Abstract
Companion diagnostics (CDx) hail promise of improving the drug development process and precision medicine. However, there are various challenges involved in the clinical development and regulation of CDx, which are considered high-risk in vitro diagnostic medical devices given the role they play in therapeutic decision-making and the complications they may introduce with respect to their sensitivity and specificity. The European Union (E.U.) is currently in the process of bringing into effect in vitro Diagnostic Medical Devices Regulation (IVDR). The new Regulation is introducing a wide range of stringent requirements for scientific validity, analytical and clinical performance, as well as on post-market surveillance activities throughout the lifetime of in vitro diagnostics (IVD). Compliance with General Safety and Performance Requirements (GSPRs) adopts a risk-based approach, which is also the case for the new classification system. This changing regulatory framework has an impact on all stakeholders involved in the IVD Industry, including Authorized Representatives, Distributors, Importers, Notified Bodies, and Reference Laboratories and is expected to have a significant effect on the development of new CDx.
Keywords: Companion diagnostics, in vitro diagnostics, IVDR, In-vitro Diagnostic Medical Devices Legislation, precision medicine, biomarkers, laboratory developed tests, diagnostic test approval, diagnostic techniques and procedures, diagnostic equipment, molecular diagnostic techniques
Introduction
Clinical practice today heavily relies on the in vitro analysis of biological specimens for information that will support and/or guide diagnosis and therapeutic efficacy monitoring. Such in vitro diagnostics (IVD) can be used alone or in combination with other devices and/or therapies.1-4 When used in combination with a therapeutic drug, they are referred to as Companion Diagnostics (CDx). 5 The development of CDx depends on companion biomarker(s), intending to stratify patients based on their predicted response to a drug and its potential toxicity levels.6-9
Precision Medicine (PM), or stratified medicine, has been the driving force of the shift from the so-called trial-and-error medicine to a concept of individualized prevention, diagnosis, and treatment.10-12 PM translates patient-specific clinical, genetic, and environmental data into patient-specific therapeutic strategies, improving response to therapy and potential remission.13,14 In many cases, the deriving treatment is based on a predictive biomarker, which can reveal “biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” 8 and requires a CDx. Although oncology drugs prevail among the therapeutics linked to FDA-approved CDx, 15 other medical conditions such as neuropathic, chronic musculoskeletal, cardiovascular, and metabolic disorders have also indirectly benefited from the ongoing research on biomarker-based therapies and efforts to develop CDx, mainly in terms of patients’ quality of life and prognostic potential and/or positive prognostic outcomes.6,16–23 In oncology, the growing understanding of cancer’s pathophysiology and the clinical complexity of its management tends to render non-specific cytotoxic drugs less attractive when compared to personalized chemotherapeutic agents and immunotherapeutic approaches. 13 This not only improves prognosis but allows for more informed prediction of response and tolerance to treatment.13,24,25
The exponential growth of PM is depicted by the fact that 25% of all new drugs approved by the FDA in 2019 were personalized medicines (42% in 2018). 26 On top of that, an average of 65% of drug approvals by the European Medicines Agency (EMA) and the FDA have at least one biomarker consideration in the drug development process between 2015 and 2019. 27 Therefore, considering the significant role that CDx plays in the clinical use of biomarkers, their development, validation, manufacturing, and distribution processes must be tightly regulated. This article sets the stage for the opportunity and challenges of in vitro diagnostics today and aims to discuss how the clinical development of CDx is impacted by ongoing regulatory reforms, especially within Europe, due to the imminent implementation of in vitro Diagnostic Medical Devices Regulation (IVDR).
State of the Art for CDx
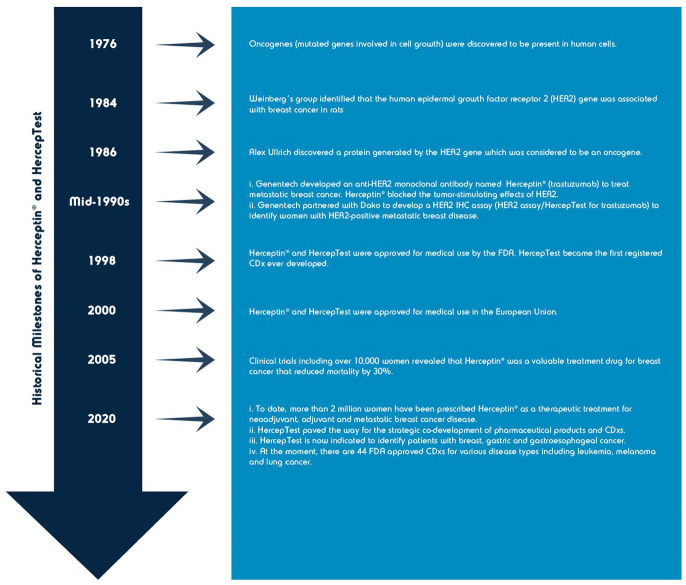
The first predictive biomarker associated with drug development was the HER2 protein, which resulted in the approval of trastuzumab (Herceptin) and HER2 immunohistochemical (IHC) assay, HercepTest (Dako) (see Figure 1) for the detection and treatment of metastatic HER2-positive breast cancer in 1998 by the FDA.2,11,28,29 Since then, the development and nomenclature of predictive biomarker assays linked to specific pharmaceutical agents has rapidly grown.1,3 At the dawn of the millennium, the term “companion diagnostic” was introduced and adopted by FDA (see Table 2 for current definition). 30
Figure 1.
Table 2.
Major differences in the regulation of CDx in the U.S. and in Europe (IVDR).
