Abstract
Background:
Up to 7% of hospitalised patients acquire at least one healthcare-associated infection (HAI). The aim of the present study was to quantify the burden of HAIs in an Italian hospital, identifying involved risk factors.
Methods:
Prevalence point study carried out from 2011 to 2018. For each recruited patient, a data entry form was compiled including information on demographics, hospital admission, risk factors, antimicrobial treatment, and infection if present.
Results:
A total of 2844 patients were included and 218 (7.03%) reported an infection. HAI prevalence rates showed a significant increase (average annual per cent change (AAPC) +33.9%; p=0.018) from 2011 to 2014 whereas from 2014 to 2018 a gradual decline was observed (AAPC –6.15%; p=0.35). Urinary tract infection was the most common HAI (25.2%) and a total of 166 (76.1%) pathogens were isolated from 218 infections. Enterococcus and Klebsiella species were the most prevalent pathogens, causing 15.1% and 14.5% of HAIs, respectively. A significant higher risk of HAIs was found in patients exposed to central catheter (adjusted odds ratio (adj-OR)=5.40), peripheral catheter (adj-OR=1.89), urinary catheter (adj-OR=1.46), National Healthcare Safety Network surgical intervention (adj-OR=1.48), ultimately fatal disease (adj-OR=2.19) or rapidly fatal disease (adj-OR=2.09) and in patients with longer hospital stay (adj-OR=1.01).
Conclusion:
Intervention programmes based on guidelines dissemination and personnel training can contribute to reduce the impact of HAI. Moreover, McCabe score can be a very powerful and efficient predictor of risk for HAI. Finally, an unexpected very high burden of disease due to Enterobacteriaceae and Gram positive cocci that could be related to the frequent use of carbapenems and third generation cephalosporins in this hospital was found.
Keywords: Infections, causative micro-organisms, prevalence, hospital, risk factors
Highlights
Urinary tract infections continue to be the most common healthcare-associated infection (HAI).
Guidelines dissemination and personnel training reduce the impact of HAI over time.
McCabe score can represent a very powerful and efficient predictor of risk for HAI.
Enterobacteriaceae and Gram positive cocci can have a very important burden.
Introduction
Healthcare-associated infections (HAIs) and antimicrobial resistance represent significant public health problems nowadays, as they result in high morbidity and mortality, as well as a reduction in quality of life worldwide (Raka et al, 2019; WHO, 2016).
At any one time, 7% of hospital patients in developed countries and 10% in developing countries have a HAI, incurring high financial costs for patients and their families as well as an important additional economic burden for the health system (Chen et al, 2017; WHO, 2016).
A recent point-prevalence survey (PPS) of HAI and antimicrobial use that was conducted in the European Union, from 2016 to 2017, showed that 6.5% of hospitalised patients in acute care hospitals had a HAI (Suetens et al, 2018). The most frequent HAIs are the lower respiratory tract infections, followed by bloodstream infections, urinary tract infections and surgical site infections (Ministero della Salute, 2018; Sinatra et al, 2013).
In Italy, the annual prevalence of HAIs has been found to range between 5% and 8% per year. A recent study conducted in France reported that patients admitted into Intensive Care Units (ICU) had an increased length of stay of five days due to HAIs (Ohannessian et al, 2018). It has been revealed that HAIs cause 16 million additional days of hospitalisation per year, with a burden of 37,000 related deaths, and cause annual financial losses of about seven billion euros in Europe (Allegranzi et al, 2011; Antonioli et al, 2016). In Italy, the national health institute “Istituto Superiore di Sanità” (ISS) estimates that HAIs could be directly responsible for 1350–2100 avoidable deaths per year (CNESP, 2019).
Antimicrobial resistance is another required challenge linked to HAIs. Excessive and inappropriate use of antimicrobials causes the increase of drug resistant micro-organisms and adverse drug reactions worldwide (Harbarth et al, 2015; Laxminarayan et al, 2013) as well as a tendency towards escalation in many countries of Europe (Versporten et al, 2014). In the European Union the average total consumption, in the community and hospitals, of antibiotics for systemic use in 2017 was 23.4 Defined Daily Doses per 1000 inhabitants per day (ECDC, 2018). A European Centre for Disease Prevention and Control (ECDC) country visit to Italy confirmed that the anti-microbial resistance situation in Italian hospitals and regions represents a major public health problem. The levels of carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii have reached widespread levels, just like meticillin-resistant Staphylococcus aureus. Due to this issue, Italy is one of the countries with the highest level of resistance in Europe (ECDC, 2017).
It should be noted that a large percentage of HAIs, including those attributable to antibiotic resistant micro-organisms, could be prevented by means of effective infection prevention and control measures and active surveillance programmes (WHO, 2016). In this sense, the ISS reported that up to 30% of HAIs in Italian hospitals are preventable.
According to the previously reported considerations, the aim of the present study was to quantify the burden of HAIs in a large Italian hospital. In particular, prevalence rates and main characteristics of HAIs were evaluated over an eight-year period, identifying risk factors involved in infection development.
Methods
The surveys took place from 2011 to 2018 at the University Hospital “P. Giaccone” of Palermo (Italy), a tertiary care hospital with 542 beds, with nearly 14,000 hospital admissions per year.
In order to monitor HAIs, PPSs were conducted every year using the ECDC protocols for PPS of all types of HAIs and antimicrobial use in European hospitals (ECDC, 2016). Due to participation in the second EU-wide PPS (PPS 2016–2017), in 2016 there were two PPSs: as well as the usual autumn–winter period, there was another PPS carried out in July, in the summer season, when staffing at hospitals is usually low, as indicated by the ECDC (Plachouras et al, 2018). The PPS protocol and codebook v.5.3 (since PPS 2016–2017), including the case definitions of HAI and PPS data entry forms, were used (ECDC, 2019).
