Abstract
Although vaginitis caused by Saccharomyces cerevisiae is extremely rare, in recent years we have experienced an increasing frequency of S. cerevisiae isolation from the vaginas of fertile-age women. In order to investigate the epidemiology of these vaginal infections, a total of 40 isolates of S. cerevisiae derived from symptomatic and asymptomatic women were characterized by two DNA typing approaches, named ribosomal DNA (rDNA) hybridization and Ty917 hybridization, based on the Southern blotting technique. After transfer, the polymorphic DNA restriction fragments were hybridized with the entire repeat of S. cerevisiae rDNA for one method and with the entire sequence of the Ty917 retrotransposon for the other. After elaboration with computer-assisted analysis, the results of each method showed that Ty917 hybridization is endowed with a discriminatory power higher than that of rDNA hybridization. With the Ty917 hybridization method, all of the S. cerevisiae isolates tested appeared very heterogeneous, with the exception of those collected from individual patients with recurrent vaginitis. This allowed us to exclude a possible common source of infection while the high relatedness among S. cerevisiae sequential isolates from recurrent-vaginitis patients could suggest a pattern of relapse rather than frequent reinfection.
Saccharomyces cerevisiae is an ascomycetous yeast used extensively in the baking and brewing industries. Like Candida spp., it colonizes the respiratory, urinary, and gastrointestinal tracts of humans, especially those with chronic underlying diseases (14). Despite the ubiquity of S. cerevisiae, invasive disease due to this yeast has been rarely reported in the past and this yeast was usually considered nonpathogenic. However, together with the recent increase in immunocompromised hosts, such as AIDS patients, transplant recipients, and debilitated patients with malignancies, there has been increasing evidence in the literature of severe clinical diseases caused by Saccharomyces species, including fungemia, endocarditis, pneumonia, and infections of the urinary tract and skin (2, 7, 12, 21, 24, 36).
Vaginal colonization and symptomatic vaginitis due to S. cerevisiae are extremely rare, and the incidence of vaginal infections caused by this yeast has been estimated to be less than 1% (23, 24). On the contrary, Agatensi et al. (1) reported a higher incidence (i.e., 5.5%) of S. cerevisiae infection in Italian women. The increased use of vaginal and systemic azole agents, particularly fluconazole, which exhibits poor activity against this organism, has been reported as one of the major factors that may contribute to disease development (34).
Recently, attempts have been made not only to elucidate the mechanisms by which S. cerevisiae causes vaginitis (5, 8, 19) but also to determine whether local or systemic predisposing factors or exposure to exogenous sources of S. cerevisiae (such as baker’s yeast) may play a role in the pathogenesis of the disease (22, 34, 38). For all of these investigations, different DNA typing methods, such as electrophoretic karyotyping and restriction fragment length polymorphism (RFLP) pattern analysis (9, 22), have been used.
In this report, we describe the use of two DNA typing approaches based on Southern blot hybridization in which probing of polymorphic DNA restriction fragments was performed with the entire repeat unit of ribosomal DNA (rDNA) and with the entire sequence of retrotransposon Ty917, both belonging to S. cerevisiae. The Ty917 probe is a class II yeast transposable element consisting of a 5.9-kb sequence including the two long terminal repeats (28). By these methods we characterized 40 S. cerevisiae clinical isolates from women who presented with either asymptomatic vaginal colonization or symptomatic vaginitis. In addition, an epidemiological investigation was carried out. Finally, the genetic relationship among vaginal and commercial S. cerevisiae isolates was assessed.
(This work was presented in part at the XIIIth Congress of the International Society of Human and Animal Mycology in Salsomaggiore Terme, Parma, Italy, 7 to 13 June 1997 [26]).
MATERIALS AND METHODS
Patients and isolates.
Patient and control groups were designated in a study population of 513 women observed for suspected vaginitis at the Microbiological Service of the University Hospital “A. Gemelli,” Università Cattolica del Sacro Cuore, Rome, Italy, from March to September 1996. Women both with and without symptoms who had cultures positive for S. cerevisiae were included. In the observation period, 30 women with the presence of S. cerevisiae in the vagina were included in the study. Among the patients who attended our service on the same day, two controls with cultures positive for etiologic agents different from S. cerevisiae were selected for each patient. Sixty controls were designated the comparison group. At the time of observation, all of the women were investigated for the assessment of social, clinical, and laboratory information. This information included each patient’s age, current symptoms, history of vaginitis, pregnancy, estroprogestinic drug therapy, urinary bacterial infections, and use of yeast products in the home, at work, or to benefit health.
