Abstract
Stem cells in different types may interact with each other to maintain homeostasis or growth and the interactions are complicated and extensive. There is increasing evidence that mesenchymal-epithelial interactions in early morphogenesis stages of both tooth and hair follicles show many similarities. In order to explore whether stem cells from one tissue could interact with cells from another tissue, a series of experiments were carried out. Here we successfully extracted and identified stem cells from human exfoliated deciduous teeth (SHED) of 8–12 years old kids, and then found that SHED could promote hair regeneration in a mouse model. In vitro, SHED shortened the hair regeneration cycle and promoted the proliferation and aggregation of dermal cells. In vivo, when SHED and skin cells of C57 mice were subcutaneously co-transplanted to nude mice, more hair was formed than skin cells without SHED. To further explore the molecular mechanism, epidermal and dermal cells were freshly extracted and co-cultured with SHED. Then several signaling molecules in hair follicle regeneration were detected and we found that the expression of Sonic Hedgehog (Shh) and Glioma-associated oncogene 1 (Gli1) was up-regulated. It seems that SHED may boost the prosperity of hairs by increase Shh/Gli1 pathway, which brings new perspectives in tissue engineering and damaged tissue repairing.
Keywords: stem cells from human exfoliated deciduous teeth, hair regeneration, cell interaction, transplantation, Sonic Hedgehog
Introduction
Regeneration of multiple tissues - including the skin, blood, stomach, and intestines - is maintained by stem cells 1 . In skin, slow-cycling and relatively undifferentiated stem cells exist within a niche known as the bulge, located below the sebaceous gland in the outer root sheath of the hair follicle 2 –4 . Hair follicle is an important structure regulating hair cycling process and this developmental process occurs over the total lifetime of a mammal, well beyond the organogenesis of other systems (e.g., the cycling lifetime of the ovary or endometrium) 5 . This cyclic regeneration is thought to require many cellular signals of morphogenetic and regenerating systems, among which, the intimate epithelial-mesenchymal interactions is the most fundamental basics 6,7 . Therefore, we suspect that the transplantation of mesenchymal stem cells from another source into the microenvironment of hair stem cells may have an effect on hair follicle stem cells (HFSCs) and then eventually affect hair regeneration.
Hair regeneration is a normal physiological process, and improvements in this process may help halt the excessive hair shedding progression. In recent years, with the increasingly fierce social competition and work pressure, excessive hair shedding or hair loss becomes an increasingly serious problem. Lots of drugs for the treatment of hair loss have been developed, including minoxidil 8 –10 . However, many of the drugs have obvious side effects or limited effect, for example, minoxidil can cause rapid heart rate, arrhythmia, and skin flushing. Effective methods for treating hair loss still needed. So far, there has been no research on promoting hair regeneration with mesenchymal stem cells. This peer research may bring a new perspective for hair follicle reconstruction and hair growth.
Stem cells from human exfoliated deciduous teeth (SHED) are considered to be mesenchymal stem cells, derived from neural crest, and can differentiate into osteoblasts, chondrocytes, hepatocytes, and neuron-like cells under appropriate conditions 11 . SHED were discovered in 2003, since then they have received extensive attention due to their convenience in obtaining, low immunogenicity and no ethical issues in experiment 12 –15 . The morphogenesis of teeth and hair follicles share many similarities. Both processes involve the interaction between mesenchymal cells and epidermal cells and finally result in a relatively independent organ 5,16,17 .
In this study, we transplanted SHED along with C57BL/6 mice skin cells into nude mice to investigate whether external stem cells could interact with hair follicle cells and promote the hair regeneration in vivo.
Materials and Methods
Animals
7-week-old male nude (BALB/c) mice, 1-day-old and 7-week-old male C57BL/6 mice were purchased from Peking University Medical College, China. Experimental animals were cared with free water and all animal experiments were completed in the laboratory of animal science department of Peking University Medical College.
Isolation and Culture of SHED
Normal exfoliated human deciduous incisors were collected from 8 to 12 years old children gifted from People’s Liberation Army No. 307 Hospital. The cells were isolated and digested to obtain single cells according to the method established by Gronthos 18 . Modified steps are as flows: (1) Cleaning: The teeth were immersed in iodine and 75% ethanol for 30 s for disinfection. The sterilized teeth were washed several times with phosphate-buffered saline (PBS, Biological Industries, Kibbutz Beit Haemek, Israel); (2) Separation: Knock the crown, grasp the pulp tissue and cut the pulp tissue at the length of about 1 mm from the apex; (3) Digestion: The pulp tissue was cut as much as possible digested in a solution of 3 mg/mL collagenase Ⅰ (Sigma-Aldrich Corp, St. Louis, MO, USA) and 4 mg/mL dispase (Solarbio, Beijing, China) for about 2 h at 37°C. Gently shake the digestive solution every 5 min to allow the tissue to be fully digested until no visible tissue blocks are existed; (4) Seeding: The single cells were obtained by centrifuging at 1200 rpm for 10 min. Collagenase Ⅰ was completely removed, and the cells were cultured in Dulbecco Modified Eagle Medium (DMEM) (Biological Industries, Kibbutz Beit Haemek, Israel) containing 15% Fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit Haemek, Israel) and 1% penicillin-streptomycin solution (Caisson, Smithfield, UT, USA). The non-adherent cells were removed by changing medium. According to the results of flow cytometry (Fig. 1A), the sixth- and seventh-passage SHED were used for all experiments.
