Abstract
Posttraumatic stress disorder (PTSD) is associated with lower gray matter volume (GMV) in brain regions critical for extinction of learned threat. However, relationships among volume, extinction learning, and PTSD symptom development remain unclear. We investigated subcortical brain volumes in regions supporting extinction learning and fear-potentiated startle (FPS) to understand brain-behavior interactions that may impact PTSD symptom development in recently traumatized individuals. Participants (N = 99) completed magnetic resonance imaging and threat conditioning two weeks following trauma exposure as part of a multisite observational study to understand the neuropsychiatric effects of trauma (AURORA Study). Participants completed self-assessments of PTSD (PTSD Checklist for DSM-5; PCL-5), dissociation, and depression symptoms two- and eight-weeks post-trauma. We completed multiple regressions to investigate relationships between FPS during late extinction, GMV, and PTSD symptom development. The interaction between thalamic GMV and FPS during late extinction at two weeks post-trauma predicted PCL-5 scores eight weeks (t(75) = 2.49, β=0.28, p=0.015) post-trauma. Higher FPS predicted higher PCL-5 scores in the setting of increased thalamic GMV. Meanwhile, lower FPS also predicted higher PCL-5 scores in the setting of decreased thalamic GMV. Thalamic GMV and FPS interactions also predicted posttraumatic dissociative and depressive symptoms. Amygdala and hippocampus GMV by FPS interactions were not associated with posttraumatic symptom development. Taken together, thalamic GMV and FPS during late extinction interact to contribute to adverse posttraumatic neuropsychiatric outcomes. Multimodal assessments soon after trauma have the potential to distinguish key phenotypes vulnerable to posttraumatic neuropsychiatric outcomes.
Keywords: posttraumatic stress disorder, gray matter volume, thalamus, extinction, fear-potentiated startle
Introduction
Posttraumatic stress disorder (PTSD) affects approximately 6.8% of the United States population (Kessler et al., 2005) with consequences for individuals and society (Kessler, 2000). Early interventions improve and mitigate the development of PTSD (Kearns et al., 2012; Rothbaum et al., 2012). However, the high rate of trauma exposures in the general population and variability in individual susceptibility to PTSD make it costly and inefficient to provide intensive early interventions to every traumatized individual (Kilpatrick et al., 2013). Early identification of individuals susceptible to PTSD following trauma exposure is thus crucial for the development of efficient early intervention strategies (Kearns et al., 2012; McLean et al., 2019).
Chronic PTSD is associated with disruptions in threat learning processes that are commonly studied with Pavlovian fear conditioning. In healthy adaptive learning, individuals both acquire new threat memories when danger is present and extinguish the memory once danger has passed. Individuals with PTSD, however, show an enhanced ability to acquire, but a reduced ability to extinguish, fear conditioned memories (Jovanovic et al., 2012; Norrholm et al., 2011).
Fear-potentiated startle (FPS) is a robust psychophysiological measure of threat learning and autonomic reactivity, indexed by an increased startle response in the presence of a danger signal. PTSD is associated with exaggerated FPS to a conditioned stimulus (CS) during acquisition that persists during extinction (Grillon & Morgan, 1999; Jovanovic et al., 2012; Norrholm & Jovanovic, 2018). However, increasing attention is being paid to heterogeneity within PTSD, with differences in extinction patterns potentially aiding in defining PTSD subgroups (Galatzer-Levy et al., 2013; Seligowski et al., 2019).
Subcortical/deep cortical neural circuitry, including the amygdala, hippocampus, and thalamus, play an important role in the acquisition, expression, and extinction of conditioned fear (Barad et al., 2006; Fanselow and Ledoux, 1999; Maren, 2001; Phelps et al., 2004). The basolateral nucleus of the amygdala receives sensory input from the thalamus and cortex (Senn et al., 2014) and connects to the central nucleus of the amygdala, which mediates autonomic responses to threat (Gafford and Ressler, 2016; Krabbe et al., 2018). Amygdala-dependent extinction learning is then modulated by the hippocampus to facilitate the formation and contextualization of extinction memories (Ji & Maren, 2007; Knight, et al., 2004; LaBar & Phelps, 2005; Liu et al., 2012; Rudy et al., 2004; Senn et al., 2014). Thalamic connections to the amygdala and hippocampus also critically relay extinction-related sensory information necessary to form an inhibitory memory (Fanselow & Ledoux, 1999; Galatzer-Levy et al., 2013; Lee et al., 2019; Lee et al., 2012; Ramanathan & Maren, 2019; Troyner et al., 2018). Together, the amygdala, hippocampus, and thalamus constitute key subcortical circuitry for extinction learning.
This set of regions show structural morphometric alterations in chronic PTSD. Decreased amygdala gray matter volume (GMV) have been seen in those with prior trauma histories and PTSD (Ganzel et al., 2008; Logue et al., 2018; O’Doherty et al., 2015). Meta-analyses have demonstrated diminished hippocampal GMV in PTSD (Bromis et al., 2018; Kitayama et al., 2005; Kühn and Gallinat, 2013; Logue et al., 2018; Woon et al., 2010). Although decreased thalamic GMV was not observed in meta-analyses of PTSD that combined military and civilian trauma (Bromis et al., 2018; Kitayama et al., 2005; Logue et al., 2018), smaller thalamic GMVs have been observed in civilians with PTSD (O’Doherty et al., 2017), dissociation (Daniels et al., 2015), and intrusion symptoms (Shucard et al., 2012). In sum, lower volumes of the amygdala, hippocampus, and thalamus have been consistently linked with trauma exposure and PTSD symptoms.
No prior work has assessed for a potential relationship between GMV and FPS during extinction in the acute aftermath of trauma or if associations between GMV and FPS relate to future posttraumatic symptom expression. We thus hypothesized that examining both behaviors and brain structures in the early aftermath of trauma exposure and evaluating their association with later PTSD symptom development would help identify posttraumatic stress vulnerability phenotypes. By focusing on neural integrity in regions critically implicated in extinction, we may gain mechanistic insight regarding neurophysiological mechanisms acutely that are relevant to the maintenance of later chronic symptoms.