A total of 40 S. cerevisiae isolates were recovered from vaginal swabs. In addition, two strains obtained from the only two commercial preparations of yeasts sold in the same city were included in the study. Reference strain S. cerevisiae ATCC 9763 was also tested. All isolates were identified by the Automicrobic Vitek System (bioMerieux, Marcy l’Etoile, France), and micromorphology was assessed by the Dalmau plate technique (37). Moreover, the identification was confirmed by the API 20C Aux (bioMerieux). In addition, all of the isolates were confirmed to belong to the species by restriction enzyme analysis of the PCR-amplified intergenic transcribed spacer regions as described by McCullough et al. (18). Isolates from the same individual or apparently different colonies of the same strain were considered related in origin. Isolates from different individuals were considered unrelated in origin. All isolates were maintained on YEPD agar (1% yeast extract, 2% Bacto Peptone, 2% glucose, 2% Bacto Agar) at room temperature. Long-term storage was done in a 20% glycerol water solution at −80°C. In some instances, yeasts were grown in YEPD broth.
DNA probes.
The 9.0-kb rDNA repeat unit of S. cerevisiae was cloned from strain AB 1380 (4) into pBluescript SK+ (Stratagene, La Jolla, Calif.) at the KpnI site and used as a probe for rDNA hybridization. With the Ty917 hybridization method, we used as a probe retrotransposon Ty917 generated as an XhoI-XhoI fragment from plasmid pR22 (30). Both DNA probes were labeled with digoxigenin dUTP by the random priming method in accordance with the manufacturer’s instructions (DNA labeling kit; Boehringer Mannheim, Mannheim, Germany) and stored at −20°C until use.
Southern blot hybridization analysis.
Genomic DNA from yeasts was extracted as described previously (31). A 10-μg sample of DNA from each strain was separately digested at 37°C for 4 h with restriction enzymes EcoRI and HindIII (Boehringer Mannheim) for the rDNA and Ty917 analyses, respectively. The products of each digestion were separated in a 0.8% (wt/vol) agarose gel in TAE buffer (40 mM Tris-acetate, 1 mM EDTA [pH 8.00]) at 27 V for 18 h at room temperature. After visualization under UV light by ethidium bromide staining, the DNA fragments were transferred to a positively charged membrane (Boehringer Mannheim) in a vacuum blotter apparatus by following the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, Calif.) with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) as the transfer buffer. DNA was fixed on the membrane by baking at 80°C for 2 h. Membranes were prehybridized at 42°C with a buffer containing 50% formamide, 5× SSC, 2% blocking reagent (Boehringer Mannheim), 0.1% N-lauroylsarcosine, and 0.02% sodium dodecyl sulfate. DNA probes were added to the hybridization solution, and the mixture was incubated overnight. The washing and revealing steps were identical to those recommended by the supplier (DIG luminescent detection kit for nucleic acids; Boehringer Mannheim). Membranes were exposed to X-Omat AR film (Eastman Kodak Company, Rochester, N.Y.) for documentation.
Computerized data analysis.
To compare the DNA fingerprints of all isolates, X-ray images of Southern blots were scanned with a Scanjet 4C scanner (Hewlett Packard, Palo Alto, Calif.) and elaborated in a computer-digitized dendrogram by Dendron software package version 2.0 (Solltech, Iowa City, Iowa). Distortions in the gel were straightened with the unwarping option of Dendron, and each lane was automatically identified and scanned. The coefficient of similarity (SAB) between the patterns of every pair of strains, designated A and B, respectively, was computed by the formula SAB = 2E/2E + a + b, where E is the number of bands shared by strains A and B, a is the number of bands unique to strain A, and b is the number of bands unique to strain B. For the present study, an SAB of 1.00 indicates that all bands in the patterns of isolates A and B completely match, an SAB of 0.00 indicates that the patterns of strains A and B had no band in common, and SABs ranging between 0.01 and 0.99 represent increasing levels of similarity. Dendrograms based on SABs were generated by the unweighted pair group method with arithmetic averages (32).
Stability tests.
To assess the stability of Southern hybridization patterns during in vitro culture, serial passages from an initial broth culture of S. cerevisiae ATCC 9763 were done in YEPD broth. At passage 15, DNA was extracted from the newly grown cells and compared to that from the parent culture by Southern hybridization analysis.
Statistical analysis.
Odds ratios (ORs) with 95% confidence intervals (CIs) for measuring the association between different characteristics at baseline and the vaginal presence of S. cerevisiae was obtained by a cross-tabulation procedure. Statistical analyses were performed by using Fisher’s exact test. In order to estimate the contribution of each independent variable to predict the event, a conditional logistic regression model with forward stepwise selection was employed, with a chosen cutoff value of 0.05 to enter into the model. Statistical tests were performed by SPSS Base on Professional Statistics 7.5 software (SPSS Inc., Chicago, Ill.). Differences with probabilities of <0.05 were considered statistically significant.
RESULTS
Clinical correlations.
Of 513 women who were referred to the Microbiological Service at the University Hospital in Rome during the experimental period, 30 patients positive for S. cerevisiae (5.8%), aged 25 to 39 years (mean age, 32.1 years), and 60 controls negative for S. cerevisiae, aged 15 to 42 years (mean age, 30.6 years), were included in this study (Table 1). S. cerevisiae was isolated on two occasions from seven patients and three times from one patient, resulting in 40 clinical isolates that were available for evaluation, including the two morphological variants isolated from the same clinical specimen (Table 2). In addition, two nonclinical S. cerevisiae strains obtained from commercial yeast preparations in the same area were included for comparison.
