Abstract
Discovered almost simultaneously with insulin, glucagon is a pleiotropic hormone with metabolic action that goes far beyond its classical role to increase blood glucose. Albeit best known for its ability to directly act on the liver to increase de novo glucose production and to inhibit glycogen breakdown, glucagon lowers body weight by decreasing food intake and by increasing metabolic rate. Glucagon further promotes lipolysis and lipid oxidation and has positive chronotropic and inotropic effects in the heart. Interestingly, recent decades have witnessed a remarkable renaissance of glucagon’s biology with the acknowledgment that glucagon has pharmacological value beyond its classical use as rescue medication to treat severe hypoglycemia. In this article, we summarize the multifaceted nature of glucagon with a special focus on its hepatic action and discuss the pharmacological potential of either agonizing or antagonizing the glucagon receptor for health and disease.
Introduction
Seeking to develop a rapid and inexpensive method to purify insulin from pancreatic homogenates, Charles Kimball and John Murlin in 1923 identified a pancreatic factor that opposes the hypoglycemic effect of insulin (203). Due to its ability to increase blood glucose, the factor was named “the glucose agonist,” or shortly glucagon. Subsequent studies by Earl Sutherland and Christian deDuve then identified the pancreatic α-cells as the origin of glucagon (101, 396). The hyperglycemic effect of glucagon resides in its ability to directly act on the liver where it stimulates de novo glucose production and glycogen breakdown (36–38, 91, 115, 357, 382). Studies by Roger Unger then showed in 1970 that glucose inhibition of glucagon secretion is diminished in patients with type-2 diabetes, suggesting that postprandial hyperglucagonemia plays a causal role in the development of type-2 diabetes (271, 386). Several clinical studies subsequently assessed the pharmacological potential of suppressing glucagon action for the treatment of type-2 diabetes, revealing that postprandial levels of glucagon are increased in patients with type-2 diabetes (5, 6, 39, 93, 122, 192, 261, 271, 281, 282, 313, 371) and that blocking of glucagon action improves glucose handling in patients with type-2 diabetes (5, 6, 189, 193). For decades, these liver-mediated hyperglycemic effects of glucagon overshadowed that glucagon is a pleiotropic hormone with metabolic effects beyond its role to buffer against hypoglycemia. In line with this notion, glucagon stimulates insulin secretion (329), lowers body weight by decreasing food intake and by enhancing energy expenditure (23, 68, 326), stimulates lipolysis, while inhibiting lipid synthesis (4, 43, 71, 86, 286, 326), slows down gastric emptying (262, 345, 401), increases cardiac output (131, 188, 224, 241, 413), and stimulates autophagy and renal glomerular filtration (270). Recent years have witnessed a remarkable renaissance of glucagon’s multifaceted biology (as reviewed elsewhere (95, 270)) with therapeutic implications not only as a life-saving rescue medication to treat severe hypoglycemia but also when combined with glucagon-like peptide-1 (GLP-1) to treat obesity and type-2 diabetes (5, 6, 53, 66, 159, 299, 377) and nonalcoholic steatohepatitis (NASH) (27). In this article, we summarize glucagon’s role in regulating systemic energy balance with a special focus on its hepatic action and highlight its multifaceted nature that led to its use to develop drugs to treat obesity and type-2 diabetes.
Transcriptional and Translational Control of Glucagon
In rodents, glucagon is the first hormone found in the developing endocrine pancreas (135, 179, 294), with detectable levels as early as embryonic (E) day E9.5. In contrast, in the human pancreas, detection of insulin-expressing cells by week 8 of gestational age precedes the detection of glucagon-positive cells by approximately one week (175). Glucagon is derived from the cleavage of proglucagon, a 160-amino acid (AA) precursor protein originating from the preproglucagon (Gcg) gene. Proglucagon gives in a tissue-selective manner rise to several other peptides, including glicentin, glicentin-related pancreatic polypeptide (GRPP), oxyntomodulin (OXM), GLP-1 and −2 (GLP-2), and the major proglucagon fragment (MGPF) (18, 82, 265). Proglucagon processing into these smaller peptide fragments is cell-type specific. While glucagon, MPGF, and GRPP are mainly produced in the pancreatic α-cells, GLP-1, GLP-2, OXM, and glicentin are the main proglucagon cleavage products of the enteroendocrine L-cells, which are predominantly located in the large intestine. Tissue specificity in preproglucagon expression is achieved by binding of specific transcription factors (TFs) to distinct DNA control elements in the preproglucagon promoter region to initiate or inhibit preproglucagon expression (135, 179) (Figure 1). The rat preproglucagon promoter includes at least six DNA control elements positioned within a 0.3 kb region upstream of the ATG start codon of Gcg (179, 294). The control elements can be separated into a critical promoter, encompassing the TATA box and the G1 and G4 elements. These are pivotal for α-cell-specific expression of preproglucagon (127, 162, 179, 296) (Figure 1).
Figure 1.
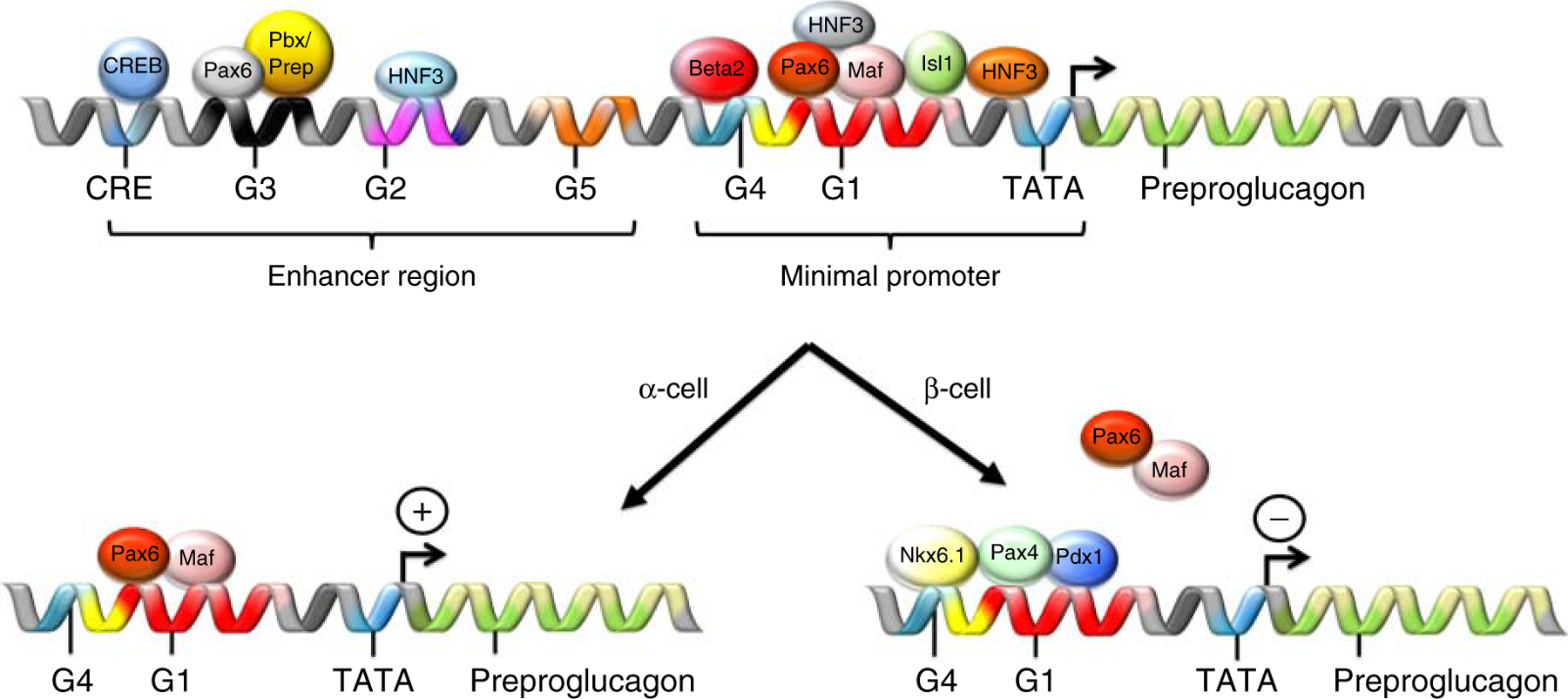
Schematic on the transcriptional regulation of preproglucagon in the pancreatic α- and β-cells. The expression of preproglucagon is regulated through interaction of home domain proteins that bind to the preproglucagon promoter region, which comprises a minimal promoter region and an enhancer region. For further explanations please see text.
The preproglucagon DNA control elements are targeted by several homeodomain proteins, which either activate or repress preproglucagon expression (179, 180, 206). In α-cells, Pax6 heterodimerizes with cMaf or MafB and induces preproglucagon expression through binding to the G1 element (117, 134). In β-cells, Pdx1, Pax4, and Nkx6.1 bind to G1 and competitively inhibit preproglucagon expression through blocking the binding of the preproglucagon activating Pax6/Maf heterodimer to the G1 element (116, 135, 316) (Figure 1). Adenoviral overexpression of Pdx1 alone, however, is not sufficient to suppress Gcg expression in α-cells (100). Pax6 stimulates preproglucagon expression through binding of Pax6 to the G3 element of the preproglucagon promoter (135). Mice devoid of Pax6 have markedly reduced levels of preproglucagon mRNA (356). In addition, Foxa1 (HNF-3α) and Foxa2 (HNF-3β) stimulate Gcg expression through binding to the G1 and G2 elements of the preproglucagon promoter (135). Mice lacking either Foxa1 or Foxa2 have a 70–90% reduction in preproglucagon mRNA levels and are hypoglycemic (85, 185).
In addition to the cell-type-specific expression of preproglucagon through direct interactions of selective TFs in the preproglucagon promotor region, preproglucagon expression is also controlled by increased levels of cAMP via the cAMP-response element (CRE) and the respective CRE-binding protein (CREB) (229), as well as the exchange protein activated by cAMP signaling pathways (Epac) (84, 127, 179, 206). Insulin inhibits preproglucagon expression in α-cells (293–295), while stimulating preproglucagon mRNA levels in the intestine (427). Finally, specific effectors of the Wnt signaling pathway have been shown to promote preproglucagon expression in the intestine but not the pancreas (275, 426, 427).
The majority of glucagon is produced in the pancreatic α-cells, with small amounts also being synthesized in a subset of neurons in the brain stem (83, 148, 178) and seemingly also in the gut (242). The latter has been subject of debate for several decades since the measurement of glucagon is challenging due to its low abundance in the circulation and cross-reactivity of glucagon detecting antibodies with oxm and glicentin, which both contain the full AA sequence of glucagon. However, more recently developed enzyme-linked immunosorbent assays (ELISAs) show reduced cross-reactivity to oxm (<5%) and glicentin (<2%) (407). Their use in combination with mass-spectrometry-based proteomics revealed that a 29 AA molecule indistinguishable from glucagon is detectable in the circulation of pancreatectomized patients and circulating levels of this molecule increase in response to oral but not intravenous administration of glucose (242). These data collectively suggest that extrahepatic glucagon secretion can, at least under conditions of α-cell dysfunction, contribute to postprandial hyperglucagonemia. Future studies need to clarify if and to which extent extrapancreatic glucagon is also produced in humans without disturbed α-cell function.
Specific prohormone convertase (PC) enzymes are responsible for tissue-specific proglucagon cleavage. In α-cells, the prohormone convertase 2 (PC2; also called PCSK2) cleaves the proglucagon protein to produce glucagon, GRPP, and MPGF. In contrast, prohormone convertase 1 (PC1; also called PCSK1)-mediated cleavage of proglucagon yields GLP-1, GLP-2, OXM, and glicentin in the brain and the intestine (12, 225, 379, 395). Consistent with the crucial role of PC2 in proglucagon cleavage, PC2 knockout (KO) mice have lower circulating glucagon levels, are hypoglycemic and display signs of α-cell hyperplasia. The latter can however be corrected by continuous intraperitoneal supplementation of glucagon (108, 402). The chaperone protein 7B2 is responsible for the maturation of PC2 as well as its enzymatic activity and thus helps to facilitate the αcell-specific processing of proglucagon to glucagon (319). While cell-specific expression of PC2 ensures that glucagon is the main proglucagon cleavage product in the α-cells, STZ-induced β-cell destruction increases PC1 expression in rat α-cells, resulting in concomitant production of Glp-1 in the islets, and plausibly in the α-cells itself (276). In line with these data, overexpression of PC1/3 in α-cells increases islet Glp-1 secretion and leads to improved glucose-stimulated insulin secretion (414). Collectively, these data suggest a potential role of the α-cells to produce Glp-1 under conditions of impaired β-cell function. The PC enzymes might thus play an important, yet underappreciated role in regulating this plasticity in islet function.
Regulation of Glucagon Secretion
Glucagon secretion is similar to insulin secretion intimately tied to circulating levels of blood glucose (318). In the β-cell, high levels of blood glucose increase the ATP over ADP ratio with the result that ATP-sensitive potassium (KATP) channels close and depolarize the cell membrane. This leads to opening of voltage-dependent Ca2+ channels (VDCC), influx of Ca2+, and exocytosis of the insulin granules (136). In the α-cells, low glucose levels lead via moderate activation of the KATP channels to a membrane potential of about ~60 mV, which entails opening of T-type Ca2+ channels, followed by depolarization of the cell membrane and opening of voltage-dependent Ca2+ and Na+ channels. The influx of Ca2+ and Na+ then triggers release of glucagon into the circulation (305). An increase in extracellular glucose increases the cytosolic ATP over ADP ratio with the result that KATP channels close and depolarize the cell membrane to level where the voltage-dependent Ca2+ and Na+ channels are inactive. The resulting lack of Ca2+ and Na+ influx then shuts down glucagon secretion (305). In support of this model, sulfonylurea-induced blockage of KATP channels mimics high glucose-mediated inhibition of glucagon secretion in isolated α-cells (137) and islets independent of changes in insulin secretion (245). In addition to glucose-dependent mechanisms, AAs and free fatty acids (FFAs) also regulate glucagon secretion. Individual intravenous administration of 20 natural AAs in dogs identified that 17 out of 20 natural AAs increase glucagon secretion (317). The branched-chain AA’s were the only ones that failed to stimulate glucagon secretion, while arginine produced the greatest stimulation (317). Consistently, high protein meals (113, 236, 248) also stimulate glucagon secretion in humans. However, hyperglycemia attenuates (309, 386) or abolishes (309) the increase in plasma glucagon following intravenous arginine or a high protein meal, suggesting AA-mediated regulation of glucagon secretion is dependent on glycemic status.
