Abstract
Introduction
Hepatocellular carcinoma (HCC) has been the second leading cause of cancer-related death in China. Radiofrequency ablation is a relatively novel treatment that may improve the treatment of HCC.
Aim
To evaluate and compare the efficacy and safety of radiofrequency ablation (RFA) versus laparoscopic liver resection (LLR) in the treatment of HCC.
Material and methods
We searched for relevant published studies in English (PubMed, Cochrane Library, EMBASE) and in Chinese (CBM, CNKI and Wanfang) from their inception until September 23, 2019. The quality of included studies was evaluated by the Newcastle-Ottawa Scale.
Results
A total of 19 retrospective studies including 2038 patients were eligible for the meta-analysis. The results of the meta-analysis demonstrated that LLR was superior to RFA in terms of 3-year overall survival rate (OR = 0.62), 1 to 3-year disease-free survival rates (OR = 0.57; OR = 0.41, respectively) and local recurrence rates (OR = 2.71).
Conclusions
The meta-analysis demonstrates that laparoscopic liver resection should be preferred in tumors of size 3–5 cm, while for < 3 cm the long term results are equal.
Keywords: radiofrequency ablation, laparoscopic liver resection, small hepatocellular carcinoma, survival
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a global public health problem [1, 2]. It prevalent in the Asia-Pacific region and increasing in Western countries, and is predicted to exceed a million cases per year by 2025 worldwide [3, 4]. It occurs often in patients with viral hepatitis, liver cirrhosis, nonalcoholic fatty liver disease, alcohol abuse, and aflatoxin exposure [5]. In China, HCC has been the fourth most common malignant tumor and the second leading cause of cancer-related death. The incidence and death of HCC have been on the rise in recent decades [6–8], mainly due to the hepatitis B virus infection epidemic in China [9].
Treatment options for HCC include liver transplantation, liver resection, and loco-regional therapies such as radiofrequency ablation (RFA) or transarterial chemoembolization or systemic treatment. Theoretically, the best treatment for HCC conforming to the Milan criteria (single HCC ≤ 5 cm or up to 3 nodules < 3 cm) is liver transplantation, which is limited in China by the underlying liver dysfunction, lack of donors, a large number of patients and economic capacity [10–12]. Thus, hepatic resection, especially laparoscopic liver resection (LLR), is still considered a viable treatment option [13, 14]. Several alternative loco-regional therapies have been developed, such as RFA, percutaneous ethanol injection, acetic acid injection, microwave coagulation, and transcatheter arterial chemoembolization, while RFA is the most frequently used because of its simplicity, safety, and minimal invasiveness [15]. Several studies had compared the clinical effects of RFA and LLR for treatment of small HCC [16–19], but the sample sizes of those studies were small and the results are still controversial. In two recent meta-analyses focused on the comparison between RFA and minimally invasive liver surgery by Si et al. and Li et al. [20, 21], Si et al. found that minimally invasive liver surgery was superior to RFA according to long-term outcomes and RFA might be an alternative treatment for HCC with a single nodule less than 3 cm [20]; Li et al. found that there was no conclusive evidence for the superiority of LLR or RFA for the treatment of HCCs [21]. Additionally, there has been accumulating evidence on the comparison of RFA and LLR for treatment of HCC in China in recent years, but the evidence is not readily accessible for the international readership.
Aim
The aim of this comprehensive meta-analysis including both English and Chinese databases was to compare the clinical outcomes of RFA and LLR for the treatment of HCC in China, providing scientific information for better clinical decisions.
Material and methods
Search strategy and selection criteria
Two investigators independently and comprehensively searched the online databases in English (PubMed, Cochrane Library, EMBASE) and in Chinese (Chinese Biological Medical Literature, Chinese National Knowledge Infrastructure and Wanfang) from their inception to September 23, 2019 to identify relevant articles. The following search terms were used: (“laparoscopic partial hepatectomy” or “laparoscopic resection” or “laparoscopic operation”) and (“radiofrequency ablation” or “radio-frequency ablation”) and (“small hepatocellular carcinoma” or “small liver carcinoma” or “small hepatic carcinoma”) and (“China” or “Chinese” or “Hongkong” or “Taiwan”). Potentially related articles in the reference lists of all the retrieved articles were manually searched. The searches were limited to human subjects, and in addition, restriction to English or Chinese language was applied. Two investigators independently reviewed, selected and extracted data of all identified studies, and any disagreement about eligibility was resolved by discussion with a third investigator. This study was conducted according to the PRISMA guidelines for the screening and selection process [22].
Inclusion and exclusion criteria
The included studies fulfilled the following criteria: (1) adult patients diagnosed with primary hepatocellular carcinoma by cytohistological evidence or radiological imaging techniques; (2) HCC meets the following criteria: “patients with single HCC nodule ≤ 5 cm in diameter or up to 3 nodules that are each ≤ 3 cm in diameter” [23], or “a single tumor ≤ 6.5 cm in diameter or up to 3 nodules that are each ≤ 4.5 cm in diameter and 8 cm in total diameter, with Child-Pugh class A/B” [24]; (3) radiofrequency ablation included percutaneous or laparoscopic RFA, LLR included laparoscopic or laparoscopic-assisted or robotic-assisted liver resection; (4) no metastases and previous treatments; and (5) patients suitable for LLR and RFA according to the guidelines. Exclusion criterion were as follows: (1) patients with liver metastases or recurrence; (2) absence of original data or comparative results between LLR and RFA; (3) abstracts, case reports, editorials, or reviews.
Data extraction
The relevant data were extracted by two independent reviewers, including first author, publication year, study period, areas in China, sex, age, number of patients, number of nodules, tumor size, Child-Pugh class and clinical outcome indicators. The follow-up clinical outcomes included local recurrence rates, 3-year OS rates, and 3-year DFS rates. The perioperative outcomes included postoperative complication rates, blood loss, transfusion rate, hospitalization duration, and overall response rate; and liver function indicators of postoperative 1 month.
Quality assessment
The methodological quality of included studies was assessed by two independent reviewers using the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies based on the population selection, comparability and outcomes. Every study was awarded a total score from 1 to 9 stars by adding all satisfied items. High quality was considered with NOS ≥ 7 stars.
Statistical analysis
All analyses were performed using R version 3.6.1 software with the “metabin” and “metacont” functions in the meta package. The odds ratio (OR) with the 95% confidence interval (CI) was calculated for dichotomous outcomes, and the standardized mean differences (SMDs) with 95% confidence intervals (CI) for continuous outcomes between RFA and LLR groups. The heterogeneity of the study was determined by the I2 statistic and Q test. There was moderate or high heterogeneity when I2 > 50% and p < 0.05 of the Q test, then the random effect model was applied; otherwise, the fixed effect model was adopted. Subgroup analyses were performed based on data in patients with different lesion sizes, RFA approaches and study areas in China. Sensitivity analyses were conducted by successively excluding each study and then the effect size was calculated based on the remaining studies for assessing which study markedly affected the pooled results. Publication bias among included studies was assessed by funnel plot analysis. A p-value < 0.05 was considered statistically significant.