TABLE 1.
Clinical data and use of yeast by the 90 patients enrolled as cases and controls in this study
| Parameter | Cases (n = 30) | Controls (n = 60) | OR (95% CI) | P value |
|---|---|---|---|---|
| Median age (yr) | 32.1 | 30.6 | 0.34 | |
| Pregnancy | 22 | 30 | 2.75 (1.0–7.1) | 0.028 |
| Estroprogestinic therapy | 1 | 0 | NCa | 0.61 |
| Previous bacterial infections of urinary tract | 14 | 8 | 5.7 (2.0–16.0) | 0.001 |
| Symptoms (vulvar pruritus, irritation, discharge) | 16 | 29 | 1.22 (0.46–3.23) | 0.65 |
| Use of yeast | ||||
| Domestic, >3 times/wk | 28 | 3 | 266 (35.2–2,744.4) | 0.0001 |
| Occupational | 0 | 0 | NC | |
| Consumption for health benefit | 2 | 0 | NC | 0.21 |
NC, not calculable.
TABLE 2.
S. cerevisiae isolates from the 30 patients
| Patient no. | Age (yrs) | S. cerevisiae isolate(s) |
|---|---|---|
| 1 | 35 | SC1, SC18 |
| 2 | 35 | SC2, SC3 |
| 3 | 32 | SC4, SC12, SC31 |
| 4 | 28 | SC5 |
| 5 | 28 | SC6 |
| 6 | 31 | SC8 |
| 7 | 28 | SC9 |
| 8 | 31 | SC10 |
| 9 | 39 | SC11 |
| 10 | 33 | SC13 |
| 11 | 33 | SC15 |
| 12 | 26 | SC17 |
| 13 | 25 | SC19 |
| 14 | 39 | SC20 |
| 15 | 30 | SC21, SC22 |
| 16 | 36 | SC23 |
| 17 | 33 | SC25, SC43 |
| 18 | 31 | SC26 |
| 19 | 32 | SC27 |
| 20 | 32 | SC28, SC29 |
| 21 | 29 | SC32 |
| 22 | 36 | SC33a, SC33b |
| 23 | 38 | SC34 |
| 24 | 38 | SC35 |
| 25 | 33 | SC37 |
| 26 | 25 | SC40 |
| 27 | 39 | SC41 |
| 28 | 31 | SC42, SC51 |
| 29 | 25 | SC44 |
| 30 | 33 | SC47, SC50 |
Sixteen (53.3%) of the 30 patients presented vulvovaginal pruritus, irritation, and a typical discharge (clumpy, cottage cheese appearance). The remaining patients, in whom symptoms and signs of inflammation were lacking, were considered to be colonized. The majority of the patients (22 of 30) were pregnant, and one was treated with estroprogestinic drugs. At the time of examination, all other causes of possible vulvovaginitis, such as trichomoniasis, bacterial infection, genital herpes, or infection with yeasts other than S. cerevisiae, were excluded, but 1 patient suffered from Candida vaginitis and 14 reported recent recurrent urinary tract infections. Twenty-eight of 30 patients reported the use of large amounts of this yeast as alimentary products (self-prepared pizza and more than three times a week) 2 of 30 ate raw baker’s yeast, but none used the yeast occupationally. Among the 60 control women, the majority were negative for vaginal carriage of yeast. Only seven controls had vaginal swabs positive for yeast, three symptomatic nonpregnant women and two symptomatic pregnant women had C. albicans in their vaginas, and two asymptomatic pregnant women had C. albicans and C. parapsilosis, respectively. None was positive for S. cerevisiae. Only three of the controls reported domestic use of yeast more than three times a week. A significant correlation with the presence of S. cerevisiae was observed for alimentary use of large amounts of yeast (OR, 266.0; 95% CI, 35.2 to 2744.4; P < 0.0001), for previous bacterial urinary tract infections (OR, 5.7; 95% CI, 2.0 to 16.0; P < 0.001), and for pregnancy (OR, 2.75; 95% CI, 1.0 to 7.1; P < 0.03) (Table 1). Multivariate logistic regression analysis showed that only alimentary use of yeast had an independent significant ability to predict S. cerevisiae isolation (OR, 351.3; 95% CI, 37.0 to 3331.8; P < 0.00001).
Initial results of Southern blot hybridization.
In preliminary experiments, DNAs extracted from reference strain S. cerevisiae ATCC 9763 and several clinical isolates were digested with a panel of restriction endonucleases (EcoRI, BamHI, ClaI, HindIII, BglII, and PstI). The DNA digests were Southern hybridized with the rDNA and Ty917 probes, respectively. Restriction enzyme EcoRI was found to be better for the method that uses probing with rDNA, while HindIII was chosen for the Ty917 method (data not shown). The reproducibility of patterns was checked by performing experiments in duplicate. EcoRI and HindIII were then used for all subsequent digestions.
