Abstract
A novel nanomaterial, bacterial cellulose (BC), has become noteworthy recently due to its better physicochemical properties and biodegradability, which are desirable for various applications. Since cost is a significant limitation in the production of cellulose, current efforts are focused on the use of industrial waste as a cost-effective substrate for the synthesis of BC or microbial cellulose. The utilization of industrial wastes and byproduct streams as fermentation media could improve the cost-competitiveness of BC production. This paper examines the feasibility of using typical wastes generated by industry sectors as sources of nutrients (carbon and nitrogen) for the commercial-scale production of BC. Numerous preliminary findings in the literature data have revealed the potential to yield a high concentration of BC from various industrial wastes. These findings indicated the need to optimize culture conditions, aiming for improved large-scale production of BC from waste streams.
Keywords: bacterial cellulose (BC), biopolymer, industrial waste, microbial cellulose, carbon source, nitrogen source
1. Introduction
As a novel nanomaterial, Bacterial Cellulose (BC) has continued to draw scholarly interests since it was first discovered due to its unique properties, such as a high degree of purity, biodegradability, biocompatibility, and ease of polymerisation [1,2], making BC a material with a wide range of applications including skin and bone tissue engineering, barrier technology, and electrical, electrochemical, and sensing applications [3,4,5,6,7,8]. Despite offering many beneficial properties, its expensive production cost bounds its industrial-scale application. Conventionally, producers utilize fructose and glycerol as conventional carbon sources, however, the costs of these materials are remarkably high. A growing research body studies methods of minimizing the BC production cost. However, it has ended up with unconvincing and inadequate findings [9]. Recent research on reducing the production costs has emphasized utilizing waste products for sources of carbon or nitrogen. At present, active research to investigate the cost-effectiveness of BC synthesis from different waste products is ongoing and needs to be elaborated.
Nevertheless, the literature analysis compiles crucial developments in the field and, hence, enables the assessment of the future practicability of this manufacturing of BC for various applications [10,11,12]. The feasibility of using waste in BC production is examined in this paper through an extensive literature review to strengthen the current phase of knowledge and analyse discernible trends and gaps in inexperience. Many industrial wastes are rich in carbon and nitrogen content; hence, utilizing them as substrates may yield high microbial cellulose concentrations with the optimization of culture conditions.
2. Overview of Bacterial Cellulose (BC) and Its Applications
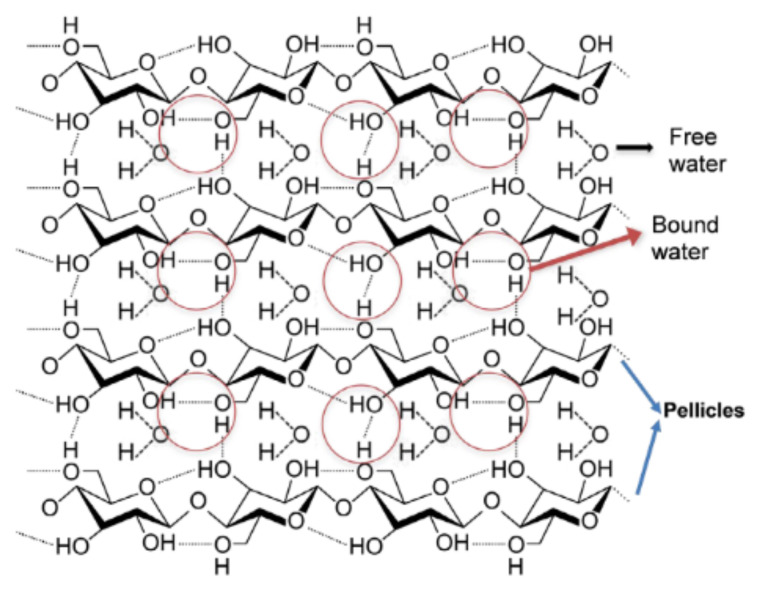
Bacterial cellulose (BC), commonly known as biocellulose, which is the purest form of cellulose, continues to receive widespread focus due to its superior physicochemical properties compared to plant cellulose, in which impurities such as hemicellulose and lignin are often found [13,14,15,16,17,18,19,20,21]. Some of the superior physicochemical properties of BC include high tensile strength, crystallinity, and water holding capacity (WHC), as well as a slow water release rate (WRR) and remarkable moldability into three-dimensional structures [22]. The water molecules are bonded through hydrogen bonds within the complex structure of BC. The unbonded free water molecules will penetrate and exit the BC molecular structure, as shown in Figure 1 [23].
Figure 1.
Schematic of the molecular structure of bacterial cellulose and its bound and free water [23].
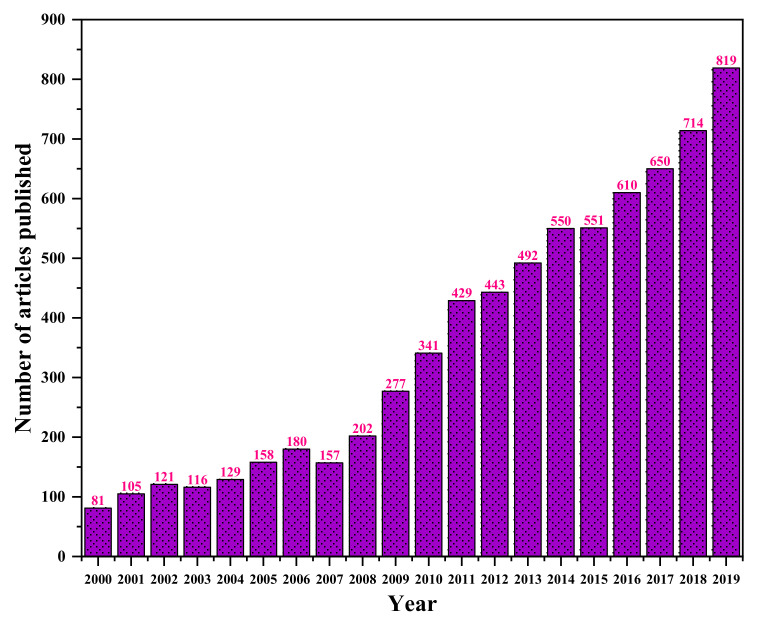
Bi et al. [24] characterized the BC synthesized from different strains in agitated culture. The macrostructure morphology of BC varied depending on the different culture methods [25]. The research used isolated bacteria, namely Komagataeibacter nataicola Y19 (BC-1) and Gluconacetobacter entanii ACCC10215 (BC-2). Both bacteria were fermented in Hestrin–Schramm medium. The BC morphology result depicts that both samples have different sizes and shapes, as shown in Figure 2. Figure 2a,b illustrates the optical image of BC-1 and BC2, while Figure 2c,d shows the morphology of BC samples. The BC-1 shows the flocky asterisk-like and solid sphere-like for BC-2. In addition, based on Pang et al. [7], BC is useful as a natural renewable polymer in many fields due to its versatility and numerous notable properties such as biocompatibility, chirality, structure-forming potential, hydrophilicity, high crystallinity, high purity, a high degree of polymerization, high porosity, large specific area, favourable permeability, flexibility, hygroscopicity, and biodegradability. BC is produced as extrusions of glucose chains from the bacterial body via small pores present on their cell envelope. These extrusions then form microfibrils that further aggregate into web-shaped cellulose ribbon networks with many empty spaces between the fibres. The well-separated non-fibrils of BC create an expanded surface area and highly porous matrix. The basic fibril structure contains a β-1→4 glucan chain with the molecular formula (C6H10O5)n and is held together by hydrogen bonds. These microfibrils are approximately 100-fold smaller than the fibrils of vegetal cellulose [26]. Until recently, much research was done on the production of BC and its modification and applications in various fields. As displayed in Figure 3, the number of BC publications has increased since 2000 from 81 to 819 publications.
Figure 2.
Optical images (a,b), scanning electron microscope (SEM) images (c) of BC samples and ultrastructural transmission electron microscopy (TEM) images (d) of BC samples [24,27,28].
Figure 3.
Number of publications on bacterial cellulose since 2000–2020 (Scopus search engine system, the search term “bacterial cellulose”).
The unique macro-physical and outstanding thermal and mechanical properties of BC make it an ideal material to be applied in various fields of applications (Figure 4). BC possesses good thermal stability and low or no chronic inflammatory response, which has attracted huge attention for BC as a novel functional material in applications such as nonwoven fabric-like products and paper [29]. BC is also used as a binder in advanced paper technology due to its nano-sized structure, a property that significantly improves the durability and strength of pulp when reinforced into paper [30]. One of the main reasons it is being used in biomedicine is its excellent biocompatibility [14]. In addition, the weight-average degree of polymerization (DPw) of BC is high, such as the DPw of BC produce by Acetobacter xylinum BPR2001, which remained in the range of 14,000 of 16,000 [7,31]. BC possesses nanofibrillar and ultrafine structured material with an excellent combination of properties such as high flexibility and tensile strength (Young modulus of 114 GPa) [32], as well as high crystallinity (84–89%) [33]. Therefore, due to its outstanding mechanical properties, BC nanocomposites had been fabricated by reinforcing it with other polymers to be developed in various applications, including paper [29], treating tympanic membrane perforation [34,35], shielding film [36], food packaging films [37], audio speaker diaphragms [38], and so on. Development of BC for paper products had been actively conducted by Ajinomoto Corporation along with Mitsubishi Paper Mills in Japan since 1995 (JP patent 63295793) [39].
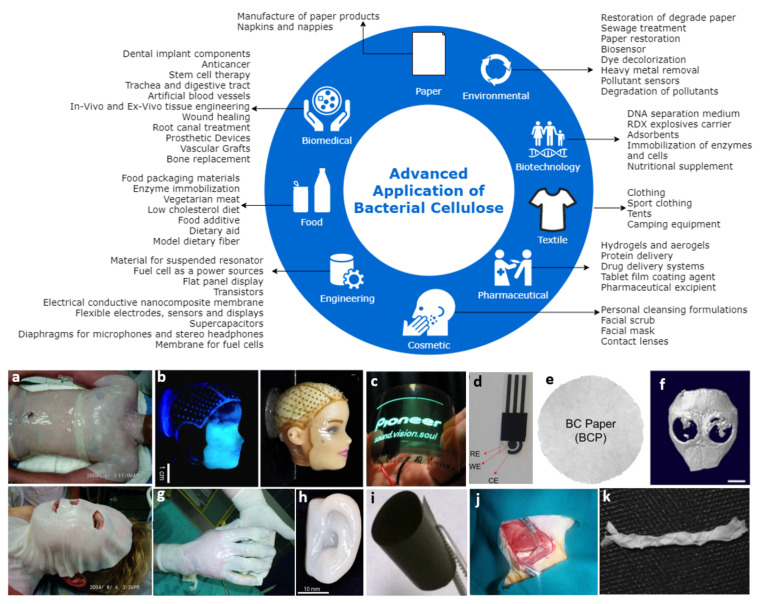
Figure 4.
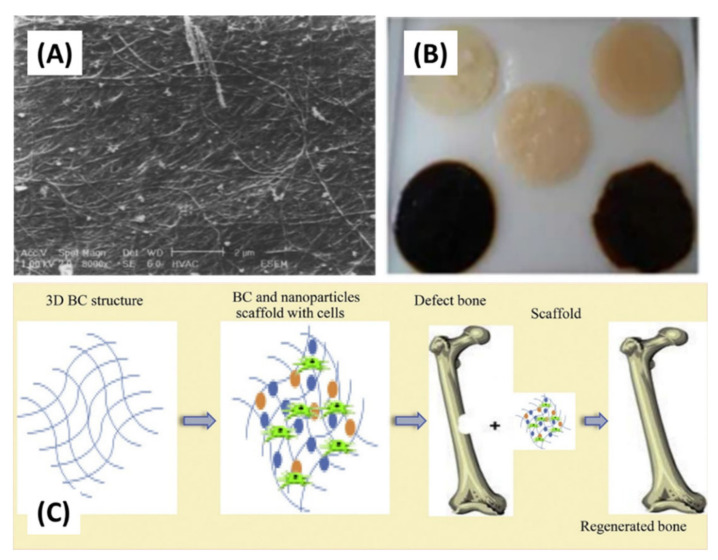
Advanced application of bacterial cellulose (BC). (a) A never-dried microbial cellulose membrane shows remarkable conformability to the various body contours, maintains a moist environment, and significantly reduces pain [74]. (b) A doll face was scanned, and a 4.5 wt % Flink containing A. xylinum was deposited onto the face using a custom-built 3D printer. In situ cellulose growth leads to the formation of a cellulose-reinforced hydrogel that, after removal of all biological residues, can serve as a skin transplant [75]. (c) Luminescence of an organic light-emitting diode deposited onto a flexible, low-CTE, and optically transparent cellulose nanocomposite [76]. (d) Screen-printed electrodes made on BC substrate [77]. (e) BC paper [78]. (f) Bone regeneration efficacy of the scaffolds [79]. (g) Microbial cellulose dressing applied on a wounded hand [80]. (h) 3D Bioprinting Human Chondrocytes with nanocellulose−alginate bioink [81]. (i) Flexible freestanding nanocellulose paper-based Si Anodes for Lithium-ion batteries [82]. (j) Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue [83]. (k) Artificial Bacterial cellulose ligament or tendons [84].
Due to the high porosity combined with a large specific area of three-dimensional structure, research on BC has opened up opportunities for it to be used as a photocatalyst [40], electronic sensing platform [41], and biosensing material [42,43] (Figure 5). BC has also been used widely in biomedical applications such as wound-dressing [44,45,46,47] (applied on the wounded torso, hand, and face) and cell culture [48,49,50,51] because of its excellent flexibility, high mechanical strength at wet state, water holding capacity, very low risk for irritation due to its ultra-high purity, hygroscopicity, liquid/gasses permeability, and ease of wound inspection due to its transparency. Biopolymer such as polylactic acid (PLA), starch, polyhydroxyalkanoate (PHA) [52,53,54,55,56], and synthetic polymer such as polyvinyl alcohol (PVA) and unsaturated polyester (UP) [57,58] are potential polymers to be reinforced with BC. The outstanding properties of BC such as biodegradability, good controllability during BC production, and possessing net-like morphology that is almost similar to human collagen as a biomimetic feature makes it favoured in the medical field and has been widely utilized in controlled drug delivery [59], medical pads [41], artificial skin [7,60], cartilage [61] and bone [62,63], bone tissue engineering scaffolds [64,65,66], hormones [72], and nerve guides for spinal cord injury treatment [73]. vascular grafting [67,68], blood vessel tubes [69,70], dental implant [71], proteins and hormones [72], and nerve guides for spinal cord injury treatment [73].
Figure 5.
Experimental images of (A) clay-needle template with needles at the centre, (B) growing BC scaffolds with aid from clay-needle templates in static cultures, and (C) clean BC pellicles with channels to be an effective hydrogel-like material for different tissue engineering applications. (D) Enlargement of the channel area in (C). The channel diameter was approximately 250 μm and the inter-distance approximately 1 mm. (E) Scanning electron images (SEM) of channeled area in (C). (F) Cross-section of channels [85].
BC possess large surface areas and have the capability to absorb liquids. Hence, a small amount of BC can be utilized for producing coating, thickening, and binding agents, especially in the food industry. Remarkably, in 1992, BC was categorized as ‘‘generally recognized as safe’’ (GRAS) by the USA Food and Drug Administration (FDA) and, hence, is suitable to be used in food industry applications [86]. The largest industrial-scale production of BC that has been produced so far is led by Cetus Co. (Emeryville, CA, USA) and Weyerhaeuser Co. (Tacoma, Washington, DC, USA). Both companies develop a Cellulon, a bulking agent with a wide range of applications such as in coating, binding, and thickening applications [87]. Besides that, BC also can be used in the oil and gas recovery sector, cosmetics, adhesives, paints, and mining. High-end audio speaker systems had been fabricated by Sony Corporation using BC. This might be due to its good acoustic properties [88].
Food packing, battery separator, transparent coating or film, pharmaceutical industries, adsorbent, cosmetics, water treatment, ethanol production, biomaterials, artificial blood vessels, electric conductors or magnetic materials, and scaffolds for tissue engineering are examples of uses of BC in industrial and medical areas [10,90]. This can be observed in Figure 6. Besides that, BC has been utilized in biomedical applications such as scaffolds and ex-situ and in situ modified through different processes [91]. The culture conditions are modified with additives or reinforcement materials via in situ modification, whereas the modification of ex-situ is performed after BC harvest. Incorporation of the additive materials can be added into a growing BC microfibril for the preparation of BC composites with required properties. A biocomposite is a material composed of two or more distinct constituent materials (one being naturally derived) which are combined to yield a new material with improved performance over single constituent materials [92,93,94,95]. This modification type can be employed in a static method for the purpose of control of properties, shape, and structure of modified BC. This application is mostly applied in bone tissue engineering, in which, in order to produce BC scaffolds with microporous structure, paraffin wax microspheres were added into culture medium via an in situ modification technique [96].
Figure 6.
(A) Network structure of ribbon-shaped fibrils of BC, (B) natural biomaterial of BC, and (C) 3D-shaped BC for bone tissue engineering [87,89].
Gonçalves-Pimentel et al. [97] conducted experiments on BC as a support for the growth of retinal pigment epithelium, showing that all surface-modified BC substrates showed similar permeation coefficients with solutes of up to 300 kDa. Surface modification of BC greatly improved the proliferation and adhesion of retinal pigment epithelium cells. All samples showed. Insignificant stress−strain behaviour was observed in all samples, of which acetylated BC showed the highest elastic modulus; however, after some period, it exhibited a slightly smaller tensile strength and elongation at break as compared to control BC [98]. A study conducted by Buruaga-Ramiro [78] on the suitability of BC matrices to prepare enzymatically active nanocomposites shows improvement in durability, reusability, and thermal stability of BC/lipase nanocomposites (Figure 7). Besides that, the enzyme immobilised onto BC/lipase nanocomposites paper retained 60% of its activity after 48 h at 60 °C. The results attained suggest that BC/lipase nanocomposites are promising biomaterials for the development of green biotechnological devices with potential applications to be used as part of biosensor devices with applications in many fields such as food quality control, environmental monitoring, and clinical diagnosis.
Figure 7.
Schematic diagram to explain the approach for bacterial cellulose matrices production [78].
The effect of BC on disintegrability in composting conditions of plasticized polyhydroxybutyrate (PHB) nanocomposites [99] shows that the compounds with BC and plasticizer presented a similar behaviour to that of control plasticized PHB. This might be due to the low dispersion and low interfacial adhesion of BC in the matrix. However, the crystallinity of PHB nanocomposites was increased. Another study conducted by Zhang et al. [100] on the reinforcement of BC with polyvinyl alcohol (PVA) coated with Bichar-Nanosilver (C-Ag) antibacterial composite membranes, shows that the BC was homogeneously mixed into the PVA gel and that the C-Ag particles were uniformly immobilized in the PVA/BC hybrid composites membrane. These hybrid composites show excellent antibacterial activity against Escherichia coli and good reusability to be used as drinking water treatment applications. Hamedi et al. [101] conducted experiments on double-network antibacterial hydrogel based on aminated BC and schizophyllan (SPG) biopolymer nanocomposites. A novel hydrogel composed of BC/SPG biopolymers shows an improvement in antibacterial, swelling, and mechanical properties. MTT assay displayed that amine-grafted BC/SPG stimulated the proliferation of normal human fibroblast cells. They concluded that this novel nanocomposite can be utilized in diverse areas such as anti-wrinkle dressing masks, wound healing, and absorption biomaterial for water treatment applications.
A hybrid of BCNCs–AgNPs/alginate–MoO3NPs was effectively developed for H2S gas sensors [102]. In this study, BC was produced by Gluconacetobacter xylinus strain under static culture. The bionanocomposites film was successfully fabricated using a solution casting method and has the ability to detect H2S gas emission. Through the shift in the oxidation number of MoO3NPs, the colour of the film was changed. Once activated by AgNPs, MoO3NPs were readily reduced to a coloured sub-oxide by atomic hydrogen that was produced and received from the reaction of H2S gas [102].
Cazón et al. [3] conducted a study on BC reinforced polyvinyl alcohol (PVOH) composite film with eco-friendly UV-protective properties. The addition of PVOH shows improvement in mechanical and transparency properties and reduced the water vapour permeability of composite films. Thus, they concluded that these novel composite films have huge advantages to be utilized in the food industry to prevent oxidation of proteins, lipids, and vitamins, as well as the degradation of antioxidants in foods. Besides that, it can be s substitute novel material to antioxidants to increase food shelf-life as well as to maintain the quality of food products [3]. AgNP produced using CUR:HPβCD (cAgNP) reinforced with BC-based hydrogels for wound dressing applications has been developed by Gupta et al. [103]. The composites show high cytocompatibility between cAgNp and BC with high moisture content and a good level of transparency. These hydrogels-based composites also showed broad-spectrum antimicrobial activity along with antioxidant properties.
In terms of electrical applications, there are a few applications of BC, as bioelectrical devices are hard to fabricate. However, several previous works have been conducted. According to Di Pasquale et al. [104], electrodes of the sensor are made of BC that has been treated with ionic solutions and coated with conducting polymers. The mechano-electric transduction properties of the composite are used to create a generating sensor. The device, which is placed in a cantilever arrangement, is used to detect anchor acceleration. On the other hand, Di Pasquale and co-researchers are testing an all-organic Bacterial Cellulose-Conducting Polymer (BC)-PEDOT:PSS composite soaked with Ionic Liquids (ILs) as a mass sensor [104]. As a result of the applied deformation, the sensor functions as a vibrating transducer in a cantilever arrangement, producing a voltage signal. The effect of the additional mass on the system’s frequency response is used to estimate the value of the measurand. The sensing system is made of low-cost, flexible, and environmentally friendly components that may be used to create smart ubiquitous sensing systems in the future.