Results
Study selection and characteristics
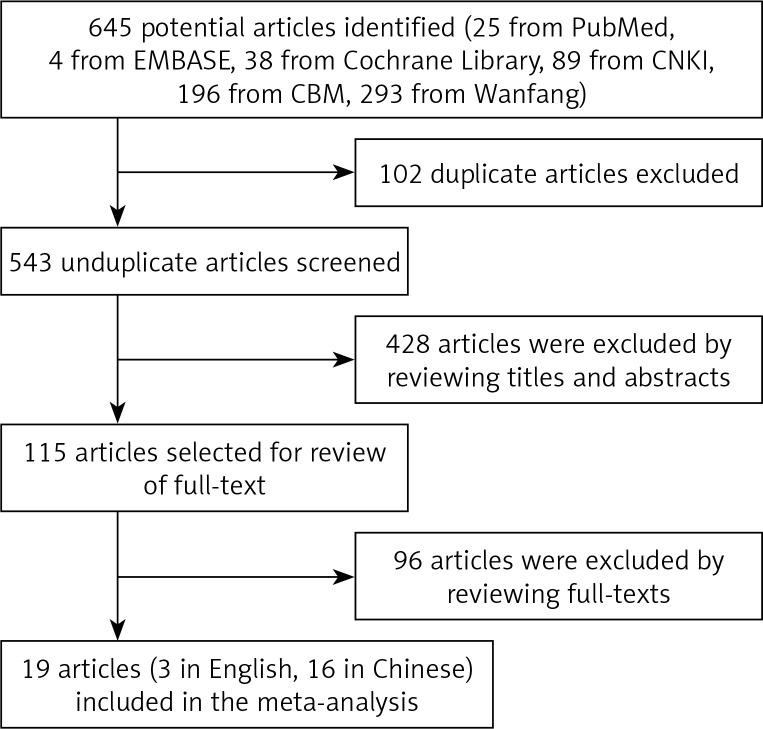
A total of 645 articles were initially identified from the electronic databases. Removing the duplicates and reviewing the titles, abstracts and full texts, 19 nonrandomized controlled studies with a total of 2038 patients (1043 for RFA and 995 for LLR) met the inclusion criteria for meta-analysis. The outcomes of the systematic literature search are shown in Figure 1. The sample size ranged from 61 to 213 and the mean age of patients ranged from 32.7 to 66.5 years. The total score of quality assessment ranges from 6 to 9. The detailed characteristics and evaluation indexes of these patients are shown in Table I.
Figure 1.
Flowchart for selection of studies
Table I.
Baseline characteristics of included studies
| Study | Region | Period | Treat | Sample size | Sex (M/F) | Age (M ± SD) | Child-Pugh A/B | Tumor number | Tumor size | NOS | Outcome indicators | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1–3 | |||||||||||
| Xu ZJ, 2017 [28] | Anhui | 2012–2015 | LLR | 30 | 25/5 | 52.8 ±9.4 | 29/1 | 65 | 0 | ≤ 3 cm | 8 | Operation time, blood loss, hospitalization duration, liver function, complication, local recurrence, overall survival rate, disease-free survival rate |
| PRFA | 35 | 27/8 | 57.1 ±12.7 | 32/3 | ||||||||
| Song JX, 2016 [26] | Chongqing | 2007–2013 | LLR | 78 | 70/8 | 48.0 ±11.1 | 78/0 | 73 | 83 | ≤ 4 cm | 9 | Operation time, blood loss, blood transfusion, hospitalization duration, complication, local recurrence, overall survival rate, disease-free survival rate |
| PRFA | 78 | 70/8 | 48.0 ±9.6 | 76/2 | ||||||||
| Song JX, 2017 [27] | Chongqing | 2012–2014 | LLR | 81 | 69/12 | 49.0 ±10.7 | 80/1 | 162 | 13 | ≤ 5 cm | 7 | Operation time, blood loss, hospitalization duration, complication, local recurrence, overall survival rate, disease-free survival rate |
| PRFA | 94 | 82/12 | 48.0 ±11.8 | 88/6 | ||||||||
| He RQ, 2016 [33] | Shanxi | 2009–2015 | LLR | 41 | 28/13 | 52.7 ±9.4 | 30/11 | 66 | 13 | ≤ 5 cm | 6 | Operation time, blood loss, hospitalization duration, complication, curative effect, life quality improvement |
| LRFA | 38 | 30/8 | 54.1 ±11.2 | 28/10 | ||||||||
| Wang XW, 2017 [34] | Chongqing | 2011–2015 | LLR | 61 | 35/26 | 65.4 ±15.6 | 46/15 | 105 | 21 | ≤ 3 cm | 7 | Complication, overall survival time, disease-free survival time, liver function |
| PRFA | 65 | 36/29 | 66.5 ±16.1 | 47/18 | ||||||||
| Cui HX, 2019 [29] | Henan | 2013–2015 | LLR | 48 | 21/28 | 53.7 ±9.2 | 21/27 | 78 | 19 | ≤ 3 cm | 7 | Operation time, blood loss, hospitalization duration, complication, curative effect, overall survival rate, liver function |
| PRFA | 49 | 29/20 | 53.3 ±9.6 | 21/28 | ||||||||
| Song CG, 2018 [43] | Shandong | 2015–2018 | LLR | 51 | 39/12 | 62.2 ±5.2 | 49/2 | – | – | ≤ 3 cm | 6 | Operation time, blood loss, hospitalization duration, curative effect, life quality improvement |
| PRFA | 51 | 38/13 | 62.6 ±5.7 | 50/1 | ||||||||
| Zhou SX, 2019 [30] | Zhejiang | 2011–2015 | LLR | 45 | 35/10 | 59.5 ±10.4 | 31/14 | 66 | 21 | ≤ 5 cm | 7 | Hospitalization duration, complication, liver function, overall survival rate, disease-free survival rate |
| LRFA | 42 | 33/9 | 58.3 ±10.7 | 27/15 | ||||||||
| Zheng XW, 2019 [35] | Henan | 2013–2013 | LLR | 31 | 23/8 | 64.2 ±2.2 | – | – | – | ≤ 5 cm | 7 | Complication, overall survival rate, disease-free survival rate |
| LRFA | 31 | 22/9 | 64.2 ±2.2 | – | ||||||||
| Fan XK, 2018 [32] | Sichuan | 2014–2016 | LLR | 31 | 25/6 | 58.6 ±7.2 | 26/5 | 52 | 17 | ≤ 5 cm | 8 | Operation time, blood loss, liver function, pain index, local recurrence, overall survival rate, |
| LRFA | 38 | 28/10 | 55.7 ±6.3 | 29/9 | ||||||||
| Ma W, 2018 [36] | Shanxi | 2015–2017 | LLR | 60 | 35/25 | 59.7 ±9.3 | – | – – |
– – |
≤ 5 cm | 6 | Operation time, hospitalization duration, complication, liver function |
| PRFA | 60 | 34/26 | 59.5 ±9.7 | – | ||||||||
| Su N, 2019 [37] | Hubei | 2017–2018 | LLR | 42 | 23/19 | 57.1 ±4.3 | – | – | – | ≤ 5 cm | 6 | Operation time, hospitalization duration, complication, liver function |
| PRFA | 42 | 22/20 | 58.6 ±4.4 | – | ||||||||
| Wang YJ, 2018 [38] | Fujian | 2013–2017 | LLR | 35 | 21/14 | 59.2 ±9.4 | – | – | – | ≤ 5 cm | 6 | Operation time, hospitalization duration, curative effect, complication, liver function |
| PRFA | 35 | 20/15 | 59.5 ±9.7 | – | ||||||||
| Wang X, 2018 [39] | Liaoning | 2016–2017 | LLR | 40 | 28/12 | 66.2 ±10.8 | – | – | – | ≤ 3 cm | 7 | Operation time, hospitalization duration, complication, liver function, overall survival time, disease-free survival time |
| PRFA | 40 | 22/18 | 64.2 ±7.6 | – | ||||||||
| Zhao J, 2018 [40] | Sichuan | 2014–2016 | LLR | 68 | 38/30 | 55.7 ±7.6 | 50/18 | 113 | 21 | ≤ 3 cm | 6 | Hospitalization duration, complication, disease-free survival time |
| PRFA | 66 | 40/26 | 55.4 ±7.3 | 49/17 | ||||||||
| Zhang HY, 2019 [41] | Fujian | 2012–2014 | LLR | 60 | 34/26 | 32.7 ±15.5 | 55/5 | 88 | 52 | ≤ 5 cm | 7 | Complication, local recurrence, overall survival, disease-free survival, liver function |
| PRFA | 80 | 45/35 | 35.2 ±17.2 | 72/8 | ||||||||
| Liu T, 2016 [42] | Hebei | 2014–2015 | LLR | 106 | 80/26 | 58.9 ±1.1 | 83/23 | 157 | 56 | ≤ 5 cm | 6 | Curative effect, complication, quality of life |
| PRFA | 107 | 98/9 | 58.8 ±0.3 | 75/32 | ||||||||
| Lai C, 2016 [25] | Zhejiang | 2006–2011 | LLR | 28 | 24/4 | 56.5 ±12.6 | 28/0 | 56 | 5 | ≤ 5 cm | 9 | Operation time, blood loss, blood transfusion, morbidity, mortality, hospital stay, local recurrence, overall survival rate, disease-free survival rate |
| PRFA | 33 | 29/4 | 62.8 ±11.3 | 29/4 | ||||||||
| Chong CC, 2019 | Xianggang | 2005–2015 | LLR | 59 | 46/13 | 57.7 ±10.5 | 59/0 | 112 | 6 | ≤ 3 cm | 9 | Overall survival rate, disease-free survival rate |
| RFA | 59 | 46/13 | 59.3 ±11.0 | 58/1 | ||||||||
LLR – laparoscopic liver resection, PRFA – percutaneous radio frequency ablation, LRFA – laparoscopic radio frequency ablation, M – male, F – female, NOS – Newcastle–Ottawa Scale, M ± SD – mean ± standard deviation.