| U.S.—FDA | Europe—IVDR | |
|---|---|---|
| Definition | An IVD companion diagnostic is an in vitro diagnostic device that provides essential information for the safe and effective use of a corresponding therapeutic product. The use of an IVD companion diagnostic device with a therapeutic product is stipulated in the instructions for use in the labeling of both the diagnostic device and the corresponding therapeutic product, including the labeling of any generic equivalents of the therapeutic product 30 | “Companion diagnostic” means a device, which is essential for
the safe and effective use of a corresponding medicinal product
to: (a) identify, before, and/or during treatment, patients who are most likely to benefit from the corresponding medicinal product; or (b) identify, before, and/or during treatment, patients likely to be at increased risk of serious adverse reactions as a result of treatment. 53 |
| Classification | In the vast majority of cases, FDA classifies CDx as Class III Medical Devices under the rationale that the risk associated with the use of the CDx is similar to the risk associated with the drug that will or will not be administered based on a CDx test. There may be cases when a Class II classification is appropriate with PMA (510(k)). | In IVDR, CDx are classified as class C IVDs under Rule 3. |
| Regulatory pathway for approval | Class III Medical Devices require a premarketing approval (PMA)
procedure according to section 515 of the FD&C
Act. Of the 44 currently FDA-approved CDx (last update: March 2021), 40 have been approved via the PMA procedure. Note: The IVD companion diagnostic device application will be reviewed and approved or cleared under the device authorities of the FD&C Act and relevant medical device regulations; the therapeutic product application will be reviewed and approved under section 505 of the FD&C Act (i.e., drug products) or section 351 of the Public Health Service Act (i.e., biological products) and relevant drug and biological product regulations, that is a PMA submission is reviewed by CDRH while a new drug application for a therapeutic is submitted and reviewed by CDER or then CBER. |
According to IVDR (Annex IX, section 5.2), the conformity
assessment process for CDx foresees a consultation procedure
between a notified body and a medical authority. This could take
place between any of the national regulatory authorities in the
E.U. or the EMA, depending on who is responsible for the
authorization of the corresponding medicinal product. Under the
current directives, the interaction between medicines
authorities, EMA, and notified bodies is limited to consultation
procedures of devices that incorporate a medicinal
substance. For the CDx consultation procedure itself, the notified body will seek a scientific opinion from a medical authority or EMA “on the basis of the draft summary of safety and performance and the draft instructions for use” regarding the suitability of the device to the medicinal product concerned. The timeframe for the consultation is 60 days with the possibility to extend once for another 60 days. |
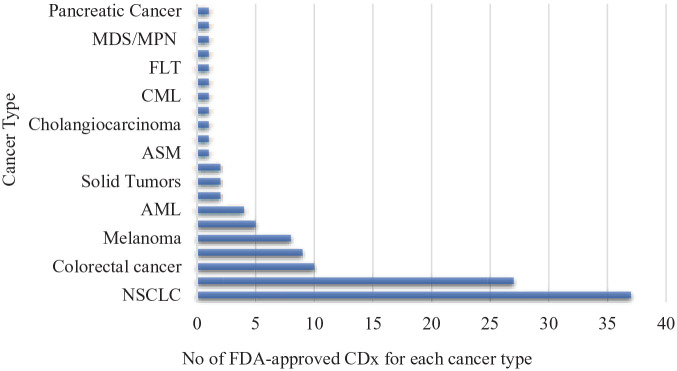
As of March 2021, the total number of FDA-approved CDx assays was 44 (see Figure 2). In fact, 43% (n = 19) of approvals have taken place between 1998 and 2015, while 57% (n = 25) of all FDA-approved CDx happened in the last 5 years. 15 Interestingly, this list only includes 2 non-cancer-related indications: Deferasirox (Exjade), a Fe-chelator indicated for the treatment of non-transfusion-dependent thalassemia which was approved in 2013 and in 2015, imatinib mesylate (Gleevec) was approved for the treatment of aggressive systemic mastocytosis. 15 The continuous expansion of indications for marketed CDx not only boosts personalized medicinal approaches but also enables basic and clinical research on both drug-response and mechanism level. This growth is also a reflection of the Regulators’ intention to adopt more innovative approaches for the regulation of these products in order to facilitate access to new therapeutic and diagnostic options.
Figure 2.
CDx approved by FDA up to 22 March 2021 by cancer type.
With respect to the analytical platforms used, polymerase chain reaction (PCR) took over for the identification of specific known mutations after the 2011 approval of Roche’s 4800 BRAF V600 Mutation Test, which was intended to be used for melanoma patients potentially eligible for treatment with vemurafenib (Zelboraf). 34 Up until then, the preferred platforms were Immunohistochemistry (IHC) and in situ hybridization (ISH)28,29,35 but overall, 36% (n = 16) of all FDA-approved assays are PCR-based.
Technical limitations of these platforms and the restrictions imposed by the limited amount of material obtained from biopsies resulted in the development of parallel multi-genic DNA sequencing platforms, such as next-generation sequencing (NGS), which enable the simultaneous analysis of hundreds of genetic alterations in a single test.36-39 The first FDA-approved assay based on NGS was the FoundationFocus CDx BRCA Assay for the detection of BRCA1 and BRCA2 alterations in tissues from ovarian cancer patients potentially eligible for treatment with rucaparib (Rubraca). In 2017, the FDA approved FoundationOne CDx (Foundation Medicine) as the first FDA-approved comprehensive genomic profiling (CGP) assay for all solid tumors incorporating multiple companion diagnostics. The test detects substitutions, insertions, deletions, and copy number alterations in 324 genes with implications to inform on therapies covering 5 different cancers: non-small cell lung cancers (NSCLC), melanoma, breast, colorectal, and ovarian cancers (see Table 1). 15 This was followed by the approval of a liquid biopsy platform of the FoundationOne CDx test in 2020. As of February 2021, 7 NGS-based assays were approved by FDA. As we move into an era of big data analytics, more resources and better-defined criteria are being considered, particularly when moving to more automated platforms such as Artificial Intelligence (AI) and Machine Learning (ML) for predictive therapeutics and prognostics.40-42
Table 1.
FDA-approved FoundationOne CDx assays until 22 March 2021.
| Cancer type | Companion diagnostic | Associated biomarker | FDA-approved drug |
|---|---|---|---|
| Non-small cell lung cancer | FoundationOne CDx | EGFR exon 19 deletions and EGFR exon 21 L858R alterations | Gilotrif® (afatinib), Iressa® (gefitinib), Tagrisso® (osimertinib), or Tarceva® (erlotinib) |
| EGFR exon 20 T790M alterations | Tagrisso® (osimertinib) | ||
| ALK rearrangements | Alecensa® (alectinib), Xalkori® (crizotinib), or Zykadia® (ceritinib) | ||
| BRAF V600E | Tafinlar® (dabrafenib) in combination with Mekinist® (trametinib) | ||
| MET single nucleotide variants (SNVs) and indels that lead to MET exon 14 skipping | TabrectaTM (capmatinib) | ||
| FoundationOne liquid CDx | ALK rearrangements | Alecensa® (alectinib) | |
| EGFR exon 19 deletions and EGFR exon 21 L858R substitution | Iressa® (gefitinib), Tagrisso® (osimertinib), or Tarceva® (erlotinib) | ||
| Melanoma | FoundationOne CDx | BRAF V600E | Tafinlar® (dabrafenib) or Zelboraf® (vemurafenib) |
| BRAF V600E or V600K | Mekinist® (trametinib) or Cotellic® (cobimetinib), in combination with Zelboraf® (vemurafenib) | ||
| Breast cancer | FoundationOne CDx | ERBB2 (HER2) amplification | Herceptin® (trastuzumab), Kadcyla® (ado-trastuzumab-emtansine), or Perjeta® (pertuzumab) |
| PIK3CA C420R, E542K, E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R, H1047L, H1047R, and H1047Y alterations | Piqray® (alpelisib) | ||
| FoundationOne liquid CDx | PIK3CA mutations C420R, E542K, E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R; and H1047L, H1047R, and H1047Y | Piqray® (alpelisib) | |
| Colorectal cancer | FoundationOne CDx | KRAS wild-type (absence of mutations in codons 12 and 13) | Erbitux® (cetuximab) |
| KRAS wild-type (absence of mutations in exons 2, 3, and 4) and NRAS wild type (absence of mutations in exons 2, 3, and 4) | Vectibix® (panitumumab) | ||
| Ovarian cancer | FoundationOne CDx | BRCA1/2 alterations | Lynparza® (olaparib) or Rubraca® (rucaparib) |
| FoundationOne Liquid CDx | BRCA1, BRCA2 alterations | Rubraca® (rucaparib) | |
| Cholamgiosarcoma | FoundationOne CDx | FGFR2 fusions and select rearrangements | Pemazyre® (pemigatinib) or Truseltiq™ (infigratinib) |
| Colon cancer | FoundationOne CDx | Homologous Recombination Repair (HRR) gene (BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, and RAD54L) alterations | Lynparza® (olaparib) |
| Prostate cancer | FoundationOne Liquid CDx | BRCA1, BRCA2, ATM alterations | Lynparza® (olaparib) |
| BRCA1, BRCA2 alterations | Rubraca® (rucaparib) |
Basic Considerations for the Development of a CDx
The principal objectives of a CDx1-3,30,43 are to:
(a) Identify the appropriate patient group who are most likely to benefit from a therapeutic product;
(b) Identify the patient groups for which the therapeutic product has been adequately proven safe and effective, allowing for adjustment of treatment to achieve optimal safety;
(c) Predict serious adverse reactions that some patients may present as an outcome of the therapeutic drug used;
(d) Monitor the response to treatment to improve/adjust the dosage scheme and to ensure continued patient safety.