Early studies in dogs (246, 338) and humans (123) revealed that FFA inhibit glucagon secretion; however, more recent in vitro studies in isolated rodent islets suggest that palmitate increases glucagon secretion in euglycemic but not hyperglycemic conditions (28, 167). These seemingly contradictory findings may depend on the type of FFA administered, or whether exogenous or endogenous FFAs were studied. Hong et al. (167) observed that FA chain length, spatial configuration, and degree of saturation influence glucagon secretion independent of glucose concentration. These data suggest that FFA may affect glucagon secretion differently depending on the source (exogenous vs. endogenous) and the FFA characteristics. Another recent study suggests that glucagon secretion is also triggered by enhanced fatty acid oxidation since loss of CPT1a lowers glucagon secretion by decreasing the pool of ATP supply for the Na+/K+ ATPase (32).
Also, paracrine factors affect glucagon secretion. Insulin receptors are present on α-cells (74) and insulin inhibits glucagon secretion under hypoglycemic conditions (58) through modulating KATP channel activity (102) in a phosphoinositide 3 kinase-dependent manner (186). Additionally, insulin may indirectly suppress glucagon secretion through increasing translocation of α-cell GABA-A receptors (420). Inhibition of GABA receptors increases glucagon secretion (404) and GABA released from β-cells (103, 367) is postulated to mediate glucose-facilitated inhibition of glucagon secretion (404). Further, zinc (Zn2+) is co-secreted with insulin (102, 231) and inhibits glucagon secretion (102). Also, somatostatin, which is secreted from δ-cells, inhibits both insulin and glucagon secretion (107, 204, 322, 353), suggesting that glucagon is tightly controlled by pancreatic factors. However, glucose is still sufficient to suppress glucagon secretion independently of insulin (143, 310, 397), Zn2+96, GABA (245), or somatostatin (393), indicating a dominant regulatory function of glucose on glucagon action, most likely via its ability to modulate KATP channel activity. Nonetheless, GABA- or somatostatin-receptor antagonism at low glucose levels increased basal glucagon secretion, suggesting a paracrine role for GABA in the regulation of glucagon release independent of glucose levels (245). These observations collectively highlight the complex interaction of glycemia and paracrine signaling in regulating glucagon secretion. It is likely that all factors play a complementary role in inhibiting glucagon secretion, thereby ensuring compensation across multiple physiological conditions.
Also, gut hormones regulate glucagon secretion. GLP-1 (60, 153, 184) and glucose-dependent insulinotropic polypeptide (GIP) (49, 96) both indirectly inhibit glucagon secretion, presumably via their ability to stimulate the secretion of insulin and Zn2+. Importantly, while GIP stimulates insulin secretion under hyperglycemic conditions, it stimulates glucagon secretion in hypoglycemic or euglycemic states (51, 254, 288), suggesting a bi-functional role to maintain euglycemia. In line with these data, GIP inhibition of glucagon secretion seems to be mediated indirectly rather than directly, since GIP treatment of αTC1 cells does not decrease (but rather increases) glucagon secretion (49). In the isolated perfused rat pancreas, GIP affects glucagon (and insulin) secretion in a glucose-dependent manner with stimulation of insulin secretion under glucose concentrations >5.5 mM and stimulating of glucagon secretion at glucose concentrations <5.5 mM (288). These data align with studies in humans in which GIP increases postprandial glucagon levels (49, 243) and ameliorates insulin-induced hypoglycemia (52).
The pancreas is highly innervated by both the sympathetic (splanchnic) and parasympathetic (vagus) nervous system (283). Vagal stimulation increases insulin secretion (106), whereas splanchnic stimulation decreases insulin and increases glucagon secretion (26, 214, 283, 364). While central regulation of glucose homeostasis has been appreciated since the mid-1800s (298, 380), it was not until 1971 that the ventromedial hypothalamus (VMH) was implicated in regulating glucagon secretion (105) and that neuronal activation of glucagon correlates with rises in blood glucose levels (249). Further, glucagon secretion has been implicated in the cephalic phase (335, 369) of feeding. Intriguingly, this regulation is observed in healthy controls but not individuals with a kidney and pancreas transplant (335), suggesting functional pancreatic innervation is necessary to mediate cephalic-induced glucagon secretion. The relative contributions of direct and/or indirect neuronal efferents to glucagon secretion, however, remain unclear. Centrally regulated glucagon secretion could be mediated via direct sympathetic innervation on the α-cell, indirectly via the sympathetic tone and signaling through the hypothalamic-adrenal-pancreas signaling axis, and/or potential indirect parasympathetic signaling (283, 363). Altogether, glucagon secretion is a complex process regulated by multiple interactions between glycemic, paracrine, endocrine, and neural factors.
α-Cell Regulation of β-Cell Function
While PCSK2 is under nonpathological conditions the predominant PC in the α-cells, PCSK1/3 expression/activity increases in α-cells under pregnancy and under conditions of metabolic stress such as insulin resistance and diabetes (198, 276, 372, 415). Increased PCSK1/3 expression with concomitant GLP-1 production has also been demonstrated in α-cell lines and in isolated islets that have been cultured at conditions of high glucose (251, 412). The production of GLP-1 in the α-cells has been linked to the action of interleukin 6 (IL-6). The IL-6 receptor is highly expressed in murine α-cells (88) and administration of IL-6 increases expression of preproglucagon and of PCSK1/3 and accelerates the production of GLP-1 in the intestinal L-cells and the α-cells (89). Consistent with these data, adenoviral overexpression of PCSK1/3 in the α-cells enhances GLP-1 production and improves glucose-stimulation of insulin secretion and islet survival in mice (414). Collectively, there is growing evidence indicating that α-cells produce GLP-1 under conditions of higher β-cell demand to improve islet function in a paracrine fashion (47, 234, 264, 374, 391). Notably, intraislet paracrine signaling also plays a role in β-cell function under nonpathological conditions. In line with this notion, glucagon stimulation of insulin secretion was already described by Ellis Samols and Vincent Marks in 1965 (329) and was later confirmed in numerous other studies (263, 269, 270). β-Cells with contact to α-cells also secrete more insulin when challenged with glucose relative to β-cells without α-cell contact (416). Glucagon amplifies glucose-stimulated insulin secretion through direct action (169) and the receptors for glucagon and insulin are expressed on both α- and β-cells (190, 196). Glucagon was also recently shown to cross-react with GLP-1R in the β-cells and interaction of glucagon with GLP-1R has been demonstrated to enhance insulin secretion (360). Other factors potentially playing a paracrine role in α-cell regulation of β-cell function include glutamate and acetylcholine (263).
Glucagon Receptor Signaling
Once glucagon is secreted into the circulation, it elicits its function intracellularly by binding to its cell surface receptor, a seven-transmembrane protein belonging to the large superfamily of G protein-coupled receptors (GPCRs) (69). The glucagon receptor (Gcgr) belongs to the class B family of GPCRs, which are peptide hormone receptors of the secretin family that are widely used drug targets for many human diseases, including diabetes, cancer, neurodegeneration, cardiovascular diseases, and others (285). Gcgr is mainly expressed in the liver. Only traces of Gcgr are found outside the liver such as in the kidney, adipose tissue, pancreas, spleen, lymphoblasts, brain, the gastrointestinal tract, and the adrenal gland (361). In the liver, Gcgr expression is zonated and occurs only at the periportal area, where also the metabolic effects of glucagon occur (213).
In liver cells, the Gcgr as a dimer induces the activation of two signaling cascades mediated by two classes of G proteins, a cAMP stimulatory G protein (Gs) and a Gq protein that signals via Ca2+ through the inositol 1,4,5-trisphosphate (IP3) pathway (174, 260). Production of IP3 is mediated by Gq-dependent activation of phospholipase C (PLC) and concomitant Ca2+ release from the endoplasmic reticulum (ER) to the cytosol and into the mitochondria. Increase of cellular calcium activates downstream signaling cascades and contributes to enhanced mitochondrial respiration observed under elevated glucagon levels (15, 34, 94). Interestingly, recent data highlight the role of the mitochondrial IP3R1 receptor in Ca2+ dependent activation of mitochondrial β-oxidation (422). The interaction between the mitochondria and the ER has received a lot of attention due to membrane contact site formation and their function in calcium flux and signaling (315). However, the effects of glucagon on this cellular interaction have not been elucidated and might represent an underappreciated site of glucagon action.
Glucagon signaling via Gs represents the canonical Gcgr signaling pathway. Here, glucagon-induced Gs activation leads to the dissociation of the Gsα subunit from the G protein α/β/γ heterotrimer and its subsequent interaction with adenylate cyclase. Activated adenylate cyclase enhances its production of cAMP and consequently activates protein kinase A (PKA), enhances signaling via Rap guanine nucleotide exchange factor 3 (RAPGEF3, also known as Epac1) and the cAMP response element-binding protein (Creb)-regulated transcription coactivator 2 (Crtc2, also called Torc2) (144, 173). Stimulated PKA translocates to the nucleus, where it initiates the nuclear localization and phosphorylation of Creb at the serine-133 residue (Ser133) (174, 208). Once phosphorylated, Creb binds to the CRE elements located in the promoter region of downstream target genes and induces their transcription. This signaling cascade causes the expression of gluconeogenic and glycogenolytic genes, such as glucose-6-phosphatase (G6Pase), phosphoenolpyruvate kinase (Pepck), Pc, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc-1α) (3, 163, 208, 306, 418). The activation of the transcriptional co-activator Crtc2 is regulated by fasting-feeding cycles and changes in ATP levels. Underfeeding conditions, when ATP is high, salt-inducible kinase 2 (Sik2) and AMP-activated protein kinase (AMPK) phosphorylate Crtc2 on Ser171 and Ser307, respectively, causing its localization in the cytosol (3, 208, 381). Upon fasting, Sik2 is inhibited causing Crtc2 dephosphorylation by calcineurin in response to elevated cAMP and calcium levels, leading to its nuclear translocation (3, 30). In the nucleus, Crtc2 binds along with Creb to the CRE element in the promoter region of target genes and thereby, for example, enhances the expression of gluconeogenic and glycogenolytic gene programs in the liver (3, 208, 323).
Glucagon Receptor Trafficking
GPCR signaling is regulated by endosomal membrane trafficking, where rapid internalization of the ligand-receptor complex contributes to signal termination and receptor desensitization (150). GPCRs are mainly internalized via clathrin-mediated endocytosis involving the β-arrestin family (14, 266). Here, arrestins are recruited to the activated GPCR, upon phosphorylation via the G protein-coupled receptor kinase (GRK) family, which results in uncoupling the GPCR from its corresponding G protein (244). Arrestins then connect the GPCR to the clathrin coat due to its dual binding function and facilitate internalization (244, 297). Importantly, GPCRs differ substantially in their way in which they engage with the GRK/arrestin/clathrin machinery. This helps to provide GPCR diversity in signaling, as only limited amounts of G protein pathways exist. In fact, class B receptors have been shown to recruit both β-arrestin-1 and 2 equally well and co-internalize with them, whereas class A receptors (e.g. β2-adrenergic receptors, β2AR) preferentially recruit arrestin-2 and co-localize only transiently on the plasma membrane with clathrin and arrestin (150). An alternative way of internalization involves caveolin-mediated endocytosis, where mainly GPCRs with primary signaling pathways via Gq are internalized (50, 279). In fact, GPCRs, G proteins, as well as arrestins, have been shown to sequester in caveolae, mediated through direct interaction of Caveolin 1 (Cav1) with Gq leading to internalization and initiation of Ca2+ signaling (50, 279, 337). These data suggest that Gq signaling is mainly mediated via caveolin-mediated endocytosis.
The Gcgr, a prototypical class B receptor, has been shown selectively interact with β-arrestin-2, and not β-arrestin-1, as only the knock-down of β-arrestin-2 lead to an impaired glucose tolerance as consequence to enhanced GPCR cell surface signaling (433). However, other in vitro overexpression studies reported the importance of both β-arrestins for Gcgr trafficking, emphasizing the differences between endogenous and exogenously overexpressed receptors (211) and the requirement for research in appropriate cell types. Gcgr has been shown to be internalized into endosomal fractions within 30 min after glucagon stimulation in vitro (Figure 1) (33, 212) and in vivo (255), causing a relatively mild decrease in membrane-localized Gcgr (8, 255, 387). Short-term activation leads to its phosphorylation through GRKs, both at the cell surface and after internalization into endosomes (255), highlighting the importance of phosphorylation for internalization and a potential endosomal contribution to signaling. In fact, endo-lysosomal transfer of Gαs subunit but not β-arrestins have been observed upon glucagon stimulation, together with increased adenylate cyclase activity, suggesting sustained Gcgr signaling at the level of endosomes (8, 255, 390). This can be achieved through the different binding properties of β-arrestins in class B versus class A GPCRs. While β-arrestins bind to the common binding pocket in the transmembrane core of the receptor in class A GPCRs, they bind class B GPCRs in the C-terminal tail, leaving the binding site for G proteins free for interactions in endosomes. In fact, a second wave of G protein-induced and β-arrestin-mediated signalling from endosomal membranes has been reported (87, 210, 378). Why β-arrestins have not been shown to traffic to endosomes upon glucagon treatment is puzzling in this regard, however, limitation of antibody sensitivity and resolution of the subcellular fractionation could have influenced this study (255).