According to the protocol indications, data had to be collected in a single day for each ward, therefore the time frame of survey data collection had not to exceed 2–3 weeks per year, considering the number of patients and wards to be monitored.
PPSs were carried out by medical residents of the Post-graduate School of Hygiene and Preventive Medicine of the University of Palermo, supported by physicians and nurses of the Hospital Network for Infection Control. All data collectors have been trained by members of the Italian Study Group of Hospital Hygiene of the Italian Society of Hygiene, Preventive medicine and Public health. All medical and surgical wards were included in the surveillance, as well as ICUs and long-term care wards (from 2016).
Ward specialties were classified into six areas: Medical area (including Allergology and Immunology, Cardiology, Dermatology, Endocrinology, Gastroenterology, Geriatrics, Haematology, Hepatology, Infectious diseases, Internal medicine, Nephrology, Neurology, Oncology, Pneumology, Rheumatology), Surgical area (including Cardiovascular surgery, General surgery, Maxillofacial surgery, Neurosurgery, Oncological surgery, Ophthalmology, Otorhinolaryngoiatry, Orthopaedic surgery, Paediatric surgery, Plastic surgery, Thoracic surgery, Urology, Vascular surgery), Intensive Care Unit (including General ICU, Critical ICU and Specialised ICU), Paediatrics, Obstetrics and Gynaecology, and Psychiatry.
Inpatients of any age were eligible for inclusion. All patients admitted to the ward before or at 08:00 h and not discharged from the ward at the time of the survey were included; patients who were transferred after 08:00 h from or to another ward were not included.
Data collected for each patient included: age, sex, date of hospital admission, consultant/patient specialty, surgery since admission (using ICD-9-CM code of the intervention listed for the surveillance of surgical site infections in the National Healthcare Safety Network (NHSN) system, McCabe score, presence of invasive devices (central vascular catheter, peripheral vascular catheter, urinary catheter, intubation) on the day of the survey and whether the patient had one or more active HAIs and/or received antimicrobial treatment. In the statistical analysis, age has been categorised into four groups using quartile cut-off.
For patients who were administered antibiotics, the type, route of administration of the antimicrobial agent, indication for antimicrobial use, purpose (treatment intention, surgical prophylaxis, medical prophylaxis, other or unknown indication), reason in notes, date of antimicrobial start, any antimicrobial changed, dosage per day were recorded. Detection of resistance pattern was determined by disc diffusion method (Kirby–Bauer method) as recommended by the Clinical and Laboratory Standards Institute.
For patients having HAIs the following variables were considered: infection site, date of onset, presence at admission, origin of the infection (associated with the current hospitalisation and/or current ward), relevant device in situ, micro-organisms and antimicrobial resistance phenotype.
Statistical analyses were carried out to determine risk factors associated with HAIs. Patients with HAI whose origin was associated with another hospitalisation or with an indeterminate origin were excluded from these analyses. Categorical data were summarised by absolute and relative frequencies, and analysed by Chi-square test. For not normally distributed continuous variables, medians were compared by Mann–Whitney U-test. Multivariate analysis was conducted using logistic regression models. Variables that were significant at p <0.1 in an univariable analysis were included into the multivariable model and excluded, with a backward stepwise approach, if not significantly associated with HAI. Breakpoint analysis has been implemented to assess trends over time for the yearly HAI prevalence rates. A joinpoint model was obtained to evaluate the time trends, direction and intensity of the trend, and the average annual per cent change (AAPC). The final model is based on linear segments connected at joinpoints that represent the best fit of observed data. Statistical analysis was performed using R statistical software version 3.6.1. and the packages “Segmented” and “Strucchange” were used for the joinpoint analysis. All tests were considered as significant at p <0.05.
Results
Patients
A total of 2844 patients were included in the PPS, from 2011 to 2018; 52.4% were males. The median patient age was 65 years (interquartile range (IQR): 47–76). As reported in Table 1, 24.2% surveyed patients were under 46 years old, 27.1% were aged between 46 and 65 years, 22.1% were 66 to 75 years old and 26.6% were aged 76 years or older. Most patients (85.5%) were hospitalised in medical and surgical areas; 7.1% were in ICUs.
Table 1.
Demographic and clinical characteristics of patients a included in the point-prevalence surveys (2011–2018).