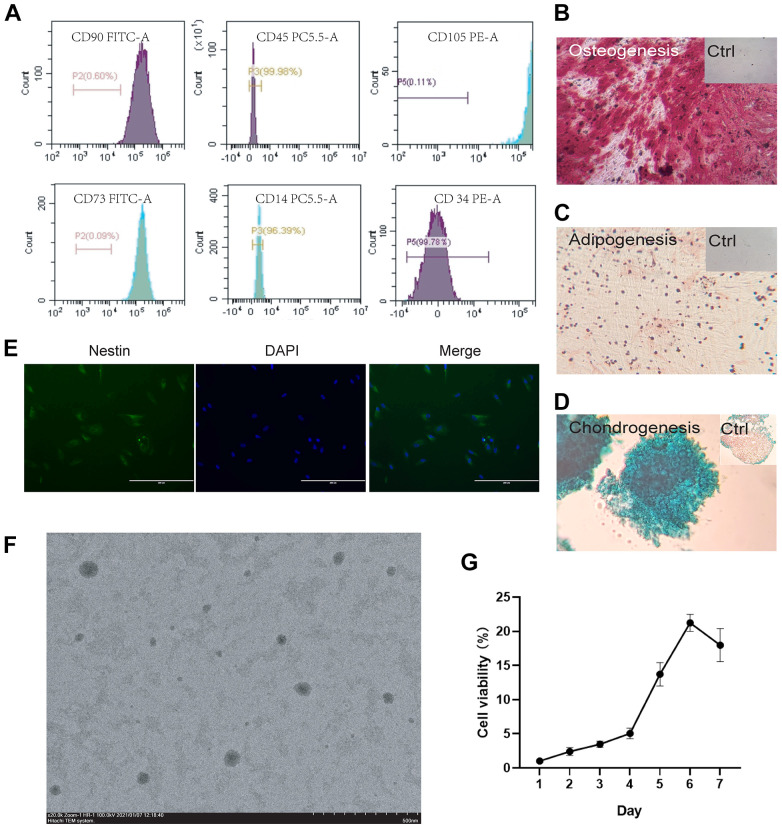
Figure 1.
Identification and characterization of SHED (A) Surface markers of extracted SHED. Flow cytometric analysis showed that isolated cell population highly expressed CD73, CD90, and CD105 while it nearly didn’t express CD14, CD34, and CD45. Isotype samples were used as negative control to delineate positive areas (marked with a horizontal line in the figure.); (B) Osteogenic of SHED. Alizarin red staining of SHED were applied after osteogenic induction for 2–4 weeks. Control group was cultured for the same time in complete medium and stained with Alizarin red; (C) Adipogenic induction of SHED. Oil red staining of SHED were applied after adipogenic induction for 3–4 weeks. Control group was cultured for the same time in complete medium and stained with Oil red; (D) Chondrogenic induction of SHED. Alcian staining of SHED were applied after chondrogenic induction for 3–4 weeks. Control group was cultured for the same time in complete medium and stained with Alcian; (E) Neural induction of SHED. After SHED were induced in neural differentiation medium (EGF/FGF/NGF/GDNF, 10mg/ml respectively) for 10 days, the neural stem cell marker nestin was detected. The antibody of nestin produces green fluorescence while DAPI binds to the nucleus and produces blue fluorescence; (F) Transmission Electron Microscope of SHED. SHED were made into cell suspension and the SEM picture showed the morphology, size and distribution of the cells; (G) Cell proliferation curve of SHED. After SHED were seeded into the plate, the absorbance is measured once a day, and the cell viability is [A(Dn)-A(blank)] / [A(D1)-A(blank)] × 100%. “A” represent the absorbance, “Dn” represents the Nth day, n = 1, 2, 3, 4, 5, 6, 7.
Flow Cytometry Analysis
Cell surface expression of CD14, CD34, CD45, CD73, CD90, and CD105 (Sino Biological, Beijing, China) was tested using flow cytometry. SHED were washed twice with cold PBS and stained with indicated PE-, PC5.5 or FITC-conjugated Abs for 30 min on ice at dark room. Samples were examined on an Accuri C6 flow cytometer (BD Biosciences, Franklin Lake, NJ, USA). Data were analyzed using FlowJo7.6.1 analysis software.
Cell Proliferation Viability Assays
SHED (passage 5) were seeded into 96-well plates, and the cell viability was tested every day by using CCK-8 (Solarbio, Beijing, China). After CCK-8 was added, the cells were incubated at 37°C for 1.5 hours and then the absorbance at 560 nm was measured. The blank group contained only medium and CCK-8, and the other groups contained cells, medium and CCK-8. The calculation formula for cell viability is A(Dn)-A(blank)/A(D1)-A(blank)×100%. “A” represent the absorbance, “Dn” represents the Nth day, n = 1, 2, 3, 4, 5, 6, 7.
Neurogenic Induction
SHED (passage 5) were seeded into 6-well plate and the induction fluid contains neural differentiation factors (EGF/FGF/NGF/GDNF, 10 mg/mL respectively). After 10 days of induction, SHED were used to detect the expression of nestin, a marker for neural stem (or progenitor) cells. The primary antibody of nestin has a green fluorescent label and can identify positive cells after co-localized with the nucleus stained by DAPI.
Osteogenic Induction
Osteogenic induction of SHED were followed by the Instruction manual of osteogenic differentiation medium Kit (Cyagen, Guangzhou, Guangdong Province, China). Alizarin red staining was applied after induction for 2–4 weeks.
Adipogenic Induction
Adipogenic induction of SHED were followed by the Instruction manual of adipogenic differentiation medium Kit (Cyagen, Guangzhou, Guangdong Province, China). Oil red staining was applied after induction for 3–4 weeks.
chondrogenic Induction
Chondrogenic induction of SHED were followed by the Instruction manual of chondrogenic differentiation medium Kit (Cyagen, Guangzhou, Guangdong Province, China). Alcian staining was applied after induction for 3–4 weeks.
Morphological Analysis of SHED
SHED were collected and washed by PBS for 3 times, and cells were collected by centrifugation at 1000 rpm for 5 min each time. 4% paraformaldehyde (Servicebio, China) was used to fix the cells at room temperature for 15 min. After that, the cells were put on the carbon-coated copper grid, stained with 3% phosphotungstic acid (Solarbio, China) and washed away. Then the copper grid was placed in a shady room for 24 hours to dry. Finally the morphology of the cells was examined on a transmission electron microscope (TEM, HT7700 Exalens, Hitachi, Japan) at an accelerating voltage of 100 kV.
