Abstract
Background
Faecal microbiota transplantation (FMT) is an emerging treatment modality, but its current clinical use and organisation are unknown. We aimed to describe the clinical use, conduct, and potential for FMT in Europe.
Methods
We invited all hospital-based FMT centres within the European Council member states to answer a web-based questionnaire covering their clinical activities, organisation, and regulation of FMT in 2019. Responders were identified from trials registered at clinicaltrials.gov and from the United European Gastroenterology (UEG) working group for stool banking and FMT.
Findings
In 2019, 31 FMT centres from 17 countries reported a total of 1,874 (median 25, quartile 10–64) FMT procedures; 1,077 (57%) with Clostridioides difficile infection (CDI) as indication, 791 (42%) with experimental indications, and 6 (0•3%) unaccounted for. Adjusted to population size, 0•257 per 100,000 population received FMT for CDI and 0•189 per 100,000 population for experimental indications. With estimated 12,400 (6,100–28,500) annual cases of multiple, recurrent CDI and indication for FMT in Europe, the current European FMT activity covers approximately 10% of the patients with indication. The participating centres demonstrated high safety standards and adherence to international consensus guidelines. Formal or informal regulation from health authorities was present at 21 (68%) centres.
Interpretation
FMT is a widespread routine treatment for multiple, recurrent CDI and an experimental treatment. Embedded within hospital settings, FMT centres operate with high standards across Europe to provide safe FMT. A significant gap in FMT coverage suggests the need to raise clinical awareness and increase the FMT activity in Europe by at least 10-fold to meet the true, indicated need.
Funding
NordForsk under the Nordic Council and Innovation Fund Denmark (j.no. 8056–00006B).
Keywords: Faecal microbiota transplantation, FMT, Stool banking, Clostridioides difficile
Keywords: Abbreviations: CDI, Clostridioides difficile infection; ECDC, European Centre for Disease Prevention and Control; FMT, Faecal microbiota transplantation; IQR, Interquartile range; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; UEG, United Gastroenterology European; REDCap, Research Data Capture software
Research in context.
Evidence before this study
FMT infrastructures have emerged internationally to meet a growing demand for safe FMT, but the current clinical use, organisation, and dissemination of FMT are unknown. We searched PubMed for all available literature on clinical FMT activity until the 24th of March 2020 using a combination of the terms “Fe(a)cal microbiota transplantation” with and without “survey”. We found several consensus guidelines, FMT centre descriptions, and three studies on national/regional FMT coverage. None provided generalisable evidence for the clinical activity at an international or continental level, and we concluded that a joint European collaboration was needed to assess the current clinical use, conduct, and potential of FMT in Europe.
Added value of this study
We document that FMT is a widespread treatment and estimate that 1874 hospital-based FMT procedures were performed in Europe in 2019. Insights from the study provide the first estimates of the current supply and demand for FMT in Europe and documents how numerous FMT centres operate across Europe with high safety standards to make the treatment accessible to patients and providers. These data may guide future clinical practice and decision-making on how to perceive, use, and regulate FMT in Europe.
Implications of all the available evidence
The results confirm that FMT has become a routine treatment that clinicians should familiarize themselves with to secure their patients the most effective treatment. Despite being recommended by clinical societies, the current clinical use covers approximately 10% of the patients with multiple, recurrent CDI and indication for FMT, indicating a significant underuse that emphasises the need to raise clinical awareness and increase the European FMT activity by 10-fold.
Alt-text: Unlabelled box
1. Introduction
Faecal microbiota transplantation (FMT) is an emerging treatment to target and modulate the human intestinal microbiota [1]. The use of FMT is highly effective in patients with recurrent Clostridioides difficile infection (CDI) [2] and is embedded in international guidelines and consensus reports [3], [4], [5]. Promising results indicate that FMT may also be effective in other diseases such as ulcerative colitis, multidrug resistant organism carriage, irritable bowel syndrome, hepatic encephalopathy, and other conditions where the intestinal microbiota may contribute to disease pathogenesis [1,[6], [7], [8]].
The use of FMT has surged since the first randomised clinical trial in 2013 demonstrated that FMT was superior to antibiotics for recurrent CDI [9]. FMT has since undergone drastic technological improvements, and infrastructures are now being implemented internationally to serve a growing demand for safe FMT, especially for the large group of patients suffering from multiple CDI recurrences after failing antibiotics and where FMT is the only effective treatment [10], [11], [12], [13], [14], [15], [16]. International consensus reports guide FMT practices [3], [4], [5], but the actual dissemination of FMT in clinical practice, the potential to provide it, and how it is operated and regulated are heterogenous and largely unknown.
The aim of the present study was to describe the clinical use, conduct and potential for FMT in Europe.
2. Methods
In this Europe-wide, cross-sectional survey conducted in March 2020, we invited hospital-based FMT centres across Europe to answer questions specific to their clinical FMT activities in 2019. The survey was organised by the United Gastroenterology European (UEG) working group for stool banking and FMT [4]. All working group members agreed and approved of the study conduct.
2.1. The questionnaire
The online survey (Supplementary file 1) comprised items designed to cover six overall domains related to (i) demographics (ii) activity, treatment modalities, and indications used in 2019, (iii) organisation of donor recruitment and screening, (iv) organisation of laboratory preparation facilities, (v) organisation of clinical application and follow-up, and (vi) regulation and auditing.
Prior to launch, a pilot survey was conducted in May 2019 in Iceland, Norway, Sweden, Finland, and Denmark. Based on the feedback from the pilot, a revised survey was validated by six members of the UEG working group to form the final survey. Following approval by the working group, the survey was constructed as a web-based questionnaire using the Research Data Capture software (REDCap) (www.redcap.au.dk), hosted by Aarhus University, Denmark.
2.2. Eligibility criteria and definitions
We defined an FMT site as any site with ongoing or previous clinical FMT activity, and we classified each FMT site according to its operation and activity as either an FMT clinic/service or an FMT centre:
-
•
An FMT clinic/service was defined as an FMT site that solely offers a clinical service for providing FMT with preparations distributed to them from external providers and has no donor activity nor stool preparation.
-
•
An FMT centre was defined as an FMT site that actively recruits and screens donors, prepares stool for clinical use (routine and/or experimental) and performs local procedures with or without distribution.
Only FMT centres were included to avoid potential double count of procedures. As a harmonised definition, FMT was defined as the procedure of transferring intestinal microbiota from processed stool donated by a single donor.
2.3. Participating FMT centres
Active FMT centres in the European Council member states were identified within the UEG working group[4] and from completed or on-going clinical trials, registered at www.clinicaltrials.gov by February 2nd, 2020. FMT sites registered at clinicaltrials.gov were identified using the search string “f(a)ecal microbiota transplantation”, and contact information for investigators and sponsors was extracted and reviewed. Hospital-based, European FMT sites that had provided contact information were contacted for potential participation in the survey.
Following initial consent to participate, dedicated members from each FMT centre answered the survey. A unique participation link was e-mailed to every participant. All participants had a four-week window to respond to the survey with biweekly automated reminders. Non-responders were contacted by email and telephone.
2.4. Statistical analysis
All data were entered in REDCap. All working group members were offered access to all data. Institution-specific data are presented anonymously.
For statistical analyses, we used R version 3.6.1 with the “dplyr” extension package. The statistics were descriptive, and were presented as counts for proportional data with rounded percentages of totals, and as medians with 25–75% interquartile ranges (IQR) for continuous, numerical data. Proportional data was derived from the total number of participating FMT centres if not stated otherwise, and missing data were counted as no where appropriate. Answers not listed among the original options was added as categorical options. When applicable, data were grouped based on country and standardised towards a population size of 100,000 according to each country's population size. Population data were obtained from the World Bank Open Data hub (data.worldbank.org) and based on the population sizes of 2019.
2.5. Role of the funding source
The funder of the study had no role in study design, data acquisition, analysis, and interpretation, nor decision to submit the manuscript.
3. Results
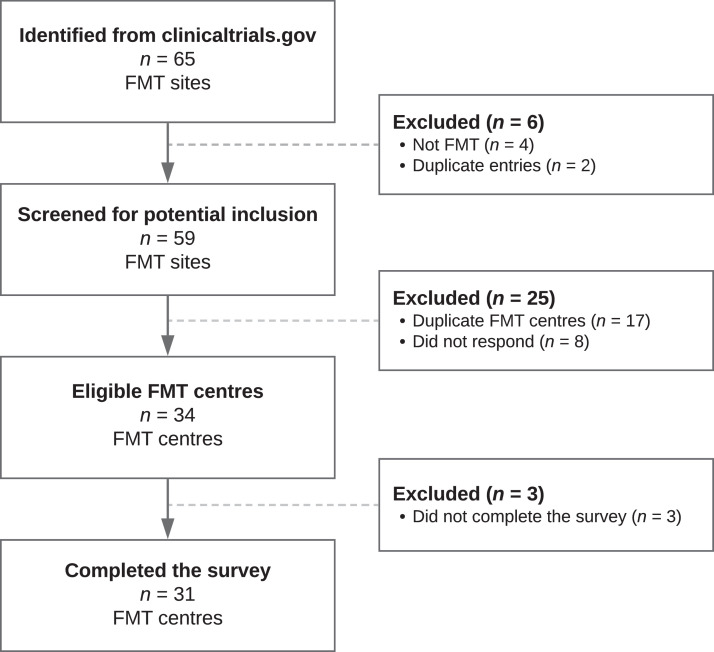
From the clinicaltrials.gov registrations, we identified 65 European FMT sites. Fig. 1 presents the inclusion process for screening the FMT centres included in the study. Of the 42 FMT sites eligible for contact and screening as an FMT centre, 33 were already part of, or identified by, the UEG working group collaboration, and 9 FMT sites were unrelated. Eight FMT sites did not respond to the initial contact.
Fig. 1.
Study flow chart of the screened FMT centres included in the study and reason for exclusion.
Legend: Definitions: FMT site: any site with ongoing or previous clinical activity; FMT centre: a FMT site that had donor recruitment, stool preparation, and clinical activity. With or without distribution.
Abbreviations: FMT: Faecal Microbiota Transplantation.