Follow-up clinical outcomes
Overall survival (OS)
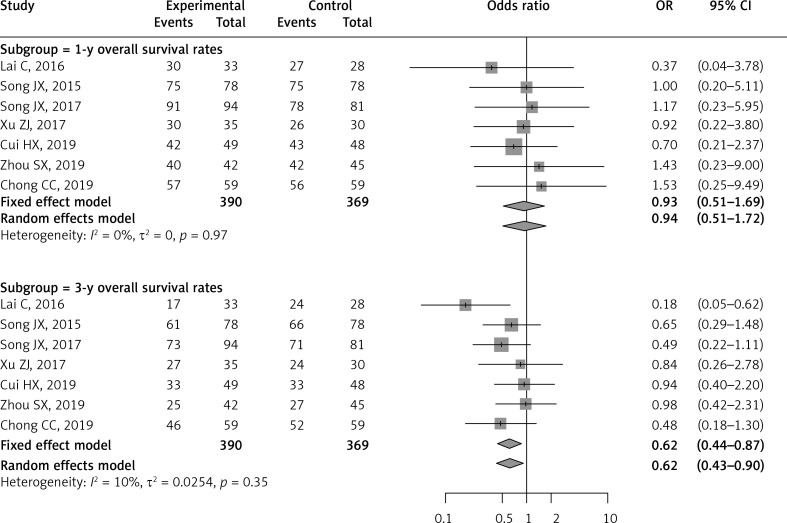
Seven of the included studies reported and compared the 1-year and 3-year overall survival rates [25–31]. There was no significant difference between RFA and LLR groups for the 1-year OS rate (OR = 0.93; 95% CI: 0.51–1.69; p = 0.809). However, meta-analysis of these seven studies demonstrated a significantly lower 3-year OS rate in the RFA patients compared to the LLR patients (OR = 0.62; 95% CI: 0.44–0.87; p = 0.006). There was no significant heterogeneity for pooling the 1-year and 3-year OS rates (I2 = 0%, p = 0.97; I2 = 0%, p = 0.25, respectively) (Table II, Figure 2).
Table II.
Subgroup analyses based on RFA approach, areas in China and single nodule size between RFA and LH
| Subgroup | Categories | Study | Patients | Statistical method | Effect estimate (95% CI) | I2 (%) | P-value |
|---|---|---|---|---|---|---|---|
| Postoperative local recurrence: | |||||||
| Total | 5 | 583 | OR (M-H, Fixed) | 2.71 (1.84–3.99) | 39.0 | < 0.0001 | |
| RFA approach: | |||||||
| LRFA | 1 | 69 | OR (M-H, Fixed) | 1.67 (0.14–19.29) | – | 0.682 | |
| PRFA | 3 | 396 | OR (M-H, Fixed) | 2.05 (1.30–3.23) | 0.0 | 0.002 | |
| Mixed | 1 | 118 | OR (M-H, Fixed) | 6.77 (3.01–15.23) | – | < 0.0001 | |
| Areas: | |||||||
| East | 1 | 118 | OR (M-H, Fixed) | 6.77 (3.01–15.23) | – | < 0.0001 | |
| Mid-west | 4 | 465 | OR (M-H, Fixed) | 2.04 (1.3–3.19) | 0.0 | 0.002 | |
| Overall survival: | |||||||
| Total | 1-y | 7 | 759 | OR (M-H, Fixed) | 0.93 (0.51–1.69) | 0.0 | 0.809 |
| 3-y | 7 | 759 | OR (M-H, Fixed) | 0.62 (0.44–0.87) | 10.0 | 0.006 | |
| Single nodule: | |||||||
| ≤ 5 cm | 1-y | 4 | 479 | OR (M-H, Fixed) | 0.96 (0.40–2.31) | 0.0 | 0.929 |
| 3-y | 4 | 479 | OR (M-H, Fixed) | 0.56 (0.36–0.86) | 41.0 | 0.008 | |
| ≤ 3 cm | 1-y | 3 | 280 | OR (M-H, Fixed) | 0.90 (0.40–2.04) | 0.0 | 0.805 |
| 3-y | 3 | 280 | OR (M-H, Fixed) | 0.73 (0.42–1.29) | 0.0 | 0.278 | |
| RFA approach: | |||||||
| LRFA | 1-y | 1 | 87 | OR (M-H, Fixed) | 1.43 (0.23–9.0) | – | 0.704 |
| 3-y | 1 | 87 | OR (M-H, Fixed) | 0.98 (0.42–2.31) | – | 0.964 | |
| PRFA | 1-y | 5 | 554 | OR (M-H, Fixed) | 0.81 (0.41–1.61) | 0.0 | 0.556 |
| 3-y | 5 | 554 | OR (M-H, Fixed) | 0.58 (0.38–0.87) | 24.0 | 0.009 | |
| Mixed | 1-y | 1 | 118 | OR (M-H, Fixed) | 1.53 (0.25–9.49) | – | 0.649 |
| 3-y | 1 | 118 | OR (M-H, Fixed) | 0.48 (0.18–1.3) | – | 0.146 | |
| Areas: | |||||||
| East | 1-y | 3 | 266 | OR (M-H, Fixed) | 1.02 (0.35–3.02) | 0.0 | 0.966 |
| 3-y | 3 | 266 | OR (M-H, Fixed) | 0.52 (0.3–0.91) | 60.0 | 0.022 | |
| Mid-west | 1-y | 4 | 493 | OR (M-H, Fixed) | 0.89 (0.43–1.83) | 0.0 | 0.750 |
| Disease-free survival: | |||||||
| Total | 1-y | 6 | 662 | OR (M-H, Fixed) | 0.57(0.40–0.83) | 43.0 | 0.003 |
| 3-y | 6 | 662 | OR (M-H, Fixed) | 0.41 (0.30–0.57) | 59.0 | < 0.0001 | |
| Single nodule: | |||||||
| ≤ 5 cm | 1-y | 4 | 479 | OR (M-H, Fixed) | 0.68 (0.44–1.06) | 20.0 | 0.089 |
| 3-y | 4 | 479 | OR (M-H, Fixed) | 0.46 (0.32–0.66) | 31.0 | < 0.0001 | |
| ≤ 3 cm | 1-y | 2 | 183 | OR (M-H, Random) | 0.43 (0.11–1.60) | 70.0 | 0.208 |
| 3-y | 2 | 183 | OR (M-H, Random) | 0.37 (0.07–1.95) | 85.0 | 0.24 | |
| RFA approach: | |||||||
| LRFA | 1-y | 1 | 87 | OR (M-H, Fixed) | 0.91 (0.34–2.48) | – | 0.86 |
| 3-y | 1 | 87 | OR (M-H, Fixed) | 0.85 (0.36–1.99) | – | 0.708 | |
| PRFA | 1-y | 4 | 457 | OR (M-H, Fixed) | 0.67 (0.43–1.05) | 17.0 | 0.08 |
| 3-y | 4 | 457 | OR (M-H, Fixed) | 0.44 (0.31–0.65) | 24.0 | < 0.0001 | |
| Mixed | 1-y | 1 | 118 | OR (M-H, Fixed) | 0.23 (0.09–0.57) | – | 0.002 |
| 3-y | 1 | 118 | OR (M-H, Fixed) | 0.16 (0.07–0.36) | – | < 0.0001 | |
| Areas: | |||||||
| East | 1-y | 3 | 266 | OR (M-H, Fixed) | 0.44 (0.24–0.78) | 51.0 | 0.005 |
| 3-y | 3 | 266 | OR (M-H, Random) | 0.31 (0.11–0.91) | 76.0 | 0.032 | |
| Mid-west | 1-y | 3 | 396 | OR (M-H, Fixed) | 0.69 (0.43–1.12) | 42.0 | 0.133 |
| 3-y | 3 | 396 | OR (M-H, Fixed) | 0.49 (0.33–0.74) | 0.0 | < 0.001 | |
1-y – 1-year, 3-y – 3-year, LRFA – laparoscopic, PRFA – percutaneous, OR – odds ratio, M-H – Mantel-Haenszel method.