Wang et al. [105] created a novel wound care system that uses an aligned bacterial cellulose (BC)/gelatin membrane in combination with EF stimulation to direct cell migration and improve wound healing. The produced BC/gelatin membranes had a well-aligned fibre structure, a strong mechanical property, a high thermal stability, good light transmittance, foldability, and surface roughness, and great biocompatibility. Especially, the 40% stretched BC/gelatin membrane promoted the adhesion, orientation, and migration of NIH3T3 cells in vitro. For further increase in electrical conductivity and cell survival of polyaniline (PANI) coated BC nanocomposites, BC fibres are chemically functionalized with a poly(4-vinylaniline) (PVAN) interlayer [106]. PVAN was discovered to have increased PANI yield by promoting the creation of a uniform PANI layer with nanofiber- and nanorod-like supramolecular structures. These new electrically conductive BC/PVAN/PANI nanocomposites have the potential to enable a wide range of biomedical applications, including bioelectronic interfaces and the manufacturing of biosensors. Table 1 displays BC and its biocomposites yielded in static and agitation/shaking culture bioreactor and their various applications.
Table 1.
Fabrication of BC and BC-based biocomposites under static and agitation culture methods, their properties, and applications.
| Bacterial Cellulose and Bacterial Cellulose-Based Biocomposites | Applications | Structure and Properties | References |
|---|---|---|---|
| Fabrication of BC and BC-based composites under static culture methods | |||
| BC | BC mask | Fast healing, high moisture donation, and high conformability | Saxena et al. [107] |
| Blood vessel; Vascular grafts | Excellent mechanical properties, thin layers, dense | Putra et al. [108] | |
| Implant material for auricular cartilage regeneration and for ear cartilage replacement | Compatible mechanical strength and patient-specific shapes | Nimeskern et al. [109] | |
| Potential meniscus implant | High compression strain and mechanical strength | Bodin et al. [110] | |
| Replacement of blood vessels and diseased arteries | High water holding capacity and mechanical strength | Charpentier et al. [111] | |
| Artificial blood vessels for microsurgery | The smooth inner surface, moldability, and high mechanical properties | Klemm et al. [112] | |
| Artificial cornea and eye bioengineering Retinal pigment epithelium (RPE) |
High elastic modulus, tensile strength and elongation at break, high initial cell adhesion, porous, permeable up to 300 kDa, and dimensionally stable | Padra et al. [98] | |
| BC/polycaprolactone biocomposites | Tissue substitutes in rabbits’ cornea | Signs of the moderate inflammatory process, pro- tected ocular surface and remained stable in corneal tissue during the 45-day follow-up |
Sepúlveda et al. [113] |
| BC/polycaprolactone (PCL) biocomposites | Biodegradable food packaging | Good transparency of the BC/PCL, smooth surface morphology | Barud et al. [114] |
| BC/benzoyltrifluoroacetone | Phosphors and UV to Visible energy converting devices | Improvement of the luminescence characteristics | Caiut et al. [115] |
| BC/ AgNPs/ lginate-molybdenum trioxide nanoparticles (MoO3NPs) | Hydrogen sulfide (H2S) gas sensor | Successfully detected H2S gas | Sukhavattanakul et al. [102] |
| BC/chitosan biocomposites | Wound dressing | The improved proliferation and fibroblast adhesion | Kim et al. [116] |
| BC/Lipase nanocomposites | Bioactive paper for developing a simple, handheld, and disposable devices; industrials bio- processes of detergents and food industry and biomedicine | Specific activity was higher for BC/ Lipase suspension (4.2 U/mg), improved thermal stability, reusability, and durability | Buruaga-Ramiro [78] |
| BC/ SOD (Procel-Super) and poviargol (Procel-PA) biocomposites | Skin regeneration scaffold; Membranes for skin tissue regeneration | Highly transparency, antibacterial activity | Legeza et al. [117] |
| BC/ PVOH | The food industry, food packaging | Improved mechanical properties; UV-light barrier properties; Reduce WVP and porosity value | Cazón et al. [3] |
| BC/ PHB | Food packaging applications | low dispersion of BC in the matrix; increased crystallinity of the incubated samples; low interfacial adhesion | Seoane et al. [99] |
| BC/ciprofloxacin biocomposites | Contact lens for better tissue regeneration, enhance the recovery of ocular burns, replacement for antibiotics eye drops, wound dressing after eye surgery or protection against bacteria. | No mutagenicity, genotoxicity and cytotoxicity effects | Messaddeq et al. [118] |
| BC/ polyvinyl alcohol coated biochar nanosilver biocomposites | Drinking water treatment | BC was uniformly mixed into the PVA gel; PVA/BC/C-Ag composite membranes exhibited excellent antibacterial activity; good reusability | Zhang et al. [100] |
| BC/polycaprolactone biocomposites | Tissue substitutes in rabbit cornea | High transparency and mechanical properties | Sepúlveda et al. [113] |
| BC/polyvinyl alcohol biocomposites | BC gloves | Skin cell support and fabrication of optimal moist condition | Osorio et al. [119] |
| BC/ cAgNP | Wound dressing | High cytocompatibility; high moisture content and; good level of transparency; broad-spectrum antimicrobial activity along with antioxidant properties | Gupta et al. [103] |
| Fabrication of BC and BC-based composites under agitation/shaking culture method | |||
| BC | Sewage treatment; Immobilized reaction; Adsorption of Pb2+ bio-separation and bovine serum albumin | Porous and loose structure, BC adhering to each other; diameter of composites with a size range of 0.5–6 mm | Zhu, Jia, Yang, et al. [120] |
| The production of immobilized glucoamylase was supported by BC spheres for industrial applications usage | BC spheres were produced with various range of sizes such as 0.5–1.5, 2–3, and 4–5 mm; Large functional group, as well as great surface area to connect with enzymes, resulted to the higher activity of small spheres. | Wu & Li, [121] | |
| For good viability and adhesion on the surface of the material | Sphere formation was affected by temperature; solid structure formed; diameter of composites with a size range of 2–8 mm formed | Hu et al. [122] | |
| Fermentation | IR: 6.52–3.85; Crystallinity: 81.43–84.35 %; Flocky asterisk-like; diameter of composites with a size range of 5–10 mm, | Bi et al. [24] | |
| Food, healthcare, and materials applications | Diameter is less than 1–8 mm at 150 rpm; Form solid structure however the central region is not layered; Layer spacing 10 μm (150 rpm) and 20 μm (125 rpm) | Hu & Catchmark [123] | |
| Good production yield | Thinner microfibrils structure; IR: 4.48; crystallinity: 84%; large and unique spheres; diameter of composites with a size range of 5–10 mm | Czaja et al. [124] | |
| High-efficiency lipase-immobilization system for large-scale industrial hydrolysis of fats and oils Suitable for enzymatic immobilization. | High hydrolytic activity; High operational activity; Lipase immobilized BC sphere; Size of diameter between 3–9 mm. | Cai et al. [125] | |
| Pectin and xyloglucan can be used to enhance cellulose growth and cellulose assembly. | Xyloglucan: Layered structure, densely packed cellulose bundles with the layered structure were formed; Central core is not obviously seen; diameter of composites with a size range of 4–5 mm; aster-likePectin: Densely packed cellulose bundles with layered structure; diameter of composites with a size range of 5–6 mm; aster-like Xylan: Pore structure of cellulose bundles with a few tails formed on the surface of sphere; diameter of composites with a size range of 7–8 mm; layered structure Arabinogalactan: Cellulose linkage between layered structure; diameter of composites with a size range of 4–6 mm; Sphere |
Gu & Catchmark [126] | |
| BC/ schizophyllan (SPG) biopolymers | Anti-wrinkle dressing masks, wound healing and absorption materials | Mechanical, swelling and antibacterial properties were improved; moderate antibacterial activity | Hamedi et al. [101] |
| BC/CNT biocomposites | -- | BC: IR index 2.23, crystallinity 67.2%; snow like structuredBC/CNT composites: IR index 2.56; crystallinity 76.2%, the diameter of composites with a size range of 2–5 mm, rice-like structured | Yan et al. [127] |
| BC/Fe3O4 biocomposites | Elution: Mn2+ > Pb2+ > Cr3+
Superparamagnetic Adsorption: Pb2+ > Mn2+ > Cr3+ |
Iron(II,III) oxide (Fe3O4) particles with a size of 15 nm were distributed uniformly in spheres The diameter of composites with a size range of 3–5 mm |
Zhu, Jia, Wan, et al. [128] |
| BC/GO biocomposites | Superabsorbent for water environmental protection | Superior absorption capacity; Interconnected structure with a honeycomb-like surface pattern; diameter of composites with a size range of 3–7 mm | Hu [129] |
3. Principal Pathways of Cellulose Production
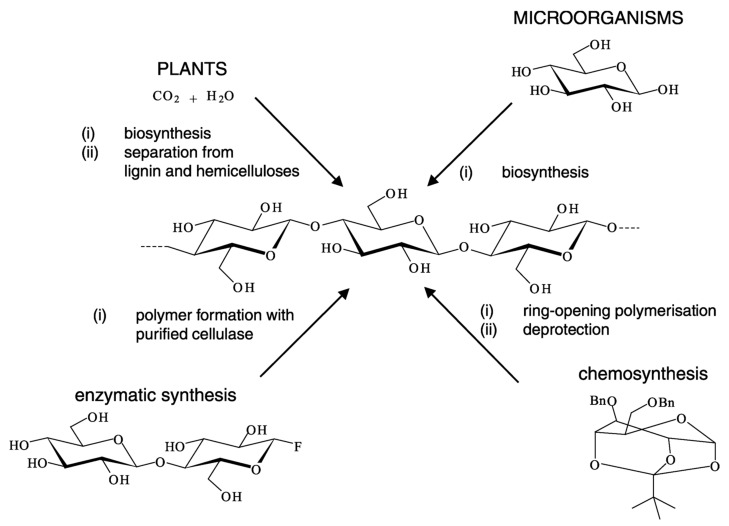
Biopolymer cellulose can be produced using four distinguishing methods, including cellulose extraction, cellulose biosynthesis, enzymatic synthesis, and chemosynthesis. The most well-known method is cellulose extraction from plants, including the elimination of lignin and hemicelluloses using alkali or acid treatments. According to Klemm et al. [112], there are two main sources in cellulose production including plants and microorganisms, as shown in Figure 1. Extensive research has been conducted on the extraction of cellulose fibre from various plant fibre, i.e., sugar palm fibre [130,131,132,133,134,135,136,137,138], water hyacinth [139], ginger fibre [140,141], kenaf [142], sugarcane [143,144], lemongrass [145], cassava, corn, oat, palm oil fibre, and others [146,147,148]. Next is cellulose biosynthesis by using different types of microorganisms; (i) bacteria (gram-negative: Alcaligenes [149], Salmonella, Enterobacter, Pseudomonas [150], Gluconacetobacter xylinus [151], Agrobacterium [152], Komagataeibacter Medellinensis [153], Aerobacter, Achromobacter insuavis [154], Rhizobium leguminosarum [155], Acetobacter spp. [156], Acetobacter xylinum [157], Zoogloea [97], and gram-positive: Sarcina ventriculi [158], Leifsonia sp [159], Rhizosphere bacterium, Bacillus subtilis [70,160]); (ii) fungi (Aspergillus ornatus [161], Penicillium sp. [162,163], Aspergillus terreus MS105 [164], Aspergillus terreus M1 [165], Aspergillus niger, Rhizopus sp. [166,167], Aspergillus niger [168], Trichoderma longibrachiatum [169], Beauveria Bassiana [170], Ascomycota [171,172], or Basidiomycota [173]); (iii) algae (Gelidium elegans [174], Posidonia oceanica [175], Aegagropila Linnaei [176], Komagataeibacter hansenii [177], Cladophora glomerata [178]). However, extracellular synthesized cellulose as fibres is not achievable in some microorganisms. From the scientific viewpoint, the first enzymatic in vitro synthesis was initiated from cellobiosyl fluoride [179,180], and the earliest chemosynthesis was started from glucose via ring-opening polymerization of benzylated and pivaloylated derivatives [181]. These principle paths are schematically described in Figure 8 [112].
Figure 8.
Major pathways to the cellulose [112].
Different modified methods or additives have been applied to enhance BC production. BC gained from bioreactors have been characterized and analysed for structure, shape, and properties of BC, thermogravimetric analysis, density, porosity, yield, water holding capacity, Fourier transform infrared, purity, zeta potential, degree of polymerization, surface area, chemical structure, pore size and distribution, degree of crystallinity, and microstructure, as well as macroscopic morphology [86,182].
4. Fundamentals of Bacterial Cellulose (BC) Production Process
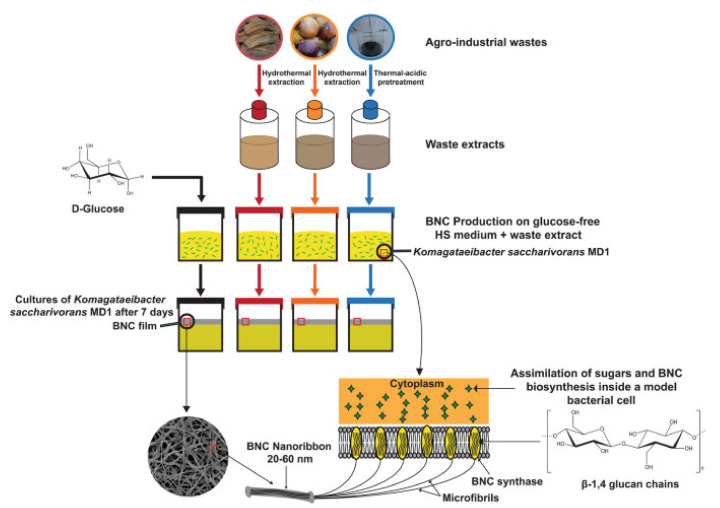
Numerous aerobic and non-pathogenic bacteria yield BC from the genera Gluconacetobacter, Sarcina, Rhizobium, and Agrobacterium either in synthetic or non-synthetic media [22]. However, these bacteria are non-photosynthetic; therefore, they need glucose or organic substrate synthesized by the photosynthetic organism to accumulate their cellulose [183]. BC production comprises fermentation in static or agitated conditions. Among the cultivation media, the most frequently used cultivation medium is a chemically defined medium known as the Hestrin–Schramm (HS) medium [22]. This medium involves somewhat expensive additional components, such as peptone, yeast extract, citric acid, glucose, and disodium phosphate, resulting in costly production. According to Abol-Fotouh et al. [184], thermal-acidic pre-treatment was proposed to enhance the characteristics of molasses, boost its (glucose-fructose) content per volume, and remove the majority of contaminants that might stifle microbial development or reduce product output, as shown in Figure 9 [185,186]. The function of thermal acidic pre-treatment of molasses in virtually complete breakdown of the contained sucrose to its original constituents, glucose and fructose, was clarified by Bae and Shoda [187].
Figure 9.
Schematic illustrations of pre-treatment of wastes for BC biosynthesis [184].
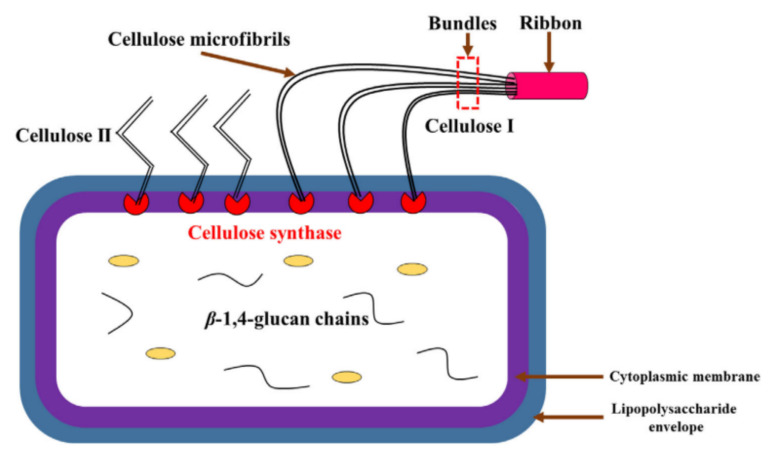
Alteration of growth conditions; temperature, pH, and sources of carbon and their concentrations influenced both the quality and quantity of BC yielded. In addition, different cultivation pathways led to the production of BC with different properties and structures [22]. Figure 10 illustrates the mechanism of bacterial cellulose synthesis from G. oxydans on the surface cell of cellulose [188].
Figure 10.
Representation of cellulose chains formation in microbial cells, and formation of micro- and macro fibrils, bundles, and ribbons [188].
The BC obtained after the fermentation process has yielded good properties for several applications. Stable and efficient bacteria strains will influence the effectiveness of bacterial cellulose (BC) production. The hydroxyl groups in the BC structure have enabled direct modification by introducing other polymers into the BC network [189]. However, some modifications can be done on BC by combining other materials into the polymeric system for a broader range of applications [188]. The modification process can be divided into two main groups, which are in situ and ex-situ modifications. An inadequate supply of oxygen causes bacteria to be inactive, which is a significant constraint in static production environments. Agitated conditions result in higher yields; however, the BC formation mechanism remains uncertain under different conditions [190].
More comprehensive applications of BC depend on practical considerations regarding production costs and scale-up capability. Recently, many studies have focused on cheap nutrient sources, diverse strains of cellulose-producing microorganisms, and supplementary components to produce value-effective BC [26]. Many waste products from different fields, such as whey, industrial waste, wastewater, and agro-industrial waste, have been examined as alternative substrates for the enhanced production of BC. Various additives or modified methods have been used to improve the production of BC. The BC harvested from other bioreactors has been characterized in terms of structure and properties such as macroscopic morphology, microstructure, degree of crystallinity, chemical structure, polymerization degree, purity, water holding capacity, porosity, and thermogravimetric ability [191]. Table 2 shows the BC production specifications, modifications, and advantages of different reactors.
Table 2.
Various modifications, product specifications, and advantages of different reactors for BC production.
| Modification | Production Specification and Advantages | BC Production | References |
|---|---|---|---|
| Enriched Oxygen Bioreactors | |||
|
Bubble column (controlled pH)
Aeration rate:1.0 vvm (30 L/min) |
Attributes: Low concentrated solution state culture; Low shear stress; Low mechanical properties: 17.15 to 11.66 MPa; Low crystallinity: 86 to 79.6%, Low degree of polymerization and molecular weight Advantages: High oxygen transfer rate |
0.07–0.09 g/L/h | Choi et al. [192] |
| High oxygen concentration |
Attribute: After 30 h the production decreased Advantages: Higher productivity; High oxygen transfer rate; Low power requirement. |
0.20 g/L/h | Chao et al. [193] |
| Internal loop airlift with controlled pH/ fresh and glucose medium |
Attribute: The highest concentration: 10.4 g/L at 60–70 g/L fructose Advantages: Formed a unique ellipse; Low mechanical strength; High hydrodynamic characteristic; High volumetric oxygen transfer |
0.22 g/L/h | Chao et al. [194] |
| Internal loop airlift with enriched oxygen |
Advantages: Unique ellipse was formed; High hydrodynamic characteristic; High volumetric oxygen transfer |
0.116 g/L/h | Chao et al. [195] |
| Shaking flask with controlled pH/ Hestrin & Schramm medium |
Attribute: A membrane-type BC was produced Advantages: Varying the net plates number would result in high Young’s modulus and water holding capacity |
- | Wu and Li [121] |
| Rotating disc bioreactors | |||
| A rotating disk bioreactor |
Attribute: A consistent product Advantages: Produced strong and intact cellulosic matrix, BC pore size of 10–15 μm; High tensile strength |
- | Mormino & Bungay [196] Zahan et al. [197] |
| Rotating disk bioreactor supported by plastic composites |
Attribute: A semi-continuous process Advantages: Low mechanical property (Young’s modulus of 372.5 MPa); Low crystallinity: 66.9%; similar thermostability and water content with BC produced by static culture |
0.01 g/L/day | Lin et al. [198] |
| Rotating disk bioreactor with different additions supported by plastic composites |
Attribute: A semi-continuous process Advantages: Similar strain but lower stress for carboxymethylcellulose and avicel, respectively; High water retention properties of 98.6–99%; Disc rotation speed and oxygen concentration improved the fermentation process; Fructose concentration was decreased from 50 to 10 g/L; No re-inoculation |
0.64 g/slice with 0.8% carboxymethylcellulose and avicel | Lin et al. [199] |
| Rotating magnetic field |
Advantages: Yield BC with an altered degree of porosity and microstructure; Increased biochemical properties; Positive impact on the growth of bacteria; Increased water retention by 26% as compared to the control sample; high density with tangled and long fibres |
- | Fijałkowski et al. [200,201,202,203] |
| Other bioreactors for BC production | |||
| Spin filter supporting bioreactor |
Advantages: BC concentration was increased from 5.65 to 11.52 g/L/140 h; An abundance of Cel + cells were converted into Cel- mutants |
0.02 to 0.06 g/L/h | Jung et al. [204] |
| Fed-batch principle |
Advantages: The gradient of a graph in the load-displacement diagram: (aerosol bioreactor = 34.7 N/10 mm, usual surface culture = 8.9 N/10 mm); High tensile strength: 114 N; High-quality cellulose; the degree of polymerization of BC is 5200; Best time interval: 6 h; BC layer or slices (3–4 cm); Culture box: low cost |
- | Hornung et al. [205] |
| Biofilm reactor |
Advantages: High crystallinity: 93% with a crystal size of 5.2 nm; high biomass density; Water retention ability up to 95 %; better thermal performance |
7.05 g/L | Cheng et al. [206] |
| Biofilm reactor with additives |
Advantages: Continuous BC production; High biomass density; High Young’s modulus and tensile strength; High crystallinity: 80% with a crystal size of 4.2 nm; potential application of BC paper sheets |
13 g/L | Cheng et al. [207] |
5. Industrial Waste Streams as Feedstock for the Production of Bacterial Cellulose
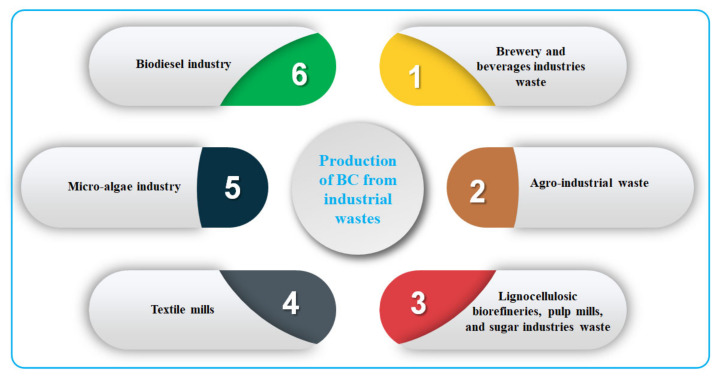
Industrial-scale applications of BC manufacturing encountered some drawbacks such as high culture medium-cost as well as low yield production. In the fermentation process, the cost of the medium for the cultivation of BC accounts for 50–65% of the total production. Thus, according to Velásquez-Riaño et al. [208] and Vazquez et al. [190], the establishment of a cost-effective culture medium for optimum product yield is important in order to enhance the process of fermentation. Some hard work has been done to minimize the production cost of BC, such as using the low-cost medium for BC cultivation and accessible and renewable sources of the nutrient. Over the last twenty years, significant global energy, environmental, and economic concerns have set the prominence of accessible and sustainable utilization of various industrial wastes such as agro-industrial waste products, wastewater treatment plants of dairy industries, brewery and beverages industries waste, waste from textile mills, waste from the micro-algae industry, etc. The innovations in clean and green technology techniques, as well as biotechnological methods, have equipped scientists and researchers with platforms for renewable natural sources consumption, i.e., using industrial waste to produce BC. The utilization of these industrial wastes for BC production helps prevent disposal and environmental pollution, aid in waste management, and, hence, reduce industrial waste disposal costs. From this novel approach, the production of BC from the industrial wastes can be categorized into six individual industrial wastes as illustrated in Figure 11: (1) brewery and beverages industries wastes; (2) agro-industrial wastes; (3) lignocellulosic biorefineries, pulp mills, and sugar industries wastes; (4) textile mills; (5) micro-algae industry wastes; (6) biodiesel industry wastes.