However, the practicality of achieving these objectives is far from ideal, as designing a validated CDx test does not guarantee accurate detection of the optimal patient population and the corresponding provision of treatment. The post-approval delay in clinical uptake of a CDx for a targeted treatment take as long as 5 years, 44 resulting in a delay in the access of patients to more effective therapies. Coordination of drug and CDx development is one of the main challenges that must be addressed, because a failure to co-develop and co-launch the drug and its CDx often results in substantial loss of revenue whilst stifling PM-focused decision-making and compromising on the clinical outcomes of some patients. A typical example of this can be seen in the case of Novartis’s Gleevec and its CDx produced by Dako Denmark over 20 years ago. The Danish diagnostic firm significantly delayed the development of the CDx trying to avoid the possibility of launching a CDx that would not be “linked” to an associated drug but this ultimately resulted in substantial revenue loss for the Swiss pharma giant which could not support the use of its approved drug with a CDx.45,46
Whilst CDx incorporation can take place at the beginning of a clinical trial, the advancement of genomic technologies and the adoption of genetic screening in clinical settings is a relatively recent addition to the traditional drug discovery process. This means that dedicated clinical trials are sometimes required to repurpose existing therapeutics in order to extract more diagnostic information in favor of more stratified CDx. Given that PM considers individual genetic variability and is an important factor of CDx design, it is worth highlighting that a disproportionate majority of data from Genome Wide Association Studies (GWAS) are extracted from individuals of European descent. In fact, 71.8% of the data was generated from only 3 countries, namely the U.S., Iceland, and the United Kingdom 47 resulting in a significant proportion of the world’s population not being represented in the genomics datasets available to pharmaceutical companies and clinicians. Additionally, most clinical trials take place in the U.S. and Europe. The combination of the lack of diversity in clinical trial participants and genomic data limits PM and CDx potential for underrepresented populations. A good example of this is seen in clopidogrel, an antiplatelet prodrug used to treat cardiovascular disease. Most drugs are metabolized by the Cytochrome P450 (CYP) enzyme family. CYP2C1, one of the principal enzymes involved in the bioactivation of clopidogrel, is highly polymorphic, with the most common loss-of-function variant, CYP2C19*2, having a frequency of 25% to 30% (European), 70% (Asian), and 14% to 20% (African). This genetic variation reduces activation of clopidogrel, increasing the risk of serious cardiovascular events in patients undergoing balloon angioplasty or stent placement, particularly in the Asian population. 16 Indeed, these findings have led FDA to subsequently issue a warning on the label for clopidogrel. 48 Whilst CYP2C19 is not a CDx to clopidogrel, using an example of how patients from different populations respond to medications metabolized by certain genes clearly illustrates the importance of improving CDx design and sensitivity in order to facilitate more accurate PM clinical decisions. Further improvements in CDx approvals should come from a better understanding of both the pathology in question and the companion drug’s mechanism of action.
The most common pitfalls related to CDx development pertain to the49-52:
❒ Determination of clinically-relevant and population-specific genetic markers;
❒ Identification of genetic variants linked to specific diseases and/or genetic variants correlating with response to treatment;
❒ Hesitance to analyze large molecule sets (i.e., analysis of proteins, lipids, metabolites, etc.) as biomarkers;
❒ Identification and stratification of the eligible patient groups;
❒ Adoption and establishment of solid regulatory measures that encourage innovation and patient safety;
❒ Reliability and consistency of the biomarker test quality;
❒ Timely availability of test results;
❒ Reimbursement options in different countries.
Changing Regulatory Framework
From a regulatory perspective, because the development of CDx combines pharmaceutical and medical devices, their regulatory requirements must reflect the complexity and costs associated with scientific, clinical, operational, and commercial decisions. This is why a straightforward regulatory and commercialization strategy is crucial and must be developed following the identification of user needs, definition of intended use, and implementation of a quality management system. 52 Up until 2017, there was no official consensus in the definition of CDx but the publication of IVDR 2017/746 has aligned the U.S. and E.U. definitions (see Table 2). 27
In the U.S., the development of IVDs is guided by the Investigational Device Exemptions (IDE) regulation, Title 21, Code of Federal Regulations (21 CFR) Part 812, which sets specific requirements for studies of investigational devices. 54 However, many IVD devices are exempt from IDE regulations if the testing is non-invasive, does not pose a significant risk in the process of sampling, or is approved for use by another product of procedure. 55
IVDs that are under consideration, or those that are exempt from IDE regulations, have to adhere to labeling requirements under 12 CFR 809, stating either “For Research Use Only,” “Not for use in diagnostic procedures,” or “For Investigational Use Only.” The performance characteristics of this product have not been established.
Investigational IVDs are classified based on the level of risk that the study presents to subjects and can be of54,56,57:
❒ Significant risk (SR), regulated by CFR Part 812—full IDE requirements, application to FDA for IDE approval;
❒ Non-significant risk (NSR), regulated by CFR Part 812.2 (b)—abbreviated IDE requirements, approval of the investigation by an institutional review board (IRB) and compliance with informed consent requirements or;
❒ Excepted devices regulated by CFR Part 812.2(c)—investigations are exempt from most of the requirements of IDE regulation.
In the vast majority of cases, CDx are classified as Class III Medical Devices by FDA because the risk associated with a CDx is similar to the risk associated with the drug that will or will not be administered based on a CDx test.9,56
Typically, class III Medical Devices require a premarketing approval (PMA) procedure according to section 515 of the FD&C Act 58 but there have been 2 CDx tests (MRDx BCR-ABL Test and FerriScan) cleared by 510(k) and 2 cleared by a Humanitarian Device Exemption (HDE) utilizing Gleevec as a companion. 59
The 2018 FDA guidance on the “Principles for Co-development of an In-vitro Companion Diagnostic Device with a Therapeutic Product” 60 set a framework for the management of potential issues sponsors may face when developing a therapeutic product and an accompanying IVD companion diagnostic, referred to as co-development, such as:
❒ Different schedules and agency interactions for therapeutic agents and CDx development and corresponding managing issues;
❒ Alignment in the goals of the therapeutic product, and how they align with the IVD;
❒ Anticipation of the complexity raised by including an IVD in a therapeutic product clinical trial design process;
❒ Decision on what data are needed for a new drug application (NDA) or a biologics licensing application (BLA).