In the present study, we investigated putative relationships among subcortical GMV, physiological reactivity (indexed via FPS), and acute PTSD symptoms following trauma exposure. Late extinction, the time when inhibitory learning should be strongest, has been utilized as a marker of treatment success for PTSD (Rousseau et al., 2019). We therefore hypothesized that FPS during late extinction would relate to lower amygdala, hippocampus, and thalamus GMV. Further, we postulated that the interaction of thalamus, amygdala, and hippocampus GMV and FPS during late extinction would be prospectively associated with PTSD symptom severity eight-weeks following trauma exposure. The interaction of GMV-FPS on dissociation and depression symptoms was also examined to consider posttraumatic neuropsychiatric dysfunction more broadly.
Methods and Materials
Participants
Volunteers were recruited as part of a larger, ongoing, multisite longitudinal study of posttraumatic outcomes – the AURORA study (McLean et al., 2019). Participants were recruited from 22 Emergency Departments (EDs) across the United States following trauma exposure. Exposures included motor vehicle collision, physical assault, sexual assault, fall greater than 10 feet, mass casualty incidents, or other plausible exposure to threatened or actual injury, violence, or death. For more details of inclusion and exclusion criteria, please refer to the methodology paper describing the AURORA study (McLean et al., 2019) and the supplement. Structural MRI and psychophysiological assessment were completed within approximately two-weeks of recruitment at one of four locations (McLean Hospital, Emory University, Temple University, or Wayne State University). All participants gave written informed consent as approved by each study site’s Institutional Review Board. Trauma-exposed adults (N = 126) with complete MRI data at the time of analysis were included in the present study. Twenty-seven participants were excluded from analyses due to noise artifact in FPS data during extinction. Therefore, 99 participants were included in the present analysis (M = 35.95 years, SD = 13.58 years, range = 18–75; n = 64 females; Table 1).
Table 1.
Sample Characteristics of included participants (N=99).
| N (%) | M (SD) | ||
|---|---|---|---|
|
| |||
| Sex | Male | 35 (35.4) | |
| Female | 64 (64.6) | ||
| Race/Ethnicity | Hispanic | 20 (20.2) | |
| Non-Hispanic White | 39 (39.4) | ||
| Non-Hispanic Black | 35 (35.4) | ||
| Non-Hispanic Other | 5 (5.1) | ||
| Educational Attainment | Some high school | 4 (4.0) | |
| High school graduate | 16 (16.2) | ||
| GED or equivalent | 13 (13.1) | ||
| Some college, no degree | 32 (32.3) | ||
| Associate degree, technical/occupational/vocational program | 5 (5.1) | ||
| Associate degree, academic program | 6 (6.1) | ||
| Bachelor’s degree | 18 (18.2) | ||
| Master’s degree | 4 (4.0) | ||
| Professional school degree | 1 (1.0) | ||
| Site | Emory University | 7 (7.1) | |
| McLean Hospital | 51 (51.5) | ||
| Temple University | 18 (18.2) | ||
| Wayne State University | 23 (23.2) | ||
| GMV ROI Proportion of Total ICV | Thalamus | 98 | 0.49 (0.04) |
| Amygdala | 95 | 0.11 (0.01) | |
| Hippocampus | 98 | 0.26 (0.03) | |
| Late Extinction FPS | CS+ | 98 | 20.18 (44.97) |
| CS− | 97 | 13.83 (43.47) | |
| (CS+) – (CS−) | 96 | 6.50 (27.28) | |
| PCL-5 Scores | 2 weeks post-trauma | 89 | 28.53 (15.42) |
| 8 weeks post-trauma | 86 | 27.63 (16.68) | |
| Dissociation total Scores | 2 weeks post-trauma | 91 | 1.43 (1.68) |
| 8 weeks post-trauma | 88 | 1.53 (2.0) | |
| PROMIS Depression T-Scores | 2 weeks post-trauma | 90 | 54.08 (8.85) |
| 8 weeks post-trauma | 87 | 55.02 (9.71) | |
GMV: gray matter volume; ROI: region of interest; ICV: intracranial volume; FPS, fear-potentiated startle; CS, conditioned stimulus
Self-report measures
The PTSD Checklist for DSM-5 (PCL-5) was used to assess the presence and severity of PTSD symptoms at two- and eight-weeks post-trauma and is scored out of 80 points (Weathers et al., 2013). Note, of participants who provided PCL-5 data at two-weeks post-trauma, 33 participants (33%) scored higher than 32 on the PCL-5, indicative of subacute PTSD. Of participants who provided PCL-5 data at eight-weeks post-trauma, 35 participants (35.4%) scored higher than 32 on the PCL-5 indicative of probable PTSD. Dissociative symptoms were examined with a modified version of the Brief Dissociative Experiences Scale scored out of eight points (DES-B; Dalenberg & Carlson, 2010), see supplement for details. Depression symptoms were assessed with the Patient-Reported Outcomes Measurement Information System (PROMIS) Depression instrument (Pilkonis et al., 2011) which is T-score converted. PTSD, dissociation, and depression symptoms were evaluated for the past two weeks at the two-week time point and for the past month at the eight-week time point. Some participants did not provide self-report data; please see the sample description in Supplemental Table 1 for more information. Additionally, the Life Events Checklist (Weathers et al., 2013) was utilized to assess for prior traumatic experiences. Responses were summed to create a composite trauma score and correlated with subcortical GMVs to consider the role of prior traumas on GMV (see supplement).
MRI acquisition and processing
Anatomical T1-weighted MRI scans were acquired on 3T Siemens MRI systems at four neuroimaging centers (Supplemental Table 2). Image processing was completed at Emory University utilizing standard procedures in fMRIPrep (Esteban et al., 2019), see supplement for details. All segmentations were visually inspected with special attention to individual regions whose volume fell outside +/− 1.5 times the interquartile range for the sample. Regional volumes were excluded from analysis if there was a clear segmentation error by Freesurfer (listed by region in Supplemental Table 1).