Figure 11.
Schematic overview of Bacterial Cellulose (BC) production from different industrial wastes.
The examples are a small number of possible limitations of more lignocellulosic, sugar, brewery, and other industrial wastes as media without any additional nutrient source or as nitrogen and carbon sources with an additional nutrient source for the production of BC. Among all industrial waste, agro-industrial wastes are seen as highly potent and can be extensively utilized for producing BC. This might be due to the higher BC productivity and large-scale accessibility. Besides that, municipal waste is becoming a progressively more prominent source of biomass waste with high organic content, especially carbon, as a result of fast urbanization around the world, especially in developing countries [209,210]. The potential of upscale production of BC at a large scale or industrial scale from all the low-cost industries waste media are elaborated here; specifically, those that do not require complicated or complex supplementation, detoxification, and pre-treatments. Currently, the production of BC from industrial waste media has been observed to have comparable yield, physical, physico-chemical, crystallinity, and mechanical properties compared to standard media.
Industrial waste is a rich source of carbon for the bacterial synthesis of cellulose. In the past few decades, the urge to achieve ‘zero waste’ in the industrial sector has led many researchers to utilize industrial waste and byproducts as potential nutrient sources for microbial cultivation. Of many such industries, wastes and byproducts from the food, agriculture, and brewery industries are the most commonly utilised and can be a rich source of carbon [86]. All confectionery products are made of varying amounts of sugar and sugar substitutes. Since waste from confectionaries is rich in carbohydrates, this suggests that it can yield substantial amounts of carbon [211].
In terms of efficacy of BC produced by different utilized wastes, numerous researchers have extensively studied this issue. Kongruang [212] mentioned that agro-industries waste is richer in proteins, carbohydrates, and trace elements. Thus, it resulted in a higher BC productivity. Furthermore, Goelzer et al. [213] had stated that brewery and beverage industries waste mainly affects BC production, and similar influence can be seen from other different wastes such as wastewater sugar industries [214,215], lignocellulosic biorefineries wastes [216,217,218], and micro-algae biomass industries waste [177]. The results showed that utilizing pre-treated orange peel medium produced seven times more BC than using standard (HS) medium. However, structural research revealed that BC made from various wastes had a thicker and denser pack of nanofibrils, but FTIR spectra revealed no significant differences [214]. Plus, according to Fan et al. [216], in comparison to BC produced from HS medium, BC created from waste medium had no significant variations in microstructure, features, FTIR peaks, crystallinity index, or color parameter. These observations are in line with results discovered by Qi et al. [218], who found that the BC samples obtained from these hydrolysates had similar physico-chemical structural characteristics (microscopic morphology, functional groups, and crystallinity), but had a high water holding capacity and low mechanical strength. Furthermore, the physico-chemical characteristics of BC generated in various media were similar. However, when compared to HS medium, the viscosity of BC formed from molasses medium is low [217]. As a result, it may be inferred that agricultural wastes from all over the world can be used as a low-cost, readily available, and abundant feedstock for BC production.
Furthermore, fiber/textile industry waste derived hydrolysate was used as growth medium for BC production and the results showed 83% higher yield (10.8 g/L) and 79% higher tensile strength (0.070 MPa) of BC as compared to the production by glucose-based HS medium [215]. Cotton-based textile wastes were treated with the ionic liquid 1-allyl-3-methylimidazolium chloride before being hydrolyzed with enzymes. This resulted in a decreasing sugar concentration of 17 g/L in the hydrolysate. Because the natural sources used in the fiber/textile sector are often high in cellulose content, the wastes generated can be used to produce a variety of value-added products such as BC after detoxification and hydrolysis treatments [219,220].
Research by Costa et al. [26] revealed that industrial debris waste, namely sugarcane molasses, corn steep liquor (CSL), and jeans laundry effluent are rich sources of carbon and nitrogen that maintain BC production using Gluconacetobacter hansenii [26]. The wastes yielded a high concentration of microbial cellulose; however, the main limitation of the study was a substantial deformation observed in the product. According to Gao et al. [221], the cracks in the polymer might be due to the existence of crazing at the tip of the crack during tensile testing. Besides, BC microfibrils and nanofibrils sustained the cracks until rupture occurred [222]. The further analysis exposed that tensile testing caused the fibres to deform, leading to the formation of nodes [223,224].
In another study, Bıyık & Çoban [225] studied the potential of a bacterial strain isolated from a wine sample called Acetobacter pasteurianus for cellulose production using industrial waste and examined its performance with different carbon and nitrogen sources. The results showed that the presence of glucose and yeast extract in the media manufactured the highest quantity of microbial cellulose of 0.45 g/L. Among the industrial wastes (CSL, molasses, and whey), molasses produced the highest amount of BC (0.31 g/L). Further analysis of the structural properties of cellulose using Thin Layer Chromatography (TLC), Scanning Electron Microscopy (SEM), and Carbon-13 NMR revealed similarities in the structural characteristics of the BC with plant cellulose, indicated by the presence of non-branched polymer with D-glucopyranose units bonded with β-1, 4 bonds. Moreover, Voon et al. [226] used Beijerinkia fluminensis WAUPM53 and Gluconacetobacter xylinus 0416 bacteria to produce BC in sago byproducts (SBM), coconut water (CWM), and the standard Hestrin–Schramm mediums (HSM). The highest BC production was recorded in HSM followed by SBM and CWM for about 0.52 g/L, 0.47 g/L, and 0.45 g/L, respectively [226].
5.1. Brewery and Beverages Industries Wastes
There is a rising interest in the production of brewery and beverage industries because of the increasing user demand worldwide. According to Uzuner et al. [227], the beverage industry has become one of the biggest food processing industries. This industry can be categorized into two main groups that are non-alcoholic (i.e., whey, tea, cordial, coffee, apple, lassi, carbonated soft drink, etc.) and alcoholic drinks (i.e., whiskey, wine, beer, etc.). Carbonated soft drinks are consumed the most compared to other drinks, which are consumed at a rate of 48.8 gal/person, followed by bottled water, coffee, and beer with a value of 29.1, 24.6, and 21.8 gal/person, respectively [228]. This industry produces a large volume of waste per day and becomes a concern for management, spurring an effort to reduce the cost of disposal. These wastes are rich in nutrients; thus, they can be used for the biological treatment to produce BC for cost-effective and efficient waste management. Table 3 shows the evaluation of several waste or byproducts generated from the brewery and beverage industries to be used for BC production.
Table 3.
Industrial wastes utilized as sustainable feedstock for the production of bacterial cellulose (BC).
| Microorganism | Production Mode | BC Production | Time | Industrial Waste | Additional Nutrients | References |
|---|---|---|---|---|---|---|
| Beverages/Brewery | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Komagataeibacter xylinus CICC No.10529 | Static | 5.7 g/L | 8 days | Citrus peel and pomace enzymolysis medium | Yeast extract, ethanol and peptone | Fan et al. [216] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 8.00 g/L | 14 days | Grape bagasse | Corn steep liquor | Vazquez et al. [190] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 7.20 g/L | 14 days | Grape bagasse | Diammonium phosphate | |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 0.35 g/L | 3 days | Makgeolli sludge filtrate | Modified HS (MHS) medium | Hyun et al. [267] |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 1.2 g/L | 3 days | Makgeolli sludge filtrate | Mixed modified HS (MMHS) | |
| Gluconacetobacter xylinus BCRC 12334 | Static | 0.90 g/L | 7 days | Thin stillage (TS) wastewater | 50% TS | Wu & Liu [232] |
| Gluconacetobacter xylinus BCRC 12334 | Static | 6.26 g/L | 7 days | Thin stillage (TS) wastewater | 50/50 TS-HS | |
| Gluconacetobacter oboediens | Shaking | 10.8 g/L | 72 h | Distillery effluent | Sucrose (carbon source) and corn steep liquor (nitrogen source) | Jahan et al. [268] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 13.95 g/L | 336 h | Untreated WBFB | 1% Glucose | Ha et al. [269] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Shaking | 1.50 g/L | 168 h | Untreated WBFB | 1% Glucose | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 7.37 g/L | 336 h | Autolyzed WBFB | Glucose | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 3.64 g/L | 336 h | Hydrolysed WBFB | 1% Glucose | |
| Waste as a complex medium without any additional nutrients | ||||||
|
Komagataeibacter saccharivorans strain BC1
(K. saccharivorans strain BC1) |
Static | 1.24 g/L | 8 days | UB breweries limited, Baikampady, Mangalore, India |
- | Gayathri et al. [270] |
| Gluconacetobacter xylinus BCRC 12334 | Static | 3.10 g/L | 7 days | Thin stillage (TS) wastewater | - | Wu & Liu [232] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 4.20 g/L | 14 days | Grape bagasse | - | Vazquez et al. [190] |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 0.30 g/L | 3 days | Makgeolli sludge filtrate | - | Hyun et al. [267] |
| Gluconacetobacter medellinensis ID13488 | Static | 1.5 g/L | 14 days | Fresh apple peel/ sugar cane ratio (w/w) (1/2.3) | - | Urbina et al. [271] |
| Gluconacetobacter medellinensis ID13488 | Static | 1.4 g/L | 14 days | Apple residue (AR)/ sugar cane (SC) ratio (w/w) (1/2.3) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 2.0 g/L | 14 days | AR/SC ratio (w/w) (0.5/2.8) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 1.2 g/L | 14 days | AR/SC ratio (w/w) (2/1.3) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 2.5 g/L | 14 days | AR/SC ratio (w/w) (1.5/2.3) | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 2.9 g/L | 9 days | Black strap molasses | - | Khattak et al. [272] |
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 3.05 g/L | 9 days | Black strap molasses | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 1.70 g/L | 9 days | Brewery molasses | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 1.75 g/L | 9 days | Brewery molasses | - | |
| Gluconacetobacter oboediens | Shaking | 8.5 g/L | 72 h | Crude effluent | - | Jahan et al. [268,273] |
| Acetobacter xylinum NRRL B-42 | Static | 6.7 g/L | 21 days | Grape pomace extract/corn steep liquor | - | Cerrutti et al. [274] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 8.46 g/L | 336 h | Untreated Waste from beer fermentation broth (WBFB) | - | Ha et al. [270] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 2.00 g/L | 336 h | Autolyzed WBFB | - | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 2.82 g/L | 336 h | Hydrolysed WBFB | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 2.40 g/L | 3 days | Hestrin and Schramm (HS) medium | - | Revin et al. [230] |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 6.19 g/L | 3 days | Thin stillage | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 5.50 g/L | 3 days | Cheese whey | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 6.19 g/L | 3 days | Thin stillage pH 3.95 | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 5.40 g/L | 3 days | Thin stillage pH 5 | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 3.50 g/L | 3 days | Thin stillage pH 6 | - | |
| Gluconacetobacter xylinus | Static | 2.90 g/L | 4 days | Acid hydrolysate of waste oleaginous yeast biomass | - | Luo et al. [275] |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 3.89 g/L | 14 days | Waste beer yeast treated with ultrasonication treatment | - | Lin et al. [237] |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 2.40 g/L | 14 days | Waste beer yeast treated with NaOH treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 2.00 g/L | 14 days | Waste beer yeast treated with high speed homogenizer treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 1.50 g/L | 14 days | Waste beer yeast treated with microwaves treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 1.20 g/L | 14 days | Waste beer yeast treated with untreatment | - | |
| Gluconacetobacter xylinus BC-11 K. | Static | 1.18 g/L | 10 days | Wastewater after pullulan polysaccharide fermentation | - | Zhao et al. [236] |
| Agro industrial waste | ||||||
| Waste as nitrogen source | ||||||
| Gluconacetobacter swingsii | Static | 2.8 g/L | 13 days | Pineapple peel juice | Glucose, fructose and sucrose | Castro et al. [235] |
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter swingsii | Static | - | 13 days | Sugar cane juice | Glucose, fructose and sucrose | Castro et al. [235] |
| Gluconacetobacter xylinum bacterium (ATCC 700178) | Shaking | 10.6 g/L | 7 days | Wheat straw | Corn steep liquor (CSL) | Goyat [266] |
| Gluconacetobacter xylinus | Static | 1.8 g/L | 9 days | Carob and haricot bean (CHb) medium | Citric acid | Bilgi et al. [276,277] |
| Komagataeibacter rhaeticus | Static | 6.0 g/L | 7 days | HS medium and Cashew tree exudates (HSCTE) |
HS medium | Pacheco et al. [278] |
| Komagataeibacter rhaeticus | Static | 6.0 g/L | 7 days | HS medium and Cashew tree exudates (HSCG) |
HS medium | |
| Acetobacter aceti ATCC 23770 | Shaking and static | 2.12 g/L | 8 days | Cheap agricultural product konjac powder | Yeast extract and tryptone | Hong & Qiu [279] |
|
Gluconacetobacter hansenii UAC09 |
Static | 8.2 g/L | 14 days | Coffee cherry husk (CCH) |
8% corn steep liquor (CSL) | Rani & Appaiah [242] |
|
Gluconacetobacter hansenii UAC09 |
Static | 6.5 g/L | 14 days | Coffee cherry husk (CCH) |
0.2% Urea | |
|
Gluconacetobacter hansenii UAC09 |
Static | 6.9 g/L | 14 days | Coffee cherry husk (CCH) |
Ethyl alcohol (EA) + Acetic acid (AA) | |
|
Gluconacetobacter hansenii UAC09 |
Static | 7.5 g/L | 14 days | Coffee cherry husk (CCH) |
8% CSL + EA + AA | |
|
Gluconacetobacter hansenii UAC09 |
Static | 6.6 g/L | 14 days | Coffee cherry husk (CCH) |
0.2% urea + EA + AA | |
| Acetobacter xylinus ATCC 23770 | Static | 8.3 g/L | 7 days | Enzymatic hydrolysate of wheat straw | Other components are same as of HS medium | Chen et al. [280] |
| Acetobacter xylinum 0416 MARDI | Static | 4.0 g/L | 8 days | Extracted date syrup (DSH-2%) | Other components are same as of HS medium | Lotfiman et al. [247] |
| Acetobacter xylinum 0416 MARDI | Static | 5.8 g/L | 8 days | Extracted date syrup (DSH-3%) | Other components are same as of HS medium | |
| Acetobacter xylinum 0416 MARDI | Static | 4.5 g/L | 8 days | Extracted date syrup (DSH-5%) | Other components are same as of HS medium | |
| Gluconacetobacter sacchari | Static | 0.1 g/L | 96 h | Grape skins aqueous extract, cheese whey, crude glycerol and sulfite pulping liquor | Organic or inorganic nitrogen | Carreira et al. [281] |
| Acinetobacter sp. BAN1 | Static | 0.3 g/L | 15 days | Pineapple juice medium (PIJM) | Other components are same as that of HS medium | Adebayo-Tayo et al. [282] |
| Acinetobacter sp. BAN1 | Static | 6.4 g/L | 15 days | Pawpaw juice medium (PAJM) | Other components are same as that of HS medium | |
| Acinetobacter sp. BAN1 | Static | 0.6 g/L | 15 days | Watermelon juice medium (WMJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 0.1 g/L | 15 days | Pineapple juice medium (PIJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 7.7 g/L | 15 days | Pawpaw juice medium (PAJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 0.4 g/L | 15 days | Watermelon juice medium (WMJM) | Other components are same as that of HS medium | |
| Gluconoacetobacter xylinus BCRC 12334 | Static | 3.40 g/L | 8 days | Orange peel fluid and orange peel hydrolysate | Acetate buffer, peptone and yeast extract | Kuo et al. [214] |
| Beijerinkia fluminensis WAUPM53 | Static | 0.47 g/L | 14 days | Sago byproduct | Other components are same as of HS medium | Voon et al. [226] |
| Gluconacetobacter xylinus 0416 | Static | 1.55 g/L | 14 days | Sago byproduct | Other components are same as of HS medium | |
| Acetobacterxylinum NBRC 13693 | Static | 4.1 g/L | 14 days | Pineapple | Disodium hydrogen phosphate buffer | Kurosumi et al. [283] |
| Acetobacterxylinum NBRC 13693 | Static | 3.95 g/L | 14 days | Apple | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 5.9 g/L | 14 days | Orange | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 3.5 g/L | 14 days | Japanese pear | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 1.8 g/L | 14 days | Grape | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 0.5 g/L | 14 days | Pineapple | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.2 g/L | 14 days | Apple | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 1.85 g/L | 14 days | Orange | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.5 g/L | 14 days | Japanese pear | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.4 g/L | 14 days | Grape | Sugar reagent (glucose, fructose and sucrose) | |
| Gluconacetobacter sacchari | Static | 1.7 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C |
Nitrogen | Gomes et al. [248] |
| Gluconacetobacter sacchari | Static | 1.4 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C |
Phosphorus | |
| Komagataeibacter hansenii MCM B-967 | Static | 125 g/L | 7 days | Pineapple and watermelon peels | Sucrose, ammonium sulfate and cycloheximide | Kumbhar et al. [284] |
| Acetobacter xylinum DSMZ2004 | Static | 8.6 g/L | 48 h | Poor quality apple residues in combination with glycerol | Apple glucose equivalents, glycerol, ammonium sulfate and citric acid | Casarica et al. [285] |
| Acetobacter xylinum BCRC 14182 (purchased) | Static | - | 3–7 days | Coconut-water | Sugar | Lin et al. [286] |
| Waste as complex medium without any additional nutrients | ||||||
|
Komagataeibacter hansenii GA2016 |
Static | 2.06 BC/100 g peel | 21 days | Lemon peels (LBC) | - | Güzel & Akpınar [287] |
|
Komagataeibacter hansenii GA2016 |
Static | 3.92 BC/100 g peel | 21 days | Mandarin peels (MBC) | - | |
|
Komagataeibacter hansenii GA2016 |
Static | 2.33 BC/100 g peel | 21 days | Orange peels (OBC) | - | |
|
Komagataeibacter hansenii GA2016 |
Static | 2.68 BC/100 g peel | 21 days | Grapefruit peels (GBC) | - | |
| Komagataeibacter xylinus | Static | 2.90 g/L | 10 days | Discarded waste durian shell | - | Luo, Huang et al. [275] |
| Gluconacetobacter xylinus CH001 | Static | 2.67 g/L | 10 days | Discarded waste durian shell | - | Luo, Huang, et al.[288] |
|
Gluconacetobacter
medellinensis |
Static | 3.24 g/L | 7 days | Sugar cane juice and pineapple residues | - | Algar et al. [289] |
|
Gluconacetobacter
medellinensis |
Dynamic | 0.82 g/L | 7 days | Sugar cane juice and pineapple residues | - | |
| Acinetobacter sp. BAN1 | Static | 0.4–0.6 g/L | 15 days | Pineapple waste medium (PIWAM) | - | Adebayo-Tayo et al. [290] |
| Acinetobacter sp. BAN1 | Static | 0.2–1.1 g/L | 15 days | Pawpaw waste medium (PAWAM) | - | |
| Acetobacterpasteurianus PW1 | Static | 0.2–1.0 g/L | 15 days | Pawpaw waste medium (PAWAM) | - | |
| Acetobacterpasteurianus PW1 | Static | 0.1–3.9 g/L | 15 days | Pineapple waste medium (PIWAM) | - | |
| Komagataeibacter rhaeticus iGEM | Static | – | 10 days | Fermented tea | - | Florea et al. [291] |
| Gluconacetobacter sacchari | - | 1.28 g/L | - | Industrial residues from olive oil production | - | Gomes et al. [248] |
| Gluconacetobacterpersimmonis GH-2 | Static | 5.75 g/L | 14 days | Watermelon + HS medium | - | Hungund et al. [292] |
| Gluconacetobacterpersimmonis GH-2 | Static | 5.98 g/L | 14 days | Orange juice + HS medium | - | |
| Gluconacetobacterpersimmonis GH-2 | Static | 6.18 g/L | 14 days | Muskmelon + HS medium | - | |
| Gluconacetobacterpersimmonis GH-2 | Static | 8.08 g/L | 14 days | Coconut water +HS medium | - | |
| Acetobacter xylinum | Static | 19.46 g/L | 15 days | Banana peel | - | Hungund et al. [245] |
| Gluconacetobacterxylinus ATCC 53582 | Static | 60 g/L | 96 h | Rotten fruit culture | - | Jozala et al. [293] |
| Gluconacetobacterxylinus CGMCC 2955 | Static | 2.25 g/L | 114 h | Waste water of candied jujube hydrolysate | - | Li et al. [252] |
| Acetobacterxylinum 0416 | Rotary disc reactor | 28.30 g/L | 4 days | Pineapple waste medium | - | Zahan et al. [197] |
| Komagataeibacter rhaeticus | Static | 2.8 g/L | 7 days | Cashew tree exudates (CTE) | - | Pacheco et al. [278] |
| Komagataeibacter rhaeticus | Static | 2.3 g/L | 7 days | Cashew gum (CG) | - | |
|
Gluconacetobacter hansenii UAC09 |
Static | 5.6 g/L | 14 days | Coffee cherry husk (CCH) |
- | Rani & Appaiah [242] |
| Gluconacetobacter sacchari | Static | 0.81 g/L | 96 h | Dry olive mill residue (DOR40) Water extraction at 40 °C |
- | Gomes et al. [248] |
| Gluconacetobacter sacchari | Static | 0.85 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C |
- | |
| Sugar industries, pulp mills and lignocellulosic biorefineries wastes | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Komagatacibacter xylinus PTCC 1734 | Static | 7.02 g/L | 10 days | Vinasse | Other components are same as of HS medium | Barshan et al. [294] |
| Acetobacter xylinum BPR2001 | Rotary shaker | 3.01 g/L | 70 h | Molasses | Corn steep liquor | Bae & Shoda [187] |
| Acetobacter xylinum BPR2001 | Rotary shaker | 5.30 g/L | 70 h | H2SO4 heat treated molasses | Corn steep liquor | |
| Gluconacetobacter xylinus | Static | 5.9 g/L | 14 days | Cane molasses | Corn steep liquor and diammonium phosphate | Vazquez et al. [190] |