Isolation of C57BL/6 Mice Skin Cells
One-day-old C57BL/6 mice were sacrificed by neck dissection, and the back skin was carefully peeled off with sterile forceps. Subcutaneous fat was removed from the skins with a scalpel and the whole skin was placed with dermis down in PBS containing 0.2% dispase overnight at 4°C. The tissue after dispase treatment was separated into epidermis and dermis. The epidermis was digested in 0.25% trypsin (Biological Industries, Kibbutz Beit Haemek, Israel) at 4°C for 30 min while dermis was digested in 0.2% collagenase Ⅰ at 37°C for 1.5 h. Subsequently, the cells were filtered with strainers (70 μm, Corning, Tewksbury, MA, USA).
Cell Co-culture
For SHED group, SHED were seeded in the upper layer of the Transwell chamber, and freshly isolated dermal cells of C57BL/6 mice were seeded in the lower layer of the Transwell chamber. For control group, only dermal cells of C57BL/6 mice were seeded in the lower layer of the Transwell chamber (the upper layer contained no cell). The medium was DMEM/F12 (Biological Industries, Kibbutz Beit Haemek, Israel) containing 15% FBS and 1% penicillin-streptomycin solution. Each well contained 1 × 105 cells.
In experiments to explore molecular mechanisms, freshly isolated epidermal and dermal cells were placed in the lower layer of the transwell while SHED were placed in the upper layer. The remaining conditions were the same as above. The co-cultivation lasted for 3 days, and then the total RNA of the lower mixed cell was extracted for RT-qPCR.
Transplantation
The 7-week-old nude mice were purchased. All procedures were performed on anaesthetized animals and the cells were subcutaneously injected into the back skin of the nude mice (6 injection sites on each mouse). In order to eliminate the individual differences of different mice, the back of each mouse was divided into left and right sides. The left side was the injection site of the control group while the right was the injection site of the SHED group. In the control group each injection site containing 1∼5 × 106 skin cells while in the SHED group each site containing 1∼5 × 106 skin cells and 1∼5 × 106 SHED. Skin cells included epidermal cells and dermal cells and the ratio is 1: 2. The experiment totally enrolled 5 mice.
Histochemical Analysis
Dehydrated skin tissue from injection site of nude mice was fixed in 4% paraformaldehyde and embedded in tissue freezing medium (Leica Biosystems, Shanghai, China). The sliced skin was stained in hematoxylin-eosin (HE).
C57BL/6 Mice Surface Smear Experiment
The 7-week-old C57BL/6 mice were randomly recruited in Minoxidil group (positive control), SHED group (test group) and saline group (negative control), and then labeled with ear tags. Each group included 3 mice. The back hair of the mice was removed with a razor and a mild depilatory cream. The reagents (commercially available minoxidil tincture for Minoxidil group, SHED extracts for SHED group and saline for saline group) were smeared at the bald area of C57BL/6 mice once a day for 7 days. Photos were taken to record results. The bald area was determined by the statistical gray value through Image J software.
RNA Extraction and RT-qPCR
The total RNA of skin cells was extracted by the TRIzon method. Briefly, after the cells were cleaved by TRIzon (CWBIO, Beijing, China), chloroform was added. Then the mixture was centrifuged at 12000 rpm for 10 min, after that the upper aqueous phase was token and isopropanol was added, after standing still the precipitate was collected by centrifugation at 12000 rpm, and then re-dissolved with absolute ethanol. To prepare cDNA, total RNA was reverse transcribed using random hexamer primers (Accurate Biology, China). Real-time PCR was performed on an OneStepPlus (Funglyn, Canada) machine using SYBR Green master mix (Beyotime, China). Primer pairs (Table 1) were designed to work at the same settings: 95°C for 2 min, 40X (95°C for 15 s, 60°C for 20 s, 72°C for 30 s). Differences were quantified based on the ΔΔCt method.
Statistical Analysis
The results were shown as mean ± SD. For the data statistics of the two experimental results, T-test was applied to calculate the variance. And for the data statistics of three groups and above, ANOVA-test was applied to obtain the variance between the groups. SPSS V21.0 was used to calculate all results. The significance level less than 0.05 were considered as statistically significant.
Results
Isolation and identification of SHED
After the cells were extracted from the medulla tissue of 6–12 years old children’s teeth, flow cytometry was used to detect the specific surface markers of SHED, meanwhile neurogenic, osteogenic, adipogenic, and chondrogenic induction were conducted to test the stemness of cells. The proliferation ability of stem cells within 7 days and the morphology under electron microscope were also tested.
The same differentiation antigen recognized by monoclonal antibodies from different cells is called cluster of differentiation (CD). CD14 is a glycosylphosphatidylinositol (GPI)-anchored receptor and is known to act as a co-receptor for multiple Toll-like receptors (TLR) on the cell surface that enhances innate immune responses 19 . CD34 is mainly expressed in stem cells derived from hematopoietic origin, and acts as a growth factor receptor to regulate early hematopoiesis 20 . CD45 is an evolutionarily highly conserved receptor protein tyrosine phosphatase, which is only expressed on all nucleated cells of the hematopoietic system 21 . CD73, also known as Ecto-5’-nucleotidase, is a ubiquitously expressed glycosylphosphatidylinositol-immobilized glycoprotein that can convert extracellular adenosine 5’-monophosphate into adenosine 22 . CD90, also known as Thy-1, is a member of the cell adhesion molecule immunoglobulin superfamily, usually used as a surface marker for mesenchymal stem cells that has an important regulatory role in osteogenic differentiation 23 . CD105 (Endoglin) is a type I transmembrane protein, which is reported to induce endothelial cell activation and proliferation 24 . SHED, as neural crest-derived mesenchymal stem cells, are define as highly express CD73, CD90, and CD105 while lowly express CD14, CD34, and CD45. Our results from flow cytometry analysis showed that the positive rate of CD73, CD90, and CD105 was higher than 99%, while the positive rate of CD34 and CD45 was lower than 1%, and the positive rate of CD14 was lower than 4%, as Fig. 1A indicated. The fluorescent confocal pictures of the surface markers of stem cells were shown in the supplement materials (S.1). The results were consistent with the flow cytometry test. Flow cytometric identification of cell surface markers showed that the cells we isolated were indeed SHED. In order to detect the stemness of cells, we induced neurogenic, osteogenic, adipogenic, and chondrogenic differentiation of SHED.