The fate of the GPCRs is decided at the level of the endosomes, which determine their re-usage or disposal. Internalized GPCRs can be either recycled back to the plasma membrane via the recycling endosomes for re-sensitization and continued signaling or can be degraded through the lysosomal system for a transient response (Figure 2) (150). These fates are determined by multiple mechanisms in the endosomal system. Targeting GPCRs to recycling usually requires sequence-directed mechanisms involving the cis-sorting sequence in their C-terminal tails (151, 375). These are recognized by multiple sorting complexes in the endosomal network, including the retromer and WASH (Wiskott-Aldrich syndrome protein and SCAR homolog) complexes (62, 252), highlighting the complexity of the recycling system. Interestingly, the recycling kinetics can be altered depending on the extracellular environment, suggesting sensitivity in the sorting machinery to external nutritional cues (151, 405).
Figure 2.
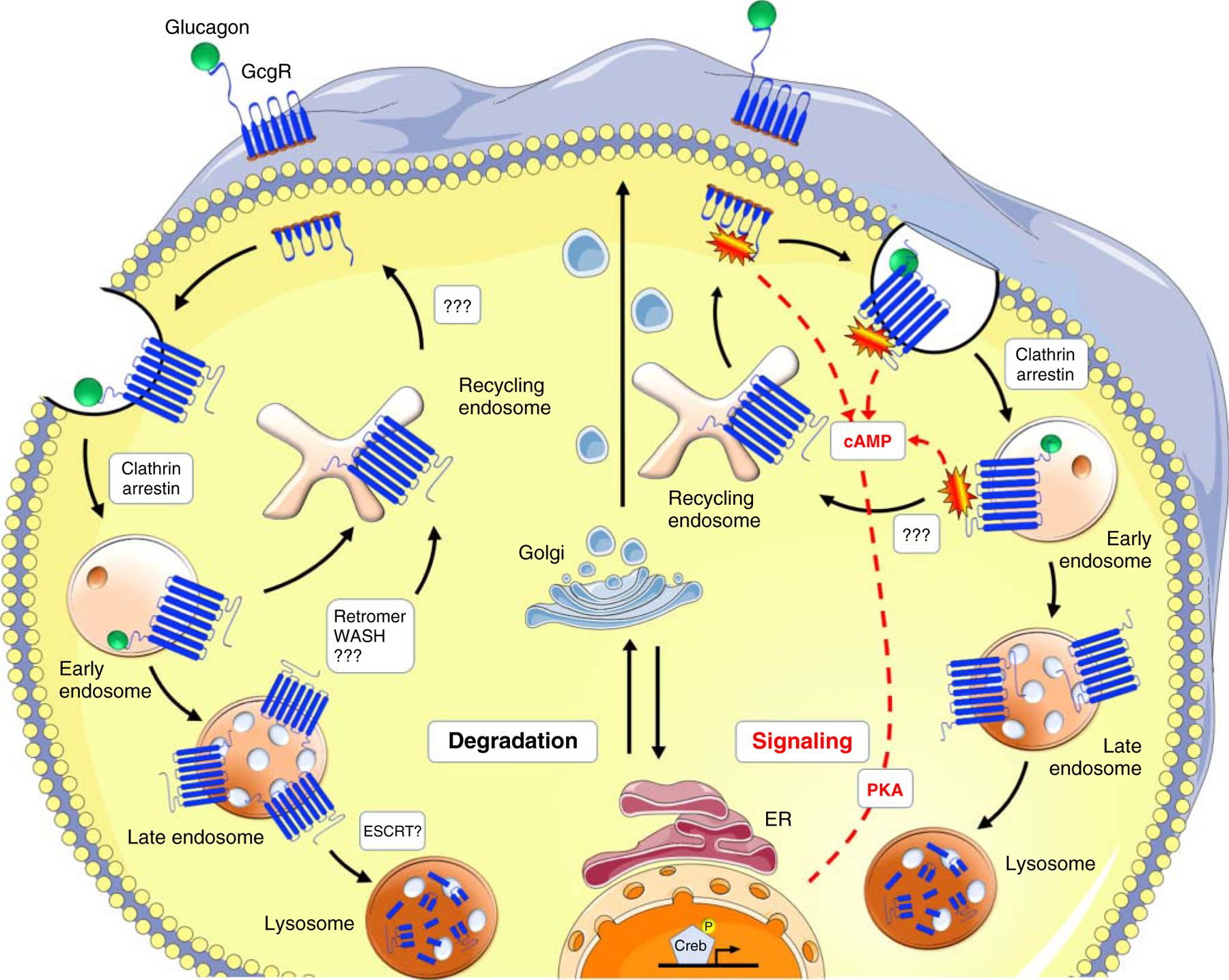
Proposed model of Gcgr trafficking and signaling. Stimulations with glucagon induce glucagon receptor recruitment into clathrin-coated vesicles on the plasma membrane through the interaction of β-arrestins with the cytoplasmic tail of the receptor and subsequent interaction with the clathrin coat. Short-term stimulations with glucagon increase glucagon receptor presence in early endosomes and enhanced signaling, followed by receptor recycling. Upon long-term treatments, reoccurrence of the receptor on the membrane is reduced, and its lysosomal degradation increases. Regulators of these sorting mechanisms on early and late endosomes, such as retromer and WASH complex for recycling and ESCRTs for degradation have been shown to be involved in other GPCR trafficking, however, the knowledge on Gcgr is still very limited and represented by question marks.
The fate of the Gcgr is dependent upon the duration of glucagon stimulation. Acute glucagon injections have no effect on Gcgr protein levels, leading to Gcgr internalization and reoccurrence after 2 h (8), suggesting activated recycling (Figure 2, right side). As for other GPCRs, this is dependent on the C-terminus of Gcgr, as its truncation causes reduced internalization, phosphorylation of the receptor, and a complete block on recycling (33, 212), resulting in presumably enhanced Gcgr localization to endosomes. Although the concept of sustained endosomal signaling of Gcgr has been proposed (8, 255, 390), the resulting consequences on signaling under these conditions have not been investigated. This would be of interest also in comparison to the fact that GPCRs can couple to G proteins even without inducing G protein signaling (150), which has also been shown for the Gcgr, as its antagonist (des-His1-[Glu9]glucagon) also induces detectable internalization of Gcgr (255).
GPCR internalization can be regulated by additional posttranslational modifications, such as palmitoylation and ubiquitination (150). Ubiquitination is a strong signal for receptor downregulation through the endo-lysosomal system (160). Lysosomal targeting is especially important for chronically activated receptors to downregulate their activity. In addition, it is also thought to play a role in drug tachyphylaxis or tolerance (411), which is particularly relevant for class B receptors that are used as drug targets. Indeed, the use of the pharmacological inhibitor, the receptor activity modifying protein (RAMP2), has been shown to co-localize with Gcgr and induces a reduction in cell surface-bound Gcgr (46). Whether enhanced degradation is achieved under these conditions need to be elucidated.
Receptor downregulation involves the trafficking through Rab7 positive late endosomes, multivesicular body formation, and concomitantly fusion with lysosomes (62, 128, 399). Membrane receptors designed for degradation are ubiquitinated at lysine residues that are recognized by the endosomal sorting complex required for transport (ESCRT) machinery, which binds ubiquitinated cargo and sequester those into intraluminal vesicles in multivesicular bodies/late endosomes, leading to receptor downregulation (160, 362). Activation of this process has been shown to be beneficial for degrading toll-like receptor 4 (TLR4) thus reducing its signaling in nonalcoholic fatty liver disease (NAFLD) to NASH progression (432). Although investigated for other GPCRs, such as chemokine receptor CXCR4 and β2AR (194, 392), this cellular mechanism has not been shown for Gcgr. In fact, some GPCRs are not ubiquitinated (80), however, arrestins are known to recruit E3 ligases to GPCRs (130, 342, 343), thereby inducing ubiquitination and potentially thereby marking the bound GPCR for downregulation. As for the Gcgr, prolonged and chronicle treatment with glucagon results in a net decrease of glucagon binding efficiency and colocalization with lysosomes (Figure 2, left side) (7, 212), suggesting receptor downregulation under these conditions. Whether chronic glucagon treatment enhances arrestin-2 ubiquitination and thus Gcgr trafficking to lysosomes has not been investigated but would be an interesting concept that could be exploited to downregulate Gcgr in conditions of type-2 diabetes, where the overactivation of glucagon signaling is contributing to enhanced glucose output.
In fact, many of the studies on GPCRs have been performed on other members of the family, hampering our knowledge of Gcgr trafficking and its connection to signaling. Most of the studies on Gcgr were performed 20–30 years ago, where the detection techniques were less developed and subcellular fractionation or overexpression studies in cell lines, which do not endogenously express the Gcgr, were used. Given the fact that a complex trafficking machinery is involved in GPCR sorting, tissues with endogenous levels of Gcgr might engage other regulatory trafficking proteins than cell lines with an overexpression of nonendogenous Gcgr. In addition, studies with iodinated glucagon might have given misleading results, as iodoglucagons have been reported to alter adenylate cyclase activity in vitro and exhibit hyperglycemia in vivo (73, 235). Thus, further studies are needed to shed light into the regulation of Gcgr trafficking and signaling under physiological conditions and to connect this to its function as a fasting-induced receptor.
Glucagon Effects on Food Intake
Albeit its classical function to increase blood glucose under conditions of hypoglycemia, glucagon also lowers food intake and body weight in rodents (23, 68, 326) and humans (118, 289, 327, 333, 359) (Figure 3). Glucagon’s anorexigenic effect is driven by the liver-vagus-hypothalamus axis and is achieved via a decrease in meal size without affecting meal frequency (119, 227), taste aversion, or postprandial behavior (120). Consistent with its role as a meal terminating factor, circulating levels of glucagon rise during food intake (70, 221, 385) and preprandial inhibition of glucagon signaling (222, 227), or antibody-based blocking of glucagon action (222), increase meal size, while stimulation of glucagon signaling during a meal terminates food intake (118). Glucagon’s role in satiety is substantiated by increases in glucagon secretion following ingestion of high carbohydrate, high protein, and high-fat meals (70, 221), while antagonism of endogenous glucagon via hepatic-infused glucagon antibodies increases spontaneous meal size (119). Early studies observed that glucagon reduces meal size in humans (333), rodents (119), and sheep (215) independent of meal frequency (119, 227). Moreover, glucagon infused directly into the hepatic portal vein of rats reduces food intake and this effect is lost in hepatic-vagotomized rats (119), suggesting liver glucagon signaling mediates satiety via vagal afferents to the brain.
Figure 3.
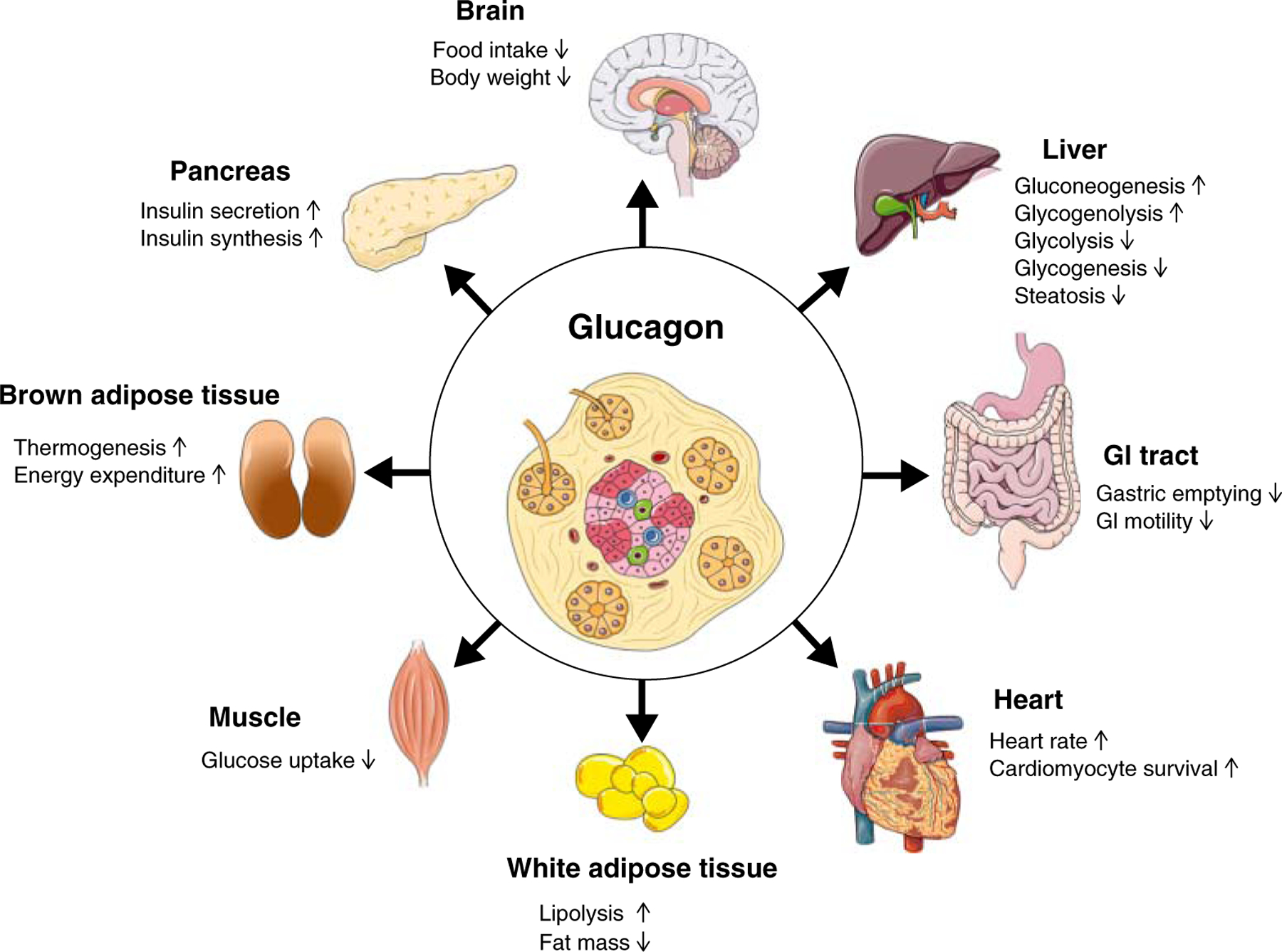
Schematic on the direct and indirect metabolic effects of glucagon.
