Abstract
Background
A trauma to the skull in the area of the pterion usually causes rupture of the middle meningeal artery leading to life- threatening epidural hematoma. The objective of the study is to assess the prevalence of different types of pterion and to determine its location using valuable bony landmarks.
Methods
On 90 dry adult human skulls of unknown sex, age and nationality the distance of different landmarks from pterion was measured using stainless steel sliding Vernier caliper. The data were analyzed using SPSS version-20 and an independent t-test analysis was implemented. A value of P< 0.05 was considered as statistically significant.
Results
A higher occurrence of sphenoparietal type of pterion with the absence of frontotemporal type was noted. About 23% and 77% of the suture types are found to be unilateral and bilateral, respectively. There was a statistically significant difference between right and left sides of the skull in distances from the center of pterion to frontozygomatic suture, root of zygomatic arch, inion and in central thickness pterion.
Conclusion
This study showed that the most prevalent type of pterion is sphenoparietal, and revealed asymmetry in the distances from center of pterion to frontozygomatic suture, root of zygomatic arch and inion, and its central thickness. Such findings could offer worthy information about the type and location of pterion, which could be relevant to anatomists, neurosurgeons, forensic medicine specialist and anthropologists.
Keywords: Frontozygomatic suture, inion, Pterion, sphenoid bone, zygomatic arch
Introduction
The pterion is an H-shaped bony neurological landmark found at the junction of the frontal, sphenoid, parietal and the squamous part of temporal bone (1). It is located approximately 4 cm superior to the zygomatic arch and 3.5 cm posterior to the frontozygomatic suture (2).
Internally, the pterion is related to various anatomical structures, namely, the anterior division of the middle meningeal vessels, middle cerebral vessels, Sylvain fissure, circle of Willis, insula and Broca's motor speech area (on the left) and optic nerve (3).
Any traumatic blow to the pterion presumably causes rupture of the anterior divisions of the middle meningeal vessels causing an epidural haematoma subsequently resulting in compression of cerebral cortex and death unless proper intervention is carried out (4,5). Surgical approach via the pterion has been quoted as the most widely implemented approach for the proper management of intracranial anterior circulation aneurysm. This approach has better advantages over the traditional surgical approach with minor tissue damage, lesser brain retraction, a superior cosmetic results and a shorter duration of surgery (6).
According to Murphy (1956), pterion can be categorized into four types, namely, sphenoparietal, frontotemporal, stellate and epipteric suture (7). The sphenoparietal type is the most common suture formed by the articulation of the greater wing of sphenoid bone with parietal bone. The frontotemporal type is a pterional sutural pattern between the frontal and temporal bone. The stellate variety of suture is the site of articulation formed by the fusion of four flat bones, sphenoid, frontal, parietal and temporal bones. The epipteric type of pterion is characterized by the presence of small sutural bones between the sphenoid and parietal bones. The presence of epipteric or wormian (sutural) bone in the area, can possibly lead to wrong radiological diagnosis and clinical management of fracture in the pterion. The presence of sutural bones could possibly complicate surgical interventions involving burr hole surgeries as their extension may lead to orbital penetration (4,8,9).
Various evidences demonstrated that the type and location of pterion exhibits significant ethnic, sex and age-related variations (3,4,10–14). Even though such evidences exist, there is no documented information in Ethiopia so far. The present study is aimed to determine the type and location of the pterion using dry adult human skulls obtained from Northwest Ethiopia. The findings of this study could provide baseline information about the type and location of the pterion in the studied population that can be useful for anatomists, neurosurgeons, forensic pathologists and anthropologists.
The objective of the present study is to assess the prevalence of different types of pterion and to determine its location using valuable bony landmarks.
Materials and Methods
A cross sectional study was conducted on ninety dried and intact adult skulls of unknown sex, age and nationality. The study was conducted from 20th of March to 20th of April, 2020 on dry skulls obtained from the anatomical museum in the Department of Human Anatomy, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Ethiopia. Sutural patterns of the pterion and its distance from selected structural landmarks were assessed macroscopically on both sides. The data collection was performed after the ethical clearance and approval obtained from the School of Medicine Ethical committee, University of Gondar (Reference SOM 876/12 dated December 24, 2019). Skulls with any pathological deformities and trauma affecting the measurements, for instance fracture of zygomatic arch were excluded.