Stimuli and task design
Psychophysiological data were collected within approximately two weeks of trauma exposure using a Pavlovian fear conditioning procedure described in prior reports (Glover et al., 2011; Jovanovic et al., 2012; Norrholm et al., 2011). Briefly, a shape on a computer screen (a blue square; CS+) was repeatedly paired with an aversive unconditioned stimulus (US) (140 psi airblast to the larynx, 250 ms duration). A different shape (a purple triangle; CS−) was never paired with the aversive stimulus. The paradigm included a 108 dB white noise startle probe that elicited the eyeblink startle response. The startle probe was presented during CS+ and CS− trials, and on its own (noise alone [NA] trials) to assess individual baseline startle response. See supplement for further details on the paradigm. Following habituation (see supplement), acquisition consisted of three conditioning blocks with four trials of each type (NA, CS+ paired with US, CS−) in each block. Ten minutes after acquisition, the extinction phase consisted of four blocks with four trials of each type (CS+, CS−, NA), wherein the airblast never occurred (20 minutes in duration). Due to its relevance in the development of PTSD symptoms, the focus of this study was on extinction, thus analyses were limited to late extinction, defined as the last two blocks of extinction.
Fear-potentiated startle response
FPS was measured using surface electromyography (EMG) of the right orbicularis oculi muscle using a Biopac MP160 physiological recording system (Biopac Systems, Inc., Aero Camino, CA). FPS was defined as the maximal orbicularis oculi contraction 20 to 200 ms following the startle probe presentation. EMG data were analyzed using MindWare software (MindWare Technologies, Inc.; Gahanna, OH). FPS was calculated by subtracting the average startle magnitude to the noise probe alone from the startle magnitude to each CS in each block of the experiment (Norrholm et al., 2011), see supplement for details. Individuals who had less than 75% usable data as identified during visual inspection were excluded from analyses, as above (Supplemental Table 1).
Statistical analysis
Statistical analyses were completed using SPSS software (IBM Corporation, version 24, Armonk, NY). Individual multiple regression analyses were completed to assess the relationship between FPS to the CS+ and subcortical GMV in each a priori region of interest (i.e., amygdala, hippocampus, and thalamus). Regional volumes were normalized as a proportion of total intracranial volume (ICV) to adjust for potential global ICV differences among participants. GMV for each region was averaged across hemispheres given our lack of a priori hypotheses on laterality and the high correlations between hemispheres per region (see supplement). Exploratory post-hoc analyses assessed the relationship with other subcortical regions including bilateral lateral ventricles, globus pallidus, caudate, putamen, and nucleus accumbens.
Second, multiple regression analyses were completed to assess the relationship between FPS during late extinction and subcortical volume on PCL-5 total scores at eight-weeks post-trauma. The regression models (type 3 sums of squares approach) included mean-centered regressors for FPS, GMV, and an FPS by GMV interaction as independent variables with PCL-5, modified DES-B, and PROMIS Depression scores at eight-weeks as the respective dependent variables. FPS and GMV were analyzed as continuous variables. However, to aid in interpretation of the interaction effects, FPS was divided into low and high groupings based on median split where noted. Age, sex, and scan site were included as covariates within the models. We applied a Bonferroni correction for multiple comparisons across the three GMV regions studied at the eight-week timepoint, as this was the primary aim of the study, with a threshold p-value of 0.017. We completed post-hoc regression analyses that included two-week scores as a covariate to determine if observed effects were specific to the eight-week timepoint. Further, supplementary follow-up analyses evaluating interactions at two-weeks post-trauma exposure were completed. Additional exploratory post-hoc analyses investigated the relationship with supplementary subcortical regions at two- and eight-weeks following trauma exposure (Supplemental Tables 4, 5). Additional exploratory analyses tested interactions with dissociation and depression symptoms.
Results
Associations between GMV and FPS
We first assessed whether GMV related to FPS during late extinction. No significant associations were observed between FPS and GMV in the amygdala, hippocampus, or thalamus (amygdala: t(92) = 0.08, β = 0.01, B = 30.76, 95% CI [−733.94, 795.46], p = 0.936; hippocampus: t(95) = −0.40, β = −0.04, B = −61.77, 95% CI [−366.69, 243.15], p = 0.688; thalamus: t(95) = −0.20, β = −0.02, B = −20.62, 95% CI [−229.46, 188.22], p = 0.845). Exploratory analyses examining the interaction between FPS and nucleus accumbens, caudate, putamen, globus pallidus, and lateral ventricles GMV did not reach significance (Supplemental Table 3).