Stability of RFLP patterns over time.
S. cerevisiae ATCC 9763 was tested by the method described above to assess the stability of the patterns generated by hybridization with the rDNA and Ty917 probes. A single ATCC 9763 colony was inoculated into liquid medium. The DNA was analyzed with the rDNA and Ty917 probes and compared to DNA extracted from a broth culture of the same parent strain after several passages on solid medium. The hybridization patterns were found to be identical, with no appreciable changes.
Analysis by rDNA hybridization.
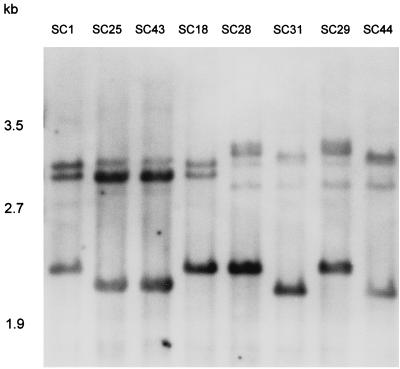
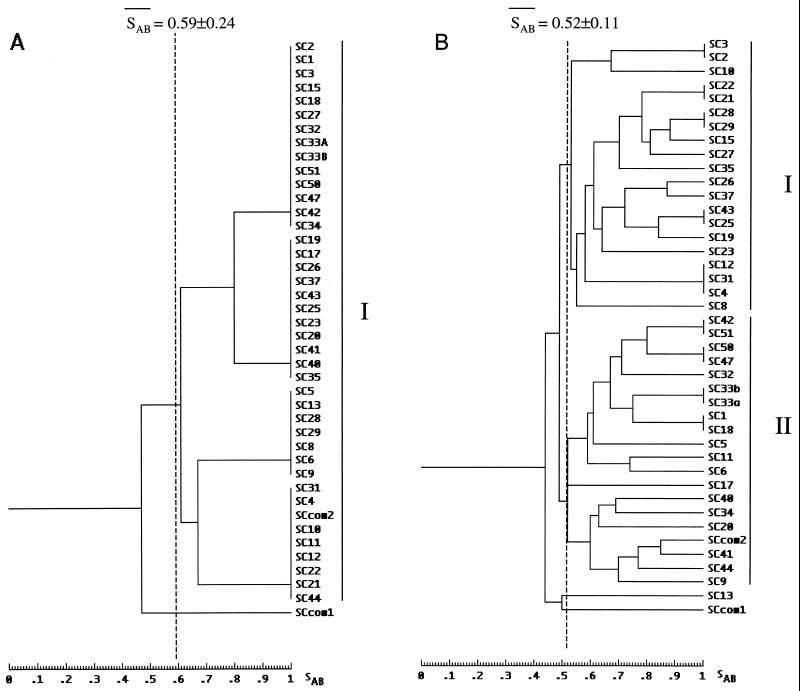
All 40 of the S. cerevisiae clinical isolates in this study have been fingerprinted with an rDNA probe. rDNA generated patterns of three to five bands in the range of 2 to 3 kb for each isolate. Examples of the patterns obtained by hybridization with rDNA are presented in Fig. 1. Among the 42 isolates analyzed, including the two commercial strains, four different rDNA hybridization profiles were observed. A dendrogram of relatedness for the 42 isolates based on SABs computed from band positions is presented in Fig. 2A. The average SAB for the 42 isolates analyzed was 0.55 ± 0.29, and the average for the 30 isolates from unrelated hosts was 0.59 ± 0.24. The lowest SAB in the dendrogram generated by hybridization with rDNA was 0.40. The analysis with rDNA grouped the following known related isolates as identical: SC1 and SC18; SC2 and SC3; SC4, SC12, and SC31; SC21 and SC22; SC25 and SC43; SC28 and SC29; SC42 and SC51; and SC47 and SC50. In addition, SC33a and SC33b were also found to be identical; they represent morphological variations of the same strain. However, rDNA also grouped as identical several isolates unrelated in origin. In fact, with an SAB of 1, all of the clinical isolates are distinguished in four subgroups (Fig. 2A). With an average of 0.59 as a threshold, rDNA analysis generated a unique cluster (Fig. 2A). All isolates but one were grouped in this cluster.
FIG. 1.
Examples of hybridization patterns obtained with rDNA for S. cerevisiae isolates. Molecular sizes are presented on the left. The designations at the top refer to patients’ S. cerevisiae isolates.
FIG. 2.
Dendrograms depicting the relatedness of the 40 clinical isolates and the 2 commercial isolates (SCcom1 and SCcom2) fingerprinted by Southern blot hybridization with rDNA (A) and Ty917 (B). In each case, the average SAB is indicated as a dashed line.
Analysis by Ty917 hybridization.