Figure 2.
Forest plot of 1-year and 3-year overall survival rates between RFA and LLR
Disease-free survival (DFS)
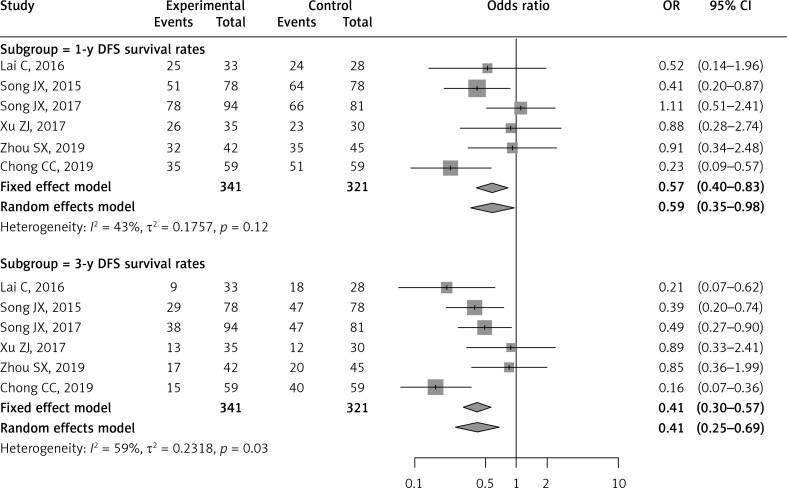
The 1-year and 3-year disease-free survival rates were reported and compared in six of the included studies [25–28, 30, 31]. The LLR group had a higher DFS rate than the RFA group for 1-year DFS rate (OR = 0.57; 95% CI: 0.40–0.83; p = 0.003) and 3-year DFS rate (OR = 0.41; 95% CI: 0.30–0.57; p < 0.0001). No significant heterogeneity was observed in 1-year DFS rate (I2 = 43%, p = 0.12), and moderate heterogeneity in 3-year DFS rate (I2 = 59%, p = 0.03) (Table II, Figure 3).
Figure 3.
Forest plot of 1-year and 3-year disease-free survival rates between RFA and LLR
Local recurrence rate (LRR)
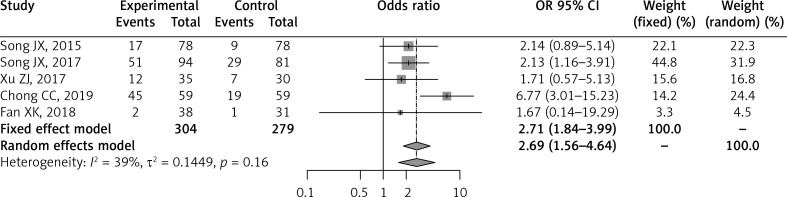
Five of the included studies compared the postoperative local recurrence rates during median follow-up times [26–28, 31, 32]. Meta-analysis of these six studies using the fixed effect model demonstrated that the RFA-treated group had a significantly higher local recurrence rate than the LLR-treated group (OR = 2.71; 95% CI: 1.84–3.99; p < 0.0001) with no statistically significant heterogeneity in the analysis (I2 = 39%, p = 0.16) (Table II, Figure 4).
Figure 4.
Forest plot of postoperative local recurrence between RFA and LLR
Perioperative outcomes
Postoperative complications (POC)
Seventeen of the included studies reported the postoperative complications [25–31, 33–42]. Markedly, the pooled meta-analysis showed that the RFA-treated group had a significantly lower complication than the LLR-treated group (OR = 0.28; 95% CI: 0.21–0.37; p < 0.0001). Notably, there was no significant statistical heterogeneity in the analysis (I2 = 0%, p = 0.95) (Table III, Figure 5).
Table III.
Meta-analyses of perioperative indicators between RFA versus LH
| Categories | Study | Patients | Statistical method | Effect estimate (95% CI) | I2 (%) | P-value | |
|---|---|---|---|---|---|---|---|
| Perioperative index: | |||||||
| Postoperative complications: | 17 | 1867 | OR (M–H, Fixed) | 0.28 (0.21–0.37) | 0.0 | < 0.0001 | |
| RFA approach: | |||||||
| LRFA | 3 | 228 | OR (M–H, Fixed) | 0.31 (0.14–0.67) | 0.0 | 0.002 | |
| PRFA | 13 | 1521 | OR (M–H, Fixed) | 0.27 (0.20–0.38) | 0.0 | < 0.0001 | |
| Mixed | 1 | 118 | OR (M–H, Fixed) | 0.32 (0.03–3.19) | – | 0.332 | |
| Areas: | |||||||
| East | 7 | 769 | OR (M–H, Fixed) | 0.31 (0.19–0.51) | 0.0 | < 0.0001 | |
| Mid-west | 10 | 1098 | OR (M–H, Fixed) | 0.26 (0.18–0.38) | 0.0 | < 0.0001 | |
| Operation time: | 11 | 1078 | SMD (I–V, Random) | –2.8 (–3.41– –2.20) | 92.0 | < 0.0001 | |
| RFA approach: | |||||||
| LRFA | 2 | 148 | SMD (I–V, Random) | –4.63 (–7.24– –2.03) | 93.0 | < 0.001 | |
| PRFA | 9 | 930 | SMD (I–V, Random) | –2.46 (–3.02– –1.89) | 90.0 | < 0.0001 | |
| Areas: | |||||||
| East | 3 | 233 | SMD (I–V, Random) | –2.19 (–3.99– –0.95) | 95.0 | 0.0014 | |
| Mid-west | 8 | 845 | SMD (I–V, Random) | –2.93 (–3.62– –2.24) | 92.0 | < 0.0001 | |
| Blood loss: | 8 | 817 | SMD (I–V, Random) | –3.57 (–4.67– –2.46) | 96.0 | < 0.0001 | |
| RFA approach: | |||||||
| LRFA | 2 | 148 | SMD (I–V, Random) | –5.06 (–7.06– –3.06) | 88.0 | < 0.0001 | |
| PRFA | 6 | 669 | SMD (I–V, Random) | –3.07 (–4.19– –1.94) | 96.0 | < 0.0001 | |
| Areas: | |||||||
| East | 2 | 163 | SMD (I–V, Random) | –2.91 (–5.47– –0.34) | 97.0 | 0.026 | |
| Mid-west | 6 | 654 | SMD (I–V, Random) | –3.80 (–5.17– –2.44) | 97.0 | < 0.0001 | |
| Transfusion rate: | 4 | 671 | OR (M–H, Fixed) | 0.08 (0.02–0.37) | 0.0 | 0.001 | |
| RFA approach: | |||||||
| LRFA | 1 | 79 | OR (M–H, Fixed) | 0.07 (0.01–0.60) | – | 0.015 | |
| PRFA | 3 | 391 | OR (M–H, Fixed) | 0.09 (0.01–0.82) | 0.0 | 0.032 | |
| Areas: | |||||||
| East | 1 | 61 | OR (M–H, Fixed) | 0.09 (0.01–0.82) | – | 0.032 | |
| Mid-west | 3 | 410 | OR (M–H, Fixed) | 0.07 (0.01–0.60) | 0.0 | 0.015 | |
| Hospitalization duration: | 13 | 1348 | SMD (I–V, Random) | –1.68 (–2.07– –1.30) | 89.0 | < 0.0001 | |
| RFA approach: | |||||||
| LRFA | 2 | 166 | SMD (I–V, Fixed) | –1.37 (–1.71– –1.03) | 0.0 | < 0.0001 | |
| PRFA | 10 | 1064 | SMD (I–V, Random) | –1.57 (–2.28– –1.26) | 92.0 | < 0.0001 | |
| Mixed | 1 | 118 | SMD (I–V, Fixed) | –1.51 (–1.02– –1.10) | – | < 0.0001 | |
| Areas: | |||||||
| East | 5 | 438 | SMD (I–V, Random) | –1.31 (–1.66– –0.96) | 64.0 | < 0.0001 | |
| Mid-west | 8 | 910 | SMD (I–V, Random) | –1.94 (–2.52– –1.35) | 93.0 | < 0.0001 | |
| Overall response rate: | 6 | 630 | OR (M–H, Random) | 2.09 (0.98–4.45) | 60.0 | 0.056 | |
| RFA approach: | |||||||
| LRFA | 2 | 148 | OR (M–H, Fixed) | 0.99 (0.41–2.44) | 0.0 | 0.988 | |
| PRFA | 4 | 482 | OR (M–H, Random) | 2.96 (1.09–8.04) | 68.0 | 0.033 | |
| Areas: | |||||||
| East | 3 | 385 | OR (M–H, Fixed) | 4.68 (2.31–9.44) | 0.0 | < 0.0001 | |
| Mid-west | 3 | 245 | OR (M–H, Fixed) | 0.97 (0.53–1.77) | 0.0 | 0.915 | |
| Postoperative liver function (1-m): | |||||||
| ALT | 9 | 841 | SMD (I–V, Random) | –0.52 (–1.1–0.07) | 94.0 | 0.081 | |
| AST | 9 | 841 | SMD (I–V, Random) | –1.42 (–2.35– –0.49) | 97.0 | 0.003 | |
| TBIL | 4 | 344 | SMD (I–V, Random) | –0.09 (–0.53–0.35) | 75.0 | 0.685 | |
| ALB | 2 | 134 | SMD (I–V, Random) | 1.11 (0.03–2.2) | 88.0 | 0.044 | |
| AFP | 3 | 296 | SMD (I–V, Random) | 0.28 (–0.09–0.64) | 58.0 | 0.137 | |