Interaction of GMV and FPS on Posttraumatic Symptoms
PCL-5 scores at eight-weeks post-trauma
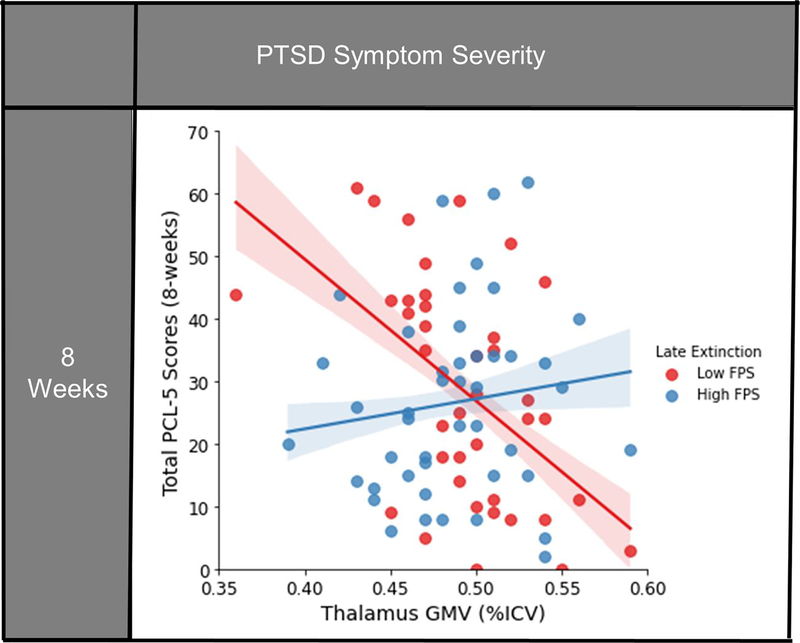
We next investigated the effect of GMV and FPS during late extinction on PCL-5 scores eight-weeks following trauma exposure. (Table 2). We observed a significant interaction between thalamus GMV and FPS on PCL-5 scores at eight weeks post-trauma after controlling for multiple comparisons (t(75) = 2.49, β = 0.28, B = 2.90, 95% CI [0.58, 5.22], p = 0.015). No significant main effects of GMV or FPS on PCL-5 scores were observed (GMV: t(75) = −0.99, β = −0.13, B = −51.93, 95% CI [−156.96, 53.10], p = 0.328, FPS: t(75) = −0.16, B = −0.01, 95% CI [−0.09, 0.07], β = −0.02, p = 0.870). Both greater thalamic GMV with higher FPS, as well as decreased thalamic GMV with lower FPS during late extinction related to greater PCL-5 scores following trauma exposure (Figure 1). Follow-up correlation analyses were completed separately for low and high FPS groups, defined by median split, to disentangle the interaction effects. The high FPS group show a non-significant positive correlation (r = 0.22, p = 0.18) while the low FPS group showed a significant negative correlation (r = −0.43, p = 0.010), between thalamic GMV and PCL-5 scores at eight-weeks post-trauma. There was no interaction between amygdala or hippocampus GMV and FPS on eight-week PCL-5 scores (amygdala: t(72) = 1.61, β = 0.19, B = 7.48, 95% CI [−1.76, 16.71], p = 0.111; hippocampus: t(75) = 1.50, β = 0.17, B = 2.98, 95% CI [−0.98, 6.95], p = 0.138) (Table 2).
Table 2.
Interaction of subcortical GMV and FPS to the CS+ during late extinction on PCL-5 scores eight-weeks post-trauma.
| FPS | Eight Weeks |
|
|---|---|---|
| β | t (p) | |
|
| ||
| Amygdala | 0.19 | 1.61 (0.111) |
| Hippocampus | 0.17 | 1.50 (0.138) |
| Thalamus | 0.28 | 2.50 (0.015*) |
p < .05, two-sided test
FPS, fear-potentiated startle; CS, conditioned stimulus
Figure 1: Significant interaction of FPS to the CS+ during late extinction and thalamic GMV on posttraumatic symptom severities at eight-weeks following trauma exposure.
FPS is median split here for visual clarity, but analyzed as a continuous variable in the analysis. Confidence intervals shown represent the 68% CI (+/−1 SE of the regression line). For PCL-5 scores, greater thalamic GMV coupled with higher FPS to danger cue (CS+) during late extinction was associated with greater symptom severity at eight-weeks following trauma exposure; conversely decreased thalamic GMV and low FPS to CS+ during late extinction also related to higher scores post-trauma. FPS: Fear-potentiated startle; GMV: Gray matter volume; ICV: Intracranial volume; PCL-5: PTSD Checklist for DSM-5.
When two-week PCL-5 scores were included in the eight-week model, the GMV-FPS interaction term lost significance, likely due to the high correlation of PCL-5 scores between timepoints (r = 0.65, p < 0.001). To delve further into these findings, additional analyses focused on the two-week timepoint were undertaken. In brief, a similar interaction between thalamus GMV and FPS on PCL-5 scores two-weeks post-trauma emerged (see supplemental results). Overall, these data suggest that the interaction between thalamic GMV and FPS on PCL-5 scores develops two weeks following trauma exposure, with significant effects persisting weeks later.
Additional analyses involving supplemental subcortical structures demonstrated that at eight weeks post-trauma, an interaction between nucleus accumbens volume and FPS on PCL-5 scores emerged (t(76) = 2.12, β = 0.28, B = 17.18, 95% CI [1.04, 33.32], p = 0.037) as did an interaction between lateral ventricle volume and FPS on PCL-5 scores (t(76) =−2.03, β = −0.32, B = −0.54, 95% CI [−1.08, −0.01]p = 0.046) (Supplemental Table 6).
Dissociation/depression symptoms at eight-weeks post-trauma
To establish if the above brain-behavior profiles were associated with other posttraumatic outcomes, we also assessed dissociation and depression symptoms using multiple regressions. PCL-5 and modified DES-B scores were highly correlated at each time point (two-week: r = 0.62, p < 0.001; eight-week: r = 0.73, p < 0.001). Similar to PCL-5 scores, the thalamic GMV-FPS interaction was associated with modified DES-B scores at eight weeks following trauma exposure (t(77) = 2.54, β = 0.27, B = 0.34, 95% CI [0.07, 0.61], p = 0.013). No significant main effects of GMV or FPS on DES-B scores were observed (GMV: t(77) = −1.36, β = −0.17, B = 8.11, 95% CI [−20.02, 3.81], p = 0.179, FPS: t(77) = −0.56, β = −0.06, B = 0.00, 95% CI [−0.01, 0.01], p = 0.576). Greater thalamic GMV with higher FPS during late extinction and decreased thalamic GMV with lower FPS were both related to higher modified DES-B scores (Figure 2a).
Figure 2: Interaction of FPS to the CS+ during late extinction and thalamic GMV on dissociation and depression symptom severities at eight-weeks following trauma exposure.