While the liver-hypothalamus axis regulates this process, direct neuronal glucagon signaling may also play a role. Acute intracerebroventricular (ICV) administration of glucagon decreases food intake acutely (1–4 h) in male mice in a dose-dependent manner and this effect was lost >6 h postadministration (307). Inhibition of PKA, the main downstream mediator of glucagon signaling, blunted glucagon’s hypophagic effects, by decreasing Ca2+-calmodulin-dependent protein kinase β (CaMKKβ) levels and AMPK activity (307). Further, ICV glucagon decreased expression of Agouti-related protein (AgRP) without changes in POMC, NPY, and CART; suggesting glucagon may decrease food intake via modulation of AgRP levels (307).
Genetically modified mice deficient for whole body Gcgr (Gcgr−/−) also support glucagon’s regulation of food intake. Gcgr−/− mice are resistant to diet-induced obesity (DIO), most likely due to a decrease in food intake compared to control animals (56). Interestingly, mice deficient for hepatic Gcgr (Gcgrliver) are not resistant to DIO. Chronic Gcgr agonism via the long-acting Gcgr agonist, IUB288, in DIO mice reduces food intake (201, 274) in addition to increasing energy expenditure (201). However, the same glucagon-receptor agonism stimulates a similar suppression of food intake in Gcgrliver mice as compared to littermate controls (201), further supporting hepatocyte-independent regulation and potentially implicating central Gcgr signaling in regulating HFD-food intake. Interestingly, chronic Gcgr agonism in lean male mice stimulates hyperphagia and a defense of their body weight (145), most likely to offset the increase in energy expenditure. Together these data suggest differential effects of glucagon on food intake depending on energy balance status.
While there is convincing data to support that central and liver-specific glucagon signaling both act to regulate food intake, the respective contributions of each pathway remain unclear. It is likely that both pathways work in concert with each other or selective pathways may dominate in a specific nutrient milieu. Regardless, further studies are needed to tease apart the contributions of central glucagon versus liver-mediated reductions in food intake.
CNS Regulation of Glucagon Action
While liver-regulated glucose homeostasis is well established (344), the hypothalamus likewise comprises a glucose-sensing network that is sensitive to hormonal signaling and known to modify peripheral glucose homeostasis (320, 347). Shimazu et al. (346) were the first to uncover that electrical stimulation, specifically in the VMH, resulted in an increase in blood glucose levels, accompanied by a decrease in liver glycogen. It is now appreciated that within the VMH there are both glucose excitatory (GE) neurons, which control peripheral glucose utilization, and glucose inhibitory (GI) neurons, which control hepatic glucose production (347). Insulin and glucagon are well characterized in targeting peripheral tissues to mediate glucose homeostasis. However, there is a growing body of evidence that insulin is an important neuroregulatory peptide, involved in energy balance and glucose homeostasis (76). Despite greater appreciation for glucagon in energy balance beyond glucose metabolism, little attention has been given to its central actions.
Historically considered a diabetogenic hormone, glucagon signaling increases blood glucose levels via PKA-dependent signaling in the liver. However, this glycemic effect is transient, despite continuous intravenous glucagon infusion and lack of insulin (29, 92). This suggests an insulin-independent compensatory mechanism may be triggered to restore glucose homeostasis. A possible explanation for this effect may involve a negative feedback loop involving glucagon. Glucagon crosses the blood-brain barrier (13) and glucagon immunoreactivity has been identified in the hypothalamus (332), suggesting a potential physiological role for central glucagon signaling. Consistently, administration of glucagon to the mediobasal hypothalamus (MBH) decreases hepatic glucose production in clamped rats and improves glucose tolerance in nonclamped rats mediated via PKA-dependent signaling and hepatic vagal efferents (256). Similarly, central glucagon infusion also decreases hepatic glucose production in control, but not Gcgr−/− mice (256). While these data support that central glucagon signaling is sufficient to regulate hepatic glucose production, the physiological role of endogenous central glucagon signaling remains unclear.
Intriguingly, a high protein meal (65.4% protein) improves glucose tolerance, despite increasing glucagon signaling in the dorsal vagal complex (DVC) of the brainstem (223). DVC administration of either a Gcgr antagonist or a glucagon mAb blunts high protein diet-induced improvements in glucose tolerance, highlighting a role for endogenous central glucagon signaling in the regulation of glucose homeostasis. Interestingly, inhibition of Gcgr signaling on a normal protein diet (21.5% protein) did not alter glucose production, suggesting that DVC glucagon signaling may be important in specific nutrient states (e.g. high protein consumption).
Hormone resistance is common in rodents (67, 97) and humans with obesity (90, 425). Consistently, acute (3d) and chronic (3w) HFD-feeding resulted in Gcgr resistance in the MBH (256), indicating that hypothalamic Gcgr resistance may play a role in diet-induced hyperglycemia. Data support that the brain is sensitive to glucagon; however, most studies to date involve central glucagon administration, which may not reflect endogenous glucagon action. Further studies utilizing neuronal Gcgr knockout or central Gcgr-antagonist models will be essential for dissecting the endogenous role of central glucagon signaling. The focus of these studies will likely involve both the hypothalamus and the brainstem and the respective contributions of direct neuronal glucagon signaling versus indirect liver-brain communication. In addition, further studies are warranted to uncover whether central glucagon signaling mediates other facets of energy balance beyond peripheral glucose homeostasis.
Glucagon Effects on Energy Expenditure
Glucagon was first shown to increase energy expenditure in rats in 1960 (65) and has since then been confirmed in several human studies (205) (Figure 3). The energy expenditure effect in patients is rapid, with oxygen consumption elevated within minutes after intravenous glucagon infusion (366). In the fed state, glucagon’s ability to stimulate energy expenditure is less effective compared to a robust increase by 100–200 kcal per day when administered in the fasted state (205). The magnitude of glucagon’s energy expenditure effect in humans is similar to that of the β3-adrenergic receptor agonist mirabegron, which primarily targets the brown adipose tissue (BAT) (+203 kcal/day) (63), and to the energy expenditure increase detected during acute cold exposure (+193 kcal/day) (325).
Early studies investigating how glucagon leads to rapid increases in energy expenditure pointed to the BAT as the main responsible organ; this was based on studies showing that glucagon increases oxygen consumption in isolated BAT cells and BAT tissue explants from rats (181, 216), albeit at supraphysiological doses. In different animal models, glucagon elevates the temperature over interscapular BAT and augments blood flow into BAT (54, 157, 421). Moreover, in cold-adapted mice, which have more BAT glucagon’s effect on energy expenditure is potentiated (77).
Substantial literature indicating that glucagon affects energy expenditure via BAT-dependent (181, 216) and - independent mechanisms. In animals with little (adult dog) or no functional BAT (pig) glucagon is still able to acutely increase energy expenditure (172, 403). Moreover, while BAT thermogenesis relies predominantly on the uncoupling protein 1 (UCP1), glucagon injection in mice lacking UCP1 increases energy expenditure to similar extent as in wild-type controls (17). In addition, mice with selective deletion of the Gcgr in BAT also increase their energy expenditure normally following glucagon injection (17). Collectively, this suggests that in vivo neither BAT per se nor Gcgr signaling in BAT are required for the acute energy expenditure effect of glucagon in mice. Fittingly, in humans, glucagon was recently shown to increase energy expenditure without increasing BAT activity in subjects specifically screened for functional BAT (325). An alternative, BAT-independent explanation for how glucagon mediates increased energy expenditure could comprise the engagement of multiple metabolic (predominantly catabolic) pathways. For example, liver oxygen consumption has been shown to increase by up to 20% during glucagon infusion in rats (57).
In addition to its acute effects, glucagon can also elevate energy expenditure chronically. Notably, glucagon fails to promote body weight loss in mice lacking liver glucagon receptor (Gcgrliver) (201), suggesting that liver Gcgr-signaling is necessary for the energetic response to glucagon. In the liver, glucagon stimulates the synthesis and release of fibroblast growth factor 21 (Fgf21) (64, 145), a circulating peptide hormone that regulates energy homeostasis (99, 129) via centrally mediated mechanisms (81, 284). Chronic glucagon treatment fails to augment energy expenditure and to prevent HFD-induced obesity in Fgf21 null mice, suggesting that glucagons effect on energy expenditure requires Ffg21 signaling (145). Similarly, in obese liver-specific Fgf21-deficient mice, glucagon-mediated body weight loss is blunted (201), suggesting that specifically hepatic Fgf21 secretion contributes to the chronic effects of glucagon on energy expenditure. In addition to Fgf21, prolonged glucagon treatment increases circulating levels of bile acids in DIO mice (201). Bile acids are ligands for the farnesoid X receptor (FXR) (201) and both, bile acids and FXR, regulate energy expenditure (370). In liver-specific FXR knockout mice, the body weight lowering effects of glucagon is blunted, despite normal Fgf21 secretion (201); indicating that in addition to Fgf21, a hepatic bile acid—FXR axis contributes to the chronic effects of glucagon on energy expenditure.
It remains possible, that other factors that have been shown to be regulated by glucagon, like epinephrine, cortisol, and thyroid hormone (205) may play a role in glucagon’s prolonged thermogenic effect. Also, glucagon can cross the blood-brain barrier (13) and the Gcgr is expressed in hypothalamus and brainstem regions, two sites known to modulate energy metabolism (168, 332). However, chronic ICV studies assessing the role of glucagon on energy expenditure are still missing.
Glucagon Action in the Heart
Traces of the Gcgr are expressed in the heart (1). Whole-body knockout of the Gcgr results in a lower intrinsic heart rate (268), whereas glucagon administration increases heart rate (chronotropic effect), contraction force (inotropic effect), and stroke volume in animals and humans (241, 287) (Figure 3). Glucagon fails to increase heart rate in Gcgr null mice (268). Mechanistically, glucagon triggers adenylyl cyclase activation through Gs protein-coupled signaling. Glucagon-mediated adenylyl cyclase activation occurs independently of the β-adrenergic system and its activation leads to an increase in cAMP levels, which engage the cyclic nucleotide-gated channels to elevate calcium concentrations in cardiac conduction tissue like the sinoatrial (SA) node (291). These effects are transient, lasting only several minutes rather than hours (287), because adenylyl cyclase quickly becomes uncoupled from the Gcgr (424), cAMP is rapidly broken down by phosphodiesterase (183), and receptor internalization reduces the number of available Gcgrs (164).
In the context of cardiac health following injury, like myocardial infarction, the role of augmenting versus diminishing cardiac glucagon signaling has been investigated in several preclinical studies. In mice, glucagon treatment impairs survival after myocardial infarction, whereas cardiac-specific deletion of the Gcgr markedly improves survival rates compared to wild-type mice (1). Similarly, treatment with monoclonal Gcgr antagonistic antibody ameliorates onset and progression of heart failure (114, 187, 341). Whether Gcgr antagonism improves heart health in humans has not been tested.
Glucagon Regulation of Hepatic Metabolism
The fundamental aspect of liver glucagon action is its function on increasing hepatic glucose output (308). Initially recognized as a hormonal factor that counter-regulates the hypoglycemic effects of insulin, glucagon was later identified to increase hepatic glucose production through stimulation of glycogenolysis and gluconeogenesis, while at the same time inhibiting glycogenesis and glycolysis (308). Glucagon’s role on hepatic glucose production is most prominent via intra-portal injection and is absent in hepatectomized rats (25). Consistent with this observation, glucagon has been identified to be secreted into the portal vein from the pancreas and reaches the liver at a much higher concentration than the in the systemic circulation (365), indicating an acute and preferential effect on the liver.
Regulation of glycogenolysis
Upon short-term starvation, glucagon induces rapid mobilization of hepatic glycogen stores leading to an immediate increase in hepatic glucose output (176). This is achieved via glucagon signaling through PKA (144) and activation of glycogen phosphorylase kinase (GPK), which leads to phosphorylation and activation of glycogen phosphorylase (GP) initiating glycogen breakdown (Figure 4) (144). Besides this, glucagon has also been shown to reduce acetylation of hepatic GP thereby enhancing its activity (430). In addition to stimulating glycogen breakdown, glucagon also inhibits the activity of glycogen synthase, causing an overall net increase in glycogenolysis (292), thereby channeling glucose into the plasma. Glucagon thus becomes an important counter regulatory hormone during conditions of hypoglycemia as a direct access to release hepatic glycogen (352). Thus, the glucose releasing effect of glucagon is directly proportional to glycogen levels, as seen in fasted animals or patients with liver cirrhosis (61).
Figure 4.
