-epiafzelechin-(4α→8)-epicatechin | AA | Legu | [132] |
| 338 | Ent-epiafzelechin-(4α→8)-ent-epicatechin | AA | Legu | [134] |
| 339 | Ichangol | AA | Adox | [143] |
| 340 | Epicatechin-(4β→8)-epicatechin-(4β→2)-phloroglucinol | AA | Legu, Pina | [109,144] |
| 341 | Epigallocatechin-(4β→8)-epicatechin-(4β→2)-phloroglucinol | AA | Legu | [109] |
| 342 | Epigallocatechin-(4β→8)-epigallocatechin-(4β→2)-phloroglucinol 3,3′-digallate | AA | Cist | [145] |
| 343 | Catechin-(4α→8)-epicatechin-(4β→2)-phloroglucinol | AA | Pina | [144] |
| 344 | Gallocatechin-(4α→8)-epigallocatechin-(4β→2)-phloroglucinol | AA | Cist | [145] |
| 345 | Epirobinetinidol-(4β→8)-catechin | AA | Legu | [146] |
| 346 | Robinetinidol-(4β→8)-epigallocatechin | AA | Mimo | [147] |
| 347 | Robinetinidol-(4β→8)-epigallocatechin-3′-gallate | AA | Mimo | [147] |
| 348 | Robinetinidol-(4α→8)-epigallocatechin | AA | Mimo | [147] |
| 349 | Robinetinidol-(4α→8)-epigallocatechin-3′-gallate | AA | Mimo | [147] |
| 350 | Robinetinidol-(4β→8)-catechin | AA | Legu | [148] |
| 351 | Robinetinidol-(4α→8)-gallocatechin | AA | Legu | [149] |
| 352 | Robinetinidol-(4α→8)-catechin | AA | Legu | [149] |
| 353 | Prodelphinidin B2 | AA | Phyl, Legu, Myri | [100] |
| 354 | 3′-O-(4-hydroxybenzoyl)prodelphinidin B2 | AA | Legu | [150] |
| 355 | 3-O-galloylprodelphinidin B2 | AA | Cist, Poly | [99,151] |
| 356 | 3′-galloylprodelphinidin B2 | AA | Cist, Myri, Thea | [6,99,100,152] |
| 357 | Rhodisin | AA | Myri, Cras | [100,153] |
| 358 | Rhodisinoside | AA | Cras | [153] |
| 359 | Epicatechin-(4β→8)-epigallocatechin-3-O-gallate | AA | Thea | [98] |
| 360 | Epicatechin-3-O-gallate-(4β→8)-epigallocatechin-3-O-gallate | AA | Thea | [98] |
| 361 | Epicatechin-(4β→8)-4′-O-methylepigallocatechin | AA | Cela | [154] |
| 362 | Epigallocatechin-(4β→8)-epicatechin-3-O-gallate | AA | Thea | [155] |
| 363 | Prodelphinidin B1 | AA | Cist, Legu | [150,156] |
| 364 | 3-galloylprodelphinidin B1 | AA | Cist, Hama | [117,156] |
| 365 | 3,3′-digalloylprodelphinidin B1 | AA | Myri | [100] |
| 366 | Epigallocatechin-(4β→8)-4′-O-methylgallocatechin | AA | Legu | [157] |
| 367 | Epicatechin-(4β→8)-gallocatechin | AA | Phyl | [158] |
| 368 | Epicatechin-(4β→8)-4′-O-methylgallocatechin | AA | Legu | [157] |
| 369 | Epigallocatechin-(4β→8)-catechin | AA | Legu, Pina | [117,159] |
| 370 | 3′′′-Deoxy, 3-O-(3,4,5-trihydroxybenzoyl)epigallocatechin-(4β→8)-catechin | AA | Hama | [117] |
| 371 | Prodelphinidin B4 | AA | Thea, Phyl, Gros | [160] |
| 372 | Gallocatechin-(4α→8)-epigallocatechin-3-O-(4-hydroxybenzoate) | AA | Mimo | [150] |
| 373 | 3′-galloylprodelphinidin B4 | AA | Thea | [98] |
| 374 | 4′′,4′′′-di-me ether-prodelphinidin B4 | AA | Legu | [150] |
| 375 | Catechin-(4α→8)-epigallocatechin | AA | Thea | [161] |
| 376 | Catechin-(4α→8)-epigallocatechin-3-O-gallate | AA | Thea | [161] |
| 377 | Gallocatechin-(4α→8)-epicatechin | AA | Thea | [161] |
| 378 | Prodelphinidin B3 | AA | Faga, Rham | [162,163] |
| 379 | 4′′,4′′′-di-O-methylprodelphinidin B3 | AA | Legu | [157] |
| 380 | Catechin-(4α→8)-gallocatechin | AA | Cist | [156] |
| 381 | Prodelphinidin C | AA | Hama, Myri, Faga, Sali | [117,160,163,164] |
| 382 | Epifisetinidol-(4β→8)-epicatechin | AA | Legu | [135] |
| 383 | Epifisetinidol-(4β→8)-catechin | AA | Legu | [165] |
| 384 | Fisetinidol-(4β→8)-epicatechin | AA | Legu | [166] |
| 385 | Fisetinidol-(4α→8)-epicatechin | AA | Legu | [166,167] |
| 386 | Fisetinidol-(4β→8)-catechin | AA | Legu | [166] |
| 387 | Fisetinidol-(4α→8)-catechin | AA | Legu | [168] |
| 388 | Fisetidinol-(4α→8)-3-O-galloylcatechin | AA | Legu | [166] |
| 389 | Ent-fisetinidol-(4β→8)-epicatechin | AA | Anac | [149] |
| 390 | Ent-fisetinidol-(4β→8)-catechin | AA | Anac, Legu | [149,169] |
| 391 | Ent-fisetinidol-(4α→8)-catechin | AA | Anac, Legu | [149] |
* Adox: Adoxaceae; Cela: Celastraceae; Cras: Crassulaceae; Gros: Grossulaceae; Mimo: Mimosaceae; Phyl: Phyllanthaceae; Rhiz: Rhizophoraceae; and Rubi: Rubiaceae.
Table 8.
The 4′-8′′, 5-5′′, 6-6′′, 6-γ, 6-8′′, 7-7′′, and 8-8′′ subtypes of biflavonoids.
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| 4′-8′′ | 392 | 5,3′,5′′,4′′′-tetrahydroxy-3′′′,5′′′-dimethoxy-biflavone(4′→8′′)-7-O-(2-rhamnoside)rhamnoside | BB | Apia | [170] |
| 5-5′′ | 393 | 8,8′-bis(7,8-dihydroxy-2-C-methyl-2H-1-benzopyran-5-yl)-4,4′-dimethoxy-[5,5′-bi-6H-furo[3,2-h][1]benzopyran]-6,6′-dione | BB | Legu | [171] |
| 394 | 3-C-(6-deoxy-α-L-mannopyranosyl)-3′-C-α-d-glucopyranosyl-2,2′-bis(4-hydroxyphenyl)-7,7′-dimethyl-[5,5′-bi-4H-1-benzopyran]-4,4′-dione | BB | Legu | [171] | |
| 6-6′′ | 395 | Succedaneaflavanone | AA | Anac | [1] |
| 396 | 6,6′′-bigenkwanin | BB | Ochn | [172] | |
| 6-γ | 397 | 8-methylsocotrin-3′-methoxy-4′-ol | AG | Drac | [1] |
| 398 | 8-methylsocotrin-4′-methoxy-3′-ol | AG | Drac | [1] | |
| 399 | 8-methylsocotrin-3-methoxy-4-ol | AG | Drac | [1] | |
| 400 | 8-methylsocotrin-4-methoxy-3-ol | AG | Drac | [1] | |
| 401 | 8-methylsocotrin-4-ol | AG | Drac | [1] | |
| 6-8′′ | 402 | 6,8′′-bigenkwanin | BB | Arau, Ochn | [172,173] |
| 403 | Agathisflavone | BB | Anac, Ochn | [173] | |
| 404 | 7,7′′,4′′′-tri-O-methylagathisflavone | BB | Arau | [1,174] | |
| 405 | 7,4′′′-di-O-methylagathisflavone | BB | Arau | [1] | |
| 406 | Agathisflavone A | BB | Arau | [173,174] | |
| 407 | Ouratine A | BB | Ochn | [1,175] | |
| 408 | Agatisflavone | AB | Ochn | [1] | |
| 409 | 7,4′,7′′,4′′′-tetra-O-methylcupressuflavone | AB | Sela | [7] | |
| 410 | 7,4′,7′′-tri-O-methylcupressuflavone | AB | Arau | [1] | |
| 411 | 7,7′′-di-O-methylcupressuflavone | AB | Arau | [1] | |
| 412 | Rhusflavone | AB | Anac | [176] | |
| 413 | Lateriflavanone | AB | Clus | [1] | |
| 414 | 4′′′-O-methylagatisflavone | AB | Clus, Ochn | [54,177] | |
| 415 | Rhusflavanone | AA | Anac | [178] | |
| 416 | 6,8′′-binaringenin | AA | Clus | [178] | |
| 417 | 3′′′,4′,4′′′,5,5′′,7,7′′-hepta-me ether-3,3′′,3′′′,4′,4′′′, 5,5′′,7,7′′-nonahydroxy-6,8′′-biflavanone | AA | Ochn | [179] | |
| 418 | Ouratine B | BB | Ochn | [175] | |
| 419 | 4′′′-O-methylagathisflavone | BB | Ochn | [177] | |
| 420 | 7′′-O-methylagathisflavone | BB | Ochn | [180] | |
| 421 | Agathisflavone B | BB | Arau | [174] | |
| 7-7′′ | 422 | 4′-methoxy-7,7′′-biflavone | BB | Legu | [181] |
| 8-8′′ | 423 | Cupressuflavone | AA | Anac, Arau, Cupr | [182] |
| 424 | 3,3′′-dihydroxycupressuflavone | BB | Thea | [183] | |
| 425 | 4′-O-methylcupressuflavone | BB | Clus | [54] | |
| 426 | Mesuaferrone B | AB | Anac, Clus | [178,184] | |
| 427 | 4′,4′′′-di-O-methylcupressuflavanone | AA | Comp | [185] | |
| 428 | (R)4′-O-β-D-glucopyranoside-cupressuflavone | AA | Cupr | [186] | |
| 429 | (S)4′-O-β-D-glucopyranoside-cupressuflavone | AA | Cupr | [186] | |
| 430 | 7-me ether-cupressuflavone | BB | Arau | [173,187] | |
| 431 | 8,8′′-bigenkwanin | BB | Arau, Cupr | [186,187] | |
| 432 | W11 | BB | Arau, Phyl | [188] | |
| 433 | 4′,7,7′′-tri-O-methylcupressuflavone | BB | Arau | [187] | |
| 434 | WB1 | BB | Arau | [173,187] | |
| 435 | Moghatin | BB | Malv | [189] | |
| 436 | Neorhusflavanone | AA | Anac, Calo | [184,190] |
* Apia: Apiaceae; Calo: Calophllaceae; Comp: Compositae; Drac: Dracaenaceae; and Rham: Rhamnaceae.
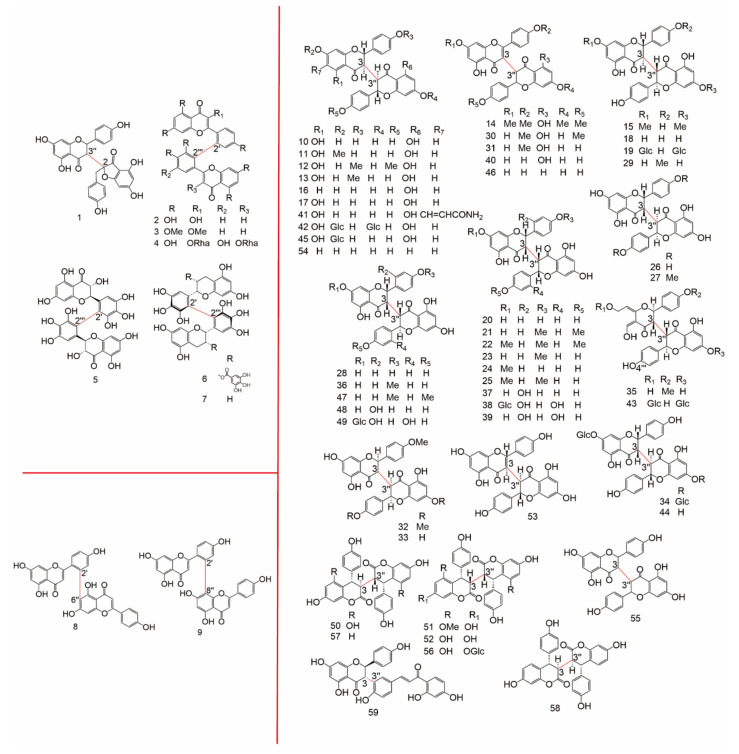
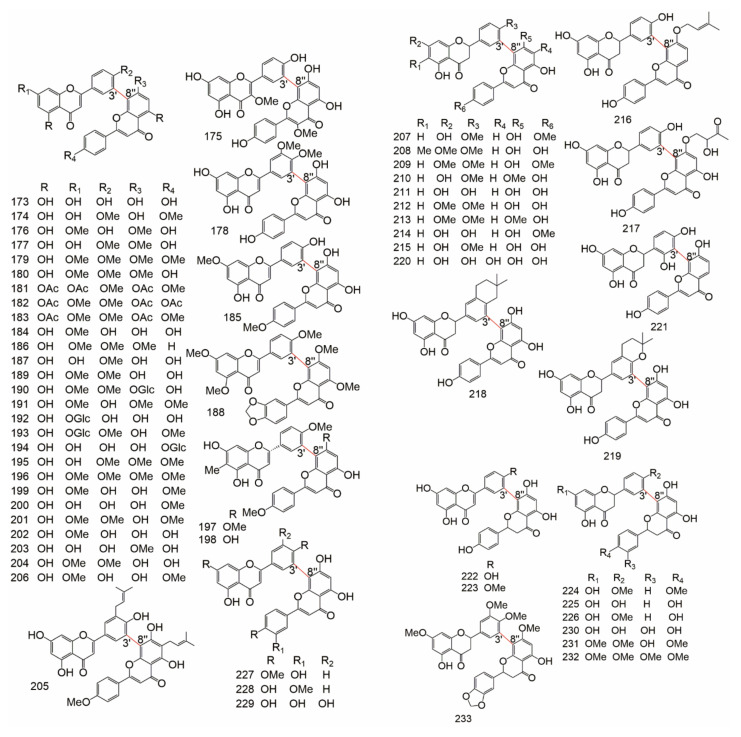
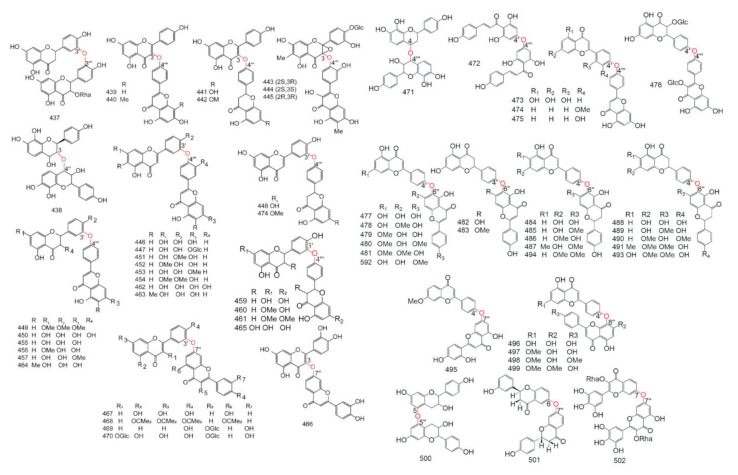
The detailed data about subtypes, No., monomer types, origin families and references of 2-3′′, 2′-2′′′, 2′-6′′, 2′-8′′, 3-3′′ type biflavonoids were showed in Table 1, the structure of them were drew in Figure 2.
Figure 2.
The structure of 2-3′′, 2′-2′′′, 2′-6′′, 2′-8′′, and 3-3′′ type.
The detailed data of 3-3′′′, 3′-3′′′, 3′-4′′′,3′-5′′, 3-6′′, 3′-6′′, 3-7′′, 3′-7′′ type biflavonoids were showed in Table 2, the structure of them were drew in Figure 3.
Figure 3.
The structure of 3-3′′′, 3′-3′′′, 3′-4′′′,3′-5′′, 3-6′′, 3′-6′′, 3-7′′, and 3′-7′′type.
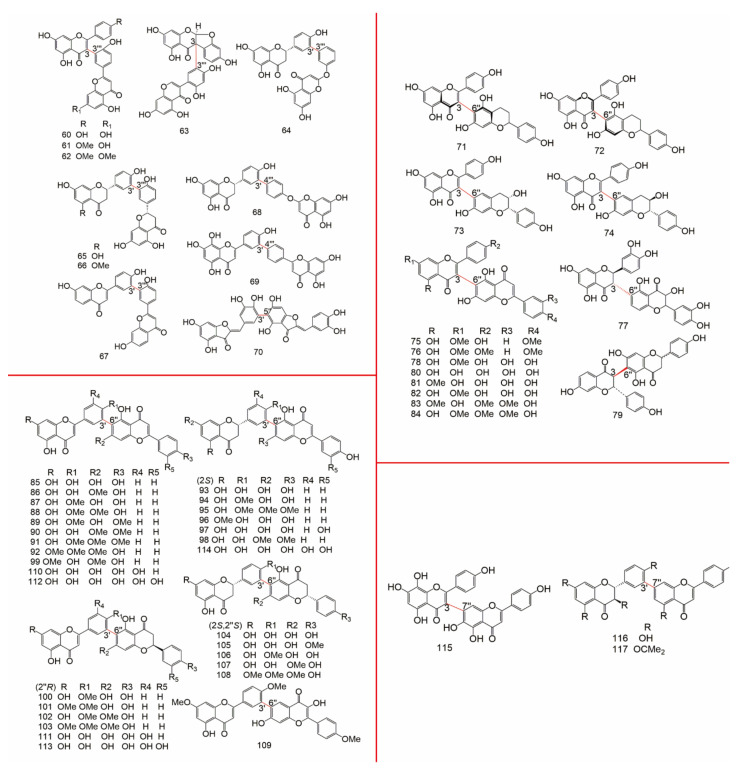
The data of 3-8′′ type biflavonoids were showed in Table 3, the structure of them were drew in Figure 4.
Figure 4.
The structure of 3-8′′ type biflavonoids.
The data of 3′-8′′ type biflavonoids were showed in Table 4, the structure of them were drew in Figure 5.
Figure 5.
The structure of 3′-8′′ type biflavonoids.
The data of 4-6′′ type biflavonoids were showed in Table 5, the structure of them were drew in Figure 6.
Figure 6.
The structure of 4-6′′ type biflavonoids.
The data of 4-8′′ type biflavonoids were showed in Table 6 and Table 7, the structure of them were drew in Figure 7.
Figure 7.
The structure of 4-8′′ type biflavonoids.
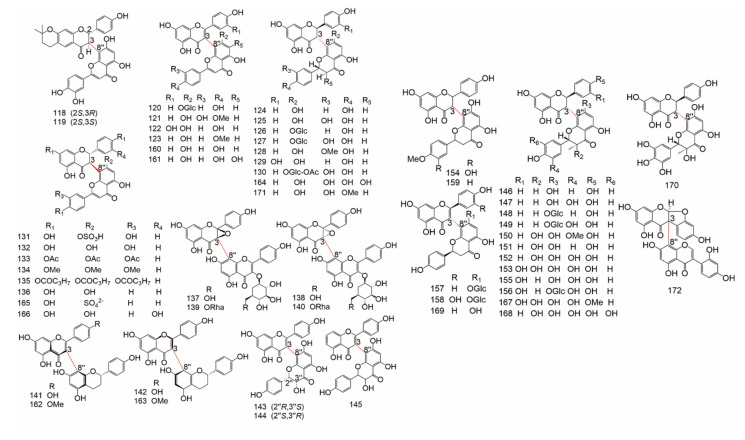
The data of 4′-8′′, 5-5′′, 6-6′′, 6-γ, 6-8′′, 7-7′′, and 8-8′′ type biflavonoids were showed in Table 8, the structure of them were drew in Figure 8.
Figure 8.
The structure of 4′-8′′, 5-5′′, 6-6′′, 6-γ, 6-8′′, 7-7′′, and 8-8′′ type biflavonoids.
4.2. C-Linear Fragment-C Type
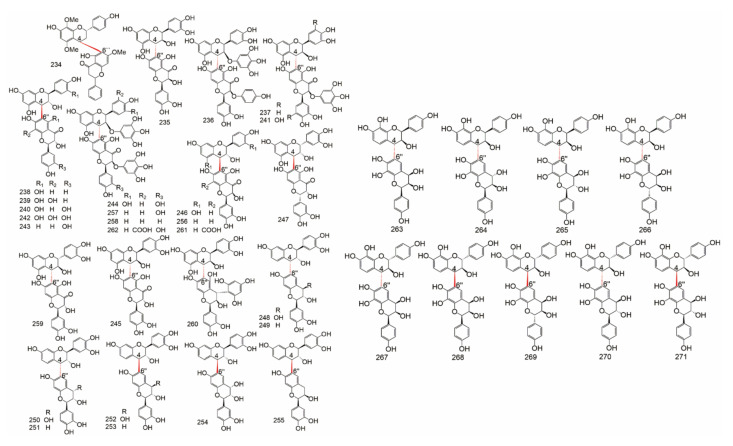
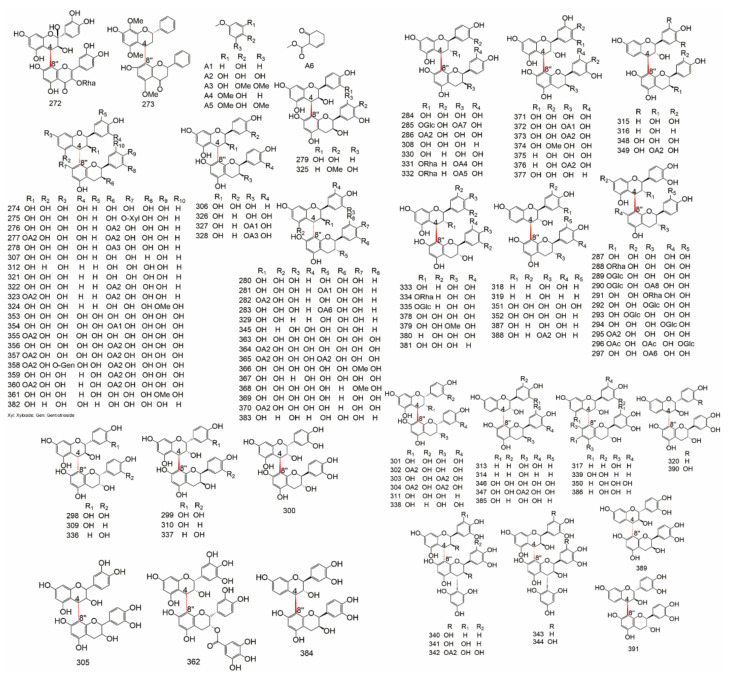
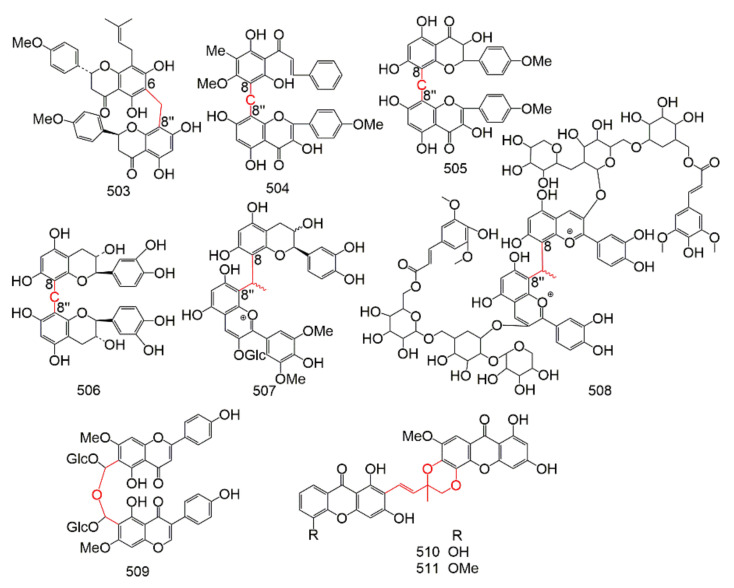
Group B (Table 9) is consist of C-O-C connections, C-C-C connections and other linear fragment connections, including: 3′-O-3′′′, 3-O-4′′, 3-O-4′′′, 3′-O-4′′′, 3-O-7′′, 3′-O-7′′, 4-O-4′′, 4′-O-4′′′, 4′-O-6′′, 4′-O-7′′, 4′-O-8′′, 5-O-5′′, 6-O-7′′, 7-O-7′′, 6-C-8′′, and 8-C-8′′. The structure of C-linear fragment-C biflavonoids were showed in Figure 9 and Figure 10.
Table 9.
The C-linear fragment-C subtypes of biflavonoids.
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| 3′-O-3′′′ | 437 | Sparinaritin | AB | Chry | [191] |
| 3-O-4′′ | 438 | Epioritin(4β-3)-epioritin-4β-ol | AA | Legu | [20] |
| 3-O-4′′′ | 439 | Delicaflavone | BB | Sela | [7] |
| 440 | 5,7,4′,5′′-tetrahydroxy-7′′-metroxy-[3-O-4′′′]-biflavone | BB | Sela | [7] | |
| 441 | Chrysocauloflavone I | AB | Sela | [7] | |
| 442 | Chrysocauloflavone II | AB | Sela | [7] | |
| 443 | Baeckein E | AB | Myrt | [192] | |
| 444 | Baeckein C | AB | Myrt | [192] | |
| 445 | Baeckein D | AB | Myrt | [192] | |
| 3′-O-4′′′ | 446 | Ochnaflavone | BB | Ochn | [7,193] |
| 447 | Ochnaflvone 7′′-O-β-D-glucopyranoside | BB | Ochn | [194] | |
| 448 | 2′′,3′′-dihydroochnaflavone | AB | Ochn | [7,195] | |
| 449 | 2,3-dihydro-4′,7,7′′-tri-O-methylochnaflavone | AB | Ochn | [7,196] | |
| 450 | Sulcatone A | AB | Ochn | [197] | |
| 451 | 4′-me ether-ochnaflavone | BB | Ochn | [193] | |
| 452 | 7-O-methylochnaflavone | BB | Ochn | [198] | |
| 453 | 7′′-O-methylochnaflavone | BB | Ochn | [199] | |
| 454 | 4′,7-di-O-methylochnaflavone | BB | Ochn | [193] | |
| 455 | 2,3-dihydroochnaflavone | AB | Ochn | [200] | |
| 456 | 2,3-dihydro-7-O-methylochnaflavone | AB | Ochn | [200] | |
| 457 | 2,3-dihydro-7′′-O-methylochnaflavone | AB | Ochn | [9] | |
| 458 | 2′′,3′′-dihydro-7′′-O-methylochnaflavone | AB | Ochn | [9] | |
| 459 | 2,2′′,3,3′′-tetrahydroochnaflavone | AA | Para | [201] | |
| 460 | 2,2′′,3,3′′-tetrahydro-7-O-methylochnaflavone | AA | Ochn, Para | [201] | |
| 461 | 2,2′′,3,3′′-tetrahydro-7,7′′-di-O-methylochnaflavone | AA | Para | [202] | |
| 462 | 3′′′-hydroxyochnaflavone | BB | Rubi | [203] | |
| 463 | 6,6′′-dimethylochnaflavone | BB | Sela | [1] | |
| 464 | 2,3-dihydro-6,6′′-dimethylochnaflavone | AB | Sela | [196] | |
| 465 | Hypnumbiflavanoid B | AA | Hypn, Ochn | [197,204,205] | |
| 3-O-7′′ | 466 | 3-O-7′′-biluteolin | BB | Aste | [206] |
| 3′-O-7′′ | 467 | Lophirone L | BB | Ochn | [52] |
| 468 | Lophirone penta-acetate | BB | Ochn | [52] | |
| 469 | 5,7,4′,5′′,3′′′,4′′′′-hexahydroxy-3′′-O-β-glucosyl-3′,7′′-O-biflavone | BB | Legu | [207] | |
| 470 | 5,7,4′,5′′,3′′′,4′′′′-hexahydroxy-3,3′′-di-O-β-glucosyl-3′,7′′-O-biflavone | BB | Vita | [208] | |
| 4-O-4′′ | 471 | Epimesquitol(4β-4)-epioritin-4β-ol | AA | Legu | [20] |
| 4′-O-4′′′ | 472 | Achyrobichalcone | GG | Aste | [209] |
| 473 | Loniflavone | BB | Capr | [1] | |
| 474 | 3′-O-methylloniflavone | BB | Capr | [210] | |
| 475 | Oniflavone | BB | Capr | [210] | |
| 476 | Ericoside | AB | Eric | [211] | |
| 4′-O-6′′ | 477 | Hinokiflavone | BB | Cupr, Psil, Sela, Taxo, Cyca | [212,213,214,215,216] |
| 478 | Isocryptomerin | BB | Cupr, Sela, Taxo | [7,213,214,215,217] | |
| 479 | Neocryptomerin | BB | Podo | [7] | |
| 480 | Cryptomerin B | BB | Taxo | [7,214,218] | |
| 481 | Chamaecyparin | BB | Cupr, Sela | [7,217,219] | |
| 482 | 2,3-dihydrohinokiflavone | AB | Cupr, Cyca | [7,215,216] | |
| 483 | (2S)-2,3-dihydroisocryptomerin | AB | Sela | [7,37] | |
| 484 | 2′′,3′′-dihydrohinokiflavone | AB | Sela | [7,220] | |
| 485 | 2′′,3′′-dihydroisocryptomerin | AB | Sela | [7,221] | |
| 486 | 7-O-methyl-2′′,3′′-dihydroisocryptomerin | AB | Sela | [221] | |
| 487 | Taiwaniaflavone B | AB | Capr | [7,79] | |
| 488 | (2S,2S’’)-2,3,2′′,3′′-tetrahydrohinokiflavone | AA | Cyca | [7,222] | |
| 489 | 7′′-O-methyl-2,3,2′′,3′′-tetrahydrohinokiflavone | AA | Sela | [7] | |
| 490 | 7,4′′-di-O-methyl-2,3,2′′,3′′-tetrahydrohinokiflavone | AA | Sela | [7] | |
| 491 | Oliveriflavone A | AA | Capr | [85] | |
| 492 | Cryptomerin A | BB | Taxo | [215,218] | |
| 493 | 2,2′′,3,3′′-tetrahydro-7,7′′-di-O-methylhinokiflavone | AA | Cyca | [223] | |
| 494 | 2,3-dihydrochamaecyparin | AB | Sela | [39] | |
| 4′-O-7′′ | 495 | Brevipedicelone E | BB | Clus | [224] |
| 4′-O-8′′ | 496 | Lanaroflavone | BB | Anac, Lana | [225,226] |
| 497 | 7-O-methyllanaroflavone | BB | Ochn | [227] | |
| 498 | 4′′′,7-di-O-methyllanaroflavone | BB | Ochn | [227] | |
| 499 | 7,7′′-di-O-methyllanaroflavone | BB | Ochn | [228] | |
| 5-O-5′′ | 500 | Potifulgene | AA | Rosa | [1] |
| 6-O-7′′ | 501 | Masazinoflavanone | AA | Anac | [229] |
| 7-O-7′′ | 502 | (myricetin-3-O-α-L-rhamnoside(C7I-O-C7II)myricetin-3-O-α-L-rhamnoside | BB | Legu | [230] |
| 6-C-8′′ | 503 | Bosistoabiflavanone | AA | Ruta | [231] |
| 8-C-8′′ | 504 | Ttrianguletin | BG | Adia | [20] |
| 505 | Pentagrametin | AB | Adia | [20] | |
| 506 | Di(8-catechinyl)methane | AA | Malv | [232] | |
| 507 | 3-O-β-D-glucopyranoside-malvidin 8-(8-ethylcatechin) | AC | Red wine | [233] | |
| 508 | 3,3′-bis-O-[β-d-xylopyranosyl-(1→2)-[4-hydroxy-3,5-dimethoxy-E-cinnamoyl-(→6)-β-d-glucopyranosyl-(1→6)]-β-d-galactopyranoside] | CC | Apia | [234] | |
| Others | 509 | Carinoside A | BE | Gent | [235] |
| 510 | Mesuferrol A | II | Clus | [236] | |
| 511 | Mesuferrol B | II | Clus | [236] |
* Adia: Adiantaceae; Chry: Chrysobalanaceae; Gent: Gentianaceae; Hypn: Hypnaceae; Para: Paracryphiaceae; Lana: Lanariaceae; Psil: Psilotaceae and Ruta: Rutaceae.
Figure 9.
The structure of C-O-C type biflavonoids.
Figure 10.
The structure of C-C-C and others type biflavonoids.
4.3. Complex Biflavonoids
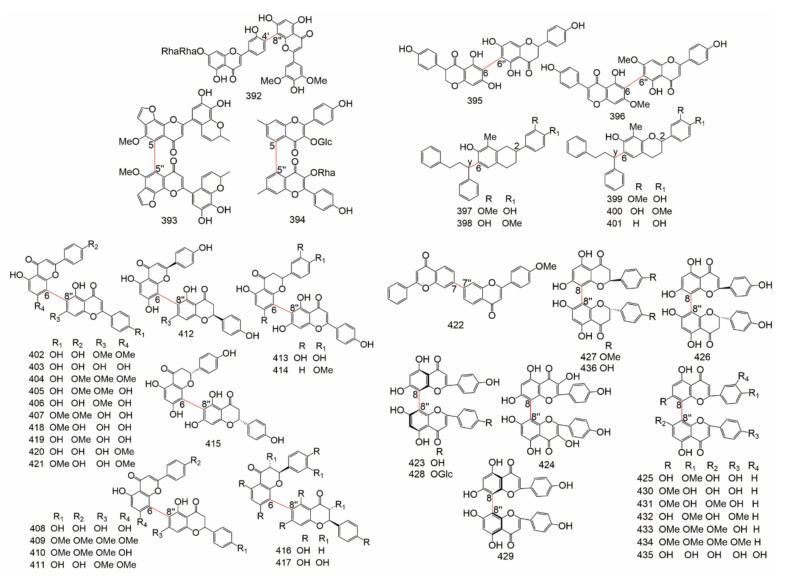
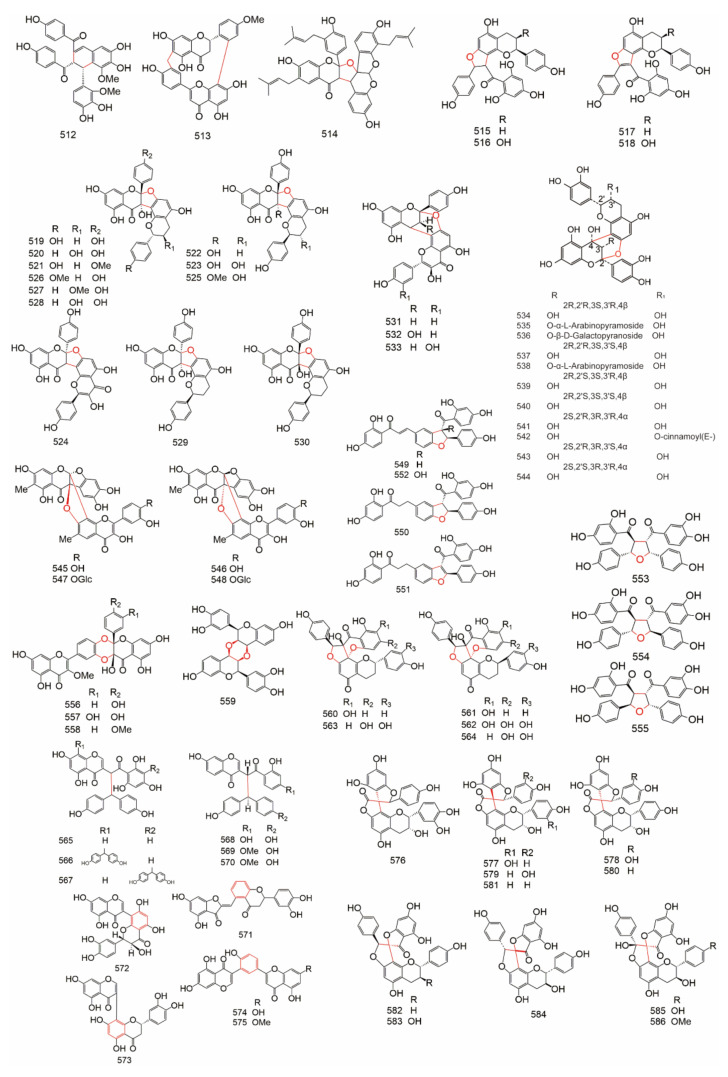
Group C belongs to the complex biflavonoids (Table 10 and Table 11). They include the simple-ring type (C-C & C-C, C-C & C-C-C, C-C & C-O-C, C-O-C & C-O-C), the bicyclic type, the atom-shared type, and spirobiflavonoids. The structure of complex biflavonoids were showed in Figure 11.
Table 10.
Complex biflavonoids. (Simple-ring type).
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| C-C & C-C | 512 | Licobichalcone | GG | Legu | [237] |
| C-C & C-C-C | 513 | Selacyclicbifkavone A | AB | Sela | [7] |
| C-C & C-O-C | 514 | Licoagrodin | AD | Legu | [20] |
| 515 | Daphnodorin A | AG | Thym | [29,238] | |
| 516 | Daphnodorin B | AG | Thym | [29,238] | |
| 517 | Dihydrodaphnodorin B | AG | Thym | [239] | |
| 518 | Daphnodorin J | AG | Thym | [29,238,240] | |
| 519 | Daphnodorin E | AA | Thym | [29] | |
| 520 | Daphnodorin H | AA | Thym | [29] | |
| 521 | 4′-methoxydaphnodorin E | AA | Thym | [241] | |
| 522 | Daphnodorin F | AA | Thym | [29] | |
| 523 | Daphnodorin G | AA | Thym | [29] | |
| 524 | Lawsonia biflavone A | AB | Lyth | [230] | |
| 525 | 3′-O-methyldaphnodorin G | AA | Thym | [242] | |
| 526 | 4′′-O-methyldaphnodorin E | AA | Thym | [241] | |
| 527 | 3-O-methyldaphnodorin H | AA | Thym | [242] | |
| 528 | 3′-O-methyldaphnodorin H | AA | Thym | [242] | |
| 529 | Daphnogirin A | AA | Thym | [243] | |
| 530 | Daphnogirin B | AA | Thym | [243] | |
| 531 | Ephedrannin B | AB | Ephe, Daph | [244,245] | |
| 532 | Ephedrannin A | AB | Ephe, Daph, Vita | [244,245] | |
| 533 | Ent-epiafzelechin-(2α→7, 4α→8)-quercetin | AB | Rosa | [246] | |
| 534 | Proanthocyanidin A5′ | AA | Ephe, Rosa | [247,248] | |
| 535 | 3-O-α-L-arabinopyranosylproanthocyanidin A5′ | AA | Malv | [248] | |
| 536 | 3-O-β-D-galactopyranosylproanthocyanidin A5′ | AA | Malv | [248] | |
| 537 | Pavetannin A2 | AA | Ephe, Rosa, Rubi | [249] | |
| 538 | 3-O-α-L-arabinopyranosylpavetannin A2 | AA | Malv | [232] | |
| 539 | Ent-epicatechin-(2α→7,4α→8)-ent-catechin | AA | Rubi | [250] | |
| 540 | Ent-epicatechin-(2α→7,4α→8)-ent-epicatechin | AA | Ephe | [248] | |
| 541 | Proanthocyanidin A2 | AA | Sapi, Legu, Laur | [251,252,253] | |
| 542 | 3′-O-trans-cinnamoylproanthocyanidin A2 | AA | Legu | [254] | |
| 543 | Proanthocyanidin A1 | AA | Sapi, Legu, Laur | [253] | |
| 544 | Proanthocyanidin A4 | AA | Sapi, Rubi | [250] | |
| 545 | Baeckein F | AB | Myrt | [1] | |
| 546 | Baeckein G | AB | Myrt | [255] | |
| 547 | Baeckein H | AB | Myrt | [1] | |
| 548 | Baeckein I | AB | Myrt | [255] | |
| 549 | Lophirone C | GG | Ochn | [256,257] | |
| 550 | Dihydrolophirone C | GG | Ochn | [257] | |
| 551 | Isolophirone C | GG | Ochn | [257] | |
| 552 | Lophirone K | GG | Ochn | [258] | |
| 553 | Lophirone F | GG | Ochn | [259] | |
| 554 | Lophirone G | GG | Ochn | [259] | |
| 555 | Lophirone L‡ | GG | Ochn | [260] | |
| C-O-C & C-O-C | 556 | Dysoverine D | AB | Berb | [261] |
| 557 | Dysoverine F | AB | Berb | [261] | |
| 558 | Dysoverine A | AB | Berb | [261] | |
| 559 | (2R,2′R,3S,3′S,4α,4′α)-3′,4′,7-trihydroxyflavan-(3→O→4)(4→O→3)-3′,4′,7-trihydroxyflavan | AA | Legu | [262] |
* Berb: Berberidaceae; Daph: Daphniphyllaceae; Ephe: Ephedraceae; Laur: Lauraceae; and Lyth: Lythraceae.
Table 11.
The other types of bioflavonoids.
| Subtype | No. | Compounds Name | Monomer Type | Origin (Family *) | References |
|---|---|---|---|---|---|
| Bicyclic type | 560 | Daphnodorin M | AG | Thym | [29] |
| 561 | Daphnodorin N | AG | Thym | [29] | |
| 562 | Stelleranol | AG | Thym | [1] | |
| 563 | Genkwanol B | AG | Thym | [1] | |
| 564 | Genkwanol C | AG | Thym | [1] | |
| Atom-shared type | 565 | Chamaechromone | EG | Thym | [63] |
| 566 | Mohsenone | EG | Thym | [63] | |
| 567 | Isomohsenone | EG | Thym | [63] | |
| 568 | Lophirone A | EG | Ochn | [263] | |
| 569 | Calodenone | EG | Ochn, Anac | [264] | |
| 570 | Afzelone D | EG | Ochn | [265] | |
| 571 | Campylopusaurone | AH | Clus | [266] | |
| 572 | Preussianone | AB | Clus | [267] | |
| 573 | Paucinervin K | AE | Clus | [268] | |
| 574 | Lancedatin A | BD | Legu | [20] | |
| 575 | Lancedatin B | BD | Legu | [20] | |
| Spirobiflavonoids | 576 | Absienol A | AA | Mora | [60] |
| 577 | Absienol B | AA | Pina | [1] | |
| 578 | Absienol C | AA | Mora | [60] | |
| 579 | Absienol D | AA | Pina | [1] | |
| 580 | Absienol E | AA | Mora | [60] | |
| 581 | Absienol F | AA | Pina | [1] | |
| 582 | Daphnodorin C | AA | Thym | [29] | |
| 583 | Daphnodorin I | AA | Thym | [29] | |
| 584 | Genkwanol A | AA | Thym | [269] | |
| 585 | 2′′-hydroxygenkwanol A | AA | Thym | [1] | |
| 586 | 4′-methylgenkwanol A | AA | Thym | [1] | |
| 587 | Olgensisinol A | AA | Pina | [270] | |
| 588 | Olgensisinol B | AA | Pina | [270] | |
| 589 | Olgensisinol C | AA | Pina | [270] | |
| 590 | Olgensisinol D | AA | Pina | [270] | |
| 591 | Vitisinol | AA | Pina | [270] | |
| 592 | Larixinol | AA | Pina | [271] |
Figure 11.
The structure of Complex biflavonoids.
5. Pharmacology of Biflavonoids
5.1. Antioxidant
Andrade et al. [272] conducted an antioxidant test on agathisflavone in 2018. Trolox was used as a control, and agathisflavone was extracted and isolated from the fresh leaves of Caesalpinia pyramidalis Tull. In the experiment of DPPH radical scavenging, it was found that agathisflavone scavenged DPPH free radicals in a concentration-dependent manner; the EC50 of agathisflavone was 0.474 mM, and for Trolox it was 0.149 mM, within the 95% confidence interval. The ABTS scavenging assay data found that agathisflavone was EC50 = 0.179 mM, while for Trolox, it was EC50 = 0.311 mM. In the OH radical scavenging assay, agathisflavone also showed a concentration-dependent hydroxyl radical scavenging ability, while agathisflavone and Trolox both showed a concentration-dependent reduction in the three iron ions to ferrous iron. Through structural analysis of agathisflavone, it was found that the hydroxyl groups at positions 4′,7,7′′,4′′′ in its structure can provide free radical hydrogen to reduce free radicals. In addition, agathisflavone can also inhibit the production of TBARS, and has a significant ability to protect against oxidative damage, indicating that agathisflavone is likely to be a good antioxidant.
The antioxidant effect of Garcinia kola is mainly based on the biflavonoids in the extract. Through the DPPH method and the ATBS method, Lixian et al., studied the antioxidant capacity of garcinianin, kolaflavanone, GB1a, GB2, and panciflavanon. The antioxidant activity of different compounds determined by the DPPH method was garcinianin > panciflavanon > GB2 > kolaflavanone > GB1a, and the antioxidant activity of different compounds determined by the ABTS method was garcinianin > panciflavanon > GB1a > kolaflavanone > GB2. Among them, the antioxidant effect of garcinianin was more obvious [273].
In a study of the antioxidant mechanism of the neuroprotective biflavonoids, hinokiflavone, isocryptomerin, amentoflavone, ginkgetin, amentoflavone, and ginkgetin have good antioxidant capacities, can inhibit the activity of SOD, GR, Gpx, CAT, and other oxidases, reduce the content of GSH, and achieve an antioxidant effect. Ginkgetin can also act on the ERK1/2 target for antioxidants [274]. In 2013, Jia et al. [192] extracted baeckein E from Baeckea frutescens and six other known compounds, and its IC50 value ranged from 11.8–16.1 μM in the DPPH free radical scavenging test. Baeckein A and B (IC50 = 23.5 μM, IC50 = 26.2 μM) showed cytotoxicity and could not be used in H2O2-induced oxidation experiments. The treatment rates of biflavonoid baeckein E, baeckein C, and baeckein D were 31.8%, 34.8%, and 36.0%, respectively, which were lower than those of nonbiflavonoids (43.0~44.7%).
5.2. Anti-Inflammatory Properties
The anti-inflammatory activity of biflavonoids is mainly detected by inhibiting the expression of cyclooxygenase 2 (COX-2) and iNOS. In 2006, Park et al. [275] looked for C-C linked biflavonoids as anti-inflammatory drugs and examined the production of PGE2 and nitric oxide (NO) of synthetic biflavonoids in RAW cells treated with lipopolysaccharide (LPS). The results showed that 3′-6′′, 6-6′′, and 3-4′′′ linked biflavonoids showed resistance to COX-2 -mediated significant inhibition of PGE2 production (IC50 = 17.3 μM; IC50 = 3.7 μM; IC50 = 7.0 μM, respectively). Western blot and reverse transcription-polymerase chain reaction analyses showed that these compounds are not COX-2 downregulation mediated, but are instead COX-2 inhibition mediated. Among them, 6-6′′ biflavonoids have the strongest PGE2 production inhibitory activity. To ensure accuracy, PGE2 and NO tests were performed after LPS pretreatment. The IC50 of the 6-6′′ is < 3.0μM, and it can be used as a synthetic leader of new anti-inflammatory agents. However, the biflavonoids 4′-6′′ and 3-4′′′ can have cytotoxic effects on RAW cells.
In 2002, the anti-inflammatory mechanism of amentoflavone as a natural biflavonoid was studied. Banerjee et al. [276] found that amentoflavone can inhibit TNF-α-mediated COX-2 expression through the NF-κB pathway, thereby showing anti-inflammatory effects. In 2019, Li et al. [277] also studied the anti-inflammatory mechanism of the natural biflavonoid ginkgetin, and found that it can produce anti-inflammatory effects through the TLR4/NF-κB signaling pathway and improve ischemia/regeneration perfusion injury.
Jia et al. [255] extracted and separated root products from Baeckea frutescens in 2014 and discovered four new natural biflavonoids of baeckeins F-I. It was found that the four biflavonoids are the cyclic biflavonoids. The conformations of baeckein F, baeckein H (2S, 3S), baeckein G, and baeckein I (2R, 3R) are different, while baeckein H and baeckein I are glycosyl substituted biflavonoids. An anti-inflammatory activity test was performed in the RAW264.7 cell line induced by LPS to produce NO. It was found that the IC50 values of baeckein F, baeckein G, baeckein H, and baeckein I were 54.7 ± 5.26 μM, 25.4 ± 2.78 μM, 43.8 ± 3.30 μM, and 15.2 ± 1.34 μM, respectively, while the IC50 of the control indomethacin was 13.8 ± 1.29 μM, and there was no cytotoxicity. Data analysis showed that baeckein H and baeckein I had glycosylated biflavonoids that had more anti-inflammatory activity than the nonglycosylated biflavonoids. The anti-inflammatory activity of baeckein I was not much different from that of indomethacin, and it can be developed as a new anti-inflammatory drug.
There are many mechanisms for the anti-inflammatory activity of biflavonoids. There have been reviews summarizing the anti-inflammatory targets of natural biflavonoids including: ICAM-1, PPAR-γ, COX-2, NF-κB, iNOS, ERK1/2, MMP-9, TIMP-1, and PI3K/Akt, etc. [278]. These are all targets of conventional anti-inflammatory pathways. In addition, predictive pathways such as arachidonic acid metabolism are also new anti-inflammatory mechanisms of biflavonoids.
5.3. Antiviral Activities
To find new molecules against dengue fever virus (DV), Coulerie et al. [279] extracted four biflavonoids from the ethyl acetate extract of Dacrydium balansae, including amentoflavone, podovarpusflavone A, isoginkgetin, and hinokiflavone, and found that the biflavonoid compounds were the strongest inhibitors of the full activity of DV-NS5 RDRP and DV-NS5, with IC50s lower than 3.1 and 5.3 μM. The IC50 values were as follows: hinokiflavone (IC50 = 0.26 µM) > podovarpusflavone A (IC50 = 0.75 µM) > amentoflavone (IC50 = 1.40 µM) > isoginkgetin (IC50 = 3.10 µM). Hinokiflavone was the most active biflavonoid with IC50 = 0.26μM, but podocarpusflavone A was the strongest non-cytotoxic DV-NS5 inhibitor and could inhibit polymerase activity in the DV replicon, so podocarpusflavone A can be used as a template for the development of drugs against dengue fever virus. In addition, amentoflavone can also be developed as an antiviral drug for herpes simplex virus (HSV-1) [280], and agathisflavone can produce an anti-influenza virus effect [281].
5.4. Antibacterial and Antifungal Activities
Although the antibacterial and antifungal effects are different in mechanism, this review describes them to facilitate the summary of biflavonoids. Tang et al. [282] isolated six biflavonoids from the bark of Ochna macrocalyx. Dehydroxyhexaspermone C, and hexaspermone C are the C-C linked biflavonoids, and ochnone, cordigol, calodenin B, and 2,3-dihydrocalodenin B are all different from general biflavonoids. Calodenin B and 2,3-dihydrocalodenin B have a certain cytotoxicity, but also show strong antibacterial effects. Compared with the control drug, the antibacterial activities of calodenin B and 2,3-dihydrocalodenin B were more obvious. In addition, fukugiside can inhibit the activity of Streptococcus pyogenes [283].
The antifungal activity test mainly uses Candida albicans to test the antifungal effect of the biflavonoids. Lee et al. [284] used bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)], a traditional membrane potential dye, in a regeneration test with fungal protoplasts to study the mechanism of isocryptomerin by depolarization. In this study, amphotericin B was used as a positive control, and isocryptomerin had an MIC value of 18.11 μM, which showed antifungal activity against human pathogenic fungi (such as Candida albicans and Trypanosoma beige). The cumulative amount of the DiBAC4(3) in isocryptomerin is small and less than the value of amphotericin B, which proves that it destroys the plasma membrane of Candida albicans and causes cell death. In addition, fungal arthritis, caused by Candida albicans, and ochnaflavone can promote the expression of IL-2 and IL-10 through the T cell immune system, and inhibit the expression of inflammatory mediators such as IFN-γ and IL-2, but it does not cause hemolysis, kill redundant macrophages, or improve fungal arthritis [285].
5.5. Anti-Diabetic and Anti-Atherosclerosis
A biflavonoid composed of two molecules of kaemferol was isolated from the seeds of Semecarpus anacardium and its antihyperglycemic mechanism in diabetic mice induced by a high-fat diet plus streptozotocin, was studied showing it could reduce the content of plasma glucose and increase the level of plasma insulin [286]. At a dose of 80 mg/kg b.wt, the biflavonoid’s effect is basically the same as that of metformin, and when the biflavonoid is combined with metformin, they can significantly increase liver and muscle glycogen content, maintain hemoglobin levels, and restore the glycosynthase and glycogen phosphorylase close to normal levels. The glucose metabolism is also maintained at a normal level, and it can significantly increase enzymatic antioxidants (SOD, CAT, GPx, and GST) and nonenzymatic antioxidants (vitamin C, vitamin E, and GSH) and improve the activity of enzymes, thereby curing hyperglycemia. Liu et al. [287] indicated that biflavonoids (isoginkgetin, bilobetin, ginkgetin, and sciadopitysin), which are extracted from Ginkgo biloba, have the potential to become pancreatic lipase inhibitors. Four natural biflavonoids had a strong inhibitory effect on pancreatic lipase, and their residual activities were isoginkgetin = 35.7%, bilobetin = 22.3%, ginkgetin = 41.6%, and sciadopitysin = 58.6%. Through the lipase of a concentration-dependent inhibitor of 4-MUO hydrolysis, each IC50 value was isoginkgetin = 2.90 ± 0.98 μM, bilobetin = 3.57 ± 0.53 μM, ginkgetin = 6.90 ± 1.60 μM, and sciadopitysin = 12.78 ± 2.30 μM, showing a degree of medium to strong inhibition. Isoginkgetin can also improve the healing of foot ulcer wounds in diabetic rats [288].
There are many pathological mechanisms of atherosclerosis, but they are related to hypertension, hyperlipidemia, and other mechanisms. Therefore, the treatment of atherosclerosis is basically inseparable from the antioxidant and anti-inflammatory effects [289]. Tabares-Guevara et al. performed oxygen radical absorbance capacity (Orac) and lDl oxidation inhibition assays on three natural biflavonoids: morelloflavone, volkensiflavone, and fukugiside, and found that all of them were effective reactive oxygen scavengers, inhibited the production of reactive oxygen species and the secretion of proinflammatory factors (IL-6, IL-12p70, MCP-1, TNF-α, MIP-1α, and NLRP3, etc.) in macrophages, and they reduced the circulating levels of cholesterol and the lipid peroxidation product propylene glycol, showing the antioxidation, anti-inflammatory, hypolipidemic, and anti-atherosclerotic effects of biflavonoids in the body [290].
5.6. Alzheimer’s Disease and Parkinson’s Disease
Alzheimer’s disease in terms of anti-inflammatory, antioxidative stress, and neurodegenerative damage overlaps to a large extent with the treatment pathway of biflavonoids [291] so biflavonoids have great potential in the treatment of Alzheimer’s disease [292]. Moreover, due to the aromatic interaction of biflavonoids, their therapeutic effect is better than that of a flavonoid [293], indicating that biflavonoids can be used as lead compounds for the development of treatments for Alzheimer’s disease. In particular, the amentoflavone type includes amentoflavone (1) and its monomethoxy derivatives. They can inhibit the formation and accumulation of amyloid β, thereby preventing Alzheimer’s disease [294].
Choi et al. used the peptide of Aβ1-42 to inhibit the aggregation of Aβ1-42 in vitro by thioflavin T fluorescence analysis of biflavonoids (amentoflavone, bilobetin, sequoiaflavone, sotetsuflavone, podocarpuflavone, ginkgetin, isoginkgetin, and sciadopitysin), and found that amentoflavone has the strongest comprehensive strength in inhibiting the formation of Aβ1-42 fibers and reducing the formation of Aβ1-42 fibers among the eight biflavonoids, and it has great potential as a lead compound for treating Alzheimer’s disease [295]. CGY-1 [82], GB1, and other gambogic biflavonoids [296] also have the potential to treat Alzheimer’s disease.
Biflavonoids extracted from Impatiens balsamina can prevent the production of NO, have neuroprotective activity, and improve neurodegenerative diseases [61]. Amentoflavone can improve Parkinson’s disease through the PI3K/Akt and ERK signaling pathways [297], while ginkgetin can improve Parkinson’s disease nerve damage through neuroprotection [298].
5.7. Cytotoxic Activity and Antitumor Activities
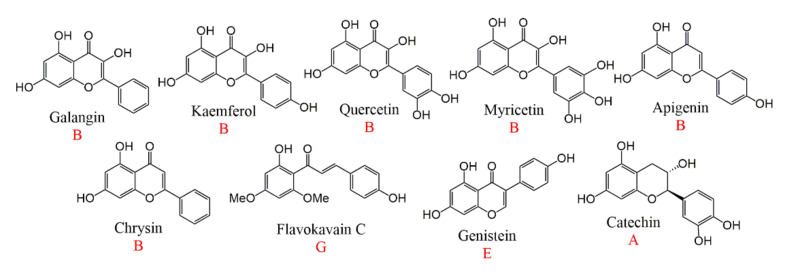
The cytotoxicity of flavonoids with different structures is also different. A review had summarized that the flavonoids with flavone(B) units (galangin, kaempferol, quercetin, myricetin, apigenin, and chrysin) had the ability to antihepatoma; the flavonoids with chalcone(G) units (flavokavain C) could cause hepatic failure; the flavonoid with isoflavone(E) units (genistein) had an antiestrogen, increasing the risk of breast cancer and the flavonoids with flavan(A) units (catechin) had no effect on tumor cells, but had the hemolytic anemia thrombocytopenia [299]. Biflavonoids are composed of two flavone monomers, so the toxicity study of flavonoids is also helpful to the toxicity activity of biflavonoids. The structure of these flavonoids are shown in Figure 12.
Figure 12.
The structure of flavonoids.
For the toxicity of biflavonoids, a study found that amentoflavone, sciadopitysin, ginkgetin, isoginketin, and bilobetin extracted from ginkgo can reduce the cell viability of human renal tubular epithelial cells (HK-2 cells) in a dose-dependent manner. Ginkgetin, isoginkgetin, and bilobetin showed the cell viability of HK-2 cells were less than 50% at 10 and 100 μg/mL. At the dose of 100 μg/mL, amentoflavone, ginkgetin, isoginkgetin, and bilobetin injured the human normal hepatocytes (L-02 cells), moreover, the cell viability of isoginkgetin and bilobetin were less than 50%. After HE staining of mouse liver sections, it was found that bilobetin and ginkgetin were more toxic to hepatocytes. In renal tissue, these five biflavonoids caused acute renal injury, and renal interstitial hemorrhage was a common pathological phenomenon [300]. Therefore, Ginkgo biloba extract preparation should pay attention to its hepatorenal toxicity. A study found that hinokiflavone, as the cytotoxic principle, its ED50 value was 2.0 μg/mL in KB cells. It was proven that the ether bond between the two flavonoid monomers had a significant cytotoxicity. However, other biflavonoids with C-C linkages, being hexamethyl ethers of ring C/A-linked dimers between two flavonoid units, also showing the cytotoxic activity (the ED50 value was 3.0~4.0 μg/mL) [301]. A non-clinical toxicological study in 2019 revealed that there were no reported fatalities after agethisflavone acted on the female mice, and it has an LD50 larger than 2000 mg/kg [302].
Adem et al. [10] used the caspase-Glo assay to test the cytotoxicity of three biflavonoids (chamaejasmin, 7,7′′-di-O-methylchamaejasmin, and campylospermone A) and other compounds. The cell cycle, apoptosis, mitochondrial membrane sites, and reactive oxygen species were analyzed by flow cytometry. The model cells were CCRF-CEM leukemia cells and CEM/ADR5000 cells and seven other cancer cells including U87MG. = EGFR glioblastoma, HepG2 liver cancer cells, U87MG. = EGFR cells, MDA-MB-231/BCRP breast cancer cells, MDA-MB-231 cells, and HepG2 cells. The IC50 values of chamaejasmin in CCRF-CEM cells and CEM/SDR5000 cells were both greater than 61 μM, and the IC50 value of campylospermone A to CEM/ADR5000 cells was also greater than 61 μM. Therefore, chamaejasmin and campylospermone A were considered to be less cytotoxic. However, 7, 7′′-di-O-methylchamaejasmin had an IC50 = 3.58 ± 0.09 μM for CCRF-CEM cells, and an IC50 = 5.69 ± 0.51 μM for CEM/ADR5000 cells, and the IC50 values of the other cancer cells were less than 8 μM, indicating that it had greater cytotoxicity, and could inhibit the growth of cancer cells.
Due to the cytotoxicity of biflavonoids, they have great potential in the treatment of cancer. For example, delicaflavone can inhibit the PI3K/Akt/mTOR and Ras/MEK/Erk signaling pathways in rectal cancer cells through the mitochondrial ROS pathway [303], inhibit the MSPK signaling pathway in HeLa cervical cancer cells, and induce cell apoptosis in G2/M phase [304]; hinokiflavone inhibits the induction of apoptosis of the NF-κB signaling pathway in liver cancer cells by activating the mitochondrial ROS/JNK/caspase pathway [305]. However, the spirobiflavonoids of abiesinolA-F extracted from Abies sachalinensis can effectively inhibiting the activation of NOR1, thereby inhibiting the activity of skin cancer [306]. In the literature on the toxicology of biflavonoids, only the toxicological experiments of biflavonoids with Aunits, B units, and spirobiflavones were included. For instance, 6-8″ linkage biflavonoid (agathisflavone) had no cytotoxic activity [302], but 3′-8″ linkage biflavonoids (amentoflavone, ginkgetin, isoginkgetin, and bilobetin) impaired the liver and renal cells [300] and 3-3″ linkage biflavonoids (chamaejasmin, 7,7″-di-O-methylchamaejasmin, and campylospermone A) had the capacity to inhibit the growth of cancer cells [10]. The biflavonoids with the ether bond between two flavonoid monomers (delicaflavone [301,304], hinokiflavone [305], and spirobiflavone [306]) had the ability of anticancer. Additionally, the biflavonoids with the hexamethyl ether substituents could reduce cell activity [301].
5.8. Anti-Angiogenesis
Li et al. [307] correlated zebrafish angiogenesis measurement with ultra-performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF/MS) as the base chemometric analysis to identify the potential antiangiogenic active compounds of Garcinia xanthochymus. Preliminary biological activity results showed that amentoflavone can significantly inhibit the growth of subintestinal vessels at 10 and 20 μM, and downregulate the expression of the Angpt2 and Tie2 genes in zebrafish embryos. In addition, the zebrafish model was used to evaluate the structure-activity relationship of seven biflavonoids (volkensiflavone, fukugetin, fukugeside, GB 1a, GB 1a with glycosides, GB 2a and GB 2a with glycosides) isolated from Garcinia. Fukugetin, which has anticancer effects, and can effectively inhibit the growth of subintestinal vessels. Both amentoflavone and fukugetin showed antiangiogenic effects on zebrafish for the first time [308].
5.9. Other
In addition, the other pharmacological effects of biflavonoids are: morelloflavone has 63% preventive inhibition of PLA2-induced myotoxic activity, its 38% cure rate inhibits myotoxicity, and it can inhibit edema formation and anticoagulation in a concentration-dependent manner, proving that morelloflavone can be developed as an inhibitor of secretory PLA2 such as in snake venom [309]; GB1 can inhibit α-glucosidase (IC50 = 0.90 ± 0.01 mM) and aromatase (IC50 = 0.28 ± 0.02 mM), and produce anti-plasmodium activity [310]; robustaflavone-4′-dimethyl ether can inhibit the accumulation of inflammatory cells by inhibiting the AKT and APK pathways, improve lung tissue damage, and reduce pulmonary edema [311]; rhusflavone, from Rhus parviflora, has a sedative and hypnotic effect, significantly binds to the GABAA-BZD receptor (IC50 = 0.045 mM), and induces sleep [178]; II-3, I-5, II-5, II-7, I-4′,II-4′-hexahydroxy-(I-3,II-8)-flavonylflavanonol, from G arcinia nervosa var. pubescens King can produce 73.9% in 18.2 μg/mL Platelet-Activating-Factor Inhibition (IC50 = 20.4 μM) [312]; and GB-2a-II-4′-OMe has a certain analgesic effect on the pain sensation induced by Marfrine, and its mechanism from analgesic effect is different of morphine [64]; amentoflavone can reduce the influence of gamma rays [313], and six biflavonoids of Araucaria angustifolia can improve DNA damage caused by ultraviolet radiation, including: amentoflavone, mono-O-methylamentoflavone, di-O-methylamentoflavone, ginkgetin, tri-O-methylamentoflavone, and tetra-O-methylamentoflavone [314]; GB-2a can inhibit the formation of melanin [315]; studies have shown that isoginkgetin is an inhibitor of mRNA splicing [316]; and chamaejasmine and ginkgetin can improve chronic dermatitis through anti-inflammatory effects [317,318,319]. All of the above are the pharmacological effects discovered and studied in recent years for biflavonoids, indicating that biflavonoids have great developmental prospects.
6. Pharmacokinetics
LC-MS/MS is a sensitive method used in pharmacokinetics, and it is also used in the pharmacokinetics of biflavonoids. It is used in the study of amentoflavone pharmacokinetics by different drug intake modes, including oral gavage (p.o.), intravenous (i.v.), or intraperitoneal (i.p.) injection in rat models. As a result, 90.7% ± 8.3% of the total amount of amentoflavone (300 mg/kg) by p.o., 73.2% ± 6.29% of amentoflavone (10 mg/kg) by i.v., and 70.2% ± 5.18% of the total amentoflavone (10 mg/kg) by i.p. could be detected. The total amentoflavone was found to circulate as conjugated metabolites in the plasma of rats after different modes of administration [320].
Amentoflavone was used as the standard of the study of pharmacokinetics of biflavonoids in LC-MS/MS. For instance, the pharmacokinetics of total hinokiflavone in rat plasma was studied by LC-MS/MS. It was discovered that T1/2 was 6.10 ± 1.86 h [321].
However, there are other ways to calculate the main index of pharmacokinetics. The main components of Platycladus orientalis leaf extract include amentoflavone and hinokiflavone. Therefore, their pharmacokinetics in the plasma of a rat model were evaluated by UFLC-MS/MS. Their T1/2 and Tmax were 2.60 ± 1.34 h and 1.5 ± 0.00 h (amentoflavone), and 2.11 ± 0.29 h and 1.92 ± 0.20 h (hinokiflavone), respectively [322]. All the pharmacokinetics data of biflavonoids were showed in Table 12.
Table 12.
The pharmacokinetics of biflavonoids.
| Name | Testline | Delivery Route | Doses(mg/kg) | Method | T1/2(h) | References |
|---|---|---|---|---|---|---|
| Amentoflavone | Rat plasma | i.p. | 10 | LC-MS/MS | 3.42 ± 1.45 | [320] |
| Amentoflavone | Rat plasma | i.v. | 10 | LC-MS/MS | 5.88 ± 1.78 | [320] |
| Amentoflavone | Rat plasma | p.o. | 300 | LC-MS/MS | 11.3 ± 3.61 | [320] |
| Amentoflavone | Rat plasma | p.o. | 4.31 | UFLC-MS/MS | 2.60 ± 1.34 | [322] |
| Hinokiflavone | Rat plasma | p.o. | 4.30 | UFLC-MS/MS | 2.11 ± 0.29 | [322] |
| Hinokiflavone | Rat plasma | i.v. | 1.0 | LC-MS/MS | 6.10 ± 1.86 | [321] |
7. The Biosynthesis and Synthesis of Biflavonoids
7.1. The Biosynthesis of Biflavonoids
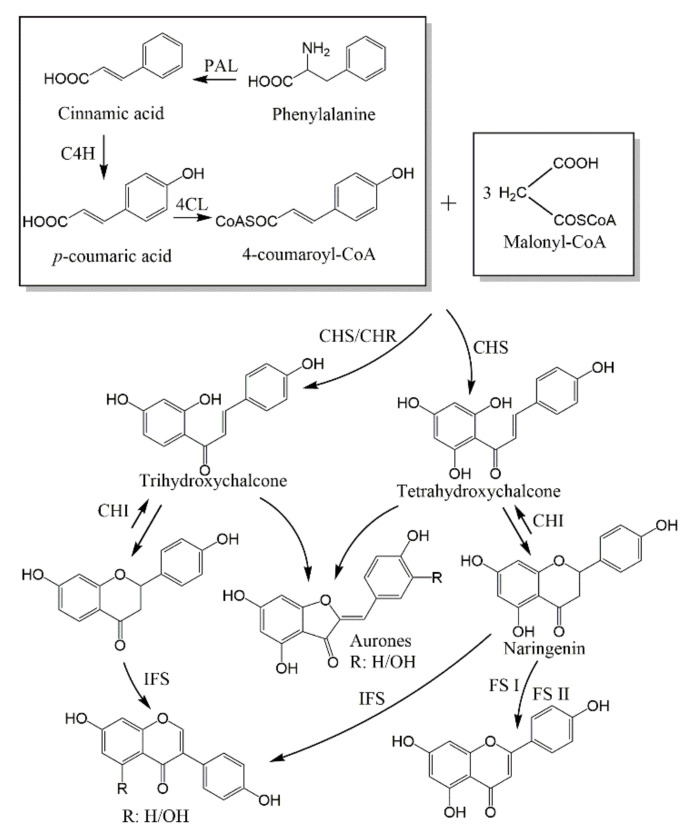
There were few references about the biosynthesis of biflavonoids, but it involves the oxidative coupling of two flavonoid units; therefore, the biosynthesis of flavonoids was a significant step to shape biflavonoids in plants. Alzand et al. [323] had reviewed the major pathways of flavonoid biosynthesis. Starting from phenylpropanoid metabolism and then giving the chalcone (trihydroxychalcone and tetrahydroxychalcone). The tetrahydroxychalcone is isomerised to naringenin, a key intermediate, which can transform to several end-flavonoids (Figure 13).
Figure 13.
The biosynthesis of flavonoids. Enzyme names are abbreviated as follows: cinnamate-4-hydroxylase (C4H), chalcone isomerase (CHI), chalcone reductase (CHR), chalcone synthase(CHS), 4-coumaroyl:CoA-ligase (4CL), flavone synthase (FS I and FS II), isoflavone synthase (IFS), and Phe ammonia-lyase (PAL).
Furthermore, promoting the biosynthesis of biflavonoids can improve the yield of biflavonoids in plants by changing different catalytic enzymes or elicitors. Kicia Karinne Pereira Gromes-Copeland et al. [324] had converted the elicitors of 30 g/L of sucrose and 5 mg/L of 2,4-dichlorophenoxyacetic acid in Poincianella pyramidalic. Providing a higher accumulation of amentoflavone (16.44 mg/L) and agathisflavone (0.58 mg/L). Subsequently, they found that the amentoflavone biosynthesis is superior to agatisflavone. It seems to be related to the linkage type between two flavonoid units.
7.2. The Synthesis of Biflavonoids
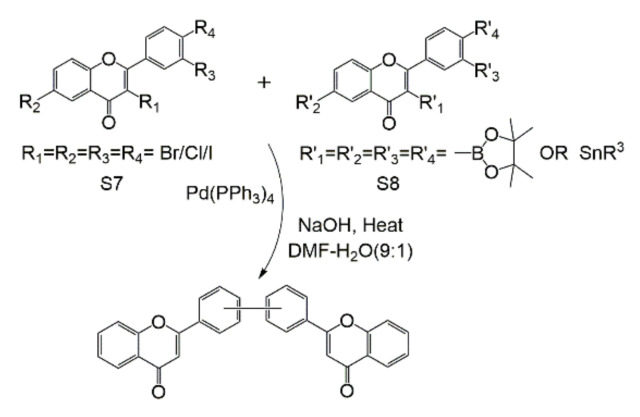
Biflavonoids have great medicinal value and great development prospects. Therefore, the quantity needed in treatment and research will increase. However, it is impossible to obtain a large number of single and high-quality biflavonoids by simply extracting and separating the biflavonoids. In the process of synthesizing biflavonoids, Xue Ying et al. [325] reviewed the previous synthesis methods of biflavonoids in 2010, compared the differences between the various methods, and concluded that the synthesis method of biflavonoids is mainly to synthesize a flavonoid monomer. Then, two molecules of flavonoids coupled with boron-containing flavonoids are chosen by Suzuki or iodide-biflavonoids to obtain the final product, or the two molecules are coupled with the catalyst. The related C-C biosynthesis and reverse synthesis analysis, and the Ullmann ether condensation reaction of C-O-C, are also introduced. In the case of the literature that has been previously summarized, this review will conduct a general analysis of the new biflavonoid synthesis method, and compare the old method with the new one, so that readers can be more intuitive.
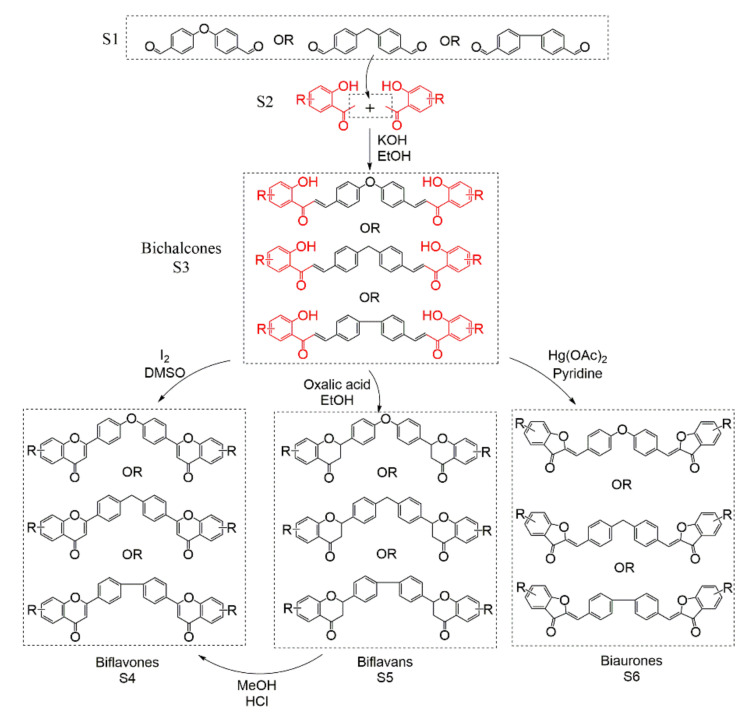
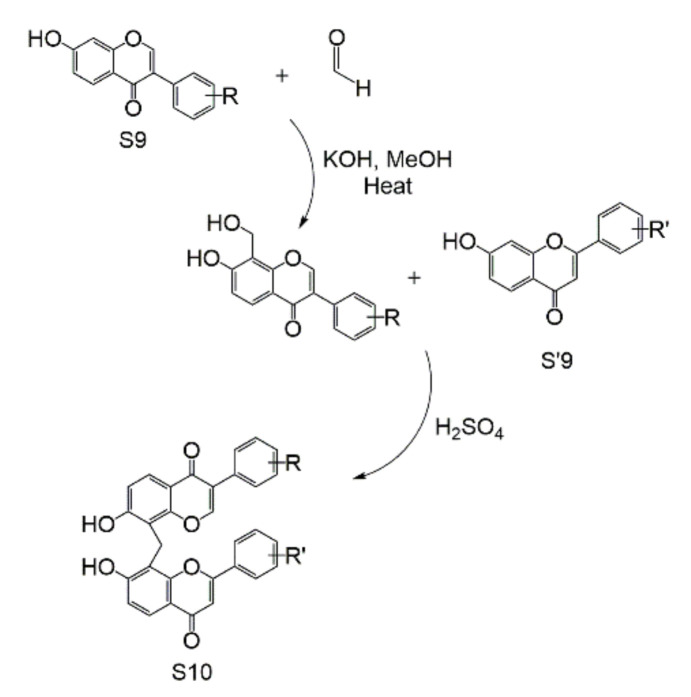
Until 2017, the syntheses of biflavonoids were the construction of a biflavonoid skeleton, and different types of dimers were synthesized under different synthesis conditions. First, the biflavonoid skeleton, bichalcones (S3) is obtained by Claisen–Schmidt aldol condensation from the different dialdehyde molecules (S1) with the corresponding acetophenone (S2). Second, the bichalcone skeleton can obtain biflavones (S4), through iodine-mediated or produce biaurones (S6) by mercury acetate oxidation [27]. Biflavans can be obtained by oxalic acid with EtOH [326], but biflavans will change to biflavones in MeOH with HCl [327]. (Scheme 1). These methods can synthesize different types of biflavonoids as long as different dialdehydes can be provided. For example, the dialdehydes S1 are 4,4′-biphenyldicarboxaldehyde, 4,4′-diaryletherdicarboxaldehyde, or 4,4′-bitoluenedicarboxaldehyde, and the biflanonoids are C-C, C-O-C, or C-C-C. It can be said that this Claisen–Schmidt aldol condensation of dialdehyde and acetophenone can be the synthesis route of most symmetric biflavonoids. According to the difference in the final product, bichalcones, biflavones, biflavans, and biaurones can also be obtained by autonomously controlling the conditions.
Scheme 1.
Total synthesis of C-O-C, C-C-C, and C-C biflavonoids.
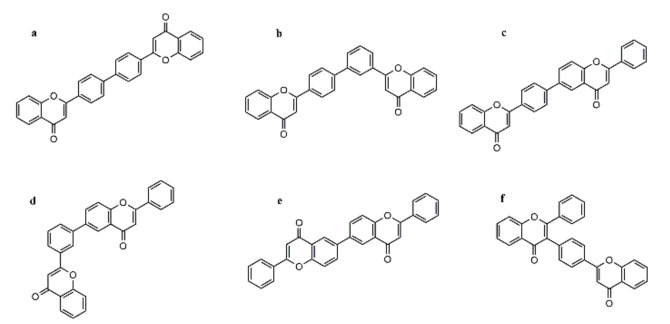
Due to the large number and types of biflavonoids connected to C-C, there are many related synthetic studies. Among them, Chen et al., achieved the synthesis of C-C biflavonoids through the construction of two flavonoid analogs in 2006: one flavonoid analog substituted by a halogen atom (bromide), and the other substituted by a group coupled by a transition metal-catalyzed cross-coupling method, namely two typical methods: the Suzuki coupling reaction and the Stille coupling reaction. The two flavonoid monomers are connected through the biaryl group. In addition, they synthesized a series of C-C 4’-4’linkage biflavonoids a–f and compared the inhibition of sPLA2-IIA among them. Amentoflavone and ochnaflavone were used as controls. Subsequently, they found that the inhibitory potency of the synthesis biflavonoid a(IC50 = 3.0 + 0.9 M) was slightly better than ochnaflavone(IC50 = 3.5 + 0.6 M), the biflavonoids b(IC50 = 15.5 + 3.7 M), d(IC50 = 19.9 + 4.6 M), and f(IC50 = 23.2 + 3.1 M) possessed the comparative inhibitory potency with amentoflavone(IC50 = 23.8 + 3.4 M) [328]. The C-C 4’-4’ linkage biflavonoids a–f are shown in Figure 14.
Figure 14.
The structure of C-C 4′-4′ linkage biflavonoids.
However, due to the low yield of the above method, it is impossible to obtain high-yield biflavonoids on a large scale; as a result, researchers have found other ways to synthesize C-C type biflavonoids. Brominated, iominated, or chlorine substituted flavones (S7) and commercially available bis(pinacolato) diboron are reacted to obtain the corresponding pinacolato boronates (S8), and then S8 (120 mol%) and S7 under standard conditions (Pd(PPh3)4 (5 mol%), NaOH (400 mol%), and DMF-water (9:1), 100 °C) are reacted to obtain C-C biflavones. R1, R2, R3, R4, R′1, R′2, R′3, and R′4 are the positions attacked by brominated, iominated, chlorine, or bis(pinacolato) diboron. Moon et al., adjusted the reaction conditions to catalytic PdCl2(dppf) and K2CO3 in DMF at 90 °C, to reduce the loss of products [329] (Scheme 2).
Scheme 2.
The synthesis of C-C biflavonoids.
According to the above method, Lim et al., processed chrysin into the precursor product required for the reaction and then performed the relevant synthesis under standard conditions to obtain a C-C (6-6′′) anti-inflammatory biflavonoid G168 [330], which had the potency of inhibiting COX-2 mediated PGE2 production. For G168, the IC50 value of inhibiting PGE2 production and againsting iNOS-mediated NO production were 0.1 μM and 50 μM. Furthermore, 5 mg/kg G168 was able to inhibit the paw edema in mice (30% inhibition) and 1~5 mg/kg G168 had the capacity to inhibit writhing in mice (57.3~82.9%). It has been proven that this method can synthesize amentoflavone-type biflavonoids [331] and Wikstrol A and B [332].
In addition to the synthesis shown in Scheme 1, C-C-C-type biflavonoids can be obtained by the Ullmann condensation reaction of the corresponding flavonoid monomers; assuming the relevant conditions are controlled, and the yield is generally high. For instance, the flavonoid monomer chrysin was used to obtain 7-hydroxy-8-hydroxymethyl-4′-methoxyisoflavonoid [333]. Therefore, the synthesis of C-C-C can be summarized as follows: flavone monomer S9 reacts with formaldehyde to form an intermediate, and then it reacts with another flavone monomer S’9 to form C-C-C type biflavone S10 (Scheme 3). However, the yield of the synthesis in the above conditions is low. Thus, Xue Ying et al., modified the method in 2010; they used daidzein as the raw material, the catalyst was concentrated sulfuric acid, and the feed ratio of the raw material was daidzein. When the amount of catalyst was 10% of the molar ratio of daidzein, and the reaction temperature was 80 °C for 24 h, the highest yield of daidzein biflavonoid derivative was obtained [334]. Later, in 2011, Xue Ying et al., further improved the method: using 9% Lewis acid as a catalyst, isoflavones and formaldehyde as raw materials, and controlling the reaction temperature to 90 °C for 20 h; the yield can reach 82~85% [335].
Scheme 3.
The synthesis of C-C-C biflavonoids.
In 2015, Baron and Mead first synthesized 3-benzylidene-dihydrofurochromen2-ones (S14), a flavan-chalcone type biflavonoid [336]. This was the first time de novo synthesis was attempted. The raw material of this synthetic route was flavonoids and chromene (S11). In the presence of catalytic Rh2(S-TBSP)4, the researchers treated S11 with a diazo derivative to obtain the donor-acceptor cyclopropane, and then used Sn(OTf)2 to rearrange the donor-acceptor cyclopropane to obtain the α-carbomethoxy lactone (S12). Then, removal from the hydroxyl protection and treatment with enolate lithium will result in a mixture of stereoisomers with a high hydroxyl alcohol ratio. The alcohol base was protected by TESCl and oxidized by DDQ to selectively oxidize the protected allyl alcohol to obtain aldehyde S13. After adding the aryl lithium reagent, 71% of the final product S14 was obtained, which was an inseparable isomer mixture, but it had all of the functions of target biflavonoids (Scheme 4). Compared with the synthetic methods of Scheme 1, the required conditions are more difficult to control, but it may become one kind of synthetic method that can control the separation of intermediate stereoisomers to better obtain a pure single product and heterobiflavonoids with different types of flavonoid monomers.
Scheme 4.
The de novo synthesis of biflavonoids.
8. Conclusions
In recent years, the method of extracting active ingredients from herbs and using them in research experiments has been a key research direction, and also a huge challenge. As the components in plants are complex, there are many metabolites, and current extraction and separation technologies are still insufficient. A suitable method to efficiently extract, purify, and apply the required active ingredients is the goal we need to achieve. There are many kinds of biflavonoids, and there is an increasing number of synthetic biflavonoids; they are used as anti-inflammatory and antioxidant therapeutics, as treatments for Alzheimer’s disease and Parkinson’s disease, and for other therapeutic applications. Their use is more significant in anticancer and antiviral treatment. Moreover, Qiu-xia et al. [337] developed and applied amentoflavone based on antisolvent freeze-drying technology, and studied its stability during storage and the stable type of drug efficacy to solve the problem of poor water solubility of amentoflavone micropowder, and improve the oral availability of the drug. In particular, Ginkgo biloba has been proven to be useful in clinical treatment [338]. In summary, there is still much room for developing the pharmacology and synthesizing of biflavonoids, but there is a large gap in the research on dosage forms that needs to be supplemented by additional research. This review mainly provides a more detailed report on the classification, pharmacology, pharmacokinetics, synthesis, and other aspects of biflavonoids, to assist researchers in exploring biflavonoids.
Author Contributions
X.H. (Xinqian He) and X.H. (Xin’an Huang) designed the paper. X.H. (Xinqian He) collected literature on the phyto-chemistry, pharmacokinetics, and synthesis. F.Y. collected literature on the pharmacology. X.H. (Xinqian He) wrote the paper. X.H. (Xin’an Huang) provided some suggestions and modified the language in the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Natural Science Foundation of Guangdong Province (2018A030313731).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All the authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gontijo V.S., Dos Santos M.H., Viegas C., Jr. Biological and Chemical Aspects of Natural Biflavonoids from Plants: A Brief Review. Mini Rev. Med. Chem. 2017;17:834–862. doi: 10.2174/1389557517666161104130026. [DOI] [PubMed] [Google Scholar]
- 2.Yu S., Yan H., Zhang L., Shan M., Chen P., Ding A., Li S.F. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules. 2017;22:299. doi: 10.3390/molecules22020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parveen M., Ilyas M., Mushfiq M., Busudan O.A., Muhaisen H.M. A new biflavonoid from leaves of Garcinia nervosa. Nat. Prod. Res. 2004;18:269–275. doi: 10.1080/14786410310001620574. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya K., Satake T. Chemical constituents of Baeckea frutescens leaves inhibit copper-induced low-density lipoprotein oxidation. Fitoterapia. 2010;81:185–189. doi: 10.1016/j.fitote.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Hu J.-F., Garo E., Hough G.W., Goering M.G., O’Neil-Johnson M., Eldridge G.R. Acuminatanol, the first 2′,2‴-bis-dihydrobiflavonol from the aqueous extract of Trichoscypha acuminata. Tetrahedron Lett. 2007;48:5747–5749. doi: 10.1016/j.tetlet.2007.06.094. [DOI] [Google Scholar]
- 6.Nonaka G., Kawahara O., Nishioka I. Tannins and Related Compounds. XV. A New Class of Dimeric Flavan-3-ol Gallates, Theasinensins A and B, and Proanthocyanidin Gallates from Green Tea Leaf. (1) Chem. Pharm. Bull. 1983;31:3906–3914. doi: 10.1248/cpb.31.3906. [DOI] [Google Scholar]
- 7.Yang X., Kang-Ping X., Zhen-Xing Z., Gui-Shan T. Advances in chemodiversity from Selaginella. Cent. South. Pharm. 2017;15:129–142. [Google Scholar]
- 8.Yun-Yun Y., Lu H., Ping W., Guo-Zhu S., Tian-Tian S., Chang-Cai B. Advances on chemical constituents and bioactivities of genus Stellera. China J. Chin. Mater. Med. 2015;40:4324–4332. [PubMed] [Google Scholar]
- 9.Reddy B.A.K., Reddy N.P., Gunasekar D., Blond A., Bodo B. Biflavonoids from Ochna lanceolata. Phytochem. Lett. 2008;1:27–30. doi: 10.1016/j.phytol.2007.12.005. [DOI] [Google Scholar]
- 10.Adem F.A., Mbaveng A.T., Kuete V., Heydenreich M., Ndakala A., Irungu B., Yenesew A., Efferth T. Cytotoxicity of isoflavones and biflavonoids from Ormocarpum kirkii towards multi-factorial drug resistant cancer. Phytomedicine. 2019;58:152853. doi: 10.1016/j.phymed.2019.152853. [DOI] [PubMed] [Google Scholar]
- 11.Manga S.S.E., Tih A.E., Ghogomu R.T.A., Blond B.B. Biflavonoid constituents of Campylospermum mannii. Biochem. Syst. Ecol. 2009;37:402–404. doi: 10.1016/j.bse.2009.04.002. [DOI] [Google Scholar]
- 12.Chen L.Y., Chen I.S., Peng C.F. Structural elucidation and bioactivity of biflavonoids from the stems of Wikstroemia taiwanensis. Int. J. Mol. Sci. 2012;13:1029–1038. doi: 10.3390/ijms13011029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyandat E., Hassanali A., Vicente Y.D., Multari G., Galeffi C. The 7,7″-β-diglucoside of (2S,3R)-chamaejasmin from Ormocarpum kirkii. Phytochemistry. 1990;29:2361–2364. doi: 10.1016/0031-9422(90)83077-E. [DOI] [Google Scholar]
- 14.Kim A.R., Jin Q., Jin H.G., Ko H.J., Woo E.R. Phenolic compounds with IL-6 inhibitory activity from Aster yomena. Arch. Pharm. Res. 2014;37:845–851. doi: 10.1007/s12272-013-0236-x. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Lu L.Y., Zeng L.H., Zhang C., Hu J.L., Li X.R. Sikokianin D, a new C-3/C-3”-biflavanone from the roots of Wikstroemia indica. Molecules. 2012;17:7792–7797. doi: 10.3390/molecules17077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y.-J., Foubert K., Dhooghe L., Lemière F., Maregesi S., Coleman C.M., Zou Y., Ferreira D., Apers S., Pieters L. Rapid isolation and identification of minor natural products by LC–MS, LC–SPE–NMR and ECD: Isoflavanones, biflavanones and bisdihydrocoumarins from Ormocarpum kirkii. Phytochemistry. 2012;79:121–128. doi: 10.1016/j.phytochem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Dhooghe L., Maregesi S., Mincheva I., Ferreira D., Marais J.P.J., Lemière F., Matheeussen A., Cos P., Maes L., Vlietinck A., et al. Antiplasmodial activity of (I-3,II-3)-biflavonoids and other constituents from Ormocarpum kirkii. Phytochemistry. 2010;71:785–791. doi: 10.1016/j.phytochem.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Li X.-Q., Rahman K., Zhu J.-Y., Zhang H. Chemical Constituents and Pharmacological Activities of Stellera chamaejasme. Curr. Pharm. Des. 2018;24:2825–2838. doi: 10.2174/1381612824666180903110802. [DOI] [PubMed] [Google Scholar]
- 19.NISHIMUTA S., Taki M., Takaishi S., Iijima Y., Akiyama T. Structures of 4-aryl-coumarin (neoflavone) dimers isolated from Pistacia chinensis BUNGE and their estrogen-like activity. Chem. Pharm. Bull. 2000;48:505–508. doi: 10.1248/cpb.48.505. [DOI] [PubMed] [Google Scholar]
- 20.Zhi X., Jian-hui S. Research Progress of Biflavonoids. China J. Mod. Med. 2004;14:88–91. [Google Scholar]
- 21.Chien S.-C., Liu H.-K., Kuo Y.-H. Two New Compounds from the Leaves of Calocedrus microlepic var. formosana. Chem. Pharm. Bull. 2004;52:762–763. doi: 10.1248/cpb.52.762. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.-W., Choi H.-J., Kim H.-S., Kim D.-H., Chang I.-S., Moon H.T., Lee S.-Y., Oh W.K., Woo E.-R. Biflavonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fibroblasts. Bioorg. Med. Chem. 2008;16:732–738. doi: 10.1016/j.bmc.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Kamil M., Ilyas M., Rahman W., Hasaka N., Okigawa M., Kawano N. Taiwaniaflavone and its derivatives: A new series of biflavones from Taiwania cryptomerioides Hayata. J. Chem. Soc. Perkin Trans. 1. 1981;12:553–559. doi: 10.1039/p19810000553. [DOI] [Google Scholar]
- 24.Sakasai M., Fukui H., Yamane H., Kyaw A.N., Tahara S. A New Class of Biflavonoids: 2′-Hydroxy genistein Dimers from the Roots of White Lupin. Z. Nat. C. 2000;55:165–174. doi: 10.1515/znc-2000-3-406. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X., Jiang H.-X., Huang H., Zhu R.-L., Jiang B. Ring-B Linked Bidihydroflavonoids from Thuidium kanedae Sak. Chin. J. Chem. 2006;24:393–395. doi: 10.1002/cjoc.200690075. [DOI] [Google Scholar]
- 26.Hamada T. Studies on the medicinal plant in the “Sambutsu-cho” of Bungo Province possessed by the Kumamoto Clan (II); studies on the medicinal herbs. Yakushigaku Zasshi. 1992;27:117–124. [PubMed] [Google Scholar]
- 27.Wan H., Ge L., Li J., Zhang K., Wu W., Peng S., Zou X., Zhou H., Zhou B., Zeng X. Effects of a novel biflavonoid of Lonicera japonica flower buds on modulating apoptosis under different oxidative conditions in hepatoma cells. Phytomedicine. 2019;57:282–291. doi: 10.1016/j.phymed.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 28.Sum T.H., Sum T.J., Collins S., Galloway W., Twigg D.G., Hollfelder F., Spring D.R. Divergent synthesis of biflavonoids yields novel inhibitors of the aggregation of amyloid beta (1-42) Org. Biomol. Chem. 2017;15:4554–4570. doi: 10.1039/C7OB00804J. [DOI] [PubMed] [Google Scholar]
- 29.Zheng R., Rui-jie C., Yan-ying Y., Shu-wen C. Research Progresses on Chemical Constituents of Genus Daphne genus and Their Bioactivities. Food Sci. 2009;30:249–258. [Google Scholar]
- 30.Duanrui S., Shouxun Z. Non-alkaloid constituents from aerial parts of Stephania tetrandra. J. Jining Med. Coll. 1993;2:1–5. [Google Scholar]
- 31.Pegnyemb D.E., Tih R.G., Sondengam B.L., Blond A., Bodo B. Flavonoids of Ochna afzelii. Phytochemistry. 2003;64:661–665. doi: 10.1016/S0031-9422(03)00267-X. [DOI] [PubMed] [Google Scholar]
- 32.Machado M.B., Lopes L.M.X. Tetraflavonoid and biflavonoids from Aristolochia ridicula. Phytochemistry. 2008;69:3095–3102. doi: 10.1016/j.phytochem.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Machado M.B., Lopes L.M.X. Chalcone–flavone tetramer and biflavones from Aristolochia ridicula. Phytochemistry. 2005;66:669–674. doi: 10.1016/j.phytochem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Carneiro F.J.C., Boralle N., Silva D.H.S., Lopes L.M.X. Bi- and tetraflavonoids from Aristolochia ridicula. Phytochemistry. 2000;55:823–832. doi: 10.1016/S0031-9422(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee N.-Y., Min H.-Y., Lee J., Nam J.-W., Lee Y.-J., Han A.-R., Wiryawan A., Suprapto W., Lee S.K., Seo E.-K. Identification of a new cytotoxic biflavanone from Selaginella doederleinii. Chem. Pharm. Bull. 2008;56:1360–1361. doi: 10.1248/cpb.56.1360. [DOI] [PubMed] [Google Scholar]
- 36.Park S.Y., Nguyen P.H., Kim G., Jang S.N., Lee G.H., Phuc N.M., Wu Z., Liu K.H. Strong and Selective Inhibitory Effects of the Biflavonoid Selamariscina A against CYP2C8 and CYP2C9 Enzyme Activities in Human Liver Microsomes. Pharmaceutics. 2020;12:343. doi: 10.3390/pharmaceutics12040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin L.-C., Kuo Y.-C., Chou C.-J. Cytotoxic biflavonoids from Selaginella delicatula. J. Nat. Prod. 2000;63:627–630. doi: 10.1021/np990538m. [DOI] [PubMed] [Google Scholar]
- 38.Kassem M.E.S., El-Desoky S.K., Sharaf M. Biphenyl esters and biflavonoids from the fruits of Schinus terebenthefolus. Chem. Nat. Compd. 2004;40:447–450. doi: 10.1007/s10600-005-0008-z. [DOI] [Google Scholar]
- 39.Chen J.-J., Duh C.-Y., Chen J.-F. New cytotoxic biflavonoids from Selaginella delicatula. Planta Med. 2005;71:659–665. doi: 10.1055/s-2005-871273. [DOI] [PubMed] [Google Scholar]
- 40.Gu S., Xu L., Sun N. Studies on chemical compositions of Podocarpus imbricatus. China J. Chin. Matera Med. 1995;20:105–106. [PubMed] [Google Scholar]
- 41.Bahia M.V., Santos J.B.D., David J.P.D.L., David J.M. Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae) J. Braz. Chem. Soc. 2005;16:1402–1405. doi: 10.1590/S0103-50532005000800017. [DOI] [Google Scholar]
- 42.Aguilar M.I., Romero M.G., Chávez M.I., King-Díaz B., Lotina-Hennsen B. Biflavonoids Isolated from Selaginella lepidophylla Inhibit Photosynthesis in Spinach Chloroplasts. J. Agric. Food Chem. 2008;56:6994–7000. doi: 10.1021/jf8010432. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J., Wang N., Fan M., Chen H., Liu H., Yao X. A new biflavonoid from Selaginella uncinata. Asian J. Tradit. Med. 2007;2:92–97. [Google Scholar]
- 44.Zheng J.-X., Wang N.-L., Liu H.-W., Chen H.-F., Li M.-M., Wu L.-Y., Fan M., Yao X.-S. Four new biflavonoids from Selaginella uncinata and their anti-anoxic effect. J. Asian Nat. Prod. Res. 2008;10:945–952. doi: 10.1080/10286020802181166. [DOI] [PubMed] [Google Scholar]
- 45.Cane H., Saidi N., Yahya M., Darusman D., Erlidawati E., Safrida S., Musman M. Macrophylloflavone: A New Biflavonoid from Garcinia macrophylla Mart. (Clusiaceae) for Antibacterial, Antioxidant, and Anti-Type 2 Diabetes Mellitus Activities. Sci. World J. 2020;2020:2983129. doi: 10.1155/2020/2983129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee A., Kotoky J., Das K.K., Banerji J., Chakraborty T. Abiesin, a biflavonoid of abies webbiana. Phytochemistry. 1984;23:704–705. doi: 10.1016/S0031-9422(00)80421-5. [DOI] [Google Scholar]
- 47.Seeger T., Zinsmeister H.D., Geiger H. The Biflavonoid Pattern of Rhytidiadelphus squarrosus (Hedw.) Warnst. Z. Nat. C. 1990;45:583–586. doi: 10.1515/znc-1990-0603. [DOI] [Google Scholar]
- 48.Anhut S., Seeger T., Zinsmeister H.D., Geiger H. New Dihydrobiflavones from the Moss Plagiomnium cuspidatum. Z. Nat. C. 1989;44:189–192. doi: 10.1515/znc-1989-3-403. [DOI] [Google Scholar]
- 49.Markham K.R., Andersen Ø.M., Viotto E.S. Unique biflavonoid types from the moss Dicranoloma robustum. Phytochemistry. 1988;27:1745–1749. doi: 10.1016/0031-9422(88)80436-9. [DOI] [Google Scholar]
- 50.Rampendahl C., Seeger T., Geiger H., Zinsmeister H.D. The biflavonoids of Plagiomnium undulatum. Phytochemistry. 1996;41:1621–1624. doi: 10.1016/0031-9422(95)00804-7. [DOI] [Google Scholar]
- 51.Matamela T., Green I.R., Mtunzi F.M. A Novel Biflavonoid from Rhus leptodictya. Nat. Prod. Commun. 2016;11:1279–1280. doi: 10.1177/1934578X1601100922. [DOI] [PubMed] [Google Scholar]
- 52.Tih A.E., Ghogomu R.T., Sondengam B.L., Caux C., Bodo B. Minor biflavonoids from Lophira alata leaves. J. Nat. Prod. 2006;69:1206–1208. doi: 10.1021/np050169w. [DOI] [PubMed] [Google Scholar]
- 53.Pieters L., Mbwambo Z.H., Kapingu M.C., Moshi M.J., Machumi F., Apers S., Cos P., Ferreira D., Marais J.P.J., Berghe D.V., et al. Antiparasitic Activity of Some Xanthones and Biflavonoids and Identification of a New Biflavanoid from the Root Bark of Garcinia livingstonei. Planta Med. 2006;72:P_003. doi: 10.1055/s-2006-949803. [DOI] [PubMed] [Google Scholar]
- 54.Al-Shagdari A., Alarcon A.B., Cuesta-Rubio O., Piccinelli A.L., Rastrelli L. Biflavonoids, main constituents from Garcinia bakeriana leaves. Nat. Prod. Commun. 2013;8:1237–1240. doi: 10.1177/1934578X1300800913. [DOI] [PubMed] [Google Scholar]
- 55.Min Y. Current Status of Research on Biflavonoids in Garcinia. Guangdong Pharm. 2004;14:5–8. [Google Scholar]
- 56.Konoshima M., Ikeshiro Y. Fukugiside, the first biflavonoid glycoside from garcinia spicata hook. f. Tetrahedron Lett. 1970;11:1717–1720. doi: 10.1016/S0040-4039(01)98064-5. [DOI] [PubMed] [Google Scholar]
- 57.Terashima K., Aqil M., Niwa M. Garcinianin, a novel biflavonoid from the roots of garcinia kola. Heterocycles. 1995;41:2245–2250. [Google Scholar]
- 58.Osorio E., Londono J., Bastida J. Low-density lipoprotein (LDL)-antioxidant biflavonoids from Garcinia madruno. Molecules. 2013;18:6092–6100. doi: 10.3390/molecules18056092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saelee A., Phongpaichit S., Mahabusarakam W. A new prenylated biflavonoid from the leaves of Garcinia dulcis. Nat. Prod. Res. 2015;29:1884–1888. doi: 10.1080/14786419.2015.1010087. [DOI] [PubMed] [Google Scholar]
- 60.Ren Y., de Blanco E.J.C., Fuchs J.R., Soejarto D.D., Burdette J.E., Swanson S.M., Kinghorn A.D. Potential Anticancer Agents Characterized from Selected Tropical Plants. J. Nat. Prod. 2019;82:657–679. doi: 10.1021/acs.jnatprod.9b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joshi B.S., Kamat V.N., Viswanathan N. The isolation and structure of two biflavones from Garcinia talboti. Phytochemistry. 1970;9:881–888. doi: 10.1016/S0031-9422(00)85197-3. [DOI] [Google Scholar]
- 62.Kim C.S., Bae M., Oh J., Subedi L., Suh W.S., Choi S.Z., Son M.W., Kim S.Y., Choi S.U., Oh D.C., et al. Anti-Neurodegenerative Biflavonoid Glycosides from Impatiens balsamina. J. Nat. Prod. 2017;80:471–478. doi: 10.1021/acs.jnatprod.6b00981. [DOI] [PubMed] [Google Scholar]
- 63.Bao-Min F., Yue-Hu F., Yong-Qi W. Distribution of Biflavonoids in Six Species of Thymelaeaceae. J. Dalian Univ. 2003;24:95–98+112. [Google Scholar]
- 64.Iwu M., Igboko O. Flavonoids of Garcinia kola Seeds. J. Nat. Prod. 1982;45:650–651. doi: 10.1021/np50023a026. [DOI] [Google Scholar]
- 65.Cechinel Filho V., da Silva K.L., de Souza M.M., Oliveira A.E., Yunes R.A., Guimaraes C.L., Verdi L.G., Simionatto E.L., Delle Monache F. I3-naringenin-II8--4′OMe-eriodictyol: A new potential analgesic agent isolated from Rheedia gardneriana leaves. Z. Nat. C J. Biosci. 2000;55:820–823. doi: 10.1515/znc-2000-9-1024. [DOI] [PubMed] [Google Scholar]
- 66.Konoshima M., Ikeshiro Y., Miyahara S., Yen K.-Y. The constitution of biflavonoids from Garcinia plants. Tetrahedron Lett. 1970;11:4203–4206. doi: 10.1016/S0040-4039(01)98703-9. [DOI] [PubMed] [Google Scholar]
- 67.Stark T.D., Germann D., Balemba O.B., Wakamatsu J., Hofmann T. New Highly in Vitro Antioxidative 3,8″-Linked Biflav(an)ones and Flavanone-C-glycosides from Garcinia buchananii Stem Bark. J. Agric. Food Chem. 2013;61:12572–12581. doi: 10.1021/jf404783y. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira R.F., Camara C.A., Agra M.D.F., Silva T.M.S. Biflavonoids from the unripe fruits of Clusia paralicola and their antioxidant activity. Nat. Prod. Commun. 2012;7:1597–1600. doi: 10.1177/1934578X1200701215. [DOI] [PubMed] [Google Scholar]
- 69.Kitanov G.M. Biflavone, flavonol, and xanthone glycosides from Hypericum aucheri. Chem. Nat. Compd. 1988;24:390–391. doi: 10.1007/BF00598599. [DOI] [Google Scholar]
- 70.Terashima K., Kondo Y., Aqil M., Waziri M., Niwa M. A study of biflavanones from the stems of garcinia kola (GUTTIFERAE) Heterocycles. 1999;50:283–290. [Google Scholar]
- 71.Loo P.V., Bruyn A.D., Verzele M. On the liquid chromatography and identification of the flavonoids, present in the “sumach tannic acid” extracted fromRhus coriaria. Chromatographia. 1988;25:15–20. [Google Scholar]
- 72.Zhang X., Wang G., Huang W., Ye W., Li Y. Biflavonoids from the Roots of Wikstroemia indica. Nat. Prod. Commun. 2011;6:1111–1114. doi: 10.1177/1934578X1100600815. [DOI] [PubMed] [Google Scholar]
- 73.Ito C., Itoigawa M., Miyamoto Y., Rao K.S., Takayasu J., Okuda Y., Mukainaka T., Tokuda H., Nishino H., Furukawa H. A New Biflavonoid from Calophyllum panciflorum with Antitumor-Promoting Activity. J. Nat. Prod. 1999;62:1668–1671. doi: 10.1021/np990065j. [DOI] [PubMed] [Google Scholar]
- 74.Li X.-C., Joshi A.S., Tan B., ElSohly H.N., Walker L.A., Zjawiony J.K., Ferreira D. Absolute configuration, conformation, and chiral properties of flavanone-(3→8″)-flavone biflavonoids from Rheedia acuminata. Tetrahedron. 2002;58:8709–8717. doi: 10.1016/S0040-4020(02)01096-7. [DOI] [Google Scholar]
- 75.Babu V., Ali S.M., Sultana S., Ilyas M. A biflavonoid from Garcinia nervosa. Phytochemistry. 1988;27:3332–3335. doi: 10.1016/0031-9422(88)80062-1. [DOI] [Google Scholar]
- 76.Ferrari J., Terreaux C., Kurtán T., Szikszai-Kiss A., Antus S., Msonthi J.D., Hostettmann K. Isolation and On-Line LC/CD Analysis of 3,8”-Linked Biflavonoids from Gnidia involucrata. Helv. Chim. Acta. 2003;86:2768–2778. doi: 10.1002/hlca.200390226. [DOI] [Google Scholar]
- 77.Niwa M., Terashima K., Ishida T., Furukawa T., Takaya Y. Constituents of green and ripened fruit of Garcinia subelliptica. Heterocycles. 2008;75:407–413. doi: 10.3987/COM-07-11216. [DOI] [Google Scholar]
- 78.Jingxian P., Huyi Z., Xianbin Y., Meifang H. Biflavones from the testa of Ginkgo biloba L. J. Plant Resour. Environ. 1995;4:17–21. [Google Scholar]
- 79.Wenli M., Jiao W., Haofu D. Advances in studies on chemical constituents in plants of Cephalotaxus Sieb. et Zucc. and their pharmacological activities. Chin. Tradit. Herb. Drugs. 2006;37:452–458. [Google Scholar]
- 80.Chun Y., Jun-Song W., Ling-Yi K. A new biflavone from needles of Taxus canadensis. China J. Chin. Mater. Med. 2016;41:443–445. doi: 10.4268/cjcmm20160314. [DOI] [PubMed] [Google Scholar]
- 81.Murthy S.S.N. A biflavanone from Semecarpus anacardium. Phytochemistry. 1983;22:2636–2638. doi: 10.1016/0031-9422(83)80192-7. [DOI] [Google Scholar]
- 82.Zhang R.R., Lin Z.X., Lu X.Y., Xia X., Jiang R.W., Chen Q.B. CGY-1, a biflavonoid isolated from cardiocrinum giganteum seeds, improves memory deficits by modulating the cholinergic system in scopolamine-treated mice. Biomed. Pharm. 2019;111:496–502. doi: 10.1016/j.biopha.2018.12.100. [DOI] [PubMed] [Google Scholar]
- 83.Xia X.H., Zhang Y., Xi Y.B., Wang G.H., Yang L.Q., Xue K.F. Advances in Studies on Chemical Constituents and Bioactivites Actions of Ginkgo Biloba, L. Chin. J. Exp. Tradit. Med. Formulae. 2009;15:100–104. [Google Scholar]
- 84.Li M., Li B., Xia Z.M., Tian Y., Zhang D., Rui W.J., Dong J.X., Xiao F.J. Anticancer Effects of Five Biflavonoids from Ginkgo Biloba, L. Male Flowers In Vitro. Molecules. 2019;24:1496. doi: 10.3390/molecules24081496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao S., Mu Z.Q., Cheng C.R., Ding J. Three new biflavonoids from the branches and leaves of Cephalotaxus oliveri and their antioxidant activity. Nat. Prod. Res. 2019;33:321–327. doi: 10.1080/14786419.2018.1448817. [DOI] [PubMed] [Google Scholar]
- 86.Liu T.X., Wang S.H. Research Progress on Use of Cycas Revolute. J. MUC (Natural Sciences Edition) 2016;25:49–54. [Google Scholar]
- 87.Das B., Mahender G., Koteswara Rao Y., Prabhakar A., Jagadeesh B. Biflavonoids from Cycas beddomei. Chem. Pharm. Bull. 2005;53:135–136. doi: 10.1248/cpb.53.135. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y., Huang J., Hua H., Sun B., Gao H., Wu L. A new biflavone from the twigs and leaves of Taxus cuspidata Sieb et Zucc. Asian J. Tradit. Med. 2007;2:235–238. [Google Scholar]
- 89.Sun M., Feng X., Yin M., Chen Y., Zhao X., Dong Y. A biflavonoid from stems and leaves of Lonicera macranthoides. Chem. Nat. Compd. 2012;48:231–233. doi: 10.1007/s10600-012-0211-7. [DOI] [Google Scholar]
- 90.Rao N.S.P., Row L.R., Brown R.T. Phenolic constituents of Semecarpus anacardium. Phytochemistry. 1973;12:671–681. doi: 10.1016/S0031-9422(00)84463-5. [DOI] [Google Scholar]
- 91.Murthy S.S., Rao N.S., Anjaneyulu A.S., Row L.R. Confirmation of structures of semecarpus biflavanones A1 and A2. Planta Med. 1981;43:46–50. doi: 10.1055/s-2007-971471. [DOI] [PubMed] [Google Scholar]
- 92.Murthy S.S.N. New biflavonoid from Semercarpus anacardium Linn. Chim. Acta Turc. Istanb. 1992;20:33. [Google Scholar]
- 93.Thompson R.S., Jacques D., Haslam E., Tanner R.J.N. Plant proanthocyanidins. Part I. Introduction; the isolation, structure, and distribution in nature of plant procyanidins. J. Chem. Soc. Perkin Trans. 1. 1972:1387–1399. doi: 10.1039/p19720001387. [DOI] [Google Scholar]
- 94.Ölschläger C., Regos I., Zeller F.J., Treutter D. Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum×F. homotropicum. Phytochemistry. 2008;69:1389–1397. doi: 10.1016/j.phytochem.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Kashiwada Y., Nonaka G.-I., Nishioka I. Tannins and Related Compounds. XLVIII.: Rhubarb. (7). Isolation and Characterization of New Dimeric and Trimeric Procyanidins. Chem. Pharm. Bull. 1986;34:4083–4091. doi: 10.1248/cpb.34.4083. [DOI] [PubMed] [Google Scholar]
- 96.Wang J.-N., Hano Y., Nomura T., Chen Y.-J. Procyanidins from the seeds of Vitis amurensis. Phytochemistry. 2000;53:1097–1102. doi: 10.1016/S0031-9422(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 97.Nilanonta C., Isaka M., Kittakoop P., Palittapongarnpim P., Kamchonwongpaisan S., Pittayakhajonwut D., Tanticharoen M., Thebtaranonth Y. Antimycobacterial and Antiplasmodial Cyclodepsipeptides from the Insect Pathogenic Fungus Paecilomyces tenuipes BCC 1614. Planta Med. 2000;66:756–758. doi: 10.1055/s-2000-9776. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto F., Nonaka G.-i., Nishioka I. Tannins and Related Compounds. XC: 8-C-Ascorbyl (−)-Epigallocatechin 3-O-Gallate and Novel Dimeric Flavan -3-ols, Oolonghomobisflavans A and B, from Oolong Tea. (3) Chem. Pharm. Bull. 1989;37:3255–3263. doi: 10.1248/cpb.37.3255. [DOI] [Google Scholar]
- 99.Danne A., Petereit F., Nahrstedt A. Flavan-3-ols, prodelphinidins and further polyphenols from Cistus salvifolius. Phytochemistry. 1994;37:533–538. doi: 10.1016/0031-9422(94)85094-1. [DOI] [PubMed] [Google Scholar]
- 100.Nonaka G.-I., Muta M., Nishioka I. Myricatin, a galloyl flavanonol sulfate and prodelphinidin gallates from Myrica rubra. Phytochemistry. 1983;22:237–241. doi: 10.1016/S0031-9422(00)80097-7. [DOI] [Google Scholar]
- 101.Geiss F., Heinrich M., Hunkler D., Rimpler H. Proanthocyanidins with (+)-epicatechin units from Byrsonima crassifolia bark. Phytochemistry. 1995;39:635–643. doi: 10.1016/0031-9422(94)00934-L. [DOI] [Google Scholar]
- 102.Viviers P.M., Young D.A., Botha J.J., Ferreira D., Roux D.G., Hull W.E. Synthesis of condensed tannins. Part 6. The sequence of units, coupling positions and absolute configuration of the first linear [4,6:4,6]-triflavanoid with terminal 3,4-diol function. J. Chem. Soc. Perkin Trans. 1. 1982;13:535–540. doi: 10.1039/p19820000535. [DOI] [Google Scholar]
- 103.Steenkamp J.A., Malan J.C.S., Roux D.G., Ferreira D. Oligomeric flavanoids. Part 1. Novel dimeric profisetinidins from Colophospermum mopane. J. Chem. Soc. Perkin Trans. 1. 1988;6:1325–1330. doi: 10.1039/p19880001325. [DOI] [Google Scholar]
- 104.Ferreira D., Cornelius du Preez I., Wijnmaalen J.C., Roux D.G. Biflavanoid proguibourtinidin carboxylic acids and their biflavanoid homologues from Acacia luederitzii. Phytochemistry. 1985;24:2415–2422. doi: 10.1016/S0031-9422(00)83054-X. [DOI] [Google Scholar]
- 105.Malan E., Swinny E., Ferreira D., Steynberg P. The structure and synthesis of proguibourtinidins from Cassia abbreviata. Phytochemistry. 1996;41:1209–1213. doi: 10.1016/0031-9422(95)00656-7. [DOI] [Google Scholar]
- 106.Park K.H., Kim S.K., Choi S.E., Kwon J.H., Oh M.H., Lee M.W. Three New Stereoisomers of Condensed Tannins from the Roots of Rosa multiflora. Chem. Pharm. Bull. 2010;58:1227–1231. doi: 10.1248/cpb.58.1227. [DOI] [PubMed] [Google Scholar]
- 107.Hemingway R.W., Foo L.Y., Porter L.J. Linkage isomerism in trimeric and polymeric 2,3-cis-procyanidins. J. Chem. Soc. Perkin Trans. 1. 1982;13:1209–1216. doi: 10.1039/p19820001209. [DOI] [Google Scholar]
- 108.Yeap Foo L., Karchesy J.J. Procyanidin dimers and trimers from Douglas fir inner bark. Phytochemistry. 1989;28:1743–1747. doi: 10.1016/S0031-9422(00)97836-1. [DOI] [Google Scholar]
- 109.Foo L.Y., Newman R., Waghorn G., McNabb W.C., Ulyatt M.J. Proanthocyanidins from Lotus corniculatus. Phytochemistry. 1996;41:617–624. doi: 10.1016/0031-9422(95)00602-8. [DOI] [Google Scholar]
- 110.Malan E., Sireeparsad A. The structure and synthesis of the first dimeric proteracacinidins from acacia galpinii. Phytochemistry. 1995;38:237–239. doi: 10.1016/0031-9422(94)00561-7. [DOI] [Google Scholar]
- 111.Bennie L., Coetzee J., Malan E., Ferreira D. (4→6)-Coupled proteracacinidins and promelacacinidins from Acacia galpinii and Acacia caffra. Phytochemistry. 2002;60:521–532. doi: 10.1016/S0031-9422(02)00124-3. [DOI] [PubMed] [Google Scholar]
- 112.Li J., Xu P.-S., Zou Z.-X., Zou H., Long H.-P., Tan L.-H., Liu R.-H., Wang Y.-K., Xu K.-P., Tan G.-S. Three new compounds from the roots of Juglans mandshurica Maxim. Phytochem. Lett. 2017;20:40–44. doi: 10.1016/j.phytol.2017.03.014. [DOI] [Google Scholar]
- 113.Lou H., Yuan H., Ma B., Ren D., Ji M., Oka S. Polyphenols from peanut skins and their free radical-scavenging effects. Phytochemistry. 2004;65:2391–2399. doi: 10.1016/j.phytochem.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 114.Nonaka G., Nishioka I., Nagasawa T., Oura H. Tannins and Related Compounds. I. Rhubarb (1) Chem. Pharm. Bull. 1981;29:2862–2870. doi: 10.1248/cpb.29.2862. [DOI] [Google Scholar]
- 115.Bekker M., Bekker R., Brandt V.E. Two flavonoid glycosides and a miscellaneous flavan from the bark of Guibourtia coleosperma. Phytochemistry. 2006;67:818–823. doi: 10.1016/j.phytochem.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 116.Cheng H.-Y., Yang C.-M., Lin T.-C., Shieh D.-E., Lin C.-C. ent-Epiafzelechin-(4alpha-->8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J. Med. Microbiol. 2006;55:201–206. doi: 10.1099/jmm.0.46110-0. [DOI] [PubMed] [Google Scholar]
- 117.Hartisch C., Kolodziej H. Galloylhamameloses and proanthocyanidins from Hamamelis virginiana. Phytochemistry. 1996;42:191–198. doi: 10.1016/0031-9422(96)00926-0. [DOI] [Google Scholar]
- 118.Nonaka G.-I., Miwa N., Nishioka I. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum. Phytochemistry. 1982;21:429–432. doi: 10.1016/S0031-9422(00)95282-8. [DOI] [Google Scholar]
- 119.Hsu F.-L., Nonaka G.-I., Nishioka I. Acylated flavanols and procyanidins from Salix sieboldiana. Phytochemistry. 1985;24:2089–2092. doi: 10.1016/S0031-9422(00)83128-3. [DOI] [Google Scholar]
- 120.Lokvam J., Coley P.D., Kursar T.A. Cinnamoyl glucosides of catechin and dimeric procyanidins from young leaves of Inga umbellifera (Fabaceae) Phytochemistry. 2004;65:351–358. doi: 10.1016/j.phytochem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 121.Zhang B., Nonaka G.-I., Nishioka I. Potentillanin, a biflavanoid and a procyanidin glycoside from Potentilla viscosa. Phytochemistry. 1988;27:3277–3280. doi: 10.1016/0031-9422(88)80042-6. [DOI] [Google Scholar]
- 122.Ishimaru K., Nonaka G.-I., Nishioka I. Flavan-3-ol and procyanidin glycosides from quercus miyagii. Phytochemistry. 1987;26:1167–1170. doi: 10.1016/S0031-9422(00)82371-7. [DOI] [Google Scholar]
- 123.Tanaka T., Nonaka G.-I., Nishioka I. 7-O-Galloyl-(+)-catechin and 3-O-galloylprocyanidin B-3 from Sanguisorba officinalis. Phytochemistry. 1983;22:2575–2578. doi: 10.1016/0031-9422(83)80168-X. [DOI] [Google Scholar]
- 124.Abe I., Seki T., Noguchi H., Kashiwada Y. Galloyl Esters from Rhubarb are Potent Inhibitors of Squalene Epoxidase, a Key Enzyme in Cholesterol Biosynthesis. Planta Med. 2000;66:753–756. doi: 10.1055/s-2000-9781. [DOI] [PubMed] [Google Scholar]
- 125.Liimatainen J., Karonen M., Sinkkonen J. Procyanidin xylosides from the bark of Betula pendula. Phytochemistry. 2012;76:178–183. doi: 10.1016/j.phytochem.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 126.Cho Y.J. Isolation of 3-Galloylprocyanidin B3, a Glucosyltransferase Inhibitor from the Korean Green Tea Leaves. J. Appl. Biol. Chem. 2000;43:273–276. [Google Scholar]
- 127.Cong H.J., Zhang S.W., Zhang C., Huang Y.J., Xuan L.J. A novel dimeric procyanidin glucoside from Polygonum aviculare. Chin. Chem. Lett. 2012;23:820–822. doi: 10.1016/j.cclet.2012.04.021. [DOI] [Google Scholar]
- 128.Ozawa T., Hiroto M., Imagawa H. Procyanidins from Sago Palm Pith. Agric. Biol. Chem. 1990;54:217–218. [Google Scholar]
- 129.Reddy K.R.S., Srimannarayana G., Rao N.V.S. Ein proanthocyanidin-dimenes aus cassia auriculata-blumen. Cheminform. 1973;4:291. [Google Scholar]
- 130.Ariga T., Asao Y. Isolation, Identification and Organoleptic Astringency of Dimeric Proanthocyanidins Occurring in Azuki Beans. Agric. Biol. Chem. 1981;45:2709–2712. [Google Scholar]
- 131.Middelkoop T.B., Labadie R.P. The Action of Saraca asoca Roxb. de Wilde Bark on the PGH2 Synthetase Enzyme Complex of the Sheep Vesicular Gland. Z. Nat. C. 1985;40:523–526. doi: 10.1515/znc-1985-7-812. [DOI] [PubMed] [Google Scholar]
- 132.Morimoto S., Nonaka G.-I., Chen R.-F., Nishioka I. Tannins and Related Compounds. LXI: Isolation and Structures of Novel Bi- and Triflavanoids from the Leaves of Cassia fistula L. Chem. Pharm. Bull. 1988;36:39–47. doi: 10.1248/cpb.36.39. [DOI] [Google Scholar]
- 133.Weinges K., Göritz K., Nader F. Zur Kenntnis der Proanthocyanidine, XI1) Konfigurationsbestimmung von C30H26O12-Procyanidinen und Strukturaufklärung eines neuen Procyanidins. Eur. J. Org. Chem. 1968;715:164–171. [Google Scholar]
- 134.Kashiwada Y., Iizuka H., Yoshioka K., Chen R.-F., Nonaka G.-i., Nishioka I. Tannins and Related Compounds. XCIII: Occurrence of Enantiomeric Proanthocyanidins in the Leguminosae Plants, Cassia fistula L. and C. javanica L. Chem. Pharm. Bull. 1990;38:888–893. doi: 10.1248/cpb.38.888. [DOI] [Google Scholar]
- 135.Nunes D.S., Haag A., Bestmann H.-J. Two proanthocyanidins from the bark of Dalbergia monet̊ari. Phytochemistry. 1989;28:2183–2186. doi: 10.1016/S0031-9422(00)97940-8. [DOI] [Google Scholar]
- 136.Messanga B.B., Ghogomu R., Sondengam B.L., Martin M.-T., Blond A., Brouard J.-P., Bodo B. Calodenin C: A New Guibourtinidol-(4α→8)-afzelechin from Ochna calodendron. Planta Med. 1998;64:760–761. doi: 10.1055/s-2006-957577. [DOI] [PubMed] [Google Scholar]
- 137.Bicker J., Petereit F., Hensel A. Proanthocyanidins and a phloroglucinol derivative from Rumex acetosa L. Fitoterapia. 2009;80:483–495. doi: 10.1016/j.fitote.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 138.Monache F.D., Pomponi M., Marini-Bettolo G.B., D’Albuquerque I.L., de Lima O.G. A methylated catechin and proanthocyanidins from the celastraceae. Phytochemistry. 1976;15:573–574. doi: 10.1016/S0031-9422(00)88986-4. [DOI] [Google Scholar]
- 139.Nonaka G., Nishioka I. Novel Biflavonoids, Chalcan-flavan Dimers from Gambir. Chem. Pharm. Bull. 1980;28:3145–3149. doi: 10.1248/cpb.28.3145. [DOI] [Google Scholar]
- 140.Hsu F., Nonaka G., Nishioka I. Tannins and Related Compounds. XXXI. Isolation and Characterization of Proanthocyanidins in Kandelia candel (L.) DRUCE. Chem. Pharm. Bull. 1985;33:3142–3152. doi: 10.1248/cpb.33.3142. [DOI] [Google Scholar]
- 141.Achenbach H., Benirschke G. Joannesialactone and other compounds from Joannesia princeps. Phytochemistry. 1997;45:149–157. doi: 10.1016/S0031-9422(96)00777-7. [DOI] [Google Scholar]
- 142.Karioti A., Bilia A.R., Gabbiani C., Messori L., Skaltsa H. Proanthocyanidin glycosides from the leaves of Quercus ilex L. (Fagaceae) Tetrahedron Lett. 2009;50:1771–1776. doi: 10.1016/j.tetlet.2009.01.158. [DOI] [Google Scholar]
- 143.Wu B., Wang K., Wu X. A New Phenolic Diglycoside Produced in Response to Copper Toxicity and a New Flavan Dimer from the Leaves of Viburnum ichangense (Hemsl.) Rehd. Helv. Chim. Acta. 2011;94:1677–1684. doi: 10.1002/hlca.201100177. [DOI] [Google Scholar]
- 144.Foo L.Y., Karchesy J.J. Procyanidin polymers of Douglas fir bark: Structure from degradation with phloroglucinol. Phytochemistry. 1989;28:3185–3190. doi: 10.1016/0031-9422(89)80303-6. [DOI] [Google Scholar]
- 145.Qa’dan F., Petereit F., Mansoor K., Nahrstedt A. Antioxidant oligomeric proanthocyanidins from Cistus salvifolius. Nat. Prod. Res. 2006;20:1216–1224. doi: 10.1080/14786410600899225. [DOI] [PubMed] [Google Scholar]
- 146.Kusano R., Ogawa S., Matsuo Y., Tanaka T., Yazaki Y., Kouno I. α-Amylase and Lipase Inhibitory Activity and Structural Characterization of Acacia Bark Proanthocyanidins. J. Nat. Prod. 2011;74:119–128. doi: 10.1021/np100372t. [DOI] [PubMed] [Google Scholar]
- 147.Palazzo de Mello J., Petereit F., Nahrstedt A. Prorobinetinidins from Stryphnodendron adstringens. Phytochemistry. 1996;42:857–862. doi: 10.1016/0031-9422(95)00953-1. [DOI] [Google Scholar]
- 148.Botha J.J., Ferreira D., Roux D.G. Synthesis of condensed tannins. Part 4. A direct biomimetic approach to [4,6]-and [4,8]-biflavanoids. J. Chem. Soc. Perkin Trans. 1. 1981;12:1235–1245. doi: 10.1039/p19810001235. [DOI] [Google Scholar]
- 149.Viviers P.M., Botha J.J., Ferreira D., Roux D.G., Saayman H.M. Synthesis of condensed tannins. Part 7. Angular [4,6: 4,8]-prorobinetinidin triflavanoids from black wattle (‘Mimosa’) bark extract. J. Chem. Soc. Perkin Trans. 1. 1983;14:17–22. doi: 10.1039/P19830000017. [DOI] [Google Scholar]
- 150.Palazzo de Mello J.C., Petereit F., Nahrstedt A. A dimeric proanthocyanidin from Stryphnodendron adstringens. Phytochemistry. 1999;51:1105–1107. doi: 10.1016/S0031-9422(98)00715-8. [DOI] [Google Scholar]
- 151.Makhmatkulov A.B., Kuliev Z.A., Vdovin A.D., Malikov V.M. Proanthocyanidins ofPolygonum corarium. II. Chem. Nat. Compd. 1994;30:214–222. doi: 10.1007/BF00630009. [DOI] [Google Scholar]
- 152.Dawang S., Zuchun Z., Wong H., Lai Y.F. Tannins and other phenolics from Myrica esculenta bark. Phytochemistry. 1988;27:579–583. doi: 10.1016/0031-9422(88)83145-5. [DOI] [Google Scholar]
- 153.Kwan Hu K., Kuliev Z.A., Vdovin A.D., Yagudaev M.R., Malikov V.M. Structure of rhodisin and rhodisinoside. Chem. Nat. Compd. 1989;25:618–619. doi: 10.1007/BF00598092. [DOI] [Google Scholar]
- 154.Hussein G., Nakamura N., Meselhy M.R., Hattori M. Phenolics from Maytenus senegalensis. Phytochemistry. 1999;50:689–694. doi: 10.1016/S0031-9422(98)00571-8. [DOI] [Google Scholar]
- 155.Nonaka G.-I., Sakai R., Nishioka I. Hydrolysable tannins and proanthocyanidins from green tea. Phytochemistry. 1984;23:1753–1755. doi: 10.1016/S0031-9422(00)83484-6. [DOI] [Google Scholar]
- 156.Danne A., Petereit F., Nahrstedt A. Proanthocyanidins from Cistus incanus. Phytochemistry. 1993;34:1129–1133. doi: 10.1016/S0031-9422(00)90729-5. [DOI] [Google Scholar]
- 157.Schmidt C.A., Murillo R., Heinzmann B., Laufer S., Wray V., Merfort I. Structural and Conformational Analysis of Proanthocyanidins from Parapiptadenia rigida and Their Wound-Healing Properties. J. Nat. Prod. 2011;74:1427–1436. doi: 10.1021/np200158g. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Y.-J., Tanaka T., Iwamoto Y., Yang C.-R., Kouno I. Novel Norsesquiterpenoids from the Roots of Phyllanthus emblica. J. Nat. Prod. 2000;63:1507–1510. doi: 10.1021/np000135i. [DOI] [PubMed] [Google Scholar]
- 159.Gupta R.K., Haslam E. Plant proanthocyanidins. Part 7. Prodelphinidins from Pinus sylvestris. J. Chem. Soc. Perkin Trans. 1. 1981;12:1148–1150. doi: 10.1039/p19810001148. [DOI] [Google Scholar]
- 160.Foo L.Y., Porter L.J. Prodelphinidin polymers: Definition of structural units. J. Chem. Soc. Perkin Trans. 1. 1978;10:1186–1190. doi: 10.1039/p19780001186. [DOI] [Google Scholar]
- 161.Hashimoto F., Nonaka G.-i., Nishioka I. Tannins and Related Compounds. LXXVII: Novel Chalcan-flavan Dimers, Assamicains A, B and C, and a New Flavan-3-ol and Proanthocyanidins from the Fresh Leaves of Camella sinensis L. var. assamica KITAMURA. Chem. Pharm. Bull. 1989;37:77–85. doi: 10.1248/cpb.37.77. [DOI] [Google Scholar]
- 162.Weinges K., Schick H. Dodecaacetylprodelphinidin B3 from the dried leaves of Ziziphus spina-christi. Phytochemistry. 1995;38:505–507. doi: 10.1016/0031-9422(94)00574-D. [DOI] [Google Scholar]
- 163.Sun D., Wong H., Foo L.Y. Proanthocyanidin dimers and polymers from Quercus dentata. Phytochemistry. 1987;26:1825–1829. doi: 10.1016/S0031-9422(00)82297-9. [DOI] [Google Scholar]
- 164.Krishnamoorthy V., Seshadri T.R. A new proanthocyanidin from the stem bark of Myrica nagi thumb. Tetrahedron. 1966;22:2367–2371. doi: 10.1016/S0040-4020(01)82156-6. [DOI] [Google Scholar]
- 165.Steynberg J.P., Steynberg J.P., Vincent Brandt E., Ferreira D., Hemingway R.W. Oligomeric flavanoids. Part 26. Structure and synthesis of the first profisetinidins with epifisetinidol constituent units. J. Chem. Soc. Perkin Trans. 1. 1997;13:1943–1950. doi: 10.1039/a701334e. [DOI] [Google Scholar]
- 166.Malan J.C.S., Young D.A., Steenkamp J.A., Ferreira D. Oligomeric flavanoids. Part 2. The first profisetinidins with dihydroflavonol constituent units. J. Chem. Soc. Perkin Trans. 1. 1988;9:2567–2572. doi: 10.1039/p19880002567. [DOI] [Google Scholar]
- 167.Steynberg J.P., Burger J.F.W., Malan J.C.S., Cronjé A., Young D.A., Ferreira D. Natural (−)-fisetinidol-(4,8)-(−)-epicatechin profisetinidins. Phytochemistry. 1990;29:275–277. doi: 10.1016/0031-9422(90)89049-F. [DOI] [Google Scholar]
- 168.Drewes S.E., Roux D.G., Eggers S.H., Feeney J. Three diastereoisomeric 4,6-linked bileucofisetinidins from the heartwood of Acacia mearnsii. J. Chem. Soc. C Org. 1967:1217–1227. doi: 10.1039/j39670001217. [DOI] [Google Scholar]
- 169.Hatano T., Yamashita A., Hashimoto T., Ito H., Kubo N., Yoshiyama M., Shimura S., Itoh Y., Okuda T., Yoshida T. Flavan dimers with lipase inhibitory activity from Cassia nomame. Phytochemistry. 1997;46:893–900. doi: 10.1016/S0031-9422(97)00367-1. [DOI] [Google Scholar]
- 170.Akhavan M., Shafaghat A., Salimi F. Novel acetylated chalcone and biflavonoid glycosides from Trigonosciadium brachytaenium (Boiss.) Alava. Nat. Prod. Res. 2013;27:2111–2117. doi: 10.1080/14786419.2013.791822. [DOI] [PubMed] [Google Scholar]
- 171.Yadav S., Bhadoria B.K. Two dimeric flavonoids from Baiihinia purpured. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2005;44:2604–2607. [Google Scholar]
- 172.Felício J.D.A., Gonçalez E., Braggio M.M., Costantino L., Albasini A., Lins A.P. Inhibition of lens aldose reductase by biflavones from Ouratea spectabilis. Planta Med. 1995;61:217–220. doi: 10.1055/s-2006-958059. [DOI] [PubMed] [Google Scholar]
- 173.Khan N.U., Ilyas M., Rahman W., Mashima T., Okigawa M., Kawano N. Biflavones from the leaves of Araucaria bidwillii Hooker and Agathis alba foxworthy (araucariaceae) Tetrahedron. 1972;28:5689–5695. doi: 10.1016/S0040-4020(01)88913-4. [DOI] [Google Scholar]
- 174.Ilyas M., Seligmann O., Wagner H. Biflavones from the Leaves of Araucaria rulei F. Muell. and a Survey on Biflavanoids of the Araucaria Genus. Z. Nat. C. 1977;32:206–209. doi: 10.1515/znc-1977-3-409. [DOI] [Google Scholar]
- 175.Ngo Mbing J., Enguehard-Gueiffier C., Atchadé A.d.T., Allouchi H., Gangoué-Piéboji J., Mbafor J.T., Tih R.G., Pothier J., Pegnyemb D.E., Gueiffier A. Two biflavonoids from Ouratea nigroviolacea. Phytochemistry. 2006;67:2666–2670. doi: 10.1016/j.phytochem.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 176.Chen F.-C., Lin Y.-M. Rhusflavanone, a new biflavanone from the seeds of wax-tree. J. Chem. Soc. Perkin Trans. 1. 1976;1:98–101. doi: 10.1039/p19760000098. [DOI] [Google Scholar]
- 177.Ndongo J.T., Shaaban M., Mbing J.N., Bikobo D.N., Atchadé A.d.T., Pegnyemb D.E., Laatsch H. Phenolic dimers and an indole alkaloid from Campylospermum flavum (Ochnaceae) Phytochemistry. 2010;71:1872–1878. doi: 10.1016/j.phytochem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Shrestha S., Park J.H., Lee D.Y., Cho J.G., Cho S., Yang H.J., Yong H.I., Yoon M.S., Han D.S., Baek N.I. Rhus parviflora and its biflavonoid constituent, rhusflavone, induce sleep through the positive allosteric modulation of GABA(A)-benzodiazepine receptors. J. Ethnopharmacol. 2012;142:213–220. doi: 10.1016/j.jep.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 179.D’Arc Felicio J., Rossi M.H., Park H.R., Gonçalez E., Braggio M.M., David J.M., Cordeiro I. Biflavonoids from Ouratea multiflora. Fitoterapia. 2001;72:453–455. doi: 10.1016/S0367-326X(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 180.Moreira I.C., de Carvalho M.G., Bastos A.B.F.O., Braz-Filho R. A flavone dimer from Ouratea hexasperma. Phytochemistry. 1999;51:833–838. doi: 10.1016/S0031-9422(99)00106-5. [DOI] [Google Scholar]
- 181.Sharma S.K., Vasudeva N., Rathi P., Ali M. Isolation and identification of a new phytosterol ester from tephrosia purpurea (linn.) pers. root. Int. J. Chem. Sci. 2008;6:1734–1741. [Google Scholar]
- 182.Yan X.X., Pan Z.H., Cheng L., Ning D.S., Zu-Qiang L.I., Luo L. Chemical constituents of Sabina squamata(1) Guihaia. 2015;35:428–430. [Google Scholar]
- 183.Ye Y., Guo Y., Luo Y.T., Wang Y.F. Isolation and free radical scavenging activities of a novel biflavonoid from the shells of Camellia oleifera Abel. Fitoterapia. 2012;83:1585–1589. doi: 10.1016/j.fitote.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 184.Chen F., Lin Y., Ho T., Ueng T. Synthesis of Hexa-O-methyl-8,8”-binaringenin. Cheminform. 1975;3:833–836. doi: 10.3987/R-1975-10-0833. [DOI] [Google Scholar]
- 185.Ferraro G.E., Martino V.S., Coussio J.D. 4′,4”-Dimethylcuppressuflavanone from Eupatorium subhastatum. J. Nat. Prod. 1988;51:586–587. doi: 10.1021/np50057a025. [DOI] [Google Scholar]
- 186.Miceli N., Trovato A., Dugo P., Cacciola F., Donato P., Marino A., Bellinghieri V., Barbera T.M.L., Güvenç A., Taviano M.F. Comparative analysis of flavonoid profile, antioxidant and antimicrobial activity of the berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009;57:6570–6577. doi: 10.1021/jf9012295. [DOI] [PubMed] [Google Scholar]
- 187.Ofman D.J., Markham K.R., Vilain C., Molloy B.P.J. Flavonoid profiles of New Zealand kauri and other species of Agathis. Phytochemistry. 1995;38:1223–1228. doi: 10.1016/0031-9422(94)00783-P. [DOI] [Google Scholar]
- 188.Ilyas M., Usmani J.N., Bhatnagar S.P., Ilyas M., Rahman W. WB1 and W11, the first optically active biflavones. Tetrahedron Lett. 1968;9:5515–5517. doi: 10.1016/S0040-4039(00)75549-3. [DOI] [Google Scholar]
- 189.Meselhy M.R. Constituents from Moghat, the Roots of Glossostemon bruguieri (Desf.) Molecules. 2003;8:614–621. doi: 10.3390/80800614. [DOI] [Google Scholar]
- 190.Chen F.-C., Lin Y.-M., Lin Y.-C. Neorhusflavanone, a New Biflavanone from Wax-tree. Heterocycles. 1978;9:663–668. doi: 10.3987/R-1978-05-0663. [DOI] [Google Scholar]
- 191.Adjapmoh M.F., Toze F.A., Songue J.L., Langat M.K., Kapche G.D., Hameed A., Lateef M., Shaiq M.A., Mbaze L.M., Wansi J.D., et al. A New Ceramide and Biflavonoid from the Leaves of Parinari hypochrysea (Chrysobalanaceae) Nat. Prod. Commun. 2016;11:615–620. doi: 10.1177/1934578X1601100515. [DOI] [PubMed] [Google Scholar]
- 192.Jia B.X., Ren F.X., Jia L., Chen X.Q., Yang J., Wang Q. Baeckein E, a new bioactive C-methylated biflavonoid from the roots of Baeckea frutescens. Nat. Prod. Res. 2013;27:2069–2075. doi: 10.1080/14786419.2013.778852. [DOI] [PubMed] [Google Scholar]
- 193.Okigawa M., Kawano N., Aqil M., Rahman W. Ochnaflavone and its derivatives: A new series of diflavonyl ethers from Ochna squarrosa Linn. J. Chem. Soc. Perkin Trans. 1. 1976;5:580–583. doi: 10.1039/p19760000580. [DOI] [Google Scholar]
- 194.Ma J.L., Li N., Li X. One new biflavone glucoside from the leaves of Lonicera japonica Thunb. Chin. J. Med. Chem. 2009;19:63–64. [Google Scholar]
- 195.Likhitwitayawuid K., Rungserichai R., Ruangrungsi N., Phadungcharoen T. Flavonoids from Ochna integerrima. Phytochemistry. 2001;56:353–357. doi: 10.1016/S0031-9422(00)00409-X. [DOI] [PubMed] [Google Scholar]
- 196.Jayakrishna G., Reddy M.K., Jayaprakasam B., Gunasekar D., Blond A., Bodo B. A new biflavonoid from Ochna beddomei. J. Asian Nat. Prod. Res. 2003;5:83–87. doi: 10.1080/1028602021000034100. [DOI] [PubMed] [Google Scholar]
- 197.Pegnyemb D.E., Mbing J.N., de Theodore Atchade A., Tih R.G., Sondengam B.L., Blond A., Bodo B. Antimicrobial biflavonoids from the aerial parts of Ouratea sulcata. Phytochemistry. 2005;66:1922–1926. doi: 10.1016/j.phytochem.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 198.Makhafola T.J., Samuel B.B., Elgorashi E.E., Eloff J.N. Ochnaflavone and ochnaflavone 7-O-methyl ether two antibacterial biflavonoids from Ochna pretoriensis (Ochnaceae) Nat. Prod. Commun. 2012;7:1601–1604. doi: 10.1177/1934578X1200701216. [DOI] [PubMed] [Google Scholar]
- 199.Reutrakul V., Ningnuek N., Pohmakotr M., Yoosook C., Napaswad C., Kasisit J., Santisuk T., Tuchinda P. Anti HIV-1 Flavonoid Glycosides from Ochna integerrima. Planta Med. 2007;73:683–688. doi: 10.1055/s-2007-981538. [DOI] [PubMed] [Google Scholar]
- 200.Rao K.V., Sreeramulu K., Venkata Rao C., Gunasekar D., Martin M.T., Bodo B. Two New Biflavonoids from Ochna obtusata. J. Nat. Prod. 1997;60:632–634. doi: 10.1021/np9604590. [DOI] [Google Scholar]
- 201.Jayaprakasam B., Damu A.G., Rao K.V., Gunasekar D., Blond A., Bodo B. 7-O-Methyltetrahydroochnaflavone, a New Biflavanone from Ochna beddomei. J. Nat. Prod. 2000;63:507–508. doi: 10.1021/np9902993. [DOI] [PubMed] [Google Scholar]
- 202.Ariyasena J., Baek S.-H., Perry N.B., Weavers R.T. Ether-Linked Biflavonoids from Quintinia acutifolia. J. Nat. Prod. 2004;67:693–696. doi: 10.1021/np0340394. [DOI] [PubMed] [Google Scholar]
- 203.Mbukwa E., Chacha M., Majinda R.R.T. Phytochemical Constituents of Vangueria Infausta: Their Radical Scavenging and Antimicrobial Activities. Arkivoc. 2006;2007:104–112. doi: 10.3998/ark.5550190.0008.912. [DOI] [Google Scholar]
- 204.Wild S.H., Roglic G., Green A., Sicree R., King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 205.Sievers H., Burkhardt G., Becker H., Zinsmeister H.D. Hypnogenols and other dihydroflavonols from the moss Hypnum cupressiforme. Phytochemistry. 1992;31:3233–3237. doi: 10.1016/0031-9422(92)83482-E. [DOI] [Google Scholar]
- 206.Ali D.M., Wong K.C., Lim P.K. Flavonoids from Blumea balsamifera. Fitoterapia. 2005;76:128–130. doi: 10.1016/j.fitote.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 207.Sabudak T., Demirkiran O., Ozturk M., Topcu G. Phenolic compounds from Trifolium echinatum Bieb. and investigation of their tyrosinase inhibitory and antioxidant activities. Phytochemistry. 2013;96:305–311. doi: 10.1016/j.phytochem.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 208.Tartaglione L., Gambuti A., De Cicco P., Ercolano G., Ianaro A., Taglialatela-Scafati O., Moio L., Forino M. NMR-based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia. 2018;125:13–17. doi: 10.1016/j.fitote.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 209.Carini J.P., Kaiser S., Ortega G.G., Bassani V.L. Development, optimisation and validation of a stability-indicating HPLC method of achyrobichalcone quantification using experimental designs. Phytochem. Anal. 2013;24:193–200. doi: 10.1002/pca.2399. [DOI] [PubMed] [Google Scholar]
- 210.Kumar N., Singh B., Bhandari P., Gupta A.P., Uniyal S.K., Kaul V.K. Biflavonoids from Lonicera japonica. Phytochemistry. 2005;66:2740–2744. doi: 10.1016/j.phytochem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 211.Bitchagno G.T., Tankeo S.B., Tsopmo A., Simo Mpetga J.D., Tchinda A.T., Fobofou S.A., Nkuete A.H., Wessjohann L.A., Kuete V., Tane P. Ericoside, a new antibacterial biflavonoid from Erica mannii (Ericaceae) Fitoterapia. 2016;109:206–211. doi: 10.1016/j.fitote.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 212.Nakazawa K. Syntheses of Ring-substituted Flavonoids and Allied Compounds. XI. Synthesis of Hinokiflavone. Chem. Pharm. Bull. 1968;16:2503–2511. doi: 10.1248/cpb.16.2503. [DOI] [Google Scholar]
- 213.Gadek P.A., Quinn C.J. Biflavones of the subfamily cupressoideae, cupressaceae. Phytochemistry. 1985;24:267–272. doi: 10.1016/S0031-9422(00)83535-9. [DOI] [Google Scholar]
- 214.Geiger H., de Groot-Pfleiderer W. Die biflavone von Taxodium distichum. Phytochemistry. 1973;12:465–466. doi: 10.1016/0031-9422(73)80042-1. [DOI] [Google Scholar]
- 215.Markham K.R., Sheppard C., Geiger H. 13C NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry. 1987;26:3335–3337. doi: 10.1016/S0031-9422(00)82499-1. [DOI] [Google Scholar]
- 216.Gadek P.A. Biflavonoids from the seed testa of cycadales. Phytochemistry. 1982;21:889–890. doi: 10.1016/0031-9422(82)80086-1. [DOI] [Google Scholar]
- 217.Miura H., Kawano N. The Partial Demethylation of Flavones. IV. Formation of New Bisflavones, Hinokiflavone-7, 7"-dimethyl Ether and Neocryptomerin. Chem. Pharm. Bull. 1968;16:1838–1840. doi: 10.1248/cpb.16.1838. [DOI] [Google Scholar]
- 218.Miura H., Kawano N., Anthony C.W., Jr. Cryptomerin A and B, Hinokiflavone Methyl Ethers from the Leaves of Cryptomeria japonica. Chem. Pharm. Bull. 1966;14:1404–1408. doi: 10.1248/cpb.14.1404. [DOI] [PubMed] [Google Scholar]
- 219.Meurer-Grimes B., Yu J. Chamaecyparin—A Rare Biflavone from Selaginella Species. Z. Nat. C. 1999;54:1143–1144. doi: 10.1515/znc-1999-1221. [DOI] [Google Scholar]
- 220.Swamy R.C., Kunert O., Schühly W., Bucar F., Ferreira D., Rani V.S., Kumar B.R., Appa Rao A.V.N. Structurally Unique Biflavonoids from Selaginella chrysocaulos and Selaginella bryopteris. Chem. Biodivers. 2006;3:405–414. doi: 10.1002/cbdv.200690044. [DOI] [PubMed] [Google Scholar]
- 221.Silva G.L., Chai H., Gupta M.P., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Beecher C.W.W., Douglas Kinghorn A. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry. 1995;40:129–134. doi: 10.1016/0031-9422(95)00212-P. [DOI] [PubMed] [Google Scholar]
- 222.Sobha Rani M., Venkata Rao C., Gunasekar D., Blond A., Bodo B. A biflavonoid from Cycas beddomei. Phytochemistry. 1998;47:319–321. doi: 10.1016/S0031-9422(97)00417-2. [DOI] [Google Scholar]
- 223.Jayaprakasam B., Damu A.G., Gunasekar D., Blond A., Bodo B. A biflavanone from Cycas beddomei. Phytochemistry. 2000;53:515–517. doi: 10.1016/S0031-9422(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 224.Akongwi M., Tih A.E., Nyongbela K.D., Samje M., Ghogomu R.T., Bodo B. Brevipedicelones D and E, Two C-O-C Flavonoid Dimmers from the Leaves of Garcinia brevipedicellata and Anti-onchocercal Activity. Nat. Prod. Bioprospect. 2019;9:61–68. doi: 10.1007/s13659-018-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dora G., Edwards J.M. Taxonomic Status of Lanaria lanata and Isolation of a Novel Biflavone. J. Nat. Prod. 1991;54:796–801. doi: 10.1021/np50075a007. [DOI] [Google Scholar]
- 226.Weniger B., Vonthron-Senecheau C., Arango G.J., Kaiser M., Brun R., Anton R. A bioactive biflavonoid from Campnosperma panamense. Fitoterapia. 2004;75:764–767. doi: 10.1016/j.fitote.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 227.Velandia J.R., Carvalho M.G.D., Braz-Filho R., Werle A.A. Biflavonoids and a glucopyranoside derivative from Ouratea semiserrata. Phytochem. Anal. 2002;13:283–292. doi: 10.1002/pca.656. [DOI] [PubMed] [Google Scholar]
- 228.Daniel J.F.d.S., Carvalho M.G.d., Cardoso R.d.S., Agra M.d.F., Eberlin M.N. Others flavonoids from Ouratea hexasperma (Ochnaceae) J. Braz. Chem. Soc. 2005;16:634–638. doi: 10.1590/S0103-50532005000400022. [DOI] [Google Scholar]
- 229.Mahjoub M.A., Ammar S., Mighri Z. A new biflavonoid and an isobiflavonoid from Rhus tripartitum. Nat. Prod. Res. 2005;19:723–729. doi: 10.1080/14786410412331272068. [DOI] [PubMed] [Google Scholar]
- 230.Li Q., Gao W., Cao J., Bi X., Chen G., Zhang X., Xia X., Zhao Y. New cytotoxic compounds from flowers of Lawsonia inermis L. Fitoterapia. 2014;94:148–154. doi: 10.1016/j.fitote.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 231.Parsons I.C., Gray A.I., Waterman P.G., Hartley T.G. New Triterpenes and Flavonoids from the Leaves of Bosistoa brassii. J. Nat. Prod. 1993;56:46–53. doi: 10.1021/np50091a007. [DOI] [Google Scholar]
- 232.Hatano T., Miyatake H., Natsume M., Osakabe N., Takizawa T., Ito H., Yoshida T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry. 2002;59:749–758. doi: 10.1016/S0031-9422(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 233.Lee D.F., Swinny E.E., Jones G.P. NMR identification of ethyl-linked anthocyanin–flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004;45:1671–1674. doi: 10.1016/j.tetlet.2003.12.110. [DOI] [Google Scholar]
- 234.Abe Y., Sawada A., Momose T., Sasaki N., Kawahara N., Kamakura H., Goda Y., Ozeki Y. Structure of an anthocyanin–anthocyanin dimer molecule in anthocyanin-producing cells of a carrot suspension culture. Tetrahedron Lett. 2008;49:7330–7333. doi: 10.1016/j.tetlet.2008.10.041. [DOI] [Google Scholar]
- 235.Wang Q., Han N., Wu X., Tai W., Dai N., Wu R., Wu J., Bao B. A biflavonoid glycoside from Lomatogonium carinthiacum (Wulf) Reichb. Nat. Prod. Res. 2015;29:77–81. doi: 10.1080/14786419.2014.959009. [DOI] [PubMed] [Google Scholar]
- 236.Iinuma M., Tosa H., Tanaka T., Ito T., Asai F. Chemical Constituents of Guttiferaeous Plants and Their Bioactivities. Symp. Chem. Nat. Prod. 1996;38:409–414. [Google Scholar]
- 237.Bai H., Li W., Koike K., Dou D., Pei Y., Chen Y., Nikaido T. A novel biflavonoid from roots of Glycyrrhiza uralensis cultivated in China. Chem. Pharm. Bull. 2003;51:1095–1097. doi: 10.1248/cpb.51.1095. [DOI] [PubMed] [Google Scholar]
- 238.Chen R.J., Cao S.W., Ruan Z. Isolation of chemical constituents from Daphne odora var. Margirmt by high-speed counter-current chromatography. Chem. Nat. Compd. 2009;45:534–535. doi: 10.1007/s10600-009-9389-8. [DOI] [Google Scholar]
- 239.Liang S., Tian J.-M., Feng Y., Liu X.-H., Xiong Z., Zhang W.-D. Flavonoids from Daphne aurantiaca and Their Inhibitory Activities against Nitric Oxide Production. Chem. Pharm. Bull. 2011;59:653–656. doi: 10.1248/cpb.59.653. [DOI] [PubMed] [Google Scholar]
- 240.Taniguchi M., Fujiwara A., Baba K. Three flavonoids from Daphne odora. Phytochemistry. 1997;45:183–188. doi: 10.1016/S0031-9422(96)00800-X. [DOI] [Google Scholar]
- 241.Huang W.-H., Zhou G.-X., Wang G.-C., Chung H.-Y., Ye W.-C., Li Y.-L. A new biflavonoid with antiviral activity from the roots of Wikstroemia indica. J. Asian Nat. Prod. Res. 2012;14:401–406. doi: 10.1080/10286020.2011.653963. [DOI] [PubMed] [Google Scholar]
- 242.Zheng W.-F., Shi F. Three biflavonoids from ethanol extract of the root of Daphne genkwa. Acta Pharm. Sin. 2005;40:438–442. [PubMed] [Google Scholar]
- 243.Zhou G.-X., Jiang R.-W., Cheng Y., Ye W.-C., Shi J.-G., Gong N.-B., Lu Y. Daphnogirins A and B, Two Biflavones from Daphne giraldii. Chem. Pharm. Bull. 2007;55:1287–1290. doi: 10.1248/cpb.55.1287. [DOI] [PubMed] [Google Scholar]
- 244.Xu M., Shen L., Wang K. A new biflavonoid from Daphniphyllum angustifolium Hutch. Fitoterapia. 2009;80:461–464. doi: 10.1016/j.fitote.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 245.Tao H., Wang L., Cui Z., Zhao D., Liu Y. Dimeric proanthocyanidins from the roots of Ephedra sinica. Planta Med. 2008;74:1823–1825. doi: 10.1055/s-0028-1088321. [DOI] [PubMed] [Google Scholar]
- 246.Bilia A.R., Morelli I., Hamburger M., Hostetmann K. Flavans and A-type proanthocyanidins from Prunus prostrata. Phytochemistry. 1996;43:887–892. doi: 10.1016/0031-9422(96)00291-9. [DOI] [Google Scholar]
- 247.Kolodziej H., Sakar M.K., Burger J.F.W., Engelshowe R., Ferreira D. A-type proanthocyanidins from Prunus spinosa. Phytochemistry. 1991;30:2041–2047. doi: 10.1016/0031-9422(91)85064-7. [DOI] [Google Scholar]
- 248.Porter L.J., Ma Z., Chan B.G. Cacao procyanidins: Major flavanoids and identification of some minor metabolites. Phytochemistry. 1991;30:1657–1663. doi: 10.1016/0031-9422(91)84228-K. [DOI] [Google Scholar]
- 249.Baldé A.M., Pieters L.A., Wray V., Kolodziej H., Berghe D.A.V., Claeys M., Vlietinck A.J. Dimeric and trimeric proanthocyanidins possessing a doubly linked structure from Pavetta owariensis. Phytochemistry. 1991;30:4129–4135. doi: 10.1016/0031-9422(91)83480-9. [DOI] [Google Scholar]
- 250.Baldé A.M., Pieters L.A., Gergely A., Kolodziej H., Claeys M., Vlietinck A.J. A-type Proanthocyanidins from stem-bark of Pavetta owariensis. Phytochemistry. 1991;30:337–342. doi: 10.1016/0031-9422(91)84150-Q. [DOI] [Google Scholar]
- 251.Kamiya K., Watanabe C., Endang H., Umar M., Satake T. Studies on the Constituents of Bark of Parameria laevigata MOLDENKE. Chem. Pharm. Bull. 2001;49:551–557. doi: 10.1248/cpb.49.551. [DOI] [PubMed] [Google Scholar]
- 252.Vivas N., Glories Y., Pianet I., Barbe B., Laguerre M. A complete structural and conformational investigation of procyanidin A2 dimer. Tetrahedron Lett. 1996;37:2015–2018. doi: 10.1016/0040-4039(96)00187-6. [DOI] [Google Scholar]
- 253.De Bruyne T., Pieters L., Witvrouw M., De Clercq E., Vanden Berghe D., Vlietinck A.J. Biological Evaluation of Proanthocyanidin Dimers and Related Polyphenols. J. Nat. Prod. 1999;62:954–958. doi: 10.1021/np980481o. [DOI] [PubMed] [Google Scholar]
- 254.Su B.-N., Hwang B.Y., Chai H., Carcache-Blanco E.J., Kardono L.B.S., Afriastini J.J., Riswan S., Wild R., Laing N., Farnsworth N.R., et al. Activity-Guided Fractionation of the Leaves of Ormosia sumatrana Using a Proteasome Inhibition Assay. J. Nat. Prod. 2004;67:1911–1914. doi: 10.1021/np040134g. [DOI] [PubMed] [Google Scholar]
- 255.Jia B.X., Zeng X.L., Ren F.X., Jia L., Chen X.Q., Yang J., Liu H.M., Wang Q. Baeckeins F-I, four novel C-methylated biflavonoids from the roots of Baeckea frutescens and their anti-inflammatory activities. Food Chem. 2014;155:31–37. doi: 10.1016/j.foodchem.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 256.Tih R.G., Sondengam B.L., Martin M.T., Bodo B. Structure of lophirones B and C, biflavonoids from the bark of Lophira lanceolata. Phytochemistry. 1989;28:1557–1559. doi: 10.1016/S0031-9422(00)97794-X. [DOI] [Google Scholar]
- 257.Pegnyemb D.E., Tih R.G., Sondengam B.L., Blond A., Bodo B. Biflavonoids from Ochna afzelii. Phytochemistry. 2001;57:579–582. doi: 10.1016/S0031-9422(01)00101-7. [DOI] [PubMed] [Google Scholar]
- 258.Messanga B., Tih R.G., Sondengam B.-L., Martin M.-T., Bodo B. Biflavonoids from Ochna calodendron. Phytochemistry. 1994;35:791–794. doi: 10.1016/S0031-9422(00)90607-1. [DOI] [Google Scholar]
- 259.Tih R.G., Sondengam B.L., Martin M.T., Bodo B. Structure of the chalcone dimers lophirone F, and H from Lophira lanceolata stem bark. Phytochemistry. 1990;29:2289–2293. doi: 10.1016/0031-9422(90)83054-5. [DOI] [Google Scholar]
- 260.Anuradha V., Srinivas P.V., Ranga Rao R., Manjulatha K., Purohit M.G., Madhusudana Rao J. Isolation and synthesis of analgesic and anti-inflammatory compounds from Ochna squarrosa L. Bioorg. Med. Chem. 2006;14:6820–6826. doi: 10.1016/j.bmc.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 261.Lingfang P., Lihe L., Liguo Y., Xueping L., Tao C., Zhaoyun Z. A new biflavone from Dysosma versipellis. Acta Pharm. Sin. 2016;51:1281–1284. [PubMed] [Google Scholar]
- 262.Young D.A., Ferreira D., Roux D.G. Synthesis of condensed tannins. Part 10. ‘Dioxane-linked’ profisetinidins. J. Chem. Soc. Perkin Trans. 1. 1983;14:2031–2035. doi: 10.1039/P19830002031. [DOI] [Google Scholar]
- 263.Kaewamatawong R., Likhitwitayawuid K., Ruangrungsi N., Takayama H., Kitajima M., Aimi N. Novel Biflavonoids from the Stem Bark of Ochna integerrima. J. Nat. Prod. 2002;65:1027–1029. doi: 10.1021/np010630u. [DOI] [PubMed] [Google Scholar]
- 264.Messanga B.B., Tih R.G., Kimbu S.F., Sondengam B.L., Martin M.T., Bodo B. Calodenone, a New Isobiflavonoid from Ochna calodendron. J. Nat. Prod. 1992;55:245–248. doi: 10.1021/np50080a018. [DOI] [Google Scholar]
- 265.Pegnyemb D.E., Tih R.G., Sondengam B.L., Blond A., Bodo B. Isolation and Structure Elucidation of a New Isobiflavonoid From Ochna afzelii. Pharm. Biol. 2003;41:92–95. doi: 10.1076/phbi.41.2.92.14245. [DOI] [Google Scholar]
- 266.Geiger H., Markham K.R. Campylopusaurone, an auronoflavanone biflavonoid from the mosses campylopus clavatus and campylopus holomitrium. Phytochemistry. 1992;31:4325, 4328. doi: 10.1016/0031-9422(92)80467-S. [DOI] [Google Scholar]
- 267.Messi B.B., Ndjoko-Ioset K., Hertlein-Amslinger B., Lannang A.M., Nkengfack A.E., Wolfender J.L., Hostettmann K., Bringmann G. Preussianone, a new flavanone-chromone biflavonoid from Garcinia preussii Engl. Molecules. 2012;17:6114–6125. doi: 10.3390/molecules17056114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jia C., Han T., Xu J., Li S., Sun Y., Li D., Li Z., Hua H. A new biflavonoid and a new triterpene from the leaves of Garcinia paucinervis and their biological activities. J. Nat. Med. 2017;71:642–649. doi: 10.1007/s11418-017-1092-7. [DOI] [PubMed] [Google Scholar]
- 269.Baba K., Takeuchi K., Tabata Y., Taniguchi M., Kozawa M. Chemical studies on the constituents of the thymelaeaceous plants. IV. Structure of a new spiro biflavonoid, genkwanol A, from the root of Daphne genkwa Sieb. et Zucc. Yakugaku Zasshi. 1987;107:525–529. doi: 10.1248/yakushi1947.107.7_525. [DOI] [PubMed] [Google Scholar]
- 270.Yang B.-H., Zhang W.-D., Liu R.-H., Tan C.-H., Li T.-Z., Zhang C., Xu X.-K., Su J. Spiro-biflavonoids from Larix olgensis Henry var. koreana Nakai. Helv. Chim. Acta. 2005;88:2892–2896. doi: 10.1002/hlca.200590232. [DOI] [Google Scholar]
- 271.Shen Z., Falshaw C.P., Haslam E., Begley M.J. A novel spiro-biflavonoid from Larix gmelini. J. Chem. Soc. Chem. Commun. 1985;16:1135–1137. doi: 10.1039/c39850001135. [DOI] [Google Scholar]
- 272.Andrade A.W.L., Machado K.D.C., Machado K.D.C., Figueiredo D.D.R., David J.M., Islam M.T., Uddin S.J., Shilpi J.A., Costa J.P. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem. Cent. J. 2018;12:75. doi: 10.1186/s13065-018-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lixian W., Yuanyuan Y., Meng S., Qi W., Changsheng D., Xin’an H., Jianping S. Chemical Constituents from Garcinia kola Seeds and Their Anti-Oxidant Activity. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020;31:1133–1140. [Google Scholar]
- 274.Jeong E.J., Hwang L., Lee M., Lee K.Y., Ahn M.J., Sung S.H. Neuroprotective biflavonoids of Chamaecyparis obtusa leaves against glutamate-induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 2014;64:397–402. doi: 10.1016/j.fct.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 275.Park H., Kim Y.H., Chang H.W., Kim H.P. Anti-inflammatory activity of the synthetic C-C biflavonoids. J. Pharm. Pharm. 2006;58:1661–1667. doi: 10.1211/jpp.58.12.0014. [DOI] [PubMed] [Google Scholar]
- 276.Banerjee T., Valacchi G., Ziboh V.A., van der Vliet A. Inhibition of TNFalpha-induced cyclooxygenase-2 expression by amentoflavone through suppression of NF-kappaB activation in A549 cells. Mol. Cell Biochem. 2002;238:105–110. doi: 10.1023/A:1019963222510. [DOI] [PubMed] [Google Scholar]
- 277.Li Q., Ye T., Long T., Peng X. Ginkgetin exerts anti-inflammatory effects on cerebral ischemia/reperfusion-induced injury in a rat model via the TLR4/NF-kappaB signaling pathway. Biosci. Biotechnol. Biochem. 2019;83:675–683. doi: 10.1080/09168451.2018.1553608. [DOI] [PubMed] [Google Scholar]
- 278.Kim H.P., Park H., Son K.H., Chang H.W., Kang S.S. Biochemical pharmacology of biflavonoids: Implications for anti-inflammatory action. Arch. Pharm. Res. 2008;31:265–273. doi: 10.1007/s12272-001-1151-3. [DOI] [PubMed] [Google Scholar]
- 279.Coulerie P., Eydoux C., Hnawia E., Stuhl L., Maciuk A., Lebouvier N., Canard B., Figadere B., Guillemot J.C., Nour M. Biflavonoids of Dacrydium balansae with potent inhibitory activity on dengue 2 NS5 polymerase. Planta Med. 2012;78:672–677. doi: 10.1055/s-0031-1298355. [DOI] [PubMed] [Google Scholar]
- 280.Li F., Song X., Su G., Wang Y., Wang Z., Jia J., Qing S., Huang L., Wang Y., Zheng K., et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses. 2019;11:466. doi: 10.3390/v11050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.De Freitas C.S., Rocha M.E.N., Sacramento C.Q., Marttorelli A., Ferreira A.C., Rocha N., de Oliveira A.C., de Oliveira Gomes A.M., Dos Santos P.S., da Silva E.O., et al. Agathisflavone, a Biflavonoid from Anacardium occidentale L. Inhibits Influenza Virus Neuraminidase. Curr. Top. Med. Chem. 2020;20:111–120. doi: 10.2174/1568026620666191219150738. [DOI] [PubMed] [Google Scholar]
- 282.Tang S., Bremner P., Kortenkamp A., Schlage C., Gray A.I., Gibbons S., Heinrich M. Biflavonoids with cytotoxic and antibacterial activity from Ochna macrocalyx. Planta Med. 2003;69:247–253. doi: 10.1055/s-2003-38478. [DOI] [PubMed] [Google Scholar]
- 283.Nandu T.G., Subramenium G.A., Shiburaj S., Viszwapriya D., Iyer P.M., Balamurugan K., Rameshkumar K.B., Karutha Pandian S. Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors. J. Med. Microbiol. 2018;67:1391–1401. doi: 10.1099/jmm.0.000799. [DOI] [PubMed] [Google Scholar]
- 284.Lee J., Choi Y., Woo E.R., Lee D.G. Isocryptomerin, a novel membrane-active antifungal compound from Selaginella tamariscina. Biochem. Biophys. Res. Commun. 2009;379:676–680. doi: 10.1016/j.bbrc.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 285.Lee J.H. Involvement of T-cell immunoregulation by ochnaflavone in therapeutic effect on fungal arthritis due to Candida albicans. Arch. Pharm. Res. 2011;34:1209–1217. doi: 10.1007/s12272-011-0720-0. [DOI] [PubMed] [Google Scholar]
- 286.Ramalingam S., Karuppiah M., Thiruppathi M., Palanivelu S., Panchanatham S. Antioxidant potential of biflavonoid attenuates hyperglycemia by modulating the carbohydrate metabolic enzymes in high fat diet/streptozotocin induced diabetic rats. Redox Rep. 2020;25:1–10. doi: 10.1080/13510002.2020.1722914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu P.K., Weng Z.M., Ge G.B., Li H.L., Ding L.L., Dai Z.R., Hou X.D., Leng Y.H., Yu Y., Hou J. Biflavones from Ginkgo biloba as novel pancreatic lipase inhibitors: Inhibition potentials and mechanism. Int. J. Biol. Macromol. B. 2018;118:2216–2223. doi: 10.1016/j.ijbiomac.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 288.Xianming W., Aiqiong L., Lili Z., Jian L. Study on wound healing mechanism of the foot ulcer in diabetic rats by isoginkgetin. J. Xiangnan Univ. (Med. Sci.) 2019;21:6–10. [Google Scholar]
- 289.Zhou Q., Han X., Li R., Zhao W., Bai B., Yan C., Dong X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine. 2018;51:171–180. doi: 10.1016/j.phymed.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 290.Tabares-Guevara J.H., Lara-Guzman O.J., Londono-Londono J.A., Sierra J.A., Leon-Varela Y.M., Alvarez-Quintero R.M., Osorio E.J., Ramirez-Pineda J.R. Natural Biflavonoids Modulate Macrophage-Oxidized LDL Interaction In Vitro and Promote Atheroprotection In Vivo. Front. Immunol. 2017;8:923. doi: 10.3389/fimmu.2017.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Uddin M.S., Kabir M.T., Tewari D., Mathew B., Aleya L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci. Total Environ. 2020;700:134836. doi: 10.1016/j.scitotenv.2019.134836. [DOI] [PubMed] [Google Scholar]
- 292.Thapa A., Chi E.Y. Biflavonoids as Potential Small Molecule Therapeutics for Alzheimer’s Disease. Adv. Exp. Med. Biol. 2015;863:55–77. doi: 10.1007/978-3-319-18365-7_3. [DOI] [PubMed] [Google Scholar]
- 293.Thapa A., Woo E.R., Chi E.Y., Sharoar M.G., Jin H.G., Shin S.Y., Park I.S. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-beta toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry. 2011;50:2445–2455. doi: 10.1021/bi101731d. [DOI] [PubMed] [Google Scholar]
- 294.Sirimangkalakitti N., Juliawaty L.D., Hakim E.H., Waliana I., Saito N., Koyama K., Kinoshita K. Naturally occurring biflavonoids with amyloid β aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg. Med. Chem. Lett. 2019;29:1994–1997. doi: 10.1016/j.bmcl.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 295.Choi E.Y., Kang S.S., Lee S.K., Han B.H. Polyphenolic Biflavonoids Inhibit Amyloid-Beta Fibrillation and Disaggregate Preformed Amyloid-Beta Fibrils. Biomol. Ther. 2020;28:145–151. doi: 10.4062/biomolther.2019.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Olajide O.J., Ugbosanmi A.T., Enaibe B.U., Ogunrinola K.Y., Lewu S.F., Asogwa N.T., Akapa T., Imam A., Ibrahim A., Gbadamosi I.T., et al. Cerebellar Molecular and Cellular Characterization in Rat Models of Alzheimer’s Disease: Neuroprotective Mechanisms of Garcinia Biflavonoid Complex. Ann. Neurosci. 2017;24:32–45. doi: 10.1159/000464421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cao Q., Qin L., Huang F., Wang X., Yang L., Shi H., Wu H., Zhang B., Chen Z., Wu X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharm. 2017;319:80–90. doi: 10.1016/j.taap.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 298.Wang Y.Q., Wang M.Y., Fu X.R., Peng Y., Gao G.F., Fan Y.M., Duan X.L., Zhao B.L., Chang Y.Z., Shi Z.H. Neuroprotective effects of ginkgetin against neuroinjury in Parkinson’s disease model induced by MPTP via chelating iron. Free Radic. Res. 2015;49:1069–1080. doi: 10.3109/10715762.2015.1032958. [DOI] [PubMed] [Google Scholar]
- 299.Galati G., O’Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 300.Li Y.Y., Lu X.Y., Sun J.L., Wang Q.Q., Zhang Y.D., Zhang J.B., Fan X.H. Potential hepatic and renal toxicity induced by the biflavonoids from Ginkgo biloba. Chin. J. Nat. Med. 2019;17:672–681. doi: 10.1016/S1875-5364(19)30081-0. [DOI] [PubMed] [Google Scholar]
- 301.Lin Y.-M., Chen F.-C., Lee K.-H. Hinokiflavone, a Cytotoxic Principle from Rhus succedanea and the Cytotoxicity of the Related Biflavonoids. Planta Med. 1989;55:166–168. doi: 10.1055/s-2006-961914. [DOI] [PubMed] [Google Scholar]
- 302.Lopes Andrade A.W., Dias Ribeiro Figueiredo D., Torequl Islam M., Viana Nunes A.M., da Conceicao Machado K., da Conceicao Machado K., Uddin S.J., Ahmed Shilpi J., Rouf R., de Carvalho Melo-Cavalcante A.A., et al. Toxicological evaluation of the biflavonoid, agathisflavone in albino Swiss mice. Biomed. Pharmacother. 2019;110:68–73. doi: 10.1016/j.biopha.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 303.Yao W., Lin Z., Shi P., Chen B., Wang G., Huang J., Sui Y., Liu Q., Li S., Lin X., et al. Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem. Pharmacol. 2020;171:113680. doi: 10.1016/j.bcp.2019.113680. [DOI] [PubMed] [Google Scholar]
- 304.Yao W., Lin Z., Wang G., Li S., Chen B., Sui Y., Huang J., Liu Q., Shi P., Lin X., et al. Delicaflavone induces apoptosis via mitochondrial pathway accompanying G2/M cycle arrest and inhibition of MAPK signaling cascades in cervical cancer HeLa cells. Phytomedicine. 2019;62:152973. doi: 10.1016/j.phymed.2019.152973. [DOI] [PubMed] [Google Scholar]
- 305.Mu W., Cheng X., Zhang X., Liu Y., Lv Q., Liu G., Zhang J., Li X. Hinokiflavone induces apoptosis via activating mitochondrial ROS/JNK/caspase pathway and inhibiting NF-kappaB activity in hepatocellular carcinoma. J. Cell Mol. Med. 2020;24:8151–8165. doi: 10.1111/jcmm.15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wada S., Hitomi T., Tokuda H., Tanaka R. Anti-tumor-initiating effects of spiro-biflavonoids from Abies sachalinensis. Chem. Biodivers. 2010;7:2303–2308. doi: 10.1002/cbdv.201000147. [DOI] [PubMed] [Google Scholar]
- 307.Li P., Yue G.G., Kwok H.F., Long C.L., Lau C.B., Kennelly E.J. Using Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry-Based Chemometrics for the Identification of Anti-angiogenic Biflavonoids from Edible Garcinia Species. J. Agric. Food Chem. 2017;65:8348–8355. doi: 10.1021/acs.jafc.7b02867. [DOI] [PubMed] [Google Scholar]
- 308.Tarallo V., Lepore L., Marcellini M., Dal Piaz F., Tudisco L., Ponticelli S., Lund F.W., Roepstorff P., Orlandi A., Pisano C., et al. The biflavonoid amentoflavone inhibits neovascularization preventing the activity of proangiogenic vascular endothelial growth factors. J. Biol. Chem. 2011;286:19641–19651. doi: 10.1074/jbc.M110.186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cabrini D.A., Patino A.C., Nunez V., Osorio E. The biflavonoid morelloflavone inhibits the enzymatic and biological activities of a snake venom phospholipase A2. Chem. Biol. Interact. 2014;220:94–101. doi: 10.1016/j.cbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 310.Antia B.S., Pansanit A., Ekpa O.D., Ekpe U.J., Mahidol C., Kittakoop P. Alpha-glucosidase inhibitory, aromatase inhibitory, and antiplasmodial activities of a biflavonoid GB1 from Garcinia kola stem bark. Planta Med. 2010;76:276–277. doi: 10.1055/s-0029-1186081. [DOI] [PubMed] [Google Scholar]
- 311.Wu X.-N., Yang Y., Zhang H.-H., Zhong Y.-S., Wu F., Yu B., Yu C.-H. Robustaflavone-4′-dimethyl ether from Selaginella uncinata attenuated lipopolysaccharide-induced acute lung injury via inhibiting FLT3-mediated neutrophil activation. Int. Immunopharmacol. 2020;82:106338–106342. doi: 10.1016/j.intimp.2020.106338. [DOI] [PubMed] [Google Scholar]
- 312.Jalil J., Jantan I., Ghani A.A., Murad S. Platelet-activating factor (PAF) antagonistic activity of a new biflavonoid from Garcinia nervosa var. pubescens King. Molecules. 2012;17:10893–10901. doi: 10.3390/molecules170910893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Qu X., Li Q., Zhang X., Wang Z., Wang S., Zhou Z. Amentoflavone protects the hematopoietic system of mice against γ-irradiation %J Pharmaceutical Society of Korea. Arch. Pharm. Res. 2019;42:1021–1029. doi: 10.1007/s12272-019-01187-0. [DOI] [PubMed] [Google Scholar]
- 314.Yamaguchi L.F., Kato M.J., Di Mascio P. Biflavonoids from Araucaria angustifolia protect against DNA UV-induced damage. Phytochemistry. 2009;70:615–620. doi: 10.1016/j.phytochem.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 315.Campos P.M., Prudente A.S., Horinouchi C.D., Cechinel-Filho V., Favero G.M., Cabrini D.A., Otuki M.F. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015;174:224–229. doi: 10.1016/j.jep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 316.O’Brien K., Matlin A.J., Lowell A.M., Moore M.J. The biflavonoid isoginkgetin is a general inhibitor of Pre-mRNA splicing. J. Biol. Chem. 2008;283:33147–33154. doi: 10.1074/jbc.M805556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kwak W.J., Han C.K., Son K.H., Chang H.W., Kang S.S., Park B.K., Kim H.P. Effects of Ginkgetin from Ginkgo biloba Leaves on cyclooxygenases and in vivo skin inflammation. Planta Med. 2002;68:316–321. doi: 10.1055/s-2002-26742. [DOI] [PubMed] [Google Scholar]
- 318.Lim H., Son K.H., Chang H.W., Kang S.S., Kim H.P. Effects of anti-inflammatory biflavonoid, ginkgetin, on chronic skin inflammation. Biol. Pharm. Bull. 2006;29:1046–1049. doi: 10.1248/bpb.29.1046. [DOI] [PubMed] [Google Scholar]
- 319.Kim T.Y., Park N.J., Jegal J., Choi S., Lee S.W., Hang J., Kim S.N., Yang M.H. Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice. Biomolecules. 2019;9:697. doi: 10.3390/biom9110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liao S., Ren Q., Yang C., Zhang T., Li J., Wang X., Qu X., Zhang X., Zhou Z., Zhang Z., et al. Liquid chromatography-tandem mass spectrometry determination and pharmacokinetic analysis of amentoflavone and its conjugated metabolites in rats. J. Agric. Food Chem. 2015;63:1957–1966. doi: 10.1021/jf5019615. [DOI] [PubMed] [Google Scholar]
- 321.Yin R., Xiong K., Wen S., Wang Y., Xu F. Development and validation of an LC-MS/MS method for the determination of hinokiflavone in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2017;31:3821–3840. doi: 10.1002/bmc.3821. [DOI] [PubMed] [Google Scholar]
- 322.Shan C.-X., Guo S.-C., Yu S., Shan M.-Q., Li S.F.Y., Chai C., Cui X.-B., Zhang L., Ding A.-W., Wu Q.-N. Simultaneous Determination of Quercitrin, Afzelin, Amentoflavone, Hinokiflavone in Rat Plasma by UFLC-MS-MS and Its Application to the Pharmacokinetics of Platycladus orientalis Leaves Extract. J. Chromatogr. Sci. 2018;56:895–902. doi: 10.1093/chromsci/bmy066. [DOI] [PubMed] [Google Scholar]
- 323.Alzand K.I., Mohamed M.A. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res. 2012;5:4013–4020. [Google Scholar]
- 324.Gomes-Copelanda K.K.P., Lédob A.d.S., Almeidac F.T.C.d., Moreirad B.O., Santosd D.C.d., Santosd R.A.F., Jorge Mauricio Davidd J.P.D. Effect of elicitors in Poincianella pyramidalis callus culture in the biflavonoid biosynthesis. Ind. Crop. Prod. 2018;126:421–425. doi: 10.1016/j.indcrop.2018.10.038. [DOI] [Google Scholar]
- 325.Ying X., Ling-bo Q., Jin-wei Y. Research Progress on the Extraction and Synthesis of Biflavonoid Compounds. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2010;31:78–85. [Google Scholar]
- 326.Ndoile M.M., van Heerden F.R. Total synthesis of ochnaflavone. Beilstein J. Org. Chem. 2013;9:1346–1351. doi: 10.3762/bjoc.9.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang Y., Lin S., Shi A., Yang Y., Tang W. The synthetic research of (±)-2,3,2”,3”-Tetrahydroochnaflavone. Chin. J. Org. Chem. 2015;35:2114–2118. doi: 10.6023/cjoc201503029. [DOI] [Google Scholar]
- 328.Chen J., Chang H.W., Kim H.P., Park H. Synthesis of phospholipase A2 inhibitory biflavonoids. Bioorganic Med. Chem. Lett. 2006;16:2373–2375. doi: 10.1016/j.bmcl.2006.01.117. [DOI] [PubMed] [Google Scholar]
- 329.Moon T.C., Quan Z., Kim J., Kim H.P., Kudo I., Murakami M., Park H., Chang H.W. Inhibitory effect of synthetic C-C biflavones on various phospholipase A(2)s activity. Bioorg Med. Chem. 2007;15:7138–7143. doi: 10.1016/j.bmc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 330.Lim H., Kim S.B., Park H., Chang H.W., Kim H.P. New anti-inflammatory synthetic biflavonoid with C-C (6-6”) linkage: Differential effects on cyclooxygenase-2 and inducible nitric oxide synthase. Arch. Pharm. Res. 2009;32:1525–1531. doi: 10.1007/s12272-009-2104-2. [DOI] [PubMed] [Google Scholar]
- 331.Yunchang T. Master’s Thesis. Tianjin University of Science and Technology; Tianjin, China: 2018. Synthesis of I3’,II8-apigenin Biflavone and Inhibitory Activity Evaluation as α-Glucosidase Inhibitors. [Google Scholar]
- 332.Ming L. Master’s, Thesis. Tianjin University of Science and Technology; Tianjin, China: 2018. Synthesis of Wikstrol A/B and Morelloflavone. [Google Scholar]
- 333.Zhang Z.T., Gao R.L., Zhuang S.K. Synthesis of biflavones and their interaction with DNA. Acta Pharm. Sin. 2009;44:873–878. [PubMed] [Google Scholar]
- 334.Ying X., Jinwei Y., Yongmei X., Pu M., Gonggong H. The Process of Biflavonoids’s Synthesis by Acid Catalysis; Proceedings of the Academic Annual Meeting of Henan Chemical Society and Celebration of its 70th Anniversary; Nanyang, China. 24 September 2010; p. 1. [Google Scholar]
- 335.Ying X., Jin-wei Y., Ling-bo Q. Synthesis and Reaction Mechanism of Biflavonoids; Proceedings of the The 12th National Annual Meeting of Applied Chemistry of the Chinese Chemical Society; Zhengzhou, China. 17 October 2011; p. 2. [Google Scholar]
- 336.Baron V., Mead K.T. Synthesis of 3-benzylidene-dihydrofurochromen-2-ones: Promising intermediates for biflavonoid synthesis. Heterocycl. Commun. 2015;21:225–231. doi: 10.1515/hc-2015-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ren Q.X., Zhou Z., Wang S.Q. Preparation and analytical characterization of micronized amentoflavone by antisolvent freeze-drying method. Int. J. Pharm. Res. 2013;40:237–241. [Google Scholar]
- 338.DeKosky S.T., Williamson J.D., Fitzpatrick A.L., Kronmal R.A., Ives D.G., Saxton J.A., Lopez O.L., Burke G., Carlson M.C., Fried L.P., et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.