FPS is median split here for visual clarity, but analyzed as a continuous variable in the analysis. Confidence intervals shown represent the 68% CI (+/−1 SE of the regression line). For modified DES-B and PROMIS Depression Inventory scores, greater thalamic GMV coupled with higher FPS to danger cue (CS+) during late extinction was associated with greater symptom severity at eight-weeks following trauma exposure; conversely decreased thalamic GMV and low FPS to CS+ during late extinction also related to higher scores post-trauma. A) Interaction of FPS x thalamic GMV predicting modified DES-B scores eight weeks following trauma. B) Interaction of FPS x thalamic GMV predicting PROMIS Depression T-scores eight weeks following trauma. DES-B: Dissociative Experiences Scale- Brief; FPS: Fear-potentiated startle; GMV: Gray matter volume; ICV: Intracranial volume; PROMIS: Patient-Reported Outcomes Measurement Information System
PCL-5 and PROMIS Depression scores were also highly correlated (two-week: r = 0.74, p = < 0.001; eight-week: r = 0.71, p = < 0.001). The interaction between thalamic GMV and FPS during late extinction related to depression severity at eight weeks following trauma (t(76) = 2.40, β = 0.27, B = 1.75, 95% CI [0.30, 3.20], p = 0.019). There were no significant main effects of GMV or FPS on depression scores (GMV: t(76) = −0.95, β = −0.13, B = −30.58, 95% CI [−94.91, 33.75], p = 0.347, FPS: t(76) = −0.60, β = −0.07, B = −0.02, 95% CI [−0.07, 0.04], p = 0.553). Greater thalamic GMV with higher FPS during late extinction and decreased thalamic GMV with lower FPS were both related to higher depressive symptom severity (Figure 2b).
These data suggest that the thalamic GMV-FPS interactions may reflect general posttraumatic dysfunction acutely following trauma. However, given the high degree of comorbidity of dissociation, depression, and PTSD symptoms, when PCL-5 scores were included as a covariate, the interaction terms were no longer significant (eight-week modified DES-B: (t(74) = 1.03, β = 0.08, B = 0.10, 95% CI [−0.09, 0.30], p = 0.304; eight-week PROMIS Depression: (t(73) = 0.75, β = 0.06, B = 0.42, 95% CI [−0.70, 1.53], p = 0.458).
Assessing FPS Stability Throughout Extinction
As a post-hoc analysis, we sought to determine, among participants with elevated PCL-5 scores, defined by a PCL-5 score of greater than 32, if low FPS in late extinction was reflective of enhanced learning (a change over the course of extinction) or a persistent behavioral response. Only the high FPS group had a significant intercept term for FPS, (High: t(14) = 3.88, p = 0.002; Low: t(16) = −0.76, p = 0.46), indicating only the high FPS group potentiated relative to baseline startle, consistent with fear learning. Individuals with low FPS during late extinction were persistently low throughout extinction, while those with high FPS during late extinction were also persistently increased (see supplement). Most critically, low FPS reactivity during late extinction was a marker of low initial psychophysiological reactivity maintained throughout extinction.
Discussion
We investigated subcortical GMV and extinction-related FPS in recently traumatized individuals to identify brain-behavior relationships associated with acute PTSD symptom development. Interactions between thalamic GMV and FPS during late extinction showed a significant effect on PTSD, dissociation, and depression symptom severity at two- and eight-weeks following trauma exposure. While FPS and GMV appear to be independent of one another during the acute posttraumatic phase, these measures in combination were informative of susceptibility to posttraumatic dysfunction. Among participants experiencing posttraumatic stress symptoms, two distinct brain-behavior profiles emerged, indicative that neither GMV nor FPS data alone sufficiently characterized the risk for posttraumatic symptom development.
Our findings that subcortical GMV did not vary with autonomic reactivity are inconsistent with prior human functional (Cheng et al., 2006; Harnett et al., 2015, Knight et al., 2004) and structural (Hartley et al., 2011) MRI research demonstrating relationships between the amygdala and hippocampus and skin conductance responses (SCRs) during fear acquisition (Pohlack et al., 2012). Although both SCR and FPS index psychophysiological arousal, they have differing neural substrates that may lead to different brain-behavior relationships (Abend et al., 2020; Glover et al., 2011; Lindner et al., 2015; Young et al., 2018), potentially underlying the discrepant findings. Additionally, we focused on extinction learning only, which involves new learning reliant on related, but distinct, neural mechanisms (Phelps et al., 2004).
While subcortical GMVs were independent of FPS during late extinction, an interaction between thalamic GMV and FPS was related to future (i.e., eight-week) PTSD symptom severity. Two distinct brain-behavior phenotypes were associated with greater PTSD symptoms following trauma: 1) individuals with greater thalamic GMV in combination with higher FPS, and 2) individuals with decreased thalamic GMV in combination with lower FPS. We speculate that the group high GMV/FPS may relate to the ‘classical’ concept of PTSD symptomatology, namely patients unable to extinguish the fear response during safety. Conversely, the group with low GMV/FPS may represent individuals with consistently blunted behavioral responses (e.g., a more emotionally numb or avoidant subtype). These results highlight the utility of multimodal approaches to better characterize individual PTSD phenotypes and to potentially inform optimal preventive/treatment interventions.
To contextualize these findings, prior translational evidence links thalamic activity and FPS responses to threat cues and PTSD (Davis, 2006; Lindner et al., 2015). The thalamus integrates sensory signals and projects directly and indirectly to the medial prefrontal cortex (mPFC) (Lee et al., 2019; Lindner et al., 2015; Ramanathan et al., 2018; Ramanathan & Maren, 2019), an area central to the formation of extinction memories (Mitchell, 2015; Ouhaz et al., 2018). Importantly, several thalamic subregions are heavily implicated in extinction circuitry. The nucleus reuniens is a key site where mPFC signals converge to regulate the suppression or retrieval of threat memories (Giustino and Maren, 2015). It is also linked to sleep-related memory consolidation (Hauer et al., 2019), with sleep disruptions thought to significantly contribute to the development of PTSD symptoms (Neylan et al., 2020). Additionally, the dorsal medial thalamus is tied to extinction learning. When dorsal medial thalamic projections to the amygdala are suppressed, extinction is promoted, outlining the critical function this region plays in extinction (Ramanathan et al., 2018). Our findings could point to hypertrophy or atrophy of either of these subregions, leading to dysfunction in the circuitry underlying extinction learning. While the T1w imaging used here accurately segments the thalamus en bloc, further dissection requires diffusion tensor imaging (Battistella et al., 2017) or 7T imaging (Xiao et al., 2016). As advanced methodologies become validated, further subsegmentation of the thalamus to delineate its effects on posttraumatic outcomes is warranted.