| Characteristics | All patients (N= 2844, % by column | Patients without HAI (n= 2644, % by row) | Patients with HAI (n= 200, % by row) | p-value b |
|---|---|---|---|---|
| Gender, n (%) | 0.12 | |||
| Male | 1491 (52.4%) | 1250 (92.4%) | 103 (7.6%) | |
| Female | 1353 (47.6%) | 1394 (93.5%) | 97 (6.5%) | |
| Age in years, n (%) | 0.16 | |||
| 0–45 | 687 (24.2%) | 647 (94.2%) | 40 (5.8%) | |
| 46–65 | 772 (27.1%) | 718 (93%) | 54 (7%) | |
| 66–75 | 627 (22.1%) | 579 (92.3%) | 48 (7.7%) | |
| ⩾76 | 758 (26.6%) | 700 (92.4%) | 58 (7.6%) | |
| Years of survey | 0.31 | |||
| 2011 | 339 (11.9%) | 325 (95.9%) | 14 (4.1%) | |
| 2012 | 330 (11.6%) | 311 (94.2%) | 19 (5.8%) | |
| 2013 | 327 (11.5%) | 301 (92%) | 26 (7.95%) | |
| 2014 | 329 (11.6%) | 296 (90%) | 33 (10%) | |
| 2015 | 309 (10.9%) | 284 (91.9%) | 25 (8.1%) | |
| 2016, July | 321 (11.3%) | 297 (92.5%) | 24 (7.5%) | |
| 2016, November | 266 (9.3%) | 251 (94.4%) | 15 (5.6%) | |
| 2017 | 307 (10.8%) | 291 (94.8%) | 16 (5.2 %) | |
| 2018 | 316 (11.1%) | 288 (91.1%) | 28 (8.9%) | |
| Location of patient in hospital on survey date | 0.002 | |||
| Medical area | 1495 (52.6%) | 1397 (93.4%) | 98 (6.6%) | |
| Surgical area | 936 (32.9%) | 866 (92.5%) | 70 (7.5%) | |
| Intensive Care Unit | 202 (7.1%) | 176 (87.1%) | 26 (12.9%) | |
| Paediatrics | 93 (3.3%) | 50 (98%) | 1 (2%) | |
| Obstetrics and Gynaecology | 67 (2.3%) | 88 (94.6%) | 5 (5.4%) | |
| Psychiatry | 51 (1.8%) | 67 (100%) | 0 (0%) | |
| Central catheter in place on survey date | <0.0001 | |||
| Yes | 234 (8.2%) | 169 (72.2%) | 65 (27.8%) | |
| No | 2603 (91.5%) | 2468 (94.8%) | 135 (5.2%) | |
| Peripheral catheter in place on survey date | 0.001 | |||
| Yes | 2212 (77.8%) | 2040 (92.2%) | 172 (7.8%) | |
| No | 627 (22%) | 599 (95.5%) | 28 (4.5%) | |
| Urinary catheter in place on survey date | <0.0001 | |||
| Yes | 868 (30.5%) | 764 (88%) | 104 (12%) | |
| No | 1967 (69.2%) | 1871 (95.1%) | 96 (4.9%) | |
| Intubation on survey date | <0.0001 | |||
| Yes | 76 (2.7%) | 56 (73.7%) | 20 (26.3%) | |
| No | 2765 (97.2%) | 2585 (93.5%) | 180 (6.5%) | |
| Surgery since admission | <0.0001 | |||
| No surgery | 2049 (72.1%) | 1934 (94.4%) | 115 (5.6%) | |
| Yes, NHSN surgery | 567 (19.9%) | 501 (88.4%) | 66 (11.6%) | |
| Yes, minimally invasive/non-NHSN surgery | 221 (7.8%) | 203 (91.9%) | 18 (8.1%) | |
| McCabe score | <0.0001 | |||
| Non-fatal disease | 1934 (68%) | 1848 (95.6%) | 86 (4.4%) | |
| Ultimately fatal disease | 432 (16.9%) | 422 (87.6%) | 60 (12.4%) | |
| Rapidly fatal disease | 282 (9.9%) | 246 (87.2%) | 36 (12.8%) | |
| Days from admission to survey, median (IQR) | 5 (2–10) | 5 (2–9) | 15 (10–29) | <0.0001 |
Percentages may not total 100 because of missing data.
The Chi-square test was used for calculating the p-value. The comparison excluded patients with missing data.
NHSN: National Healthcare Safety Network; IQR: interquartile range
A central vascular catheter was present in 8.2% of patients, whereas peripheral vascular catheters were present in 77.8% of patients. Urinary catheters were present in 30.5% of patients and only 2.7% of patients were intubated at the time of the survey.
Almost 27.7% of the patients had undergone surgery since their admission to the hospital: 19.9% had a NHSN surgery while 7.8% had minimally invasive surgery/not NHSN.
Overall, 9.9% of surveyed patients were classified as having diagnoses that were rapidly fatal (within one year), 16.9% as ultimately fatal and 68% as non-fatal diagnoses.
The median time from hospital admission to the survey date was five days (IQR: 2–10).
Risk factors for HAIs
The univariate analysis identified an increased risk of HAI associated with intensive care (with reference to medical area odds ratio (OR)=2.1; 95% confidence interval (CI) =1.3-3.3; p<0.001) and patients intubated (OR= 5.13; 95% CI= 3.01–8.73; p<0.001) or who had a urinary catheter (OR=2.65; 95% CI= 1.98–3.54; p<0.001) or central (OR=7.03; 95% CI= 5.03–9.82; p<0.001) or peripheral (OR=1.80; 95% CI= 1.20–2.72; p=0.001) catheter in place during hospitalisation. A higher risk was also observed in those who had undergone surgery since their admission to the hospital (p<0.001), were classified as having diagnoses that were rapidly fatal or fatal (p<0.001), had longer hospital stay at the time of the survey (p<0.001) (Table 1).
In the multivariable analysis, after adjusting for age, sex, hospital area, years of PPS and intubation, a higher risk of HAIs was found in patients exposed to central catheter (adjusted OR (adj-OR) =5.40; 95% CI: 3.59–8.13), peripheral catheter (adj-OR=1.89; 95% CI: 1.16–3.07), urinary catheter (adj-OR=1.46; 1.02–2.08), NHSN surgical intervention (adj-OR=1.48; 1.01–2.18), ultimately fatal disease (adj-OR=2.19; 1.49–3.23) or rapidly fatal disease (adj-OR=2.09; 1.32–3.30) and in patients with longer hospital length of stay (adj-OR=1.01; 95% CI: 1.01–1.02) (Table 2).
Table 2.