When the osteogenic differentiation was induced for about 4 weeks, alizarin red staining was performed and the results showed in Fig. 1B. Obvious dark red calcium nodules and red bone matrix were observed under the microscope (the bone matrix generally appeared before the formation of the bone nodules) indicating the isolated cells had osteogenic differentiation potential. Adipogenic differentiation was induced for 3 weeks, and deep red lipid droplets could be seen after oil red staining. The results are shown in Fig. 1C. Chondrogenic differentiation was confirmed by Alcian staining after 3 weeks of induction which was shown in Fig. 1D. Ten days after neural induction, SHED expressed neural stem cell marker nestin. After incubating with nestin antibody which contains green fluorescent label, positive cells could be seen under a fluorescence microscope as showed in Fig. 1E. The four-direction induction of SHED proves its high stemness.
In cell proliferation viability assays, SHED continued to proliferate in the first five days and reached the plateau after reaching the highest number on the sixth day as we can see from Fig. 1G.
TEM pictures showed the distribution and size of SHED. It can be seen from the Fig. 1F that the size and distribution of SHED were relatively uniform, and the cell diameter was 500–800 nm in the suspended state.
SHED Promotes the Growth of Dermal Cells in Vitro
Based on our guess, we first explored whether there is an interaction between mesenchymal stem cells from two sources. We co-cultured SHED with freshly extracted dermal cells of C57BL/6 suckling mice in a Transwell chamber.
After SHED and dermal cells were co-cultured for 3 days in Transwell chamber, the state of dermal cells was shown as Fig. 2A. The pore size of Transwell chamber is 0.4 μm. SHED were cultured in the upper layer and dermal cells of C57BL/6 mice was cultured at the bottle of the chamber (Fig. 2B). The cells of two layers were not in direct contact, but they could interact by secreting cytokines or signaling molecule. Three repetitions were done for each group.
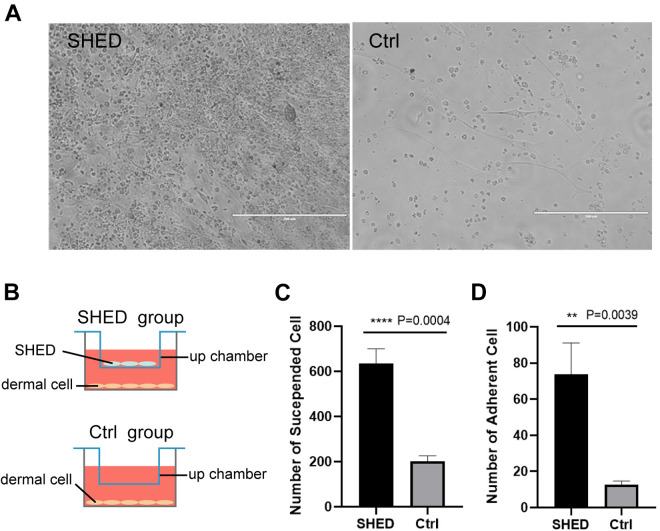
Figure 2.
SHED promote the growth of dermal cells in vitro (A) Freshly extracted C57BL/6 mouse dermal cells co-cultured with SHED for 3 days in a transwell chamber (left), and dermal cells without SHED co-culture (right); (B) Schematic diagram of co-cultivation of cells in Tranwell chamber. The gap between the upper and lower chambers is smaller than the cell diameter, and the two layers of cells can only communicate through signal molecules or cytokines; (C, D) Statistical results of the suspended and adherent cells in (A). Significance was calculated using t-test, P < 0.05 *, P < 0.01 **, P < 0.001 ***, P < 0.0001****.
The results calculated in Fig. 2C, D showed that the number of dermal cells in the experimental group co-cultured with SHED was significantly higher than that in the control group (both suspended cells and adherent cells), and there were cell aggregation in the experimental group. This phenomenon suggested that SHED could promote the proliferation of a variety of dermal cells and adherence of dermal cells by paracrine.
SHED Extracts Promotes Hair Regeneration in Vitro
The indirect communication between SHED and dermal cells made us realize that SHED may secrete some signal molecules or cytokines to promote the proliferation of dermal cells. In order to explore the role of SHED in hair regeneration, we made a cream using cracked SHED and applied it to the depilatory area on the back of C57BL/6 mice. The smear experiment lasted for 1 week, each mouse smeared once a day. The experimental design of this part was shown in Fig. 3A. At the same time, commercially available minoxidil and physiological saline were used as the positive control group and the negative control group respectively.
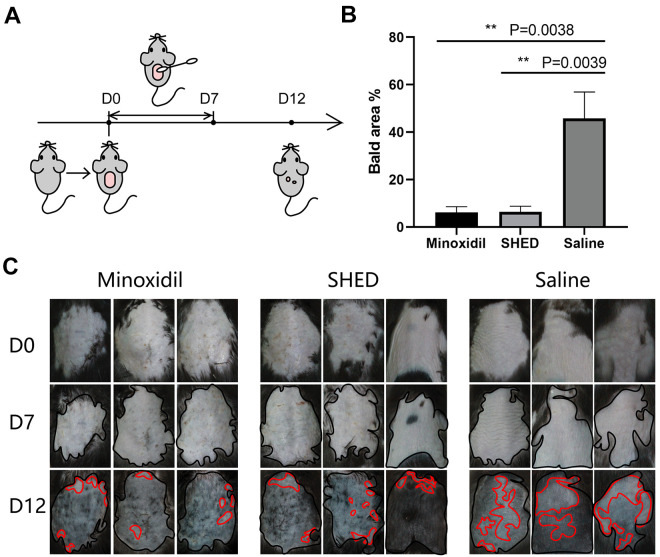
Figure 3.
