Abstract
Aim:
The aim of this study was to investigate the predictive role of lymphocyte subsets and other laboratory measurements in patients with COVID-19.
Methods:
Electronic medical records of adult patients with confirmed diagnosis of COVID-19 from the Shanghai Public Health Clinical Center were reviewed retrospectively to obtain relevant data.
Results:
The mean age of patients was 40.98 ± 15.95 years, with 58% of the patients being males. The cutoff values at the intensive care unit (ICU) admission, mechanical ventilation, and mortality were CD4+ cells (267, 198, and 405), CD8+ cells (263, 203, and 182), and CD4+ /CD8+ cells (1.4, 1.8, and 1.4). The cutoffs below these values indicate the higher chances of disease progression. Higher CD4+ cell count led to lesser chances for ICU admission [odds ratio (OR) (95% confidence interval (CI): 0.994 (0.991, 0.997); p = 0.0002] and mortality [OR (95% CI): 0.988 (0.979, 0.99); p = 0.001], higher CD8+ count was an independent risk factor for ICU admission. T-cell count positively correlated with total lymphocyte count and platelets, while negatively correlated with D-dimer and lactate dehydrogenase (LDH). Among patients with non-severe COVID-19, median CD8+ T cell, CD4+ T cell, total lymphocyte count, and platelets were 570, 362, 1.45, and 211, respectively, while median values decreased to 149, 106, 0.64, and 172, respectively, in patients with severe COVID-19.
Conclusion:
Lower T lymphocyte subsets were significantly associated with higher admission to ICU, mechanical ventilation, and mortality among patients with COVID-19. A cutoff value of ICU admission, mechanical ventilation, and mortality below CD4+ cells (267, 198, and 405), CD8+ cells (263, 203, 182), and CD4+/CD8+ cells (1.4, 1.8, 1.4) may help identify patients at high risk of disease progression. The continuous evaluation of laboratory indices may help with dismal prognosis and prompt intervention to improve outcomes.
Keywords: CD3, CD4, cellular immunity, COVID-19, lymphocyte subsets, predictive
Introduction
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which began as a cluster of pneumonia cases in Wuhan, China, in December 2019, was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. 1 According to the WHO, as of February 2021, >100 million people worldwide are infected, with 2,381,295 people succumbing to this infection. 2 The causative RNA viruses were grouped into the coronavirus (CoV) family that has already resulted in three outbreaks of pneumonia in the last two decades. SARS-CoV was responsible for the severe acute respiratory syndrome (SARS) that broke out in 2003, while the Middle East respiratory syndrome (MERS) was triggered by the MERS-CoV 3 that broke out in 2012. Subsequently, the international viral classification commission named the viral etiological agent as SARS-CoV-2, and the disease was officially named COVID-19 by the WHO. The current management strategies include rapid identification of cases, isolation, contact tracing, and self-quarantine of the exposed individuals. 4 Furthermore, there is no effective oral therapy for the outpatient treatment of COVID-19. Reducing the symptom severity and decreasing outpatient hospitalizations are the important public health prevention strategies for overcoming this pandemic. 4
SARS-CoV-2 mainly affects tissues expressing elevated angiotensin-converting enzyme 2 (ACE2) levels, which includes the lungs, heart, and gastrointestinal tract, following viremia. 5 Post onset of initial symptoms, patients experience “cytokine storm” characterized by a surge in the clinical manifestations of the disease and marked systemic increase in inflammatory mediators and cytokines. 6 At this point, significant lymphopenia is also observed. Furthermore, a cytokine storm results in elevated levels of interleukins (ILs) (mostly IL-6, IL-2, IL-7, granulocyte colony-stimulating factor, interferon-γ inducible protein 10, MCP-1, and MIP1-a) and tumor necrosis factor (TNF)-alpha, which may promote lymphocyte apoptosis.7,8 Various clinical studies have indicated the presence of lymphocytes and other laboratory abnormalities in patients with COVID-19, especially with severe symptoms compared with those with non-severe ones.9 –12 A recent meta-analysis reported a consistent decrease in lymphocytes subsets among >3000 severe/critical COVID-19–affected patients across 20 studies, of which, multivariate analyses identified CD4+ and CD8+ T cells, but not total lymphocytes, as statistically significant predictors of intensive care unit (ICU) admission, mortality, treatment recovery, and viral clearance. 13 Studies have also shown that SARS-CoV-2 can impair CD4+ and regulatory T-cell function and encourage initial hyperactivation followed by rapid exhaustion of cytotoxic CD8+ T cells.14,15
The seventh edition of the COVID-19 Diagnosis and Treatment Scheme issued by the National Health Commission of China specified that the gradual decline in peripheral blood lymphocyte count and the persistent decrease in CD4+ T-cells and CD8+ T-cells were the indicators of a deterioration in the patient’s condition. 16 The absolute count of CD4+ and CD8+ T cells can independently predict the prognosis of patients with COVID-19. 17 In addition, laboratory measurements are less expensive, faster and easier to obtain, and preferred modality to monitor and predict the outcomes and prognosis of the disease. 18 Therefore, identifying the possible immune factors and laboratory parameters is a requisite for the early identification of patients with severe COVID-19 and timely intervention for control of the disease. There is an unmet need to establish robust clinical cutoffs of lymphocyte subsets for predicting the risk associated with ICU admission, mechanical ventilation, and mortality in patients with COVID-19. This retrospective study was thus carried out to improve the knowledge and clinical utility of lymphocyte subset measurements, along with other laboratory measurements and clinical information, for understanding the prognosis in patients with COVID-19.