Multivariable logistics analysis to identify risk factors associated with healthcare-associated infections.
| Variable | adj-OR (95% CI) | p-value |
|---|---|---|
| Central catheter in place on survey date (ref. value) | 5.40 (3.59–8.13) | <0.0001 |
| Peripheral catheter in place on survey date (ref. value) | 1.89 (1.16–3.07) | 0.01 |
| Urinary catheter in place on survey date (ref. value) | 1.46 (1.02–2.08) | 0.03 |
| Surgery since admission (ref. no surgery) | ||
| Minimally invasive/non-NHSN surgery | 1.61 (0.92–2.80) | 0.09 |
| NHSN surgery | 1.48 (1.01–2.18) | 0.04 |
| McCabe score (ref. non-fatal disease) | ||
| Ultimately fatal disease | 2.19 (1.49–3.23) | <0.0001 |
| Rapidly fatal disease | 2.09 (1.32–3.30) | 0.001 |
| Number of days from admission to survey (per day increase) | 1.01 (1.01–1.02) | <0.0001 |
adj-OR: adjusted odds ratio; CI: confidence interval; ref.: reference; NHSN: National Healthcare Safety Network
Prevalence and distribution of HAI
A total of 218 HAIs were detected in 200 of 2844 surveyed patients, accounting for 7.03% of patients. Urinary tract infection was the most common HAI (25.2%), followed by pneumonia (22%), surgical site infection (21.1%) and bloodstream infection (11%) (Table 3). Device-associated infections, including central catheter in place, urinary catheters and intubation, accounted for 46.3% of all HAIs (101 out of 218 infections). The remaining 53.7% of infections were not associated with devices or operative procedures. Of 46 surgical-site infections, 30 (13.8%) were deep incisional or organ-space infections. Out of 48 patients with pneumonia, 28 (58.3%) were reported in ICUs and four (8.3%) were ventilator-associated pneumonia (data not shown in table). Among the 200 patients with HAIs, 98 patients (49%) were located in medical area, 70 (35%) in surgical area, 26 (13%) in ICU, five (2.5%) in Obstetrics and Gynaecology and one (0.5%) in Paediatrics.
Table 3.
Distribution of 218 healthcare-associated infections (HAIs) and reported causative pathogens.
| Type of infection | n | % of HAIs | HAIs per 100 patients | Isolated micro-organisms, n (% by row) a |
|---|---|---|---|---|
| Urinary tract infection | 55 | 25.2% | 1.9 | Escherichia coli, 14 (31.1%); Klebsiella spp., 8 (17.8%); Enterococcus spp., 7 (15.6%); Candida spp., 5 (11.1%); others, 11 (24.4%) |
| Pneumonia | 48 | 22% | 1.7 | Acinetobacter spp., 7 (21.9%); Klebsiella spp., 7 (21.9%); Staphylococcus spp., 3 (9.4%); Streptococcus spp., 3 (9.4%); Candida spp., 3 (9.4%); Pseudomonas spp., 2 (6.3%); Haemophilus spp., 2 (6.3%); others, 5 (15.6%) |
| Surgical-site infection | 46 | 21.1% | 1.6 |
Enterococcus spp., 7 (20.6%);
Pseudomonas spp., 5 (14.7%);
Klebsiella spp., 4
(11.8%); Staphylococcus spp., 4 (11.8%); Acinetobacter spp., 4 (11.8%); Candida spp., 3 (8.8%); Enterobacter spp., 2 (5.9%); others, 5 (14.7%) |
| Deep incisional or organ-space infection | 30 | 13.8% | 1.1 | Enterococcus spp., 5 (23.8%); Pseudomonas spp., 4 (19%); Acinetobacter spp., 3 (14.3%); Candida spp., 2 (9.5%); Enterobacter spp., 2 (9.5%); others, 5 (23.8%) |
| Superficial incisional infection | 16 | 7.3% | 0.6 | Klebsiella spp., 3 (23.1%); Staphylococcus spp., 3 (23.1%); Enterococcus spp., 2 (15.4%); others, 5 (38.5%) |
| Bloodstream infection | 24 | 11% | 0.8 | Staphylococcus spp., 5 (23.8%); Escherichia coli, 4 (19%); Enterococcus spp., 3 (14.3%); Acinetobacter spp., 2 (9.5%); Klebsiella spp., 2 (9.5%); Candida spp., 2 (9.5%); others, 2 (9.5%) |
| Other bloodstream infection | 18 | 8.3% | 0.6 | Escherichia coli, 3 (18.8%); Staphylococcus spp., 3 (18.8%); Klebsiella spp., 2 (12.5%); Candida spp., 2 (12.5%); Enterococcus spp., 2 (12.5%); Acinetobacter spp., 2 (12.5%); others, 2 (12.6%) |
| Central catheter-associated bloodstream infection | 6 | 2.7% | 0.2 | Staphylococcus spp., 2 (40%); others, 3 (60%) |
| Skin and soft tissue infection | 14 | 6.4% | 0. | Enterococcus spp., 6 (28.6%); Acinetobacter spp., 5 (23.8%); Staphylococcus spp., 4 (19%); Pseudomonas spp., 2 (9.5%); others, 3 (14.4%) |
| Gastrointestinal infection | 6 | 2.8% | 0.2 | Clostridium difficile, 2 (50%); others, 2 (50%) |
| Sepsis | 6 | 2.8% | 0.2 | Pseudomonas spp., 1 (100%) |
| Other infections | 19 | 8.7% | 0.7 | Staphylococcus spp., 2 (25%); Klebsiella spp., 2 (25%); others, 4 (50%) |
| Total | 218 | 100.0% | 7.7 |
Only micro-organisms accounting for >5% and having at least two isolates have been reported. One or more pathogens were reported for 166 of 218 infections (76.1%). No pathogens were reported for the remaining 52 infections (23.9%).
The prevalence rate of HAI varied between prevalence surveys from 4.1% (in 2011) to 10% (in 2014) although these between-year differences were not statistically significant (Table 1). However, in a breakpoint analysis to identify trends in rates (Figure 1), we found a breakpoint in 2014. In a first trend, from 2011 to 2014, the HAI prevalence showed a statistically significant increase (AAPC +33.9%; p=0.018) whereas from 2014 to 2018 a more gradual decline in HAI prevalence was found (AAPC –6.15%; p<=0.35).
Figure 1.