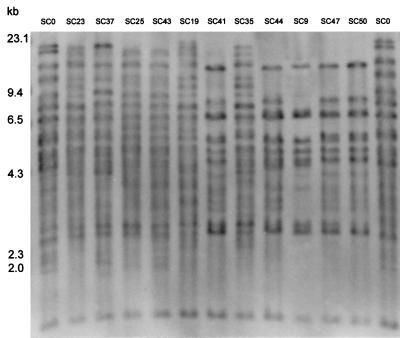
All of the isolates in this study were also analyzed with the Ty917 hybridization method. The Ty917 probe generated patterns of 10 to 22 bands in the range of 1 to >14 kb for each isolate. Examples of the patterns obtained by hybridization with probe Ty917 are presented in Fig. 3. The reproducibility of the patterns is evident in the repeat patterns for isolate SC0 (reference strain ATCC 9763) in the outermost lanes, isolate SC25 versus isolate SC43 (isolates from the same individual), and isolate SC47 versus isolate SC50 (isolates from the same individual). Ty917 hybridization yielded 32 profiles for the 42 isolates analyzed. Figure 2B shows a dendrogram based on band positions alone. The average SAB for all of the isolates analyzed was 0.53 ± 0.12, and the average for the 30 isolates from unrelated hosts was 0.52 ± 0.11. The analysis with probe Ty917 distinguished as identical the following eight pairs and one group of isolates: SC1 and SC18; SC2 and SC3; SC4, SC12, and SC31; SC21 and SC22; SC25 and SC43; SC28 and SC29; SC33a and SC33b; SC42 and SC51; and SC47 and SC50. These isolates were considered to be related in origin. In contrast to the rDNA hybridization results, with an SAB of 1, only the isolates related in origin were classified as highly related. In addition, with an average SAB threshold of 0.52, the Ty917 analysis generated two general clusters, as shown in Fig. 2B. All but two isolates were grouped in these clusters. No genetic identity between commercial and clinical strains was found.
FIG. 3.
Examples of hybridization patterns obtained with probe Ty917 for S. cerevisiae isolates. Molecular sizes are presented on the left. The designations at the top refer to some patients’ S. cerevisiae isolates and reference strain S. cerevisiae ATCC 9763 (SC0).
Discriminatory powers of the methods tested.
Both of the methods used were compared for the ability to identify identical or highly related isolates (e.g., isolates from recurrent infection in the same individual), to discriminate between unrelated isolates (e.g., from unrelated individuals in the same geographic area), and to cluster weakly related isolates. The Ty917 hybridization and rDNA hybridization techniques were able to find undistinguishable sequential isolates derived from eight patients during the recurrence of vaginitis, as well as isolates SC33a and SC33b, which were derived from the same strain and had different morphological characteristics. With regard to the capacity of the two methods to differentiate unrelated isolates, analysis with the rDNA probe revealed a lower level of discrimination. This is due to the very small number of bands obtainable by analysis with the rDNA probe. On the other hand, analysis with the Ty917 probe, through generation of a higher number of polymorphic bands, provided increased levels of discrimination; in the meantime, this method demonstrated adequate clustering capacity. Our findings show that the genetic diversity observable among S. cerevisiae isolates with Ty917 hybridization makes it an effective DNA typing method for epidemiological purposes.
DISCUSSION
The aim of this study was to demonstrate that good characterization of S. cerevisiae clinical isolates can be achieved by using a molecular method such as Southern blot hybridization analysis. Even though the development of a molecular typing method useful for the characterization of a yeast infection is a desirable goal for many mycologists, the number of studies in which the epidemiology of S. cerevisiae infections has been investigated is very limited (9, 17, 34). A universally accepted typing method for this organism has been not defined. This could be due in part to the low prevalence of diseases caused by S. cerevisiae, particularly of vaginal infections. However, in our investigation, these infections have been of considerable interest. As described above, of 513 women who visited our Microbiological Service in the study period, 5.8% had S. cerevisiae in their vaginal swabs. This was in agreement with the data reported by Agatensi et al. (1), by which the incidence of vaginal infections due to S. cerevisiae in Italian women was estimated to be greater than that observed by others (17). Moreover, the clinical data recorded 4 months after initiation of the study showed how the eradication of S. cerevisiae required a prolonged therapeutic regimen in almost of these patients.
In order to investigate the epidemiology of these infections, S. cerevisiae patient isolates were characterized by Southern blot hybridization. This report describes the first application of a DNA typing technique in which the polymorphic DNA restriction fragments derived from these isolates were hybridized with a Ty917 retrotransposon probe. In parallel, the same isolates were analyzed by hybridization with the entire repeat of rDNA as a probe.
The choice of the method used was based on a critical review of the techniques available for epidemiological studies on fungi (25, 27). Among these techniques, which include electrophoretic karyotyping, randomly amplified polymorphic DNA analysis, restriction enzyme analysis of PCR-amplified genes, and RFLP analysis (3, 6, 10, 11, 15, 16, 20, 39), the last is the most extensively used for typing of yeasts. The work of Magee et al. (16) is a valid example of how RFLP generated in the rDNA repeats of various C. albicans clinical isolates can be used to discriminate isolates at the species level. Clemons et al. (9) have successfully used the same method for strain differentiation of 60 clinical and nonclinical S. cerevisiae isolates.