Methods
Study design and population
This was a single-center, retrospective, study wherein clinical data of patients enrolled between 20 January and 28 August 2020 at the Shanghai Public Health Clinical Center (SPHCC) were collected. The patients were given standard of care treatment and followed as per Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (trial version 7) issued by the National Health Commission of China. 16
The analysis included hospitalized male or female subjects aged ⩾18 years and diagnosed with COVID-19. All patients tested positive for SARS-CoV-2 according to quantitative real-time polymerase chain reaction (qRT-PCR) were enrolled. Patients were divided at admission into mild/moderate/severe/critical according to the COVID-19 Guidelines (trial version 7). 16 In brief, the clinical classification of patients was as follows: patients with mild disease presented with mild symptoms and exhibited no signs of pneumonia on imaging; patients with moderate disease were presented with fever and respiratory symptoms with radiological findings of pneumonia; patients with severe disease presented with any one of the following: (1) respiratory distress (>30 breaths per minute), (2) oxygen saturation <93% at rest, or (3) an oxygenation index (PaO2/FiO2) <300mmHg; critically ill patients presented with any one of the following: (1) respiratory failure and requiring invasive ventilation, (2) signs of shock (circulatory failure), or (3) failure of any other organ that requires ICU care. Patients were divided into a non-severe cohort and a severe cohort according to their severity of symptoms. The non-severe cohort included patients with mild or moderate disease, and the severe cohort included patients with severe disease or who were critically ill.
The study was approved by the Institutional Ethics Committee of SPHCC (YJ-2020-S015-01) and was conducted in accordance to Declaration of Helsinki. As this study was a retrospective analysis of electronic data from SPHCC database, no informed consent was obtained. The trial was registered at www.clinicaltrials.gov (NCT04779359).
Study outcomes
The study endpoints were to demonstrate whether CD4+ and CD8+ T cell absolute counts are the prognostic markers in determining the disease outcomes (including ICU admission and/or mechanical ventilation and/or mortality), to explore whether lymphocyte subset measurements stabilized at hospital discharge or in convalescence and to explore lymphocyte subset measurements between severe and non-severe groups, at admission. In addition, the association between CD4+ and CD8+ T-cell absolute counts with cytokine levels and other laboratory parameters (neutrophil counts, total lymphocyte counts, platelet counts, C-reactive protein levels) was also assessed.
When respiratory distress and/or hypoxemia of the patient cannot be alleviated after receiving standard oxygen therapy, high-flow nasal cannula oxygen therapy or non-invasive ventilation was considered. However, when the patients’ condition did not improve or got worse within a short time (1–2 hours), tracheal intubation and timely utilization of invasive mechanical ventilation was considered. In patients, with ventilator-related lung injury, low tidal volume (6–8 ml/kg of ideal body weight) and low level of airway platform pressure (<30 cm H2O) were used to perform mechanical ventilation. While the airway platform pressure maintained ⩽30 cm H2O, high positive end-expiratory pressure (PEEP) was used to keep the airway warm and moist; to avoid long sedation and waking the patient early for lung rehabilitation. The admission criteria to ICU included oxygen requirements ⩾6–8 l/min to reach a peripheral oxygen saturation ⩾ 90–92%, respiratory failure, shock, acute organ dysfunction, and patients at high risk for clinical deterioration.
Data collection
The demographic and clinical data collected were age, sex, symptom from onset to hospital admission (fever, chills, night sweat, dizziness, headache, sore throat, nasal congestion, runny nose, cough, expectoration, hemoptysis, weakness, chest pain, chest tightness, and shortness of breath, hypoesthesia, anosmia, nausea and vomiting, abdominal pain, abdominal distention, and diarrhea) and laboratory findings at admission (lymphocyte subset counts, neutrophil counts, total lymphocyte counts, platelet counts, C-reactive protein levels, procalcitonin levels, lactate dehydrogenase levels, D-dimer levels, and ferritin levels). A Sysmex hematology analyzer was used to analyze neutrophil counts, total lymphocyte counts, platelet counts, C-reactive protein levels; Abbott automated chemiluminescence immunoassay was used for estimating procalcitonin levels, lactate dehydrogenase levels, and ferritin levels; STAGO automated coagulation analyzer for D-dimer levels, whereas BD FACSCanto II flow cytometry and BD Multitest 6-Color TBNK with Trucount were used to analyze the lymphocyte subset counts.