The sutural patterns of the pterion (sphenoparietal, frontotemporal, stellate and epipteric types) were studied on both sides of each skull using the principles of Murphy classification (7).
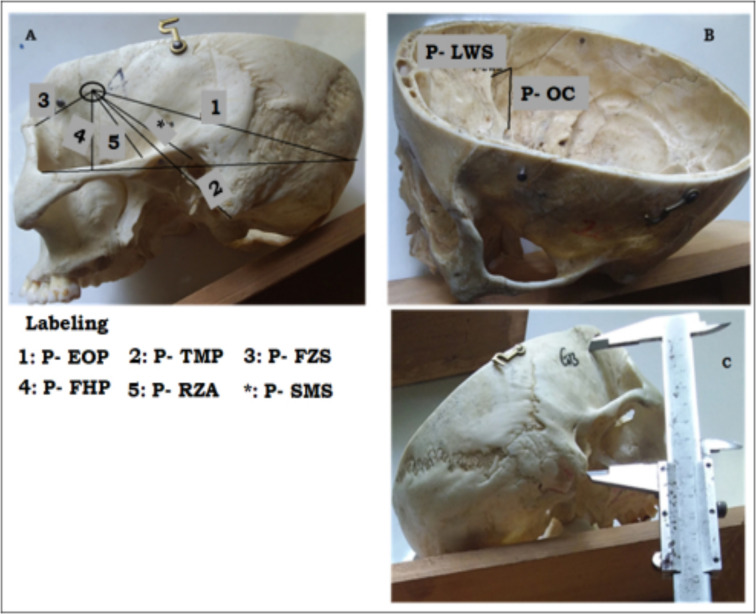
For the purpose of measurements of distances of various clinically important landmarks from the corresponding pterion, a circle of smallest radius was drawn just at the site of formation of the pterion. The center of the circle was considered as the center of pterion (Figure 1a and b). Distance measurements in centimeter were taken from the center of pterion with a stainless-steel sliding Vernier caliper (Figure 1c) twice and the average was taken as the actual measurement.
Figure 1.
Representative picture presenting the distances from the pterion to some special external (A) and internal (B) structural landmarks and measurement from the central distance of pterion to various landmarks of skulls of unknown sex age and nationality obtained from the anatomical museum in the Department of Human Anatomy, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Ethiopia using a Stainless steel Vernier caliper (C)
The following distance measurement parameters were taken on the lateral aspects of the skulls from the center of the pterion (P), 1) to the posterolateral aspect of the frontozygomatic suture (FZS), 2) to the root of zygomatic arch (RZA), 3) to the tip of the mastoid process (TMP), 4) to the suprameatal spine (SMS), 5) to the external occipital protuberance (EOP), 6) to the Frankfurt horizontal plane (FHP). Additionally, distance measurements were also taken from the internal aspect of the center of the pterion to the lateral end of the sphenoid ridge on the lesser wing of sphenoid bone (LWS) and to the lateral margin of the optic canal (OC). The thickness at the center of the pterion (TAAC) was also measured.
Statistical analysis: All the data were analyzed using SPSS version 20 statistical software. A comparison of the mean values between the sides was done using the independent t-test and a P-value less than 0.05 was considered as statistically significant. The data were presented as mean with the corresponding standard error of mean (SEM).
Results
As it is presented in Table 1 and Figure 2, three types of pterion patterns (sphenoparietal, epipteric and stellate) were identified. Sphenoparietal was the most common type with frequency of 152 (84.4%), followed by epipteric 24 (13.3%). Frontotemporal type of pterion was not observed. In all the three identified types of pteria, there was asymmetric distribution.
Table 1.
Distribution of types of pteria among skulls obtained in Northwest Ethiopia, 2020
| Type of pterion | Right side | Left side | Both sides | Total |
| Frequency | Frequency | Frequency | Frequency (%) | |
| Sphenoparietal | 14 | 6 | 132 | 152 (84.4%) |
| Frontotemporal | - | - | - | - |
| Epipteric | 6 | 12 | 6 | 24 (13.3%) |
| Stellate | 1 | 3 | - | 4 (2.2%) |
Figure 2.
Identified types of the pteria on skulls of unknown sex age and nationality obtained from the anatomical museum in the Department of Human Anatomy, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Ethiopia, 2020.
In this study, the average distances between the center of pterion and several clinically and morphologically important structural landmarks were determined using the sliding stainless steel Vernier caliper. After checking for normality of distribution and homogeneity of variance, independent sample t-test was done to determine whether the central distance of the pterion from various bony landmarks differs with the sides of the skull as presented in Table 2. The mean distances from the center of pterion to FZS on the right side was 2.92 ± 0.05 cm and on the left side was 2.75 ± 0.05 cm. The distances were relatively shorter on the left side than on the right side and the difference was statistically significant (P= 0.021). However, the actual difference of the two sides was found to be small (Eta squared= 0.03). Similarly, the average distance from the RZA to the pterion on the right and left sides was 3.55 ± 0.04 cm and 3.30 ± 0.05 cm, respectively, and the difference was statistically significant (P= 0.000) with small actual difference between the two sides (Eta squared= 0.076).
Table 2.
Comparison of the mean central distances from pterion (P) to various bony landmarks on the two sides
| Bony Landmarks |
Side | Mean | SEM | t | F | P-value | Eta squared |
| P-FZS | Right | 2.919 | .0534 | 2.33 | 1.92 | .021* | .030 |
| Left | 2.746 | .0516 | |||||
| P-RZA | Right | 3.553 | .0380 | 3.82 | 12.52 | .000* | .076 |
| Left | 3.302 | .0537 | |||||
| P-LWS | Right | 1.693 | .0233 | -.98 | 8.60 | .327 | .005 |
| Left | 1.733 | .0334 | |||||
| P-OC | Right | 3.842 | .0149 | 1.64 | .39 | .102 | .015 |
| Left | 3.801 | .0201 | |||||
| P-TMP | Right | 7.690 | .0459 | .63 | .12 | .531 | .002 |
| Left | 7.649 | .0466 | |||||
| P-Inion | Right | 12.518 | .0669 | -2.41 | 3.43 | .017* | .031 |
| Left | 12.726 | .0546 | |||||
| P-SMS | Right | 4.968 | .0380 | -1.92 | 2.66 | .057 | .020 |
| Left | 5.063 | .0323 | |||||
| P-EOP | Right | 11.686 | .0799 | -1.60 | 5.90 | .112 | .014 |
| Left | 11.847 | .0614 | |||||
| P-FHP | Right | 3.491 | .0439 | 1.76 | 1.63 | .080 | .017 |
| Left | 3.387 | .0399 | |||||
| P-Lambda | Right | 13.213 | .1189 | .28 | .02 | .784 | .000 |
| Left | 13.167 | .1214 | |||||
| P-TAAC | Right | .587 | .0139 | 4.92 | .24 | .000* | .120 |
| Left | .491 | .0136 |
The distance between the center of pterion and inion was found to be 12.52 ± 0.07 cm on the right and 12.73 ± 0.05 cm on the left side being shorter on the right side as compared to the left side and the difference was statistically significant (P<0.05) but the effect of distance to the sides was small (Eta squared= 0.031) (Table 2). The central thickness of pterion was found to be 0.59 ± 0.01 cm and 0.41 ± 0.01 cm on the right and left sides, respectively. The left side of pterion was significantly thinner than the corresponding right sides (P<0.05, Eta squared=0.120) (Table 2).
Discussion
Surgical approach via pterion has been cited as the most widely implemented approach to the proper management of intracranial anterior circulation aneurysmus. This approach has more advantages than the traditional surgical approach with minor tissue damage, less brain retraction, a superior cosmetic results and a shorter duration of surgery (6). Fundamentally, the knowledge of the pterion sutural patterns and its relationship to the bony landmarks is useful for specialist in various field of medical professions particularly for neurosurgeons (17, 18). The main findings of this study are notably the higher occurrence of the sphenoparietal type of pterion with the absence of frontotemporal type. About 23% and 77% of the suture types are found to be unilateral and bilateral, respectively. Furthermore, the central distances of the pterion to the FZS, RZA and inion, and the thickness at its center had statistically significant difference between the right and left sides of the skull.
The incidence of the different types of pteria among a diversified population of different countries were compared (1, 3, 4, 7, 8, 10–12, 14, 19–23) and illustrated in Table 3.
Table 3.
Comparison of the percentage of pteria types with other population Type of pterion
| Author | Population | Sample size |
Sphenoparietal (%) |
Frontotemporal (%) |
Epipteric (%) |
Stellate (%) |
| Murphy (1956) | Australian | 368 | 73.23 | 7.75 | 18.34 | 0.68 |
| Ersoy et al (2003) | Turkish | 300 | 96 | 3.8 | 9 | 0.2 |
| Oguz et al (2004) | Turkish | 26 | 88 | 10 | 2 | - |
| Mwachaka et al (2008) |
Kenyans | 50 | 66 | 15 | 12 | 7 |
| Zalawadia et al (2010) |
West India | 42 | 91.7 | 2.4 | 4.8 | 1.2 |
| Ilayperuma et al (2010) |
Sri Lanka | 52 | 74.04 | 4.81 | 21.15 | - |
| Ma et al (2012) | New Zealandian | 76 | 78.3 | 5.2 | 16.4 | - |
| Ukoha et al (2013) | South East Nigerian | 56 | 75.5 | 19.6 | 3.6 | 1.8 |
| Sunday et al (2013) | Nigerian | 62 | 86.1 | 8.3 | - | 5.6 |
| Cimen et al (2019) | Middle and South |
75 | 82 | 4.66 | 10.66 | 2.66 |
| Anatolian | ||||||
| Kamath et al (2016) | India | 72 | 79.25 | 10.25 | 6.3 | 4.2 |
| Oguz et al (2004) | Turkish | 26 | 88 | 0 | 2 | 10 |
| Shenoy et al (2012) | India | 75 | 77.33 | 0 | 21.33 | 1.34 |
| Lee et al (2001) | Korean | 149 | 76.5 | 0 | 40.3 | 0 |
| Present Study | Unknown | 90 | 70.6 | 0 | 15.6 | 2.8 |
Wang et al, (2006) explained the influence of environmental and/or genetic factors on the sutural patterns of pterion (13). A longitudinal study done using 368 Australian skulls reported four different stutural patterns of pterion (7). The incidence of the various types of pterion was 73.23%, 7.75%, 18.34% and 0.68% for sphenoparietal, frontotemporal, epipteric and stellate types, respectively. A study conducted in Turkey using 300 dried human skulls stated sphenoparietal type (96%), frontotemporal (3.7%), epipteric (9%) and stellate type (0.2%) (8). They revealed the existence of an epipteric or wormian bone at the pterion may complicate surgical orientation leading to complication during burr hole surgeries like orbital penetration. As it has been reported in another study on 26 dry adult skulls of Turks, 88% were sphenoparietal, 10% were frontotemporal and 2% were epipteric while the stellate type was absent (12). Different studies unanimously, reported that sphenoparietal type of pterion was found to be pre-dominant type of suture (3, 4, 10–12, 17, 20, 24). A study done on 52 dried adult Sri Lankan skulls reported the sphenoparietal type (74.04%) as the most common type followed by epipteric type (21.15%) and frontotemporal type (4.181%). Similarly, they did not find any stellate variety of pterion in their study (19). A Nigerian study also reported sphenoparietal type (77.33%), frontotemporal type (8.3%) and stellate type (5.6%) patterns of pterion without epipteric type (1). A study in India population showed sphenoparietal type (77.33%), epipteric type (21.33%), stellate type (1.34%) but a frontotemporal type of pterion was not seen (23). In another study done on 149 dried Korean skulls, the most frequent type of pterion found was sphenoparietal type (76.5%) followed by the epipteric type (40.3%) without the occurrence of stellate and frontotemporal types (20). In the present study, sphenoparietal (84.4%), epipteric (13.3%) and stellate (2.2%) types of pterion were identified. Of the observed patterns of pterion 23.3% and 76.7% were present unilaterally and bilaterally, respectively but no frontotemporal variety of pterion was observed. The presence or absence of frontotemporal pattern of pterion clearly indicates the contribution of genetic factor to the variation of occurrence ranged from zero in a British seventeenth century cementery to 9.8% in Nigerian crania (25).
The mean distance measurements between the center of pterion and different landmarks among different studies conducted elsewhere were compared (1, 3, 9–12, 14, 19, 21, 26, 27) and are depicted in Table 4. A study done on cone beam CR scans of 50 adult craniums and 76 adult dry skulls in New Zealand reported the significant clinical relationship of the anterior division of middle meningeal artery to the center of the pterion in the Frankfurt plane (21). As it was reported forty years ago the pterion is situated 3.0 – 3.5 cm posterior to the FZS (2). The mean distance of the center of the pterion from the posterolateral margin of the FZS in adult dry skulls was 3.1 ± 0.4 cm on the right side and 3.09 ± 0.4 cm on the left side (4). Studies conducted elsewhere reported a mean pterion to FZS distance ranging from 3.0 cm to 3.7 cm (10–12, 14, 27). However, Nigerian (3) and New Zealandian (21) studies stated shorter mean center of pterion to FZS distance on the right and left sides, respectively as (2.74 ± 0.17cm and 2.7 ± 0.06 cm; 2.6 ± 0.4 cm and 2.5 ± 0.4 cm). In the present study, the center of the pterion was found to be 2.92 ± 0.05 cm on the right side and 2.75 ± 0.05 cm on the left side above the posterolateral margin of the FZS (P<0.05, Eta squared= 0.03).
It has been reported that the center of the pterion is found to be 3 – 4 cm above the zygomatic arch (2). Similarly, studies conducted in a diversified population demonstrated the mean pterion - RZA distance in as presented in Table 4. In this study, the pterion was 3.55 ± 0.04 cm on the right side and 3.30 ± 0.05 cm on the left side superior to the RZA (P<0.05, Eta squared= 0.076).
The lesser wing of the sphenoid bone (LWS) is a common site for meningiomas and it can be approached through the pterional surgical technique. In this case, the distance between the internal portion of the pterion and the lateral margin of sphenoid bone is crucial. An Indian study done on 42 dry adult human skulls reported the mean distance between the pterion and LWS as 1.36 ± 0.35 cm on the right side and 1.33 ± 0.22 cm on the left side (14). Kenyans study reported that the distance of the pterion center from the lateral margin of LWS was 1.4 ± 0.33 cm on the right and 1.48 ± 0.32 cm on the left side far from the lateral margin of LWS (11). However, in the present study, the internal portion of pterion center was far from the lateral margin of LWS with a mean distance of 1.69 ± 0.02 cm on the right side and 1.73 ± 0.03 cm on the left side (P>0.05). The discrepancy of the mean distance is probably due to environmental and genetic variabilities.
Pterional approach is useful to reach to the optic canal containing optic nerve (CN II) and ophthalmic artery. In such a case, the distance measurements between the internal aspect of pterion and optic canal (OC) is decisive. Studies from India and Kenya reported that the internal surface of pterion center is far from the OC with a mean measurement of 4.52 ± 0.32 cm and 4.39 ± 0.4 cm on the right side and 4.37 ± 0.23 cm and 4.36 ± 0.4 cm on the left side, respectively (11, 14). In the present study, the mean measurement between the internal portion of pterion center and OC was 3.84 ± 0.01 cm on the right side and 3.80 ± 0.02 cm on the left side.
A very recent study done in Turkey using 75 dry adult skulls reported the mean measurement between the center of the pterion and the inion to be 13.55 ± 0.62 cm in male and 12.62 ± 0.63 cm in female (10). In another report based on skulls of male subjects of the Byzantine period, the distance between the pterion and the inion was found to be 13.80 ± 0.5 cm on the right side and 13.70 ± 0.40 cm on the left side (28). However, in this study, the mean measurement between pterion and inion was 12.52 ± 0.07 cm and 12.73 ± 0.05 cm on the right and left sides, respectively (P=0.017, Eta squared= 0.031).
A comparative study done on human skulls from 13th to 20th century using manual measurements revealed that the mean distance between pterion and the tip of mastoid process (TMP) in male subjects as 8.30 ± 0.34 cm on the right side and 8.50 ± 0.26 cm on the left side (28). In a study done in Turkey, the mean distance between pterion and TMP was found to be 8.02 ± 0.6 cm (10). On the other hand, in this study, the mean measurement between the center of the pterion and the TMP was 7.69 ± 0.05 cm on the right side and 7.65 ± 0.05 cm on the left side (P>0.05).
Cimen and collaborators (2019) measured the mean distance between pterion and external acoustic meatus as 5.71 ± 0.77 cm and 5.34 ± 0.36 cm in male and female skulls, respectively (10). In the present study, the measured mean distance between the center of pterion and the supra meatal spine was 4.97 ± 0.04 cm on the right side and 5.06 ± 0.03 cm on the left side (P>0.05). The difference may be due to geographical, genetic or methodological variations.
A study done in India on 100 dry skulls reported the mean thickness at the center of the pterion to be 0.352±0.145 cm (9). In addition, an Asian scholar reported mean different thicknesses at the center of the pterion as 0.513±0.167 cm in Thai skulls (26), 0.39 to 0.41 cm in Turks (12), and 0.319±0.085 cm in Korean skulls (29). In this study the central thickness of the pterion was 0.59 ± 0.01 cm on the right and 0.41 ± 0.01 cm on the left side (P<0.05, Eta squared= 0.12). Clinically, the knowledge of the thickness of pterion is very important for neurosurgeons which could be applied during internal and external neurosurgical fixation procedures.
In conclusion, according to the finding in this current study, sphenoparietal type of suture is the most frequent variety of pterion. The mean measurements between the center of pterion and FZS, RZA and inion and central thickness of the pterion had statistically significant difference between the right and left sides of the skulls. The findings of this study may, presumably, be useful for the anatomists, neurosurgeons, forensic pathologies and anthropologists in the area of the studied population. Further investigation on the skulls of identified sex, age and nationality, particularly Ethiopian skulls, using computed scan, X-ray and dry human skulls is strongly recommended.
Acknowledgments
I thank the expert technical assistance of Mr. Wagnew Miteku.
References
- 1.Sunday AA, Funmilayo EO, Modupe B. Study of the location and morphology of the pterion in adult Nigerian skulls. ISRN Anatomy. 2013:1–4. doi: 10.5402/2013/403937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KL, Dalley AF. Clinically oriented anatomy. 4 ed. Baltimore: Lippincott Williams & Wilkins; 1999. pp. 836–842. [Google Scholar]
- 3.Ukoha U, Oranusi CK, Okafor1 JI, Udemezue OO, Anyabolu AE, Nwamarachi1 TC. Anatomic study of the pterion in Nigerian dry human skulls. Niger J Clin Pract. 2013;16(3):325–328. doi: 10.4103/1119-3077.113455. [DOI] [PubMed] [Google Scholar]
- 4.Kamath V, Asif M, Bhat S, Avadhani R. A study on the pterion position variation and its neurosurgical implications. J Anat Soc India. 2016;65(1):S33–S39. [Google Scholar]
- 5.Alper S, Eren Ö, Günes A, Nurettin O, Muzaffer S. Morphometric study of pterion. Int J Anat Res. 2015;4(1):1954–1957. [Google Scholar]
- 6.Yasargil MG, Fox JL, Ray MW. The operative approach to aneurysms of the anterior communicating artery. Adv Tech Stand Neurosurg. 1975;2:113–117. [Google Scholar]
- 7.Murphy T. The pterion in Australian aborigine. Am J Phys Anthropol. 1956;14:225–244. doi: 10.1002/ajpa.1330140218. [DOI] [PubMed] [Google Scholar]
- 8.Ersoy M, Evliyaoglu C, Bozkurt MC, Konuksan B, Tekdemir I, Keskil IS. Epipteric bones in the pterion may be surgical pitfall. Minim Invasive Neurosurg. 2003;46:364–365. doi: 10.1055/s-2003-812434. [DOI] [PubMed] [Google Scholar]
- 9.Kamath VG, Hande M. Reappraising the neurosurgical significance of the pterion location, morphology, and its relationship to optic canal and sphenoid ridge and neurosurgical implications. Anat Cell Biol. 2019;52(4):406–413. doi: 10.5115/acb.18.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimen K, Otag I, Cimen M. Pterion types and morphometry in Middle and South Anatolian adult skulls. Rev Arg de Anat Clin. 2019;11(1):8–17. [Google Scholar]
- 11.Mwachaka P, Hassanali J, Odula P. Anatomic position of the pterion among Kenyans for lateral skull approaches. Int J Morphol. 2008;26:931–933. [Google Scholar]
- 12.Oguz O, Sani SG, Bozkir MG, Soames RW. The pterion in Turkish male skulls. Surg Radiol Anat. 2004;26:220–224. doi: 10.1007/s00276-003-0210-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang O, Opperman LA, Havil LM, Carlson DS, Dechow PC. Inheritance of sutural pattern at the pterion in Rhesus Monkey skulls. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1042–1049. doi: 10.1002/ar.a.20373. [DOI] [PubMed] [Google Scholar]
- 14.Zalawadia A, Vadgama J, Ruparelia S, Patel S, Rathod SP, Patel SV. Morphometric Study Of Pterion In Dry Skull Of Gujarat Region. NJIRM. 2010;1(4):25–29. [Google Scholar]
- 15.Urzi F, Iannello A, Torrisi A, Foti P, Mortellaro NF, Cavallaro M. Morphological variability of pterion in the human skull. Ital J Anat Embryol. 2003;108(2):83–117. [PubMed] [Google Scholar]
- 16.Twinkle Francis MS, Ganesh Lakshmanan T. Morphological study of the pterion: An important landmark for neurosurgery and to determine its clinical significance. Drug Invent Today. 2019;11(8):1857–1860. [Google Scholar]
- 17.Nayak S, Soumya KV. Unusual sutural bones at pterion. Int J Anat Var. 2008;1:19–20. [Google Scholar]
- 18.Nayak G, Mohanty BB, Das RS. Morphometric study of pterion and its clinical significance. Asian J Pharm Clin Res. 2017;10(10):142–144. [Google Scholar]
- 19.Ilayperuma I, Nanayakkora BG, Palahepitiya KN. Types of pterion in Sri Lankan skulls. Ceylon Med J. 2010;53:9–14. [Google Scholar]
- 20.Lee UY, Park DK, Kwon SO, Paik DJ, Han SH. Morphological analysis of pterion in Korea. Korean J Phys Anthropol. 2001;14(4):281–289. [Google Scholar]
- 21.Ma S, Baillie LJ, Stringer MD. Reappraising the surface anatomy of the pterion and its relationship to the middle meningeal artery. Clin Anat. 2012;25:330–339. doi: 10.1002/ca.21232. [DOI] [PubMed] [Google Scholar]
- 22.Oguz O, Sanli SG, Bozkir MG, Soames RW. The pterion in Turkish male skulls. Surg Radiol Anat. 2004;26(3):220–224. doi: 10.1007/s00276-003-0210-2. [DOI] [PubMed] [Google Scholar]
- 23.Shenoy V, Saraswathi P, Siva T, Jagadeesh D. A study on sutural morphology and anatomical position of pterion. Int J Curr Res Rev. 2012;4(9):67–75. [Google Scholar]
- 24.Hussainsaheb S, Mavishettar GF, Thomas ST, Prasanna LC, Muralidhar P. A study of sutural morphology of the pterion & asterion among human adult Indian skulls. Biomed Res. 2011;22(1):73–75. [Google Scholar]
- 25.Sowmya S, Meenakshi B, Priya R. Study of pterion: Its clinical and morphological aspects. IJCAP. 2017;4(2):247–249. [Google Scholar]
- 26.Apinhasmit W, Chompoopong S, Chaisuksunt V, Thiraphatthanavong P, Phasukdee N. Anatomical consideration of pterion and its related references in Thai dry skulls for pterional surgical approach. J Med Assoc Thai. 2011;94(2):205–214. [PubMed] [Google Scholar]
- 27.Ilknur A, Mustafa KI, Sinan B. A comparative study of variation of the pterion of human skulls from 13th and 20th century Anatolia. Int J Morphol. 2009;27:1291–1298. [Google Scholar]
- 28.Ari I, Kafa IM, Bakirci SA. Comparative study of variation of the pterion of human skulls from 13th and 20th century anatolia. Int J Morphol. 2009;27:1291–1298. [Google Scholar]
- 29.Hwang K, Kim JH, Baik SH. The thickness of the skull in Korean adults. J Craniofac Surg. 1999;10(5):395–399. doi: 10.1097/00001665-199909000-00004. [DOI] [PubMed] [Google Scholar]