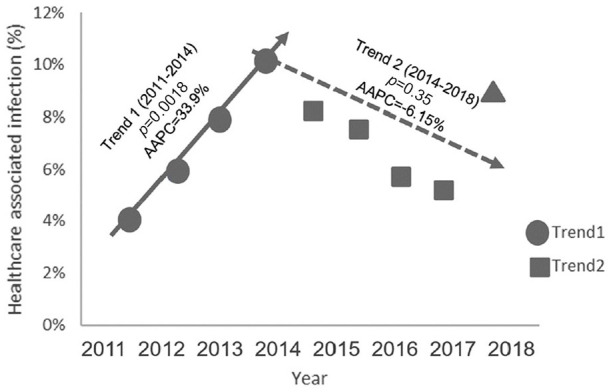
Joinpoint analysis on trends of HAI prevalence rates observed from 2011 to 2014 (+33.9%) and from 2014 to 2018 (–6.15%).
HAI: healthcare-associated infection; AAPC: average annual per cent change
Pathogens causing HAI
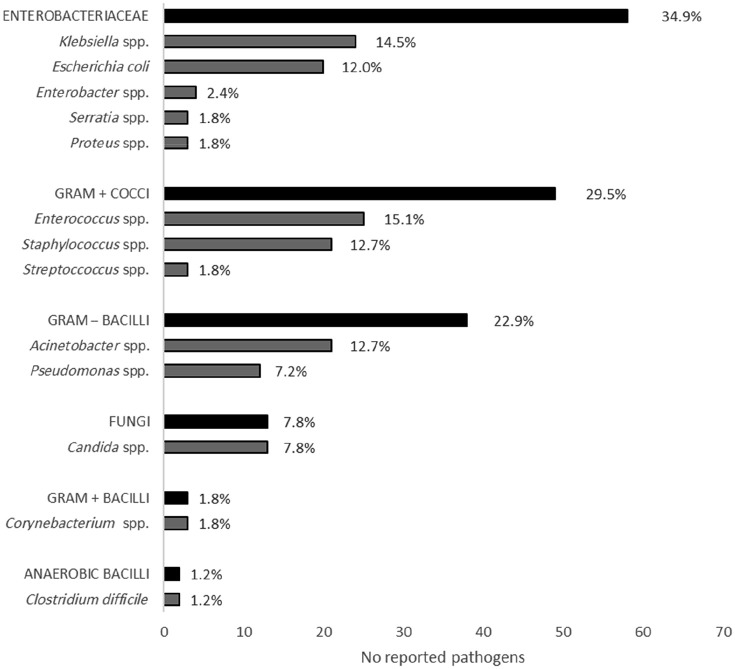
A total of 166 pathogens were isolated from 218 infections (76.1%). Enterococcus species were the most prevalent pathogens, causing 15.1% of HAIs with reported microbiological isolation. Klebsiella species were the second most prevalent pathogens, causing 14.5% of HAIs with reported microbiological isolation, followed by Staphylococcus species (12.7%), Acinetobacter species (12.7%) and Escherichia coli (12%). Overall, these five pathogens caused 50.9% of all the HAIs (111 HAIs with reported microbiological isolation of 218 HAIs) (Figure 2). Antimicrobial susceptibility was tested on 105 (63.2%) isolated micro-organisms and revealed that 63.6% (seven out of 11) of Acinetobacter species were resistant to carbapenem, 42.8% (six out of 14) of E. coli and 40.9% (nine out of 22) of Klebsiella species were resistant to third generation cephalosporins and 22.7% (five out of 22) of Klebsiella species were resistant to both third generation cephalosporins and carbapenem. Instead, only 5.9% (one out of 17) of Enterococcus species were resistant to glycopeptides.
Figure 2.
Absolute and relative frequencies of isolated pathogens* according to microbe species.
*Only micro-organisms having at least two isolates have been reported.
Discussion
To increase and update knowledge about hospital infections represents a major goal for implementing effective public health preventive strategies. Consistent with this mandate, in this study we have reported findings obtained from prevalence surveys carried out in a major Italian hospital over an eight year period.
The main message from our results is that healthcare infections, to date, can have a non-trivial morbidity burden in hospitalised patients and preventive interventions might represent a substantial public health answer for reducing the impact of these infectious diseases.
The important health burden is consistent with the finding that in our hospital setting, on average, one patient out of 14 was affected by HAI and this risk has been significantly higher in some years, in presence of extreme clinical frailty, in critical hospital areas or when some invasive medical procedures were performed. In particular, overall prevalence rates of HAIs in the study period seem to be slightly higher than values reported in other, similar, studies (Behnke et al, 2013; Chen et al, 2017; Magill et al, 2014, 2018; Sinatra et al, 2013) as well as the ECDC’s report (ECDC, 2013), which in 2011 estimated a prevalence rate of 6.0% (95% CI: 5.7–6.3%) in European acute-care hospitals, and the 2016–2017 Italian report, which observed a prevalence rate of 6.5% (95% CI: 5.22–7.78%) (Ministero della Salute, 2018).
This difference could be at least in part due to the fact that our surveys have been carried out in a tertiary care hospital whereas the European survey has collected results from primary, secondary, tertiary care and specialised hospitals in different countries. It is well known that tertiary hospitals take care of more critical patients that could have an increased risk of HAI and, consequently, higher prevalence rates. Prevalence rate of HAIs increases with rising numbers of beds and days of hospitalisation, as in highly intensive care facilities, where hospitalised patients are in critical conditions with intensive care and specialised assistance (Ministero della Salute, 2018; Valentino et al, 1987). In this sense, it should be highlighted that the prevalence rate of HAIs in this survey was similar to that in tertiary care hospitals (7.2%) (Antonioli et al, 2016; Labi et al, 2019; Weinstein et al, 1999). However, our prevalence rate could be higher also because it includes data obtained before 2015, when recommendations for preventing hospital infections were not applied in our hospital.