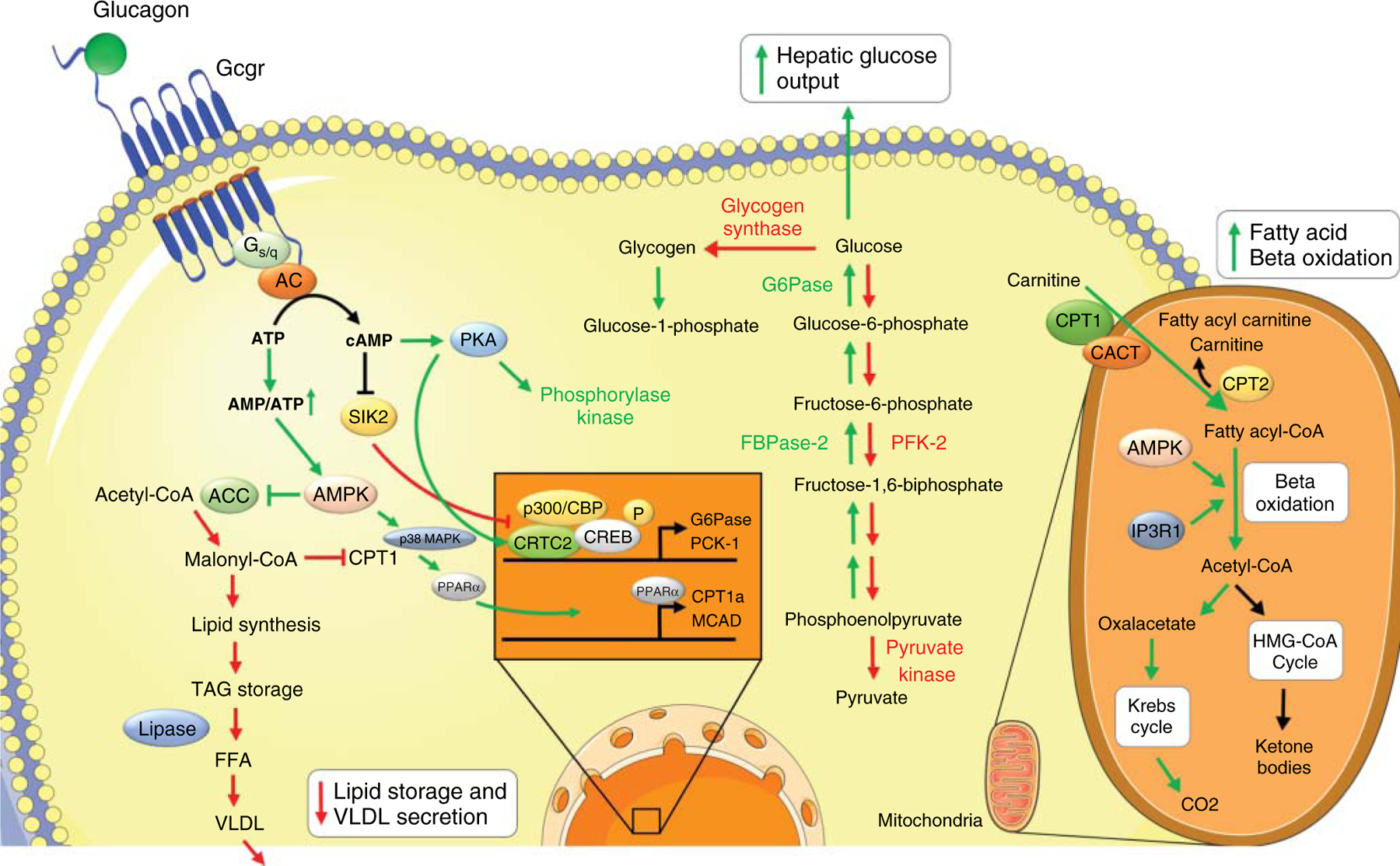
Glucagon effects on hepatic glucose and lipid metabolism. Activation of glucagon receptor by glucagon in hepatocyte stimulates adenylate cyclase-/cAMP-/PKA-dependent phosphorylation of Creb and dephosphorylation/nuclear translocation of Crtc2. p-Creb induces transcription of gluconeogenic genes G6Pase and Pck1. PKA activates phosphorylase synthase and inhibits glycogen synthase, thus stimulating glycogen breakdown. In addition, PKA activates FBPase2 and inhibits PFK-1 and pyruvate kinase, thereby enhancing gluconeogenesis and inhibiting glycolysis. By AC dependent inhibition of SIK2, glucagon stimulates activation of p300, which facilitates transcription of gluconeogenic genes. p-CREB induces transcription of Ppar-α that enhances transcription of β-oxidation genes Cpt1 and Mcad. ATP to cAMP conversion leads to enhanced AMP/ATP ratio leading to AMPK activation and inhibition of ACC. This results in inhibiting the conversion of acetyl-CoA to malonyl-CoA by ACC and subsequent decreases the lipid synthesis pathway. As a consequence malonyl-CoA formation is reduced which induces an accumulation of Cpt1. Cpt1 enhances fatty acyl-CoA transport into mitochondria and induces β-oxidation. In addition, glucagon stimulates AMPK and mitochondrial IP3R1 further activating β-oxidation. Acetyl-CoA subsequently enters Krebs cycle for ketone body formation during prolonged starvation. Abbreviations, AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; Creb, cAMP-responsive element-binding protein; G6Pase, glucose 6 phosphatase; Pck1, phosphoenol pyruvate carboxykinase 1; FBPase 2, fructose 2,6-bisphosphatase; PFK-1, phospho-fructokinase 1; Crtc2, Creb-regulated transcription coactivator 2; SIK2, salt-inducible kinase 2; Ppar-α, peroxisome proliferator-activated receptor alpha; Cpt1, carnitine palmitoyltransferase 1; Mcad, medium-chain acyl-CoA dehydrogenase; ATP, adenosine triphosphate; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; IP3R1, inositol triphosphate receptor 1; FFA, free fatty acid; TAG, tri-acyl glycerol; VLDL, very low-density-lipoprotein; LCAD, long-chain acyl-CoA dehydrogenase.
Regulation of gluconeogenesis
After depletion of glycogen stores upon longer starvation, glucagon activates gluconeogenesis to increase hepatic glucose output and to maintain blood glucose levels (292). This is achieved by allosterically modulating the activity of several enzymes shifting the metabolic flux from glycolysis to gluconeogenesis (176). Glucagon binding to its receptor induces the production of cAMP causing PKA activation. Then, PKA phosphorylates and inhibits the activity of phospho-fructokinase 2 (PFK-2), a bifunctional enzyme acting on fructose 2,6-bisphosphatase (FBPase 2) and 6-phosphofructo-2 kinase (409). Inhibition of PFK-2 activates FBPase 2 and inhibits 6-phospho fructo-2-kinase, causing a rapid reduction in the secondary metabolite fructose-2,6-bisphosphate [F(2,6)P2], shifting the flux toward gluconeogenesis (409). PKA also phosphorylates pyruvate kinase causing a reduction in its activity. This enhances fructose-1,6-bisphosphate, which lowers pyruvate levels leading again to reduced glycolysis and redirection of substrate toward gluconeogenesis (Figure 4) (176, 409).
Activation of PKA strongly depends on maintaining high cAMP levels. Thus, controlling cAMP amounts is crucial for downstream signaling. Interestingly, a recent paper has shown another level of regulation of cAMP-PKA signaling, through controlling phosphodiesterase 4B (Pde4b) transcription (431). Pde4b is responsible for the degradation of cAMP thereby terminating signaling (182). Glucagon-stimulated nuclear factor-kappa B2 (NF-κb2) (p52) binding to PDE4B promotor inhibits its transcription, thus strengthening cAMP action (431).
Besides direct modulation of enzyme activity by phosphorylation, transcriptional regulation by glucagon also enhances gluconeogenesis. Glucagon signaling increases phosphorylation of Creb at serine residue 133 and dephosphorylation and nuclear translocation of its co-activator, Creb-regulated transcription coactivator 2 (Crtc2) (see above) (237). Phosphorylated Creb binds to DNA and promotes expression of its target gluconeogenic genes G6Pase, Pepck, Pgc1α, and hepatocyte nuclear factor 4 (Hnf4), thereby enhancing glucose output (Figure 4).
In addition to the transcriptional regulation, glucagon has been shown to facilitate gluconeogenic gene transcription by regulating histone modifications that alter chromatin environment for gene induction. Other than Crtc2, Creb is also associated with coactivators—histone acyl transferase p300 and Creb-binding protein (Cbp) (237). Glucagon dephosphorylates p300 at Ser89, thereby increasing its activity (3). This is achieved by adenylyl cyclase-mediated inhibition of salt-inducible kinase 2 (Sik2) (237). p300 in turn, acetylates Crtc2 at Lys628, enhancing the transcription of G6Pase and Pepck (154, 237). Importantly, Crtc2 interaction with p300/Cbp is essential for their recruitment to Creb target genes and subsequent transcription (Figure 4) (312). Cbp and p300 are known to acetylate H3K27 lysine residue at enhancers, facilitating a chromatin environment more accessible to TF binding (48) and an enhanced transcript elongation rate of RNA polymerase 2 (354). Furthermore, p300/Cbp also directly acetylate lysine residues in TFs such as p53 (314). Substantiating this, p53 has been shown to promote gluconeogenic gene expression (133). Aside from p300, glucagon stimulation also recruits other histone acyltransferases. Glucagon induced nuclear translocation of Crtc2 has been shown to recruit lysine acetyltransferase 2B (Kat2b/Pcaf) to gluconeogenic genes (311). Kat2b then enhances histone H3 acetylation at Lys9 (H3K9Ac) promoting gene transcription and further potentiating Crtc2 occupancy at Creb-binding sites. Along with Kat2b, WD repeat-containing protein 5 (Wdr5), a core subunit of histone methyltransferase (HMT) is also recruited and exhibits concerted action with Kat2b on enhancing H3K9Ac (311).
Besides Creb, the Forkhead box protein O1 (Foxo1) is a major transcriptional regulator of gluconeogenic gene expression. Foxo1’s nuclear binding activity is modulated by acetylation/deacetylation cycles, where acetylation reduces and de-acetylation enhances binding of Foxo1 to gluconeogenic gene promoters (303). Inhibition of Foxo1 activity by E26 oncogene homolog 1 (Ets-1) (230) is attenuated by glucagon-mediated downregulation of Ets-1, reducing its acetylation (230). In addition, glucagon rapidly dephosphorylates class IIa histone deacetylases (HDACs), facilitating their translocation to the nucleus and concomitant deacetylation of Foxo1 (257). Sirtuins, another class of deacetylases involved in metabolic control, are also regulated by glucagon. Here, sirtuin 6 (Sirt6) deacetylates and thus activates the general control nonrepressed protein 5 (Gcn5/Kat2a), causing Pgc1α acetylation and reduction of its gluconeogenic gene transcriptional activity (79). By reducing the expression of Sirt6, glucagon indirectly enhances the activation of Pgc1α (78). Altogether, glucagon activates gluconeogenic gene transcription by modulating the deacetylation of transcriptional co-regulators. For a more detailed description on glucagon-mediated histone acetylation and its implication in glucagon biology visit a recent review by Zhang et al. (429).
In addition to acetylation, gluconeogenic gene expression is enhanced through histone methylation by protein arginine methyltransferase 5 (Prmt5) (376). Glucagon stimulates Crtc2 interaction with Prmt5 thereby increasing the methylation (H3R2me2) of gluconeogenic genes, while the downregulation of Prmt5 reduces gluconeogenic gene expression and circulating glucose levels (376). Altogether, these studies show the importance of histone modifications, chromatin environment, and TF binding/interaction for Creb activity and adds to the complexity in the regulation of gene transcription by glucagon (132).
Regulation of amino acid metabolism
Gluconeogenesis is a substrate driven process, wherein substrates from other tissues such as adipose-derived glycerol or muscle-derived AAs contribute to gluconeogenesis in the liver, also known as the Cori cycle (161). There is no indication of Gcgr expression in the skeletal muscle or adipose tissue in humans (238), indicating that glucagon may not directly mobilize precursors for gluconeogenesis from these tissues (240). Thus other mechanisms, such as catecholamines and cortisol, participate in precursor mobilization from muscle and fat.
However, glucagon stimulates AA influx into hepatocytes providing AA substrates for gluconeogenesis (2). This is achieved by glucagon-stimulated expression of the AA transporters for alanine (A system) and glutamine, histidine, and asparagine (N system) in the liver, resulting in increased AA uptake (197, 233). After influx, these AAs are further processed to be used as precursors for gluconeogenesis. Essential for this is their deamination, after which the amine groups enter the urea cycle for excretion (334). For this, glucagon induces the rapid deamination of glutamine, resulting in an immediate increase in ureagenesis and AA metabolism (16, 217). How the deamination is acutely regulated is unclear. The rapid increase in ureagenesis by glucagon is induced by allosteric activation of Sirt3 and Sirt5, which in turn increases the activity of ornithine transcarbamylase (Otc) and carbamoyl phosphate synthetase 1 (Cps1), critical enzymes in ureagenesis (146, 273). In addition, glucagon also induces the transcript levels of enzymes involved in the urea cycle through the cAMP-PKA-Creb mediated pathway (349). Particularly, enzyme N-acetyl glutamate synthetase (Nags) transcription is enhanced by glucagon, driving AA flux toward ureagenesis (156). Altogether, glucagon primes the hepatocytes for the uptake of AAs from the circulation, which are used as precursors of gluconeogenesis in periods of long-term starvation (61, 240).
The importance of hepatic glucagon signaling in AA metabolism is further supported by studies, where Gcgr antagonism causes hyper-aminoacidemia, due to reduced uptake of circulating AAs and decreased ureagenesis (112, 267). Hyper-aminoacidemia has been suggested to be a factor for increase in α-cell mass (350). In fact, interfering with liver glucagon signaling through liver-specific deletion of Gcgr results in pancreatic α-cell hyperplasia (112), suggesting a liver to α-cell axis. Importantly, the increase in circulating AAs upon Gcgr ablation then further stimulates glucagon secretion from α-cells (410), creating a vicious cycle of overproduction of glucagon. This partly explains the hyperglucagonemia observed after ablation of liver Gcgr signaling. In particular, arginine, alanine, and proline have been shown to stimulate the secretion of glucagon from α-cells (110), while glutamine induces α-cell mass (72). In addition, the pancreatic amino-acid transporter, Slc38a5 was found to play a vital role in α-cell hyperplasia induced by liver Gcgr inhibition in mice and its absence prevented hyperplasia development (72). The occurrence of hyperglucagonemia and hyperaminoacidemia is observed in type-2 diabetes patients (313) and patients with NAFLD (406, 408), underlining the association between these conditions and metabolic diseases. These data emphasize the need to further clearly characterize the role of glucagon on hepatic AA metabolism and to delineate the underlying mechanisms regulating the liver-α-cell axis.
Regulation of mitochondrial metabolism and hepatic calcium signaling
Gluconeogenic flux is deeply linked to respiration rate and ATP production (302). Indeed, glucagon stimulates mitochondrial oxygen consumption correlating well with the physiological requirement for energy production during gluconeogenesis (31, 422). Interestingly, glucagon via signaling through Gq and PLC-mediated IP3 formation increases intracellular and mitochondrial calcium levels as one of the ways to enhance mitochondrial respiration (55). This is achieved by release of intracellular calcium stores from the ER (94), through cAMP-mediated regulation of inositol triphosphate receptor (IP3R) by several independent mechanisms (reviewed in (368)). This includes cAMP-activated PKA phosphorylation of IP3R2, the major subtype expressed in hepatocytes, at serine residue 937 resulting in enhanced burst of IP3R channel gating (21). More importantly, cAMP directly delivered to IP3R2 signaling junctions on the ER potentiates its response to IP3 independent of PKA or Epac, as observed through nuclear patch-clamp recordings (373), suggesting a direct role for cAMP in sensitizing the IP3R2. Subsequent increase in cytosolic calcium stimulates gluconeogenesis either by directly modulating enzyme activity of pyruvate carboxylase and Pepck or by modulating the expression of gluconeogenic genes (3, 278). The later is mediated by cytosolic calcium sensors such as calmodulin-dependent kinases and calcineurin, which together increase the nuclear transcription of Foxo1, Creb, and Crtc2, thereby enhancing gluconeogenesis (3, 278). Additionally, cytosolic calcium also regulates glycogenolysis through stimulation of the phosphorylase kinase and activation of GP (3, 278). Recently, Perry et al. (290) have shown that glucagon stimulates hepatic gluconeogenesis through activation of mitochondria localized IP3R1-mediated stimulation of mitochondrial fat oxidation and lipolysis, indicating the physiological importance of this process in glucagon biology.
Calcium release from the ER occurs either directly into the cytosol, as described above, or can be taken up into the mitochondria thought mitochondria/ER contact sites (42). Mitochondrial calcium influx stimulates mitochondrial oxidative metabolism and electron transport. This is mediated by increasing the activity of calcium-sensitive dehydrogenases of the TCA cycle: pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase (258). Additionally, direct activation of the mitochondrial ATPase through calcium stimulates ATP synthesis (258). Besides this, glucagon stimulated mitochondrial calcium influx accumulates adenine nucleotides via the mitochondrial ATP-Mg/Pi carrier (SCaMC-3/slc25a23), which serve as precursors for gluconeogenesis (358).
Carbon source for glucose production during gluconeogenesis is provided by pyruvate and acetate as well as alanine, glutamine, and glycerol. Recently, the carbon share from glutamine has been shown to be enriched upon glucagon stimulation in hepatocytes (72). It is proposed that mitochondrial calcium influx following glucagon treatment stimulates the activity of α-ketoglutarate dehydrogenase paving the way to increased anaplerotic flux from glutamine. Consistent with this, deletion of glutaminase (Gls2), the enzyme involved in conversion of glutamine to glutamate, results in reduced glucagon stimulated glutamine turnover and decreased fasting blood glucose levels in mice (72). Importantly, a mutation at human GLS2 locus causes enhanced glutaminase activity stimulating glutamine influx and is connected with higher fasting blood glucose in humans (259). Altogether, these data reveal the main function of glucagon on calcium influx and mitochondrial respiration is to tune the system for maximal gluconeogenic capacity.
Glucagon action on lipid metabolism in the adipose tissue and liver
Consistent with glucagon’s main function during fasting, where lipid mobilization is needed to provide energy through β-oxidation and production of ketone bodies (321), glucagon has been connected to lipid metabolism since the 1960s (44, 286). Subsequently, glucagon has been shown to reduce plasma cholesterol (43, 141), triglycerides (43, 141, 240), and esterified fatty acid levels (45). The involvement of the adipose tissue in these effects has been investigated in mice, where small amounts of Gcgr are also detectable (35). Here, glucagon stimulates lipolysis through cAMP-PKA-hormone sensitive lipase (HSL)-mediated pathway thereby, however, increasing circulating FFA levels (155, 348, 361). Despite this, there has been no solid evidence of Gcgr expression in human adipocytes (417) and glucagon induced lipolysis was only obtained at supra-physiological concentration in human adipocytes (possibly through glucagon stimulated catecholamine secretion) rather than at physiological levels (124). Consistent with the fact that Gcgr expression is highest in the liver, these data indicate hepatic Gcgr signaling to be the primary regulator of lipid metabolism by glucagon.
Glucagon affects liver lipid metabolism through inhibition of lipogenesis and stimulation of lipolysis (111). In hepatocytes, glucagon activates AMPK and p38 MAPK which leads to nuclear translocation and transcriptional activation of peroxisome proliferator-activated receptor alpha (Ppar-α) that in turn increases the transcript level of fatty acid oxidation gene-carnitine palmitoyltransferase-1a (Cpt-1a) (240, 355). Cpt-1a enables catabolism of long-chain fatty acids by converting them to acyl-carnitines (240, 355). These acyl-carnitines are then transported into mitochondria thereby activating β-oxidation wherein fatty acids are degraded to acetate. Acetate and CoA combine to form acetyl-CoA, which then condenses with oxaloacetate to form citrate ultimately entering citric cycle. This process enhances fatty acid catabolism and inhibits glycolysis (Figure 4).
Apart from transcriptional activation, glucagon also regulates lipid metabolism by acetylation and deacetylation, similar to its control of gluconeogenesis. Here, the activity of forkhead transcription factor A2 (Foxa2) is increased upon its acetylation (394) via adenylyl cyclase mediated inhibition of Sik2 and subsequent enhancement of p300 activity (237). Foxa2 then induces the transcription of β-oxidation genes such as Cpt-1 and medium-chain acyl-CoA dehydrogenase (Mcad) (394). Recruitment of Kat2b/Pcaf by glucagon acetylates cAMP-responsive element-binding protein H (Crebh) at Lys294 (199), which induces its nuclear localization and interaction with PPARα, leading to increased transcription of fibroblast growth factor 21 (Fgf21). Fgf21 then increases energy expenditure and inhibits lipogenesis (200) as described above. In addition, glucagon induces the expression of Sirt3 (207), that in-turn deacetylates and enhances activity of long-chain acyl-CoA dehydrogenase (Lcad) (166). Lcad, a key mitochondrial fatty acid oxidation enzyme, reduces triglyceride accumulation and stimulates fatty acid oxidation.
Glucagon-induced cAMP formation shifts the intracellular AMP/ATP ratio to an energy-depleted state sufficient to activate AMPK (19). This leads to phosphorylation and inactivation of acetyl-CoA carboxylase, causing a reduction in malonyl-CoA formation. As accumulation of Malonyl-CoA inhibits Cpt-1 induced β-oxidation, reducing its production will redirect FFAs from re-esterification as triglyceride to β-oxidation (75). FFAs are either stored as triglycerides or are processed by lipases to be released as very-low-density-lipoprotein (VLDL) into circulation. As FFAs are used for β-oxidation, VLDL secretion is also downregulated in this process (Figure 4) (22, 24).
Consistent with the allosteric activations, acute and long-term administration of glucagon in mice in vivo showed reduced plasma FFA (111), TG (111, 140), and phospholipid (140) concentrations along with decreased hepatic triglyceride content (111, 158), which is dependent upon Gcgr, as Gcgr−/− mice and glucagon antagonists do not show this effect (121, 240). Also, Gcgr knockdown in db/db mice increases plasma low-density-lipoprotein (LDL) cholesterol, liver triglycerides, and liver cholesterol, which is accompanied by increases in lipogenic genes including fatty acid synthase, acetyl-CoA carboxylase, stearoyl-CoA desaturase 1, and elongation of very long-chain fatty acids protein (147), further supporting evidence for a role of glucagon in lipid metabolism. In fact, humans with hyperglucagonemia exhibit a decrease in lipoprotein particle turnover and induced β-oxidation (301, 419), confirming its clinical relevance. These observations have hampered the pharmacotherapeutic use of Gcgr antagonists as treatment options for the hyperglycemia in type-2 diabetic patients (see below) (142, 189, 328). Thus, there is a pressing need for identification of clear mechanisms and pathways mediating the glucose and lipid metabolic effects downstream of Gcgr upon ligand activation.
Regulation of ketone body metabolism
During prolonged starvation, the liver produces ketone bodies that provide energy fuel for the brain. Glucagon functions to stimulate ketogenesis, a process occurring in the mitochondria of perivenous hepatocytes, which transforms fatty acids (FAs) into acetoacetate (AcAc) and 3-hydroxy butyrate (3HB) (152). FAs shuttled into the mitochondria via Cpt-1 undergo β-oxidation to form acetyl-CoA, to enter the citric cycle or for utilization in ketone body formation. Since the activity of the citric cycle is reduced under long-term starvation, as all intermediates are used for gluconeogenesis, acetyl-CoA becomes available for ketone body formation (220). Glucagon stimulates the activity of hepatic mitochondrial HMG-CoA synthase, a key rate-limiting enzyme for AcAc formation, and thereby enhances ketone body production (Figure 4) (304). This is achieved by lowering the concentration of succinyl-CoA, which inactivates HMG-CoA synthase, thus increasing ketogenesis (304). Interestingly, elevated blood glucagon levels have been shown to contribute to increased circulating ketone bodies and metabolic acidosis in diabetic ketoacidosis and alcoholic ketoacidosis, suggesting its human relevance (220). In fact, in uncontrolled insulin-deficient diabetic patients hyperglucagonemia was found to be essential for ketosis rather than hyperglycemia (191, 253). However, recent conflicting data implies a limited role for glucagon in ketogenesis, since interruption of glucagon signaling has no effect on fasting stimulated ketosis (41), emphasizing the need to revisit the direct role of glucagon in ketone body metabolism.
Regulation of bile acid metabolism
Synthesized from cholesterol in hepatocytes, bile acids have emerged as pivotal modulators of lipid, glucose, and energy metabolism in the liver (340). Cyp7a1 is the first and rate-limiting enzyme in bile acid biosynthetic pathway. Glucagon represses the gene expression of CYP7A1 in human and rat hepatocytes, through PKA-dependent phosphorylation and inactivation of HNF4α (351). Importantly, chronic Gcgr agonism increases circulating bile acid levels in DIO mice and induces body weight reduction (140, 201). While bile acids are ligands for FXR and induce energy expenditure, the weight lowering effect of chronic Gcgr agonism was reduced in liver-specific-FXR knockout mice (201). This opens the possibility for bile acid-FXR axis in hepatocytes mediating the glucagon-stimulated effects on energy expenditure.
Glucagon and Fgf21
Fibroblast growth factor 21 (Fgf21) was first described in 2000 as a novel FGF with high homology to the endocrine Fgf19 (277). Fgf21 is secreted via coat protein complex II vesicles (400) in response to diverse nutritional stressors including fasting (11, 109, 171), a ketogenic diet (10, 11) a low protein diet (165, 218, 219), and carbohydrate refeeding (170, 330). First reported as a novel metabolic regulator in 2005 (195), Fgf21 has been shown to have pluripotent effects, including regulating energy expenditure (59, 331), thermogenesis (98, 398), fatty acid oxidation (300), glucose metabolism (40, 247, 250, 331), and body weight (59) in rodents. As such, Fgf21 has emerged as an appealing therapeutic for the metabolic syndrome (9, 171, 177).
Consistent with fasting-induced Fgf21 secretion, acute glucagon administration increases plasma FGF21 levels in rodents (64, 145) and humans (145, 149). This is a direct effect of liver glucagon signaling, as glucagon treatment in mouse primary hepatocytes increases both Fgf21 gene expression and Fgf21 in the cultured media (145). Consistently, this effect is lost in hepatocytes isolated from mice deficient for Gcgrs (Gcgr; Gcgr−/− and Gcgr−liver) (145, 201). This regulation is consistent and more robust in mice treated either acutely (145) or chronically with the potent Gcgr agonist IUB288 (145, 201). Fasting-induced Fgf21 is regulated by Ppar-α (109, 171) and glucagon signaling regulates Ppar-α transcriptional activity (240). While it is logical to assume glucagon regulates Fgf21 in a Ppar-α dependent manner, this has yet to be definitively elucidated. Glucagon also regulates Fgf21 secretion in rat primary hepatocytes via posttranslational modifications mediated in a PKA and Epac-dependent manner, with no differences in gene expression (64). This model-specific difference in glucagon-mediated Fgf21 gene regulation may be a result of differences in the model organism, time of treatment, or culture conditions. While Fgf21 is regulated by multiple factors, Gcgr−/− mice are refractive to fasting-induced liver Fgf21 expression, suggesting glucagon is the primary stimulator of Fgf21 in a fasted state (240).
