Abstract
BACKGROUND:
With the increasing prevalence of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has emerged as the most common cause of pediatric chronic liver disorder. Factors underlying the pathophysiology of NAFLD remain poorly defined.
OBJECTIVE:
This study aimed to describe the metabolic characteristics of children with NAFLD differing in race/ethnicity and to test associations between dyslipidemia and NAFLD.
METHODS:
A retrospective chart review was conducted at a tertiary referral university hospital among 309 children with a diagnosis of NAFLD.
RESULTS:
Participants (mean age 12.5 ± 3.4 years) were 64% male, 63% white, 23% Hispanic, and 14% black. Hispanic children were diagnosed with NAFLD at a significantly younger age (10.6 ± 3.1 years, P < .0001) and lower body mass index (31.5 ± 6.8 kg/m2, P < .0001) than their white and black counterparts. For the entire cohort, prevalence of systolic hypertension was 41%, diabetes 14%, elevated cholesterol 42%, elevated non–high-density lipoprotein cholesterol (non–HDL-C) 58%, elevated low-density lipoprotein cholesterol 36%, elevated triglycerides (TG) 88%, and low high-density lipoprotein cholesterol 77%. Whites had elevated non–HDL-C, low-density lipoprotein cholesterol, and TG compared to blacks or Hispanics. Serum TG and non–HDL-C were significantly correlated to alanine aminotransferase (r = 0.18, P = .01; r = 0.16, P = .02), respectively, and persisted after adjusting for age and body mass index.
CONCLUSION:
Cardiometabolic derangements, especially dyslipidemia, are highly prevalent in children with NAFLD and differ based on race/ethnicity. Serum TG and non–HDL-C may play an important role in the pathophysiology of pediatric NAFLD.
Keywords: Nonalcoholic fatty liver disease, Metabolic syndrome, Dyslipidemia, Childhood obesity, Pediatric
Introduction
With the alarming growth of the obesity epidemic, nonalcoholic fatty liver disease (NAFLD) has emerged as the most common chronic liver disorder among children and adolescents in the developed world.1–3 While prevalence estimates vary due to differences in diagnostic methods, studies using the most conservative approach suggest that NAFLD is prevalent in over one-third of all obese children.3
NAFLD is defined as fat accumulation in ≥5% of hepatocytes due to causes other than alcohol.3 It exists on a spectrum, ranging from mild fat accumulation in the liver, hepatic steatosis, to nonalcoholic steatohepatitis (NASH), a progressive medical condition involving extensive hepatic inflammation, cellular injury, and the possibility of fibrosis.4 NASH is associated with significant mortality and morbidity, including cirrhosis, hepatocellular carcinoma, and end-stage liver disease, and can require liver transplantation.5–9
Often regarded as the hepatic manifestation of metabolic syndrome, NAFLD in children is strongly correlated with abdominal obesity, insulin resistance, hypertension (HTN), and other characteristics of the syndrome.10–12 Dyslipidemia, a component of metabolic syndrome, is characterized by elevated triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C). Although dyslipidemia is frequently seen in pediatric NAFLD and may play a role in its etiology,13 lipid profiles are not routinely measured in the evaluation and management of pediatric NAFLD.12,14,15
The role of dyslipidemia in the development and progression of NAFLD is not well established. The pathophysiology of NAFLD involves a cascade of molecular events related to obesity and insulin resistance.16,17 The hyperinsulinemic hyperglycemic state contributes to fat accumulation in the liver through increased hepatic very low-density lipoproteins synthesis and secretion from increased release of free fatty acids from adipocytes and hepatic de novo lipogenesis and elevated serum TG concentrations.16,18,19 Furthermore, in insulin-resistant states, TG hydrolysis and subsequent HDL-C formation is attenuated, leading to increased circulating TG and lower HDL-C.20 We hypothesized that dyslipidemia may serve as an important factor influencing the severity of and metabolic derangements present in NAFLD.
The etiology of racial and ethnic differences in pediatric NAFLD is not well known. Although insulin resistance and obesity, 2 strong predictors of NAFLD, are more common in blacks than in whites, paradoxically, the prevalence of NAFLD is lowest among blacks.3,21 Blacks are known to have lower serum TG and higher HDL concentrations than whites. In light of the observed lower prevalence of NAFLD in blacks despite the higher body mass index (BMI) and insulin resistance, a potential role of TG in development of NAFLD could be postulated.
The primary objective of this study was to describe the demographic, anthropometric, and metabolic characteristics of children with NAFLD stratified by race/ethnicity. Secondary aims were (1) to identify the prevalence of cardiometabolic risk factors such as systolic or diastolic HTN, diabetes, dyslipidemia (ie, elevated non–HDL-C, TG, and low HDL-C) and (2) to evaluate the relationship between serum TG and non–HDL-C to indicators of liver dysfunction (ie, alanine aminotransferase [ALT], gamma-glutamyl transferase [GGT]) and cardiometabolic risk variables in children with NAFLD.
Methods
This was a cross-sectional electronic medical record (EMR) review of pediatric patients diagnosed with NAFLD, who were followed at the Children’s Hospital of Alabama, University of Alabama at Birmingham. The research protocol was approved by University of Alabama at Birmingham’s Institutional Review Board before data collection. Potential subjects were first identified from the EMR using International Classification of Diseases-9 medical billing codes indicating nonalcoholic fatty liver disease and elevations in liver transaminases (571.8 or 790.4) and then manually reviewed to determine eligibility. Patient records over a 14-year period of time, 2003–2017, were abstracted.
Participants with physician-ascertained diagnosis of NAFLD were included if they met the following inclusion criteria: (1) age at diagnosis ≤19 years; (2) diagnosis of NAFLD confirmed through ultrasound, computed tomography scan, or liver biopsy findings (“confirmed” NAFLD) or, for those without histologic or radiologic evidence of fatty liver, diagnosis of overweight or obesity (BMI ≥ 85th percentile), and elevations in ALT 1.5 times greater than the reference range (“suspected” NAFLD).22–24 Patients were excluded if they had insufficient radiological or biochemical testing, hepatitis (hepatitis A, B, C, D, E, and G; cytomegalovirus; and Epstein-Barr virus), autoimmune liver disease, metabolic liver disease, Wilson’s disease, and genetic conditions (eg, glycogen storage disorders) leading to hepatic steatosis, fatty liver from uncontrolled type I diabetes, fatty liver from alcohol consumption, or used medications known to induce steatosis.
Race and ethnicity were reported by the subjects’ parents and documented in the EMR as black, white, or Hispanic. Subjects who identified as other than black, white or Hispanic, particularly Asian participants (n = 4), were excluded due to insufficient sample size. For participants with biochemical testing at multiple time points, tests conducted closest to the most recent radiologically or histologically confirmed NAFLD were included in this study. “Age at onset” was defined as the age at which a patient had elevated ALT levels, radiological, and/or histological findings suggestive of NAFLD. “Age at diagnosis” was defined as the age at which a patient was diagnosed with NAFLD (either by the general pediatrician or specialist).
Systolic blood pressure (SBP) or diastolic blood pressure (DBP) ≥ 95th percentile for age, gender, and height was defined as HTN and an SBP or DBP between the 90th and 95th percentiles was defined as pre-HTN.25 Hemoglobin A1c (HbA1c) ≥ 6.5% was classified as diabetes, whereas an HbA1c between 5.7% and 6.4% was classified as prediabetes.26 Individual lipid measurements were classified based on the expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents27 and defined as: total cholesterol (TC): acceptable <170 mg/dL, borderline-high between 170–199 mg/dL, and high ≥200 mg/dL; non–HDL-C: acceptable <120 mg/dL, borderline-high between 120–144 mg/dL, and high ≥145 mg/dL; low-density lipoprotein cholesterol (LDL-C): acceptable <110 mg/dL, borderline-high between 110–129 mg/dL, and high ≥130 mg/dL; HDL-C: acceptable >45 mg/dL, borderline-low between 40–45 mg/dL, and low <40 mg/dL; total serum TG: acceptable <75 mg/dL, borderline-high between 75–99 mg/dL, and high ≥100 mg/dL for children ≤ 9 years; and total TG: acceptable <90 mg/dL, borderline-high between 90–129 mg/dL, and high ≥130 mg/dL for children ages ≥ 10 years. Elevated non–HDL-C was defined as ≥120 mg/dL; elevated TG was defined as ≥75 mg/dl for children ≤ 9 years and ≥90 mg/dL for children ages ≥ 10 years. Non–HDL-C was calculated by subtracting TC from HDL-C. This measure, encompassing LDL, very low-density lipoproteins, and intermediate-density lipoproteins, is becoming increasingly recognized as an important cardiovascular disease (CVD) risk factor and strong predictor of NAFLD severity in adults and children.28–30
The following assays were used to measure reported metabolic variables: DCA2000 assay for HbA1c, VITROS ALT Slide method for ALT, and VITROS 5,1 FS Chemistry System for TC, HDL-C, and LDL-C. TC was determined by standard enzymatic methods, HDL-C by a modified enzymatic assay, and LDL-C by an elimination-detergent and selective assay. The reference range for ALT in the Children’s Hospital laboratory was between 5 and 55 U/L, depending on the age and sex of the subject.
Statistical analysis
Data were evaluated using JMP software package (Version 12.0; SAS Institute Inc, Cary, NC). Descriptive statistics were computed for all subjects and stratified by race. Normality of data was assessed graphically using the normal quantile plot. Unpaired independent sample t-tests, one-way analysis of variance, and Tukey’s tests were conducted to evaluate significant differences between continuous variables. Chi-squared and Fisher’s exact tests (for groups with n < 5) were used to determine significance of differences in categorical variables. To summarize relationships between ALT, TG, non–HDL-C, and metabolic variables, we estimated partial Pearson’s correlation coefficients, adjusting for age at NAFLD onset and BMI. Although not statistically significant in the generalized linear model, these covariates were selected as most likely to confound the reported correlations based on prior knowledge. All statistical tests were two-sided, and an alpha level of 0.05 was considered significant.
Results
This cross-sectional study included 309 participants, 208 with radiological or histological evidence of NAFLD, and 101 participants with elevated ALT levels suggestive of NAFLD. Among the 208 patients with “confirmed” NAFLD, 120 were diagnosed via ultrasound, 36 via liver biopsy, 3 via computed tomography scan, and 49 had both ultrasound and liver biopsy findings indicating NAFLD. Severe obesity, defined as a BMI ≥99th percentile, was present in 222 participants (72%).
Table 1 portrays differences in demographic, anthropometric, and metabolic characteristics between participants by race. Among the 309 participants, 195 were white, 71 Hispanic, and 43 black. Hispanic subjects had a significantly lower age at diagnosis and onset (P < .0001). Relative to whites and blacks, Hispanics also weighed less (P < .0001) and had a lower BMI (P < .0001), although differences in BMI percentile and z-score were not significant. Mean SBP and DBP were also significantly lower in Hispanics than whites or blacks (P = .0006 and P = .0101, respectively).
Table 1.
Descriptive characteristics of NAFLD study population by race
| Variables | All subjects (n = 309) | White (n = 195) | Hispanic (n = 71) | Black (n = 43) | P value |
|---|---|---|---|---|---|
| Age at diagnosis, y | 12.5 (3.4) | 12.8 (3.2) | 10.6 (3.1) | 14.0 (3.8) | <.0001 |
| Age at onset, y | 12.8 (3.4) | 13.1 (3.2) | 10.9 (3.1) | 14.5 (3.5) | <.0001 |
| Sex (n, %) | |||||
| Male | 198 (64) | 129 (66) | 45 (63) | 24 (56) | .44 |
| Female | 111 (36) | 66 (34) | 26 (37) | 19 (44) | |
| Diagnosis of NAFLD (n, %) | |||||
| Confirmed | 208 (67) | 132 (68) | 53 (75) | 23 (54) | .07 |
| Suspected | 101 (33) | 63 (32) | 18 (25) | 20 (47) | |
| Weight, kg | 90.9 (34.7) | 93.4 (33.7) | 73.6 (25.4) | 108.5 (40.8) | <.0001 |
| Height, cm | 159.2 (16.1) | 161.0 (15.9) | 150.7 (14.6) | 165.5 (14.5) | <.0001 |
| BMI | |||||
| BMI, kg/m2 | 34.9 (8.7) | 35.1 (8.2) | 31.5 (6.8) | 40.0 (11.2) | <.0001 |
| BMI percentile | 98.4 (3.7) | 98.5 (3.9) | 98.4 (3.3) | 98.3 (3.7) | .96 |
| BMI z-score | 2.4 (0.5) | 2.4 (0.5) | 2.4 (0.5) | 2.5 (0.6) | .58 |
| Systolic BP, mmHg | 123.0 (14.2) | 125.1 (14.3) | 115.7 (12.7) | 126.2 (12.6) | .0006 |
| Diastolic BP, mmHg | 67.3 (9.6) | 68.2 (8.9) | 63.5 (9.0) | 69.6 (11.3) | .0101 |
| Liver enzymes, U/L | |||||
| ALT | 88.1 (65.5) | 88.3 (62.2) | 95.8 (65.8) | 75.0 (78.0) | .26 |
| AST | 59.7 (43.0) | 58.4 (38.2) | 59.7 (30.3) | 65.9 (72.3) | .59 |
| GGT | 49.9 (49.0) | 49.6 (44.1) | 42.1 (25.1) | 66.3 (88.6) | .09 |
| Insulin, mIU/L | 48.4 (43.8) | 54.1 (51.9) | 35.5 (21.1) | 53.1 (43.4) | .26 |
| Glucose, mg/dL | 130.8 (59.6) | 133.4 (45.8) | 140.5 (113.4) | 106.0 (37.3) | .74 |
| HbA1c, % | 5.9 (1.6) | 5.8 (1.5) | 5.8 (1.3) | 6.4 (2.1) | .17 |
| Total cholesterol, mg/dL | 169.7 (34) | 175.2 (34.3) | 162.8 (31.4) | 159.5 (34.4) | .0200 |
| Non-HDL-C, mg/dL | 130.4 (34.7) | 136.2 (34.7) | 124.0 (32.1) | 118.3 (35.0) | .01 |
| HDL-C, mg/dL | 39.8 (9.6) | 39.8 (10.0) | 38.9 (8.7) | 41.2 (9.3) | .58 |
| LDL-C, mg/dl | 101.8 (29.7) | 105.3 (30.6) | 98.1 (28.5) | 94.6 (26.8) | .13 |
| Triglycerides, mg/dL | 184.4 (102.6) | 197.4 (95.6) | 179.2 (93.2) | 143.3 (131.2) | .03 |
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; non–HDL-C, non–high-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data are presented as mean (standard deviation).
Significance considered at P ≤ .05 and is in bold.
Prevalence of select cardiometabolic risk factors by race is illustrated in Figure 1. In the entire sample, 41% had systolic HTN, 14% had systolic pre-HTN, 6% had diastolic HTN, and 6% had diastolic pre-HTN. A total of 14% had diabetes and an additional 14% had prediabetes. The white subgroup had a higher prevalence of systolic HTN, while systolic pre-HTN, diastolic HTN, prediabetes, and diabetes were most common among black children. Hispanic children had the lowest prevalence of all forms of HTN but had higher rates of prediabetes and diabetes compared to white children.
Figure 1.
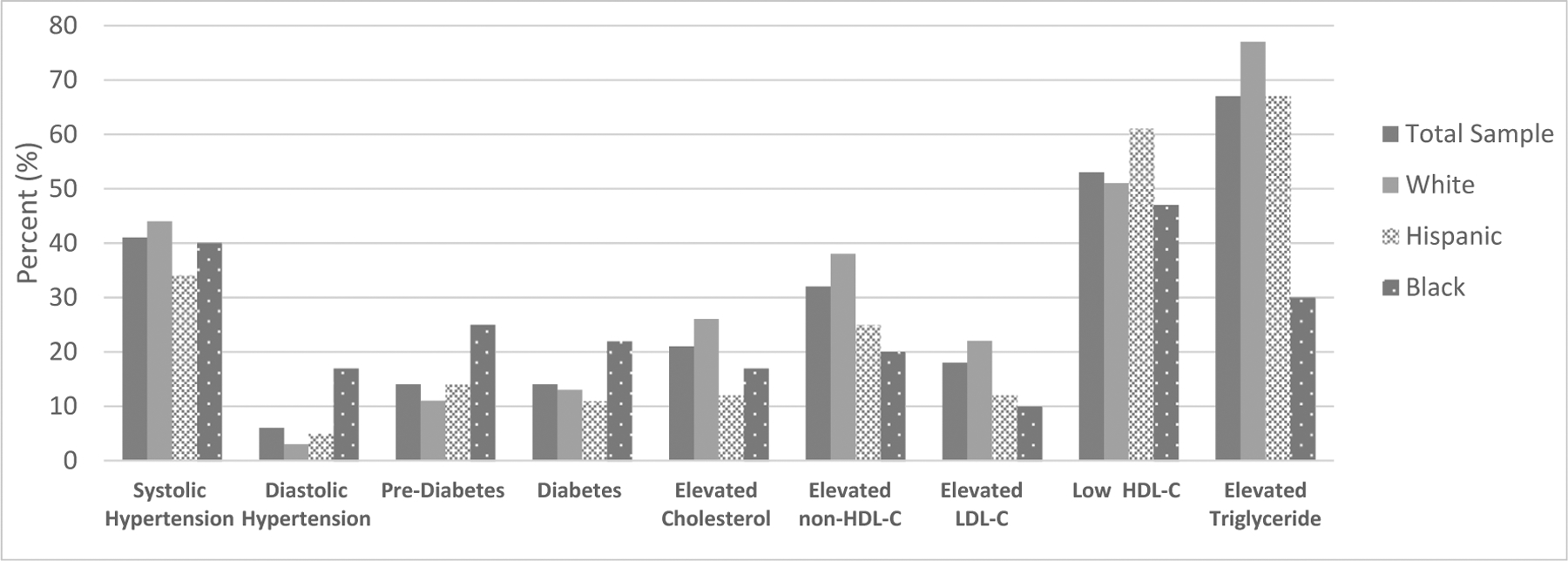
Prevalence of cardiometabolic risk factors by race. Non–HDL-C, non–high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Prevalence of dyslipidemia in the sample population was as follows: 42% had elevated TC, 58% had elevated non–HDL-C, 36% had elevated LDL-C, 77% had low HDL-C, and 88% had elevated TG. Racial differences evident in the lipid profile included significantly different TC between Whites, Hispanics, and Blacks (P = .02). In addition, whites had significantly higher non–HDL-C and serum TG compared to the other racial/ethnic groups (P = .0134, P = .0325, respectively).
Table 2 illustrates differences between subjects with elevated and normal TG and non–HDL-C. Those with elevated TG had a significantly higher mean BMI percentile (P = .0038), ALT (P = .01), GGT (P = .035), TC (P < .0001), non–HDL-C (P < .0001), LDL-C (P < .0001), and lower HDL-C (P = .0001) than those with normal TG. Similarly, subjects with elevated non–HDL-C had significantly higher mean ALT (P = .03), GGT (P = .02), TC (P < .0001), LDL-C (P < .0001), TG (P < .0001), and lower HDL-C (P = .03) than subjects with normal non–HDL-C.
Table 2.
Anthropometric and metabolic characteristics of NAFLD study population by abnormal TG and non–HDL-C
| Variables | Elevated TG (n = 170) | Normal (n = 24) | P value | Elevated non-HDL-C (n = 113) | Normal (n = 83) | P value |
|---|---|---|---|---|---|---|
| Weight, kg | 91.1 (35.8) | 95.8 (49.2) | .58 | 92.8 (33.2) | 90.3 (42.9) | .64 |
| Height, cm | 158.0 (16.1) | 160.4 (15.4) | .51 | 159.5 (14.7) | 156.6 (17.6) | .22 |
| BMI | ||||||
| BMI, kg/m2 | 35.3 (9.2) | 36.4 (13.7) | .63 | 35.5 (8.6) | 35.4 (11.1) | .93 |
| BMI percentile | 98.7 (2.5) | 95.8 (10.9) | .0038 | 98.6 (2.8) | 98.0 (5.9) | .31 |
| SystoLic BP, mm Hg | 122.5 (14.1) | 124.9 (18.7) | .56 | 124.7 (14.1) | 120.3 (14.9) | .09 |
| DiastoLic BP, mm Hg | 66.9 (9.9) | 67.1 (11.1) | .94 | 67.8 (10.7) | 65.6 (8.9) | .23 |
| Liver enzymes, U/L | ||||||
| ALT | 90.5 (64.0) | 56.0 (35.8) | .01 | 94.0 (66.3) | 74.9 (54.3) | .03 |
| AST | 61.3 (45.4) | 44.7 (18.8) | .08 | 61.6 (43.5) | 55.8 (42.9) | .35 |
| GGT | 50.1 (39.9) | 30.2 (19.3) | .035 | 53.9 (43.4) | 38.4 (27.6) | .02 |
| InsuLin, mIU/L | 47.0 (41.9) | 34.1 (19.8) | .35 | 45.1 (39.4) | 45.3 (40.8) | .98 |
| HbA1c, % | 5.9 (1.6) | 6.0 (2.1) | .90 | 6.0 (1.6) | 5.9 (1.7) | .65 |
| TotaL choLesteroL, mg/dL | 173.6 (33.5) | 139.4 (21.1) | <.0001 | 191.0 (27.3) | 140.8 (16.6) | <.0001 |
| Non-HDL-C, mg/dL | 134.8 (32.3) | 92.8 (20.9) | <.0001 | 153.3 (25.9) | 99.2 (15.8) | <.0001 |
| HDL-C, mg/dL | 38.8 (9.1) | 46.6 (10.3) | .0001 | 38.4 (9.5) | 41.5 (9.5) | .03 |
| LDL-C, mg/dL | 104.9 (29.4) | 79.0 (21.2) | <.0001 | 119.9 (23.4) | 77.5 (17.3) | <.0001 |
| TrigLycerides, mg/dL | 201.7 (97.8) | 62.5 (17.5) | <.0001 | 220.5 (104.8) | 136.2 (77.0) | <.0001 |
NAFLD, nonalcoholic fatty liver disease; TG, triglyceride; non–HDL-C, non–high-density lipoprotein cholesterol; BMI, body mass index; BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data are presented as mean (standard deviation).
Significance considered at P ≤ .05 and is in bold.
Table 3 depicts correlations between ALT, TG, non–HDL-C, and metabolic variables, adjusted for age at onset of NAFLD and BMI. Serum ALT was positively correlated with HbA1c (r = 0.19, P = .02), non–HDL-C (r = 0.16, P = .02), and total TG (r = 0.18, P = .01). Serum TG concentrations were positively correlated with ALT (r = 0.18, P = .01), GGT (r = 0.18, P = .04), insulin (r = 0.37, P = .002), glucose (r = 0.64, P = .01), and HbA1c (r = 0.35, P < .0001). Non–HDL-C concentrations were positively correlated with ALT (r = 0.16, P = .02) and insulin (r = 0.24, P = .05).
Table 3.
Correlations between ALT, TG, non–HDL-C, and metabolic variables, adjusted for age and BMI
| Variables | ALT | P value | Triglyceride | P value | Non-HDL-C | P value |
|---|---|---|---|---|---|---|
| Systolic BP | −.05 | .56 | .12 | .17 | .17 | .06 |
| Diastolic BP | .05 | .52 | .08 | .36 | .09 | .31 |
| Liver enzymes | ||||||
| ALT | 1.0 | – | .18 | .01 | .16 | .02 |
| AST | .75 | <.0001 | .05 | .46 | .06 | .40 |
| GGT | .46 | <.0001 | .18 | .04 | .17 | .06 |
| Insulin | .17 | .16 | .37 | .002 | .24 | .05 |
| Glucose | .18 | .45 | .64 | .01 | −.11 | .71 |
| HbA1c | .19 | .02 | .35 | <.0001 | .11 | .20 |
| Total cholesterol | .14 | .06 | .41 | <.0001 | .96 | <.0001 |
| Non-HDL-C | .16 | .02 | .51 | <.0001 | 1.0 | – |
| HDL-C | −.13 | .08 | −.38 | <.0001 | −.14 | .06 |
| LDL-C | .10 | .15 | .19 | .0047 | .86 | <.0001 |
| Triglyceride | .18 | .01 | 1.0 | – | .51 | <.0001 |
BMI, body mass index; BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Significance considered at P ≤ .05 and is in bold.
Discussion
While dyslipidemia is a known risk factor for NAFLD in adults, evaluation and management of dyslipidemia is not routinely performed in children with NAFLD. We present the largest to date description of lipid profiles in a pediatric population with NAFLD, stratified by race and ethnicity.
Racial/ethnic differences in pediatric NAFLD
Ethnic differences in prevalence of NAFLD have been described. Schwimmer et al observed that among obese children, prevalence of abnormally elevated ALT levels was significantly higher in Hispanics and lowest in blacks.31 These ethnic differences were also present in children with biopsy-proven NAFLD and adults with radiological and/or biochemical evidence of NAFLD.11,32–34 In our study, whites had the highest prevalence of NAFLD, followed by Hispanics and blacks. Hispanic children were observed to have NAFLD at a significantly lower age, weight, and BMI and had lower blood pressure than black and white children. While more data are needed to confirm our findings, our results suggest that pediatricians may need to monitor for liver function and blood pressure abnormalities in Hispanic children at risk for obesity and insulin resistance at an earlier age.
CVD risk factors and NAFLD
CVD is the leading cause of mortality in adults with NAFLD.35 While similar long-term studies have not been conducted in the pediatric population, presence and severity of NAFLD has been linked to increased prevalence of CVD and risk of atherosclerosis in children.14,15,32,33,36,37 Findings from this study showed that specific CVD risk markers such as systolic HTN, elevated TG, non–HDL-C, LDL-C, and low HDL-C were strikingly prevalent in children with NAFLD, in accordance with existing literature.12,13 In addition, our results showed significant associations between SBP, insulin, glucose, HbA1c, indicators of liver dysfunction (ie, ALT, GGT) and serum TG and non–HDL-C. Corey et al,13 observed significant improvements in liver histopathology and resolution of NASH in children with reductions in non–HDL-C, although a similar relationship did not exist with reductions in TG. They also observed that children who had resolution of NASH had significant improvement of their non–HDL-C. Disturbed hepatic lipid metabolism may influence development of NAFLD in the presence of metabolic risk factors such as obesity, insulin resistance, hyperglycemia, and HTN. This relationship between dyslipidemia and NAFLD is likely bidirectional. These incongruencies suggest that while peripheral lipoproteins may play an important role in the etiology of NAFLD, further investigation is warranted to disentangle these complex relationships.
Dyslipidemia in NAFLD and relationship to ethnicity
Our study is the first to investigate ethnic differences in lipid abnormalities among children with NAFLD. Our results demonstrated that TC differed significantly between whites, blacks, and Hispanics, and that whites had significantly higher levels of non–HDL-C and serum TG. Lower serum TG and higher HDL-C concentrations have been reported among black children and adults.38 This observation may be due, in part, to the enhanced lipoprotein lipase activity, the enzyme responsible for hydrolyzing TG, and reduced hepatic lipase activity in blacks,39 a protein involved in the remodeling of HDL-C.22 This may account for the lower prevalence of NAFLD in black children. Hispanics have been reported to have lower TC, LDL-C, and HDL-C and higher TG levels than non-Hispanic whites.40 Hispanics also tend to have a disproportionately higher prevalence of metabolic syndrome, NAFLD, and NASH compared to non-Hispanics.34 While the causal factors explaining racial and ethnic differences in pediatric NAFLD remain unclear, these findings support the notion that elevated TG and non–HDL-C concentrations may play a role in pathophysiology of NAFLD.
Strengths of this study include a robust overall sample size, large sample population with radiologic or biopsy-confirmed NAFLD, and focus on ethnic variations in pediatric NAFLD. It is, however, not without limitations. Intrinsic to the retrospective nature of this chart review, we were unable to ascertain fasting status. However, patients were generally instructed to fast before blood draw. In addition, waist circumference was not obtained during clinic visits; as such, abdominal obesity was not included as a study measure. Furthermore, not all subjects included in this study underwent radiological or histological testing to confirm NAFLD. To address this limitation, we employed stringent inclusion criteria on those without confirmatory testing. Lack of temporality further limited our study findings by preventing inferences of causality; we were only able to test associations. Use of a chart review also resulted in missing data, although we do not have any reason to believe that missed instances were differential by disease status. Additional limitations include unbalanced racial groups and a small sample size of black patients, although this is likely related to the low incidence of NAFLD in blacks. Finally, low numbers of ethnic minorities such as Asians prevented their inclusion into this study. As such, generalizing to these populations is inadvisable.
Conclusion
Our study found significant cardiometabolic derangements in children with NAFLD that differed based on ethnicity. This study also found that serum TG and non–HDL-C were significantly associated with indicators of liver dysfunction (ie, ALT, GGT) and cardiometabolic variables in children with NAFLD.
Acknowledgments
Authors’ contributions: A.P.A., A.G., and S.D. conceived the study. S.D. collected the data from electronic medical records and wrote the article. S.D and S.A. performed statistical analysis. All authors (S.D., S.A., A.G., K.F., and A.P.A.) reviewed/edited the article. All authors were involved writing the article and had final approval of the submitted and published versions. All authors take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the article.
Footnotes
Financial disclosure
None of the authors has any conflict of interest to declare.
References
- 1.Nobili V, Alkhouri N, Alisi A, et al. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr. 2015;169:170–176. [DOI] [PubMed] [Google Scholar]
- 2.Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD: summary of a single topic conference. Hepatology. 2006;44:1344–1354. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 4.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 5.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58: 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2462. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki D, Hashimoto E, Kaneda K, Tokushige K, Shiratori K. Liver failure caused by non-alcoholic steatohepatitis in an obese young male. J Gastroenterol Hepatol. 2005;20:327–329. [DOI] [PubMed] [Google Scholar]
- 8.Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54: 700–713. [DOI] [PubMed] [Google Scholar]
- 9.Alkhouri N, Hanouneh IA, Zein NN, et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int. 2016;29: 418–424. [DOI] [PubMed] [Google Scholar]
- 10.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond). 2008;32:381–387. [DOI] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. [DOI] [PubMed] [Google Scholar]
- 12.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey KE, Vuppalanchi R, Vos M, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2015; 60:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobili V, Alkhouri N, Bartuli A, et al. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665–670. [DOI] [PubMed] [Google Scholar]
- 15.Alkhouri N, Carter-Kent C, Elias M, Feldstein AE. Atherogenic dyslipidemia and cardiovascular risk in children with nonalcoholic fatty liver disease. Clin Lipidol. 2011;6:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanyal AJ. Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2: 46–53. [DOI] [PubMed] [Google Scholar]
- 18.Fon Tacer K, Rozman D. Nonalcoholic fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids. 2011;2011:783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita K, Nozaki Y, Wada K, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deboer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 24.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164–S192. [DOI] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 26.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Expert Panel on Integrated Guidelines for Cardiovascular Health, Risk Reduction in Children and Adolescents, National Heart Lung and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corey KE, Lai M, Gelrud LG, et al. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2012;10:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkhouri N, Eng K, Lopez R, Nobili V. Non-high-density lipoprotein cholesterol (non-HDL-C) levels in children with nonalcoholic fatty liver disease (NAFLD). Springerplus. 2014;3:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cali AM, Zern TL, Taksali SE, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30:3093–3098. [DOI] [PubMed] [Google Scholar]
- 31.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicityon suspected fatty liverin obese adolescents. Pediatrics. 2005;115:e561–e565. [DOI] [PubMed] [Google Scholar]
- 32.Demircioglu F, Kocyigit A, Arslan N, Cakmakci H, Hizli S, Sedat AT. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47:68–75. [DOI] [PubMed] [Google Scholar]
- 33.Pacifico L, Cantisani V, Ricci P, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63: 423–427. [DOI] [PubMed] [Google Scholar]
- 34.Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;16:198–210.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkhouri N, Tamimi TA, Yerian L, Lopez R, Zein NN, Feldstein AE. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig Dis Sci. 2010;55:2644–2650. [DOI] [PubMed] [Google Scholar]
- 37.Targher G Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1–6. [DOI] [PubMed] [Google Scholar]
- 38.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155:S7.e7–S7.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERI-TAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20: 1932–1938. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez C, Pablos-Mendez A, Palmas W, Lantigua R, Mayeux R, Berglund L. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians > or =65 years of age living in New York City. Am J Cardiol. 2002;89:178–183. [DOI] [PubMed] [Google Scholar]