Interestingly, the GMV-FPS interaction also predicted dissociation and depression symptom severity. PTSD is often comorbid with both dissociation (Stein et al., 2014) and depression (Bleich et al., 1997; Breslau et al., 2000; Shalev et al., 1998). Due to its role in sensory integration and relay, the thalamus is heavily implicated in dissociative disorders (Krause-Utz et al., 2017), with differences observed in both structural and perfusion studies (Schlumpf et al., 2014; Shucard et al., 2012). Additionally, volumetric data shows mixed results with both greater and smaller thalamic volumes associated with depression (Ancelin et al., 2019; Young et al., 2008) compared to controls. Our data demonstrate both groups at risk for depression and dissociation symptoms following trauma, illustrating the importance of multimodal analysis when considering morphometric data in heterogeneous and complicated clinical populations.
Our findings should be considered in light of several limitations. While the comorbidity in our sample is quite common in the clinical realm and is representative of a naturalistic sample, it limited our ability to examine each symptom cluster in isolation. Thus, while our findings highlight brain-behavior interactions in relation to general posttraumatic dysfunction, we are unable to identify specific neural substrates unique to PTSD, or other related disorder, symptom development. Second, the sample included relatively low PTSD severity, as reported on the PCL-5, which may limit extrapolation to a more clinically severe population. Additionally, the dissociation scale used included only two items from the DES-B, thus likely not capturing all volunteers with dissociation symptoms. Lastly, the present study did not include a non-traumatized control group to assess whether these brain-behavior relationships are only observed in the early aftermath of trauma or may also be observed in the general population.
Conclusion
In this sample of recently traumatized individuals, subcortical GMV and FPS during late extinction interacted to significantly relate to broad posttraumatic neuropsychiatric symptoms (i.e., PTSD, dissociation, and depression) at eight-weeks following trauma exposure. Our observation of high posttraumatic symptoms in both low GMV/FPS and high GMV/FPS groups suggest different neural mechanisms mediate different subtypes of posttraumatic sequalae. An appreciation for the complex interplay of brain structure and psychophysiology may help to inform effective, individualized interventions for those at risk for adverse outcomes following trauma.
Supplementary Material
Acknowledgements:
We would like to thank the many research assistants involved in the AURORA study for their assistance with participant recruitment and data acquisition.
Disclosures: This research was supported by the National Institute of Mental Health K00 MH119603 and U01 MH110925, the US Army Medical Research and Material Command, The One Mind Foundation, and The Mayday Fund.
Dr. Lauren Lebois reports funding from NIMH K01MH118467.
Dr. Sanne J.H. van Rooij reports funding from NIMH K01MH121653.
Dr. Gari Clifford has financial interest in Alivecor Inc, receives unrestricted funding from the company, is the CTO of Mindchild Medical, and has ownership interests in Mindchild Medical, all of which is unrelated to the present work.
Dr. Sophia Sheikh has received funding from the Florida Medical Malpractice Joint Underwriter’s Association Dr. Alvin E. Smith Safety of Healthcare Services Grant; the NIH/NIA-funded Jacksonville Aging Studies Center (JAX-ASCENT; R33AG05654); and the Florida Blue Foundation.
Dr. Christopher Jones has no direct conflicts related to this paper, and no ongoing conflicts. He has been an investigator on studies funded by Roche Diagnostics, AstraZeneca, Janssen, and Hologic Inc, for which his department has received research funding
Over the past three years, Dr. Diego Pizzagalli has received consulting fees from BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Engrail Therapeutics, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals as well as one honorarium from Alkermes. In addition, he has received stock options from BlackThorn Therapeutics, and research support from National Institute of Mental Health, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Dr. James Elliot reports support from the National Institutes of Health (NIH) through Grant Numbers R01HD079076 & R03HD094577: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research. In the past 3 years, Dr. Ronald Kessler received support for his epidemiological studies from Sanofi Aventis; was a consultant for Datastat, Inc., Sage Pharmaceuticals, and Takeda. Dr. Kerry J. Ressler has received consulting income from Alkermes and Takeda, research support from NIH, Genomind and Brainsway, and he is on scientific advisory boards for Janssen and Verily, all of which is unrelated to the present work.