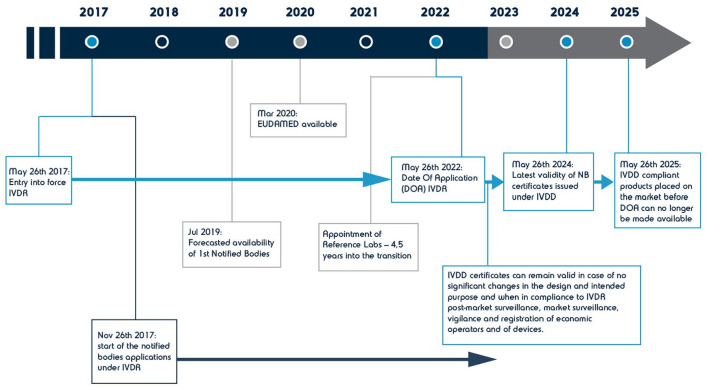
Across the Atlantic in Europe, the legal framework for IVDs and thus for CDx, is currently changing. Publication of the Regulation 2017/746 on IVDR, 53 which came into force in May 2017, is aimed to replace the IVDD 98/79/EC in a transitional period of 5 years (see Figure 3). The new Regulation applies to all IVDs and their accessories and introduces new definitions and rules not only for CDx but also for in-house tests, kits, and single-use IVDs. From May 2017 to May 2022, IVDs and associated CDx will transition from being CE-marked under IVDD, to being CE-marked under the new IVDR. During this period, IVDs can be placed under either directive and those certified by a Notified Body (NB) under IVDD may have an additional 2 years (until 2024) to place their product on the market. Those that were not supervised by a NB must adhere to the new Regulation. Along with more stringent clinical requirements for safety and performance (see Tables 3 and 4 for an overview of new requirements), and in order to ensure the active involvement of all stakeholders in the implementation of the new requirements, IVDR launched a new understanding of traceability and calls for revised, explicit roles for Authorized Representatives, Distributors, and Importers (refer to Art. 11-14 and Art. 16 of IVDR). It also mandates both Manufacturers and Authorized Representatives to have at least one person in their organization that shall hold the role of the Person Responsible for Regulatory Compliance (PRRC, refer to Art. 15 of IVDR).
Figure 3.
Timeline for the implementation of IVDR Regulation in Europe.
Table 3.
Overview of EU-Regulation IVDR 2017/746.
| Quality management system | General Safety and Performance Requirements (GSPRs) and technical documentation | Conformity assessment |
|---|---|---|
| ❒ The requirements for the Quality Management System of the
manufacturer are described in Art. 10(8) and involve the
following aspects: ❒ verification/validation for business organization, including outsourced processes, design, and development, including production process controls and quality control procedures, post-market surveillance, risk management, and performance evaluation. ❒ Although not yet harmonized with ISO 13485:2016, the new Regulation tries to align with the Standard focusing on a risk-based approach for IVD regulation. Requirements for maintaining a risk management system (refer to Art. 10(2)) differs according to IVD class and must cover the entire lifetime of the device (see Annex I) |
Technical Documentation (TD) shall be in compliance with Annexes
II and III as per Art. 10(4) in order to provide evidence of
conformity with General Safety and Performance Requirements
(GSPRs) Compared to the only 5 bullet points of Annex III in the IVD Directive, GSPRs have been extensively revised ❒ Adding completely new requirements with respect to information on device description and specification, design information, analytical performance of the device, stability, software verification and validation, PMS updating requirements to design and manufacturing information, benefit-risk analysis and risk management, performance evaluation |
❒ Conformity Assessments Routes have been updated (Art. 9 of
IVDD has been replaced by Art. 48 in IVDR) to reflect the new
classification rules and subsequent up-classification of most
IVDs. ❒ Manufacturers must select an appropriate route to conformity assessment as per Annexes IX to XI. ❒ NB involvement is required in all classes except class A (non-sterile). Class C and D IVDs now require the involvement of EMA and EU reference laboratories (refer to Chapter V, section 2 and Annexes IX, X, XI). |
| Performance evaluation | EUDAMED-related requirements | Post-market requirements |
| ❒ Performance evaluation is now required throughout the lifetime
of the IVD under the explicit requirement to collect and analyze
clinical evidence throughout its life-cycle. For this reason,
the process of performance evaluation is outlined and
performance indicators, that is scientific validity, analytical
and clinical performance are clarified. ❒ The requirement for a Performance Evaluation Plan is mandatory. ❒ Artice 10(3) mandates the conduct of performance evaluation based on clinical evidence, including a Post-Market Performance Follow-up (PMPF). ❒ Clinical performance studies are required although some exceptions apply Special provisions for interventional performance studies have been added (refer to Annex XIV) |
❒ EUDAMED will be accessible to the public and stakeholders to
enhance transparency and traceability. Issue of a Unique Device Identifier (UDI) for traceability in the supply chain is mandatory (refer to Art. 10(4) and 24) |
❒ The performance evaluation and its documentation shall be
updated throughout the life cycle of the IVD with data obtained
from the implementation of the manufacturer’s PMS and PMPF plans
(refer to Art. 56(6)) ❒ Annual updates of the PER for class C and D IVDs plans (refer to Art. 56(6)) Incident reporting and trending has become more stringent. Although the current 2-day and 10-day deadlines are retained for the report of a serious public health threat and for reporting a death or serious health deterioration respectively, a new 15-day reporting deadline is introduced for the report of all other serious incidents, replacing the 30-day requirement for reporting all other reportable incidents of the Directive. |
Table 4.
Clinical evidence requirements for IVDR.
| Performance evaluation plan | Annex XIII, part A, 1.1 |
|---|---|
| Performance evaluation report ❒ Scientific validity report ❒ Analytical performance report Clinical performance report |
Annex XIII, 1.3.2 ❒ Annex XIII, part A, 1.2.1 ❒ Annex XIII, part A, 1.2.2 Annex XIII, part A, 1.2.3 |
| Final assessment of the clinical evidence | Art. 56.3, Annex XIII, 1.3.1 |
| Clinical Performance studies ✓ Obligatory unless justification can be provided why relying on other sources is sufficient (applicable to self-test and near-patient-test IVDs) |
Annex XIII, part A, 1.2.3 |
| Special requirements | Art. 58 |
| Interventional clinical performance studies | Annex XIV |
| Performance studies on incapacitated subjects | Art. 60 |
| Performance studies in minor subjects | Art. 61 |
| Performance studies on pregnant and/or breastfeeding women | Art. 62 |
| Performance studies in emergency situations | Art. 64 |
The Regulation stipulates that clinical evidence used for the approval of an IVD must represent the sum of evidence collected after co-evaluating scientific validity, analytical performance, and clinical performance.5,61,62
The IVDR establishes four risk classes A, B, C, and D in order of lowest to highest risk class. For example, Class D covers general life-threatening conditions or transmissible agents in blood, whilst class A covers laboratory devices and instrumentation. In terms of classification, 7 risk-based rules (see Table 5) are introduced, which result in the up-classification of 80% to 90% of IVDs currently marketed in Europe. This automatically translates into a need for Quality Management Systems (QMS) remediation and consequently to the extensive revision of Technical Documentation (TD). CDx, most software that is a part of IVD instruments (SaMD), single-use IVDs, and genetic tests will fall into class C. However, software for the interpretation of automated readings of line immunoassay for the confirmation and determination of antibodies to HIV-1, HIV-1 group O. and HIV-2 in human serum and plasma, shall fall be in class D per Rule 1.53,63
Table 5.
Classification of in-vitro diagnostic medical devices per IVDR.
| Rule 1: Blood screening/high-risk diseases | |
| ❒ detection of the presence of, or exposure to, a transmissible
agent in blood, blood components, cells, tissues or organs, or
in any of their derivatives, in order to assess their
suitability for transfusion, transplantation, or cell
administration; ❒ detection of the presence of, or exposure to, a transmissible agent that causes a life-threatening disease with a high or suspected high risk of propagation; determining the infectious load of a life-threatening disease where monitoring is critical in the process of patient management. |

|
| Rule 1 does not apply? ⇒ Consider Rule 2 | |
| Rule 2: Blood or tissue compatibility | |
| Devices intended to be used for blood grouping, or tissue typing to ensure the immunological compatibility of blood, blood components, cells, tissue, or organs that are intended for transfusion or transplantation or cell administration | |
| except when intended to determine any of the following
markers: ❒ ABO system [A (ABO1), B (ABO2), AB (ABO3)]; ❒ Rhesus system [RH1 (D), RHW1, RH2 (C), RH3 (E), RH4 (c), RH5 (e)]; ❒ Kell system [Kel1 (K)]; ❒ Kidd system [JK1 (Jka), JK2 (Jkb)]; ❒ Duffy system [FY1 (Fya), FY2 (Fyb)]; ⇒ (high-risk blood groups) | |
| Rule 2 does not apply? ⇒ Consider Rule 3 | |
| Rule 3: infectious diseases—cancer testing—companion diagnostics—genetic testing—congenital screening | |
| ❒ for detecting the presence of, or exposure to, a sexually
transmitted agent; ❒ for detecting the presence in cerebrospinal fluid or blood of an infectious agent without a high or suspected high risk of propagation; ❒ for detecting the presence of an infectious agent, if there is a significant risk that an erroneous result would cause death or severe disability to the individual, foetus or embryo being tested, or to the individual’s offspring; ❒ for prenatal screening of women in order to determine their immune status toward transmissible agents; ❒ for determining infective disease status or immune status, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring; ❒ to be used as companion diagnostics; ❒ to be used for disease staging, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring; ❒ to be used in screening, diagnosis, or staging of cancer; ❒ for human genetic testing; ❒ for monitoring of levels of medicinal products, substances, or biological components, when there is a risk that an erroneous result will lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient’s offspring; ❒ for management of patients suffering from a life-threatening disease or condition; ❒ for screening for congenital disorders in the embryo or foetus; for screening for congenital disorders in new-born babies where failure to detect and treat such disorders could lead to life-threatening situations or severe disabilities. | |
| Rule 3 does not apply? ⇒ Consider Rule 4 | |
| Rule 4: self testing/near-patient testing | |
| ❒ Devices intended for self-testing Devices intended for near-patient testing are classified in their own right. | |
| Devices intended for self-testing except for
devices ❒ for the detection of pregnancy ❒ for fertility testing ❒ for determining cholesterol levels, devices for the detection of glucose, erythrocytes, leukocytes, and bacteria in urine | |
| Rule 4 does not apply? ⇒ Consider Rule 5 | |
| Rule 5: the only self-certified devices | |
| ❒ products for general laboratory use, accessories which possess
no critical characteristics, buffer solutions, washing
solutions, and general culture media and histological stains,
intended by the manufacturer to make them suitable for in vitro
diagnostic procedures relating to a specific
examination; ❒ instruments intended by the manufacturer specifically to be used for in vitro diagnostic procedures; specimen receptacles. | |
| None of the other Rules apply? Classify per Rule 6 in class B | |
| Rule 6 Devices not covered by the above-mentioned classification rules are classified as class B | |
| Rule 7 Devices which, are controls without a quantitative or qualitative assigned value | |
The IVDR establishes four risk classes D, C, B, and A, with D being the highest risk class (highlighted in red) and A the lowest (highlighted in green).
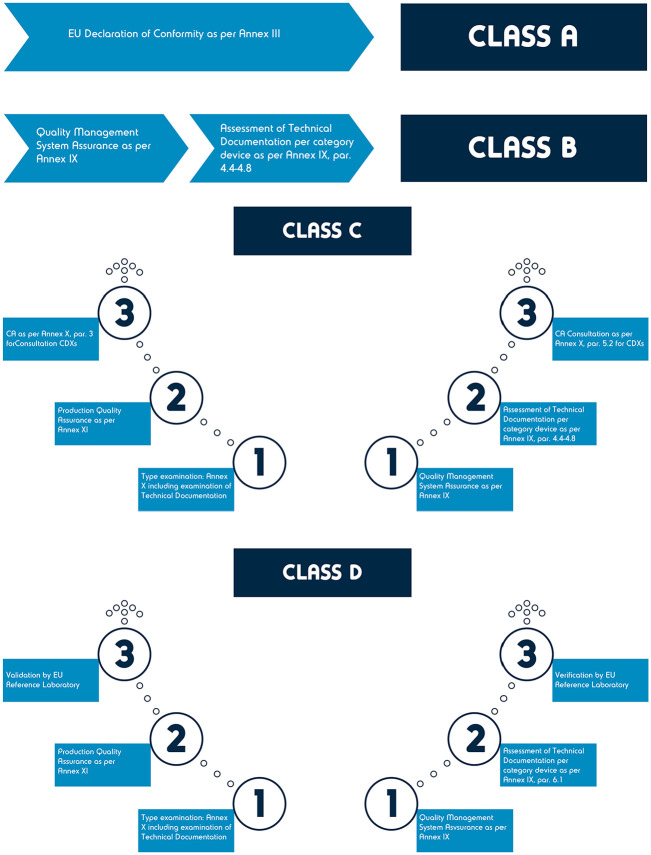
The conformity assessment concept (see Figure 4) outlined in IVDR (Annex IX, section 5.2 53 ), which is not new but is largely affected by the imminent up-classification, inevitably results in the involvement of an NB, and the assessment of the manufacturer’s QMS. 61
Figure 4.
IVDR conformity assessment routes.
The conformity assessment for CDx (see Figure 5) foresees a consultation procedure between the NB and a medical authority, depending on who is responsible for the authorization of the corresponding medicinal product. 62 Manufacturers are expected to provide a summary of safety and performance with Instructions For Use (IFU), and to evaluate the IVD for the associated medicinal product. However, because a CDx is dependent on a therapeutic agent, it is expected that new CDx consultations will primarily be performed in collaboration with the EMA. The Competent Authority must provide its opinion within 60 days, but this period may be extended once for a further 60 days if there is sufficient justification for this extension. This is the reason why manufacturers of CDx should allow for 120 days for the regulatory procedure to take place. Unlike reference laboratory testing, if the scientific and technical feedback is unfavorable, the certification process may continue based on the recommendation of the NB, provided there is a justification for this overruling.
Figure 5.
Overview of conformity assessment of a CDx in Europe under IVDR.
Any potential clinical trial application, including those needed for biomarker and CDx establishment, is regulated by national agencies and the procedure differs in each country according to individual laws. The development process for investigational IVDs and CDx is thus required to be agreed by the sponsor, the National Competent Authority, and the chosen NB on a case-by-case basis.27,62
Another challenge introduced by IVDR revolves around Laboratory Developed Tests (LDT), which are regulated for the first time in the context of IVDs in Europe. Although many requirements are not introduced by IVDR, their incorporation into the regulation mandates quality management, accreditation, and conformity with GSPRs. A significant number of these tests will now require CE IVD marking regardless of the location of the analytical laboratory. 64 IVDR allows an in-house exemption, meaning that healthcare institutions may still manufacture, modify, and use LDTs on a small scale for targeted patient groups, as long as an equivalent device that is already on the market fails to adequately perform. Practically, this exemption will only be applicable to a very limited number of LDTs which come out of laboratories that do not require CE marking.65,66
Other Regulatory Considerations: Japan, China, Australia, and WHO’s IVD Prequalification Program
The U.S., E.U., and Japan are the largest markets for IVDs, respectively. 67 More recently, other markets have emerged, such as China, which has become of great interest due to the rapid growth in its oncology market. 67 Australia’s IVD market remains very small, accounting for approximately 1.35% of global IVD sales, however, imports of IVDs make up 95% of the Australian market, the majority of which are from the U.S. and E.U. 68
Japan, China, and Australia have their regulatory authorities that assess IVD applications, including CDx (see Table 6 for an overview). In 2013, the Japanese Pharmaceutical and Medical Devices Agency (PMDA) published guidance on the approval of CDx. 69 The guidance states that an application for an unapproved CDx and the corresponding therapeutic should be made simultaneously. This is to encourage entities developing CDx and those with the corresponding drugs to work together from an early stage, enabling the approval of the CDx before the therapeutic.69,70 This simultaneous application is also strongly recommended by Australia’s Therapeutic Goods Administration (TGA), 4 which recently introduced new regulations that will be fully enforced by 2022, including the use of a Unique Product Identifier (UPI) for every CDx.