Statistical analysis
Stepwise logistic regression was carried out to measure the association among CD4+, CD8+, neutrophil counts, total lymphocyte counts, platelet counts, C-reactive protein levels affecting ICU admission, mechanical ventilation, and/or mortality. The measurement data with normal distribution were described as mean ± standard deviation, and the skewed data with non-normal distribution were described as median (Q1-Q3). The chi-square test and Fisher’s exact test were applied, wherever applicable. The Spearman rank test was used to analyze the correlation between the measurement data. Receiver operating characteristic (ROC) curves were used to calculate the cutoff points for classifying the CD4+ and CD8+ T cells as prognostic markers for determining the disease-related outcomes (including ICU admission and/or mechanical ventilation and/or mortality). Wilcoxon signed-rank test was applied to evaluate whether lymphocyte subsets recovered at the time of discharge post treatment. All analyses were performed using R software 3.6.2 with p values <0.05 were considered significant.
Results
Baseline demographics
Of 816 patients enrolled, 770 patients were included in the study after excluding patients aged <18 years and without confirmed diagnosis of the disease (Figure 1). The mean age of the patient population was 40.98 ± 15.95 years with 58% of the patients being males. At the time of ICU admission, 14 patients (1.8%) had non-severe symptoms, whereas 21 patients (84%) had severe symptoms of the disease. Similarly, five patients (0.6%) with non-severe symptoms and 10 patients (40%) with severe symptoms were observed with mechanical ventilation (Supplementary Table 1). The median value of CD4+ count, CD8+ count, IL-6, and IL-8 levels was 558 cells/µl, 362 cells/µl, 0.21 cells/µl, and 1.295 cells/µl, respectively, at the time of ICU admission. Compared with patients with the non-severe disease, those with the severe disease were more likely to have chills, cough, expectoration, weakness, tightness in chest, and shortness of breath (Table 1).
Figure 1.
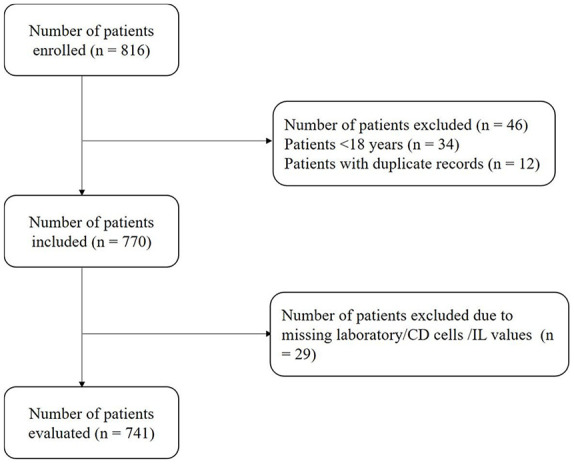
Patient disposition data.
Table 1.
Baseline clinical features of patients with COVID-19.
| Variables | Total, N (%) | Non-severe, N (%) | Severe, N (%) |
|---|---|---|---|
| Age, mean ± SD, years | 40.98 ± 15.95 | ||
| Female | 324 (42) | 315 (40.9) | 9 (11.69) |
| Male | 446 (58) | 428 (55.58) | 18 (23.37) |
| Signs and symptoms at admission | |||
| Fever | 402 (52.20) | 380 (51.9) | 22 (88.0) |
| Chills | 51 (66.23) | 49 (6.7) | 2 (8) |
| Night sweat | 6 (0.78) | 6 (0.8) | 0 (0) |
| Dizziness | 28 (36.36) | 28 (3.8) | 0 (0) |
| Headache | 66 (85.71) | 64 (8.7) | 2 (8) |
| Sore throat | 79 (10.26) | 79 (10.8) | 0 (0) |
| Dry and itchy throat | 83 (10.78) | 81 (11.1) | 2 (8) |
| Nasal congestion | 65 (84.41) | 65 (8.9) | 0 |
| Runny nose | 48 (6.23) | 48 (6.6) | 0 |
| Cough | 316 (41.04) | 303 (41.4) | 13 (52) |
| Expectoration | 153 (19.87) | 144 (19.7) | 9 (36) |
| Hemoptysis | 757 (98.31) | 732 | 25 |
| Weakness | 117 (15.19) | 109 (14.9) | 8 (32) |
| Whole body ache | 50 (6.5) | 50 (6.8) | 0 |
| Chest pain | 20 (2.6) | 17 (2.3) | 3 (12) |
| Chest tightness and shortness of breath | 72 (9.4) | 62 (8.5) | 10 (40) |
| Hypoesthesia | 17 (2.21) | 17 (2.3) | 0 (0) |
| Anosmia | 30 (3.89) | 30 (4.1) | 0 (0) |
| Nausea, vomiting | 26 (3.37) | 25 (3.4) | 1 (4) |
| Abdominal pain | 4 (0.5) | 4 (0.5) | 0 (0) |
| Abdominal distention | 8 (1.04) | 8 (1.1) | 0 (0) |
| Diarrhea | 52 (6.75) | 52 (7.1) | 0 (0) |
| Others | 729 (94.67) | 704 (96.3) | 25 (100) |
N, number of patients; SD, standard deviation.
Correlation of CD4+ and CD8+ T-cell absolute counts to disease outcomes
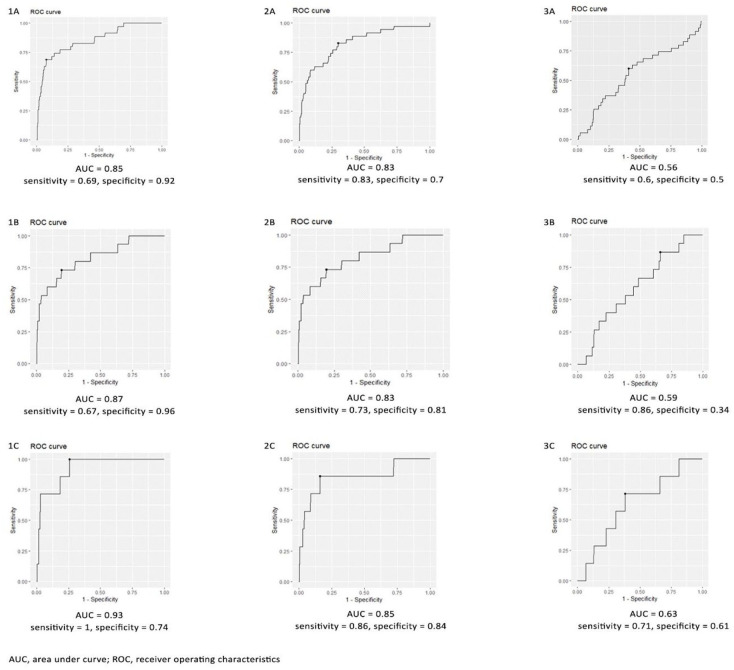
For the evaluation of CD4+, CD8+, cytokine levels, and other laboratory parameters, data of 741 patients were considered after accounting for missing values. The area under the ROC curve (AUC) was used to evaluate the prediction of disease endpoints such as ICU admission, mechanical ventilation and mortality in patients with COVID-19. The AUC, sensitivity, and specificity were 0.8524, 0.6857, and 0.9226 for CD4+ T cell counts, 0.825, 0.83, and 0.7 for CD8+ cell counts, and 0.56, 0.6, and 0.5 for CD4+/CD8+ cell counts, respectively, for predicting the ICU admission as an endpoint. Similarly, AUC, sensitivity, and specificity of 0.865, 0.67, and 0.96 for CD4+ T cell counts, 0.826, 0.733, and 0.81 for CD8+ cell counts, and 0.59, 0.86, and 0.34 for CD4+/CD8+ cell counts, respectively, were significant predictors of mechanical ventilation, while CD4+ T cell counts, CD8+ cell counts, and CD4+ /CD8+ cell counts for predicting mortality were 0.924, 1, and 0.742; 0.85, 0.857, and 0.84; and 0.63, 0.71, 0.61, respectively (Figure 1). The cutoffs for CD4+ cells (267, 198, and 405), CD8+ cells (263, 203, and 182), and CD4+/CD8+ cells (1.4, 1.8, and 1.4) at the ICU admission, mechanical ventilation, and mortality are presented in Figure 2. A lower value below these cutoffs indicates the higher chances of disease progression.
Figure 2.
ROC curve of (1) CD4+ cell count, (2) CD8+ cell count, (3) CD4+/CD8+ count for ICU, mechanical ventilation, and mortality.
Stepwise logistic regression was carried out to measure the potential variables affecting disease outcomes. The results showed that higher CD4+ cell count led to lesser chances for ICU admission [odds ratio (OR) (95% confidence interval (CI): 0.994 (0.991, 0.997); p = 0.0002] and mortality [OR (95% CI): 0.988 (0.979, 0.99); p = 0.001], while higher CD8+ count was an independent risk factor for ICU admission in patients with COVID-19 (Table 2).
Table 2.
Association of CD4+ and CD8+ cell count with disease outcomes by stepwise logistic regression.
| Outcomes | Variables | OR(CI) | p value |
|---|---|---|---|
| ICU admission | CD4+ | 0.994 (0.991, 0.997) | 0.0002 |
| CD8+ | 0.995 (0.99, 0.998) | 0.013 | |
| Mechanical ventilation | CD4+ | 0.994 (0.988, 1.0) | 0.055 |
| Mortality | CD4+ | 0.988 (0.979, 0.99) | 0.001 |
CD, cluster of differentiation; ICU, intensive care unit; OR, odds ratio.
Correlation of CD4+ and CD8+ T cell absolute counts with cytokine levels
A negative correlation was observed among CD4+ (-0.292 and -0.17), CD8+ cell counts (-0.33 and -01.8), and CD4+ /CD8+ cell count groups with IL-6 and IL-8 levels (Table 3).
Table 3.
Correlation of CD4+ and CD8+ cell counts groups with IL-6 and IL-8 levels.
| Variables | Rho, p value | |||
|---|---|---|---|---|
| CD4+ | p value | CD8+ | p value | |
| IL-6 | –0.292 | <0.001 | –0.33 | <0.001 |
| IL-8 | –0.17 | <0.001 | –0.18 | <0.001 |
CD, cluster of differentiation; IL, interleukin.
Assessment of lymphocyte subset measurements recovery at hospital discharge or in convalescence
The median cell count values of CD4+ (558 cells/µl vs 828 cells/µl), CD8+ (362 cells/µl vs 514 cells/µl), platelets (208 cells/µl vs 245 cells/µl) and total lymphocyte (1.4 cells/µl vs 1.94 cells/µl) significantly increased at the time of discharge (p < 0.001), while there was a statistically significant decrease in IL-8 count (1.295 cells/µl vs 0.98 cells/µl), neutrophil count (3.315 cells/µl vs 3.26 cells/µl), D-dimer (0.32 cells/µl vs 0.27 cells/µl), lactate dehydrogenase (LDH) (203 cells/µl vs 187 cells/µl) during discharge (p = 0.001) (Table 4).
Table 4.
Association of lymphocyte subsets measurements at discharge.
| Variables | Median (Q1, Q3) | p value | |
|---|---|---|---|
| Admission | Discharge | ||
| CD4+ | 558 (403.5, 745) | 828 (654, 1039) | <0.001 |
| CD8+ | 362 (229, 500) | 514 (372, 670) | <0.001 |
| IL-6 | 0.21 (0, 1.26) | 0 (0, 0.755) | 0.0012 |
| IL-8 | 1.295 (0.56, 3.26) | 0.98 (0.31, 2.24) | <0.001 |
| Total lymphocyte count | 1.4 (1.025, 1.835) | 1.94 (1.57, 2.405) | <0.001 |
| Neutrophil count | 3.315 (2.52, 4.4575) | 3.26 (2.5425, 4.1) | <0.001 |
| D-dimer | 0.32 (0.22, 0.53) | 0.27 (0.21, 0.45) | <0.001 |
| LDH | 203 (179, 242) | 187 (162, 216.25) | <0.001 |
| Platelets | 208 (161, 253) | 245 (205, 298.5) | <0.001 |
CD, cluster of differentiation; IL, interleukin, LDH, Lactate dehydrogenase; Q1, first quartile; Q3, third quartile.
Correlation of CD4+ and CD8+ T-cell absolute counts with other laboratory test results
There was a significant positive correlation between T-cell count (CD4+, 0.83 and CD8+, 0.8, p < 0.001) with total lymphocyte count. Similarly, a significant positive correlation was observed between T-cell count and platelets (CD4+, 0.43 and CD8+, 0.35; p < 0.001). A significantly negative correlation was observed between D-dimer (CD4+, -0.316 and CD8+, -0.37; p < 0.001), LDH (CD4+, -0.303 and CD8+, -0.33; p < 0.001), and T-cell count. CD4+ /CD8+ cell count revealed a negative correlation with total lymphocyte count, whereas a positive correlation was observed with D-dimer (Table 5).
Table 5.
Correlation of CD4+ and CD8+ T-cell absolute counts with other laboratory test results.
| Variables | CD4+ | p value | CD8+ | p value |
|---|---|---|---|---|
| Total lymphocyte count | 0.83 | <0.001 | 0.8 | <0.001 |
| Neutrophil count | 0.135 | 0.00018 | 0.097 | 0.007 |
| D-dimer | –0.316 | <0.001 | –0.37 | <0.001 |
| LDH | –0.303 | <0.001 | –0.33 | <0.001 |
| Platelets | 0.43 | <0.001 | 0.35 | <0.001 |
CD, cluster of differentiation; LDH, Lactate dehydrogenase.