Mice deficient for Fgf21 (Fgf21−/−) are likewise refractive to Gcgr-mediated increases in EE and prevention of DIO with no genotypic differences in food intake, suggesting glucagon regulates energy balance via Fgf21. Further, overexpression of liver Fgf21 and administration of recombinant Fgf21 increases EE in a brain-dependent manner (331). While it has yet to be elucidated, it is plausible that glucagon regulates energy balance via central Fgf21 action. Chronic Gcgr agonism additionally decreases plasma cholesterol, liver triglycerides, and increases day-time locomotor activity (145). Fgf21 likewise regulates plasma cholesterol and locomotor activity, highlighting Fgf21 as an important mediator of specific glucagon actions. While Fgf21 is sufficient to mediate Gcgr-prevention of body weight gain on high-fat diet, hepatic Fgf21 is only partially responsible for the weight loss effects of Gcgr-agonism in DIO mice (201). These observations may be due to differential glucagon-mediated mechanisms regulating obesity prevention versus treatment.
Pharmacological Actions of Glucagon in Type-1 and Type-2 Diabetes
Insulin deficiency is traditionally viewed as the major culprit in diabetes. In the early 1970s, however, Roger Unger proposed that elevated postprandial glucagon levels are an equally critical factor underpinning diabetes (272, 384). Indeed, postprandial glucagon levels are higher in all forms of diabetes, including type-1 and type-2 diabetes (383). It is postulated that in patients with diabetes, a relative excess of glucagon compared to the decrease in insulin drives excessive hepatic glucose production, contributing to fasting hyperglycemia (313) and greater postprandial glucose excursion (339). In support, in patients with type-2 diabetes, hepatic gluconeogenesis is increased compared to age- and BMI-matched nondiabetic control subjects. Therefore, attenuating glucagon action has been investigated as a treatment of diabetes. The first seminal study to explore this concept used somatostatin to inhibit endogenous glucagon production in patients with type-1 diabetes and observed a decrease in blood glucose levels (125). Similarly, somatostatin administration ameliorated hyperglycemia in dogs rendered diabetic by either alloxan or by removal of the pancreas (126, 324). Subsequently, genetic mouse models have been used to explore the metabolic consequences of lack of glucagon signaling. Glucagon-receptor knock-out mice (Gcgr−/−), in which the ratio of insulin to glucagon signaling is shifted entirely to the side of insulin, have lower blood glucose levels, are more glucose tolerant, and are resistant to HFD-induced insulin resistance (56, 228). Remarkably, Gcgr−/− mice are even resistant to STZ-induces hyperglycemia and β-cell destruction (56), without exhibiting signs of hypoglycemia (121).
In light of these observations, strategies to pharmacologically suppress Gcgr signaling have received a lot of attention in recent years for the potential treatment of diabetes. In the preclinical models, Gcgr antagonists improve glucose tolerance in mouse models of diabetes (104, 226, 267). Similarly, Gcgr antibodies decrease glucose levels and improve glucose tolerance in diabetic rodents and monkeys (138, 202, 280, 423) and anti-sense oligonucleotide-mediated reduction of hepatic Gcgr expression similarly ameliorated hyperglycemia in diabetic mice (232).
In healthy humans, single administration of a Gcgr antagonist reduced glucagon-induced glucose production. In longer-term trials, Gcgr antagonists lower fasting and postprandial blood glucose concentrations, as well as HbA1c levels in patients with type-2 diabetes (63, 189, 388). Similarly, antisense oligonucleotides also improve HbA1c in people with diabetes (389), while monoclonal antibodies against the Gcgr diminish glucagon-induced glucose excursions (209).
These encouraging clinical data, however, have been associated with significant side effects that have thwarted the clinical use of these agents. Increased hepatic transaminases have been seen with Gcgr antagonists (20, 142, 189, 193, 388) and humanized monoclonal antibodies (209), suggesting adverse effects on the liver. Gcgr antagonists increase LDL cholesterol (20, 139) and liver fat (142). Another concern pertains to pre-clinical studies showing that Gcgr antibodies cause α-cell hyperplasia (138, 280), which also has been observed in global Gcgr−/− mice (121) as well as in liver-specific Gcgr−/− mice (239). Whether this translates into serious clinical side effects will have to be determined in longer-term studies, but the concern that this hyperplasia may become malignant has to be carefully assessed.
However, in light of the support of glucagon in the regulation of lipid metabolism, Gcgr agonism may be useful for treatment of hepatic steatosis (336), to which there are no FDA-approved therapeutics (428). Chronic Gcgr agonism decreases liver triglycerides and plasma cholesterol in DIO mice (145, 201, 274). This effect is dependent on liver Gcgr signaling, as the benefits of Gcgr agonism on dyslipidemia are lost in mice deficient for hepatic Gcgr (Gcgrliver) (145, 201, 274). These pharmacological effects are consistent with results from clinical trials utilizing GLP1R and Gcgr dual agonist. Treatment with the dual agonist reduces liver triglycerides and plasma cholesterol (6). While glucagon monotherapy has not been tested directly in clinical trials, the dual agonists are superior to GLP1 agonism alone in reducing hepatic steatosis in rodent models (66, 299). These additional metabolic actions of glucagon warrant further study as promising avenues for the treatment of obesity and hepatic steatosis.
Outlook and Future Questions
Identified nearly a century ago in a process to optimize insulin purification, glucagon has ever since been stigmatized for its hepatic effects to increase blood glucose. Long overshadowed by the monumental importance of insulin, recent years have witnessed a renaissance of glucagon pharmacology with acknowledged applications that go far beyond its initial use as a life-saving rescue medication for severe hypoglycemia. A plethora of studies nowadays testify glucagon pharmacological value to improve body weight and lipid metabolism and dual-agonists targeting the receptors for glucagon and GLP-1 are in clinical development for the treatment of type-2 diabetes.
Acknowledgments
The figures were made using material provided by Servier Medical Art (Servier), under consideration of a Creative Commons Attribution 3.0 Unported License. This work was supported in part by the Helmholtz Alliance ICEMED and Helmholtz Initiative on Personalized Medicine iMed by Helmholtz Association, and Helmholtz cross-program topic “Metabolic Dysfunction” (Timo D. Müller). This work was further supported by German Research Foundation Grants SFB TRR152-P23 and SFB TRR296 and German Center for Diabetes Research (DZD e.V.) to Timo D. Müller and the DFG Grants ZE1037/1-3, ZE1037/3-1, and the BMBF Grant 031L0114A to Anja Zeigerer and by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01-DK-112934 to Kirk M. Habegger.
References
- 1.Ali S, Ussher JR, Baggio LL, Kabir MG, Charron MJ, Ilkayeva O, Newgard CB, Drucker DJ. Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction. Mol Metab 4 (2): 132–143, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almdal TP, Holst JJ, Heindorff H, Vilstrup H. Glucagon immunoneutralization in diabetic rats normalizes urea synthesis and decreases nitrogen wasting. Diabetes 41 (1): 12–16, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Altarejos JY, Montminy M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 12 (3): 141–151, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amatuzio DS, Grande F, Wada S. Effect of glucagon on the serum lipids in essential hyperlipemia and in hypercholesterolemia. Metabolism 11: 1240–1249, 1962. [PubMed] [Google Scholar]
- 5.Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, Jermutus L, Hirshberg B. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, phase 1 study. Br J Clin Pharmacol 84 (10): 2325–2335, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai L-F, Robertson D, Jain M, Petrone M, Rondinone C, Hirshberg B, Jermutus L. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 391 (10140): 2607–2618, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Authier F, Desbuquois B. Glucagon receptors. Cell Mol Life Sci 65 (12): 1880–1899, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Authier F, Desbuquois B, De Galle B. Ligand-mediated internalization of glucagon receptors in intact rat liver. Endocrinology 131 (1): 447–457, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Babaknejad N, Nayeri H, Hemmati R, Bahrami S, Esmaillzadeh A. An overview of FGF19 and FGF21: The therapeutic role in the treatment of the metabolic disorders and obesity. Horm Metab Res 50 (6): 441–452, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150 (11): 4931–4940, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5 (6): 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132 (6): 2131–2157, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Kastin AJ. Peptides and the blood-brain barrier: Lipophilicity as a predictor of permeability. Brain Res Bull 15 (3): 287–292, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Barki-Harrington L, Rockman HA. Beta-arrestins: Multifunctional cellular mediators. Physiology (Bethesda) 23: 17–22, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett PJ, Gaspers LD, Pierobon N, Thomas AP. Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium 55 (6): 306–316, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Battezzati A, Simonson DC, Luzi L, Matthews DE. Glucagon increases glutamine uptake without affecting glutamine release in humans. Metabolism 47 (6): 713–723, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Beaudry JL, Kaur KD, Varin EM, Baggio LL, Cao X, Mulvihill EE, Stern JH, Campbell JE, Scherer PE, Drucker DJ. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol Metab 22: 37–48, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature 304 (5924): 368–371, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Berglund ED, Lee-Young RS, Lustig DG, Lynes SE, Donahue EP, Camacho RC, Meredith ME, Magnuson MA, Charron MJ, Wasserman DH. Hepatic energy state is regulated by glucagon receptor signaling in mice. J Clin Invest 119 (8): 2412–2422, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman A, Tan B, Somayaji VR, Calle RA, Kazierad DJ. A 4-week study assessing the pharmacokinetics, pharmacodynamics, safety, and tolerability of the glucagon receptor antagonist PF-06291874 administered as monotherapy in subjects with type 2 diabetes mellitus. Diabetes Res Clin Pract 126: 95–104, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Betzenhauser MJ, Fike JL, Wagner LE, Yule DI. Protein kinase A increases type-2 inositol 1, 4, 5-trisphosphate receptor activity by phosphorylation of serine 937. Journal of Biological Chemistry 284 (37): 25116–25125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beynen AC, Haagsman HP, Van Golde LM, Geelen MJ. The effects of insulin and glucagon on the release of triacylglycerols by isolated rat hepatocytes are mere reflections of the hormonal effects on the rate of triacylglycerol synthesis. Biochim Biophy Acta 665 (1): 1–7, 1981. [DOI] [PubMed] [Google Scholar]
- 23.Billington CJ, Briggs JE, Link JG, Levine AS. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Physiol 261 (2 Pt 2): R501–R507, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Bjornsson OG, Sparks JD, Sparks CE, Gibbons GF. Regulation of VLDL secretion in primary culture of rat hepatocytes: Involvement of cAMP and cAMP-dependent protein kinases. Eur J Clin Invest 24 (2): 137–148, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Blauw H, Wendl I, DeVries J, Heise T, Jax T, Consortium P. Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes, Obes Metab 18 (1): 34–39, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Bloom SR, Edwards AV, Hardy RN. The role of the autonomic nervous system in the control of glucagon, insulin and pancreatic polypeptide release from the pancreas. J Physiol 280: 9–23, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boland ML, Laker RC, Mather K, Nawrocki A, Oldham S, Boland BB, Lewis H, Conway J, Naylor J, Guionaud S, Feigh M, Veidal SS, Lantier L, McGuinness OP, Grimsby J. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab 2 (5): 413–431, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bollheimer LC, Landauer HC, Troll S, Schweimer J, Wrede CE, Schölmerich J, Buettner R. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53 (11): 1443–1448, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Bomboy JD Jr, Lewis SB, Lacy WW, Sinclair-Smith BC, Liljenquist JE. Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man. Diabetes 26 (3): 177–174, 1977. [DOI] [PubMed] [Google Scholar]
- 30.Bonni A, Ginty DD, Dudek H, Greenberg ME. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci 6 (2): 168–183, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Breton L, Clot JP, Baudry M. Effects of glucagon on basal metabolic rate and oxidative phosphorylation of rat liver mitochondria. Horm Metab Res 15 (9): 429–432, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Briant LJB, Dodd MS, Chibalina MV, Rorsman NJG, Johnson PRV, Carmeliet P, Rorsman P, Knudsen JG. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep 23 (11): 3300–3311, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buggy JJ, Heurich RO, MacDougall M, Kelley KA, Livingston JN, Yoo-Warren H, Rossomando AJ. Role of the glucagon receptor COOH-terminal domain in glucagon-mediated signaling and receptor internalization. Diabetes 46 (9): 1400–1405, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Burcelin R, Katz EB, Charron MJ. Molecular and cellular aspects of the glucagon receptor: Role in diabetes and metabolism. Diabetes Metab 22 (6): 373–396, 1996. [PubMed] [Google Scholar]
- 35.Burcelin R, Li J, Charron MJ. Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene 164 (2): 305–310, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Bürger M. Über den hepatischen Angriffspunkt des Insulins. Die primäre Paradoxe Insulinhyperglykämie. Z Ges Exp Med 65: 487, 1929. [Google Scholar]
- 37.Bürger M. Primäre Hyperglykämie und Glykogenveränderung der Leber als Folge intraportaler Insulininjektion nach Untersuchungen am Hund. Z Ges Exp Med 67: 441, 1929. [Google Scholar]
- 38.Bürger M. Über das Glukagon (die hyperglykämisierende Substanz der Pankreas). Z ges exp Med 96: 375, 1935. [Google Scholar]
- 39.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes 40 (1): 73–81, 1991. [PubMed] [Google Scholar]
