Abstract
Identification of Actinomyces spp. by conventional phenotypic methods is notoriously difficult and unreliable. Recently, the application of chemotaxonomic and molecular methods has clarified the taxonomy of the group and has led to the recognition of several new species. A practical and discriminatory identification method is now needed for routine identification of clinical isolates. Amplified 16S ribosomal DNA restriction analysis (ARDRA) was applied to reference strains (n = 27) and clinical isolates (n = 36) of Actinomyces spp. and other gram-positive rods. Clinical strains were identified initially to the species level by conventional biochemical tests. However, given the low degree of confidence in conventional methods, the findings obtained by ARDRA were also compared with those obtained by pyrolysis-mass spectrometry. The ARDRA profiles generated by the combination of HaeIII and HpaII endonuclease digestion differentiated all reference strains to the species or subspecies level. The profiles correlated well with the findings obtained by pyrolysis-mass spectrometry and by conventional tests and enabled the identification of 31 of 36 clinical isolates to the species level. ARDRA was shown to be a simple, rapid, cost-effective, and highly discriminatory method for routine identification of Actinomyces spp. of clinical origin.
The genus Actinomyces comprises a heterogeneous group of anaerobic and facultatively anaerobic, asporogenous, nonmotile, non-acid-fast, gram-positive rods with a G+C content of 55 to 71 mol% (2). Many Actinomyces spp. are known to be indigenous to mucous membranes, particularly those in the oral cavity, in humans and other animals. Members of the genus are known to cause actinomycosis and may be found in polymicrobial infections arising from tissue invasion by oral anaerobes (16). Some species are significant in periodontal disease (16).
Detection of the presence of Actinomyces spp. in clinical specimens may affect the prognosis and patient management, but identification by conventional phenotypic methods is notoriously difficult and unreliable (6). Problems arise from the slow and granular growth of some isolates (particularly Actinomyces israelii), the poor reproducibilities of biochemical tests, and the lack of discriminatory power of biochemical tests. The lack of discriminatory power may indicate heterogeneity within species. Indeed, for some species, subdivisions based on biochemical or serological differences have been described (17, 18), and combination in a single genospecies of some serotypes of Actinomyces naeslundii and Actinomyces viscosus has been proposed (8).
Recently, the application of chemotaxonomic and molecular methods has clarified the taxonomy of the group and has led to the recognition of several new species. These include Actinomyces europaeus (4), Actinomyces graevenitzii (12), Actinomyces neuii (5), Actinomyces radingae (22), and Actinomyces turicensis (22). Other new species have been assigned to other, similar genera: Arcanobacterium bernardiae (13), Arcanobacterium phocae (13), Arcanobacterium pyogenes (13), Actinobaculum schaalii (9), and Actinobaculum suis (9). Currently, little is known of the natural habitats, clinical prevalence, and pathogenic potential of these species. A discriminatory, reproducible, and practical method for characterization of clinical isolates may help elucidate their occurrence and significance.
Pyrolysis mass-spectrometry (PMS) has been shown to be a useful tool in differentiating groups of organisms to the species or subspecies level (10) and represents a whole-cell analysis approach independent of conventional biochemical tests. However, PMS is a fingerprinting method and is best suited to examination of large batches of organisms. Barsotti et al. (1) demonstrated the potential of rRNA gene (rDNA) restriction patterns as taxonomic tools for Actinomyces. This approach may be enhanced by initial amplification of rDNA by PCR. Amplified rDNA restriction analysis (ARDRA) has been used to identify various fungi (21) and bacteria (19, 20).
In this study, ARDRA was applied to reference strains and clinical isolates of Actinomyces spp. and some other gram-positive rods. Clinical isolates were identified initially to the species level by conventional biochemical tests and comparison of the results with those for reference strains. However, given the low degree of confidence in this method, the findings were also compared with those obtained by PMS.
MATERIALS AND METHODS
Bacterial strains.
A total of 63 strains were examined (Table 1). Reference strains (n = 27) represented 17 species of Actinomyces and 5 species of other, similar genera. Clinical strains comprised 35 human isolates and 1 animal isolate from the collection held by the Anaerobe Reference Unit, Public Health Laboratory Service. Strains were selected to represent the range of species and sites of isolation of strains referred for identification from laboratories throughout England and Wales. Strains were stored at −80°C on Microbank beads (Pro-lab Diagnostics, Wirral, United Kingdom) and were recovered on Fastidious Anaerobe Agar (FAA; LabM, Bury, United Kingdom) incubated anaerobically at 37°C for 48 h.
TABLE 1.
Sources of bacterial strainsa
| Clinical isolates
|
Reference strainsb
|
||||
|---|---|---|---|---|---|
| Strain | Organism | Source | Strain | Organism | Reference |
| VH20 | A. denticolens | Abdominal abscess | VH45 | A. israelii | ATCC 12102 |
| VH29 | A. georgiae | Cerebral abscess | VH34 | A. israelii | NCTC 10236 |
| VH24 | A. georgiae | IUCD | VH28 | A. gerencseriae | ATCC 23860 |
| VH48 | A. gerencseriae | Facial abscess | VH14 | A. naeslundii serotype I | NCTC 10301 |
| VH16 | A. gerencseriae | Eyelid puncta | VH55 | A. naeslundii serotype II | ATCC 44339 |
| VH4 | A. gerencseriae | Jaw abscess | VH56 | A. naeslundii serotype III | ATCC 44340 |
| VH32 | A. gerencseriae | Vertebral abscess | VH36 | A. viscosus serotype I | NCTC 10951 |
| VH15 | A. israelii | Pleural pus | VH54 | A. viscosus serotype II | ATCC 27044 |
| VH5 | A. israelii | IUCD | VH6 | A. odontolyticus | NCTC 09935 |
| VH1 | A. israelii | Mandibular sinus | VH10 | A. meyeri | ATCC 35568 |
| VH38 | Most like A. israelii | Perinephric abscess | VH11 | A. georgiae | ATCC 49285 |
| VH21 | A. israelii | IUCD | VH47 | A. denticolens | NCTC 11490 |
| VH44 | A. meyeri | IUCD | VH43 | A. slackii | NCTC 11923 |
| VH35 | A. meyeri | IUCD | VH25 | A. bovis | NCTC 11535 |
| VH22 | A. meyeri | Groin abscess | VH7 | Actinomyces hordeovulneris | ATCC 35275 |
| VH41 | A. meyeri | Peritoneal pus | VH30 | A. howellii | NCTC 11636 |
| VH12 | A. meyeri | Pleural effusion | VH51 | A. graevenitzii | CCUG 27294 |
| VH8 | A. naeslundii | Gingival swab | VH52 | A. europaeus | CCUG 32789A |
| VH26 | Most like A. naeslundii | Blood culture | VH57 | A. neuii subsp. neuii | DSM 8576 |
| VH42 | Most like A. naeslundii | Parotid duct | VH58 | A. neuii subsp. anitratus | DSM 8577 |
| VH2 | A. naeslundii | IUCD | VH60 | A. turicensis | DSM 9168 |
| VH39 | A. naeslundii | IUCD | VH61 | A. radingae | DSM 9169 |
| VH27 | Most like A. odontolyticus | Jaw pus | VH53 | A. schaalii | CCUG 27420 |
| VH50 | Most like A. odontolyticus | Liver abscess | VH63 | A. suis | DSM 20639 |
| VH13 | A. odontolyticus | Oral bone plate | VH19 | A. pyogenes | NCTC 05224 |
| VH40 | A. odontolyticus | Blood culture | VH59 | A. bernardiae | DSM 9152 |
| VH33 | Most like A. odontolyticus | IUCD | VH62 | A. phocae | DSM 10002 |
| VH37 | Most like A. pyogenes | Cow jaw | |||
| VH18 | A. viscosus | Submandibular abscess | |||
| VH3 | Most like A. viscosus | Groin | |||
| VH49 | Most like A. viscosus | IUCD | |||
| VH46 | Most like A. viscosus | Fractured mandible | |||
| VH23 | Most like A. viscosus | IUCD | |||
| VH17 | Most like A. viscosus | Lacrimal fluid | |||
| VH9 | Actinomyces species | Dental abscess | |||
| VH31 | Actinomyces species | Osteomyelitis | |||
Abbreviations: IUCD, intrauterine contraceptive device; ATCC, American Type Culture Collection, Rockville, Md.; NCTC, National Collection of Type Cultures, London, United Kingdom; CCUG, Culture Collection, University of Goteborg, Goteborg, Sweden; DSM, Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany.
Reference strains other than A. israelii NCTC 10236 represent type strains for species or subspecies.
Conventional tests.
Strains were determined to be members of the genus Actinomyces on the basis of volatile and nonvolatile fatty acid end products of glucose metabolism, as detected by gas-liquid chromatography as described elsewhere (7).
Cell and colonial morphologies, pigment production, fluorescence under long-wave UV illumination, ability to grow in air and in air plus 5% CO2, and production of catalase and indole were recorded. Hydrolysis of esculin and starch and production of acid from amygdalin, arabinose, cellobiose, glucose, mannitol, raffinose, ribose, salicin, sucrose, trehalose, and xylose were tested by the method of Phillips (14). Production of nitrate reductase, urease, pyrazinamidase, β-galactosidase, α-glucosidase, and β-N-acetyl-glucosaminidase was detected after incubation for 18 to 24 h with Rosco diagnostic tablets (BioConnections, Leeds, United Kingdom). Identifications were made initially by reference to the scheme of Brazier and Hall (3) and subsequently by reference to the publications of Funke et al. (5), Pascual Ramos et al. (12), Funke et al. (4) and Lawson et al. (9).
PMS.
Pyrolysis was performed with colonies from 48-h anaerobic FAA cultures, and the mass spectra were analyzed as described elsewhere (11).
ARDRA.
All strains were tested in triplicate. Strains were cultured anaerobically on FAA for 48 h.