As a second point of view, our study suggests the importance of several risk factors in determining hospital infections. Some of these risk factors are comparable to other previous studies, in particular to those reported by the ECDC (ECDC, 2013). In accordance with the international literature, we have found that central and/or peripheral catheters in place during hospitalisation were highly correlated with occurrence of hospital-acquired infections (Agodi et al, 2013, 2018). Similarly, urinary catheterisation during hospitalisation was also a relevant risk factor associated with possible onset of HAIs, in coherence with Italian and international literature (Agodi et al, 2013; Tandogdu and Wagenlehner, 2016).
Surprisingly, in the study intubation was not statistically significant, in contrast to results from other studies (Chen et al, 2017; Magill et al, 2018). This finding could be due, at least in part and according to other authors, to the relatively low number (N=4) of ventilator-associated pneumonia in our hospital (Giuliano et al, 2018; Klompas, 2016). Otherwise, it is possible that the increased risk of HAIs in intubated patients is associated with their usually very critical health conditions. In this sense, in our multivariable analysis intubation could have lost its significance because of other factors (e.g. McCabe score, surgical intervention, etc.) that represent best predictors of critical health conditions and, thus, hospital infections.
In this sense, it should be noted that undergoing invasive surgery and having McCabe score “rapidly fatal disease” or “ultimately fatal disease”, in accordance to national and international data (Ministero della Salute, 2018; Zarb et al, 2012), are two strong risk factors increasing the odds of acquiring HAIs from 1.5- to 2-fold.
Overall, our results show that the risk of developing HAIs can be associated to a mix of variables associated with weak immune system (McCabe score), medicalisation and rupture of the physiological barriers (presence of invasive devices and surgery), in association with a prolonged exposure to the hospital environment (length of stay since hospital admission) (Ministero della Salute, 2018).
The most frequent infection types in this study were similar to those of the Italian ECDC report in 2016 (Ministero della Salute, 2018) and results from Europe and America (Magill et al, 2014; Suetens et al, 2018), but there was a difference in the distribution of infections results. Pneumonia, which is one of the most prevalent HAIs in the literature, is the second most frequent infection in our hospital, following urinary tract infections. Numerous studies have reported that the presence of urinary catheters is one of the most important factors in developing HAIs (Giuliano et al, 2018), as well as prolonged hospital stay (Laupland et al, 2002). Both these factors probably contribute to urinary infection directly or indirectly, through factors related to the patient’s immune system, pathogen virulence, healthcare intervention and healthcare environment (Tandogdu and Wagenlehner, 2016). Some of these risk factors are relatively common in our hospital and this suggests the need for training programmes and awareness-raising among our healthcare workers as a priority in order to prevent these infection types. Among these last factors the presence of recurrent micro-organisms as sources of infection could play a major role in increasing prevalence rates.
In particular, our data show a higher prevalence of Enterobacteriaceae when compared with the ECDC’s report data (ECDC, 2013), although this latter finding could be explained with the higher frequency of urinary tract infection in our hospital. According to the international literature the most frequent micro-organisms that have been isolated were Enterococcus spp., Klebsiella spp., Staphylococcus spp., Acinetobacter spp. and E. coli (Chen et al, 2017; Ministero della Salute, 2018). On the contrary, Clostridium difficile, despite its being the most frequently reported pathogen in a survey in the USA, in our study was found in only two infected patients, although we cannot exclude a contribution of underdiagnosis (Davies et al, 2014; Magill et al, 2014).
Noteworthy, Enterococci represented the most common pathogen isolated from our patients. The international literature documented an increasing role of these micro-organisms as responsible for HAI, accounting for approximately 10% of hospital acquired infections (Schmidt-Hieber et al, 2007), and their spreading is usually supported from person to person trasmission by hands or medical devices (Olawale et al, 2011). In our study they represented about 10% of all HAIs in each year but 2011 (when they represented 37.5% of all HAIs) and 2019 (when they accounted for 22% of all HAIs). Although we suppose that these higher prevalences in some years could be related to mini outbreaks, future investigations, including molecural analyses, could be required for confirming this hypothesis. Similarly, further analyses could be required for understanding the reasons for the high rates of resistance to carbapenems and third generation cephalosporins that we have observed. This latter finding could be due, at least in part, to the fact that these antibiotics are used a great deal in our hospital, being the second and the fifth most used groups, respectively, and it is well known that antibiotic usage could be a major determinant of antibiotic resistance of isolates in hospitalised patients (Pedersen et al, 1999).
Finally, one datum should be, at our advice, considered to be of particular interest. After an increasing temporal trend from 2011 to 2014, from 2015 HAIs decreased, year after year, their prevalence. In particular, by considering the joinpoint analysis, it has been possible to identify two different trends with a breakpoint just during 2014. This breakpoint, identified by statistical procedures, seems to confirm that the following decreasing trend, although non-statistically significant due to the high prevalence rate found in 2018, has been possibly related to infection prevention and control practices that have been implemented from 2014 in our hospital (Agodi et al, 2018). In particular, these interventions included guidelines regarding improvement in infection control procedures, such as antibiotic peri-surgical prophylaxis in adults, ambulance hygiene, handwashing (application of WHO guidelines on the hand hygiene “Clean Care is Safer Care”) (Moro et al, 2017), isolation measures and management of patients with colonisation/infection by multi-resistant pathogens, prevention of surgical site infection, and implementation of bundle for prevention of HAIs. Moreover, training programmes for healthcare workers were carried out to improve adherence to guidelines (Lanini et al, 2009).
This survey has some limitations that must be considered. First, since the survey considers data of a single tertiary care hospital, this may not be representative of all the other acute care hospitals and other settings. Furthermore, the study has some limitations inherent to the study design, including reduced periods of observation, cross-sectional study design and presence of missing data (e.g. laboratoristic identification of micro-organisms, antibiograms, etc.). Finally, it should be pointed out that we have considered the 2018 HAI prevalence rate as outlier since 11 (39.3%) out of 28 infections observed in that year were due to outbreaks in two wards. Other point-prevalence studies could be required for evaluating whether this latter observation was due only to the case or effectively attributable to a reduced effect of infection prevention and control practices with time.
Conclusions
Despite these limitations, this study has had the strength of using standardised protocols for data collection. By the use of the same protocol, the comparability over time and between similar facilities on a regional or national scale can be assured. Moreover, our data confirm the burden of hospital infections in contributing to morbidity of hospitalised patients and suggest that preventive interventions could be of paramount importance for reducing the impact of infectious diseases in populations that increase mean age and presence of chronic comorbosities.
Acknowledgments
The authors are fully indebted to medical residents of the Specialty School of the University of Palermo for their contribution to the surveys.
Footnotes
Declaration of conflicting interest: The authors have no conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Emanuele Amodio  https://orcid.org/0000-0001-5482-5268
https://orcid.org/0000-0001-5482-5268
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Agodi A, Auxilia F, Barchitta M, Brusaferro S, D’Alessandro D, Grillo OC, Montagna MT, Pasquarella C, Righi E, Tardivo S, Torregrossa V, Mura Iand GISIO-SITI working group (2013) Trends, risk factors and outcomes of healthcare-associated infections within the Italian network SPIN-UTI. The Journal of Hospital Infection 84(1): 52-58. [DOI] [PubMed] [Google Scholar]
- Agodi A, Barchitta M, Mura I, Pasquarella C, Torregrossa MV. (2018) The commitment of the GISIO-SItI to contrast Healthcare-Associated Infections and the experience of prevalence studies in Sicily. Annali di igiene: medicina preventive e di comunita 30(4 Suppl. 1): 38-47. [DOI] [PubMed] [Google Scholar]
- Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. (2011) Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. The Lancet 377(9761): 228-241. [DOI] [PubMed] [Google Scholar]
- Antonioli P, Manzalini MC, Stefanati A, Bonato B, Verzola A, Formaglio A, Gabutti G. (2016) Temporal trends of healthcare associated infections and antimicrobial use in 2011–2013, observed with annual point prevalence surveys in Ferrara University Hospital, Italy. Journal of Preventive Medicine and Hygiene 57(3): E135-E141. [PMC free article] [PubMed] [Google Scholar]
- Behnke M, Hansen S, Leistner R, Diaz LA, Gropmann A, Sohr D, Gastmeier P, Piening B. (2013) Nosocomial infection and antibiotic use: A second national prevalence study in Germany. Deutsches Arzteblatt International 110: 627-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao JY, Shan X, Han XL, Tian SG, Chen FY, Su XT, Sun YS, Huang LY, Han Land Chinese Group on Point-Prevalence Survey of Healthcare-Associated Infections (2017) A point-prevalence survey of healthcare-associated infection in fifty-two Chinese hospitals. The Journal of Hospital Infection 95(1): 105-111. [DOI] [PubMed] [Google Scholar]
- CNESP (2019) Centro Nazionale di Epidemiologia, Sorveglianza e Promozione della Salute. Infezioni correlate all’assistenza, aspetti epidemiologici. Available at: http://www.epicentro.iss.it/problemi/infezioni_correlate/epid.asp (accessed 2 July 2019).
- Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, Delmée M, Fitzpatrick F, Ivanova K, Kuijper E, Macovei IS, Mentula S, Mastrantonio P, von Müller L, Oleastro M, Petinaki E, Pituch H, Norén T, Nováková E, Nyč O, Rupnik M, Schmid D, Wilcox MH. (2014) Underdiagnosis of Clostridium difficile across Europe: The European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). The Lancet. Infectious Diseases 14(12): 1208-1219. [DOI] [PubMed] [Google Scholar]
- ECDC (2013) Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- ECDC (2016) Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals – Protocol version 5.3. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- ECDC (2017) ECDC Country Visit to Italy to Discuss Antimicrobial Resistance Issues. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- ECDC (2018) Antimicrobial consumption. In: ECDC. Annual Epidemiological Report 2017. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- ECDC (2019) Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals – ECDC PPS Validation Protocol Version 3.1.2. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- Giuliano KK, Baker D, Quinn B. (2018) The epidemiology of nonventilator hospital-acquired pneumonia in the United States. American Journal of Infection Control 46: 322-327. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Balkhy HH, Goossens H, Jarlier V, Kluytmans J, Laxminarayan R, Saam M, Van Belkum A, Pittet Dand the World Healthcare-Associated Infections Resistance Forum participants (2015) Antimicrobial resistance: One world, one fight! Antimicrobial Resistance & Infection Control 4: 49. [Google Scholar]
- Klompas M. (2016) Hospital-acquired pneumonia in nonventilated patients: The next frontier. Infection Control and Hospital Epidemiology 37(7): 825-826. [DOI] [PubMed] [Google Scholar]
- Labi AK, Obeng-Nkrumah N, Owusu E, Bjerrum S, Bediako-Bowan A, Sunkwa-Mills G, Akufo C, Fenny AP, Opintan JA, Enweronu-Laryea C, Debrah S, Damale N, Bannerman C, Newman MJ. (2019) Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. The Journal of Hospital Infection 2019; 101(1): 60-68. [DOI] [PubMed] [Google Scholar]
- Lanini S, Jarvis WR, Nicastri E, Privitera G, Gesu G, Marchetti F, Giuliani L, Piselli P, Puro V, Nisii C, Ippolito G. and INF-NOS Study Group (2009) Healthcare-associated infection in Italy: Annual point-prevalence surveys, 2002–2004. Infection Control and Hospital Epidemiology 30(7): 659-665. [DOI] [PubMed] [Google Scholar]
- Laupland KB, Zygun DA, Davies HD, Church DL, Louie T, Doig CJ. (2002) Incidence and risk factors for acquiring nosocomial urinary tract infection in the critically ill. Journal of Critical Care 17(1): 50–57. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. (2013) Antibiotic resistance – the need for global solutions. The Lancet. Infectious Diseases 13: 1057-1098. [DOI] [PubMed] [Google Scholar]
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SKand Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team (2014) Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. New England Journal of Medicine 370(13): 1198-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JRand Emerging Infections Program Hospital Prevalence Survey Team (2018) Changes in prevalence of health care-associated infections in U.S. Hospitals. New England Journal of Medicine 379(18): 1732-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministero della Salute (2018) Secondo studio di prevalenza italiano sulle infezioni correlate all’assistenza e sull’uso di antibiotici negli ospedali per acuti – Protocollo ECDC. Dipartimento Scienze della Salute Pubblica e Pediatriche, Università di Torino. Available at: http://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2791 (accessed 2 July 2019).