| Acetobacter sp. V6 | Agitated | 3.12 g/L | 168 h | Molasses and corn steep liquor | Acetic acid | Jung et al. [204] |
| Acetobacter xylinum ATCC 10245 | Static | 223% as compared to 100% in HS medium | 7 days | Sugar cane molasses | Carbohydrates, minerals, vitamins and amino acids | Premjet et al. [295] |
| Komagataeibacter rhaeticus | Static | 3.90 g/L | 120 h | Sugarcane molasses (SCM) 10 g/L of SCM |
40 g/L of glucose | Machado et al. [296] |
| Komagataeibacter rhaeticus | Static | 4.01 g/L | 120 h | 20 g/L of SCM | 30 g/L of glucose | |
| Komagataeibacter rhaeticus | Static | 3.7 g/L | 120 h | 30 g/L of SCM | 20 g/L of glucose | |
| Komagataeibacter rhaeticus | Static | 3.50 g/L | 120 h | 40 g/L of SCM | 10 g/L of glucose | |
| Gluconacetobacter xylinus ATCC 23770 | Static | 11 g/L | 7 days | Waste fiber sludge sulfate | Yeast extract and tryptone | Cavka et al. [241] |
| Gluconacetobacter xylinus ATCC 23770 | Static | 10 g/L | 7 days | Waste fiber sludge sulfite | Yeast extract and tryptone | |
| Acetobacter xylinum ATCC 10245 | Static | 20.6 % | 7 days | Softwood purified water-soluble (SPWS) | Other components are same as of HS medium | Uraki et al. [297] |
| Acetobacter xylinum ATCC 10245 | Static | 33 % | 7 days | Hardwood purified water-soluble (HPWS) | Other components are same as of HS medium | |
| Acetobacter xylinum ATCC 53582 | Static | 5.4 % | 7 days | Softwood purified water-soluble (SPWS) | Other components are same as of HS medium | |
| Acetobacter xylinum ATCC 53582 | Static | 8.9 % | 7 days | Hardwood purified water-soluble (HPWS) | Other components are same as of HS medium | |
| Waste as carbon source without any additional nutrients | ||||||
| Acetobacter xylinus 23769 | 0.15 g/L | Hot water extract | - | Erbas Kiziltas et al. [298] | ||
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 2.51 g/L | 10 days | Scum of sugarcane jaggery or gur (JS) | - | Khattak, Khan, Ul-Islam, Wahid, et al. [299] |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 2.13 g/L | 10 days | Scum of sugarcane jaggery or gur (JS) | - | |
| Komagataeibacter europaeus SGP37 | Static | 6.30 g/L | 16 days | Sweet lime pulp waste | - | Dubey et al. [300] |
| G. persimmonis GH-2 | Static | 5.75 g/L | 14 days | Molasses + HS medium | - | Hungund et al. [292] |
| G. intermedius SNT-1 | Static | 12.6 g/L | 10 days | Molasses pretreated with hea | - | Tyagi et al. [301] |
| Gluconacetobacter xylinus (PTCC, 1734) | Static | 4.35 g/L | 336 h | Date syrup | - | Moosavi-Nasab [246] |
| Komagataeibacter rhaeticus | Static | 1.90 g/L | 120 h | 50 g/L of SCM | - | Machado et al. [296] |
| Gluconaceterxylinus CH001 | Static | 0.66 g/L | 5 days | Lipid fermentation wastewater | - | Huang et al. [302] |
| Gluconaceter xylinus | Static | 1·34 g/L | 7 days | Acetone-butanol-ethanol(ABE) fermentation wastewater | - | Huang et al. [303] |
| Gluconaceter xylinus BC-11 | Static | 1.177 g/L | 10 days | Wastewater after pullulan polysaccharide fermentation | - | Zhao et al. [236] |
| Acetobacterxylinum 23769 | Static | 0.15 g/L | 672 h | Wood hot water extract | - | Erbas Kiziltas et al. [298] |
| Textile mills | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter xylinus ATCC 23770 | Static | 10.8 | 14 days | Cotton-based waste textiles | Glucose, yeast extract and peptone | Hong et al. [215] |
| Gluconacetobacter xylinus | Static | 14.2 g/L | 10 days | Waste dyed cotton fabrics hydrolysate - Purple bed sheet (PBS) |
Peptone and yeast extract | Guo et al. [257] |
| Gluconacetobacter xylinus | Static | 13.7 g/L | 10 days | Waste dyed cotton fabrics hydrolysate- rose -Red bed sheet (RRBS) | Peptone and yeast extract | |
| Gluconacetobacter xylinus | Static | 14.1 g/L | 10 days | Waste dyed cotton fabrics hydrolysate- green bed sheet (GBS) | Peptone and yeast extract | |
| Gluconacetobacter xylinus | Static | 1.59 g/L | 7 days | Coloured hydrolysate | Peptone and yeast extract | Kuo et al. [256] |
| Gluconacetobacter xylinus | Static | 1.88 g/L | 7 days | Discoloured hydrolysate | Peptone and yeast extract | Kuo et al. [256] |
| Biodiesel industry | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconaceter xylinus BNKC19 | Static | 12.31 g/L | 7 days | Non-detoxified crude glycerol | Pineapple and in combination with HS medium components | Soemphol et al. [264] |
| Gluconacetobacter xylinus DSM 46604 | Agitated | 2.87 g/L | 5 days | 20 g/L glycerol | Yeast extract, ammonium sulphate, potassium hydrogen orthophosphate and magnesium sulphate | Adnan [304] |
| Gluconacetobacter xylinus DSM 46604 | Agitated | 2.87 g/L | 5 days | 50 g/L glucose | Yeast extract, ammonium sulphate, potassium hydrogen orthophosphate and magnesium sulphate | Adnan [304] |
| Gluconacetobacter xylinus | Static | 10 g/L | 14 days | Glycerol from biodiesel | Diammonium phosphate and corn steep liquor | Vazquez et al. [190] |
| Gluconacetobacter intermedius NEDO-01 | Static | 3.4 g/L | 4 days | Waste glycerol | Carboxymethyl Cellulose | Kose et al. [305] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 3.2 g/L | 15 days | Crude glycerol from biodiesel | Yeast extract and peptone | Tsouko et al. [259] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 13.3 g/L | 15 days | Crude glycerol from biodiesel | Sunflower meal hydrolysates | Tsouko et al. [259] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 13 g/L | 15 days | Crude glycerol from biodiesel | Flour-rich hydrolysates | Tsouko et al. [259] |
| Waste as carbon source without additional nutrients | ||||||
| Gluconacetobacter xylinus | Static | 3.5 g/L | 14 days | Glycerol from biodiesel | - | Vazquez et al. [190] |
| Micro-algae industry | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter xylinum bacterium (ATCC 700178) | Shaking | 4.86 g/L | 7 days | Algae | Corn steep liquor (CSL) | Goyat [266] |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 77% | 7 days | Chlorella vulgaris | Glucose/yeast extract | Chen et al. [306] |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 94% | 7 days | Scenedesmus obliqnus | Glucose/yeast extract | |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 85% | 7 days | Chlamydomonas reinhardtii | Glucose/yeast extract | |
| Komagataeibacter hansenii DSMZ | Static | 1.104 g/L | 7 days | Algae (Chlorella vulgaris) algae based glucose | Meat extract, peptone, NaCl and ethanol | Uzyol & Saçan [177] |
| Waste as carbon source without additional nutrients | ||||||
| Komagataeibacter saccharivorans | Static | 85.1% | 14 days | Algae (Chlamydomonas debaryana) (BEA0067) | - | Nóbrega et al. [307] |
Whey is known to be rich in various nutritional components; hence, a growing literature body examines the feasibility of utilizing waste products as low-cost substrates for improved BC production [229]. Specifically, whey protein functions as an excellent source of nutrients. Revin et al. [230] examined the utilization of the dairy and alcohol industries acidic wastes, stillage (TS) and cheese whey, for the economical manufacturing of BC with Gluconacetobacter sucrofermentans. The findings revealed that, in three days of cultivation, the bacterial strain in whey produced up to 5.45 g/L of B and C structural properties analysis showed similarities between the synthesised cellulose with plant cellulose, despite morphological differences associated with crystallinity. The findings also indicated that acidic byproducts of dairy industries, such as wheat stillage and whey, are potential affordable sources of nitrogen and carbon for BC production.
Thin stillage (TS), a liquid byproduct produced after microbial fermentation of carbohydrates by yeast, contains various organic compounds. Hence, it is a potential source of nitrogen and carbon for BC synthesis. TS quantification via NMR methods showed that whey TS is rich in nutrition, containing high concentrations of lactic acid (7.41 g/L), dextrin (11.65 g/L), ethanol (1.31 g/L), acetic acid (2.72 g/L), and glycerol (7.87 g/L) [231]. TS wastewater from rice wine distilleries demonstrated the capability of producing BC with a concentration of 6.26 g/L in a seven days period of Gluconacetobacter xylinus cultivation [232]. From the study, it is confirmed that low-cost production of BC using TS as a substitute for HS medium is possible and the best alternative. Furthermore, the research revealed a facile and more practical approach for wastewater disposal. There have been efforts to enhance BC formation under static conditions by evaluating BC formation using Gluconacetobacter xylinus and a combination of whey and fruits as a culture medium by Jozala et al. [233]. The findings were in good correspondence to results achieved in other studies using Gluconacetobacter sucrofermentans [151].
5.2. Agro-Industry Waste
Several studies on the practicability of using different sources of agro-industry waste in BC production are reported. For instance, using Komagataeibacter hansenii for BC manufacturing from sisal juice as the substrate [234]. The researchers evaluated the effects of various variables on the potential of production, including the sugar concentration, pH, duration of cultivation, and nitrogen supplementation. From the findings, the best BC yield achieved from sisal waste was 3.38 g/L, which was yielded after 10 days of cultivation at a pH of 5. The study recommended that sisal waste is a precious resource for BC production; however, concerns arise regarding the ease of availability of sisal waste for large-scale manufacturing.
In a related study, Castro et al. [235] characterized the structural properties of BC obtained from agrochemical wastes of sugarcane and pineapple using Gluconacetobacter swingsii. HS medium was used as the reference standard for the comparisons. The results revealed that pineapple peel juice produced BC of higher quality than the reference standard, with values of 2.8 g/L and 2.1 g/L, respectively. The findings were parallel with other studies that concluded that utilizing agro-industry waste in general, and pineapple and sugarcane substrates in particular, are feasible for BC production. Whereas, when HS medium was utilized, some structural similarities were observed using SEM, while ATR-FR-IR spectra displayed chemical similarities in the microfibrils.
Zhao et al. [236] evaluated the potential of using yeast lees from fermentation vessels during fruit production using Glucoacetobacter xylinum for BC production. From the findings, yeast residue was identified as a potential substrate for economic BC production. However, for optimum production, modifications to the medium component and culture conditions of the bacterial strain are necessary. This is particularly important, given that the BC yield decreased with loading volume into cultivation vessels, which could have been associated with a reduced concentration of oxygen in the media [237].
For the yeast lees, researchers determined that mango pulp could supply essential substrate during BC production. Mango and guava purees displayed similar results due to the significant increase in water vapor permeability of the product [238]. Additional alterations in the produced BC included enhanced elongation and tensile strength reduction. Several studies suggested the addition of hydrophobic compounds [239] as a method to improve water resistance through cross-linking mechanisms [240].
Recent attempts to manufacture BC by Gluconacetobacter xylinus using pulp mill and lignocellulosic biorefinery waste fibre sludge displayed the potential to generate close to 11 g/L cellulose [241]. Producing high-quality BC at a low cost by utilizing sequential fermentation of residual streams from pulp mills and biorefinery processes is the most crucial contribution of this paper. The findings are in good agreement with the results achieved in other studies using various substrates.
There have been efforts to evaluate the possibility of utilizing other agricultural wastes for carbon sources in BC production, including corn products, coffee cherry husk (CCH), date fruits, and banana peel. CCH waste is an abundant agro-industrial waste. This method, using CCH as a substrate to produce BC achieved up to 8.2 g/L of BC using 8% of CCH extract combined with steep corn liquor under optimized conditions [242]. The findings were parallel with research evidence that steep corn liquor is rich in nutrition, which supplied organic content during BC production, such as carbon and nitrogen [243,244].
Banana peel is another potential agricultural waste being studied for a carbon source in BC production using Acetobacter xylinum [245]. The concentration of BC produced was 19.46 g/L of the product in a period of 15 days and a temperature of 30 oC. Similar results were achieved with coconut water and pineapple juice as substrates for the same bacteria [245]. The date is a fruit with a potential carbon source for BC production. Date trees are grown mainly in tropical and arid areas of North Africa and Southwest Asia. Date syrup consists of essential nutrients that are sufficient for the growth of numerous microorganisms [246]. However, date processing is accompanied by massive loss and wastage, which can be converted to useful byproducts. Lotfiman et al. [247] assessed the viability of producing BC from date syrup using A. Xylinum. The researchers examined sugar content in the waste sample using HPLC and tested different concentrations of the fruit syrup at different culture times. BC production was attained with 3% (w/v) date in the medium cultivated for a duration of eight days. Alteration of the HS medium resulted in an increase in BC yield of up to 68%. It was determined that date waste is a potential source of carbon. Other cellulosic non-food wastes have also been utilized for BC formation with reduced BC yields, such as olive mill residues that produced 0.81 g/L of BC [248]. This BC yield was lower compared to date syrup. The findings indicated that agricultural waste could be used as a potential carbon source substitute compared to non-food sources.
Besides the substrates explained above, date industry waste is another possible substrate for the economic production of BC. One such byproduct of the industry is date syrup (DS), which is rich in carbohydrates [249]. A study utilized low-quality DS with very little commercial value; Moosavi-Nasab and Yousefi [246] found that BC production displayed a steady increment up to day 14 compared to sucrose, for which the production of BC remained almost constant. At the end of the cultivations, cellulose yield from DS (4.35 g/L) were more than two-folds that of sucrose (1.69 g/L). This is associated with the DS consisting of reducing sugars in abundance compared to sucrose, a disaccharide [246]. The same substrate was studied by Lotfiman et al. (2018) for the investigation of BC production by A. xylinum. Results of their study showed that A. xylinum produced up to 5.8 g/L of BC that was 68% higher compared to that of the standard HS medium [247].
Coffee cherry husk (CCH), a byproduct that is present in abundance from coffee cherry processing, is seen as a potential substrate for BC production [242]. Results of research attempts for BC production from CCH showed that the production capacity of up to 8.2 g/L was attained using 8% CCH extract combined with steep corn liquor under optimized conditions.
Besides the examples listed, banana peel is also being studied as a potential substrate for the economic production of BC. Acetobacter xylinum generated 19.46 g/L of BC in a cultivation period of 15 days at 30 °C [245]. Similar results were achieved when coconut water and pineapple juice were used as a substrate for the same microorganism [250].
5.3. Wastewater Sugar Industries, Pulp Mills and Lignocellulosic Biorefineries Wastes
Zhao et al. [236] studied the use of fermented wastewater as a substrate that showed a BC yield of 1.177 g/L, which much lower than HS medium (1.757 g/L). This could be ascribed to a low concentration of nitrogen and carbon contents in the substrate. However, BC yield from wastewater was sufficient to maintain large-scale commercial applications, with the low costs of the production process taken into consideration. These results are in good agreement with the existing evidence, which indicated that the ideal system for cellulose biosynthesis does not exist, even with gram-negative bacterium such as Gluconacetobacter xylinus that is able to secrete large volumes of cellulose as microfibrils from different waste products [251]. Other cellulosic wastes from non-food wastes are also being studied and have resulted in reduced BC yields [248].
In another related study, Li et al. [252] determined that jujube processing industry wastewater could provide an inexpensive raw material for BC production using Gluconacetobacter xylinum [252]. The experiments exhibited the potential to yield 2.25 g/L of BC in hydrolysate with acid treatment. However, the setup involves the usage of special filters of between 3 and 14 nm to produce nanostructures. This study showed the possibility of improving BC yield by adjusting the level of crystallinity and manipulating ammonium citrate concentration. Further research revealed that candied jujube consists of various nutritional compounds, such as amino acids, saccharides, and vitamins, which make an ideal substrate for BC synthesis of considerable quantities [253]. However, the crystallinity of the microbial cellulose is an important factor to be taken into consideration when utilizing jujube for BC, which was altered significantly in different cultivation media as a result of the effect of fibre size distribution. This effect is also observed during BC production using other waste feedstocks [254].
5.4. Textile Industries Waste
The growth of industrialization worldwide is affected by the increasing number of the world population. This phenomenon has resulted in an increase in the utilization of fabric and textiles to produce clothes and other materials related to textile-based products [90]. The growing demand for these products has led to the generation of tons of waste produced by the textile industries and consumers. Natural fibre resources such as cotton are commonly used in the textile industry to produce the fabric. According to Estur [255], world textile fibre consumption is projected to expand at an annual average rate of 4% to reach 70 million tons in 2010 and by 2.8% per year to reach 87 million tons in 2020. Used cotton fabric is not recycled because it does not provide a satisfactory level of use. It is usually dumped at the landfilled or garbage collection station or disposed of by incineration.
These waste cotton textiles have the potential to be used as an effective alternative to producing high-value products at low cost through enzymatic hydrolysis and microbial conversion processes. In addition, these wastes also have the potential to reduce environmental problems and save natural resources. Kuo et al. [256] conducted an experiment on enzymatic saccharification of dissolution pretreated cellulosic waste fabrics for BC production by Gluconacetobacter xylinus, which has shown that the BC produced from discoloured hydrolysate (1.88 g/L) by G. xylinus in static cultivation of seven days was about 20% higher compared to that in the coloured hydrolosate (1.59 g/L). This might be attributed to the fact that the coloured reducing sugars that were removed by chitosan adsorption prevent the fermentation activity of Gluconacetobacter xylinus for BC production.
Guo et al. [257] showed that BC could be successfully produced using waste dyed cotton fabrics cellulose through pretreatment with the ionic liquid (IL) 1-allyl-3-methyl-imidazolium chloride ([AMIM]Cl) with Gluconacetobacter xylinus followed by the production of enzymes with Trichoderma reesei via enzymatic saccharification. They found that the BNC yield obtained from the purple bed sheet (14.2 g/L) by Gluconacetobacter xylinus in static cultivation of 10 days was higher compared to that in the red bed sheet (13.7 g/L) and green bed sheet (14.1 g/L) [257]. Moreover, according to Guo et al. [257], this is due to the supplementation of calcium ions during treatment of Ca(OH)2 detoxification as well as the removal of dyes from the enzymatic hydrolysates.
Previous studies by Hong et al. [215] reported on production of high-quality carbon sources for BC from cotton-based waste textiles by Gluconacetobacter xylinus. These fabrics were pre-treated with the ([AMIM]Cl) followed by enzymatic hydrolysis. The results show that the yield and tensile strength of BC are 83% (10.8 g/L) and 79% (0.07 MPa) higher compared to a culture grown on a glucose-based medium [215]. The studies describing the use of textile mills waste for BC production are displayed in Table 3.
5.5. Biodiesel Industry Waste
BC is well known as a natural biomaterial with a broad range of applications. However, high-cost production in terms of raw materials, as well as low yields, have limited the industrial and commercial applications of BC. Hence, the usage of low cost-alternative raw materials as fermentation media would enhance BC production’s cost-competitiveness. The worldwide biodiesel production was more than 2.8 billion liters in 2018, and it has increased by 933% over the last 20 years [258,259]. It is estimated that crude glycerol is generated as a 10% (w/w) byproduct from transesterification of triglycerides with alcohol, most frequently methanol, which is equivalent to 0.28 billion liters.
In one of the studies, Tsouko et al. [259] investigated the feasibility of using fermentation media obtained from the confectionery industry and sunflower-based biodiesel industries waste streams and found that confectionary industry waste provides rich sources for carbon and nitrogen required for highly efficient BC production [259]. Batch fermentations using Komagataeibacter sucrofermentans (DSM) in synthetic media yielded BC concentrations of up to 13.3 g/L. The experimental results showed similar yields using both waste streams. The findings determined the significance of Komagataeibacter sucrofermentans DSM strain for high concentrations of BC production from the confectionery and biodiesel industry wastes. More importantly, the findings of this study on the water holding capacity (WHC) of the BC were parallel with the existing literature [260,261,262,263].
Previous studies reported the production and characterization of BC produced from non-detoxified crude glycerol as an alternative medium by Gluconacetobacter xylinus strain [264]. The highest BC production is 12.31 g/L. However, increasing crude glycerol has resulted in decreased BC production. This phenomenon might be due to the impurities in crude glycerol that might affect the activity of the cell. Besides that, from the research conducted by Soemphol et al. [264], it was shown that production of BC could improve by the addition of pineapple peel extract (PPE) into crude glycerol without any supplementation, and the optimal BC production was seen at acidic pH. The usage of these wastes or byproducts from biodiesel industries will not only produce value-added materials, it will also reduce environmental pollution and non-renewable energy consumption. The studies describing the use of biodiesel wastes for the production of BC are displayed in Table 3.
5.6. Micro-Algae Biomass Industries
Bioactive compounds such as carotenoids, polyunsaturated fatty acids, protein, vitamins, and minerals can be found in various commercial forms of micro-algal biomass (i.e., capsule, tablet, oil, liquid, flour, or powder forms). They play essential roles in numerous applications such as cosmetic products, pharmaceutical chemicals, feed product for animals (for fish, shellfish, poultry, and cattle) or functional food (i.e., supplements, dye, oil-derivatives, pastas, dairy products, and dessert) or with favorable outcomes upon human health, including antiviral, antimicrobial, anti-inflammatory, and antioxidant effects, as well as prevention of hypertension, diabetes, anaemia, constipation, and gastric ulcers [265]. Starch is one of the valuable constituents of microalgae biomass. Low-cost starch biomass products can be yielded from outdoor photobioreactors of Chlorella cultures microalgae [177]. Besides that, there are several studies that have been conducted to increase the starch content of algal biomass under different conditions (i.e., light intensities, nitrogen starvation, and sulphur). Freshwater algae Chlorella vulgaris can produce low-cost starch in large quantities (Dragone et al., 2011). This starch can be utilized as a promising alternative carbon source medium for the production of BC. Several studies reported the use of a byproduct of the micro-algae medium as a carbon source for BC. Several byproducts of micro-algae industries have been evaluated for BC production, as stated in Table 3.
Uzyol & Saçan [177] produced BC with Komagataeibacter hansenii using algae-based glucose, and showed that the BC production yields were 1.202 g/L and 1.104 g/L from glucose and algae-based glucose, respectively. The morphological structure of algae-based BC was observed to be similar to those of glucose-based BC. Another study, conducted on the production of green BC by utilizing renewable resources of algae with corn steep liquor [266], shows that the maximum BC production is 4.86 g/L. Therefore, based on the literature review, it can be summarized that integrating the metabolic components in algal biomass (i.e., corn steep liquor, glucose, yeast, starch, peptone, etc.) in the production of BC with the biorefinery concept would bring economic and environmental benefits, including the achievement of large scale production at low cost, and protecting the environment.
6. Future Perspectives
Different industrial sectors produce a large amount of waste on a daily basis. More brewery, sugar, lignocellulosic, and other industrial wastes could be valorized as complex media without additional nutrient sources or as carbon and nitrogen sources with additional nutrient sources for BC production. Due to their large-scale availability and increased BC productivity, agro-industrial wastes can be widely utilised for BC production. Due to increased urbanization around the world, particularly in economically developing nations, municipal waste is anticipated to become an increasingly major source of waste biomass with higher organic content. All of the low-cost waste media of the industries discussed here, especially those that do not require complex pre-treatments, detoxification, or supplementing, have a lot of potential for upscale production of BC on an industrial scale. When compared to regular media, BC created from waste media has similar physico-chemical characteristics and a higher yield.
Because these wastes are available in huge quantities, waste producers may be able to sell them to commercial BC producers or academic institutions. The BC that was obtained could be used as a raw material by a variety of biomedical enterprises for commercial purposes as well as by scientists for study. Since BC derived from some agro-wastes might be colored and absorb unwanted compounds, proper purification is required. These facts may justify limiting the use of BC in industries with stringent regulatory standards, such as biomedicine, pharmaceutics, cosmetics, or the food industry. In terms of the environment, eliminating these industrial outputs will allow for proper waste management, lowering the environmental and health risks associated with these wastes. This will be a realistic option for dealing with pollution issues.
7. Conclusions
Bacterial cellulose (BC) is considered a desirable biomaterial for various applications across many fields due to its unique structural features and desirable properties. This review mainly discusses the technical and economic feasibility of producing microbial cellulose from industrial wastes from agro-industry, textile, biodiesel, micro-algae biomass, wastewater sugar, and lignocellulosic biorefineries, breweries, and beverages. The overarching conclusion is that most industrial wastes have the potential to produce high concentrations of BC. The production of high concentrations of BC can be obtained by optimizing bacterial culture conditions, such as temperature and pH. More importantly, the findings demonstrate that the produced microbial cellulose would have desirable chemical, physical, and mechanical properties, which suit various advanced applications. This review shows that the production of BC from industrial waste is successful. The future of using industrial wastes for BC production seems promising, since the source of nutrients in BC production from industrial wastes has reduced the production cost. Moreover, tonnes of industrial waste are generated daily, and using some of these wastes in BC production can mitigate waste disposal problems. The high yield and low production cost of BC is the main challenge that needs to be contemplated. A lot of progress can be made by developing new fermentation methods, new bioreactor design, and using a cheaper waste media that aims to increase the yield of BC at a lower cost. The BC has been used in various industries in manufacturing products as well as advanced applications. Products such as BC masks, BC gloves, paper, biodegradable food packaging, and wound dressing have been on the market. More advanced BC applications have shown promising results, such as never-dried microbial cellulose membranes, skin transplants, optically transparent cellulose nanocomposites, and artificial bacterial cellulose ligaments. Overall, large-scale commercial production and demand of microbial cellulose using waste as a carbon and energy source can lower the biomaterial production cost and help eliminate or reduce the economic and environmental burden of industrial waste.
Acknowledgments
The authors would like express gratitude for the financial support received from Universiti Teknologi Malaysia, project CRG 30.3: Retardant coating using graphene/bamboo aerogel mixtures on SAR robotics system, grant number PY/2020/03495—R.J130000.7351.4B534”. The research has been carried out under program Research Excellence Consortium (JPT (BPKI) 1000/016/018/25 (57)) provided by Ministry of Higher Education Malaysia (MOHE). The author also would like to thank Universiti Teknologi Malaysia (UTM) for work and facilities support. The article processing fee of this manuscript is funded by Research Management Centre (RMC), Univesiti Putra Malaysia.
Author Contributions
Conceptualization, A.K. and R.A.I.; methodology, A.K. and R.A.I.; investigation, A.K. and R.A.I.; writing—original draft preparation, A.K., R.A.I., J.W., M.R.M.H., N.H., R.Y., S.M.S., M.M.H. and M.N.M.A.; writing—review and editing, R.I., M.S.N.A., K.C., M.A.I., S.S., S.P., A.R., M.R.M.A. and M.R.I.; visualization, A.K. and R.A.I.; supervision, A.K. and R.A.I.; project administration, A.K. and R.A.I.; funding acquisition, M.R.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like express gratitude for the financial support received from Universiti Teknologi Malaysia, project CRG 30.3: Retardant coating using graphene/bamboo aerogel mixtures on SAR robotics system, grant number PY/2020/03495—R.J130000.7351.4B534. The research has been carried out under program Research Excellent Consortium (JPT)(BKI)1000/016/018/25(57) provided by Ministry of Higher Education Malaysia (MOHE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abral H., Pratama A.B., Handayani D., Mahardika M., Aminah I., Sandrawati N., Sugiarti E., Muslimin A.N., Sapuan S.M., Ilyas R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021;2021:1–11. doi: 10.1155/2021/6641284. [DOI] [Google Scholar]
- 2.Abral H., Chairani M.K., Rizki M.D., Mahardika M., Handayani D., Sugiarti E., Muslimin A.N., Sapuan S.M., Ilyas R.A. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021;11:896–904. doi: 10.1016/j.jmrt.2021.01.057. [DOI] [Google Scholar]
- 3.Cazón P., Velazquez G., Vázquez M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020;99:105323. doi: 10.1016/j.foodhyd.2019.105323. [DOI] [Google Scholar]
- 4.Lin C.-M., Chang Y.-C., Cheng L.-C., Liu C.-H., Chang S.C., Hsien T.-Y., Wang D.-M., Hsieh H.-J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose. 2020;27:2651–2667. doi: 10.1007/s10570-019-02940-w. [DOI] [Google Scholar]
- 5.Kamiński K., Jarosz M., Grudzień J., Pawlik J., Zastawnik F., Pandyra P., Kołodziejczyk A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose. 2020;27:5353–5365. doi: 10.1007/s10570-020-03128-3. [DOI] [Google Scholar]
- 6.Galdino C.J.S., Maia A.D., Meira H.M., Souza T.C., Amorim J.D.P., Almeida F.C.G., Costa A.F.S., Sarubbo L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020;91:288–296. doi: 10.1016/j.procbio.2019.12.020. [DOI] [Google Scholar]
- 7.Pang M., Huang Y., Meng F., Zhuang Y., Liu H., Du M., Ma Q., Wang Q., Chen Z., Chen L., et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020;122:109365. doi: 10.1016/j.eurpolymj.2019.109365. [DOI] [Google Scholar]
- 8.Norizan M.N., Harussani M.M., Demon S.Z.N., Halim N.A., Samsuri A., Mohamad I.S., Knight V.F., Abdullah N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020:43704–43732. doi: 10.1039/D0RA09438B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esa F., Tasirin S.M., Rahman N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia. 2014;2:113–119. doi: 10.1016/j.aaspro.2014.11.017. [DOI] [Google Scholar]
- 10.Andriani D., Apriyana A.Y., Karina M. The optimization of bacterial cellulose production and its applications: A review. Cellulose. 2020;27:6747–6766. doi: 10.1007/s10570-020-03273-9. [DOI] [Google Scholar]
- 11.Ilyas R.A., Sapuan S.M., Harussani M.M., Atikah M.S.N., Ibrahim R., Asyraf M.R.M., Radzi A.M., Nadlene R., Kian L.K., Mali S. Roselle. Elsevier; Amsterdam, The Netherlands: 2021. Development and characterization of roselle nanocellulose and its potential in reinforced nanocomposites; pp. 285–317. [Google Scholar]
- 12.Omran A.A.B., Mohammed A.A.B.A., Sapuan S.M., Ilyas R.A., Asyraf M.R.M., Koloor S.S.R., Petrů M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers. 2021;13:231. doi: 10.3390/polym13020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sari N.H., Pruncu C.I., Sapuan S.M., Ilyas R.A., Catur A.D., Suteja S., Sutaryono Y.A., Pullen G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020;91:106751. doi: 10.1016/j.polymertesting.2020.106751. [DOI] [Google Scholar]
- 14.Picheth G.F., Pirich C.L., Sierakowski M.R., Woehl M.A., Sakakibara C.N., de Souza C.F., Martin A.A., da Silva R., de Freitas R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017;104:97–106. doi: 10.1016/j.ijbiomac.2017.05.171. [DOI] [PubMed] [Google Scholar]
- 15.Aisyah H.A., Paridah M.T., Sapuan S.M., Ilyas R.A., Khalina A., Nurazzi N.M., Lee S.H., Lee C.H. A Comprehensive Review on Advanced Sustainable Woven Natural Fibre Polymer Composites. Polymers. 2021;13:471. doi: 10.3390/polym13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norizan M.N., Moklis M.H., Alias A.H., Rushdan A.I., Norrrahim M.N.F., Abdan K., Abdullah N. Treatments of Natural Fibre as Reinforcement in Polymer Composites-Short Review. Funct. Compos. Struct. 2021;3:024002. doi: 10.1088/2631-6331/abff36. [DOI] [Google Scholar]
- 17.Norrrahim M.N.F., Huzaifah M.R.M., Farid M.A.A., Shazleen S.S., Misenan M.S.M., Yasim-Anuar T.A.T., Naveen J., Nurazzi N.M., Rani M.S.A., Hakimi M.I., et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers. 2021;13:2971. doi: 10.3390/polym13172971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azman M.A., Asyraf M.R.M., Khalina A., Petrů M., Ruzaidi C.M., Sapuan S.M., Wan Nik W.B., Ishak M.R., Ilyas R.A., Suriani M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers. 2021;13:1917. doi: 10.3390/polym13121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurazzi N.M., Asyraf M.R.M., Khalina A., Abdullah N., Aisyah H.A., Rafiqah S.A., Sabaruddin F.A., Kamarudin S.H., Norrrahim M.N.F., Ilyas R.A., et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers. 2021;13:646. doi: 10.3390/polym13040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsubari S., Zuhri M.Y.M., Sapuan S.M., Ishak M.R., Ilyas R.A., Asyraf M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers. 2021;13:423. doi: 10.3390/polym13030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayu R.S., Khalina A., Harmaen A.S., Zaman K., Isma T., Liu Q., Ilyas R.A., Lee C.H. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers. 2020;12:1571. doi: 10.3390/polym12071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullah H., Santos H.A., Khan T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose. 2016;23:2291–2314. doi: 10.1007/s10570-016-0986-y. [DOI] [Google Scholar]
- 23.Rebelo A.R., Archer A.J., Chen X., Liu C., Yang G., Liu Y. Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci. Technol. Adv. Mater. 2018;19:203–211. doi: 10.1080/14686996.2018.1430981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi J.-C., Liu S.-X., Li C.-F., Li J., Liu L.-X., Deng J., Yang Y.-C. Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J. Appl. Microbiol. 2014;117:1305–1311. doi: 10.1111/jam.12619. [DOI] [PubMed] [Google Scholar]
- 25.Evans B.R., O’Neill H.M. Effect of surface attachment on synthesis of bacterial cellulose. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2005;121:439–450. doi: 10.1385/ABAB:121:1-3:0439. [DOI] [PubMed] [Google Scholar]
- 26.Costa A.F.S., Almeida F.C.G., Vinhas G.M., Sarubbo L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres F.G., Commeaux S., Troncoso O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012;3:864–878. doi: 10.3390/jfb3040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boisset C., Fraschini C., Schülein M., Henrissat B., Chanzy H. Imaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A. Appl. Environ. Microbiol. 2000;66:1444–1452. doi: 10.1128/AEM.66.4.1444-1452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basta A.H., El-Saied H. Performance of improved bacterial cellulose application in the production of functional paper. J. Appl. Microbiol. 2009;107:2098–2107. doi: 10.1111/j.1365-2672.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- 30.El-Saied H., Basta A.H., Gobran R.H. Research Progress in Friendly Environmental Technology for the Production of Cellulose Products (Bacterial Cellulose and Its Application) Polym. Plast. Technol. Eng. 2004;43:797–820. doi: 10.1081/PPT-120038065. [DOI] [Google Scholar]
- 31.Tahara N., Tabuchi M., Watanabe K., Yano H., Morinaga Y., Yoshinaga F. Degree of Polymerization of Cellulose from Acetobacter xylinum BPR2001 Decreased by Cellulase Produced by the Strain. Biosci. Biotechnol. Biochem. 1997;61:1862–1865. doi: 10.1271/bbb.61.1862. [DOI] [PubMed] [Google Scholar]
- 32.Grande C.J., Torres F.G., Gomez C.M., Troncoso O.P., Canet-Ferrer J., Martínez-Pastor J. Development of self-assembled bacterial cellulose-starch nanocomposites. Mater. Sci. Eng. C. 2009;29:1098–1104. doi: 10.1016/j.msec.2008.09.024. [DOI] [Google Scholar]
- 33.Gorgieva S., Trček J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials. 2019;9:1352. doi: 10.3390/nano9101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lou Z.C. A better design is needed for clinical studies of chronic tympanic membrane perforations using biological materials. Eur. Arch. Oto Rhino-Laryngol. 2016;273:4045–4046. doi: 10.1007/s00405-016-4019-0. [DOI] [PubMed] [Google Scholar]
- 35.Biskin S., Damar M., Oktem S.N., Sakalli E., Erdem D., Pakir O. A new graft material for myringoplasty: Bacterial cellulose. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:3561–3565. doi: 10.1007/s00405-016-3959-8. [DOI] [PubMed] [Google Scholar]
- 36.Lang N., Merkel E., Fuchs F., Schumann D., Klemm D., Kramer F., Mayer-Wagner S., Schroeder C., Freudenthal F., Netz H., et al. Bacterial nanocellulose as a new patch material for closure of ventricular septal defects in a pig model. Eur. J. Cardio-Thorac. Surg. 2014;47:1013–1021. doi: 10.1093/ejcts/ezu292. [DOI] [PubMed] [Google Scholar]
- 37.Azeredo H.M.C., Rosa M.F., Mattoso L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017;97:664–671. doi: 10.1016/j.indcrop.2016.03.013. [DOI] [Google Scholar]
- 38.Gellner P.E.L., Dang A.E., Jay H. Acoustic Diaphragm and Method for Producing Same. US005274199A. US Patent. 1993 December 28;:3–7.
- 39.Hioki N., Hori Y., Watanabe K., Morinaga Y., Yoshinaga F., Hibino Y., Ogura T. Bacterial cellulose; as a new material for papermaking. Jpn. TAPPI J. 1995;49:718–723. doi: 10.2524/jtappij.49.718. [DOI] [Google Scholar]
- 40.Yang J., Yu J., Fan J., Sun D., Tang W., Yang X. Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011;189:377–383. doi: 10.1016/j.jhazmat.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 41.Benito-González I., López-Rubio A., Gómez-Mascaraque L.G., Martínez-Sanz M. PLA coating improves the performance of renewable adsorbent pads based on cellulosic aerogels from aquatic waste biomass. Chem. Eng. J. 2020;390:124607. doi: 10.1016/j.cej.2020.124607. [DOI] [Google Scholar]
- 42.Morales-Narváez E., Golmohammadi H., Naghdi T., Yousefi H., Kostiv U., Horák D., Pourreza N., Merkoçi A. Nanopaper as an Optical Sensing Platform. ACS Nano. 2015;9:7296–7305. doi: 10.1021/acsnano.5b03097. [DOI] [PubMed] [Google Scholar]
- 43.Yuen J.D., Shriver-Lake L.C., Walper S.A., Zabetakis D., Breger J.C., Stenger D.A. Microbial nanocellulose printed circuit boards for medical sensing. Sensors. 2020;20:2047. doi: 10.3390/s20072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portela R., Leal C.R., Almeida P.L., Sobral R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019;12:586–610. doi: 10.1111/1751-7915.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X., Peng X., Zhang S.W. Biodegradable medical polymers. In: Zhang X., editor. Science and Principles of Biodegradable and Bioresorbable Medical Polymers. Elsevier; Duxford, UK: 2017. pp. 1–33. [Google Scholar]
- 46.Czaja W., Krystynowicz A., Kawecki M., Wysota K., Sakiel S., Wróblewski P., Glik J., Nowak M., Bielecki S. Cellulose: Molecular and Structural Biology. Springer; Dordrecht, The Netherlands: 2007. Biomedical Applications of Microbial Cellulose in Burn Wound Recovery; pp. 307–321. [DOI] [Google Scholar]
- 47.Sulaeva I., Hettegger H., Bergen A., Rohrer C., Kostic M., Konnerth J., Rosenau T., Potthast A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C. 2020;110:110619. doi: 10.1016/j.msec.2019.110619. [DOI] [PubMed] [Google Scholar]
- 48.Blanco Parte F.G., Santoso S.P., Chou C.C., Verma V., Wang H.T., Ismadji S., Cheng K.C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020;40:397–414. doi: 10.1080/07388551.2020.1713721. [DOI] [PubMed] [Google Scholar]
- 49.Żur J., Piński A., Michalska J., Hupert-Kocurek K., Nowak A., Wojcieszyńska D., Guzik U. A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. Int. Biodeterior. Biodegrad. 2020;149:104919. doi: 10.1016/j.ibiod.2020.104919. [DOI] [Google Scholar]
- 50.Zhang S., He H., Guan S., Cai B., Li Q., Rong S. Bacterial cellulose-alginate composite beads as yarrowia lipolytica cell carriers for lactone production. Molecules. 2020;25:928. doi: 10.3390/molecules25040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feil G., Horres R., Schulte J., Mack A.F., Petzoldt S., Arnold C., Meng C., Jost L., Boxleitner J., Kiessling-Wolf N., et al. Bacterial cellulose shifts transcriptome and proteome of cultured endothelial cells towards native differentiation. Mol. Cell. Proteom. 2017;16:1563–1577. doi: 10.1074/mcp.RA117.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan J.X., Wong J.F., Petrů M., Hassan A., Nirmal U., Othman N., Ilyas R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers. 2021;13:2867. doi: 10.3390/polym13172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Punia Bangar S., Nehra M., Siroha A.K., Petrů M., Ilyas R.A., Devi U., Devi P. Development and Characterization of Physical Modified Pearl Millet Starch-Based Films. Foods. 2021;10:1609. doi: 10.3390/foods10071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumari N., Bangar S.P., Petrů M., Ilyas R.A., Singh A., Kumar P. Development and Characterization of Fenugreek Protein-Based Edible Film. Foods. 2021;10:1976. doi: 10.3390/foods10091976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilyas R.A., Sapuan S.M., Harussani M.M., Hakimi M.Y.A.Y., Haziq M.Z.M., Atikah M.S.N., Asyraf M.R.M., Ishak M.R., Razman M.R., Nurazzi N.M., et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers. 2021;13:1326. doi: 10.3390/polym13081326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ilyas R.A., Sapuan S.M. Biopolymers and Biocomposites: Chemistry and Technology. Curr. Anal. Chem. 2020;16:500–503. doi: 10.2174/157341101605200603095311. [DOI] [Google Scholar]
- 57.Abral H., Atmajaya A., Mahardika M., Hafizulhaq F., Kadriadi, Handayani D., Sapuan S.M., Ilyas R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020;9:2477–2486. doi: 10.1016/j.jmrt.2019.12.078. [DOI] [Google Scholar]
- 58.Sapuan S.M., Aulia H.S., Ilyas R.A., Atiqah A., Dele-Afolabi T.T., Nurazzi M.N., Supian A.B.M., Atikah M.S.N. Mechanical properties of longitudinal basalt/woven-glass-fiber-reinforced unsaturated polyester-resin hybrid composites. Polymers. 2020;12:2211. doi: 10.3390/polym12102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pötzinger Y., Kralisch D., Fischer D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017;8:753–761. doi: 10.4155/tde-2017-0059. [DOI] [PubMed] [Google Scholar]
- 60.Unal S., Gunduz O., Uzun M. Tissue Engineering Applications of Bacterial Cellulose Based Nanofibers. In: Ahmed S., Ali W., editors. Green Nanomaterials. Advanced Structured Materials. Springer; Singapore: 2020. pp. 319–346. [Google Scholar]
- 61.Wang K., Ma Q., Zhang Y.M., Han G.T., Qu C.X., Wang S.D. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose. 2020;27:1845–1852. doi: 10.1007/s10570-019-02869-0. [DOI] [Google Scholar]
- 62.Klinthoopthamrong N., Chaikiawkeaw D., Phoolcharoen W., Rattanapisit K., Kaewpungsup P., Pavasant P., Hoven V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020;149:51–59. doi: 10.1016/j.ijbiomac.2020.01.158. [DOI] [PubMed] [Google Scholar]
- 63.Junka A., Bartoszewicz M., Dziadas M., Szymczyk P., Dydak K., Żywicka A., Owczarek A., Bil-Lula I., Czajkowska J., Fijałkowski K. Application of bacterial cellulose experimental dressings saturated with gentamycin for management of bone biofilm in vitro and ex vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:30–37. doi: 10.1002/jbm.b.34362. [DOI] [PubMed] [Google Scholar]
- 64.Svensson A., Nicklasson E., Harrah T., Panilaitis B., Kaplan D.L., Brittberg M., Gatenholm P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials. 2005;26:419–431. doi: 10.1016/j.biomaterials.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 65.Codreanu A., Balta C., Herman H., Cotoraci C., Mihali C.V., Zurbau N., Zaharia C., Rapa M., Stanescu P., Radu I.C., et al. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials. 2020;13:1433. doi: 10.3390/ma13061433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W., Wang X.C., Li X.Y., Zhang L.L., Jiang F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020;236:116043. doi: 10.1016/j.carbpol.2020.116043. [DOI] [PubMed] [Google Scholar]
- 67.Lina F., Chandra P., Adrianna M., Wankei W. Bacterial cellulose production using a novel microbe. Front. Bioeng. Biotechnol. 2016;4:1–2. doi: 10.3389/conf.FBIOE.2016.01.00552. [DOI] [Google Scholar]
- 68.Bodea I.M., Cătunescu G.M., Stroe T.F., Dîrlea S.A., Beteg F.I. Applications of bacterial-synthesized cellulose in veterinary medicine—A review. Acta Vet. Brno. 2019;88:451–471. doi: 10.2754/avb201988040451. [DOI] [Google Scholar]
- 69.Lin N., Dufresne A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014;59:302–325. doi: 10.1016/j.eurpolymj.2014.07.025. [DOI] [Google Scholar]
- 70.Kim Y.-K., Lee S.-C., Cho Y.-Y., Oh H.-J., Ko Y.H. Isolation of Cellulolytic Bacillus subtilis Strains from Agricultural Environments. ISRN Microbiol. 2012;2012:1–9. doi: 10.5402/2012/650563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoue B.S., Streit S., dos Santos Schneider A.L., Meier M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020;148:1098–1108. doi: 10.1016/j.ijbiomac.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 72.Li N., Yang L., Pan C., Saw P.E., Ren M., Lan B., Wu J., Wang X., Zeng T., Zhou L., et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020;102:298–314. doi: 10.1016/j.actbio.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Stumpf T.R., Tang L., Kirkwood K., Yang X., Zhang J., Cao X. Production and evaluation of biosynthesized cellulose tubes as promising nerve guides for spinal cord injury treatment. J. Biomed. Mater. Res.-Part A. 2020;108:1380–1389. doi: 10.1002/jbm.a.36909. [DOI] [PubMed] [Google Scholar]
- 74.Czaja W.K., Young D.J., Kawecki M., Brown R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules. 2007;8:1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 75.Schaffner M., Rühs P.A., Coulter F., Kilcher S., Studart A.R. 3D printing of bacteria into functional complex materials. Sci. Adv. 2017;3:eaao6804. doi: 10.1126/sciadv.aao6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okahisa Y., Yoshida A., Miyaguchi S., Yano H. Optically transparent wood-cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009;69:1958–1961. doi: 10.1016/j.compscitech.2009.04.017. [DOI] [Google Scholar]
- 77.Gomes N.O., Carrilho E., Antonio S., Machado S., Sgobbi F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta. 2020;349:136341. doi: 10.1016/j.electacta.2020.136341. [DOI] [Google Scholar]
- 78.Buruaga-Ramiro C., Valenzuela S.V., Valls C., Roncero M.B., Pastor F.I.J., Díaz P., Martínez J. Bacterial cellulose matrices to develop enzymatically active paper. Cellulose. 2020;27:3413–3426. doi: 10.1007/s10570-020-03025-9. [DOI] [Google Scholar]
- 79.Park S., Park J., Jo I., Cho S.P., Sung D., Ryu S., Park M., Min K.A., Kim J., Hong S., et al. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials. 2015;58:93–102. doi: 10.1016/j.biomaterials.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 80.Czaja W., Krystynowicz A., Bielecki S., Brown J.R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 81.Markstedt K., Mantas A., Tournier I., Martínez Ávila H., Hägg D., Gatenholm P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X., Liu D., Yang L., Zhou L., You T. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A. 2015;3:10031–10037. doi: 10.1039/C5TA00355E. [DOI] [Google Scholar]
- 83.Naseri-Nosar M., Salehi M., Hojjati-Emami S. Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int. J. Biol. Macromol. 2017;103:701–708. doi: 10.1016/j.ijbiomac.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 84.Mathew A.P., Oksman K., Pierron D., Harmand M.F. Biocompatible Fibrous Networks of Cellulose Nanofibres and Collagen Crosslinked Using Genipin: Potential as Artificial Ligament/Tendons. Macromol. Biosci. 2013;13:289–298. doi: 10.1002/mabi.201200317. [DOI] [PubMed] [Google Scholar]
- 85.Sämfors S., Karlsson K., Sundberg J., Markstedt K., Gatenholm P. Biofabrication of bacterial nanocellulose scaffolds with complex vascular structure. Biofabrication. 2019;11:45010. doi: 10.1088/1758-5090/ab2b4f. [DOI] [PubMed] [Google Scholar]
- 86.Hussain Z., Sajjad W., Khan T., Wahid F. Production of bacterial cellulose from industrial wastes: A review. Cellulose. 2019;26:2895–2911. doi: 10.1007/s10570-019-02307-1. [DOI] [Google Scholar]
- 87.Vandamme E.J., De Baets S., Vanbaelen A., Joris K., De Wulf P. Improved production of bacterial cellulose and its application potential. Polym. Degrad. Stab. 1998;59:93–99. doi: 10.1016/S0141-3910(97)00185-7. [DOI] [Google Scholar]
- 88.Bajpai P. Biobased Polymers: Properties and Applications in Packaging. 1st ed. Elsevier Inc.; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 89.Torgbo S., Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today. 2018;11:34–49. doi: 10.1016/j.apmt.2018.01.004. [DOI] [Google Scholar]
- 90.Mohd Nurazzi N., Asyraf M.R.M., Khalina A., Abdullah N., Sabaruddin F.A., Kamarudin S.H., Ahmad S., Mahat A.M., Lee C.L., Aisyah H.A., et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers. 2021;13:1047. doi: 10.3390/polym13071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stumpf T.R., Yang X., Zhang J., Cao X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C. 2018;82:372–383. doi: 10.1016/j.msec.2016.11.121. [DOI] [PubMed] [Google Scholar]
- 92.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N., Ilyas R.A., Rafidah M., Razman M.R. Potential Application of Green Composites for Cross Arm Component in Transmission Tower: A Brief Review. Int. J. Polym. Sci. 2020;2020:1–15. doi: 10.1155/2020/8878300. [DOI] [Google Scholar]
- 93.Asyraf M.R.M., Rafidah M., Ishak M.R., Sapuan S.M., Ilyas R.A., Razman M.R. Integration of TRIZ, Morphological Chart and ANP method for development of FRP composite portable fire extinguisher. Polym. Compos. 2020;41:2917–2932. doi: 10.1002/pc.25587. [DOI] [Google Scholar]
- 94.Nazrin A., Sapuan S.M., Zuhri M.Y.M., Tawakkal I.S.M.A., Ilyas R.A. Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites. Nanotechnol. Rev. 2021;10:431–442. doi: 10.1515/ntrev-2021-0033. [DOI] [Google Scholar]
- 95.Tarique J., Sapuan S.M., Khalina A., Sherwani S.F.K., Yusuf J., Ilyas R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021;13:1191–1219. doi: 10.1016/j.jmrt.2021.05.047. [DOI] [Google Scholar]
- 96.Zaborowska M., Bodin A., Bäckdahl H., Popp J., Goldstein A., Gatenholm P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010;6:2540–2547. doi: 10.1016/j.actbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Gonçalves-Pimentel C., Moreno G.M.M., Trindade B.S., Isaac A.R., Rodrigues C.G., Savariradjane M., de Albuquerque A.V., de Andrade Aguiar J.L., Andrade-da-Costa B.L.d.S. Cellulose exopolysaccharide from sugarcane molasses as a suitable substrate for 2D and 3D neuron and astrocyte primary cultures. J. Mater. Sci. Mater. Med. 2018;29:139. doi: 10.1007/s10856-018-6147-0. [DOI] [PubMed] [Google Scholar]
- 98.Padra J., Silva P., Sencadas V. Bacterial Cellulose as a Support for the Growth of Retinal Pigment Epithelium. Biomacromolecules. 2015;16:1341–1351. doi: 10.1021/acs.biomac.5b00129. [DOI] [PubMed] [Google Scholar]
- 99.Seoane I.T., Manfredi L.B., Cyras V.P., Torre L., Fortunati E., Puglia D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on disintegrability in composting conditions of Plasticized PHB Nanocomposites. Polymers. 2017;9:561. doi: 10.3390/polym9110561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L., Zheng S., Hu Z., Zhong L., Wang Y., Zhang X., Xue J. Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes. Appl. Sci. 2020;10:752. doi: 10.3390/app10030752. [DOI] [Google Scholar]
- 101.Hamedi S., Shojaosadati S.A., Najafi V., Alizadeh V. A novel double-network antibacterial hydrogel based on aminated bacterial cellulose and schizophyllan. Carbohydr. Polym. 2020;229:115383. doi: 10.1016/j.carbpol.2019.115383. [DOI] [PubMed] [Google Scholar]
- 102.Sukhavattanakul P., Manuspiya H. Fabrication of hybrid thin film based on bacterial cellulose nanocrystals and metal nanoparticles with hydrogen sulfide gas sensor ability. Carbohydr. Polym. 2020;230:115566. doi: 10.1016/j.carbpol.2019.115566. [DOI] [PubMed] [Google Scholar]
- 103.Gupta A., Briffa S.M., Swingler S., Gibson H., Kannappan V., Adamus G., Kowalczuk M., Martin C., Radecka I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules. 2020;21:1802–1811. doi: 10.1021/acs.biomac.9b01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Pasquale G., Graziani S., Pollicino A., Trigona C. Performance characterization of a biodegradable deformation sensor based on bacterial cellulose. IEEE Trans. Instrum. Meas. 2019;69:2561–2569. doi: 10.1109/TIM.2019.2961497. [DOI] [Google Scholar]
- 105.Wang L., Mao L., Qi F., Li X., Ullah M.W., Zhao M., Shi Z., Yang G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021;424:130563. doi: 10.1016/j.cej.2021.130563. [DOI] [Google Scholar]
- 106.Rebelo A., Liu Y., Liu C., Schäfer K.-H., Saumer M., Yang G. Poly (4-vinylaniline)/polyaniline bilayer functionalized bacterial cellulose membranes as bioelectronics interfaces. Carbohydr. Polym. 2019;204:190–201. doi: 10.1016/j.carbpol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Saxena I.M., Brown R.M. Cellulose: Molecular and Structural Biology. Springer; Dordrecht, The Netherlands: 2007. A Perspective on the Assembly of Cellulose-Synthesizing Complexes: Possible Role of KORRIGAN and Microtubules in Cellulose Synthesis in Plants; pp. 169–181. [Google Scholar]
- 108.Putra A., Kakugo A., Furukawa H., Gong J.P., Osada Y. Tubular bacterial cellulose gel with oriented fibrils on the curved surface. Polymer. 2008;49:1885–1891. doi: 10.1016/j.polymer.2008.02.022. [DOI] [Google Scholar]
- 109.Nimeskern L., Martínez Ávila H., Sundberg J., Gatenholm P., Müller R., Stok K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013;22:12–21. doi: 10.1016/j.jmbbm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 110.Bodin A., Concaro S., Brittberg M., Gatenholm P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007;1:406–408. doi: 10.1002/term.51. [DOI] [PubMed] [Google Scholar]
- 111.Charpentier P.A., Maguire A., Wan W. Surface modification of polyester to produce a bacterial cellulose-based vascular prosthetic device. Appl. Surf. Sci. 2006;252:6360–6367. doi: 10.1016/j.apsusc.2005.09.064. [DOI] [Google Scholar]
- 112.Klemm D., Schumann D., Udhardt U., Marsch S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001;26:1561–1603. doi: 10.1016/S0079-6700(01)00021-1. [DOI] [Google Scholar]
- 113.Sepúlveda R.V., Valente F.L., Reis E.C.C., Araújo F.R., Eleotério R.B., Queiroz P.V.S., Borges A.P.B. Bacterial cellulose and bacterial cellulose/polycaprolactone composite as tissue substitutes in rabbits’ cornea. Pesqui. Veterinária Bras. 2016;36:986–992. doi: 10.1590/s0100-736x2016001000011. [DOI] [Google Scholar]
- 114.Barud H.S., Ribeiro S.J.L., Carone C.L.P., Ligabue R., Einloft S., Queiroz P.V.S., Borges A.P.B., Jahno V.D. Optically transparent membrane based on bacterial cellulose/polycaprolactone. Polimeros. 2013;23:135–138. doi: 10.1590/S0104-14282013005000018. [DOI] [Google Scholar]
- 115.Caiut J.M.A., Barud H.S., Santos M.V., Oliveira U.L., Menezes J.F.S., Messaddeq Y., Ribeiro S.J.L. Luminescent multifunctional biocellulose membranes; Proceedings of the Nanostructured Thin Films IV; San Diego, CA, USA. 21–25 August 2011; p. 81040. [DOI] [Google Scholar]
- 116.Kim J., Cai Z., Lee H.S., Choi G.S., Lee D.H., Jo C. Preparation and characterization of a Bacterial cellulose/Chitosan composite for potential biomedical application. J. Polym. Res. 2011;18:739–744. doi: 10.1007/s10965-010-9470-9. [DOI] [Google Scholar]
- 117.Legeza V.I., Galenko-Yaroshevskii V.P., Zinov’ev E.V., Paramonov B.A., Kreichman G.S., Turkovskii I.I., Gumenyuk E.S., Karnovich A.G., Khripunov A.K. Effects of new wound dressings on healing of thermal burns of the skin in acute radiation disease. Bull. Exp. Biol. Med. 2004;138:311–315. doi: 10.1007/s10517-005-0029-4. [DOI] [PubMed] [Google Scholar]
- 118.Messaddeq Y., Ribeiro S.J.L., Thomazini W. Contact Lens for Therapy, Method and Apparatus for Their Production and Use. BR PI0603704-6. Brazil Patent. 2008
- 119.Osorio M., Velásquez-Cock J., Restrepo L.M., Zuluaga R., Gañán P., Rojas O.J., Ortiz-Trujillo I., Castro C. Bioactive 3D-Shaped Wound Dressings Synthesized from Bacterial Cellulose: Effect on Cell Adhesion of Polyvinyl Alcohol Integrated In Situ. Int. J. Polym. Sci. 2017;2017:1–10. doi: 10.1155/2017/3728485. [DOI] [Google Scholar]
- 120.Zhu H., Jia S., Yang H., Jia Y., Yan L., Li J. Preparation and Application of Bacterial Cellulose Sphere: A Novel Biomaterial. Biotechnol. Biotechnol. Equip. 2011;25:2233–2236. doi: 10.5504/BBEQ.2011.0010. [DOI] [Google Scholar]
- 121.Wu S.-C., Li M.-H. Production of bacterial cellulose membranes in a modified airlift bioreactor by Gluconacetobacter xylinus. J. Biosci. Bioeng. 2015;120:444–449. doi: 10.1016/j.jbiosc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 122.Hu Y., Catchmark J.M., Vogler E.A. Factors Impacting the Formation of Sphere-Like Bacterial Cellulose Particles and Their Biocompatibility for Human Osteoblast Growth. Biomacromolecules. 2013;14:3444–3452. doi: 10.1021/bm400744a. [DOI] [PubMed] [Google Scholar]
- 123.Hu Y., Catchmark J.M. Formation and Characterization of Spherelike Bacterial Cellulose Particles Produced by Acetobacter xylinum JCM 9730 Strain. Biomacromolecules. 2010;11:1727–1734. doi: 10.1021/bm100060v. [DOI] [PubMed] [Google Scholar]
- 124.Czaja W., Romanovicz D., malcolm Brown R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose. 2004;11:403–411. doi: 10.1023/B:CELL.0000046412.11983.61. [DOI] [Google Scholar]
- 125.Cai Q., Hu C., Yang N., Wang Q., Wang J., Pan H., Hu Y., Ruan C. Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol. 2018;109:1174–1181. doi: 10.1016/j.ijbiomac.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 126.Gu J., Catchmark J.M. Impact of hemicelluloses and pectin on sphere-like bacterial cellulose assembly. Carbohydr. Polym. 2012;88:547–557. doi: 10.1016/j.carbpol.2011.12.040. [DOI] [Google Scholar]
- 127.Yan Z., Chen S., Wang H., Wang B., Jiang J. Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr. Polym. 2008;74:659–665. doi: 10.1016/j.carbpol.2008.04.028. [DOI] [Google Scholar]
- 128.Zhu H., Jia S., Wan T., Jia Y., Yang H., Li J., Yan L., Zhong C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011;86:1558–1564. doi: 10.1016/j.carbpol.2011.06.061. [DOI] [Google Scholar]
- 129.Hu D. Master’s Thesis. Tianjin University; Tianjin, China: 2014. Study on Structural Modulation and Compounding with Graphene of Bacterial Cellulose for Adsorption of Organics. [Google Scholar]
- 130.Ilyas R.A., Sapuan S.M., Ishak M.R., Zainudin E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018;202:186–202. doi: 10.1016/j.carbpol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Ilyas R.A., Sapuan S.M., Ishak M.R., Zainudin E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019;123:379–388. doi: 10.1016/j.ijbiomac.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 132.Ilyas R.A., Sapuan S.M., Ibrahim R., Abral H., Ishak M.R., Zainudin E.S., Atikah M.S.N., Mohd Nurazzi N., Atiqah A., Ansari M.N.M., et al. Effect of sugar palm nanofibrillated celluloseconcentrations on morphological, mechanical andphysical properties of biodegradable films basedon agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019;8:4819–4830. doi: 10.1016/j.jmrt.2019.08.028. [DOI] [Google Scholar]
- 133.Hazrol M.D., Sapuan S.M., Ilyas R.A., Othman M.L., Sherwani S.F.K. Electrical properties of sugar palm nanocrystalline cellulose reinforced sugar palm starch nanocomposites. Polimery. 2020;65:363–370. doi: 10.14314/polimery.2020.5.4. [DOI] [Google Scholar]
- 134.Ilyas R.A., Sapuan S.M., Ibrahim R., Abral H., Ishak M.R., Zainudin E.S., Asrofi M., Atikah M.S.N., Huzaifah M.R.M., Radzi A.M., et al. Sugar palm (Arenga pinnata (Wurmb.) Merr) cellulosic fibre hierarchy: A comprehensive approach from macro to nano scale. J. Mater. Res. Technol. 2019;8:2753–2766. doi: 10.1016/j.jmrt.2019.04.011. [DOI] [Google Scholar]
- 135.Ilyas R.A., Sapuan S.M., Atiqah A., Ibrahim R., Abral H., Ishak M.R., Zainudin E.S., Nurazzi N.M., Atikah M.S.N., Ansari M.N.M., et al. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2019;41:459–467. doi: 10.1002/pc.25379. [DOI] [Google Scholar]
- 136.Ilyas R.A., Sapuan S.M., Atikah M.S.N., Asyraf M.R.M., Rafiqah S.A., Aisyah H.A., Nurazzi N.M., Norrrahim M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr) Text. Res. J. 2021;91:152–167. doi: 10.1177/0040517520932393. [DOI] [Google Scholar]
- 137.Rozilah A., Jaafar C.N.A., Sapuan S.M., Zainol I., Ilyas R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers. 2020;12:2605. doi: 10.3390/polym12112605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Syafiq R., Sapuan S.M., Zuhri M.Y.M., Ilyas R.A., Nazrin A., Sherwani S.F.K., Khalina A. Antimicrobial Activities of Starch-Based Biopolymers and Biocomposites Incorporated with Plant Essential Oils: A Review. Polymers. 2020;12:2403. doi: 10.3390/polym12102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Syafri E., Sudirman, Mashadi, Yulianti E., Deswita, Asrofi M., Abral H., Sapuan S.M., Ilyas R.A., Fudholi A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019;8:6223–6231. doi: 10.1016/j.jmrt.2019.10.016. [DOI] [Google Scholar]
- 140.Abral H., Ariksa J., Mahardika M., Handayani D., Aminah I., Sandrawati N., Pratama A.B., Fajri N., Sapuan S.M., Ilyas R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020;98:105266. doi: 10.1016/j.foodhyd.2019.105266. [DOI] [Google Scholar]
- 141.Abral H., Ariksa J., Mahardika M., Handayani D., Aminah I., Sandrawati N., Sapuan S.M., Ilyas R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2019;81:106186. doi: 10.1016/j.polymertesting.2019.106186. [DOI] [Google Scholar]
- 142.Sabaruddin F.A., Paridah M.T., Sapuan S.M., Ilyas R.A., Lee S.H., Abdan K., Mazlan N., Roseley A.S.M., Abdul Khalil H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers. 2020;13:116. doi: 10.3390/polym13010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Asrofi M., Sapuan S.M., Ilyas R.A., Ramesh M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type) Mater. Today Proc. 2020;46:1626–1630. doi: 10.1016/j.matpr.2020.07.254. [DOI] [Google Scholar]
- 144.Asrofi M., Sujito, Syafri E., Sapuan S.M., Ilyas R.A. Improvement of Biocomposite Properties Based Tapioca Starch and Sugarcane Bagasse Cellulose Nanofibers. Key Eng. Mater. 2020;849:96–101. doi: 10.4028/www.scientific.net/KEM.849.96. [DOI] [Google Scholar]
- 145.Kamaruddin Z.H., Jumaidin R., Selamat M.Z., Ilyas R.A. Characteristics and Properties of Lemongrass (Cymbopogan Citratus): A Comprehensive Review. J. Nat. Fibers. 2021:1–18. doi: 10.1080/15440478.2021.1958439. [DOI] [Google Scholar]
- 146.Wahab M., Sapuan S.M., Harussani M.M., Zuhri M.Y.M., Saleh A.A. Conceptual Design of Glass/Renewable Natural Fibre-Reinforced Polymer Hybrid Composite Motorcycle Side Cover. J. Renew. Mater. 2021;9:1973–1989. doi: 10.32604/jrm.2021.016221. [DOI] [Google Scholar]
- 147.Ilyas R.A., Sapuan S.M., Sanyang M.L., Ishak M.R., Zainudin E.S. Nanocrystalline cellulose as reinforcement for polymeric matrix nanocomposites and its potential applications: A Review. Curr. Anal. Chem. 2018;14:203–225. doi: 10.2174/1573411013666171003155624. [DOI] [Google Scholar]
- 148.Harussani M.M., Sapuan S.M., Rashid U., Khalina A. Development and Characterization of Polypropylene Waste from Personal Protective Equipment (PPE)-Derived Char-Filled Sugar Palm Starch Biocomposite Briquettes. Polymers. 2021;13:1707. doi: 10.3390/polym13111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jung H.I., Jeong J.H., Lee O.M., Park G.T., Kim K.K., Park H.C., Lee S.M., Kim Y.G., Son H.J. Influence of glycerol on production and structural-physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour. Technol. 2010;101:3602–3608. doi: 10.1016/j.biortech.2009.12.111. [DOI] [PubMed] [Google Scholar]
- 150.Rangaswamy B.E., Vanitha K.P., Hungund B.S. Microbial Cellulose Production from Bacteria Isolated from Rotten Fruit. Int. J. Polym. Sci. 2015;2015:280784. doi: 10.1155/2015/280784. [DOI] [Google Scholar]
- 151.Singh O., Panesar P.S., Chopra H.K. Response surface optimization for cellulose production from agro industrial waste by using new bacterial isolate Gluconacetobacter xylinus C18. Food Sci. Biotechnol. 2017;26:1019–1028. doi: 10.1007/s10068-017-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan C., Xu X., Song L., Guan W., Li J., Liu B., Shi P., Zhang W. The use of Agrobacterium-mediated insertional mutagenesis sequencing to identify novel genes of Humicola insolens involved in cellulase production. 3 Biotech. 2018;8:153. doi: 10.1007/s13205-018-1166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Molina-Ramírez C., Castro M., Osorio M., Torres-Taborda M., Gómez B., Zuluaga R., Gómez C., Gañán P., Rojas O.J., Castro C. Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials. 2017;10:639. doi: 10.3390/ma10060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reese C. Master’s Thesis. Wilfrid Laurier University; Waterloo, ON, Canada: 2019. Characterization of WssF; a Putative Acetyltransferase from Achromobacter insuavis and Pseudomonas fluorescens. [Google Scholar]
- 155.Ahmed S.A., Kazim A.R., Hassan H.M. Increasing Cellulose Production from Rhizobium leguminosarum bv. viciae. J. Al-Nahrain Univ. 2017;20:120–125. doi: 10.22401/JNUS.20.1.17. [DOI] [Google Scholar]
- 156.Anusuya R.S., Anandham R., Kumutha K., Gayathry G., Mageshwaran V. Characterization and optimization of bacterial cellulose produced by Acetobacter spp. J. Environ. Biol. 2020;41:207–215. doi: 10.22438/jeb/41/4/MRN-994. [DOI] [Google Scholar]
- 157.Sun B., Zi Q., Chen C., Zhang H., Gu Y., Liang G., Sun D. Study of specific metabolic pattern of Acetobacter xylinum NuST4.2 and bacterial cellulose production improvement. Cellul. Chem. Technol. 2018;52:795–801. [Google Scholar]
- 158.Canale-Parola E., Wolfe R.S. Synthesis of cellulose by Sarcina ventriculi. Biochim. Biophys. Acta-Gen. Subj. 1964;82:403–405. doi: 10.1016/0304-4165(64)90314-9. [DOI] [PubMed] [Google Scholar]
- 159.Rastogi A., Banerjee R. Production and characterization of cellulose from Leifsonia sp. Process Biochem. 2019;85:35–42. doi: 10.1016/j.procbio.2019.06.008. [DOI] [Google Scholar]
- 160.Sreena C.P., Sebastian D. Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J. Genet. Eng. Biotechnol. 2018;16:9–16. doi: 10.1016/j.jgeb.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Toor Y., Ilyas U. Optimization of cellulase production by Aspergillus ornatus by the solid state fermentation of Cicer arietinum. Am. J. Res. 2014;2:125–141. [Google Scholar]
- 162.Picart P., Diaz P., Pastor F.I.J. Cellulases from two Penicillium sp. strains isolated from subtropical forest soil: Production and characterization. Lett. Appl. Microbiol. 2007;45:108–113. doi: 10.1111/j.1472-765X.2007.02148.x. [DOI] [PubMed] [Google Scholar]
- 163.Prasanna H.N., Ramanjaneyulu G., Rajasekhar Reddy B. Optimization of cellulase production by Penicillium sp. 3 Biotech. 2016;6:162. doi: 10.1007/s13205-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sohail M., Ahmad A., Khan S.A. Production of cellulase from Aspergillus terreus MS105 on crude and commercially purified substrates. 3 Biotech. 2016;6:103. doi: 10.1007/s13205-016-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao J., Weng H., Zhu D., Yuan M., Guan F., Xi Y. Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour. Technol. 2008;99:7623–7629. doi: 10.1016/j.biortech.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 166.Santos T.C.D., Abreu Filho G., Brito A.R.D., Pires A.J.V., Bonomo R.C.F., Franco M. Production and characterization of cellulolytic enzymes by Aspergillus niger and Rhizopus sp. by solid state fermentation of prickly pear. Rev. Caatinga. 2016;29:222–233. doi: 10.1590/1983-21252016v29n126rc. [DOI] [Google Scholar]
- 167.Lee C.K., Darah I., Ibrahim C.O. Production and Optimization of Cellulase Enzyme Using Aspergillus niger USM AI 1 and Comparison with Trichoderma reesei via Solid State Fermentation System. Biotechnol. Res. Int. 2011;2011:1–6. doi: 10.4061/2011/658493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qurat-Ul-Ain, Baig S., Saleem M. Production and characterization of cellulases of Aspergillus niger by using rice husk and saw dust as substrates. Pak. J. Bot. 2012;44:377–382. [Google Scholar]
- 169.Pachauri P., Aranganathan V., More S., Sullia S.B., Deshmukh S. Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels. 2020;11:85–91. doi: 10.1080/17597269.2017.1345357. [DOI] [Google Scholar]
- 170.Petlamul W., Sripornngam T., Buakwan N., Buakaew S., Mahamad K. The Capability of Beauveria Bassiana for Cellulase Enzyme Production; Proceedings of the 7th International Conference on Bioscience, Biochemistry and Bioinformatics–ICBBB ’17; Bangkok Thailand. 21–23 January 2017; New York, NY, USA: ACM Press; 2017. pp. 62–66. [Google Scholar]
- 171.Hernández C., Milagres A.M.F., Vázquez-Marrufo G., Muñoz-Páez K.M., García-Pérez J.A., Alarcón E. An ascomycota coculture in batch bioreactor is better than polycultures for cellulase production. Folia Microbiol. 2018;63:467–478. doi: 10.1007/s12223-018-0588-1. [DOI] [PubMed] [Google Scholar]
- 172.Schuerg T., Gabriel R., Baecker N., Baker S.E., Singer S.W. Thermoascus aurantiacus is an Intriguing Host for the Industrial Production of Cellulases. Curr. Biotechnol. 2017;6:89–97. doi: 10.2174/2211550105666160520123504. [DOI] [Google Scholar]
- 173.Baldrian P., Valášková V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 174.Chen Y.W., Lee H.V., Juan J.C., Phang S.-M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016;151:1210–1219. doi: 10.1016/j.carbpol.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 175.Tarchoun A.F., Trache D., Klapötke T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019;138:837–845. doi: 10.1016/j.ijbiomac.2019.07.176. [DOI] [PubMed] [Google Scholar]
- 176.Sebeia N., Jabli M., Ghith A., Elghoul Y., Alminderej F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019;135:152–162. doi: 10.1016/j.ijbiomac.2019.05.128. [DOI] [PubMed] [Google Scholar]
- 177.Uzyol H.K., Saçan M.T. Bacterial cellulose production by Komagataeibacter hansenii using algae-based glucose. Environ. Sci. Pollut. Res. 2017;24:11154–11162. doi: 10.1007/s11356-016-7049-7. [DOI] [PubMed] [Google Scholar]
- 178.Xiang Z., Gao W., Chen L., Lan W., Zhu J.Y., Runge T. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose. 2016;23:493–503. doi: 10.1007/s10570-015-0840-7. [DOI] [Google Scholar]
- 179.Kobayashi S., Kashiwa K., Shimada J., Kawasaki T., Shoda S. Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase. Makromol. Chem. Macromol. Symp. 55 doi: 10.1002/masy.19920540138. [DOI] [Google Scholar]
- 180.Kobayashi S., Kashiwa K., Kawasaki T., Shoda S. Novel method for polysaccharide synthesis using an enzyme: The first in vitro synthesis of cellulose via a nonbiosynthetic path utilizing cellulase as catalyst. J. Am. Chem. Soc. 1991;113:3079–3084. doi: 10.1021/ja00008a042. [DOI] [Google Scholar]
- 181.Nakatsubo F., Kamitakahara H., Hori M. Cationic Ring-Opening Polymerization of 3,6-Di-O-benzyl-α-d-glucose 1,2,4-Orthopivalate and the First Chemical Synthesis of Cellulose. J. Am. Chem. Soc. 1996;118:1677–1681. doi: 10.1021/ja953286u. [DOI] [Google Scholar]
- 182.Wu H., Williams G.R., Wu J., Wu J., Niu S., Li H., Wang H., Zhu L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018;180:304–313. doi: 10.1016/j.carbpol.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 183.Keshk S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014;4:2. doi: 10.4172/2155-9821.1000150. [DOI] [Google Scholar]
- 184.Abol-Fotouh D., Hassan M.A., Shokry H., Roig A., Azab M.S., Kashyout A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-60315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bae S.O., Sugano Y., Ohi K., Shoda M. Features of bacterial cellulose synthesis in a mutant generated by disruption of the diguanylate cyclase 1 gene of Acetobacter xylinum BPR 2001. Appl. Microbiol. Biotechnol. 2004;65:315–322. doi: 10.1007/s00253-004-1593-7. [DOI] [PubMed] [Google Scholar]
- 186.Cakar F., Özer I., Aytekin A.Ö., Şahin F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014;106:7–13. doi: 10.1016/j.carbpol.2014.01.103. [DOI] [PubMed] [Google Scholar]
- 187.Bae S.O., Shoda M. Production of bacterial cellulose by Acetobacter xylinum BPR2001 using molasses medium in a jar fermentor. Appl. Microbiol. Biotechnol. 2005;67:45–51. doi: 10.1007/s00253-004-1723-2. [DOI] [PubMed] [Google Scholar]
- 188.Poddar M.K., Dikshit P.K. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Sel. 2021;2:1605–1628. doi: 10.1002/nano.202100044. [DOI] [Google Scholar]
- 189.Shi Z., Li Y., Chen X., Han H., Yang G. Double network bacterial cellulose hydrogel to build a biology-device interface. Nanoscale. 2014;6:970–977. doi: 10.1039/C3NR05214A. [DOI] [PubMed] [Google Scholar]
- 190.Vazquez A., Foresti M.L., Cerrutti P., Galvagno M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013;21:545–554. doi: 10.1007/s10924-012-0541-3. [DOI] [Google Scholar]
- 191.Wang J., Tavakoli J., Tang Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019;219:63–76. doi: 10.1016/j.carbpol.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 192.Choi C.N., Song H.J., Kim M.J., Chang M.H., Kim S.J. Properties of bacterial cellulose produced in a pilot-scale spherical type bubble column bioreactor. Korean J. Chem. Eng. 2009;26:136–140. doi: 10.1007/s11814-009-0021-1. [DOI] [Google Scholar]
- 193.Chao Y.P., Sugano Y., Kouda T., Yoshinaga F., Shoda M. Production of bacterial cellulose by Acetobacter xylinum with an air-lift reactor. Biotechnol. Tech. 1997;11:829–832. doi: 10.1023/A:1018433526709. [DOI] [Google Scholar]
- 194.Chao Y., Sugano Y., Shoda M. Bacterial cellulose production under oxygen-enriched air at different fructose concentrations in a 50-L, internal-loop airlift reactor. Appl. Microbiol. Biotechnol. 2001;55:673–679. doi: 10.1007/s002530000503. [DOI] [PubMed] [Google Scholar]
- 195.Chao Y., Ishida T., Sugano Y., Shoda M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000;68:345–352. doi: 10.1002/(SICI)1097-0290(20000505)68:3<345::AID-BIT13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 196.Mormino R., Bungay H. Composites of bacterial cellulose and paper made with a rotating disk bioreactor. Appl. Microbiol. Biotechnol. 2003;62:503–506. doi: 10.1007/s00253-003-1377-5. [DOI] [PubMed] [Google Scholar]
- 197.Zahan K.A., Pa’e N., Muhamad I.I. An evaluation of fermentation period and discs rotation speed of rotary discs reactor for bacterial cellulose production. Sains Malays. 2016;45:393–400. [Google Scholar]
- 198.Lin S.-P., Hsieh S.-C., Chen K.-I., Demirci A., Cheng K.-C. Semi-continuous bacterial cellulose production in a rotating disk bioreactor and its materials properties analysis. Cellulose. 2014;21:835–844. doi: 10.1007/s10570-013-0136-8. [DOI] [Google Scholar]
- 199.Lin S.-P., Liu C.-T., Hsu K.-D., Hung Y.-T., Shih T.-Y., Cheng K.-C. Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose. 2016;23:367–377. doi: 10.1007/s10570-015-0855-0. [DOI] [Google Scholar]
- 200.Fijałkowski K., Żywicka A., Drozd R., Junka A.F., Peitler D., Kordas M., Konopacki M., Szymczyk P., Rakoczy R. Increased water content in bacterial cellulose synthesized under rotating magnetic fields. Electromagn. Biol. Med. 2017;36:192–201. doi: 10.1080/15368378.2016.1243554. [DOI] [PubMed] [Google Scholar]
- 201.Fija K., Anna Ż., Junka A.F., Kordas M., Rakoczy R., Fijałkowski K., Drozd R., Żywicka A., Junka A.F., Kordas M., et al. Biochemical and cellular properties of Gluconacetobacter xylinus cultures exposed to different modes of rotating magnetic field. Pol. J. Chem. Technol. 2017;19:107–114. doi: 10.1515/pjct-2017-0036. [DOI] [Google Scholar]
- 202.Fijałkowski K., Rakoczy R., Żywicka A., Drozd R., Zielińska B., Wenelska K., Cendrowski K., Peitler D., Kordas M., Konopacki M., et al. Time Dependent Influence of Rotating Magnetic Field on Bacterial Cellulose. Int. J. Polym. Sci. 2016;2016:7536397. doi: 10.1155/2016/7536397. [DOI] [Google Scholar]
- 203.Fijałkowski K., Żywicka A., Drozd R., Niemczyk A., Junka A.F., Peitler D., Kordas M., Konopacki M., Szymczyk P., El Fray M., et al. Modification of bacterial cellulose through exposure to the rotating magnetic field. Carbohydr. Polym. 2015;133:52–60. doi: 10.1016/j.carbpol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 204.Jung J.Y., Khan T., Park J.K., Chang H.N. Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J. Chem. Eng. 2007;24:265–271. doi: 10.1007/s11814-007-5058-4. [DOI] [Google Scholar]
- 205.Hornung M., Ludwig M., Schmauder H.P. Optimizing the Production of Bacterial Cellulose in Surface Culture: A Novel Aerosol Bioreactor Working on a Fed Batch Principle (Part 3) Eng. Life Sci. 2007;7:35–41. doi: 10.1002/elsc.200620164. [DOI] [Google Scholar]
- 206.Cheng K.-C., Catchmark J.M., Demirci A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009;3:12. doi: 10.1186/1754-1611-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cheng K.-C., Catchmark J.M., Demirci A. Effects of CMC Addition on Bacterial Cellulose Production in a Biofilm Reactor and Its Paper Sheets Analysis. Biomacromolecules. 2011;12:730–736. doi: 10.1021/bm101363t. [DOI] [PubMed] [Google Scholar]
- 208.Velásquez-Riaño M., Bojacá V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose. 2017;24:2677–2698. doi: 10.1007/s10570-017-1309-7. [DOI] [Google Scholar]
- 209.Harussani M.M., Salit M.S., Rashid U., Abdan K. Plastic Waste Conversion into Electrical, Thermal and Fuel Energy via Incinerations and Pyrolysis amidst COVID-19 Pandemic; Proceedings of the AIUE Proceedings of the 2nd Energy and Human Habitat Conference; Cape Town, South Africa. 26–27 July 2021. [Google Scholar]
- 210.Harussani M.M., Sapuan S.M., Khalina A., Rashid U., Tarique J. Proceedings of the International Symposium on Applied Sciences and Engineering ISASE2021, Erzurum, Turkey, 7–9 April 2021. Office of International Affairs, Atatürk University; Erzurum, Turkey: 2021. Slow pyrolysis of disinfected COVID-19 non-woven polypropylene (PP) waste; pp. 310–312. [Google Scholar]
- 211.Miah J.H., Griffiths A., McNeill R., Halvorson S., Schenker U., Espinoza-Orias N.D., Morse S., Yang A., Sadhukhan J. Environmental management of confectionery products: Life cycle impacts and improvement strategies. J. Clean. Prod. 2018;177:732–751. doi: 10.1016/j.jclepro.2017.12.073. [DOI] [Google Scholar]
- 212.Kongruang S. Biotechnology for Fuels and Chemicals. Springer; Berlin/Heidelberg, Germany: 2007. Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products; pp. 763–774. [DOI] [PubMed] [Google Scholar]
- 213.Goelzer F.D.E., Faria-Tischer P.C.S., Vitorino J.C., Sierakowski M.-R., Tischer C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater. Sci. Eng. C. 2009;29:546–551. doi: 10.1016/j.msec.2008.10.013. [DOI] [Google Scholar]
- 214.Kuo C.-H.H., Huang C.-Y.Y., Shieh C.-J.J., Wang H.-M.M.D., Tseng C.-Y.Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization. 2019;10:85–93. doi: 10.1007/s12649-017-0034-7. [DOI] [Google Scholar]
- 215.Hong F., Guo X., Zhang S., Han S.F., Yang G., Jönsson L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012;104:503–508. doi: 10.1016/j.biortech.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 216.Fan X., Gao Y., He W., Hu H., Tian M., Wang K., Pan S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016;151:1068–1072. doi: 10.1016/j.carbpol.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 217.Keshk S., Sameshima K. The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl. Microbiol. Biotechnol. 2006;72:291–296. doi: 10.1007/s00253-005-0265-6. [DOI] [PubMed] [Google Scholar]