Dr. Tanja Jovanovic reports funding from NIH and Brain and Behavior Research Foundation
Dr. Nathaniel G. Harnett reports funding from NIMH K00MH119603
Footnotes
The authors declare no competing conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier: NIMH Data Archive DOI 10.15154/1521263. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA
References
- Abend R, Gold AL, Britton JC, Michalska KJ, Shechner T, Sachs JF, Winkler AM, Leibenluft E, Averbeck BB, Pine DS, 2020. Anticipatory threat responding: Associations with anxiety, development, and brain structure. Biol. Psychiatry 1–10. 10.1016/j.biopsych.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin ML, Carrière I, Artero S, Maller J, Meslin C, Ritchie K, Ryan J, Chaudieu I, 2019. Lifetime major depression and grey-matter volume. J. Psychiatry Neurosci 44, 45–53. 10.1503/jpn.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Gean PW, Lutz B, 2006. The Role of the Amygdala in the Extinction of Conditioned Fear. Biol. Psychiatry 60, 322–328. 10.1016/j.biopsych.2006.05.029 [DOI] [PubMed] [Google Scholar]
- Battistella G, Najdenovska E, Maeder P, Ghazaleh N, Daducci A, Thiran JP, Jacquemont S, Tuleasca C, Levivier M, Bach Cuadra M, Fornari E, 2017. Robust thalamic nuclei segmentation method based on local diffusion magnetic resonance properties. Brain Struct. Funct 222, 2203–2216. 10.1007/s00429-016-1336-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, Lerer B, 1997. Post-traumatic stress disorder and depression: An analysis of comorbidity. Br. J. Psychiatry 170, 479–482. 10.1192/bjp.170.5.479 [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR, 2000. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder-major depression connection. Biol. Psychiatry 48, 902–909. 10.1016/S0006-3223(00)00933-1 [DOI] [PubMed] [Google Scholar]
- Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ, 2018. MetaAnalysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am. J. Psychiatry 175, 989–998. 10.1176/appi.ajp.2018.17111199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ, 2006. Human amygdala activity during the expression of fear responses. Behav. Neurosci 120, 1187–1195. 10.1037/0735-7044.120.5.1187 [DOI] [PubMed] [Google Scholar]
- Dalenberg C, Carlson E (2010). Severity of Dissociative Symptoms - Adult (Brief Dissociative Experiences Scale (DES-B) – Modified). American Psychiatric Association: Online Assessment Measures. Retrieved from https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. [Google Scholar]
- Daniels J, Gaebler M, Lamke JP, Walter H, 2015. Grey matter alterations in patients with depersonalization disorder: A voxel-based morphometry study. J. Psychiatry Neurosci 40, 19–27. 10.1503/jpn.130284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1999. Neural systems involved in fear and anxiety measured With fear-potentiated startle. J. Neurosci 22, 2343–2351. [DOI] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ, 2019. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Ledoux JE, 1999. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Ressler KJ, 2016. Mouse models of fear-related disorders: Cell-type-specific manipulations in amygdala. Neuroscience 321, 108–120. 10.1016/j.neuroscience.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bonanno GA, Bush DEA, LeDoux JE, 2013. Heterogeneity in threat extinction learning: Substantive and methodological considerations for identifying individual difference in response to stress. Front. Behav. Neurosci 7, 1–7. 10.3389/fnbeh.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Glover GH, Temple E, 2008. Resilience after 9/11: Multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage 40, 788–795. 10.1016/j.neuroimage.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S, 2015. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci 9, 1–20. 10.3389/fnbeh.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T, 2011. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety 28, 1058–1066. 10.1002/da.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C Iii, C.A.M., 1999. Fear-Potentiated startle conditioning to explicit and contextual cues in Gulf War veterans With posttraumatic stress disorder. J. Abnorm. Psychol 108, 134–142. [DOI] [PubMed] [Google Scholar]
- Harnett NG, Wheelock MD, Wood KH, Ladnier JC, Mrug S, Knight DC, 2015. Affective state and locus of control modulate the neural response to threat. Neuroimage 121, 217–226. 10.1016/j.neuroimage.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA, 2011. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb. Cortex 21, 1954–1962. 10.1093/cercor/bhq253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer BE, Pagliardini S, Dickson CT, 2019. The reuniens nucleus of the thalamus has an essential role in coordinating slow-wave activity between neocortex and hippocampus. eNeuro 6. 10.1523/ENEURO.0365-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S, 2007. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 17, 749–758. 10.1002/hipo.20331 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO, 2012. Early interventions for PTSD: A review. Depress. Anxiety 29, 833–842. 10.1002/da.21997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, 2000. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry 61, 4–12. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kilpatrick Dean G.; Resnick Heidi S.; Miller Mark W.; Keyes Katherine M.; Friedman MJ, 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26, 537–547. 10.1002/jts [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD, 2005. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J. Affect. Disord 88, 79–86. 10.1016/j.jad.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Knight David C., Smith Christine C., Cheng Dominic T., Stein Elliot A., Helmstetter FJ, 2004. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn. Affect. Behav. Neurosci 4, 317–325. [DOI] [PubMed] [Google Scholar]
- Krabbe S, Gründemann J, Lüthi A, 2018. Amygdala inhibitory circuits regulate associative fear conditioning. Biol. Psychiatry 83, 800–809. 10.1016/j.biopsych.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Frost R, Winter D, Elzinga BM, 2017. Dissociation and alterations in brain function and structure: Implications for borderline personality disorder. Curr. Psychiatry Rep 19. 10.1007/s11920-017-0757-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J, 2013. Gray matter correlates of posttraumatic stress disorder: A quantitative meta-analysis. Biol. Psychiatry 73, 70–74. 10.1016/j.biopsych.2012.06.029 [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA, 2005. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci 119, 677–686. 10.1037/0735-7044.119.3.677 [DOI] [PubMed] [Google Scholar]
- Lee JH, Latchoumane CFV, Park J, Kim J, Jeong J, Lee KH, Shin HS, 2019. The rostroventral part of the thalamic reticular nucleus modulates fear extinction. Nat. Commun 10. 10.1038/s41467-019-12496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Sukchan, Ahmed T, Lee Soojung, Kim H, Choi S, Kim DS, Kim SJ, Cho J, Shin HS, 2012. Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat. Neurosci 15, 308–314. 10.1038/nn.2999 [DOI] [PubMed] [Google Scholar]