Even though RFLP is able to differentiate a high number of strains, some problems are encountered when the system is used. The major limitation of this method is the difficulty in comparing complex profiles which consist of a large number of bands. This was in part circumvented by the availability of computerized systems for automated reading of the patterns (17). In contrast, Southern blot analysis can simplify this reading and allows the detection of only particular restriction fragments associated with specific chromosomal loci. The ease of interpreting Southern blots can be directly affected by the choice of the enzymes used to prepare the genomic DNA digests and the choice of probes. However, it is noteworthy that the discriminatory power of a typing method decreases with a concomitant decrease in the number of the obtainable fragments. For all of these reasons, defining the optimal strategy for Southern blot analysis has required considerable effort.
With regard to the probe to be used, our attention was focused on a Ty917 transposable element (13, 29) because Southern blot analysis in which insertion sequences and/or transposons are used as probes have proven to be reproducible and highly discriminatory for epidemiological typing of pathogenic bacteria (35). To our knowledge, a method in which retrotransposon Ty917 is used as a probe to detect polymorphism generated by random insertion of this element into the yeast genome has never been applied to yeasts. The patterns generated by this method were compared with those obtained by rDNA polymorphism analysis at the same similarity coefficient, and in each case, dendrograms based upon the same unweighted pair group method were generated. Ty917 hybridization was demonstrated to have the ability to differentiate between unrelated isolates and, as a consequence, to be applicable for molecular epidemiological analyses. The great capacity of Ty917 hybridization to discriminate S. cerevisiae isolates could be judged detrimental for a DNA typing method, because strains with high or weak relatedness could be considered unrelated. However, we verified that Ty917 hybridization was able to assess genetic relatedness between highly related S. cerevisiae isolates such as those derived from the same patient. The fact that S. cerevisiae strains isolated from individual patients at intervals of up 1 year showed the same Ty917 pattern provided proof of the in vivo stability of this transposable element, according to its low frequency of transposition into the S. cerevisiae genome (13). In addition, the great genetic difference observed among all of the other isolates correlates with the epidemiological findings on the infections, for which we can exclude a possible common source or transmission by person-to-person contact. Moreover, the molecular analysis performed on S. cerevisiae strains from commercial preparations of yeasts showed no identity to clinical isolates. These findings seem not to agree with some data published previously (38) that suggested bread making as a possible cause for S. cerevisiae vaginal infections. In particular, Nyirjesy et al. (22) demonstrated in a woman with acute vaginitis due to S. cerevisiae infection, the disease can result from inoculation of yeast from exogenous sources. By molecular analysis, this author found a strong correlation between the isolates obtained from the patient’s vagina, her husband’s fingers, and the yeast used in his pizza shop. By contrast, the results of our molecular investigation suggest that each patient had been infected with and/or had harbored in her vagina a unique individual strain and that the repeated infections occurring in some women might be due to the same infecting organism, which has been suppressed only temporarily by antifungal therapy. Other laboratory findings (data not shown) on these women caused us to suspect that the vaginal relapse was due to recurrent infections and not an intestinal reservoir.
Another aim of this study was to understand the pathogenesis of vaginitis caused by S. cerevisiae. Although several risk factors (34) have been previously identified, such as treatment with corticosteroids and broad-spectrum antibiotics, the increased use of vaginal and systemic azole agents, and high-level exposure to an environmental source of S. cerevisiae, this study failed to clearly define the pathogenesis of S. cerevisiae vaginitis. With regard to our patients, statistical analysis was performed to evaluate whether factors such as pregnancy, previous urinary tract bacterial infections, or weekly contact with an exogenous source of yeasts could be considered to predispose women to vaginal colonization or symptomatic vaginitis due to S. cerevisiae. Statistically significant differences were observed when patients and controls groups were compared either for urinary tract infections and frequent (more than three times a week) consumption of yeast products. For the last variable, an OR of 266 was observed. In fact, when multivariate analysis was applied by a logistic regression model, a very strong correlation only between the presence of S. cerevisiae and frequent domestic use of yeast was found. This allowed us to hypothesize that direct exposure of our patients to the yeast used for food preparation could occur, although the molecular analysis carried out on two products containing commercial yeast failed to demonstrate an epidemiologic link with the S. cerevisiae clinical isolates. On the other hand, we hypothesized that the excess of carbohydrates in the diets of these women, together with the high glycogen content of pregnant women (33), might produce alterations of the vaginal environment and facilitate the adherence of S. cerevisiae cells to the vaginal epithelium. However, it remains unclear how, in some cases, this microorganism has been able to cause true infections with clear symptoms of vaginitis.
To summarize, in the light of the increasing presence of S. cerevisiae in the vaginas of fertile-age women and the difficult eradication of this organism, we think that the molecular method we propose has successfully supported our effort to clarify the epidemiology of S. cerevisiae infections.