SMD – standardized mean difference, OR – odds ratio, I-V – inverse variance method, M-H – Mantel-Haenszel method, 1-m – 1 month.
Figure 5.
Forest plot of perioperative outcomes between RFA and LLR
Operation times (OT)
Moreover, eleven studies reported and be pooled the operation times [25–29, 32, 33, 36–38, 43]. The pooled meta-analysis showed that the RFA-treated group had significantly shorter operation times than the LLR-treated group (SMD = –2.80; 95% CI: –3.41– –2.20; p < 0.0001). Otherwise, a higher statistical heterogeneity was found in the analysis (I2 = 92%, p < 0.01) and a random-effect model was performed (Table III, Figure 5).
Blood loss (BL) and transfusion (TR)
Eight studies reported blood loss [25–29, 32, 33, 43] and four studies reported transfusion during the operation [25–27, 33]. The pooled meta-analysis showed that the RFA-treated group had significantly lower blood loss and transfusion rate than the LLR-treated group (SMD = –3.57; 95% CI: –4.67– –2.46; p < 0.0001; OR = 0.08; 95% CI: 0.02–0.37; p = 0.001, respectively). However, there was higher significant heterogeneity in pooled blood loss analysis (I2 = 97%, p < 0.01); but no heterogeneity in transfusion rate (I2 = 0%, p = 0.88) (Table III, Figure 5).
Hospitalization duration (HD)
Thirteen studies compared the hospitalization duration between the RFA-treated group and LLR-treated group [25–31, 33, 36–38, 40, 43]. The pooled results suggested that the RFA group had significantly shorter hospitalization time than the LLR group (SMD = –1.68; 95% CI: –2.07– –1.30; p < 0.0001). Also, there was significant heterogeneity in our analysis (I2 = 89%, p < 0.01) (Table III, Figure 5).
Overall response rate
Six studies reported the curative effect of patients [29, 32, 33, 38, 42, 43]. Our pooled results suggested that the RFA-treated group had a significantly higher overall response rate than the LLR-treated group (OR = 2.09; 95% CI: 0.98–4.45; p = 0.056). Moreover, significant heterogeneity was found in our analysis (I2 = 60%, p = 0.03) (Table III, Figure 5).
Postoperative liver function index
In the analysis, nine studies reported ALT and AST levels of postoperative 1 month [28, 30, 32, 34, 36–39, 41], four reported TBIL levels [28, 32, 38, 41], three reported AFP levels [30, 32, 41] and two reported ALB level [28, 32]. However, there was statistically significant heterogeneity among the pooled analyses in postoperative liver function indexes (I2 > 50%, p < 0.05). Markedly, the pooled meta-analyses by the random-effect model showed that the RFA-treated group had a significantly lower AST level and higher ALB level than the LLR-treated group (SMD = –1.42; 95% CI: –2.35– –0.49; p = 0.003; SMD = 1.11; 95% CI: 0.03–2.2; p = 0.044, respectively), and no significant differences in ALT, TBIL and AFP levels between RFA and LLR groups (Table III).
Subgroup analysis
Subgroup analyses of perioperative outcomes were performed based on the RFA approach and areas of studies in China. The results showed that there were significant differences in all perioperative outcomes between the RFA group and LLR group, and the same effect as the overall pooled results (Table III).
Subgroup analyses of the local recurrence rate were performed based on the RFA approach and areas of studies in China under median follow-up time. The results showed that for laparoscopic radiofrequency ablation (LRFA) treatment approach, no significant difference was found in the local recurrence rate between the two groups. However, the percutaneous radiofrequency ablation (PRFA) group had a significantly higher local recurrence rate than the LLR group (OR = 2.05; 95% CI: 1.30–3.23; p = 0.002). The results showed that for areas in China, the RFA group had significantly higher local recurrence rates than the LLR group both in the east and mid-west of China (OR = 6.77; 95% CI: 3.01–15.23; p < 0.0001; OR = 2.04; 95% CI: 1.30–3.19; p = 0.002, respectively) (Table II).
Subgroup analyses of OS and DFS rates based on the single nodule size, RFA approach treatment and study areas in China were performed. The results showed that there was no significant difference in 1-year OS and DFS rates between RFA and LLR groups in patients with single nodule size ≤ 5 cm. However, the RFA group had significantly lower 3-year OS and DFS rates than the LLR group with single nodule size ≤ 5 cm (OR = 0.56; 95% CI: 0.36–0.86; p = 0.008; OR = 0.46; 95% CI: 0.32–0.66; p < 0.0001, respectively). There were no significant differences in OS and DFS rates between RFA and LLR groups in patients with single nodule size ≤ 3 cm or using the LRFA approach. Moreover, using the PRFA approach, no significant differences were observed in 1-year OS and DFS rates between groups; however, the PRFA group had significantly lower 3-year OS and DFS rates than the LLR group (OR = 0.58; 95% CI: 0.38–0.87; p = 0.009; OR = 0.44; 95% CI: 0.31–0.65; p < 0.0001, respectively). Our results based on study areas in China showed that for eastern China, there was no significant difference in 1-year OS rate between RFA and LLR groups; but the RFA group had significantly lower 3-year OS and DFS rates than the LLR group. For mid-western China, there were no significant differences in 1-year OS and DFS rates between groups; however, the RFA group had a significantly lower 3-year DFS rate than the LLR group (OR = 0.49; 95% CI: 0.33–0.74; p < 0.0001) (Table II).
Sensitivity analysis and publication bias
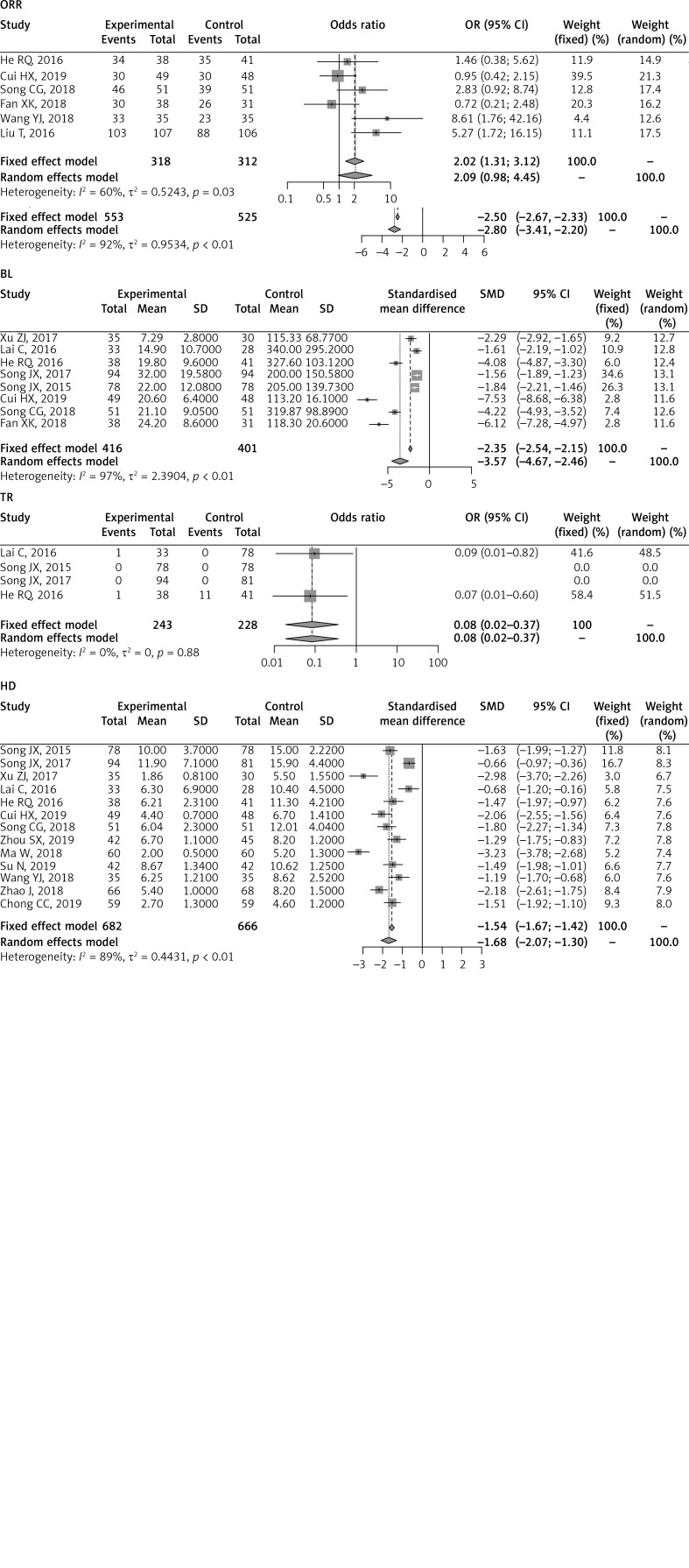
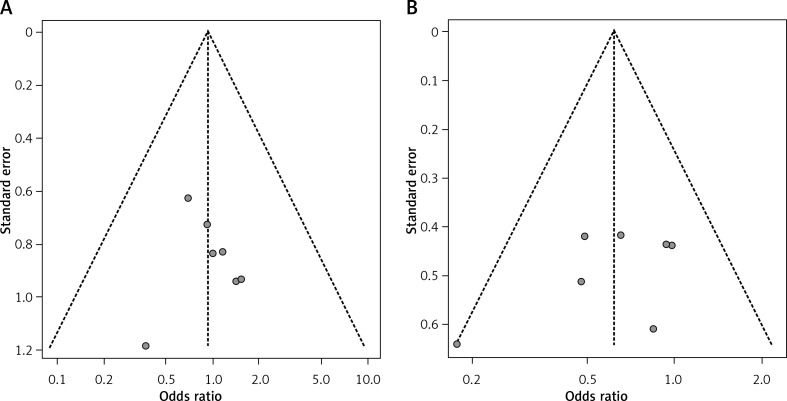
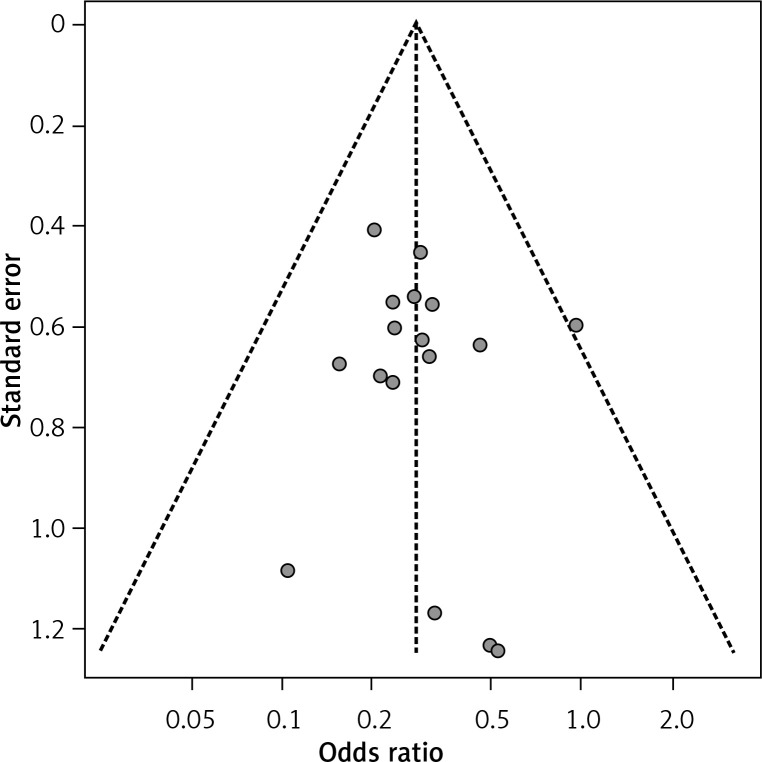
The sensitivity analyses of the above comparisons confirmed the stability of our results, and did not find any study that statistically significantly affected the pooled ORs or SMDs in either group. No publication bias was detected by funnel plot for the comparison of outcomes in the meta-analysis (Figures 6–8).
Figure 6.
Funnel plot of 1-y (A) and 3-y (B) overall survival rates
Figure 8.
Funnel plot of postoperative complications
Figure 7.
Funnel plot of 1-y (A) and 3-y (B) DFS rates
Discussion
To the best of our knowledge, our study is the first comprehensive meta-analysis to evaluate the efficacy of RFA versus LLR for hepatocellular carcinoma in China. The present meta-analysis found that HCC patients treated with LLR had significantly higher 3-year OS and 1 to 3-year DFS rates compared to those treated with RFA, but there was no significant difference in 1-year OS rate between the two groups. Meanwhile, the LLR-treated group had a significantly lower local recurrence rate during the median follow-up period. However, the RFA-treated group outperformed the LLR-treated group in terms of fewer complications, higher overall response rate, shorter operation time, less blood loss, lower transfusion rate and shorter hospitalization duration. In addition, this study found that the RFA group had lower AST and higher ALB levels of liver function compared to the LLR group, with no significant differences in other liver function indicators.
In recent years, advances in laparoscopic instruments and techniques have made LLR safe and feasible [44, 45]. There is growing evidence that the long-term clinical outcomes of LLR for HCC are similar to those of the open approach [46, 47]. However, LLR is not feasible in all cases. LLR is mainly indicated for tumors located in the peripheral portion of the anterolateral liver segments (segments 2, 3, 5 and 6, and the inferior part of segment 4 according to the classification of Couinaud) and easily accessible lesions [48]. In addition, LLR for lesions located in the posterior or superior region of the liver (segments 1, 7, and 8, and the superior part of segment 4) is considered to be technically challenging due to the difficulty in controlling bleeding and limited visualization [49]. Moreover, LLR is considered the first line choice for small HCC if feasible, even in cirrhotic cases, as the curative effect is comparable to that of open surgery [50].
RFA is a target-selective, minimally invasive technique, which is useful in the local control of HCC. Over the years, the indications for RFA have greatly expanded due to developments in artificial hydrothorax, imaging-guided localization, and probes. RFA can be carried out under conscious sedation and is associated with much less intraoperative blood loss, shorter hospitalization, and minor perioperative complications [51]. In addition, RFA can be repeated subsequently and acts as an effective supplementary therapy without causing much damage to the cirrhotic liver. However, development of local recurrence at the RFA treatment site is frequent. Due to this drawback, the long-term survival outcomes of RFA are inferior to LLR. Additionally, the lower 3-year DFS and OS of RFA may be due to the following reasons: First, patients who underwent RFA were not eligible for surgery because of inadequate liver functional reserve, multiple tumors, or poor health conditions. These factors can independently lead to lower 3-year survival. Second, LLR allows better pathological evaluation and in-depth intraoperative exploration, which are not possible with RFA. Third, RFA patients are more likely to have local recurrence due to incomplete ablation of the lesion or the heat sink effect [52]. However, the underlying molecular mechanisms responsible for the higher recurrence rate and lower survival outcome with RFA remain to be determined.
Our subgroup analyses showed that RFA and LLR had a similar effect on 1 to 3-year OS and DFS rates in HCC patients with single nodules of size lower than 3 cm and 1-year OS and DFS rates with single nodules of size lower than 5 cm. According to the RFA approach’s advantages, RFA treatments would be the reliable choice for patients with single nodules size lower than 3–5 cm. That is consistent with previous studies [13, 20]. The LLR-treated group had significantly higher 3-year OS and DFS rates than the PRFA group, and showed a similar effect on survival rate and 1-year DFS rate as the LRFA group only including 1 study with 87 patients. Moreover, previous studies have already proven that the LRFA treatment was superior to the PRFA in severe liver disease [53].
Studies performed in eastern China showed significantly higher 3-year OS and 1 to 3-year DFS rates in the LLR group compared to those treated with RFA, but no significant difference in 1-year OS rate between the two groups. However, the LLR group in those performed in mid-west China only had a significantly higher 3-year DFS rate than the RFA group. This may be due to the association of health care and economic factors with cancer survival [54]. The socioeconomic conditions in China (especially in eastern China) have changed rapidly in the past decade and health care had been improved by increasing insurance coverage and access to care [55], which facilitated the modification of health behaviors and quality of life among cancer survivors.
Hepatocellular carcinoma is common in China, and this is the first comprehensive meta-analysis assessing the clinical effects of RFA versus LLR treatments in China. There was no or low heterogeneity for most observed clinical outcomes in the meta-analysis and subgroup analyses reduced the potential bias between groups. Sensitivity analyses revealed that the pooled results are reliable and stable. Nonetheless, the meta-analysis has some limitations. First, our analysis included a limited number of quality studies, and all of the included studies were retrospective. Randomized controlled trials (RCTs) are difficult to perform because of patient baseline characteristics, choice of procedure, and tumor status. In addition, RFA in the original studies included both percutaneous RFA (PRFA) and laparoscopic RFA (LRFA), which might have impacted the pooled results. Though subgroup analyses were performed by the RFA approach, the results should be treated with caution, as there was only included 1 study on survival outcomes of 87 patients who underwent LRFA treatments. Lastly, there was variation in the palliative methods used during the 3-year follow-up among the studies, including chemotherapy, Traditional Chinese Medicine, and others, that may have affected the clinical outcomes. Traditional Chinese Medicine (TCM) has been used for prevention and treatment of HCC, which is the more widely used form of complementary and alternative medicine in modern China and achieved curative effects. Even though western medicine and treatment are the main treatment strategies of HCC, the poor prognosis outcomes suggest that prevention is important for HCC. TCM is based on empirically accumulated knowledge, including a theoretical system and Chinese herbal medicine with abundant therapeutic resources, but the principle of TCM actually is preventative treatment. TCM theory suggests that yin and yang disequilibrium and vital qi (energy) deficiency are related to cancers, and the key of TCM theory is etiology according to the whole condition to study cancer syndromes. TCM treatments for HCC include removing blood stasis, regulating flow of qi and strengthening the spleen or clearing heat and detoxifying [56].
Conclusions
The findings suggest that patients with small HCC in China who underwent RFA treatments had lower DFS and 3-year OS rates and higher local recurrence rates than LLR treatments, although with better preoperative clinical outcomes and liver function improvement. Similar effects on OS and DFS rates exist in patients with single nodules less than 3 cm in size between the two groups. Future high-quality prospective studies are required to validate the findings of this meta-analysis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC; No. 81802805), the Natural Science Foundation of Jilin Province (20200201490JC) and the translation-clinical joint foundation of the First Hospital of JIlin University (No. 2020-ZL-04).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zhu ZX, Huang JW, Liao MH, et al. Treatment strategy for hepatocellular carcinoma in China: radiofrequency ablation versus liver resection. Jpn J Clin Oncol. 2016;46:1075–80. doi: 10.1093/jjco/hyw134. [DOI] [PubMed] [Google Scholar]
- 2.Crocetti L. Radiofrequency ablation versus resection for small hepatocellular carcinoma: are randomized controlled trials still needed? Radiology. 2018;287:473–5. doi: 10.1148/radiol.2018172822. [DOI] [PubMed] [Google Scholar]
- 3.Zhu GQ, Sun M, Liao WT, et al. Comparative efficacy and safety between ablative therapies or surgery for small hepatocellular carcinoma: a network meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:935–45. doi: 10.1080/17474124.2018.1503531. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Zhang J, Liu X, et al. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroen Hepatol. 2012;27:51–8. doi: 10.1111/j.1440-1746.2011.06947.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang B, Yan XF, Zhang JH. Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res. 2018;48:635–63. doi: 10.1111/hepr.13061. [DOI] [PubMed] [Google Scholar]
- 6.Lan L, Zhao F, Cai Y, et al. Epidemiological analysis on mortality of cancer in China, 2015. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:32. doi: 10.3760/cma.j.issn.0254-6450.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Zheng R, Sun K, Zhang S, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 9.Blum HE, Moradpour D. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 10.Hirohisa O, Tomoharu Y, Yo-Ichi Y, et al. Histological architectural classification determines recurrence pattern and prognosis after curative hepatectomy in patients with hepatocellular carcinoma. PLoS One. 2018;13:e0203856. doi: 10.1371/journal.pone.0203856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamora-Valdes D, Taner T, Nagorney DM. Surgical treatment of hepatocellular carcinoma. Cancer Control. 2017;24:1073274817729258. doi: 10.1177/1073274817729258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong H, Lee J, Lee M, et al. Early experience with laparoscopic treatment of liver tumors using a separable cluster electrode with a no-touch technique. Videosurgery Miniinv. 2020 doi: 10.5114/wiitm.2020.95065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: hepatobiliary cancers, Version 1.2017. J Natl Compr Canc Ne. 2017;15:563–73. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48:S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Kobayashi S, Ye X, et al. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4 doi: 10.1038/srep07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casaccia M, Santori G, Bottino G, et al. Laparoscopic resection vs laparoscopic radiofrequency ablation for the treatment of small hepatocellular carcinomas: a single-center analysis. World J Gastroenterol. 2017;23:653–60. doi: 10.3748/wjg.v23.i4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita YI, Imai K, Kaida T, et al. Multimodal radiofrequency ablation versus laparoscopic hepatic resection for the treatment of primary hepatocellular carcinoma within Milan criteria in severely cirrhotic patients: long-term favorable outcomes over 10 years. Surg Endosc. 2019;33:46–51. doi: 10.1007/s00464-018-6264-3. [DOI] [PubMed] [Google Scholar]
- 18.Yazici P, Akyuz M, Yigitbas H, et al. A comparison of perioperative outcomes in elderly patients with malignant liver tumors undergoing laparoscopic liver resection versus radiofrequency ablation. Surg Endosc. 2016;31:1269–74. doi: 10.1007/s00464-016-5105-5. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Tanaka S, Iwai S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case-control study with propensity score matching. Hepatol Res. 2016;46:565–74. doi: 10.1111/hepr.12592. [DOI] [PubMed] [Google Scholar]
- 20.Si MB, Yan PJ, Hao XY, et al. Efficacy and safety of radiofrequency ablation versus minimally invasive liver surgery for small hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33:2419–29. doi: 10.1007/s00464-019-06784-0. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Wu YS, Chen D, et al. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:5711–24. doi: 10.2147/CMAR.S189777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 24.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 25.Lai C, Jin RA, Liang X, et al. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B. 2016;17:236–46. doi: 10.1631/jzus.B1500322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song J, Wang Y, Ma K, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc. 2016;30:4249–57. doi: 10.1007/s00464-015-4737-1. [DOI] [PubMed] [Google Scholar]
- 27.Song J, Chen J, Li J, et al. Clinical analysis of laparoscopic hepatectomy and radiofrequency ablation in the treatment of early hepatocellular carcinoma. J Hepatobiliary Surg. 2017;25:349–53. [Google Scholar]
- 28.Xu Z, Xu G, Ma J, et al. Ultrasound guided percutaneous radiofrequency ablation versus laparoscopic hepatectomy for small primary liver cancer. Chin J Gen Surg. 2017;26:18–24. [Google Scholar]
- 29.Cui H, Wang Y, Gao Y, et al. Ultrasound-guided radipfrequency ablation combined with anhydrous ethanol injection versus laparoscopic hepatectomy for small hepatocellular carcinoma. J Chin Pract Diagn Ther. 2019;33:700–3. [Google Scholar]
- 30.Zhou S, Chen Y, Yin X, et al. The comparison of efficacy between laparoscopic radiofrequency ablation and laparoscopic hepatectomy in the treatment of small hepatic carcinoma. J Laparosc Surg. 2019;24:258–62. [Google Scholar]
- 31.Chong CC, Lee KF, Chu CM, et al. Laparoscopic Hepatectomy (with or without robotic assistance) versus radiofrequency ablation as a minimally invasive treatment for very early-stage or early-stage hepatocellular carcinoma. Dig Surg. 2020;37:65–71. doi: 10.1159/000497112. [DOI] [PubMed] [Google Scholar]
- 32.Fan X, Shi L, Guo L. Comparison of laparoscopic-guided radiofrequency ablation and laparoscopic hepatectomy for the treatment of hepatocellular carcinoma. Int J Clin Exp Med. 2018;17:1540–4. [Google Scholar]
- 33.He R, Xu H. Clinical efficacy and quality of life in treatment of small hepatocellular carcinoma:laparoscopic hepatectomy versus radiofrequency ablation. J Chin Oncol. 2016;22:1048–51. [Google Scholar]
- 34.Wang X, Wang W, Zhao H, et al. Comparison of percutaneous radiofrequency ablation and laparoscopic liver resection in treatment of small hepatocellular carcinoma. Med J West China. 2017;29:183–6. [Google Scholar]
- 35.Zheng X, Liu D, Li Z, et al. Comparison of the short-term and long-term efficacy of laparoscopic radiofrequency ablation and laparoscopic hepatectomy for hepatocellular carcinoma. Clin Med. 2019;39:73–4. [Google Scholar]
- 36.Ma W. Clinical effect of percutaneous radiofrequency ablation versus laparoscopic hepatectomy on small hepatocellular carcinoma. Clin Med. 2018:59–60. [Google Scholar]
- 37.Su N. Comparative analysis between percutaneous radiofrequency ablation and laparoscopic hepatectomy for small hepatocellular carcinoma. Diet Care. 2019;6:18–9. [Google Scholar]
- 38.Wang Y, Huang T, Zhou W, et al. Curative effect of percutaneous radiofrequency ablation and laparoscopic hepatectomy in treatment of small liver cancer. China&Foreign Med Treat. 2018;36:16–8. [Google Scholar]
- 39.Wang X. Comparison of clinical efficacy between percutaneous radiofrequency ablation and laparoscopic hepatectomy for small hepatocellular carcinoma. Med J Chin People’s Health. 2018;30:57–8. [Google Scholar]
- 40.Zhao J. Clinical efficacy analysis of percutaneous radiofrequency ablation and laparoscopic hepatectomy for small hepatocellular carcinoma. Diet Care. 2018;5:52. [Google Scholar]
- 41.Zhang H, Zhuang Z, Lin C, et al. Efficacy and prognosis comparison of percutaneous radiofrequency ablation and laparoscopic hepatectomy in treatment of small hepatocellular carcinoma. Chin J Gen Surg. 2019;28:24–30. [Google Scholar]
- 42.Liu T, Li Z, Jin Z. Comparison of efficacy between radiofrequency ablation and laparoscopic hepatectomy for primary hepatocellular carcinoma. J Clin Med. 2016;3:833–6. [Google Scholar]
- 43.Song C. Analysis of clinical curative effect and prognosis of laparoscopic liver resection and radiofrequency ablation in treatment of early liver cancer. Syst Med. 2018;3:7–9. [Google Scholar]
- 44.Buell JF, Thomas MT, Rudich S, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–86. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 45.Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surgeons. 2010;211:16–23. doi: 10.1016/j.jamcollsurg.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Jiang S, Wang Z, Ou M, et al. Laparoscopic Versus open hepatectomy in short- and long-term outcomes of the hepatocellular carcinoma patients with cirrhosis: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2019;29:643–54. doi: 10.1089/lap.2018.0588. [DOI] [PubMed] [Google Scholar]
- 47.Deng ZC, Jiang WZ, Tang XD, et al. Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 157 patients: a case controlled study with propensity score matching at two Chinese centres. Int J Surg. 2018;56:S174391911831519X. doi: 10.1016/j.ijsu.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Hikspoors JPJM, Peeters MMJP, Kruepunga N, et al. Human liver segments: role of cryptic liver lobes and vascular physiology in the development of liver veins and left-right asymmetry. Sci Rep. 2017;7:17109. doi: 10.1038/s41598-017-16840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian ZQ, Su XF, Lin ZY, et al. Meta-analysis of laparoscopic versus open liver resection for colorectal liver metastases. Oncotarget. 2016;7:84544–55. doi: 10.18632/oncotarget.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, Wang X, Wang Y, et al. Effective viral suppression is necessary to reduce hepatocellular carcinoma development in cirrhotic patients with chronic hepatitis B: results of a 10-year follow up. Medicine. 2017;96:e8454. doi: 10.1097/MD.0000000000008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Zhang J, Liu X, et al. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51–8. doi: 10.1111/j.1440-1746.2011.06947.x. [DOI] [PubMed] [Google Scholar]
- 52.Kong J, Kong L, Kong J, et al. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med. 2012;10:230. doi: 10.1186/1479-5876-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Podnos YD, Henry G, Ortiz JA, et al. Laparoscopic ultrasound with radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: technique and technical considerations. Am Surg. 2001;67:1181–4. [PubMed] [Google Scholar]
- 54.Underwood JM, Townsend JS, Stewart SL, et al. Surveillance of demographic characteristics and health behaviors among adult cancer survivors--Behavioral Risk Factor Surveillance System, United States, 2009. MMWR CDC Surveill Summ. 2012;61:1–23. [PubMed] [Google Scholar]
- 55.Si L, Chen M, Palmer AJ. Has equity in government subsidy on healthcare improved in China? Evidence from the China’s National Health Services Survey. Int J Equity Health. 2017;16:6. doi: 10.1186/s12939-017-0516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes Diseases. 2020;7:370–9. doi: 10.1016/j.gendis.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]