- Lindner K, Neubert J, Pfannmöller J, Lotze M, Hamm AO, Wendt J, 2015. Fear-potentiated startle processing in humans: Parallel fMRI and orbicularis EMG assessment during cue conditioning and extinction. Int. J. Psychophysiol 98, 535–545. 10.1016/j.ijpsycho.2015.02.025 [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S, 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. 10.1038/nature11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O’Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, Morey RA, 2018. Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC Study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 83, 244–253. 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, 2001. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci 24, 897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, Clifford GD, Zeng D, An X, Linnstaedt S, 2019. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, 2015. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Neylan TC, Kessler RC, Ressler KJ, Clifford G, Beaudoin FL, An X, Stevens JS, Zeng D, Linnstaedt SD, Germine LT, Sheikh S, Storrow AB, Punches BE, Mohiuddin K, Gentile NT, McGrath ME, van Rooij SJH, Haran JP, Peak DA, Domeier RM, Pearson C, Sanchez LD, Rathlev NK, Peacock WF, Bruce SE, Joormann J, Barch DM, Pizzagalli DA, Sheridan JF, Harte SE, Elliott JM, Hwang I, Petukhova MV, Sampson NA, Koenen KC, McLean SA, 2020. Prior sleep problems and adverse post-traumatic neuropsychiatric sequelae of motor vehicle collision in the AURORA study. Sleep 1–11. 10.1093/sleep/zsaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, 2018. Fear processing, psychophysiology, and PTSD. Harv. Rev. Psychiatry 26, 129–141. 10.1097/HRP.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biol. Psychiatry 69, 556–563. 10.1016/j.biopsych.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J, 2015. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. - Neuroimaging 232, 1–33. 10.1016/j.pscychresns.2015.01.002 [DOI] [PubMed] [Google Scholar]
- O’Doherty DCM, Tickell A, Ryder W, Chan C, Hermens DF, Bennett MR, Lagopoulos J, 2017. Frontal and subcortical grey matter reductions in PTSD. Psychiatry Res. - Neuroimaging 266, 1–9. 10.1016/j.pscychresns.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Ouhaz Z, Fleming H, Mitchell AS, 2018. Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and mediodorsal thalamus. Front. Neurosci 12, 1–8. 10.3389/fnins.2018.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE, 2004. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43, 897–905. 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, 2011. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): Depression, anxiety, and anger. Assessment 18, 263–283. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Liebscher C, Cacciaglia R, Diener SJ, Ridder S, Woermann FG, Flor H, 2012. Hippocampal but not amygdalar volume affects contextual fear conditioning in humans. Hum. Brain Mapp 33, 478–488. 10.1002/hbm.21224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan KR, Jin J, Giustino TF, Payne MR, Maren S, 2018. Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat. Commun 9. 10.1038/s41467-018-06970-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan KR, Maren S, 2019. Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav. Brain Res 374. 10.1016/j.bbr.2019.112114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, Lang D, Houry D, 2012. Early intervention may prevent the development of posttraumatic stress disorder: A randomized pilot civilian study with modified prolonged exposure. Biol. Psychiatry 72, 957–963. 10.1016/j.biopsych.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau PF, El Khoury-Malhame M, Reynaud E, Boukezzi S, Cancel A, Zendjidjian X, Guyon V, Samuelian JC, Guedj E, Chaminade T, Khalfa S, 2019. Fear extinction learning improvement in PTSD after EMDR therapy: an fMRI study. Eur. J. Psychotraumatol 10. 10.1080/20008198.2019.1568132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P, 2004. Understanding contextual fear conditioning: Insights from a two-process model, in: Neuroscience and Biobehavioral Reviews. pp. 675–685. 10.1016/j.neubiorev.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Schlumpf YR, Reinders AATS, Nijenhuis ERS, Luechinger R, Van Osch MJP, Jäncke L, 2014. Dissociative part-dependent resting-state activity in dissociative identity disorder: A controlled fMRI perfusion study. PLoS One 9. 10.1371/journal.pone.0098795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski AV, Lebois LAM, Hill SB, Kahhale I, Wolff JD, Jovanovic T, Winternitz SR, Kaufman ML, Ressler KJ, 2019. Autonomic responses to fear conditioning among women with PTSD and dissociation. Depress. Anxiety 36, 625–634. 10.1002/da.22903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SBE, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A, 2014. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437. 10.1016/j.neuron.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK, 1998. Prospective study of posttraumatic stress disorder and depression following trauma. Am. J. Psychiatry 155, 630–637. 10.1176/ajp.155.5.630 [DOI] [PubMed] [Google Scholar]
- Shucard JL, Cox J, Shucard DW, Fetter H, Chung C, Ramasamy D, Violanti J, 2012. Symptoms of posttraumatic stress disorder and exposure to traumatic stressors are related to brain structural volumes and behavioral measures of affective stimulus processing in police officers. Psychiatry Res. - Neuroimaging 204, 25–31. 10.1016/j.pscychresns.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Stein DJ, Koenen KC, Friedman MJ, Hill E, Mclaughlin KA, Petukhova M, Ruscio AM, Shahly V, Spiegel D, Borges G, Bunting B, Caldas-de-almeida JM, Girolamo G. De, Florescu S, Haro JM, Karam EG, Kovess-masfety V, Lee S, Matschinger H, Mladenova M, Posada-villa J, Tachimori H, Viana MC, Kessler RC, 2014. Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biol. Psychiatry 73, 302–312. 10.1016/j.biopsych.2012.08.022.Dissociation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyner F, Bicca MA, Bertoglio LJ, 2018. Nucleus reuniens of the thalamus controls fear memory intensity, specificity and long-term maintenance during consolidation. Hippocampus 28, 602–616. 10.1002/hipo.22964 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM 2013. The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD; at www.ptsd.va.gov. [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP (2013). The PTSD Checklist for DSM-5 (PCL-5). National Center for Posttraumatic Stress Disorder. Retrieved from https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- Woon FL, Sood S, Hedges DW, 2010. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: A meta-analysis. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34, 1181–1188. 10.1016/j.pnpbp.2010.06.016 [DOI] [PubMed] [Google Scholar]
- Xiao YZ, Zitella LM, Duchin Y, Teplitzky BA, Kastl D, Adriany G, Yacoub E, Harel N, Johnson MD, 2016. Multimodal 7T imaging of thalamic nuclei for preclinical deep brain stimulation applications. Front. Neurosci 10, 1–15. 10.3389/fnins.2016.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DA, Chao L, Neylan TC, O’Donovan A, Metzler TJ, Inslicht SS, 2018. Association among anterior cingulate cortex volume, psychophysiological response, and PTSD diagnosis in a Veteran sample. Neurobiol. Learn. Mem 155, 189–196. 10.1016/j.nlm.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Bonkale WL, Holcomb LA, Hicks PB, German DC, 2008. Major depression, 5HTTLPR genotype, suicide and antidepressant influences on thalamic volume. Br. J. Psychiatry 192, 285–289. 10.1192/bjp.bp.107.039180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier: NIMH Data Archive DOI 10.15154/1521263. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA