Abstract
COVID‐19 should be considered as a new triggering factor for autoimmune disorders like DM‐lupus overlap syndrome. We recommend that patients presenting with dermatomyositis during this pandemic be screened for COVID‐19.
Keywords: COVID‐19, dermatomyositis, lupus erythematosus, myositis, trigger
COVID‐19 should be considered as a new triggering factor for autoimmune disorders like DM‐lupus overlap syndrome. We recommend that patients presenting with dermatomyositis during this pandemic be screened for COVID‐19.
1. INTRODUCTION
Here, we reported a 58‐year‐old woman who presented with dermatomyositis‐lupus overlap syndrome complicated with cardiomyopathy after coronavirus disease 2019 (COVID‐19). COVID‐19 may be considered a potential triggering factor for dermatomyositis. However, it needs to be determined whether cardiac myopathy in patients with COVID‐19‐associated dermatomyositis is directly related to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) or is a complication of dermatomyositis itself.
The coronavirus disease 2019 (COVID‐19) pandemic has spread all over the world at an unexpected rate, leading to a variety of manifestations ranging from a lack of symptoms to death. 1 Although the most common manifestations include dyspnea, cough, fever, malaise, myalgia, and weakness, other organs like the skin may also be involved; therefore, COVID‐19 is a challenge for dermatologists as well as other physicians. 2 , 3 , 4
Some of the medications used in the treatment of autoimmune rheumatologic diseases also appear to be effective in the treatment of COVID‐19 (especially severe cases), indicating some similarities in the pathogenesis of COVID‐19 and autoimmune diseases. 5 On the contrary, recent publications have shown that the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is involved in the aggravation of several autoimmune diseases like subacute thyroiditis, Kawasaki disease, coagulopathies, antiphospholipid syndrome, immune thrombocytopenic purpura, Guillain–Barré syndrome, dermatomyositis (DM), and lupus. 6 , 7 Hence, SARS‐CoV‐2 infection can perhaps be considered as a new triggering factor for some autoimmune diseases.
It should be noted that some clinical symptoms of several autoimmune diseases like DM may be similar to COVID‐19, indicating the importance of appropriate differentiation. For suspected patients complaining of proximal muscle weakness and myositis accompanied with fever, COVID‐19 should be distinguished from the myositis component of DM. 8 Here, we report a patient with dermatomyositis‐lupus overlap syndrome complicated with cardiomyopathy after SARS‐CoV‐2 infection.
2. CASE PRESENTATION
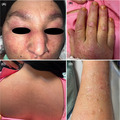
A 58‐year‐old female patient with a history of diabetes mellitus, hypothyroidism, and coronary artery disease presented to our dermatology clinic with malaise, weakness, and skin lesions. The patient had a history of COVID‐19 affliction about two months earlier in the form of fever, cough, malaise, anosmia, and ageusia, diagnosed via the PCR test and treated at home via supportive care. Forty‐five days later, she started complaining of periorbital erythema and edema together with intense pruritic erythema on the malar area and cheeks, gradually progressing to involve the hands, neck, and buttocks. After a few days, she developed malaise and loss of energy; soon, the patient was not even able to complete routine daily activities. The patient's medications included losartan, aspirin, metformin, levothyroxine, and doxepin. She was admitted to our dermatology ward. On physical examination, she had pink‐violet papules on the knuckles, ragged cuticles, midface erythema involving the nasolabial folds, an erythematous plaque involving the upper back, and multiple vesicles with an erythematous base on both upper extremities (Figure 1). There was no lymphadenopathy or hepatosplenomegaly. She had hoarseness, mild dyspnea, a low‐grade fever (38.5˚C), a pulse rate of 90/min, a blood pressure of 100/70 mm Hg, a respiratory rate of 26/min, and an oxygen saturation level of 94% under ambient conditions. The neurological examination showed proximal motor muscle weakness with a power of 2/5 at the shoulder and hip joints.