ACKNOWLEDGMENTS
This work was supported by National Research Program on AIDS-1997 grant 50A.0.15 from the Istituto Superiore di Sanità, Ministero della Sanità, Italy.
We thank Saveria Pastore for critical reading of the manuscript and Andrea Antinori for assistance with the statistical analyses.
REFERENCES
- 1.Agatensi L, Franchi F, Mondello F, Bevilacqua R L, Ceddia T, De Bernardis F, Cassone A. Vaginopathic and proteolytic Candida species in outpatients attending a gynecology clinic. J Clin Pathol. 1991;44:826–830. doi: 10.1136/jcp.44.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aucott J N, Fayen J, Grossnicklas H, Morrissey A, Lederman M M, Salata R A. Invasive infection with Saccharomyces cerevisiae: report of three cases and review. Rev Infect Dis. 1990;12:406–411. doi: 10.1093/clinids/12.3.406. [DOI] [PubMed] [Google Scholar]
- 3.Baleiras Couto M M, Eijsma B, Hofstra H, Huis in’t Veld J H J, van der Vossen J M B M. Evaluation of molecular typing techniques to assign genetic diversity among Saccharomyces cerevisiae strains. Appl Environ Microbiol. 1996;62:41–46. doi: 10.1128/aem.62.1.41-46.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke D T, Carle G F, Olson M V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987;236:806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- 5.Byron J K, Clemons K V, McCusker J H, Davis R W, Stevens D A. Pathogenicity of Saccharomyces cerevisiae in complement factor five-deficient mice. Infect Immun. 1995;63:478–485. doi: 10.1128/iai.63.2.478-485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlotti A, Grillot R, Couble A, Villard J. Typing of Candida krusei clinical isolates by restriction endonuclease analysis and hybridization with CkF1,2 DNA probe. J Clin Microbiol. 1994;32:1691–1699. doi: 10.1128/jcm.32.7.1691-1699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimolai N, Gill M J, Church D. Saccharomyces cerevisiae fungemia: case report and review of the literature. Diagn Microbiol Infect Dis. 1987;8:113–117. doi: 10.1016/0732-8893(87)90158-1. [DOI] [PubMed] [Google Scholar]
- 8.Clemons K V, McCusker J H, Davis R W, Stevens D A. Comparative pathogenesis of clinical and non-clinical isolates of Saccharomyces cerevisiae. J Infect Dis. 1994;169:859–867. doi: 10.1093/infdis/169.4.859. [DOI] [PubMed] [Google Scholar]
- 9.Clemons K V, Park P, McCusker J H, McCullough M J, Davis R W, Stevens D A. Application of DNA typing methods and genetic analysis to epidemiology and taxonomy of Saccharomyces isolates. J Clin Microbiol. 1997;35:1822–1828. doi: 10.1128/jcm.35.7.1822-1828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS. New perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. doi: 10.3109/10408419309113523. [DOI] [PubMed] [Google Scholar]
- 11.Doebbeling B N, Hollis R J, Isenberg H D, Wenzel R P, Pfaller M A. Restriction fragment analysis of a Candida tropicalis outbreak of sternal wound infections. J Clin Microbiol. 1991;29:1268–1270. doi: 10.1128/jcm.29.6.1268-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eng R H, Drehmel R, Smith S M, Goldstein E J C. Saccharomyces cerevisiae in man. Sabouraudia. 1984;22:403–407. [PubMed] [Google Scholar]
- 13.Garfinkel D J, Curcio M J, Youngren S D, Sanders N J. The biology and exploitation of the retrotransposon Ty in Saccharomyces cerevisiae. Genome. 1989;31:909–919. doi: 10.1139/g89-162. [DOI] [PubMed] [Google Scholar]
- 14.Kiehn T E, Edwards F F, Armstrong D. The prevalence of yeasts in clinical specimens from cancer patients. Am J Clin Pathol. 1980;73:518–521. doi: 10.1093/ajcp/73.4.518. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann P L, Lin D, Lasker B A. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J Clin Microbiol. 1992;30:3249–3254. doi: 10.1128/jcm.30.12.3249-3254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magee B B, D’Souza T M, Magee P T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987;169:1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough M J, Clemons K C, Farina C, McCusker J H, Stevens D A. Epidemiological investigation of vaginal Saccharomyces cerevisiae isolates by a genotypic method. J Clin Microbiol. 1998;36:557–562. doi: 10.1128/jcm.36.2.557-562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough M J, Clemons K C, McCusker J H, Stevens D A. Intergenic transcribed spacer PCR ribotyping for differentiation of Saccharomyces species and interspecific hybrids. J Clin Microbiol. 1998;36:1035–1038. doi: 10.1128/jcm.36.4.1035-1038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCusker J H, Clemons K V, Stevens D A, Davis R W. Genetic characterization of pathogenic Saccharomyces cerevisiae isolates. Genetics. 1994;136:1261–1269. doi: 10.1093/genetics/136.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merz W G, Connelly C, Hieter P. Variation of electrophoretic karyotypes among clinical isolates of Candida albicans. J Clin Microbiol. 1988;26:842–845. doi: 10.1128/jcm.26.5.842-845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen H, Stenderup J, Bruun B. Fungemia with Saccharomycetaceae. Scand J Infect Dis. 1990;22:581–584. doi: 10.3109/00365549009027100. [DOI] [PubMed] [Google Scholar]
- 22.Nyirjesy P, Vazquez J A, Ufberg D D, Sobel J D, Boikov D A, Buckley H R. Saccharomyces cerevisiae vaginitis: transmission from yeast used in baking. Obstet Gynecol. 1995;86:326–329. doi: 10.1016/0029-7844(95)00174-P. [DOI] [PubMed] [Google Scholar]
- 23.Odds F C. Ecology of Candida and epidemiology of candidiasis. In: Odds F C, editor. Candida and candidiasis. London, United Kingdom: Bailliere Tindall; 1988. pp. 85–86. [Google Scholar]
- 24.Oriol A, Ribera J M, Arnal J, Milla F, Batlle M, Feliu E. Saccharomyces cerevisiae septicemia in a patient with myelodisplastic syndrome. Am J Hematol. 1993;43:325–326. doi: 10.1002/ajh.2830430424. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller M A. Epidemiology of fungal infections: the promise of molecular typing. Clin Infect Dis. 1995;20:1535–1539. doi: 10.1093/clinids/20.6.1535. [DOI] [PubMed] [Google Scholar]
- 26.Posteraro B, Sanguinetti M, D’Amore G, Morace G, Fadda G. Abstracts of the 13th Congress of the International Society for Human and Animal Mycology 1997. Parma, Italy: Salsomaggiore Terme; 1997. Molecular typing of Saccharomyces cerevisiae clinical isolates, abstr. O48; p. 88. [Google Scholar]
- 27.Reiss E, Tanaka K, Bruker G, Chazalet V, Coleman D, Debeaupais J P, Hanazawa R, Latgé J P, Lortholary J, Makimura K, Morrison C, Murayama S Y, Naoe S, Paris S, Sarfati J, Shibuya K, Sullivan D, Uchida K, Yamaguchi H. Molecular diagnosis and epidemiology of fungal infections. Med Mycol. 1998;36:249–257. [PubMed] [Google Scholar]
- 28.Roeder G S, Fink G R. Movement of yeast transposable elements by gene conversion. Proc Natl Acad Sci USA. 1982;79:5621–5625. doi: 10.1073/pnas.79.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandmeyer S B. Yeast retrotransposons. Curr Opin Genet Dev. 1992;2:705–711. doi: 10.1016/s0959-437x(05)80130-3. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti M, Posteraro B, Rossolini G M, Satta G. Development of a yeast retrotransposon-based system useful for screening of potentially active anti-retroviral compounds. Microbiologica. 1995;18:117–125. [PubMed] [Google Scholar]
- 31.Scherer S, Stevens D A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987;25:675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneath P H, Sokal R R. Numerical taxonomy. The principles and practice of numerical classification. San Francisco, Calif: W. H. Freeman & Co.; 1973. pp. 230–234. [Google Scholar]
- 33.Sobel J D. Pathophysiology of vulvovaginal candidiasis. J Reprod Med. 1989;34:572–580. [PubMed] [Google Scholar]
- 34.Sobel J D, Vazquez J A, Lynch M, Meriwether C, Zervos M J. Vaginitis due to Saccharomyces cerevisiae: epidemiology, clinical aspects, and therapy. Clin Infect Dis. 1993;16:93–99. doi: 10.1093/clinids/16.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Stanley J, Saunders N. DNA insertion sequences and the molecular epidemiology of Salmonella and Mycobacterium. J Med Microbiol. 1996;45:236–251. doi: 10.1099/00222615-45-4-236. [DOI] [PubMed] [Google Scholar]
- 36.Tawfik O W, Papasian C J, Dixon A Y, Potter L M. Saccharomyces cerevisiae pneumonia in a patient with acquired immune deficiency syndrome. J Clin Microbiol. 1989;27:1689–1691. doi: 10.1128/jcm.27.7.1689-1691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren N G, Hazen K C. Candida, Cryptococcus and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. Washington, D.C: American Society for Microbiology; 1995. pp. 723–737. [Google Scholar]
- 38.Wilson J D, Jones B M, Kinghorn G R. Bread-making as a source of vaginal infection with Saccharomyces cerevisiae: report of a case in a woman and apparent transmission to her partner. Sex Transm Dis. 1988;15:35–36. doi: 10.1097/00007435-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Zerva L, Hollis R J, Pfaller M A. In vitro susceptibility testing and DNA typing of Saccharomyces cerevisiae clinical isolates. J Clin Microbiol. 1996;34:3031–3034. doi: 10.1128/jcm.34.12.3031-3034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]