Table 6.
CDx definitions and regulatory requirements around the world.
| Country | Regulatory authority | Definition | Classification | Submission and assessment | Requirements |
|---|---|---|---|---|---|
| Australia 4 | The Therapeutic Goods Administration (TGA) | “An IVD companion diagnostic is an in vitro diagnostic (IVD)
medical device which provides information that is essential for
the safe and effective use of a corresponding medicine or
biological.” Australia’s definition will align with the FDA and EU regulation 2017/746 definition. |
Class III IVDs or Class III in-house IVDs | Concurrent submission and assessment of CDx with the corresponding therapeutic is highly recommended (but not required). | • Aligned to EU 2017/746 and FDA regulatory
requirements. • UPI*. • Application audit*. CDx claims for an IVD cannot be approved in the absence of an application for, or approval of, the corresponding medicine or biological. |
| China35,67,71 | The National Medical Products Administration (NMPA) | No formal definition. | Class III IVD | IVD and therapeutic reviews are conducted independently by their corresponding centers. | • Panel review of CDx dossier (may involve key opinion leader
meeting for further discussion and comment). • A technical review is often conducted within 60 days of an application (to assess safety and effectiveness). • Only NMPA-certified national testing centers in China can perform the testing. • IVD clinical trials must comply with the Guidelines on Clinical Trial Technology for In Vitro Diagnostic Reagents. The clinical utility of a CDx is usually validated in the clinical trial of the corresponding therapeutic product where the CDx is used. For new CDx, this data is unlikely to exist, therefore applicants are encouraged to discuss strategies to obtain clinical evidence with the NMPA. |
| Japan69-73 | Pharmaceutical and Medical Devices Agency (PMDA), Ministry of Health, Labor and Welfare (MHLW) | “A CoDx refers to an in vitro diagnostic agent or a medical
device that is used to improve the efficacy or safety of a
specific therapeutic product, is essential for using the
pertinent therapeutic product, and corresponds to either of the
following (except in vitro diagnostic agents or medical devices
intended simply for disease diagnosis, etc.) (1) An in vitro diagnostic agent or a medical device that is used to identify patients who are expected to respond better to a specific therapeutic product. (2) An in vitro diagnostic agent or a medical device that is used to identify patients who are likely to be at high risk of developing adverse events associated with a particular therapeutic product. (3) An in vitro diagnostic agent or a medical device that is necessary for optimizing the treatment including dose, schedule, and discontinuation of a particular therapeutic product.” |
Usually, class III and often require review by PMDA and outside advisory experts | PMDA guidance stresses that therapeutic products and corresponding CDx should be submitted and assessed simultaneously. However, this process is still being implemented. | • Clinical significance and the clinical cut-off are usually
performed using clinical trial results from the corresponding
therapeutic, conducted in patients identified by the
CDx. If the CDx is not used in the confirmatory clinical studies, the concordance between the measurement method used in the clinical trials and the proposed CDx must be evaluated. |
Required as of 1 February 2020.
The Chinese regulatory authority, the National Medical Products Administration (NMPA), has not yet published any guidance for CDx. 67 However, as CDx are classified as class III IVD products they follow IVD regulations, which stipulate that applications are to be supported by clinical trials conducted in China. 67 Currently, the regulatory assessment process for CDx is challenging, due to different regulations and independent review centers for IVDs and therapeutics, with limited interaction/collaboration between the two. However, in 2017 new procedures for approval were proposed to facilitate the development/approval of IVDs, including CDx. For instance, the NMPA may now accept clinical data from global trials outside of China (for other proposed changes see Xu et al. 67 ).
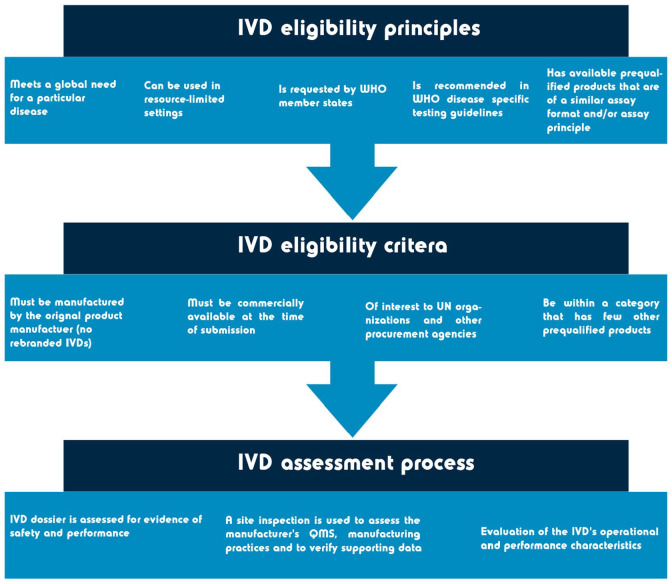
For regulatory authorities that do not have an established assessment procedure, and/or lack the experience and resources to assess IVDs, help is available from the World Health Organization (WHO) prequalification (WHO-PQ) assessment. United Nations agencies and WHO member states can use the WHO-PQ as a guide to determine whether IVDs meet the required safety and performance requirements. To be eligible for the WHO-PQ assessment, manufactures must submit a pre-assessment form and meet several requirements (see Figure 6).
Figure 6.