SHED promote hair regeneration in vitro (A) The schematic diagram of the smearing experiment. After the mice had adapted to the environment for a period of time, the back hair of mice were removed on D0 and the reagent were applied once a day during D0-D7. Pictures were taken to record the hair growth each day. The experiment ends on D12; (B) The percentage of the bald area to the total depilation area in (C) on D12. Compared with the saline group, SHED and minoxidil were significantly reduced, while there was no significant difference between SHED and minoxidil; (C) Record photos of C57BL/6 mice during the smear experiment after depilation. The total depilation area was marked with a black line, and the bald area was marked with a red line. Bald areas were distinguished by statistical gray value. Significance was calculated using ANOVA-test, P < 0.05 *, P < 0.01 **, P < 0.001 ***, P < 0.0001 ****.
In vitro smear experiment, there were significant differences in the regenerated hair area among the three groups on D12 (Fig. 3C). The depilated area on the back of the mouse was marked with a black line, and the bald area on D12 was marked with a red line. The area calculated in Fig. 3B is the percentage of the bald area to the depilated area. The percentage of bald areas in the saline group was the largest, which was significantly different from the minoxidil group and the SHED group. Compared with the positive control group (minoxidil), there was no significant difference in the SHED group. The results indicated that compared with the saline group, SHED extracts could shorten the cycle of hair follicle regeneration and promote the hair regeneration, showing a similar effect to minoxidil.
SHED promotes the Hair de Novo Regeneration in Vivo
To determine if SHED could exert an effect in vivo, the skin cells (both epidermis and dermis) of C57BL/6 mice were subcutaneously transplanted into the nude mice for 2–3 weeks, and the bulges were formed under the epidermis of the nude mice. We checked the hair development in the tissues of the injection site on the 14th (D14) and 19th days (D19) after the injection of the cells (Fig. 4A). On D14, a small amount of short hair had formed in the injection sites (Fig. 4B). Via calculation we found that the number of hairs formed at the injection site where SHED were added was significantly more than that of the control group (Fig. 4D). On D19 after the injection, we checked the hair growth at the injection site again (Fig. 4C). At this time, the hair formed at the injection site added with SHED had grown significantly, compared with the control group, the number of hairs in the experimental group increased to 1.5 times. and the length of the hair shaft has also increased (the length is more than 1 cm). The number of hairs in the control group was significantly less than that in the SHED group, and the hair shaft length was also shorter. The statistics are shown in Fig. 4E.
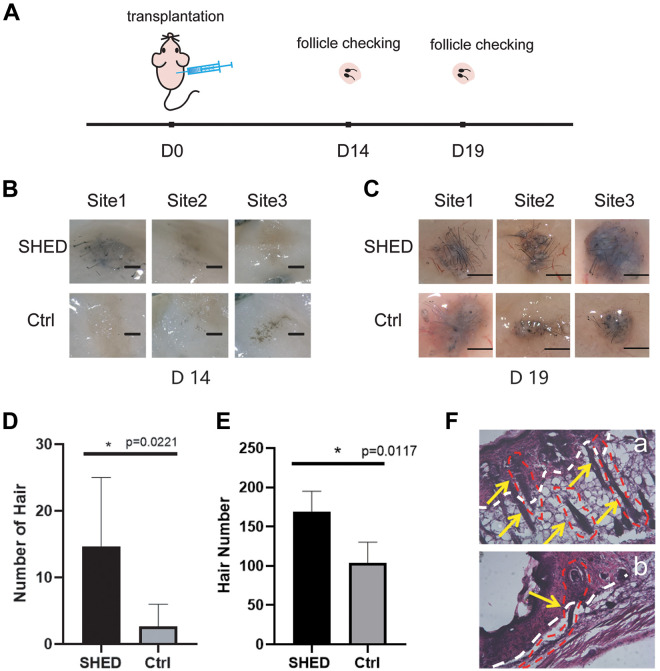
Figure 4.
Hair de novo regeneration in nude mice (A) Schematic diagram of nude mouse hair transplantation experiment. Nude mice were subcutaneously transplanted with mixed epidermal and dermal cells of C57BL/6 mice on D0, then the nude mice were sacrificed on D14 and D19, and the development of hair follicle was checked; (B) The cells transplanted subcutaneously in nude mice developed into hair on D14. The injection sites co-transplanted with SHED contained more hair; (C) Hair development of transplanted cells in nude mice on D19. Compared with D14, both the length and number of hair had increased. There were still significant differences between the SHED group and the control group. Compared with the control group, the number of hairs in the SHED group increased to 1.5 times; (D-E) Statistical results of (B) and (C). The hair under the skin was counted after being pulled out; (F) Microstructure of nude mice’s own hair (a) and hair formed by transplanted cells (b) after HE staining. The epidermis and dermis were separated by a white dotted line, and the epidermis showed darker color. The outline of the hair follicle was circled by a red dotted line pointed by a yellow arrow. The hair formed by nude mice had directionality, which grew from the dermis layer to the epidermis layer (a), while the hair growth formed by the transplanted cells had no directionality (b). Significance was calculated using t-test, P < 0.05*.
We then explored the hair structure formed at the injection site. The bulges of injection sites were sliced and stained with HE, the dermis and epidermis were separated by a white dotted line in the picture, and the epidermis was densely stained (Fig. 4F). Since nude mice would grow sparse white hair themselves, we selected C57 mice with black hair as the donor for epidermal cell transplantation. The black hair generated at the transplanted site can be distinguished from the hair of the nude mouse itself. In addition, from the results of section staining, the hair formed by the nude mice grew from the dermis to the epidermis (Fig. 4Fa), while the hairs formed by the transplanted skin cells were disordered and grew from the epidermis to the dermis (Fig. 4F).
SHED Regulate Hair Regeneration Mainly through Shh/Gli1 Signaling Pathway
The previous results of our work showed that SHED do affect hair regeneration, and we wonder whether it is to promote hair de novo regeneration in vivo or to promote periodic regeneration in vitro. This is a very interesting phenomenon. In order to clarify the underlying mechanism, we further explored how SHED promoted hair regeneration.