In total, 34 FMT sites (Fig. 1) from 17 countries across Europe agreed to participate in the survey and confirmed FMT activity consistent with being FMT centres in 2019. In total, 31/34 (91%) FMT centres completed the survey (Table 1) that was sent on 10 March 2020. Among these, 12 (39%) were part of the UEG working group, 18 (58%) were identified by UEG working group members, and 1 (3%) from clinicaltrials.gov.
Table 1.
The clinical use of faecal microbiota transplantation (FMT) in Europe in 2019 according to country, indication, and population size.
| Country | Centres no. | Total FMT procedures |
Indication CDI no. |
FMT for CDI per 100·000* |
Indication Non-CDI no. |
FMT for non-CDI per 100·000* |
|---|---|---|---|---|---|---|
| United Kingdom | 3 | 690 | 279 | 0·417 | 411 | 0·615 |
| Denmark | 5 | 305 | 294 | 5·053 | 11 | 0·189 |
| Italy | 1 | 150 | 120 | 0·199 | 30 | 0·050 |
| Sweden | 2 | 96 | 66 | 0·642 | 30 | 0·292 |
| Finland | 1 | 90 | 60 | 1·087 | 30 | 0·543 |
| France | 4 | 88 | 68 | 0·101 | 14 | 0·021 |
| Germany | 3 | 86 | 39 | 0·047 | 47 | 0·057 |
| Czech Republic | 1 | 83 | 3 | 0·028 | 80 | 0·750 |
| Netherlands | 1 | 82 | 42 | 0·242 | 40 | 0·231 |
| Norway | 2 | 61 | 31 | 0·580 | 30 | 0·561 |
| Austria | 1 | 60 | 8 | 0·090 | 52 | 0·586 |
| Belgium | 2 | 27 | 15 | 0·131 | 12 | 0·104 |
| Switzerland | 1 | 20 | 16 | 0·187 | 4 | 0·047 |
| Lithuania | 1 | 18 | 18 | 0·646 | 0 | 0·000 |
| Iceland | 1 | 8 | 8 | 2·214 | 0 | 0·000 |
| Bulgaria | 1 | 5 | 5 | 0·072 | 0 | 0·000 |
| Spain | 1 | 5 | 5 | 0·011 | 0 | 0·000 |
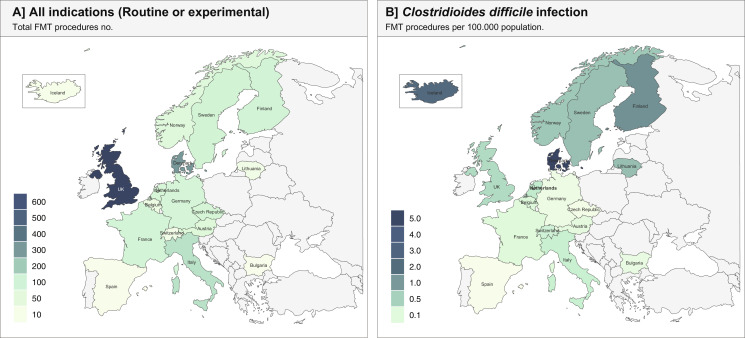
| Total | 31 | 1874 | 1077 | 0·257 | 791 | 0·189 |
Per 100·000 population
Abbreviations: CDI: Clostridioides difficile infection, no: Number.
3.1. Clinical activity of FMT in Europe
Across all FMT centres, a total of 1874 hospital-based FMT procedures (median 25, IQR: 10–64 per FMT centres) were reported for 2019 (Fig. 2, Table 1): 1077 (58%) with CDI as the indication and 791 (42%) with non-CDI indications. Six (0.3%) procedures had unknown indications. Adjusted to population size, 0.257 per 100,000 population received FMT for CDI and 0.189 per 100,000 population for non-CDI indications (Table 1). Ten FMT centres reported that they distributed FMT preparations to other FMT clinics, and this accounted for 244 preparations in total with a median of 9 (IQR: 5–35) distributed per centre for the year 2019.
Fig. 2.
Total clinical activity of faecal microbiota transplantation (FMT) across Europe and for Clostridioides difficile infections adjusted to per 100,000 population.
FMT was used for routine purposes in 24 (77%) of the 31 participating FMT centres, and/or for research purposes in 23 (74%). This research activity was performed within clinical trials in 18 (78%) of the 23 centres using FMT for research purposes and/or according to research protocols (non-clinical trials) in 11/23 (48%). Table 2 describes the specified routine and investigational (within trials) indications for using FMT.
Table 2.
Reported routine and investigational indications in faecal microbiota transplantation (FMT) in Europe, 2019.
| Indication | n* | % |
|---|---|---|
| Routine clinical indications. (n = 30 centres) | ||
| Clostridioides difficile infection (CDI): | ||
| Recurrent CDI | 30 | 100% |
| Antibiotic refractory CDI | 27 | 90% |
| Critical CDI | 14 | 47% |
| Experimental (outside trials) indications. (n = 30 centres) | ||
| Ulcerative colitis | 4 | 13% |
| Multidrug resistant organisms carriage | 3 | 10% |
| Graft versus host disease | 2 | 7% |
| Irritable bowel syndrome | 1 | 3% |
| Pouchitis | 1 | 3% |
| Antibiotic-associated diarrhoea, not CDI. | 1 | 3% |
| Investigational (within trials) indications. (n = 24 centres) | ||
| Ulcerative colitis | 11 | 46% |
| Irritable bowel syndrome | 7 | 30% |
| Multidrug resistant organisms carriage | 5 | 21% |
| Recurrent CDI | 3 | 13% |
| Index CDI | 3 | 13% |
| Refractory CDI | 2 | 8% |
| Crohn's disease | 2 | 8% |
| Pouchitis | 2 | 8% |
| Graft versus host disease | 2 | 8% |
| Obesity | 2 | 8% |
| Spondyloarthropathy | 2 | 8% |
| Liver cirrhosis, hepatic encephalopathy | 2 | 8% |
| Critical CDI | 1 | 4% |
| Antibiotic-associated diarrhoea, not CDI | 1 | 4% |
| Parkinson's disease | 1 | 4% |
| Chemotherapy-related diarrhoea | 1 | 4% |
| Non-alcoholic fatty liver disease (NAFLD) | 1 | 4% |
| Chronic fatigue syndrome | 1 | 4% |
| Microscopic colitis | 1 | 4% |
1 unanswered response in total.
Abbreviations: CDI: Clostridioides difficile infection, NAFLD: Non-alcoholic fatty liver disease.
Among delivery methods, colonoscopy was preferred by most FMT centres (11/31, 36%), followed by rectal enema (8/31, 26%) and upper gastrointestinal tract tube insertion (8/31, 26%). Administration by capsules was the most common delivery method at 3 (10%) of the 31 centres while capsules were available at 6/31 (19%) centres as glycerol-based and at 3/31 (10%) centres as lyophilised formulations. 1/31 (3%) centre preferred gastroscopy. Repeat FMT administration as part of the same treatment was practiced at 23/31 (74%) centres, either by predefined criteria at 12/23 (52%) or without predefined criteria at 11/23 (48%) centres. Indication for repeat administration included (i) severe/fulminant CDI, (ii) refractory CDI unresponsive to antibiotics, (iii) recurrent CDI following previous FMT failure, and/or (iv) within trials for experimental indications.
3.2. Donor recruitment and screening practices
Donations for FMT relied solely on anonymous donors at 18 (58%), both related (known to the patient) and anonymous donors at 12 (39%), and related donors only at 1 (3%) of the 31 centres. For donor recruitment, most FMT centres used restricted advertising e.g., among students or local societies (17/31, 55%) or personal recruitment e.g., among blood donors (13/31, 42%). Health care professionals were allowed as donors in 14 (45%) of the 31 centres, was a deferral criterion in 16 (52%) centres and unknown in 1 (3%). Reimbursement was not offered in 18/31 (58%), whereas 6/31 (19%) provided routine reimbursement per donation, and 4/31 (13%) provided a reimbursement per donation when used in clinical trials.
The selection of donors for FMT was based solely on the absence of risk factors or screening/testing parameters (clinical, biochemical, and faecal) in 25/31 (81%) centres, while 6/31 (19%) had additional positive selection criteria based on intestinal microbiota patterns. A questionnaire-based, pre-screening of all donor candidates was performed at 28 (90%) of the 31 centres, and 23 (74%) performed complete re-screening of all donors following each donation period. The median number of donations per donation period was 5 within a median duration of 35 days (Table 3). Twelve (38%) FMT centres had no specified limit for a maximum length of donation period (the period between two consecutive screening rounds [4]).
Table 3.
Summary of the reported preparation practices across the participating FMT centres for preparing stool for faecal microbiota transplantation (FMT).
| Preparation conduct | Responders | n | % |
|---|---|---|---|
| Fresh or frozen FMT used | |||
| Only frozen | 31 | 19 | 61% |
| Both fresh and frozen | 31 | 8 | 26% |
| Only fresh | 31 | 3 | 10% |
| Unanswered | 31 | 1 | 13% |
| Quarantined FMT preparations until fulfilment of release criteria | |||
| Yes | 31 | 26 | 84% |
| No | 31 | 5 | 16% |
| Routine quality controls instated | |||
| No | 31 | 16 | 52% |
| Yes | 31 | 14 | 45% |
| Unanswered | 31 | 1 | 3% |
| Preparation details | Responders | Median | [IQR] |
|---|---|---|---|
| Maximum time from defecation to initiation of processing (hours) | 29 | 4 | [2 - 6] |
| Average donation weight (gr) | 31 | 120 | [65- 150] |
| Average amount of faeces pr FMT preparation (gr.) | 31 | 50 | [50–60] |
| Average no. of donations per donation cycle (no.) | 27 | 5 | [5- 8•5] |
| Maximum duration of donation period, average (days) | 19 | 35 | [22•5 - 60] |
| - Had no defined limits for the length of donation period. | 12 | •• | •• |
| Maximum average storage at 20⁰C (months) | 4 | 6 | [5 - 6] |
| Maximum average storage at 80⁰C (months) | 26 | 12 | [10•5 - 22•5] |
Abbreviations: FMT: Faecal microbiota transplantation, Gr: Grams, IQR: Interquartile range; No: number.
3.3. Processing and preparation of stool for FMT
Most FMT centres (19/31, 61%) reported using frozen FMT preparations or a combination of both fresh and frozen preparations (8/31, 26%). Fresh preparations only were used at 3 (10%) centres. One (3%) FMT centre did not respond to this question. The processing and preparation of donor faeces for FMT preparations were handled within the facilities of clinical microbiology or immunology departments (14/31, 45%), research laboratories (12/31, 39%), certified FMT laboratories (2/31, 6%), clinical departments on site (2/31, 6%), or pharmacies (1/31, 3%). The specific summary of preparation details is presented in Table 3.
3.4. Organisation of the FMT centres
Maintaining the FMT centres engaged a variety of multi-disciplinary personnel that included physicians, nurses, laboratory technicians, pharmacists, production managers, and research assistants. Follow-up of all patients was documented in 27 (87%) of the 31 FMT centres and was practiced largely through clinical face-to-face appointments (20/27, 74%), telephone calls (20/27, 74%), medical record follow-up (16/27, 59%) and/or by written questionnaires (2/27, 7%). The longest median follow-up time for patients who received an FMT for CDI was 26 months (range 12–52 months).
Eight (26%) of the 31 centres had a formal auditing system, and 26/31 (84%) maintained a centralised database for their FMT activities and procedures. Standardised recording of adverse events was done at 19/31 (61%) centres, and 12/31 (39%) reported them to an external party, i.e., national authorities or central national registries. Fourteen of 29 (48%, 2 unanswered) centres had defined contraindications for FMT.
3.5. Safety
Most centres (26/31, 84%) had quarantine measures to prevent release of FMT preparations until fulfilment of defined release criteria (27 FMT centres used frozen preparations allowing for these practices), and 14/31 (45%) performed routine quality controls. Accreditation of FMT preparation and/or clinical trial conduct was done in 20 (65%) of the 31 FMT centres. Thirteen (42%) of 31 centres had a Good Manufacturing Practice (GMP) accreditation of the FMT preparation, and 18 of 26 centres (69%, 5 centres had no on-going trials) conducted their clinical trials according to the principles for Good Clinical Practice (GCP). Seven of 30 (23%, 1 unanswered) had specific protocols for FMT preparations for use in immunocompromised patients.
3.6. Regulation
Regulation varied across countries and centres. Formal regulation was in place at 12/31 (39%) centres; of whom 7/31 (23%) were regulated by the national medicines’ authorities, 4/31 (13%) were regulated by the national tissue authorities, and 1/31 (3%) were regulated locally by the hospital administration. Informal regulation where the centres were in dialogue with the national health authorities were present at 9/31 (29%) centres, and 3/31 (10%) centres reported having ethics approval only. Seven (23%) centres reported having no regulation.
4. Discussion
In this Europe-wide survey of clinical FMT activity and conduct, we estimate that approximately 2000 hospital-based FMT procedures were performed across the European countries in 2019. The main indication for FMT was Clostridioides difficile infection. In addition, FMT was widely used as an experimental treatment for several conditions, both within and outside of clinical trials. The survey results provide novel insights into how hospital-based FMT centres operate with high safety standards across Europe, and it represent the first complete estimate of the current supply and demand for FMT in Europe. These data are pivotal to guide future clinical practices and decision-making regarding how to perceive, use, and regulate FMT in Europe.
The survey points to a significant unmet potential for the use of FMT. Currently, FMT is recommended for patients with multiple recurrences of CDI, i.e. three or more infections [17, 18]. The European Centre for Disease Prevention and Control (ECDC) has since 2016 collected surveillance data on the CDI incidence within acute care hospitals in the European countries and estimates that 124,000 (95% CI: 61,000–285,000) patients are diagnosed with CDI annually in Europe [19]. With an anticipated 10% occurrence of multiple, recurrent CDI [20, 21], the approximately 12,400 (95% CI: 6,100–28,500) patients with multiple, recurrent CDI and indication for FMT are far from matched by the current annual 1077 FMT procedures performed for CDI. This amounts to a significant gap in FMT coverage. Despite being a conservative measure not accounting for CDI-attributable mortality nor community-acquired CDI, it suggests that FMT is currently reserved for the most severe instances and that there is a need to increase clinical awareness and scale the European FMT activity by a 10-fold factor to meet the true demand, even in countries with frequent use of FMT.
To enable a large-scale use that meets the estimated need, distribution of FMT preparations to clinicians at treatment sites without the extensive infrastructure required for preparing FMT is required. In the survey, most of the FMT centres reported operating locally, and the 250 FMT preparations distributed to FMT clinics in 2019 indicate that the capacity for most FMT centres to distribute to other regional/national clinics is developing. Improving this capacity is essential to secure equal access for all patients. Rather than promoting the formation of new local FMT centres, scaling the established FMT centres to reach the critical capacity enabling them to become centralised stool banks capable of widespread distribution may prove the best model for making FMT widely accessible in the immediate future [22]. Similar to blood centres, the ideal for stool banks is to provide clinicians access to safe, ready-to-use FMT preparations while the stool banks handle the extensive logistics and documentation [10, 23].
The degree to which widespread access to FMT is achieved depends on how easily an FMT is performed locally. Technological refinements have now simplified the FMT procedure, especially the use of encapsulated FMT containing frozen/freeze-dried stool sourced from anonymous donors have made the operations highly scalable [24, 25]. Most FMT centres in this survey reported colonoscopy as their preferred method of application, and although colonoscopy is suggested to be most effective in recurrent CDI [2], colonoscopy is logistically demanding. Transitioning to other treatment modalities with an increased ease of use such as encapsulated FMT, enema or similar, holds the potential to improve capacity.
International consensus guidelines exist to guide FMT centres [3], [4], [5], and the responses to the current survey indicate the degree to which these guidelines are adhered to. Most participating centres had defined practices for donor recruitment and screening as well as the laboratory preparations similar to those described in the consensus guidelines, indicating high adherence. Nonetheless, fresh donations were still used at some FMT centres, which per se cannot fulfil international requirements for donor screening. This practice may be needed for new, novel indications or represent a novel FMT centre in development.
As a next step in the continuous harmonisation of FMT in Europe, the present survey points to the need for shared, consensus-based definitions of operational metrics for measuring and comparing FMT centres and later stool banks. These could be similar to those used in endoscopy centres [26] and should (i) help indicate the FMT centre's current level of development, (ii) endorse quality measures for auditing, (iii) facilitate improvement, and (iv) assist in identifying current barriers to dissemination. For FMT, these metrics could include measurable performance indicators such as donor deferral rates, number of released, ready-to-use FMT preparations, number of procedures for certain indications, waste of preparations, as well as quality indicators such as adherence to the international standards for donor screening and laboratory handling or the level of implementation of e.g., stool bank capacity.
The safety of FMT remains a pivotal aspect of its use in clinical practice. While the vigorous screening of donors drastically reduces the short-term infectious risks following FMT, the long-term consequences of FMT are still unknown [27]. A united European approach is necessary to respond to future adverse events and inform clinical practices [28]. Most participating FMT centres maintained a centralised database and recorded adverse events in a predefined manner, which allows for long-term donor/recipient traceability. Compiled, annual summaries of the long-term follow-up from these registries may prove valuable in addressing the long-term safety of FMT and indicate whether the risk profile changes with indication.
Regulation of FMT is handled differently across Europe and greatly influences the national conduct and access to FMT. About 40% had formal regulation from either the national medical or tissue authorities, and most others reported they were in dialogue with their respective national competent authorities, although without formal regulation. Regulation is pivotal to the safety and creditability of FMT, but it remains controversial which jurisdictions should be applied. Currently, common consensus emphasise FMT should be considered a transfer of tissue if the transferred stool has not been subjected to substantial modifications rendering it comparable to a medicinal product [29]. A common European legislative framework for FMT is warranted and may include criteria for applying tissue and cells standards and potentially also medicines legislation [30].
The use of FMT will likely increase as a standard treatment for recurrent CDI and other indications where FMT is currently investigational. The results of this survey consolidate that FMT should be considered a routine treatment. Still, maintaining an FMT centre is not without challenges. As highlighted by the recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the subsequent COVID-19 pandemic, unknown adverse circumstances arise, which forces FMT centres to temporarily cease activity and reorganise while adapting in order to maintain their clinical activity [31,32]. The future success of the FMT centres in part depends on this ability to adapt locally and make strong collaborations internationally.
Important limitations apply to the study. Despite a high response rate, three centres did not respond and other FMT centres may be present in Europe, which we did not reach in this survey. Only hospital-based FMT centres were included, and the estimated activity is a conservative measure, subject to recall bias and accuracy limitations. By including all known FMT centres as well as those from the most commonly used public trial registry, we targeted the institutions with the largest capacity to provide FMT. However, we could not evaluate geographical variations, e.g., access in rural versus urban areas, nor the total population covered by FMT centres in each country. While the estimates and reporting of organisations in this manuscript reflects the reported activity in 2019, the current practice may have changed, and the emergence of FMT centres in the Eastern Europe has changed the regional access in Europe.
In conclusion, FMT has become a routine treatment for multiple, recurrent CDI and is a common investigational treatment for other diseases where the intestinal microbiota may be a contributing factor. Widespread FMT centres with high safety standards operate across Europe and may emerge to serve an increasing demand for FMT. A significant gap exists between the current number of FMT procedures and the European estimates for multiple, recurrent CDI, suggesting the need to increase clinical awareness and scale the European FMT activity at least by a 10-fold increase to meet the true, indicated need.
Contributors
Study concept and design: CLH, SMDB, JJK, EMT, PA, MJGTV, HS, PKK, JFD, GI, AG, GC.
Acquisition of data: SMDB, EMT, JFD, CE, PA, MJGTV, GI, AG, HS, PKK, RS, DDL, SV, RN, JB, MH, JK, AAR, SJK, LA, CTP, JS, AL, AS, JK, PHJ, KG, ESR, LS, RJB, KTG, SDG, BHM, HRTW, THI, CP, EJK, GC, JJK, CLH. Statistical analysis of data: SMDB. Analysis and interpretation of data: CLH, SMDB, JFD, CE, EMT, JJK. First draft: SMDB, CLH. Critical revision of manuscript for important intellectual content: SMDB, EMT, JFD, CE, PA, MJGTV, GI, AG, HS, PKK, RS, DDL, SV, RN, JB, MH, JK, AAR, SJK, LA, CTP, JS, AL, AS, JK, PHJ, KG, ESR, LS, RJB, KTG, SDG, BHM, HRTW, THI, CP, EJK, GC, JJK, CLH. All authors revised and approved the final manuscript. SMDB and CLH had primary access to all the data in the study, all other were offered access. JFD also had full access and verified it. All authors accepted final responsibility for the decision to submit for publication.
Declaration of interest
Andreas Stallmach reports consulting fees from Institut Allergosan, MSD, Norgine, lecture fees and travel support from Astellas, Ferring, Janssen, MSD.
Benjamin H. Mullish reports consultancy fees from Finch Therapeutics Group.
Simon D. Goldenberg reports Consultancy fees from Astellas, Enterobiotix, Menarini, MSD, Pfizer, Shionogi and research grants from Shionogi.
Laurent Alric reports consultant/speaker/investigator fees from AbbVie, BMS, Gilead, Janssen, and Merck.
Cyriel Ponsioen reports grant support form Takeda, consultancy fees from Takeda, Shire, and Pliant, and speaker's fees from Tillotts and Pfizer.
Caroline Trang-Poisson reports lecture fees from AbbVie, Amgen, Janssen, MaaT Pharma, MSD, takeda, advisory board fees from Arena, CT scout, MSD and meeting from AbvVie, takeda, MSD, and Janssen.
Maria Vehreschild reports research grants from 3 M, Astellas Pharma, Biontech, DaVolterra, Evonik, Gilead Sciences, Glycom, Immunic, MaaT Pharma, Merck/MSD, Organobalance, Seres Therapeutics, Takeda Pharmaceutical, and speaker fees and/or consulting from Alb Fils Kliniken GmbH, Arderypharm, Astellas Pharma, Basilea, Bio-Mérieux, DaVolterra, Farmak International Holding GmbH, Ferring, Gilead Sciences, Immunic AG, MaaT Pharma, Merck/MSD, Pfizer, Roche, Organobalance, and SocraTec R&D GmbH.
Severine Vermeire reports consulting fees from Prodigest/MRM Health.
Elisabeth Terveer and Ed Kuijper reports research grants from Vedanta Bioscience, Boston.
Simon Mark Dahl Baunwall, Christian Lodberg Hvas, Jens Frederik Dahlerup, Christian Erikstrup report research grant from the Innovation Fund Denmark (j.no. 8056–00006B).
All other authors declare no competing interest.
Acknowledgments
Acknowledgments
The authors thank Stefan Haraldsson, MD, at the Department of Gastroenterology, Landspitali University Hospital, Reykjavik, Iceland for contributing data to the study.
Data availability statement
Data are available upon request in blinded format, respecting the anonymity of each institution.
Funding
This study was funded by NordForsk under the Nordic Council and the Innovation Fund Denmark (j.no. 8056–00006B). The funding was independent of the study conduct. BHM is the recipient of an NIHR Academic Clinical Lectureship (CL-2019-21-002). The Department of Metabolism, Digestion and Reproduction at Imperial College London receives financial and infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.lanepe.2021.100181.
Appendix. Supplementary materials
Supplementary file 1. Copy of the questionnaire send to all participating faecal microbiota transplantation (FMT) centres.
Supplementary file 2. Filled Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for the study.
References
- 1.Allegretti J.R., Mullish B.H., Kelly C., Fischer M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet. 2019;394:420–431. doi: 10.1016/S0140-6736(19)31266-8. [DOI] [PubMed] [Google Scholar]
- 2.Baunwall S.M.D., Lee M.M., Eriksen M.K. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;29-30 doi: 10.1016/j.eclinm.2020.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cammarota G., Ianiro G., Kelly C.R. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keller J.J., Ooijevaar R.E., Hvas C.L. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG working group. United Eur Gastroenterol J. 2021;9:229–247. doi: 10.1177/2050640620967898. 2050640620967898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullish B.H., Quraishi M.N., Segal J.P. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect. 2018;100(Suppl 1):S1–s31. doi: 10.1016/j.jhin.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Ianiro G., Eusebi L.H., Black C.J., Gasbarrini A., Cammarota G., Ford A.C. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240–248. doi: 10.1111/apt.15330. [DOI] [PubMed] [Google Scholar]
- 7.Ghani R., Mullish B.H., McDonald J.A.K. Disease prevention not decolonization - a model for fecal microbiota transplantation in patients colonized with multidrug-resistant organisms. Clin Infect Dis. 2021;72:1444–1447. doi: 10.1093/cid/ciaa948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj J.S., Salzman N.H., Acharya C. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70:1690–1703. doi: 10.1002/hep.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Nood E., Vrieze A., Nieuwdorp M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 10.Terveer E.M., van Beurden Y.H., Goorhuis A. How to: establish and run a stool bank. Clin Microbiol Infect. 2017;23:924–930. doi: 10.1016/j.cmi.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Nakov R., Lyutakov I., Mitkova A. Establishment of the first stool bank in an Eastern European country and the first series of successful fecal microbiota transplantations in Bulgaria. Eur Rev Med Pharmacol Sci. 2021;25:390–396. doi: 10.26355/eurrev_202101_24406. [DOI] [PubMed] [Google Scholar]
- 12.Costello S.P., Tucker E.C., La Brooy J., Schoeman M.N., Andrews J.M. Establishing a fecal microbiota transplant service for the treatment of clostridium difficile infection. Clin Infect Dis. 2016;62:908–914. doi: 10.1093/cid/civ994. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen S.M.D., Hansen M.M., Erikstrup C., Dahlerup J.F., Hvas C.L. Faecal microbiota transplantation: establishment of a clinical application framework. Eur J Gastroenterol Hepatol. 2017;29:e36–e45. doi: 10.1097/MEG.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 14.McCune V.L., Quraishi M.N., Manzoor S. Results from the first English stool bank using faecal microbiota transplant as a medicinal product for the treatment of Clostridioides difficile infection. EClinicalMedicine. 2020;20 doi: 10.1016/j.eclinm.2020.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rode A.A., Bytzer P., Pedersen O.B., Engberg J. Establishing a donor stool bank for faecal microbiota transplantation: methods and feasibility. Eur J Clin Microbiol Infect Dis. 2019;38:1837–1847. doi: 10.1007/s10096-019-03615-x. [DOI] [PubMed] [Google Scholar]
- 16.Kragsnaes M.S., Nilsson A.C., Kjeldsen J. How do I establish a stool bank for fecal microbiota transplantation within the blood- and tissue transplant service? Transfusion. 2020;60:1135–1141. doi: 10.1111/trf.15816. [DOI] [PubMed] [Google Scholar]
- 17.McDonald L.C., Gerding D.N., Johnson S. Clinical practice guidelines for Clostridium difficile Infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debast S.B., Bauer M.P., Kuijper E.J. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 19.ECDC. Annual epidemiological report for 2016. ECDC; Stockholm: 2018. European centre for disease prevention and control. Clostridium difficile infections. [Google Scholar]
- 20.Smits W.K., Lyras D., Lacy D.B., Wilcox M.H., Kuijper E.J. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie T.J., Miller M.A., Mullane K.M. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 22.Quraishi M.N., Segal J., Mullish B. National survey of practice of faecal microbiota transplantation for Clostridium difficile infection in the UK. J Hosp Infect. 2017;95:444–445. doi: 10.1016/j.jhin.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen S.M.D., Hvas C.L., Dahlerup J.F. Banking feces: a new frontier for public blood banks? Transfusion. 2019;59:2776–2782. doi: 10.1111/trf.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao D., Roach B., Silva M. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staley C., Hamilton M.J., Vaughn B.P. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota; pragmatic cohort study. Am J Gastroenterol. 2017;112:940–947. doi: 10.1038/ajg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisschops R., Rutter M.D., Areia M. Overcoming the barriers to dissemination and implementation of quality measures for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and United European Gastroenterology (UEG) position statement. Endoscopy. 2021;53:196–202. doi: 10.1055/a-1312-6389. [DOI] [PubMed] [Google Scholar]
- 27.Vendrik K.E.W., Terveer E.M., Kuijper E.J. Periodic screening of donor faeces with a quarantine period to prevent transmission of multidrug-resistant organisms during faecal microbiota transplantation: a retrospective cohort study. Lancet Infect Dis. 2021;21:711–721. doi: 10.1016/S1473-3099(20)30473-4. [DOI] [PubMed] [Google Scholar]
- 28.Kuijper E.J., Allegretii J., Hawkey P. A necessary discussion after transmission of multidrug-resistant organisms through faecal microbiota transplantations. Lancet Infect Dis. 2019;19:1161–1162. doi: 10.1016/S1473-3099(19)30545-6. [DOI] [PubMed] [Google Scholar]
- 29.Keller J.J., Vehreschild M.J., Hvas C.L. Stool for fecal microbiota transplantation should be classified as a transplant product and not as a drug. United Eur Gastroenterol J. 2019;7:1408–1410. doi: 10.1177/2050640619887579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hvas C.L., Baunwall S.M.D., Erikstrup C. Faecal microbiota transplantation: a life-saving therapy challenged by commercial claims for exclusivity. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ianiro G., Mullish B.H., Kelly C.R. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69:1555–1563. doi: 10.1136/gutjnl-2020-321829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ianiro G., Mullish B.H., Hvas C.L. SARS-CoV-2 vaccines and donor recruitment for FMT. Lancet Gastroenterol Hepatol. 2021;6:264–266. doi: 10.1016/S2468-1253(21)00032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. Copy of the questionnaire send to all participating faecal microbiota transplantation (FMT) centres.
Supplementary file 2. Filled Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for the study.
Data Availability Statement
Data are available upon request in blinded format, respecting the anonymity of each institution.