Overview of the WHO-PQ scope and procedure. Information obtained from Mbunkah et al. 61 and World Health Organization. 74
Although the regulatory authorities differ slightly in their assessment of CDx, they all face a common issue: the lack of a global reimbursement scheme. Currently, reimbursement procedures differ by country, even within E.U. member states.75,76 This means that CDx that are funded in the U.S. may not be reimbursed in the E.U. and vice versa. For emerging and smaller markets, such as China and Australia, this may cause a barrier to the development of new CDx. 43
Concluding Remarks
Development, opportunity, and growth of the CDx market have the potential to further personalize therapeutic strategies and improve patient access, outcomes and their response to innovative pharmaceutical agents and/or diagnostic methods. To meet the complicated, growing regulatory demands of IVD technologies, an international consensus on the basic regulatory requirements for their approval and continuous monitoring is a sine qua non and should be seen as a step forward for public health. Currently, the lack of standardization and use of multiple platforms for the same biomarker raises concerns on reproducibility and sensitivity of the specific analytical assay the platform assesses. Therefore, approval of CDx must be aligned with the scrutinized assessment of their analytical validity. Implementation of IVDR 2017/746, although a challenging regulatory shift for CDx, opts to optimize the field through the alignment of European requirements with the American and Japanese ones and the continuous, dynamic monitoring of the real-world use of IVDs.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Vasiliki Valla  https://orcid.org/0000-0001-8301-7868
https://orcid.org/0000-0001-8301-7868
Saba Alzabin  https://orcid.org/0000-0003-3474-1846
https://orcid.org/0000-0003-3474-1846
References
- 1. Jørgensen JT. Clinical application of companion diagnostics. Trends Mol Med. 2015;21:405-407. [DOI] [PubMed] [Google Scholar]
- 2. Jørgensen JT. Companion and complementary diagnostics: an important treatment decision tool in precision medicine. Expert Rev Mol Diagn. 2020;20:557-559. [DOI] [PubMed] [Google Scholar]
- 3. Jørgensen JT, Hersom M. Companion diagnostics-a tool to improve pharmacotherapy. Ann Transl Med. 2016;4:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. TGA. IVD companion diagnostics – guidance on regulatory requirements. Published 2020. https://www.tga.gov.au/sites/default/files/ivd-companion-diagnostics.pdf
- 5. Beeler JF. Integrating diagnostic products into the drug development workflow: applications for companion diagnostics. In: Shimasaki C, ed. Biotechnology Entrepreneurship. Academic Press; 2020:359-370. [Google Scholar]
- 6. Gibson DS, Bustard MJ, McGeough CM, et al. Current and future trends in biomarker discovery and development of companion diagnostics for arthritis. Expert Rev Mol Diagn. 2015;15:219-234. [DOI] [PubMed] [Google Scholar]
- 7. Ptolemy AS, Rifai N. What is a biomarker? Research investments and lack of clinical integration necessitate a review of biomarker terminology and validation schema. Scand J Clin Lab Invest Suppl. 2010;242:6-14. [DOI] [PubMed] [Google Scholar]
- 8. Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor CR. Predictive biomarkers and companion diagnostics. The future of immunohistochemistry: “in situ proteomics,” or just a “stain”? Appl Immunohistochem Mol Morphol. 2014;22:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aspinall MG, Hamermesh RG. Realizing the promise of personalized medicine. Harv Bus Rev. 2007;85:108-165. [PubMed] [Google Scholar]
- 11. Barrera-Saldaña HA. Origin of personalized medicine in pioneering, passionate, genomic research. Genomics. 2020;112:721-728. [DOI] [PubMed] [Google Scholar]
- 12. Offit K. Personalized medicine: new genomics, old lessons. Hum Genet. 2011;130:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov. 2010;9:363-366. [DOI] [PubMed] [Google Scholar]
- 14. Schwaederle M, Zhao M, Lee JJ, et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33:3817-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. FDA. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Published 2021. https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools
- 16. Mpye KL, Matimba A, Dzobo K, Chirikure S, Wonkam A, Dandara C. Disease burden and the role of pharmacogenomics in African populations. Glob Health Epidemiol Genom. 2017;2:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicolaou CA, Brown N. Multi-objective optimization methods in drug design. Drug Discov Today Technol. 2013;10:e427-e435. [DOI] [PubMed] [Google Scholar]
- 18. Nikolcheva T, Jäger S, Bush TA, Vargas G. Challenges in the development of companion diagnostics for neuropsychiatric disorders. Expert Rev Mol Diagn. 2011;11:829-837. [DOI] [PubMed] [Google Scholar]
- 19. Elliott P, Cowie MR, Franke J, et al. Development, validation, and implementation of biomarker testing in cardiovascular medicine state-of-the-art: proceedings of the European Society of Cardiology-Cardiovascular round table. Cardiovasc Res. 2021;117:1248-1256. [DOI] [PubMed] [Google Scholar]
- 20. Geem D, Kugathasan S. It takes two to make it right: dual biologic and small molecule therapy for treatment-refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:1361-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iliodromiti S, Salje B, Dewailly D, et al. Non-equivalence of anti-Müllerian hormone automated assays-clinical implications for use as a companion diagnostic for individualised gonadotrophin dosing. Hum Reprod. 2017;32:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. John A, Shah RA, Wong WB, Schneider CE, Alexander M. Value of precision medicine in advanced non-small cell lung cancer: real-world outcomes associated with the use of companion diagnostics. Oncologist. 2020;25:e1743-e1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meinert E, Alturkistani A, Luo D, et al. Chapter 25 – current status and future direction of companion diagnostics. In: Jørgensen JT, ed. Companion and Complementary Diagnostics. Academic Press; 2019;455-472. [Google Scholar]
- 24. Comment LA, Ward AF, Schrock AB, et al. Evidence-Based development and clinical use of precision oncology therapeutics. Clin Pharmacol Ther. 2020;108:440-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papadopoulos N, Kinzler KW, Vogelstein B. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat Biotechnol. 2006;24:985-995. [DOI] [PubMed] [Google Scholar]
- 26. FDA. New drug therapy approvals 2019. Published 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019.
- 27. Gromova M, Vaggelas A, Dallmann G, Seimetz D. Biomarkers: opportunities and challenges for drug development in the current regulatory landscape. Biomark Insights. 2020;15:1177271920974652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell MR. Update on molecular companion diagnostics – a future in personalized medicine beyond Sanger sequencing. Expert Rev Mol Diagn. 2020;20:637-644. [DOI] [PubMed] [Google Scholar]
- 29. Poulsen TBG, Karamehmedovic A, Aboo C, et al. Protein array-based companion diagnostics in precision medicine. Expert Rev Mol Diagn. 2020;20:1183-1198. [DOI] [PubMed] [Google Scholar]
- 30. FDA. In vitro companion diagnostic devices guidance for industry and Food and Drug Administration Staff, Published August 2014. https://www.fda.gov/media/81309/download [Google Scholar]
- 31. Scheerens H, Malong A, Bassett K, et al. Current status of companion and complementary diagnostics: strategic considerations for development and launch. Clin Transl Sci. 2017;10:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. A brief history of Herceptin® and the Her2 cancer gene. Published February 2021. https://www.breastcenter.com/2020/03/04/a-brief-history-of-herceptin-and-the-her2-cancer-gene/
- 33. Hurvitz S, McCann K. Chapter 2 – HER2 testing in the era of changing guidelines. In: Hurvitz S, McCann K, eds. Her2-Positive Breast Cancer. Elsevier; 2019;13-39. [Google Scholar]
- 34. Khoury JD, Catenacci DV. Next-generation companion diagnostics: promises, challenges, and solutions. Arch Pathol Lab Med. 2015;139:11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dailey PJ, Elbeik T, Holodniy M. Companion and complementary diagnostics for infectious diseases. Expert Rev Mol Diagn. 2020;20:619-636. [DOI] [PubMed] [Google Scholar]
- 36. Schluckebier L, Caetano R, Garay OU, et al. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer. 2020;20:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cree IA. Progress and potential of RAS mutation detection for diagnostics and companion diagnostics. Expert Rev Mol Diagn. 2016;16:1067-1072. [DOI] [PubMed] [Google Scholar]