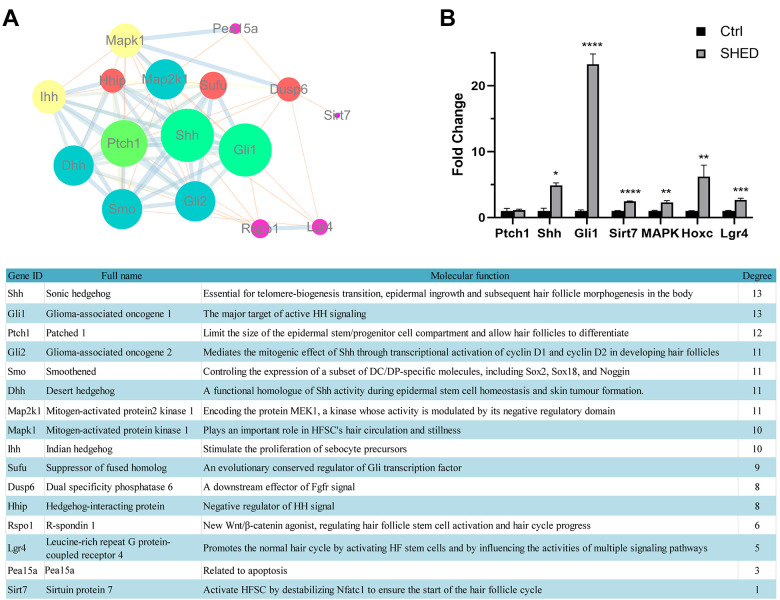
The mechanisms of hair de novo regeneration and cyclic regeneration have been studied relatively mature. Therefore, we selected several important molecules reported in the literature for PPI analysis (Figure 5A). Then three molecules with the highest PPI score (Shh, Gli1, Ptch1) and three other important molecules (Sir7, MAPK, Lgr4) reported in the literature were used as detection indicators. By detecting the expression of mRNA in the mixture of freshly extracted epidermal and dermal cells, we found that the expression of genes that promote hair regeneration in the mixed cells co-cultured with SHED were significantly higher than those in the control group (Figure 5B). In particular, the gene expression of Shh/Gli1 signaling pathway has increased dramatically by SHED.
Figure 5.
The molecular mechanism of SHED regulating hair regeneration (A) PPI Analysis of protein network interaction related to hair regeneration. Genes are marked as circles of different sizes and colors according to their scores. The molecular functions and scores of these genes were listed in the table below; (B) The mRNA expression of hair regeneration genes in SHED and control group. The detected genes include the three highest-scoring genes in the PPI analysis and the important hair regeneration genes reported in the literature. Significance was calculated using ANOVA-test, P < 0.05 *, P < 0.01 **, P < 0.001 ***, P < 0.0001 ****.
Shh signal transmission is controlled by the membrane receptor Ptch on the target cell. The receptor Ptch is encoded by the tumor suppressor gene Patched, which can directly bind to the ligand and play a negative regulatory role on Shh signaling. The addition of SHED kept Ptch unchanged and up-regulated the expression of Shh, indicating that SHED may activate the Shh signaling pathway through other pathways. When the Shh signaling pathway is activated, its downstream transcription factor Gli1 will be up-regulated and enter the nucleus to activate downstream target gene transcription. The finally activated signal pathway regulates skin cells including hair follicles, which manifests as hair regeneration.
Discussion
Since 2000, dental stem cells showed powerful application potential in multiple tissue repairing 25,26 . The similarities in the development of teeth and hair follicles sparked us to explore the cellular interactions between the two tissues. Hence, we put dermal cells together with SHED in Transwell chamber where they were not in direct contact, but could communicate indirectly through paracrine signal moleculars or other cytokines. Surprisingly we found dermal cells co-cultured with SHED had better proliferation and more clusters compared with the control group, which implied that SHED may secret some important molecules which played a part in dermal cell proliferation. Various nutritional factors secreted by SHED have been reported to play a key role in tissue repairing, include bone tissues 27,28 , liver tissues 29 , but there are few reports spotlight on hair. Herein we believe these growth factors may have a positive effect in hair follicle regulation via anti-apoptosis and provide nutritious microenvironment.
In order to determine whether extracellular factors secreted by SHED have effect on dermal cells or even hair regeneration, we smeared the extract of SHED onto the depilated dorsal skin of C57BL/6 mice. Minoxidil is an FDA-approved drug for the treatment of hair loss hence it was used as a positive control in our experiment. The results indicated SHED group had less bald area compared with the control group, which mean that SHED extracts could shorten hair regeneration cycle and boost hair regeneration.
In previous reports, chemical reagents had a certain effect on hair regeneration. Among them, minoxidil has a better hair growth effect and was approved as a drug decades ago. However minoxidil and its inactive ingredient propylene glycol can induce allergic contact dermatitis which is a severe side effect commonly appeared in chemical drugs 30 . SHED are a kind of mesenchymal stem cells in the human body with low immunogenicity 18 . Therefore, based on the similar effect of promoting hair regeneration, SHED have become a more potential therapeutic drug due to its better safety.
While SHED showed apparent effect on hair regeneration in vitro, we would like to further explore its role in vivo. Hereafter we injected skin cells (include dermal cells and epidermal cells) with or without SHED into nude mice and after 2 weeks new hairs grew out. It was found that skin cells injected with SHED produced more hair which may due to the secretion of certain factors by SHED that promoted the increase of dermal papilla cells (DPs) resulting in a larger number of hair follicles. Recent studies have shown that the conditioned medium of dental stem cells can significantly increase the number of hair follicles in the growth phase and stimulate hair growth rapidly which is consistent with our research results 31 .
To further explore the molecular mechanism, we co-cultured SHED with epidermal and dermal mixed cells through Transwell to detect the expression of hair regeneration-related genes. Through PPI analysis, we found several key genes in hair regeneration, including Shh, Pach1, and Gli1. In addition, we also include the reported important genes (Sirt7, MAPK, Hoxc and Lgr4) that regulate the hair cycle as detection indicators. Sonic hedgehog (Shh) signals regulate the proliferation and development patterns of many tissues including HF 32 . In the Shh signal pathway of hair follicle development, Ptch1, as the receptor of Shh, mainly exerts a tumor suppressor effect and negatively regulates the Shh signal pathway 33,34 . Gli1 is the nuclear factor of Shh signal transduction. Shh/Gli regulates the development of hair follicles in embryonic development and adult animals, and affects the circulation and growth of adult hair follicles by promoting the telogen-to-anagen transition of follicular cells 32,35,36 . In our experimental results, SHED did not affect the expression of Ptch1 in the epidermis and dermis mixture, while Shh and Gli1 were both upregulated. This indicates that SHED activate the Shh signaling pathway to promote hair regeneration and periodic circulation through another pathway (may up-regulate Smo, another positive regulator of Shh) instead of reducing the expression of negative regulator Ptch1. We believe that regulating effect of SHED on hair regeneration is multifaceted, which is also a major feature of biologically active drugs that are different from single-component chemical drugs. Therefore, in addition to the Shh pathway, we also tested the expression of a variety of important genes related to hair regeneration. Sirt7 is a newly discovered molecule related to the periodic circulation of hair. Research by Guo Li et al 37 . showed that the upregulation of Sirt7 can promote the transition of hair from telogen phase to anagen phase, and accelerate hair growth. MAPK1 is a classic gene that regulates hair regeneration. It can promote the proliferation of HFSCs 38 . Hoxc gene expression in the dermis of adult skin is closely related to the regional HF regeneration pattern. Zhou Yu et al 39 . found that a single Hoxc gene is sufficient to activate the dormant DPs niches and promote regional HF regeneration by regulating Wnt signaling. Lgr4 is also a factor that regulates the hair cycle. LGR4 promotes the normal hair cycle by activating HF stem cells and affecting the activity of various signaling pathways known to regulate HF stem cells 40 . In our experimental results, these signal molecules that promote the proliferation of HFSCs and the transition of hair to the anagen phase are significantly up-regulated, indicating that SHED achieve a positive regulation of hair follicle regeneration through a variety of ways.
In summary, we found that SHED could interact with dermal cells and regulate cell proliferation in a paracrine manner; SHED extracts could boost hair regeneration at the depilatory area on the back of C57 mice growth. Precisely speaking, SHED extracts shortened the hair growth cycle of C57 mice; Co-transplantation of SHED and skin cells of C57 mice could increase the number of newly formed hair in nude mice. Although our experimental results showed that SHED had a benign effect on hair regeneration, our pioneering research was not yet complete. More in-depth research is needed to promote clinical applications. SHED have been proved effective in the treatment of multiple oral diseases 26, 41 and multiple tissue repairing (e.g., nervous tissue repairing 42,43 , bone tissue repairing 44,45 ). With cautious optimism, it may have a wider application in hair regeneration field.
Supplemental Material
Supplemental Material, sj-tif-1-cll-10.1177_09636897211042927 for Stem Cells from Human Exfoliated Deciduous teeth Promote Hair Regeneration in Mouse by Xiaoshuang Zhang, Tong Lei, Peng Chen, Lei Wang, Jian Wang, Donghui Wang, Wenhuan Guo, Yabin Zhou, Quanhai Li and Hongwu Du in Cell Transplantation
Abbreviations
- SHED
Stem cells from human exfoliated deciduous teeth
- HFSCs
Hair follicle stem cells
- DPs
Dermal papilla cells
- Shh
Sonic Hedgehog
- Gli1
Glioma-associated oncogene 1
- Ptch1
Patched 1
- Sirt7
Sirtuin protein 7
- MAPK
Mitogen-activated protein kinase
- Lgr4
Leucine-rih repeat G protein-coupled receptor 4
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Medical Ethics Committee of Academy of Military Medical Sciences, Beijing, China.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the approved protocols of the Department of Laboratory Animal Science, Peking University Health Science Center, Beijing, China.
Statement of Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Hebei Provincial Department of Science and Technology [grant number 19942410G].
ORCID iD: Hongwu Du  https://orcid.org/0000-0001-7772-8444
https://orcid.org/0000-0001-7772-8444
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Clevers H, Loh K M, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: WNT signaling and stem cell control. Science. 2014;346(6205):1248012. [DOI] [PubMed] [Google Scholar]
- 2. Cotsarelis G, Sun TT, Lavker R M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. [DOI] [PubMed] [Google Scholar]
- 3. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104(2):233–245. [DOI] [PubMed] [Google Scholar]
- 4. Taylor G, lehrer MS, Jensen PJ, Sun TT, Lavker RM-. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. [DOI] [PubMed] [Google Scholar]
- 5. Stenn K S, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. [DOI] [PubMed] [Google Scholar]
- 6. Hogan BL. Morphogenesis. Cell. 1999;96(2):225–233. [DOI] [PubMed] [Google Scholar]
- 7. Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg. 2006;25(1):2–10. [DOI] [PubMed] [Google Scholar]
- 8. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. [DOI] [PubMed] [Google Scholar]
- 9. Hirai Y, Takebe K, Nakajima K. Structural optimization of pep7, a small peptide extracted from epimorphin, for effective induction of hair follicle anagen. Exp Dermatol. 2005;14(9):692–699. [DOI] [PubMed] [Google Scholar]
- 10. Rahmani W, Liu Y, Rosin NL, Kline A, Raharjo E, Yoon J, Stratton JA, Sinha S, Biernaskie J. Macrophages promote wound-induced hair follicle regeneration in a CX3CR1- and TGF-beta1-dependent manner. J Invest Dermatol. 2018;138(10):2111–2122. [DOI] [PubMed] [Google Scholar]
- 11. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1(1):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petridis X, Diamanti E, Trigas G, Kalyvas D, Kitraki E. Bone regeneration in critical-size calvarial defects using human dental pulp cells in an extracellular matrix-based scaffold. J Craniomaxillofac Surg. 2015;43(4):483–490. [DOI] [PubMed] [Google Scholar]
- 14. Huang AH, Snyder BR, Cheng PH, Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells, 2008;26(10):2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mao JJ. Stem cells and the future of dental care. N Y State Dent J. 2008;74(2):20–24. [PubMed] [Google Scholar]
- 16. Hay MF. The development in vivo and in vitro of the lower incisor and molars of the mouse. Arch Oral Biol. 1961;3(2):86–109. [DOI] [PubMed] [Google Scholar]
- 17. Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8(1):46–55. [DOI] [PubMed] [Google Scholar]
- 18. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Z, Zhang Z, Lei Z, Lei P.CD14: biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019;48:24–31. [DOI] [PubMed] [Google Scholar]
- 20. Sidney LE, Branch MJ, Dunphy S E, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rheinlander A, Schraven B, Bommhardt U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018;196:22–32. [DOI] [PubMed] [Google Scholar]
- 22. Minor M, Alcedo KP, Battaglia RA, Snider NT. Cell type- and tissue-specific functions of ecto-5’-nucleotidase (CD73). Am J Physiol Cell Physiol. 2019;317(6):C1079–C1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Picke AK, Campbell GM, Bluher M, Krügel U, Schmidt FN, Tsourdi E, Winzer M, Rauner M, Vukicevic V, Busse B, Salbach-Hirsch J. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med. 2018;10(453):eaao6806 [DOI] [PubMed] [Google Scholar]
- 24. Kauer J, Schwartz K, Tandler C, Hinterleitner C, Roerden M, Jung G, Salih HR, Heitmann JS, Märklin M. CD105 (Endoglin) as negative prognostic factor in AML. Sci Rep. 2019;9(1):18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalisserry EP, Nam SY, Park SH, Anil S. Therapeutic potential of dental stem cells. J Tissue Eng. 2017;8:2041731417702531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455):eaaf3227. [DOI] [PubMed] [Google Scholar]
- 27. Hiraki T, Kunimatsu R, Nakajima K, Abe T, Yamada S, Rikitake K, Tanimoto K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promotes bone regeneration. Oral Dis. 2019;26(2):381–390. [DOI] [PubMed] [Google Scholar]
- 28. Novais A, Lesieur J, Sadoine J, Slimani L, Baroukh B, Saubaméa B, Schmitt A, Vital S, Poliard A, Hélary C, Rochefort GY. Priming dental pulp stem cells from human exfoliated deciduous teeth with fibroblast growth factor-2 Enhances mineralization within tissue-engineered constructs implanted in craniofacial bone defects. Stem Cells Transl Med. 2019;8(8):844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi Y, Yuniartha R, Yamaza T, Sonoda S, Yamaza H, Kirino K, Yoshimaru K, Matsuura T, Taguchi T. Therapeutic potential of spheroids of stem cells from human exfoliated deciduous teeth for chronic liver fibrosis and hemophilia A. Pediatr Surg Int. 2019;35(12):1379–1388. [DOI] [PubMed] [Google Scholar]
- 30. Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46(2):309–312. [DOI] [PubMed] [Google Scholar]
- 31. Gunawardena TN, Masoudian Z, Rahman MT, Ramasamy TS, Ramanathan A, Abu Kasim NH. Dental derived stem cell conditioned media for hair growth stimulation. PLoS One. 2019;14(5):e0216003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi BY. Hair-growth potential of ginseng and its major metabolites: a review on its molecular mechanisms. Int J Mol Sci. 2018;19(9):2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66(4):2081–2088. [DOI] [PubMed] [Google Scholar]
- 34. Shi W, Kaneiwa T, Cydzik M, Gariepy J, Filmus J. Glypican-6 stimulates intestinal elongation by simultaneously regulating Hedgehog and non-canonical Wnt signaling. Matrix Biol. 2020;88:19–32. [DOI] [PubMed] [Google Scholar]
- 35. Truong VL, Bak MJ, Lee C, Jun M, Jeong WS. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 Mice. Molecules. 2017;22(9):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J, Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol. 2012;23(8):906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li G, Tang X, Zhang S, Jin M, Wang M, Deng Z, Liu Z, Qian M, Shi W, Wang Z, Xie H. SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 2020;39(18):e104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai B, Wang X, Liu H, Ma S, Zhang K, Zhang Y, Li Q, Wang J, Yao M, Guan F, Yin G. Up-regulated lncRNA5322 elevates MAPK1 to enhance proliferation of hair follicle stem cells as a ceRNA of microRNA-19b-3p. Cell Cycle. 2019;18(14):1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Yu Z, Jiang K, Xu Z, Huang H, Qian N, Lu Z, Chen D, Di R, Yuan T, Du Z, Xie W. Hoxc-dependent mesenchymal niche heterogeneity drives regional hair follicle regeneration. Cell Stem Cell. 2018;23(4):487–500 e486. [DOI] [PubMed] [Google Scholar]
- 40. Ren X, Xia W, Xu P, Shen H, Dai X, Liu M, Shi Y, Ye X, Dang Y. Lgr4 deletion delays the hair cycle and inhibits the activation of hair follicle stem Cells. J Invest Dermatol. 2020;140(9):1706–1712 e1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sui B, Chen C, Kou X, Li B, Xuan K, Shi S, Jin Y. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98(1):27–35. [DOI] [PubMed] [Google Scholar]
- 42. Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017;35(1):61–67. [DOI] [PubMed] [Google Scholar]
- 43. Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. Int J Mol Sci. 2017;18(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ercal P, Pekozer GG, Kose GT. Dental stem cells in bone tissue engineering: current overview and challenges. Adv Exp Med Biol. 2018;1107:113–127. [DOI] [PubMed] [Google Scholar]
- 45. omasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, Campisi G, Pizzolanti G, Giordano C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 2017;8(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-tif-1-cll-10.1177_09636897211042927 for Stem Cells from Human Exfoliated Deciduous teeth Promote Hair Regeneration in Mouse by Xiaoshuang Zhang, Tong Lei, Peng Chen, Lei Wang, Jian Wang, Donghui Wang, Wenhuan Guo, Yabin Zhou, Quanhai Li and Hongwu Du in Cell Transplantation