For DNA extraction, a 1-μl loopful of bacteria was suspended in 100 μl of Chelex resin (5%; Bio-Rad Laboratories, Richmond, Calif.) in sterile distilled water, boiled for 8 min, and centrifuged at 17,000 × g for 10 min. The supernatant was decanted.
For PCR, DNA extract (5 μl) was added to 45 μl of a reaction mixture containing 1 U of Taq polymerase (Pharmacia Biotech, St. Albans, United Kingdom), each deoxynucleoside triphosphate at a concentration of 200 mM, 20 pmol of each primer (pA and pH′), 2.25 mM magnesium chloride, 10 mM Tris HCl (pH 9), 50 mM KCl, and 0.1% Triton X-100. The primer sequences were AGAGTTTGATCCTGGCTCAG (pA) and AAGGAGGTGATCCAGCCGCA (pH′). Amplification was accomplished by 31 cycles of denaturation at 92°C for 2 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min, with a final extension period of 5 min. The specific PCR product (approximately 1,600 bp) was detected by electrophoresis of a 5-μl sample for 40 min at 5 V/cm in 1% agarose in TAE (Tris-acetate-EDTA) buffer with ethidium bromide (0.5 μg/ml) and was visualized under UV illumination.
Restriction endonuclease digestion was performed with HaeIII and HpaII endonucleases in separate reaction mixtures containing 1 μl of endonuclease (10 U/μl), 1.5 μl of matched incubation buffer, and 12.5 μl of PCR product; the mixture was incubated for 1.5 h at 37°C. For electrophoresis, 3 μl of 6× loading buffer (Advanced Biotechnologies, Epsom, United Kingdom) was added to each sample, and 15 μl was electrophoresed for 1.75 h at 5 V/cm in Metaphor agarose (3.5%; FMC, Rockland, Maine) in TAE buffer with ethidium bromide (0.5 μg/ml). Molecular size markers (2 kb; Sigma, Poole, United Kingdom) were run in duplicate or triplicate alongside the samples. The gels were destained in deionized water for 10 min and photographed under UV illumination, and the images were stored on a floppy disk.
Gel data were analyzed with GelCompar software (Applied Maths, Kortrijk, Belgium). Distinct banding patterns were assigned three-digit codes, arbitrarily numbered in the order encountered, and were stored as types in HAE and HPA libraries. The combined results for each strain were recorded as, for example, 001/003, which represent the HaeIII and HpaII profiles, respectively.
RESULTS
Conventional biochemical tests.
For all strains, minor amounts of acetic acid and major amounts of lactic and succinic acids were detected as products of glucose metabolism.
(i) Reference strains.
The identities of 18 of the 27 strains were confirmed by conventional tests. Strains that gave anomalous results are listed in Table 2.
TABLE 2.
Reference strains demonstrating results anomalous to those published elsewherea
| Strain | Species and strain designation | Conventional identification | Anomaly |
|---|---|---|---|
| VH28 | A. gerencseriae ATCC 23860 | A. georgiae | Raffinose negative |
| VH36 | A. viscosus ser. I NCTC 10951 | A. naeslundii | Catalase negative |
| VH47 | A. denticolens NCTC 11490 | No identification | Trehalose positive |
| VH52 | A. europaeus CCUG 32789A | No identification | Nitrate positive |
| VH59 | A. bernardiae DSM 9152 | No identification | β-N-Acetyl-glucosaminidase positive |
| VH60 | A. turicensis DSM 9168 | Poor differentiation | Pyrazinamidase positive |
| VH61 | A. radingae DSM 9169 | No identification | Esculin negativeb |
| VH62 | A. phocae DSM 10002 | A. georgiae | Xylose positive |
| VH63 | A. suis DSM 20639 | A. meyeri or A. naeslundii | β-Galactosidase negative |
(ii) Clinical strains.
Identifications obtained by conventional tests are listed in Table 1. Interpretation of results was sometimes difficult due to insufficient growth or the poor reproducibility of reactions. When results were inconsistent with those described for recognized species, strains are designated “most like” followed by the species name.
PMS.
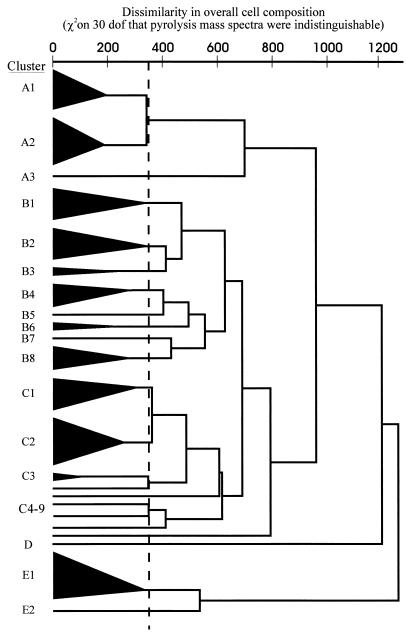
By PMS, strains VH20, VH21, and VH28 were contaminated and thus were excluded from analysis. The remaining strains formed four superclusters (superclusters A, B, C, and E) plus cluster with a single strain (cluster D) (Fig. 1; Table 3). The majority of strains clustered with members of the same species. However, five clinical strains identified by conventional tests as Actinomyces meyeri clustered closely with the type strain of A. turicensis and remotely from the type strain of A. meyeri. Also, two strains conventionally identified as Actinomyces georgiae clustered with four Actinomyces gerencseriae strains; one strain (strain VH29) was subsequently reidentified as A. gerencseriae; the identity of the other strain (strain VH24) remains uncertain. A reference strain of A. israelii (strain VH34) inexplicably clustered with this A. gerencseriae group. One strain (strain VH23), conventionally identified as most like A. viscosus, clustered with the type strain of A. neuii subsp. neuii and was subsequently reidentified as A. neuii subsp. neuii by virtue of its abilities to produce catalase, ferment mannitol, and reduce nitrate. Strains VH7, VH9, VH19, VH27, VH31, VH43, VH47, VH52, VH58, VH59, and VH61 each formed single-strain clusters.
FIG. 1.
Dendrogram of similarities between strains on the basis of PMS, which reflects whole-cell composition. Strain membership of the clusters is outlined in Table 3. dof, degrees of freedom.
TABLE 3.
Results by ARDRA, PMS, and conventional biochemical tests, arranged by HpaII profile
| ARDRA profile | PMS cluster | Strain | Conventional identification | True identification |
|---|---|---|---|---|
| 001/001 | B2 | VH6 | A. odontolyticus | A. odontolyticus NCTC 09935 |
| 001/001 | B2 | VH13 | A. odontolyticus | A. odontolyticus |
| 001/001 | B2 | VH33 | Most like A. odontolyticus | A. odontolyticus |
| 001/001 | B2 | VH50 | Most like A. odontolyticus | A. odontolyticus |
| 025/001 | B2 | VH40 | A. odontolyticus | A. odontolyticus |
| 002/002 | C9 | VH7 | A. hordeovulneris | A. hordeovulneris ATCC 35275 |
| 001/003 | B3 | VH10 | A. meyeri | A. meyeri ATCC 35568 |
| 001/004 | B3 | VH11 | A. georgiae | A. georgiae ATCC 49285 |
| 003/005 | B1 | VH2 | A. naeslundii | A. naeslundii |
| 003/005 | C1 | VH14 | A. naeslundii | A. naeslundii serotype I NCTC 10301 |
| 003/005 | C1 | VH26 | Most like A. naeslundii | A. naeslundii |
| 007/005 | B4 | VH30 | A. howellii | A. howellii NCTC 11636 |
| 009/005 | C1 | VH39 | A. naeslundii | A. naeslundii |
| 014/005 | C1 | VH8 | A. naeslundii | A. naeslundii |
| 014/005 | B4 | VH56 | A. naeslundii | A. naeslundii serotype III ATCC 44340 |
| 014/005 | B4 | VH42 | Most like A. naeslundii | A. naeslundii |
| 017/005 | C2 | VH55 | A. naeslundii | A. naeslundii serotype II ATCC 44339 |
| 017/005 | C2 | VH18 | A. viscosus | A. viscosus |
| 021/005 | C2 | VH54 | A. viscosus | A. viscosus serotype II ATCC 27044 |
| 021/005 | C2 | VH17 | Most like A. viscosus | A. viscosus |
| 021/005 | C2 | VH46 | Most like A. viscosus | A. viscosus |
| 021/005 | C2 | VH49 | Most like A. viscosus | A. viscosus |
| 021/005 | C1 | VH3 | Most like A. viscosus | A. viscosus |
| 004/006 | D | VH19 | A. pyogenes | A. pyogenes NCTC 05224 |
| 005/007 | B4 | VH25 | A. bovis | A. bovis NCTC 11535 |
| 006/008 | A2 | VH4 | A. gerencseriae | A. gerencseriae |
| 006/008 | A2 | VH16 | A. gerencseriae | A. gerencseriae |
| 006/008 | A2 | VH29 | A. georgiae | A. gerencseriae |
| 006/008 | A2 | VH32 | A. gerencseriae | A. gerencseriae |
| 006/008 | A2 | VH48 | A. gerencseriae | A. gerencseriae |
| 006/008 | NDa | VH28 | A. georgiae | A. gerencseriae ATCC 23860 |
| 008/009 | A1 | VH45 | A. israelii | A. israelii ATCC 12102 |
| 008/009 | A2 | VH34 | A. israelii | A. israelii NCTC 10236 |
| 008/009 | ND | VH21 | A. israelii | A. israelii |
| 018/009 | A1 | VH5 | A. israelii | A. israelii |
| 018/009 | ND | VH20 | A. denticolens | A. israelii |
| 020/009 | A1 | VH1 | A. israelii | A. israelii |
| 020/009 | A1 | VH15 | A. israelii | A. israelii |
| 020/009 | A1 | VH38 | Most like A. israelii | A. israelii |
| 009/010 | B1 | VH36 | A. naeslundii | A. viscosus serotype I NCTC 10951 |
| 010/011 | C5 | VH43 | A. slackii | A. slackii NCTC 11923 |
| 024/011 | B7 | VH31 | Actinomyces species | Uncertain |
| 011/012 | B5 | VH47 | Uncertain | A. denticolens NCTC 11490 |
| 013/013 | B1 | VH51 | A. graevenitzii | A. graevenitzii CCUG 27294 |
| 012/014 | C6 | VH52 | Uncertain | A. europaeus CCUG 35789A |
| 004/015 | B8 | VH53 | A. schaalii | A. schaalii CCUG 27420 |
| 001/016 | E2 | VH27 | Most like A. odontolyticus | Uncertain |
| 015/016 | E1 | VH60 | A. turicensis or A. meyeri | A. turicensis DSM 9168 |
| 015/016 | E1 | VH12 | A. meyeri | A. turicensis |
| 015/016 | E1 | VH22 | A. meyeri | A. turicensis |
| 015/016 | E1 | VH35 | A. meyeri | A. turicensis |
| 015/016 | E1 | VH41 | A. meyeri | A. turicensis |
| 015/016 | E1 | VH44 | A. meyeri | A. turicensis |
| 019/017 | A3 | VH9 | Actinomyces species | Uncertain |
| 022/019 | C3 | VH57 | A. neuii subsp. neuii | A. neuii subsp. neuii DSM 8576 |
| 022/019 | C3 | VH23 | A. viscosus | A. neuii subsp. neuii |
| 022/019 | C4 | VH58 | A. neuii subsp. anitratus | A. neuii subsp. anitratus DSM 8577 |
| 023/020 | A2 | VH24 | Most like A. georgiae | Uncertain |
| 026/021 | C8 | VH59 | Uncertain | A. bernardiae DSM 9152 |
| 027/022 | B6 | VH37 | Most like A. pyogenes | Uncertain |
| 028/023 | C7 | VH61 | Uncertain | A. radingae DSM 9169 |
| 029/024 | B6 | VH62 | A. georgiae | A. phocae DSM 10002 |
| 030/025 | B8 | VH63 | A. meyeri or A. naeslundii | A. suis DSM 20639 |
ND, no data available.
ARDRA.
All strains were cleaved by both endonucleases, yielding 6 to 10 bands by HaeIII and 5 to 12 bands by HpaII typing (Fig. 2 and 3, respectively). The resulting HaeIII and HpaII profiles were found to be highly reproducible, allowing the assignation of permanent types to each strain (Table 3). Clinical strains yielding HaeIII and HpaII profiles indistinguishable from those of a reference strain were assigned to that species. The types obtained for A. naeslundii reference serovars indicated subspecies variation in HaeIII profiles and the possible species specificity of the HpaII profiles. Therefore, clinical strains were assigned to species for which the HpaII profile was indistinguishable from that for a reference strain and identification by conventional biochemical tests concurred. When the latter did not concur or when strains yielded distinct profiles with both enzymes, the identity was considered to be uncertain. This approach was supported by the findings obtained by conventional biochemical tests and PMS.
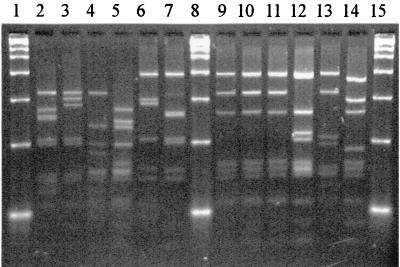
FIG. 2.
HaeIII restriction profiles for some reference strains. Lanes 1, 8, and 15, 2-kb marker; lane 2, A. israelii ATCC 12102; lane 3, A. gerencseriae ATCC 23860; lane 4, A. naeslundii serotype I NCTC 10301; lane 5, A. viscosus serotype II ATCC 27044; lane 6, A. neuii subsp. neuii DSM 8576; lane 7, A. graevenitzii CCUG 27294; lane 9, A. odontolyticus NCTC 09935; lane 10, A. georgiae ATCC 49285; lane 11, A. meyeri ATCC 35568; lane 12, A. turicensis DSM 9168; lane 13, A. radingae DSM 9169; lane 14, A. europaeus CCUG 32789A.
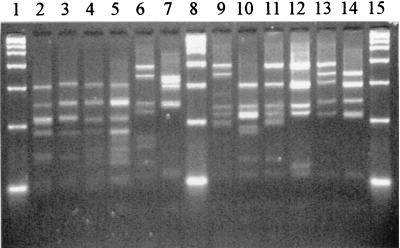
FIG. 3.
HpaII restriction profiles for some reference strains. Lanes 1, 8, and 15, 2-kb marker; lane 2, A. israelii ATCC 12102; lane 3, A. gerencseriae ATCC 23860; lane 4, A. naeslundii serotype I NCTC 10301; lane 5, A. viscosus serotype II ATCC 27044; lane 6, A. neuii subsp. neuii DSM 8576; lane 7, A. graevenitzii CCUG 27294; lane 9, A. odontolyticus NCTC 09935; lane 10, A. georgiae ATCC 49285; lane 11, A. meyeri ATCC 35568; lane 12, A. turicensis DSM 9168; lane 13, A. radingae DSM 9169; lane 14, A. europaeus CCUG 32789A.
(i) Reference strains.
By HaeIII typing, 17 of the 22 species represented and the three serotypes of A. naeslundii and two of A. viscosus were clearly differentiated. Actinomyces odontolyticus, A. meyeri, and A. georgiae strains were indistinguishable (type 001), as were A. pyogenes and A. schaalii (type 004). Both A. israelii strains were type 008. The two A. neuii subspecies were type 022.
By HpaII typing, 20 species were clearly differentiated; but A. naeslundii serotypes I, II, and III, A. viscosus serotype II, and A. howellii were indistinguishable (type 005). Both A. israelii strains were type 009. The two A. neuii subspecies were type 019. The combination of HaeIII and HpaII types allowed differentiation of all 22 species represented by reference strains.
(ii) Clinical strains.
In reactions with both endonucleases, 16 of the 36 strains were indistinguishable from reference strains of the same species, as determined by conventional tests, and were assigned to species with confidence. Eight strains were indistinguishable by ARDRA from the reference strains of a species other than that identified by conventional tests. These comprised the five strains conventionally identified as A. meyeri, which were indistinguishable from A. turicensis; VH23, which was originally most like A. viscosus but which was redesignated A. neuii subsp. neuii and which gave the same profile as the A. neuii strains; VH29, which was originally considered to be A. georgiae but which was indistinguishable from A. gerencseriae; and VH18 (A. viscosus), which gave the same profile as A. naeslundii serotype II. These were assigned to the species determined by ARDRA, with strain VH18 being deemed a member of A. naeslundii genospecies 2, which includes A. viscosus serotype II.
Seven strains had distinct HaeIII profiles but gave HpaII profiles indistinguishable from those of reference strains of the species determined by conventional tests and were assigned to that species. One strain (strain VH31), which had a distinct HaeIII profile and which was indistinguishable from the reference strain of Actinomyces slackii by HpaII typing, was not clearly identified by conventional tests and was deemed to be of uncertain identity.
One strain (strain VH27) that was identified by conventional tests as most like A. odontolyticus gave profiles indistinguishable from those of reference strains of A. odontolyticus by HaeIII typing and A. turicensis by HpaII typing. The remaining three strains (strains VH9, VH24, and VH37) gave unique profiles in both reactions. The true identities of these four strains remain uncertain.
DISCUSSION
The application of chemotaxonomic and molecular methods has clarified the taxonomy of the genus Actinomyces, but the description of novel species has rendered previously published identification schemes obsolete. When few strains of a species have been studied, the reliability of identification by conventional tests is questionable. Furthermore, given the diversity within the genus, further modification of the taxonomic status of strains may be warranted.
Currently, the Public Health Laboratory Service Anaerobe Reference Unit receives over 100 referrals each year from clinical laboratories throughout England and Wales for confirmation of isolates presumptively identified as Actinomyces spp. There is a need for a practical and discriminatory method for the identification of clinical isolates.
The identification of Actinomyces to the species level by conventional biochemical tests is beset with problems. These include technical difficulties, poorly discriminatory tests, and heterogeneity within described species. In this study, nine reference strains demonstrated reactions anomalous from those published elsewhere, and this resulted in mis- or nonidentification (Table 2). Notably, A. europaeus was found to reduce nitrate to nitrite, and A. bernardiae produced β-N-acetyl-glucosaminidase. Anomalies, particularly in enzyme reactions, may be due to differences in test methodologies.
PMS has been shown to be highly discriminatory for a wide range of organisms but is best suited to the testing of large batches of isolates. Direct comparisons between batches are not readily made. Thus, PMS would be impractical for examination of occasional clinical isolates. However, given the low degree of confidence in results obtained by conventional tests for the identification of Actinomyces, evaluation of the efficacy of novel methods is difficult. In this study, PMS provided a valuable independent approach with which to compare findings. It is noteworthy and reassuring that the dendrogram derived from PMS analysis reflects the taxonomic relationships generated by 16S rDNA sequencing (4, 9, 13). A. israelii and A. gerencseriae strains formed a supercluster (supercluster A) remote from other species. Superclusters B and C were linked and contained strains identified as A. odontolyticus, A. naeslundii, A. viscosus, and several other species, largely represented by single reference strains. Within this group, the type strains of A. meyeri and A. georgiae formed a cluster (cluster B3) closely linked to A. odontolyticus strains (cluster B2). The remoteness of cluster E (A. turicensis strains and strain VH27) from other species raises doubts as to their place in the genus Actinomyces.
ARDRA has proven to be useful for discrimination of various bacterial species. In this study, the types generated by the combination of HaeIII and HpaII endonuclease digestion profiles correlated well with the findings obtained by PMS and conventional tests and enabled the identification of 31 of 36 clinical strains to the species level. The remaining five strains were not clearly identified by conventional tests, and three strains formed distinct clusters by PMS; the latter strains may well represent novel species or other genera.
Seven strains were identified by ARDRA and PMS as species other than those initially assigned by conventional tests. Five of these seven strains initially identified as A. meyeri clustered tightly with the A. turicensis type strain by PMS and were indistinguishable from A. turicensis by ARDRA. Review of conventional test results showed that the two species are poorly differentiated by the range of biochemical tests performed. The identity of one of the five strains (strain VH12) was confirmed as A. turicensis by 16S rDNA sequence analysis (data not shown). The strain that was apparently misidentified by PMS and ARDRA as A. gerencseriae had been designated A. georgiae by conventional tests by virtue of its inability to ferment raffinose; the morphology and other reactions were consistent with A. gerencseriae. This property was also demonstrated by the A. gerencseriae type strain; thus, raffinose fermentation appears to be an unreliable determinant in the identification of this species. The strain originally designated most like A. viscosus and identified as A. neuii by PMS and ARDRA was subsequently redesignated A. neuii subsp. neuii by virtue of catalase production, nitrate reduction, and fermentation of mannitol.
A. gerencseriae and A. turicensis showed homogeneous, distinct ARDRA profiles. The subdivisions observed within A. israelii, A. odontolyticus, A. naeslundii, and A. viscosus may correspond to those previously recognized by other workers (8, 17, 18). One strain of A. viscosus was indistinguishable from A. naeslundii serotype II by PMS and ARDRA, adding to the body of evidence that A. naeslundii and A. viscosus isolates form a heterogeneous complex that requires further investigation.
When species were represented by single reference strains, all strains gave distinct profiles, and none was misidentified. However, no further conclusions regarding the efficacy of ARDRA for identification of these species can be drawn without data derived from additional strains.
Current consumable costs, based on a batch of 10 isolates, were £2.80 (as of April 1999, £1 is equal to $1.60) per isolate. Each batch required approximately 3.5 h of labor spread over 1.5 days.
In conclusion, ARDRA was shown to be a simple, rapid, cost-effective, and highly discriminatory method for identification of Actinomyces spp. of clinical origin. Application of the method to further clinical and veterinary isolates may confirm its usefulness, and an extensive investigation of strains referred to the Anaerobe Reference Unit is in progress. Within the limitations of the current study, identification of a strain as a member of the genus Actinomyces by gas-liquid chromatography is a prerequisite. However, preliminary investigations of strains of Propionibacterium spp., Lactobacillus spp., and Bifidobacterium spp. have demonstrated the clear differentiation of strains by ARDRA (unpublished data), indicating potential for identification to the species level of members of these genera and obviating the need for gas-liquid chromatography.
Identification of clinical isolates of Actinomyces to the species level may be important for patient management. Additionally, a reliable identification system is essential for the discovery of the natural habitats, prevalence, and pathogenicity of recently described species. With increasing knowledge of these aspects, species-level identification of clinical isolates may become more relevant to patient management. Some species, e.g., A. turicensis, have been shown to be identifiable in commercial biochemical systems (15). However, genotypic methods may be advantageous in their ability to detect novel species as well as those listed in commercial databases. A practical, highly discriminatory, and cost-effective method such as ARDRA applied to many strains may greatly aid in the elucidation of the ecology and clinical spectra of Actinomyces species and may further clarify the taxonomy of the genus.
ACKNOWLEDGMENTS
We thank Margaret Heginbotham and Paul Talbot for technical assistance with PMS and conventional tests.
REFERENCES
- 1.Barsotti O, Decoret D, Benay G, Carlotti A, Freney J, Guerin-Faublee V, Morrier J. rRNA gene restriction patterns as possible taxonomic tools for the genus Actinomyces. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1994;281:433–441. doi: 10.1016/s0934-8840(11)80329-4. [DOI] [PubMed] [Google Scholar]
- 2.Bowden G H W. Actinomyces. In: Balows A, Duerden B I, editors. Topley and Wilson’s microbiology and microbial infections. 9th ed. Vol. 2. London, United Kingdom: Edward Arnold; 1998. pp. 445–462. [Google Scholar]
- 3.Brazier J S, Hall V. Actinomyces. In: Emmerson A M, Hawkey P M, Gillespie S H, editors. Principles and practice of clinical bacteriology. Chichester, United Kingdom: John Wiley & Sons; 1997. pp. 625–639. [Google Scholar]
- 4.Funke G, Alvarez N, Pascual C, Falsen E, Akervall E, Sabbe L, Schouls L, Weiss N, Collins M D. Actinomyces europaeus sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol. 1997;47:687–692. doi: 10.1099/00207713-47-3-687. [DOI] [PubMed] [Google Scholar]
- 5.Funke G, Stubbs S, von Graevenitz A, Collins M D. Assignment of human-derived CDC group 1 coryneform bacteria and CDC 1-like coryneform bacteria to the genus Actinomyces as Actinomyces neuii subsp. neuii sp. nov., subsp. nov., and Actinomyces neuii subsp. anitratus subsp. nov. Int J Syst Bacteriol. 1994;44:167–171. doi: 10.1099/00207713-44-1-167. [DOI] [PubMed] [Google Scholar]
- 6.Hall V, Brazier J S. Identification of actinomyces—what are the major problems? In: Eley A R, Bennett K W, editors. Anaerobic pathogens. Sheffield, United Kingdom: Sheffield Academic Press; 1997. pp. 187–192. [Google Scholar]
- 7.Holdeman L V, Cato E P, Moore W E C. Anaerobe laboratory manual. 4th ed. Blacksburg: Virginia Polytechnic Institute and State University; 1977. Chromatographic procedures for analysis of acid and alcohol products; pp. 134–136. [Google Scholar]
- 8.Johnson J L, Moore L V H, Kaneko B, Moore W E C. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990;40:273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- 9.Lawson P, Falsen E, Åkervall E, Vandamme P, Collins M D. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997;47:899–903. doi: 10.1099/00207713-47-3-899. [DOI] [PubMed] [Google Scholar]
- 10.Magee J. Whole-organism fingerprinting. In: Goodfellow M, O’Donnell A G, editors. Handbook of new bacterial systematics. London, United Kingdom: Academic Press; 1993. pp. 383–427. [Google Scholar]
- 11.Magee J T, Hindmarch M J, Bennett K W, Duerden B I, Aries R E. A pyrolysis mass spectrometry study of fusobacteria. J Med Microbiol. 1989;28:227–236. doi: 10.1099/00222615-28-3-227. [DOI] [PubMed] [Google Scholar]
- 12.Pascual Ramos C, Falsen E, Alvarez N, Åkervall E, Sjödén B, Collins M D. Actinomyces graevenitzii sp. nov. isolated from human clinical specimens. Int J Syst Bacteriol. 1997;47:885–888. doi: 10.1099/00207713-47-3-885. [DOI] [PubMed] [Google Scholar]
- 13.Pascual Ramos C, Foster G, Collins M D. Phylogenetic analysis of the genus Actinomyces based on 16S rRNA gene sequences: description of Arcanobacterium phocae sp. nov., Arcanobacterium bernardiae comb. nov., and Arcanobacterium pyogenes comb. nov. Int J Syst Bacteriol. 1997;47:46–53. doi: 10.1099/00207713-47-1-46. [DOI] [PubMed] [Google Scholar]
- 14.Phillips K D. A simple and sensitive technique for determining the fermentation reactions of non-sporing anaerobes. J Appl Bacteriol. 1976;41:325–328. doi: 10.1111/j.1365-2672.1976.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 15.Sabbe L J M, Van de Merwe D, Schoulls L, Bergmans A, Vaneechoutte M, Vandamme P. Clinical spectrum of infections due to the newly described Actinomyces species A. turicensis, A. radingae and A. europaeus. J Clin Microbiol. 1999;37:8–13. doi: 10.1128/jcm.37.1.8-13.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaal K P. Actinomycoses, actinobacillosis and related diseases. In: Hausler W J, Sussman M, editors. Topley and Wilson’s microbiology and microbial infections. 9th ed. Vol. 3. London, United Kingdom: Edward Arnold; 1997. pp. 777–798. [Google Scholar]
- 17.Schofield G M, Schaal K P. A numerical taxonomic study of members of the Actinomycetaceae and related taxa. J Gen Microbiol. 1981;127:237–259. doi: 10.1099/00221287-127-2-237. [DOI] [PubMed] [Google Scholar]
- 18.Slack J M, Gerencser M A. Actinomyces, filamentous bacteria. Biology and pathogenicity. Minneapolis, Minn: Burgess; 1975. pp. 57–64. [Google Scholar]
- 19.Vaneechoutte M, Riegel P, de Briel D, Monteil H, Verschraegen G, De Rouck A, Claeys G. Evaluation of the applicability of amplified rDNA-restriction analysis (ARDRA) to identification of species of the genus Corynebacterium. Res Microbiol. 1995;146:633–641. doi: 10.1016/0923-2508(96)81061-8. [DOI] [PubMed] [Google Scholar]
- 20.Vaneechoutte M, Cartwright C P, Williams E C, Jäger B, Tichy H-V, De Baere T, De Rouck A, Verschraegen G. Evaluation of 16S rRNA gene restriction analysis for the identification of cultured organisms of clinically important Clostridium species. Anaerobe. 1996;2:249–256. [Google Scholar]
- 21.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wust J, Stubbs S, Weiss N, Funke G, Collins M D. Assignment of Actinomyces pyogenes-like (CDC coryneform group E) bacteria to the genus Actinomyces as Actinomyces radingae sp. nov. and Actinomyces turicensis sp. nov. Lett Appl Microbiol. 1995;20:76–81. doi: 10.1111/j.1472-765x.1995.tb01290.x. [DOI] [PubMed] [Google Scholar]