- 38. Doostparast Torshizi A, Wang K. Next-generation sequencing in drug development: target identification and genetically stratified clinical trials. Drug Discov Today. 2018;23:1776-1783. [DOI] [PubMed] [Google Scholar]
- 39. Mansfield AS, Park BH, Mullane MP. Identification, prioritization, and treatment of mutations identified by next-generation sequencing. Am Soc Clin Oncol Educ Book. 2018;38:873-880. [DOI] [PubMed] [Google Scholar]
- 40. Koelzer VH, Sirinukunwattana K, Rittscher J, Mertz KD. Precision immunoprofiling by image analysis and artificial intelligence. Virchows Arch. 2019;474:511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah P, Kendall F, Khozin S, et al. Artificial intelligence and machine learning in clinical development: a translational perspective. NPJ Digit Med. 2019;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Washington P, Park N, Srivastava P, et al. Data-driven diagnostics and the potential of mobile artificial intelligence for digital therapeutic phenotyping in computational psychiatry. Biol Psychiatr Cogn Neurosci Neuroimaging. 2020;5(8):759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaufmann M, Keppens M, Blair ED. A perspective analysis: companion diagnostics: an evolving paradigm in 21st century healthcare. Per Med. 2015;12:389-402. [DOI] [PubMed] [Google Scholar]
- 44. Keeling P, Clark J, Finucane S. Challenges in the clinical implementation of precision medicine companion diagnostics. Expert Rev Mol Diagn. 2020;20:593-599. [DOI] [PubMed] [Google Scholar]
- 45. Lütkemeyer D, Heese HS, Wuttke DA. Overcoming inefficiencies in the development of personalized medicine. Eur J Oper Res. 2021;290:278-296. [Google Scholar]
- 46. Agarwal A, Ressler D, Snyder G. The current and future state of companion diagnostics. Pharmacogenomics Pers Med. 2015;8:99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peterson RE, Kuchenbaecker K, Walters RK, et al. Genome-wide association studies in ancestrally diverse populations: opportunities, methods, pitfalls, and recommendations. Cell. 2019;179:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. FDA. FDA drug safety communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug, Published March 2017. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-reduced-effectiveness-plavix-clopidogrel-patients-who-are-poor
- 49. Alyass A, Turcotte M, Meyre D. From big data analysis to personalized medicine for all: challenges and opportunities. BMC Med Genomics. 2015;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joyner MJ, Paneth N. Seven questions for personalized medicine. JAMA. 2015;314:999-1000. [DOI] [PubMed] [Google Scholar]
- 51. Parkinson DR, Johnson BE, Sledge GW. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin Cancer Res. 2012;18:619-624. [DOI] [PubMed] [Google Scholar]
- 52. Reavie L. Four important considerations for the successful development of an in vitro or companion diagnostic test. Published November 2018. https://www.biopharma-excellence.com/news/2018/11/21/four-important-considerations-for-the-successful-development-of-an-in-vitro-or-companion-diagnostic-test
- 53. IVDR. The European Parliament and the Council of the European Union: Regulation (EU) 2017/746 of the European Parliament and of the Council on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU. European Union; 2017. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0746 [Google Scholar]
- 54. FDA. Guidance for industry and FDA Staff: in vitro diagnostic (IVD) device studies – frequently asked questions, Published June 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-diagnostic-ivd-device-studies-frequently-asked-questions
- 55. FDA. FAQs about investigational device exemption. Published May 2019. https://www.fda.gov/medical-devices/investigational-device-exemption-ide/faqs-about-investigational-device-exemption
- 56. FDA. US Food and Drug Administration. Medical devices. Classify your medical device [Code of Federal Regulations] [Title 21, Volume 8] [Revised as of April 1, 2012] [CITE: 21CFR864.1860 [CITE: 21CFR864.1860]. Published 2014. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/default.htm
- 57. FDA. US Food and Drug Administration. Overview of IVD regulation. Published 2019. https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation#:~:text=Back%20to%20top-,How%20are%20IVDs%20classified%3F,determines%20the%20appropriate%20premarket%20process
- 58. Code of Federal Regulations. Title 21: Food and drugs; part 814 – premarket approval of medical devices. Published 2002. https://www.govinfo.gov/content/pkg/CFR-2002-title21-vol8/xml/CFR-2002-title21-vol8-part814.xml
- 59. FDA. Humanitarian device exemption. Published 2019. https://www.fda.gov/medical-devices/premarket-submissions/humanitarian-device-exemption
- 60. FDA. Draft guidance for industry and FDA staff: principles for codevelopment of an in vitro companion diagnostic device with a therapeutic product. Published 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/principles-codevelopment-vitro-companion-diagnostic-device-therapeutic-product
- 61. Mbunkah HA, Reinhardt J, Kafere C, Scheiblauer H, Prat I, Nübling CM. In Vitro Diagnostics for Screening the Blood Supply: The New European Regulation for IVD and the WHO IVD Prequalification Programme. Vox Sang; 2020. [DOI] [PubMed] [Google Scholar]
- 62. Ritzhaupt A, Hayes I, Ehmann F. Implementing the EU in vitro diagnostic regulation - a European regulatory perspective on companion diagnostics. Expert Rev Mol Diagn. 2020;20:565-567. [DOI] [PubMed] [Google Scholar]
- 63. MDCG 2020-16. Guidance on classification rules for in vitro diagnostic medical devices under regulation (EU) 2017/746. Published 2020. https://ec.europa.eu/health/sites/default/files/md_sector/docs/md_mdcg_2020_guidance_classification_ivd-md_en.pdf
- 64. Bank PCD, Jacobs LHJ, van den Berg SAA, et al. The end of the laboratory developed test as we know it? Recommendations from a national multidisciplinary taskforce of laboratory specialists on the interpretation of the IVDR and its complications. Clin Chem Lab Med. 2020;59:491-497. [DOI] [PubMed] [Google Scholar]
- 65. Barberis M. In vitro diagnostic medical device regulation (IVDR): the end of laboratory developed tests (LDT)? Pathologica. 2021;113:68-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vermeersch P, Van Aelst T, Dequeker EMC. The new IVD regulation 2017/746: a case study at a large university hospital laboratory in Belgium demonstrates the need for clarification on the degrees of freedom laboratories have to use lab-developed tests to improve patient care. Clin Chem Lab Med. 2020;59:101-106. [DOI] [PubMed] [Google Scholar]
- 67. Xu X, Li J, An J. Understanding the current regulatory landscape for companion diagnostic products in China. Companion Complement Diagn. 2019;335-364. [Google Scholar]
- 68. TGA. Cost recovery impact statement – regulation of in-vitro diagnostic devices. Published 2006. https://www.tga.gov.au/sites/default/files/fees-cris-ivd-060606.pdf
- 69. Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Notification on approval application for in vitro companion diagnostics and corresponding therapeutic products. Published 2013. https://www.pmda.go.jp/files/000153914.pdf
- 70. Nagai S, Urata M, Sato H, et al. Evolving Japanese regulations on companion diagnostics. Nat Biotechnol. 2016;34:141-144. [DOI] [PubMed] [Google Scholar]
- 71. Olsen D, Jørgensen JT. Companion diagnostics for targeted cancer drugs – clinical and regulatory aspects. Front Oncol. 2014;4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mattes WB, Goodsaid F. Regulatory landscapes for biomarkers and diagnostic tests: qualification, approval, and role in clinical practice. Exp Biol Med. 2018;243:256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Technical guidance on development of in vitro companion diagnostics and corresponding therapeutic product. Published 2013. https://www.pmda.go.jp/files/000153149.pdf
- 74. World Health Organization. Eligibility criteria for WHO prequalification of in vitro diagnostics. Published 2019. https://apps.who.int/iris/bitstream/handle/10665/259170/WHO-EMP-RHT-PQT2017.03-eng.pdf?sequence=1&ua=1
- 75. Govaerts L, Simoens S, Van Dyck W, Huys I. Shedding light on reimbursement policies of companion diagnostics in European countries. Value Health. 2020;23:606-615. [DOI] [PubMed] [Google Scholar]
- 76. Govaerts L, Waeytens A, Van Dyck W, Simoens S, Huys I. Evaluation of precision medicine assessment reports of the Belgian healthcare payer to inform reimbursement decisions. Int J Technol Assess Health Care. 2020;36:1-8. [DOI] [PubMed] [Google Scholar]