- Moro ML, Morsillo F, Nascetti S, Parenti M, Allegranzi B, Pompa MG, Pittet D. (2017) Determinants of success and sustainability of the WHO multimodal hand hygiene promotion campaign, Italy, 2007–2008 and 2014. Euro Surveillance: bulletin Europeen sur les maladies transmissibles 22(23) 30546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannessian R, Gustin MP, Bénet T, Gerbier-Colomban S, Girard R, Argaud L, Rimmelé T, Guerin C, Bohé J, Piriou V, Vanhems P. (2018) Estimation of extra length of stay attributable to hospital-acquired infections in adult ICUs using a time-dependent multistate model. Critical Care Medicine 46(7):1093-1098. [DOI] [PubMed] [Google Scholar]
- Olawale KO, Fadiora SO, andTaiwo SS. (2011) Prevalence of hospital-acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. African Journal of Infectious Diseases: AJID 5(2): 40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G, Schønheyder HC, Steffensen FH, Sørensen HT. (1999) Risk of resistance related to antibiotic use before admission in patients with community-acquired bacteraemia. Journal of Antimicrobial Chemotherapy 43(1):119-126. [DOI] [PubMed] [Google Scholar]
- Plachouras D, Kärki T, Hansen S, Hopkins S, Lyytikäinen O, Moro ML, Reilly J, Zarb P, Zingg W, Kinross P, Weist K, Monnet DL, Suetens Cand The Point Prevalence Survey Study Group (2018) Antimicrobial use in European acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveillance: bulletin Europeen sur les maladies transmissibles 23(46): 1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raka L, Spahija G, Gashi-Gecaj A, Hamza A, Haxhiu E, Rashiti A, Rrahimi G, Hyseni S, Petrosillo N. (2019) Point prevalence survey of healthcare-associated infections and antimicrobial use in Kosovo hospitals. Infectious Disease Reports 11(1): 7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber M, Blau IW, Schwartz S, Uharek L, Weist K, Eckmanns T, Jonas D, Rüden H, Thiel E, Brandt C. (2007) Intensified strategies to control vancomycin-resistant enterococci in immunocompromised patients. International Journal of Hematology 86(2): 158-162. [DOI] [PubMed] [Google Scholar]
- Sinatra I, Carubia L, Marchese V, Aprea L, D’Alessandro N, Mammina C, Torregrossa MV. (2013) Prevalence survey of healthcare-associated infections and antimicrobial use at the University Hospital “Paolo Giaccone”, Palermo, Italy. Journal of Preventive Medicine and Hygiene 54(4): 200-204. [PMC free article] [PubMed] [Google Scholar]
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, Jans B, Hopkins S, Hansen S, Lyytikäinen O, Reilly J, Deptula A, Zingg W, Plachouras D, Monnet DLand The Healthcare-Associated Infections Prevalence Study Group (2018) Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Euro Surveillance: bulletin Europeen sur les maladies transmissibles 23(46): 1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandogdu Z, Wagenlehner FM. (2016) Global epidemiology of urinary tract infections. Current Opinion in Infectious Diseases 29(1): 73-79. [DOI] [PubMed] [Google Scholar]
- Valentino L, Torregrossa MV, Dardanoni G. (1987) [Prevalence and incidence of hospital infections at the University Polyclinic of Palermo.] Bollettino dell’Istituto sieroterapico Milanese 66(2): 139-144. [PubMed] [Google Scholar]
- Versporten A, Bolokhovets G, Ghazaryan L, Abilova V, Pyshnik G, Spasojevic T, Korinteli I, Raka L, Kambaralieva B, Cizmovic L, Carp A, Radonjic V, Maqsudova N, Celik HD, Payerl-Pal M, Pedersen HB, Sautenkova N, Goossens H. and the WHO/Europe-ESAC Project Group (2014) Antibiotic use in eastern Europe: A cross-national database study in coordination with the WHO Regional Office for Europe. The Lancet. Infectious Diseases 14: 381-387. [DOI] [PubMed] [Google Scholar]
- Weinstein JW, Mazon D, Pantelick E, Reagan-Cirincione P, Dembry LM, Hierholzer WJ., Jr (1999) A decade of prevalence surveys in a tertiary-care center: Trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol. 20(8): 543-548. [DOI] [PubMed] [Google Scholar]
- WHO (2016) Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens M, Vaerenberg S, Hopkins S, Catry B, Monnet D, Goossens H, Suetens C. and the National Contact Points for the ECDC pilot point prevalence survey (2012) The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveillance: bulletin Europeen sur les maladies transmissibles 17(46): 20316. [DOI] [PubMed] [Google Scholar]