- 218.Qi G., Luo M., Huang C., Guo H., Chen X., Xiong L., Wang B., Lin X., Peng F., Chen X. Comparison of bacterial cellulose production by Gluconacetobacter xylinus on bagasse acid and enzymatic hydrolysates. J. Appl. Polym. Sci. 2017;134:45066. doi: 10.1002/app.45066. [DOI] [Google Scholar]
- 219.Pensupa N., Leu S.-Y., Hu Y., Du C., Liu H., Jing H., Wang H., Lin C.S.K. Chemistry and Chemical Technologies in Waste Valorization. Springer; Berlin/Heidelberg, Germany: 2017. Recent trends in sustainable textile waste recycling methods: Current situation and future prospects; pp. 189–228. [DOI] [PubMed] [Google Scholar]
- 220.Wang Y. Fiber and textile waste utilization. Waste Biomass Valorization. 2010;1:135–143. doi: 10.1007/s12649-009-9005-y. [DOI] [Google Scholar]
- 221.Gao X., Shi Z., Liu C., Yang G., Sevostianov I., Silberschmidt V.V. Inelastic behaviour of bacterial cellulose hydrogel: In aqua cyclic tests. Polym. Test. 2015;44:82–92. doi: 10.1016/j.polymertesting.2015.03.021. [DOI] [Google Scholar]
- 222.Almeida L.R., Martins A.R., Fernandes E.M., Oliveira M.B., Correlo V.M., Pashkuleva I., Marques A.P., Ribeiro A.S., Durães N.F., Silva C.J., et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater. 2013;9:8167–8181. doi: 10.1016/j.actbio.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 223.Kowalska-Ludwicka K., Cala J., Grobelski B., Sygut D., Jesionek-Kupnicka D., Kolodziejczyk M., Bielecki S., Pasieka Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013;9:527–534. doi: 10.5114/aoms.2013.33433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang Y., Zhu C., Yang J., Nie Y., Chen C., Sun D. Recent advances in bacterial cellulose. Cellulose. 2014;21:1–30. doi: 10.1007/s10570-013-0088-z. [DOI] [Google Scholar]
- 225.Bıyık H., Çoban E.P. Evaluation of different carbon, nitrogen sources and industrial wastes for bacterial cellulose production. Eur. J. Biotechnol. Biosci. 2017;5:74–80. doi: 10.5897/AJB2005.000-3087. [DOI] [Google Scholar]
- 226.Voon W.W.Y., Muhialdin B.J., Yusof N.L., Rukayadi Y., Meor Hussin A.S. Bio-cellulose Production by Beijerinckia fluminensis WAUPM53 and Gluconacetobacter xylinus 0416 in Sago By-product Medium. Appl. Biochem. Biotechnol. 2019;187:211–220. doi: 10.1007/s12010-018-2807-2. [DOI] [PubMed] [Google Scholar]
- 227.Uzuner S., Cekmecelioglu D. Enzymes in Food Biotechnology. Elsevier Inc.; Amsterdam, The Netherlands: 2018. Enzymes in the beverage industry. [Google Scholar]
- 228.Chandrasekaran M. Enzymes in Food and Beverage Processing. CRC Press; Boca Raton, FL, USA: 2015. pp. 1–518. [DOI] [Google Scholar]
- 229.Jain S. Development of Low Cost Nutritional Beverage from Whey. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013;5:73–88. doi: 10.9790/2402-0517388. [DOI] [Google Scholar]
- 230.Revin V., Liyaskina E., Nazarkina M., Bogatyreva A., Shchankin M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018;49:151–159. doi: 10.1016/j.bjm.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ratanapariyanuch K., Shen J., Jia Y., Tyler R.T., Shim Y.Y., Reaney M.J.T. Rapid NMR method for the quantification of organic compounds in thin stillage. J. Agric. Food Chem. 2011;59:10454–10460. doi: 10.1021/jf2026007. [DOI] [PubMed] [Google Scholar]
- 232.Wu J.M., Liu R.H. Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J. Biosci. Bioeng. 2013;115:284–290. doi: 10.1016/j.jbiosc.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 233.Jozala A.F., Pértile R.A.N., dos Santos C.A., de Carvalho Santos-Ebinuma V., Seckler M.M., Gama F.M., Pessoa A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015;99:1181–1190. doi: 10.1007/s00253-014-6232-3. [DOI] [PubMed] [Google Scholar]
- 234.Lima H.L.S., Nascimento E.S., Andrade F.K., Brígida A.I.S., Borges M.F., Cassales A.R., Muniz C.R., Souza Filho M.D.S.M., Morais J.P.S., Rosa M.D.F. Bacterial cellulose production by Komagataeibacter hansenii ATCC 23769 using sisal juice—An agroindustry waste. Braz. J. Chem. Eng. 2017;34:671–680. doi: 10.1590/0104-6632.20170343s20150514. [DOI] [Google Scholar]
- 235.Castro C., Zuluaga R., Putaux J.L., Caro G., Mondragon I., Gañán P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011;84:96–102. doi: 10.1016/j.carbpol.2010.10.072. [DOI] [Google Scholar]
- 236.Zhao H., Xia J., Wang J., Yan X., Wang C., Lei T., Xian M., Zhang H. Production of bacterial cellulose using polysaccharide fermentation wastewater as inexpensive nutrient sources. Biotechnol. Biotechnol. Equip. 2018;32:350–356. doi: 10.1080/13102818.2017.1418673. [DOI] [Google Scholar]
- 237.Lin D., Lopez-Sanchez P., Li R., Li Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014;151:113–119. doi: 10.1016/j.biortech.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 238.Viana R.M., Sá N.M.S.M., Barros M.O., Borges M.d.F., Azeredo H.M.C. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018;196:27–32. doi: 10.1016/j.carbpol.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 239.Manrich A., Moreira F.K.V., Otoni C.G., Lorevice M.V., Martins M.A., Mattoso L.H.C. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017;164:83–91. doi: 10.1016/j.carbpol.2017.01.075. [DOI] [PubMed] [Google Scholar]
- 240.Azeredo H.M.C., Kontou-Vrettou C., Moates G.K., Wellner N., Cross K., Pereira P.H.F., Waldron K.W. Wheat straw hemicellulose films as affected by citric acid. Food Hydrocoll. 2015;50:1–6. doi: 10.1016/j.foodhyd.2015.04.005. [DOI] [Google Scholar]
- 241.Cavka A., Guo X., Tang S.-J.J., Winestrand S., Jönsson L.J., Hong F. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels. 2013;6:1–10. doi: 10.1186/1754-6834-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rani M.U., Appaiah K.A.A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. J. Food Sci. Technol. 2013;50:755–762. doi: 10.1007/s13197-011-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xiao X., Hou Y., Liu Y., Liu Y., Zhao H., Dong L., Du J., Wang Y., Bai G., Luo G. Classification and analysis of corn steep liquor by UPLC/Q-TOF MS and HPLC. Talanta. 2013;107:344–348. doi: 10.1016/j.talanta.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 244.Joshi S., Goyal S., Reddy M.S. Corn steep liquor as a nutritional source for biocementation and its impact on concrete structural properties. J. Ind. Microbiol. Biotechnol. 2018;45:657–667. doi: 10.1007/s10295-018-2050-4. [DOI] [PubMed] [Google Scholar]
- 245.Khami S., Khamwichit W., Suwannahong K., Sanongraj W. Characteristics of Bacterial Cellulose Production from Agricultural Wastes. Adv. Mater. Res. 2014;931-932:693–697. doi: 10.4028/www.scientific.net/AMR.931-932.693. [DOI] [Google Scholar]
- 246.Moosavi-Nasab M., Yousefi A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 2011;9:94–101. [Google Scholar]
- 247.Lotfiman S., Awang Biak D.R., Ti T.B., Kamarudin S., Nikbin S. Influence of Date Syrup as a Carbon Source on Bacterial Cellulose Production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018;37:1085–1091. doi: 10.1002/adv.21759. [DOI] [Google Scholar]
- 248.Gomes F.P., Silva N.H.C.S., Trovatti E., Serafim L.S., Duarte M.F., Silvestre A.J.D., Neto C.P., Freire C.S.R. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenergy. 2013;55:205–211. doi: 10.1016/j.biombioe.2013.02.004. [DOI] [Google Scholar]
- 249.Al-Farsi M., Alasalvar C., Al-Abid M., Al-Shoaily K., Al-Amry M., Al-Rawahy F. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007;104:943–947. doi: 10.1016/j.foodchem.2006.12.051. [DOI] [Google Scholar]
- 250.Lestari P., Elfrida N., Suryani A., Suryadi Y. Study on the Production of Bacterial Cellulose from Acetobacter xylinum Using Agro-Waste. Jordan J. Biol. Sci. 2014;7:75–80. doi: 10.12816/0008218. [DOI] [Google Scholar]
- 251.Gülsah K., Gülnur K., Mikhael B., Céline P.-B., Mualla Ö. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017;89:1841. doi: 10.1515/pac-2017-0401. [DOI] [Google Scholar]
- 252.Li Z., Wang L., Hua J., Jia S., Zhang J., Liu H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015;120:115–119. doi: 10.1016/j.carbpol.2014.11.061. [DOI] [PubMed] [Google Scholar]
- 253.Lin Q.B., Che L.L., Guo J., Wang R.Z. Use of 4-chloro-3, 5-dinitrobenzotrifluoride (CNBF) Derivatization and Ultrahigh-performance Liquid Chromatography Tandem Mass Spectrometry for the Determination of 20 Free Amino Acids in Chinese Jujube Date. Food Anal. Methods. 2014;7:571–579. doi: 10.1007/s12161-013-9657-x. [DOI] [Google Scholar]
- 254.Zhang Y., Wang X., Zhao G., Wang Y. Influence of oxidized starch on the properties of thermoplastic starch. Carbohydr. Polym. 2013;96:358–364. doi: 10.1016/j.carbpol.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 255.Estur G., editor. Cotton Exporter’s Guide. 1st ed. International Trade Centre UNCTAD/WTO; University of California; Berkeley, CA, USA: 2007. [Google Scholar]
- 256.Kuo C.H., Lin P.J., Lee C.K. Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J. Chem. Technol. Biotechnol. 2010;85:1346–1352. doi: 10.1002/jctb.2439. [DOI] [Google Scholar]
- 257.Guo X., Chen L., Tang J., Jönsson L.J., Hong F.F. Production of bacterial nanocellulose and enzyme from [AMIM]Cl-pretreated waste cotton fabrics: Effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biotechnol. 2016;91:1413–1421. doi: 10.1002/jctb.4738. [DOI] [Google Scholar]
- 258.Shao Y., Yashiro T., Okubo K., Fujii T. Effect of cellulose nano fiber (CNF) on fatigue performance of carbon fiber fabric composites. Compos. Part A Appl. Sci. Manuf. 2015;76:244–254. doi: 10.1016/j.compositesa.2015.05.033. [DOI] [Google Scholar]
- 259.Tsouko E., Kourmentza C., Ladakis D., Kopsahelis N., Mandala I., Papanikolaou S., Paloukis F., Alves V., Koutinas A. Bacterial cellulose production from industrial waste and by-product streams. Int. J. Mol. Sci. 2015;16:14832–14849. doi: 10.3390/ijms160714832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schrecker S.T., Gostomski P.A. Determining the water holding capacity of microbial cellulose. Biotechnol. Lett. 2005;27:1435–1438. doi: 10.1007/s10529-005-1465-y. [DOI] [PubMed] [Google Scholar]
- 261.Moosavi-nasab M., Yousefi A.R. Investigation of Physicochemical Properties of the Bacterial. World Acad. Sci. Eng. Technol. 2010;44:1258–1263. [Google Scholar]
- 262.Gayathry G., Gopalaswamy G. Production and characterisation of microbial cellulosic fibre from Acetobacter xylinum. Indian J. Fibre Text. Res. 2014;39:93–96. [Google Scholar]
- 263.Wang B., Qi G.X., Huang C., Yang X.Y., Zhang H.R., Luo J., Chen X.F., Xiong L., Chen X.D. Preparation of Bacterial Cellulose/Inorganic Gel of Bentonite Composite by In Situ Modification. Indian J. Microbiol. 2016;56:72–79. doi: 10.1007/s12088-015-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Soemphol W., Hongsachart P., Tanamool V. Production and characterization of bacterial cellulose produced from agricultural by-product by Gluconacetobacter strains. Mater. Today Proc. 2018;5:11159–11168. doi: 10.1016/j.matpr.2018.01.036. [DOI] [Google Scholar]
- 265.Camacho F., Macedo A., Malcata F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs. 2019;17:312. doi: 10.3390/md17060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Goyat M. Ph.D. Thesis. Ryerson University; Toronto, ON, Canada: 2016. Production of Green Bacterial Cellulose Nanofibers by Utilizing Renewable Resources of Algae in Comparison with Agricultural Residue. [Google Scholar]
- 267.Hyun J.Y., Mahanty B., Kim C.G. Utilization of Makgeolli Sludge Filtrate (MSF) as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Appl. Biochem. Biotechnol. 2014;172:3748–3760. doi: 10.1007/s12010-014-0810-9. [DOI] [PubMed] [Google Scholar]
- 268.Jahan F., Kumar V., Saxena R.K.K. Distillery effluent as a potential medium for bacterial cellulose production: A biopolymer of great commercial importance. Bioresour. Technol. 2018;250:922–926. doi: 10.1016/j.biortech.2017.09.094. [DOI] [PubMed] [Google Scholar]
- 269.Ha J.H., Shehzad O., Khan S., Lee S.Y., Park J.W., Khan T., Park J.K. Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J. Chem. Eng. 2008;25:812–815. doi: 10.1007/s11814-008-0134-y. [DOI] [Google Scholar]
- 270.Gayathri G., Srinikethan G. Bacterial Cellulose production by K. saccharivorans BC1 strain using crude distillery effluent as cheap and cost effective nutrient medium. Int. J. Biol. Macromol. 2019;138:950–957. doi: 10.1016/j.ijbiomac.2019.07.159. [DOI] [PubMed] [Google Scholar]
- 271.Urbina L., Hernández-Arriaga A.M., Eceiza A., Gabilondo N., Corcuera M.A., Prieto M.A., Retegi A. By-products of the cider production: An alternative source of nutrients to produce bacterial cellulose. Cellulose. 2017;24:2071–2082. doi: 10.1007/s10570-017-1263-4. [DOI] [Google Scholar]
- 272.Khattak W.A., Khan T., Ul-Islam M., Ullah M.W., Khan S., Wahid F., Park J.K. Production, characterization and biological features of bacterial cellulose from scum obtained during preparation of sugarcane jaggery (gur) J. Food Sci. Technol. 2015;52:8343–8349. doi: 10.1007/s13197-015-1936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jahan F., Kumar V., Rawat G., Saxena R.K. Production of microbial cellulose by a bacterium isolated from fruit. Appl. Biochem. Biotechnol. 2012;167:1157–1171. doi: 10.1007/s12010-012-9595-x. [DOI] [PubMed] [Google Scholar]
- 274.Cerrutti P., Roldán P., García R.M., Galvagno M.A., Vázquez A., Foresti M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016;133:1–9. doi: 10.1002/app.43109. [DOI] [Google Scholar]
- 275.Luo M.-T., Huang C., Chen X.-F., Huang Q.-L., Qi G.-X., Tian L.-L., Xiong L., Li H.-L., Chen X.-D. Efficient bioconversion from acid hydrolysate of waste oleaginous yeast biomass after microbial oil extraction to bacterial cellulose by Komagataeibacter xylinus. Prep. Biochem. Biotechnol. 2017;47:1025–1031. doi: 10.1080/10826068.2017.1373290. [DOI] [PubMed] [Google Scholar]
- 276.Bilgi E., Bayir E., Sendemir-Urkmez A., Hames E.E. Statistical optimization of bacterial cellulose production by gluconacetobacter xylinus using alternative raw materials; Proceedings of the International Biomedical Engineering Congress; Toronto, Canada. 7–12 June 2015; p. 49. [Google Scholar]
- 277.Bilgi E., Bayir E., Sendemir-Urkmez A., Hames E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016;90:2–10. doi: 10.1016/j.ijbiomac.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 278.Pacheco G., Nogueira C.R., Meneguin A.B., Trovatti E., Silva M.C.C., Machado R.T.A., Ribeiro S.J.L., da Silva Filho E.C., Barud H.d.S. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crops Prod. 2017;107:13–19. doi: 10.1016/j.indcrop.2017.05.026. [DOI] [Google Scholar]
- 279.Hong F., Qiu K. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008;72:545–549. doi: 10.1016/j.carbpol.2007.09.015. [DOI] [Google Scholar]
- 280.Chen L., Hong F., Yang X., Han S. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013;135:464–468. doi: 10.1016/j.biortech.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 281.Carreira P., Mendes J.A.S., Trovatti E., Serafim L.S., Freire C.S.R., Silvestre A.J.D., Neto C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011;102:7354–7360. doi: 10.1016/j.biortech.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 282.Adebayo-Tayo B., Akintunde M., Sanusi J. Effect of Different Fruit Juice Media on Bacterial Cellulose Production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. J. Adv. Biol. Biotechnol. 2017;14:1–9. doi: 10.9734/JABB/2017/34171. [DOI] [Google Scholar]
- 283.Kurosumi A., Sasaki C., Yamashita Y., Nakamura Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009;76:333–335. doi: 10.1016/j.carbpol.2008.11.009. [DOI] [Google Scholar]
- 284.Kumbhar J.V., Rajwade J.M., Paknikar K.M. Fruit peels support higher yield and superior quality bacterial cellulose production. Appl. Microbiol. Biotechnol. 2015;99:6677–6691. doi: 10.1007/s00253-015-6644-8. [DOI] [PubMed] [Google Scholar]
- 285.Casarica A., Campeanu G., Moscovici M., Ghiorghita A., Manea V. Improvement of bacterial cellulose production by Aceobacter xyilinum dsmz-2004 on poor quality horticultural substrates using the taguchi method for media optimization. Part I. Cellul. Chem. Technol. 2013;47:61–68. [Google Scholar]
- 286.Lin W.C., Lien C.C., Yeh H.J., Yu C.M., Hsu S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013;94:603–611. doi: 10.1016/j.carbpol.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 287.Güzel M., Akpınar Ö. Production and Characterization of Bacterial Cellulose from Citrus Peels. Waste Biomass Valorization. 2019;10:2165–2175. doi: 10.1007/s12649-018-0241-x. [DOI] [Google Scholar]
- 288.Luo M.-T., Zhao C., Huang C., Chen X.-F., Huang Q.-L., Qi G.-X., Tian L.-L., Xiong L., Li H.-L., Chen X.-D. Efficient Using Durian Shell Hydrolysate as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Indian J. Microbiol. 2017;57:393–399. doi: 10.1007/s12088-017-0681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Algar I., Fernandes S.C.M., Mondragon G., Castro C., Garcia-Astrain C., Gabilondo N., Retegi A., Eceiza A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2015;132:41237. doi: 10.1002/app.41237. [DOI] [Google Scholar]
- 290.Adebayo-Tayo B.C., Akintunde M.O., Alao S.O. Comparative effect of agrowastes on bacterial cellulose production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. Turk. J. Agri. Nat. Sci. 2014;4:145–154. [Google Scholar]
- 291.Florea M., Hagemann H., Santosa G., Abbott J., Micklem C.N., Spencer-Milnes X., de Arroyo Garcia L., Paschou D., Lazenbatt C., Kong D., et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA. 2016;113:E3431–E3440. doi: 10.1073/pnas.1522985113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hungund B., Prabhu S., Shetty C., Acharya S., Prabhu V., Gupta S.G. Production of bacterial cellulose from Gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol. 2013;5:31–33. doi: 10.4172/1948-5948.1000095. [DOI] [Google Scholar]
- 293.Jozala A.F., de Lencastre-Novaes L.C., Lopes A.M., de Carvalho Santos-Ebinuma V., Mazzola P.G., Pessoa A., Jr., Grotto D., Gerenutti M., Chaud M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016;100:2063–2072. doi: 10.1007/s00253-015-7243-4. [DOI] [PubMed] [Google Scholar]
- 294.Barshan S., Rezazadeh-Bari M., Almasi H., Amiri S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019;136:1188–1195. doi: 10.1016/j.ijbiomac.2019.06.192. [DOI] [PubMed] [Google Scholar]
- 295.Premjet S., Premjet D., Ohtani Y. The Effect of Ingredients of Sugar Cane Molasses on Bacterial Cellulose Production by Acetobacter xylinum ATCC 10245. Fiber. 2007;63:193–199. doi: 10.2115/fiber.63.193. [DOI] [Google Scholar]
- 296.Machado R.T.A., Meneguin A.B., Sábio R.M., Franco D.F., Antonio S.G., Gutierrez J., Tercjak A., Berretta A.A., Ribeiro S.J.L., Lazarini S.C., et al. Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind. Crops Prod. 2018;122:637–646. doi: 10.1016/j.indcrop.2018.06.048. [DOI] [Google Scholar]
- 297.Uraki Y., Morito M., Kishimoto T., Sano Y. Bacterial Cellulose Production Using Monosaccharides Derived from Hemicelluloses in Water-Soluble Fraction of Waste Liquor from Atmospheric Acetic Acid Pulping. Holzforschung. 2002;56:341–347. doi: 10.1515/HF.2002.054. [DOI] [Google Scholar]
- 298.Erbas Kiziltas E., Kiziltas A., Gardner D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015;124:131–138. doi: 10.1016/j.carbpol.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 299.Khattak W.A., Khan T., Ul-Islam M., Wahid F., Park J.K. Production, Characterization and Physico-mechanical Properties of Bacterial Cellulose from Industrial Wastes. J. Polym. Environ. 2015;23:45–53. doi: 10.1007/s10924-014-0663-x. [DOI] [Google Scholar]
- 300.Dubey S., Singh J., Singh R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018;247:73–80. doi: 10.1016/j.biortech.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 301.Tyagi N., Suresh S. Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: Optimization & characterization. J. Clean. Prod. 2016;112:71–80. doi: 10.1016/j.jclepro.2015.07.054. [DOI] [Google Scholar]
- 302.Huang C., Guo H.J., Xiong L., Wang B., Shi S.L., Chen X.F., Lin X.Q., Wang C., Luo J., Chen X.D. Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2016;136:198–202. doi: 10.1016/j.carbpol.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 303.Huang C., Yang X.-Y., Xiong L., Guo H.-J., Luo J., Wang B., Zhang H.-R., Lin X.-Q., Chen X.-D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015;60:491–496. doi: 10.1111/lam.12396. [DOI] [PubMed] [Google Scholar]
- 304.Adnan A.B. Ph.D. Dissertation. University of Waikato; Hamilton, New Zealand: 2015. Production of Bacterial Cellulose Using Low-Cost Media. [Google Scholar]
- 305.Kose R., Sunagawa N., Yoshida M., Tajima K. One-step production of nanofibrillated bacterial cellulose (NFBC) from waste glycerol using Gluconacetobacter intermedius NEDO-01. Cellulose. 2013;20:2971–2979. doi: 10.1007/s10570-013-0050-0. [DOI] [Google Scholar]
- 306.Chen Q., Fan Q., Zhang Z., Mei Y., Wang H. Effective in situ harvest of microalgae with bacterial cellulose produced by Gluconacetobacter xylinus. Algal Res. 2018;35:349–354. doi: 10.1016/j.algal.2018.09.002. [DOI] [Google Scholar]
- 307.Nóbrega V., Faria M., Quintana A., Kaufmann M., Ferreira A., Cordeiro N. From a basic microalga and an acetic acid bacterium cellulose producer to a living symbiotic biofilm. Materials. 2019;12:2275. doi: 10.3390/ma12142275. [DOI] [PMC free article] [PubMed] [Google Scholar]