FIGURE 1.

(A) Periorbital erythema and edema, and malar erythema; (B) Gottron papules and ragged nail cuticles; (C) Multiple bilateral vesicles with central crusting on both upper extremities; (D) Erythema and excoriations on the upper back
Three skin biopsies from different skin sites were taken with differential diagnoses of DM and lupus erythematosus; the first was sent for examination under direct immune fluorescence (DIF) and the findings were in favor of lupus erythematosus (Figure 2). The second (from a Gottron papule) and third (from a vesicle on the extremities) biopsies were evaluated using hematoxylin‐eosin staining; findings indicated dermatomyositis‐lupus overlap features and were compatible with a collagen vascular disease (Figure 2).
FIGURE 2.
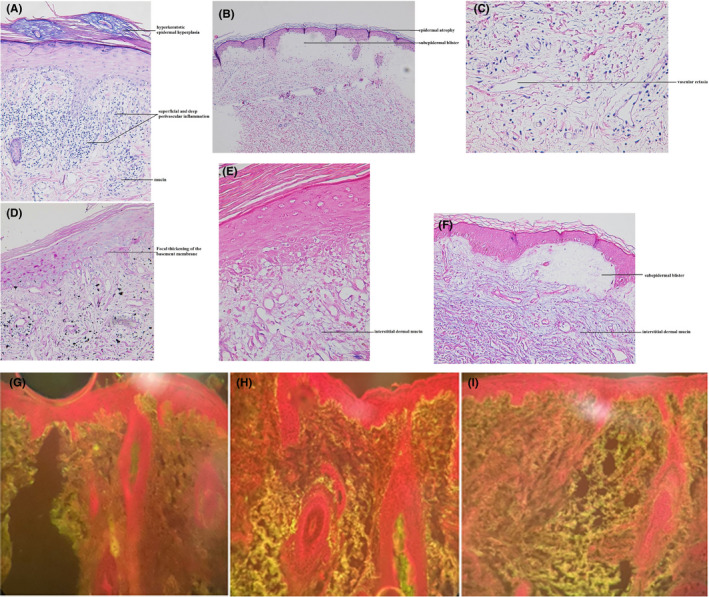
(A) Lichenoid interface reaction with superficial and deep perivascular inflammation, dermal mucin deposition, and mild hyperkeratotic epidermal hyperplasia; and (B) a subepidermal blister with mild epidermal atrophy; and (C) dermal edema and vascular ectasia in favor of collagen vascular disease including dermatomyositis (H&E stain, ×20, ×10, ×40, respectively). (D) Focal thickening of the basement membrane is present (PAS stain, ×20). (E, F) Prominent interstitial dermal mucin deposition is present (Alcian blue stain, ×40, ×10, respectively). Direct immunofluorescence showed a focal granular deposit of (G) anti‐IgG (2+), (H) anti‐IgM (3+), and (I) anti‐C3 (2+) along the dermoepidermal junction; the findings were in favor of lupus erythematosus (×10)
Laboratory evaluations revealed elevated levels of AST and ALT (67 and 66 U/L, respectively; normal range 5–40 U/L), aldolase (12.4 U/L; normal range 1–7 U/L), CPK (2611 U/L; upper limit 195 U/L), LDH (517 U/L), and ESR (57 mm/h). The complete blood count and thyroid function tests were normal, as were the levels of the CA 125, CA 19–9, C3, C4, and CH 50 markers. Serological tests including the antinuclear antibody (ANA), anti‐ds‐DNA, and anti‐Smith antibody were negative. Myositis‐specific antibodies including anti‐Mi‐2, ‐Ku, ‐PM/Scl‐100, ‐Scl‐75, ‐SRP, ‐PL‐7, ‐PL‐12, ‐EJ, ‐OJ, ‐Jo‐1, and ‐Ro‐52 were negative. The nasopharyngeal swab PCR test for SARS‐CoV‐2 was negative, whereas the SARS‐CoV‐2 IgG antibody returned positive.
Electromyography showed early inflammatory myopathy of the proximal upper and lower limbs’ muscles. The endoscopy, colonoscopy, mammography, computerized tomography (CT) of the paranasal sinuses, and ophthalmologic examination were normal. Echocardiography revealed hypokinesia of the left ventricle, septum, and anterior cardiac wall in addition to mitral and tricuspid regurgitation with an ejection fraction (EF) of 45%. Hence, metoprolol (47.5 mg daily) was initiated. The abdominopelvic CT scan was normal, while multifocal patchy consolidations on both sides with reverse halo view suggestive of the chronic phase of organizing COVID‐19 pneumonia were reported on the lung CT scan (Figure 3). According to the pulmonary consultation, cefazolin (2 g three times daily) and azithromycin (500 mg daily) were also started.
FIGURE 3.
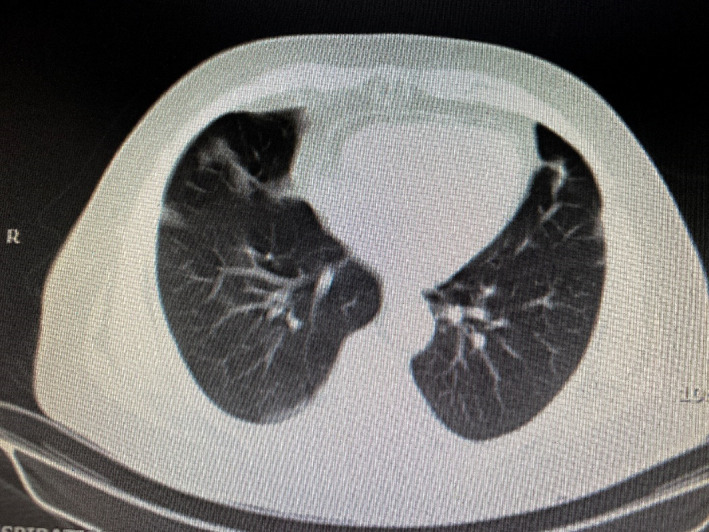
Lung CT scan shows multifocal patchy consolidations on both sides suggesting the chronic phase of organizing COVID‐19 pneumonia
Taking into account the disease severity, the high level of CPK, and the cardiac myopathy, a combination of high‐dose prednisolone (60 mg daily), methotrexate (15 mg weekly), and hydroxychloroquine sulfate (400 mg daily) was prescribed. A few days later, a gradual improvement in the patient's general condition and skin lesions was observed (Figure 4). One week later, the CPK level reached 1139 U/L; there was also an increase in EF to 55%. After 10 days, the patient was discharged with the same medications with a plan of gradual tapering of prednisolone.
FIGURE 4.

There was a significant improvement in clinical manifestations 10 days after the admission
3. DISCUSSION
Dermatomyositis (DM) is an autoimmune disorder that affects both children and adults. It is characterized by a diversity of cutaneous presentations that usually precede or accompany muscle disease. 9 Periorbital erythema and edema (heliotrope rash), Gottron papules on the metacarpophalangeal and interphalangeal joints, cuticle dystrophy, and erythema on the shoulders (shawl sign) and chest (V‐sign) comprise the characteristic cutaneous findings. 9 It is believed that DM is an immune‐mediated disease in which certain external factors (e.g., malignancies, medications, infectious agents, and UV exposure) act as stimuli in genetically predisposed individuals. 10 Exposure to SARS‐CoV‐2 in some individuals may provoke a musculoskeletal autoimmune disease or collagen vascular disorder, which may be explained by a recent publication that noted the presence of three immunogenic epitopes in DM patients featuring a high number of sequences identical to SARS‐CoV‐2 proteins. 11 , 12 The onset of DM‐lupus symptoms, in this case, was about 45 days after SARS‐CoV‐2 infection; there was clear proximal muscle weakness, periorbital and malar erythema with edema, in addition to Gottron's papules on both hands, indicating that the trigger was possibly COVID‐19. On the contrary, ANA, anti‐Ds‐DNA, and myositis‐specific antibodies were all negative, as reported in a case series of DM associated with COVID‐19. 7
An elevated level of type I interferons, which are involved in the pathogenesis of DM, is a highly sensitive marker for both DM disease activity and COVID‐19. 13 There is strong evidence of some similarities in the pathogenesis of DM and COVID‐19, including the role of type I interferons in causing myofiber and severe organ damages. This is confirmed by the use of Baricitinib, a Janus kinase (JAK) inhibitor that appears to be promising in the treatment of patients with COVID‐19‐associated severe organ damage, in addition to the use of tofacitinib in a clinical trial for the treatment of DM. 14
Furthermore, COVID‐19 leads to the production of high levels of cytokines, which may damage multiple organs including skeletal and cardiac muscles. 15 It can provoke a variety of cardiac manifestations ranging from mild cardiac injury to heart failure or even arrhythmias and cardiac arrest; these manifestations may also be seen in DM. 16 , 17 In our patient, COVID‐19 preceded the DM‐lupus presentation that was associated with cardiac myopathy in addition to muscle weakness and characteristic skin manifestations. The patient responded well to treatment including high‐dose prednisolone, methotrexate, and hydroxychloroquine sulfate. As a result of the high levels of cytokines in COVID‐19 infection and dermatomyositis, glucocorticoid agents play an important role in the treatment and can reduce cytokine levels which lead to the improvement of symptoms including myositis or cardiac myopathy. 18 In addition, MTX and hydroxychloroquine were added which are usually used in refractory and severe cases of dermatomyositis. Although MTX has many side effects, it prevents and stops the inflammatory process, leading to functional improvement. 19
In summary, interstitial pneumonia, myositis, and cardiac involvement can be seen in both DM‐lupus overlap and COVID‐19. 20 COVID‐19 should be considered a new triggering factor for autoimmune disorders like DM‐lupus overlap syndrome. 7 Proper diagnosis and prompt treatment can decrease the number of mortalities in cases with overlapping manifestations. However, further studies are required to determine whether certain manifestations such as cardiac myopathy in DM patients associated with COVID‐19 are directly related to the SARS‐CoV‐2 infection or are a complication of DM itself.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
MSD, AH, and FA were involved in the diagnosis and management of the patient and are responsible for the clinical aspect of the manuscript. AR reported the results of the histopathological evaluation. RD performed the literature review and drafted the manuscript. FA, SAH, and MSD were responsible for editing the manuscript. All authors read and approved the final manuscript.
ETHICAL APPROVAL
Not applicable.
CONSENT TO PARTICIPATE AND FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ACKNOWLEDGMENTS
We thank the patient and her family for their willingness to provide these images.
Shahidi Dadras M, Rakhshan A, Ahmadzadeh A, et al. Dermatomyositis‐lupus overlap syndrome complicated with cardiomyopathy after SARS‐CoV‐2 infection: A new potential trigger for musculoskeletal autoimmune disease development. Clin Case Rep. 2021;9:e04931. 10.1002/ccr3.4931
Mohammad Shahidi Dadras and Azadeh Rakhshan contributed equally to this work and are considered as co‐first authors.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors
Contributor Information
Reem Diab, Email: reemdiab1990@gmail.com.
Fahimeh Abdollahimajd, Email: fabdollahimajd@yahoo.com, Email: fabdollahimajd@sbmu.ac.ir.
DATA AVAILABILITY STATEMENT
All data are included in this published report.
REFERENCES
- 1. Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID‐19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. 10.1016/j.arr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferri C, Giuggioli D, Raimondo V, et al. COVID‐19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol. 2020;39:3195‐3204. 10.1007/s10067-020-05334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shahidi Dadras M, Zargari O, Abolghasemi R, Bahmanjahromi A, Abdollahimajd F. A probable atypical skin manifestation of COVID‐19 infection. J Dermatol Treat. 2020;1‐3. [DOI] [PubMed] [Google Scholar]
- 4. Shahidi Dadras M, Dadkhahfar S, Bahmanjahromi A, Seifian H, Abdollahimajd F. Skin manifestations in three cases of COVID‐19 infection from Iran and a narrative literature review. Iran J Dermatol. 2020;23:S60‐S66. [Google Scholar]
- 5. Liu Y, Sawalha AH, Lu Q. COVID‐19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33:155‐162. 10.1097/BOR.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saad MA, Alfishawy M, Nassar M, Mohamed M, Esene IN, Elbendary A. COVID‐19 and autoimmune diseases: a systematic review of reported cases. Curr Rheumatol Rev. 2021;17(2):193‐204. 10.2174/1573397116666201029155856 [DOI] [PubMed] [Google Scholar]
- 7. Gokhale Y, Patankar A, Holla U, et al. Dermatomyositis during COVID‐19 pandemic (a case series): is there a cause effect relationship? J Assoc Physicians India. 2020;68:20‐24. [PubMed] [Google Scholar]
- 8. Cao M, Zhang S, Chu D, et al. COVID‐19 or clinical amyopathic dermatomyositis associated rapidly progressive interstitial lung disease? A case report. BMC Pulm Med. 2020;20:304. 10.1186/s12890-020-01335-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalakas MC. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID‐19‐related implications. Acta Myol. 2020;39:289‐301. 10.36185/2532-1900-032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson C, Piguet V, Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2018;179:1256‐1262. 10.1111/bjd.15607 [DOI] [PubMed] [Google Scholar]
- 11. Pi Z, Chen P, Zhan Y, Xiao R. Management strategies for dermatomyositis during the coronavirus disease 2019 outbreak. Clin Dermatol. 2020;38:731‐733. 10.1016/j.clindermatol.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Megremis S, Walker TDJ, He X, et al. Antibodies against immunogenic epitopes with high sequence identity to SARS‐CoV‐2 in patients with autoimmune dermatomyositis. Ann Rheum Dis. 2020;79:1383‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baechler EC, Bilgic H, Reed AM. Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis Res Ther. 2011;13:249. 10.1186/ar3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanboon J, Nishino I. COVID‐19‐associated myositis may be dermatomyositis. Muscle Nerve. 2021;63:E9‐E10. 10.1002/mus.27105 [DOI] [PubMed] [Google Scholar]
- 15. Basu‐Ray I, Almaddah NK, Adeboye A, Soos MP, eds. Cardiac manifestations of coronavirus (COVID‐19).In StatPearls [Internet]. StatPearls Publishing; 2021. Jan. PMID: 32310612. [PubMed] [Google Scholar]
- 16. Caforio ALP. Coronavirus disease 2019 (COVID‐19): Cardiac manifestations in adults. In: Mancini D (Ed.), UpToDate, 2021. Retrieved February 1, 2021, from https://www.uptodate.com/contents/coronavirus‐disease‐2019‐covid‐19‐cardiac‐manifestations‐in‐adults
- 17. Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology. 2006;45(suppl_4):iv18‐iv21. 10.1093/rheumatology/kel311 [DOI] [PubMed] [Google Scholar]
- 18. Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID‐19 patients: a triple‐blinded randomized controlled trial. BMC Infect Dis. 2021;21:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choy EH, Isenberg DA. Treatment of dermatomyositis and polymyositis. Rheumatology. 2002;41(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 20. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS‐CoV‐2. Ann Rheum Dis. 2021;80(3):e42. 10.1136/annrheumdis-2020-217573 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this published report.