Association of lymphocyte subsets and laboratory measurements with disease outcomes
Compared to non-ICU patients, the patients admitted to ICU had a significantly lower CD4+ T-cell count [median (Q1, Q3),212 cells/µl (126.5, 345.5) vs 570 cells/µl (411.75, 762.25)] and CD8+ T-cell count [139 cells/µl (81.5, 240) vs 362.5 cells/µl (240.75, 500)]. Similarly, patients admitted to ICU had a lower lymphocyte count [0.695 cells/µl (0.5125, 1.045) vs 1.455 cells/µl (1.08, 1.88)]. There was an increase in the levels of cytokines, LDH, neutrophil count, and D-dimer in patients admitted to ICU (Table 6). Looking at the lymphocyte subsets and laboratory parameters, patients with COVID-19 on mechanical ventilation had a lower CD4+ T count [143 cells/µl (95.5, 325.5) vs 559 cells/µl (403, 752.25)], CD8+ T count [106 cells/µl (51.5, 230.5) vs 357 cells/µl (229, 497)], IL-6 [18.445 cells/µl (7.8075, 110.635) vs 0 cells/µl (0, 0.93)], and IL-8 [25.64 cells/µl (15.11, 101.04) vs 1.43 cells/µl (0.56, 3.38)]. Furthermore, there was an increase in LDH, D-dimer, and neutrophil count in mechanically ventilated patients compared with those not on mechanical ventilation who confirmed the diagnosis of COVID-19 (Table 6). The non-survivor cases of COVID-19 had a lower median CD4+ T cell count [143 cells/µl (106.5, 244.5)], CD8+ T cell count [106 cells/µl (66.5, 162.5)], total lymphocyte count [0.63 cells/µl (0.52, 0.82)], and platelets count [117 cells/µl (109, 156)]. Meanwhile, the median count of cytokines, LDH, neutrophils, and D-dimer was elevated in these patients (Table 6).
Table 6.
Association of lymphocyte subsets and laboratory measurements with ICU admission.
| Variables | ICU admission | Mechanical ventilation | Mortality | |||
|---|---|---|---|---|---|---|
| N | Median (Q1, Q3) | N | Median (Q1, Q3) | N | Median (Q1, Q3) | |
| Age | 35 | 64 (48.5, 70.5) | 15 | 70 (62.5, 79.5) | 7 | 79 (69, 81.5) |
| CD4+ | 35 | 212 (126.5, 345.5) | 15 | 143 (95.5, 325.5) | 7 | 143 (106.5, 244.5) |
| CD8+ | 35 | 139 (81.5, 240) | 15 | 106 (51.5, 230.5) | 7 | 106 (66.5, 162.5) |
| IL-6 | 33 | 8.94 (0.95, 17.6) | 14 | 18.445 (7.8075, 110.635) | 6 | 95.825 (24, 178.945) |
| IL-8 | 8 | 7.005 (4.1925, 13.0525) | 3 | 25.64 (15.11, 101.04) | 1 | 176.44 (176.44, 176.44) |
| Total lymphocyte count | 34 | 0.695 (0.5125, 1.045) | 15 | 0.63 (0.445, 1.055) | 7 | 0.63 (0.52, 0.82) |
| Neutrophil count | 35 | 3.39 (2.465, 4.7) | 15 | 4.06 (3.185, 5.53) | 7 | 4.02 (3.34, 4.305) |
| D-dimer | 35 | 0.89 (0.45, 1.145) | 15 | 0.74 (0.465, 1.195) | 7 | 0.74 (0.565, 1.195) |
| LDH | 35 | 352 (285, 460) | 15 | 343 (293, 460) | 7 | 457 (293, 561.5) |
| Platelets | 35 | 176 (141, 205.5) | 15 | 155 (119, 205.5) | 7 | 117 (109, 156) |
CD, cluster of differentiation; ICU, intensive care unit; IL, interleukin, LDH, lactate dehydrogenase; N, number of patients; Q1, first quartile; Q3, third quartile.
Comparison of lymphocyte subsets between severe and non-severe groups
Among the patients with non-severe COVID-19, the median value of CD8+ T cell, CD4+ T cell counts, total lymphocyte count, and platelets was 570, 362, 1.45, and 211, respectively, while the median value decreased to 149, 106, 0.64, and 172, respectively, in patients with severe COVID-19. The CD8+ T cell, CD4+ T cell counts, total lymphocyte count, and platelets were significantly lower in patients with the severe disease than in patients with the non-severe disease (Supplementary Table 2).
Discussion
Acute and chronic viral infections as well as non-communicable diseases have been found to be associated with lymphocytosis. However, these diseases have their own clinical features; hence, it is easy to differentiate the lymphocytopenia that exists in the non-communicable diseases from the lymphocytopenia in COVID-19. 19 Our study assessed the severity and progression of COVID-19, and the role of the lymphocyte subsets in predicting the disease progression. First, CD8+ T cell and CD4+ T cell counts could serve as risk predictors for fatal outcomes in COVID-19 patients with optimal cutoffs established for various disease outcomes. We found that T-cell counts positively correlated with lymphocyte counts and platelets, whereas negatively correlated with D-dimer and LDH levels in COVID-19 hospitalized patients. Furthermore, the lymphocyte subsets and laboratory measurements significantly improved at the time of discharge post initiation of treatment.
The lymphocyte count has been a marker of interest since the first observational study in China concerning COVID-19 infection. 10 T lymphocytes are important for viral clearance in patients with primary SARS-CoV infection; the virus-specific T lymphocytes, in particular, play a crucial role in clearing the virus and providing symptomatic relief.15,20 In particular, studies reported that COVID-19 non-survivors had a significantly lower lymphocyte count than survivors.21,22 Our study reported similar findings and established a relationship between lymphocytopenia and disease severity. In addition, we observed that patients with COVID-19 admitted to the ICU during hospital admission were more likely to have decreased lymphocyte subsets count than those not admitted to the ICU. This finding was similar to those reported in previous studies where the number of CD4+ and CD8+ T cells significantly reduced in patients with COVID-19, especially in patients requiring intensive care. 23 Although the mechanisms of lymphocytopenia in COVID-19 remain incompletely understood, we believe that it might be due to the lymphocyte sequestration in specific target organs such as lungs, gastrointestinal tract, and/or lymphoid tissues.24,25 T-cell overactivation and T-cell exhaustion are two mechanisms that can occur simultaneously in patients with COVID-19. 23 The elevated expression of T-cell immunoglobulins, mucin-domain containing-3 (Tim-3), and programmed cell death protein-1 (PD-1) levels indicates severe T-cell exhaustion and is associated with the disease severity and intensive care requirement. 26 Another hypothesis underscoring lymphocyte reduction is the presence of sepsis. Thus, lymphocytopenia might result in immune-suppressive environment that might necessitate ICU admission among patients with COVID-19. 27
We observed that increased levels of the cytokine IL-6 and IL-8 were more prominent in patients with the severe disease than in those with non-severe disease (Supplementary Table 2), and were negatively associated with CD4+ and CD8+ counts. This might be attributed to a systemic inflammatory response caused by the cytokine storm, similar to those observed in patients with SARS. Furthermore, on admission, patients with the severe disease had a lower level of lymphocytes, CD4+ T cells and CD8+ T cells than patients with the non-severe disease suggesting the role of lymphocytopenia in COVID-19 severity as well as in its prognosis. 28 Consistent to our findings, Wan et al. 29 detected elevated IL-6 levels in 33.33% of patients with mild symptoms and 75% of those with severe symptoms, concluding that IL-6 might be a prognostic factor in patients with COVID-19. Another study by Diao et al. 23 observed an inversely proportional association between elevated IL-6 levels and T-cell counts.
Thus, the study findings can be used to intervene as early as possible in patients with the severe disease so as to delay the disease progression and improve the cure rate in severe or critically ill patients. Similar to our study, Zhang et al. 30 reported that lower levels of total, CD8+, and CD4+ T cells at admission were associated with higher incidents of ICU admission, mechanical ventilation, or death in patients with COVID-19. Thus, T-cell counts could act as independent risk factors for the clinical prognosis of these patients. Early monitoring of lymphocyte subsets and cytokines can also provide early warnings of the severe disease in patients. According to an expert consensus statement from Shanghai, the early warning indicators of the severe disease include CD4+ T lymphocyte numbers <250/ml and significantly increased levels of IL-6 in blood. 30 This can be used as an adjunct in clinical practice to guide treatment and admission, helpful in improving the prognosis, and decreasing mortality rates.
Our study has certain limitations that warrant mention. First, due to the retrospective nature of the study, there could have been systematic selection bias. Second, since this study is based on a single study center in China, the finding from this study cannot be extrapolated to other geographical locations. Furthermore, some clinical data in each time period were lacking. Future studies examining the association of pro-inflammatory markers and lymphocytopenia in COVID-19 along with other lymphocyte subsets are needed to overcome these limitations. In addition, a multicenter prospective clinical study with a larger cohort in future is necessary. Nevertheless, the observation time of this study was sufficiently long and the sample size at the key time points were larger. The assessment of composite outcomes such as ICU admission, mechanical ventilation, and mortality in these patients are the major strengths of our study. Moreover, during the treatment of the disease, the pattern in the variation of cellular immune function is not influenced by the factors mentioned above. These measurements may help in early triage of high-risk patients and prevent the complications associated with poor outcomes.
Conclusion
In conclusion, this study indicates that the lower levels of T lymphocyte subsets were significantly associated with higher incidents of admission to ICU, mechanical ventilation, and mortality among patients with COVID-19. A cutoff value below CD4+ cells (267, 198, and 405), CD8+ cells (263, 203, and 182), and CD4+/CD8+ cells (1.4, 1.8, and 1.4) may help identify patients at high risk of the disease progression. Early monitoring of lymphocyte subsets and laboratory indices including LDH, D-dimer, cytokine levels, platelets, and neutrophil count in patients with COVID-19 will aid the broader community to make informed decisions and formulate appropriate treatment strategies.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666211049739 for The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: a retrospective study by Lin Wang, Jun Chen, Jun Zhao, Feng Li, Shuihua Lu, Ping Liu, Xu-hui Liu, Qin Huang, He Wang, Qing nian Xu, Xiaomin Liu, Shijun Yu, Li Liu and Hongzhou Lu in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211049739 for The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: a retrospective study by Lin Wang, Jun Chen, Jun Zhao, Feng Li, Shuihua Lu, Ping Liu, Xu-hui Liu, Qin Huang, He Wang, Qing nian Xu, Xiaomin Liu, Shijun Yu, Li Liu and Hongzhou Lu in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors acknowledge Anwesha Mandal, Dr. G Kaushik Subramanian, and Dr. Amit Bhat of Indegene Pvt. Ltd, Bangalore, India, for their medical writing assistance and editorial support. We also acknowledge Divya of Indegene Pvt. Ltd, Bangalore, India, in providing data curation and statistical analysis support for this study. Lin Wang and Jun Chen contributed equally.
Footnotes
Author contributions: Conception or design of the work: Lin Wang, Jun Chen, Jun Zhao, Feng Li
Data collection: Shuihua Lu, Ping Liu, Xu-hui Liu, Qin Huang
Data analysis and interpretation: He Wang, Qing nian Xu, Xiaomin Liu
Drafting the article: Lin Wang, Shijun Yu, Li Liu, Hongzhou Lu
Critical revision of the article: : Lin Wang, Shijun Yu, Li Liu, Hongzhou Lu
Final approval of the version to be published: All authors
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Shijun Yu was an employee of BD Biosciences, China. All the other authors declare no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by BD Biosciences (San Jose, CA, USA) and Shanghai Science and Technology Committee (20411950200, 20431900103, and 20Z11900900).
ORCID iD: Li Liu  https://orcid.org/0000-0002-8111-926X
https://orcid.org/0000-0002-8111-926X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lin Wang, Department of Nursing, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Jun Chen, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Jun Zhao, Department of Paediatrics, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Feng Li, Department of Respiratory Disease, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Shuihua Lu, Department of Tuberculosis, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Ping Liu, Department of Tuberculosis, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Xu-hui Liu, Department of Tuberculosis, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Qin Huang, Department of Infectious Diseases, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
He Wang, Department of Intensive care unit, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Qing nian Xu, Department of Hepatology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Xiaomin Liu, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, P.R. China.
Shijun Yu, Medical Affairs, BD Biosciences, San Jose, CA, USA.
Li Liu, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, P.R. China.
Hongzhou Lu, Department of Infectious Diseases, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, P.R. China; Department of Infectious Disease, Shenzhen Third People’s Hospital, Shenzhen, Guangdong Province 518112, P.R.China.
References
- 1. Coronavirus (COVID-19) events as they happen, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed 15 February 2021).
- 2. Coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 15 February 2021).
- 3. Ye ZW, Jin DY. Diagnosis, treatment, control and prevention of SARS-CoV-2 and coronavirus disease 2019: back to the future. Sheng Wu Gong Cheng Xue Bao 2020; 36: 571–592. [DOI] [PubMed] [Google Scholar]
- 4. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann Intern Med 2020; 173: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020; 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect 2020; 9: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liao Y-C, Liang W-G, Chen F-W, et al. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J Immunol 2002; 169: 4288–4297. [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol 1999; 162: 2154–2161. [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W, Berube J, McNamara M, et al. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A 2020; 97: 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng H-Y, Zhang M, Yang C-X, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 2020; 17: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7), http://en.nhc.gov.cn/2020-03/29/c_78469.htm (accessed 13 January 2021).
- 17. Huang M, Wang Y, Ye J, et al. Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19). Ann Transl Med 2020; 8: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malik P, Patel U, Mehta D, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med 2021; 26: 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deng Z, Zhang M, Zhu T, et al. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int J Infect Dis 2020; 98: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J, Zhao J, Van Rooijen N, et al. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog 2009; 5: e1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020; 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 2004; 189: 648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 9: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett 2020; 225: 31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drewry AM, Samra N, Skrupky LP, et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014; 42: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu Q, Wang Z, Yin Y, et al. Association of peripheral lymphocyte and the subset levels with the progression and mortality of COVID-19: a systematic review and meta-analysis. Front Med 2020; 7: 558545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.02.10.20021832v1
- 30. Zhang W, Li L, Liu J, et al. The characteristics and predictive role of lymphocyte subsets in COVID-19 patients. Int J Infect Dis 2020; 99: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666211049739 for The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: a retrospective study by Lin Wang, Jun Chen, Jun Zhao, Feng Li, Shuihua Lu, Ping Liu, Xu-hui Liu, Qin Huang, He Wang, Qing nian Xu, Xiaomin Liu, Shijun Yu, Li Liu and Hongzhou Lu in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211049739 for The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: a retrospective study by Lin Wang, Jun Chen, Jun Zhao, Feng Li, Shuihua Lu, Ping Liu, Xu-hui Liu, Qin Huang, He Wang, Qing nian Xu, Xiaomin Liu, Shijun Yu, Li Liu and Hongzhou Lu in Therapeutic Advances in Respiratory Disease