- 40.Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 154 (9): 3099–3109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capozzi ME, Coch RW, Koech J, Astapova II, Wait JB, Encisco SE, Douros JD, El K, Finan B, Sloop KW, Herman MA, D’Alessio DA, Campbell JE. The limited role of glucagon for ketogenesis during fasting or in response to SGLT2 inhibition. Diabetes 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung K-H, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142 (2): 270–283, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caren R. Glucagon and cholesterol metabolism. Metabolism 9: 938–945, 1960. [PubMed] [Google Scholar]
- 44.Caren R, Carbo L. Pancreatic alpha-cell function in relation to cholesterol metabolism. J Clin Endocrinol Metab 16 (4): 507–516, 1956. [DOI] [PubMed] [Google Scholar]
- 45.Caren R, Corbo L. Transfer of plasma lipid to platelets by action of glucagon. Metabolism 19 (8): 598–607, 1970. [DOI] [PubMed] [Google Scholar]
- 46.Cegla J, Jones BJ, Gardiner JV, Hodson DJ, Marjot T, McGlone ER, Tan TM, Bloom SR. RAMP2 influences glucagon receptor pharmacology via trafficking and signaling. Endocrinology 158 (8): 2680–2693, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim K-S, Gutierrez-Aguilar R, Li B, Drucker DJ, D’Alessio DA, Seeley RJ, Sandoval DA. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 25 (4): 927, e923–934, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114 (Pt 13): 2363–2373, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Chia CW, Carlson OD, Kim W, Shin YK, Charles CP, Kim HS, Melvin DL, Egan JM. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 58 (6): 1342–1349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there? J Mol Endocrinol 32 (2): 325–338, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 60 (12): 3103–3109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen MB, Calanna S, Holst JJ, Vilsboll T, Knop FK. Glucose-dependent insulinotropic polypeptide: Blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab 99 (3): E418–E426, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Clemmensen C, Chabenne J, Finan B, Sullivan L, Fischer K, Küchler D, Sehrer L, Ograjsek T, Hofmann SM, Schriever SC, Pfluger PT, Pinkstaff J, Tschöp MH, Dimarchi R, Müller TD. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes 63 (4): 1422–1427, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Cockburn F, Hull D, Walton I. The effect of lipolytic hormones and theophylline on heat production in brown adipose tissue in vivo. Br J Pharmacol Chemother 31 (3): 568–577, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Combettes L, Berthon B, Binet A, Claret M. Glucagon and vasopressin interactions on Ca2+ movements in isolated hepatocytes. Biochem J 237 (3): 675–683, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Sinha Roy R, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50 (1): 142–150, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Constantin J, Ishii-Iwamoto EL, Suzuki-Kemmelmeier F, Yamamoto NS, Bracht A. The action of glucagon infused via the hepatic artery in anterograde and retrograde perfusion of the rat liver is not a function of the accessible cellular spaces. Biochim Biophys Acta 1244 (1): 169–178, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59 (11): 2936–2940, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149 (12): 6018–6027, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19 (6): 580–586, 1996. [DOI] [PubMed] [Google Scholar]
- 61.Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 153 (3): 1039–1048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cullen PJ, Steinberg F. To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol 19 (11): 679–696, 2018. [DOI] [PubMed] [Google Scholar]
- 63.Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 21 (1): 33–38, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cyphert HA, Alonge KM, Ippagunta SM, Hillgartner FB. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9 (4): e94996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davidson IWF, Salter JM, Best CH. The effect of glucagon on the metabolic rate of rats. Am J Clin Nutr 8 (5): 540–546, 1960. [Google Scholar]
- 66.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschöp MH. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 5 (10): 749–757, 2009. [DOI] [PubMed] [Google Scholar]
- 67.de Andrade IS, Zemdegs JC, de Souza AP, Watanabe RLH, Telles MM, Nascimento CMO, Oyama LM, Ribeiro EB. Diet-induced obesity impairs hypothalamic glucose sensing but not glucose hypothalamic extracellular levels, as measured by microdialysis. Nutr Diabetes 5: e162, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Castro JM, Paullin SK, DeLugas GM. Insulin and glucagon as determinants of body weight set point and microregulation in rats. J Comp Physiol Psychol 92 (3): 571–579, 1978. [DOI] [PubMed] [Google Scholar]
- 69.de Graaf C, Song G, Cao C, Zhao Q, Wang M-W, Wu B, Stevens RC. Extending the structural view of class B GPCRs. Trends Biochem Sci 42 (12): 946–960, 2017. [DOI] [PubMed] [Google Scholar]
- 70.de Jong A, Strubbe JH, Steffens AB. Hypothalamic influence on insulin and glucagon release in the rat. Am J Physiol 233 (5): E380–E388, 1977. [DOI] [PubMed] [Google Scholar]
- 71.De Oya M, Prigge WF, Swenson DE, Grande F. Role of glucagon on fatty liver production in birds. Am J Physiol 221 (1): 25–30, 1971. [DOI] [PubMed] [Google Scholar]
- 72.Dean ED, Li M, Prasad N, Wisniewski SN, Von Deylen A, Spaeth J, Maddison L, Botros A, Sedgeman LR, Bozadjieva N, Ilkayeva O, Coldren A, Poffenberger G, Shostak A, Semich MC, Aamodt KI, Phillips N, Yan H, Bernal-Mizrachi E, Corbin JD, Vickers KC, Levy SE, Dai C, Newgard C, Gu W, Stein R, Chen W, Powers AC. Interrupted glucagon signaling reveals hepatic alpha cell axis and role for L-glutamine in alpha cell proliferation. Cell Metab 25 (6): 1362–1373.e1365, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iodoglucagon Desbuquois B.. Preparation and characterization. Eur J Biochem 53 (2): 569–580, 1975. [DOI] [PubMed] [Google Scholar]
- 74.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 280 (39): 33487–33496, 2005. [DOI] [PubMed] [Google Scholar]
- 75.DiMarco JP, Hoppel C. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J Clin Invest 55 (6): 1237–1244, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dodd GT, Tiganis T. Insulin action in the brain: Roles in energy and glucose homeostasis. J Neuroendocrinol 29 (10), e12513, 2017. DOI: 10.1111/jne.12513 [DOI] [PubMed] [Google Scholar]
- 77.Doi K, Kuroshima A. Thermogenic response to glucagon in cold-acclimated mice. Jpn J Physiol 32 (3): 377–385, 1982. [DOI] [PubMed] [Google Scholar]
- 78.Dominy JE, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1α’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim Biophys Acta (BBA)-Proteins Proteom 1804 (8): 1676–1683, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominy JE Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan H-B, Feldman J, Pierce K, Mostoslavsky R, Denu JM, Clish CB, Yang X, Shulman GI, Gygi SP, Puigserver P. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell 48 (6): 900–913, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dores MR, Trejo J. GPCR sorting at multivesicular endosomes. Methods Cell Biol 130: 319–332, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156 (7): 2470–2481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drucker DJ. Glucagon and the glucagon-like peptides. Pancreas 5 (4): 484–488, 1990. [DOI] [PubMed] [Google Scholar]
- 83.Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem 263 (27): 13475–13478, 1988. [PubMed] [Google Scholar]
- 84.Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol 8 (12): 1646–1655, 1994. [DOI] [PubMed] [Google Scholar]
- 85.Dumonteil E, Laser B, Constant I, Philippe J. Differential regulation of the glucagon and insulin I gene promoters by the basic helix-loop-helix transcription factors E47 and BETA2. J Biol Chem 273 (32): 19945–19954, 1998. [DOI] [PubMed] [Google Scholar]
- 86.Eaton RP. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J Lipid Res 14 (3): 312–318, 1973. [PubMed] [Google Scholar]
- 87.Eichel K, von Zastrow M. Subcellular organization of GPCR signaling. Trends Pharmacol Sci 39 (2): 200–208, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, Halban PA, Schuit FC, Donath MY. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A 105 (35): 13163–13168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AMK, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17 (11): 1481–1489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring) 14 (Suppl 5): 254S–258S, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Exton JH, Park CR. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3′,5′-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem 243 (16): 4189–4196, 1968. [PubMed] [Google Scholar]
- 92.Felig P, Wahren J, Hendler R. Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man. J Clin Invest 58 (3): 761–765, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Felig P, Wahren J, Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes 27 (2): 121–126, 1978. [DOI] [PubMed] [Google Scholar]
- 94.Fernando KC, Gregory RB, Barritt GJ. Protein kinase A regulates the disposition of Ca2+ which enters the cytoplasmic space through store-activated Ca2+ channels in rat hepatocytes by diverting inflowing Ca2+ to mitochondria. Biochem J 330 (Pt 3): 1179–1187, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 69 (4): 532–541, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finan B, Müller TD, Clemmensen C, Perez-Tilve D, DiMarchi RD, Tschöp MH. Reappraisal of GIP pharmacology for metabolic diseases. Trens Mol Med 22 (5): 359–376, 2016. [DOI] [PubMed] [Google Scholar]
- 97.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59 (11): 2781–2789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26 (3): 271–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol 78: 223–241, 2016. [DOI] [PubMed] [Google Scholar]
- 100.Flock G, Cao X, Drucker DJ. Pdx-1 is not sufficient for repression of proglucagon gene transcription in islet or enteroendocrine cells. Endocrinology 146 (1): 441–449, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Fodden JH, Read WO. The activity of extracted pancreatic hyperglycemic-glycogenolytic factor after cobaltous chloride and synthalin A. Endocrinology 54 (3): 303–310, 1954. [DOI] [PubMed] [Google Scholar]
- 102.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54 (6): 1808–1815, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Franklin IK, Wollheim CB. GABA in the endocrine pancreas: Its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol 123 (3): 185–190, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franklin ZJ, O’Harte FP, Irwin N. Effects of short-term chemical ablation of glucagon signalling by peptide-based glucagon receptor antagonists on insulin secretion and glucose homeostasis in mice. Biol Chem 395 (4): 433–442, 2014. [DOI] [PubMed] [Google Scholar]
- 105.Frohman LA, Bernardis LL. Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels. Am J Physiol 221 (6): 1596–1603, 1971. [DOI] [PubMed] [Google Scholar]
- 106.Frohman LA, Ezdinli EZ, Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes 16 (7): 443–448, 1967. [DOI] [PubMed] [Google Scholar]
- 107.Fujimoto WY, Williams RH. Somatostatin inhibits insulin and glucagon release by monolayer cell cultures of rat endocrine pancreas. Life Sci 15 (11): 1999–2004, 1974. [DOI] [PubMed] [Google Scholar]
- 108.Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem 273 (6): 3431–3437, 1998. [DOI] [PubMed] [Google Scholar]
- 109.Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8 (2): 169–174, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Galsgaard KD, Jepsen SL, Kjeldsen SAS, Pedersen J, Wewer Albrechtsen NJ, Holst JJ. Alanine, arginine, and proline but not glutamine are the feed-back regulators in the liver-alpha cell axis in mice. bioRxiv: 792119, 2019. [DOI] [PubMed] [Google Scholar]
- 111.Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol 10: 413–413, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galsgaard KD, Winther-Sorensen M, Orskov C, Kissow H, Poulsen SS, Vilstrup H, Prehn C, Adamski J, Jepsen SL, Hartmann B, Hunt J, Charron MJ, Pedersen J, Wewer Albre NJ. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol Endocrinol Metab 314 (1): e93–e103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 53 (9): 2375–2382, 2004. [DOI] [PubMed] [Google Scholar]
- 114.Gao C, Ren SV, Yu J, Baal U, Thai D, Lu J, Zeng C, Yan H, Wang Y. Glucagon receptor antagonism ameliorates progression of heart failure. JACC Basic Transl Sci 4 (2): 161–172, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia A, Williamson JR, Cahill GF Jr. Studies on the perfused rat liver. II. Effect of glucagon on gluconeogenesis. Diabetes 15 (3): 188–193, 1966. [DOI] [PubMed] [Google Scholar]
- 116.Gauthier BR, Gosmain Y, Mamin A, Philippe J. The beta-cell specific transcription factor Nkx6.1 inhibits glucagon gene transcription by interfering with Pax6. Biochem J 403 (3): 593–601, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gauthier BR, Schwitzgebel VM, Zaiko M, Mamin A, Ritz-Laser B, Philippe J. Hepatic nuclear factor-3 (HNF-3 or Foxa2) regulates glucagon gene transcription by binding to the G1 and G2 promoter elements. Mol Endocrinol 16 (1): 170–183, 2002. [DOI] [PubMed] [Google Scholar]
- 118.Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol 262 (6 Pt 2): R975–R980, 1992. [DOI] [PubMed] [Google Scholar]
- 119.Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol 264 (1 Pt 2): R116–R122, 1993. [DOI] [PubMed] [Google Scholar]
- 120.Geary N, Smith GP. Pancreatic glucagon and postprandial satiety in the rat. Physiol Behav 28 (2): 313–322, 1982. [DOI] [PubMed] [Google Scholar]
- 121.Gelling RW, Du XQ, Dichmann DS, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 100 (3): 1438–1443, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gerich JE, Langlois M, Noacco C, Lorenzi M, Karam JH, Korsham PH. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin-dependent diabetic subjects. Evidence for selective alpha-cell insensitivity to glucose in diabetes mellitus. J Clin Invest 58 (2): 320–325, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerich JE, Langlois M, Schneider V, Karam JH, Noacco C. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J Clin Invest 53 (5): 1284–1289, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerich JE, Lorenzi M, Bier DM, Tsalikian E, Schneider V, Karam JH, Forsham PH. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest 57 (4): 875–884, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerich JE, Lorenzi M, Schneider V, Karam JH, Rivier J, Guillemin R, Forsham PH. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med 291 (11): 544–547, 1974. [DOI] [PubMed] [Google Scholar]
- 126.Gerich JE, Lorenzi M, Schneider V, Kwan CW, Karam JH, Guillemin R, Forsham PH. Inhibition of pancreatic glucagon responses to arginine by somatostatin in normal man and in insulin-dependent diabetics. Diabetes 23 (11): 876–880, 1974. [DOI] [PubMed] [Google Scholar]
- 127.Gevrey JC, Malapel M, Philippe J, Mithieux G, Chayvialle J-A, Abello J, Cordier-Bussat M. Protein hydrolysates stimulate proglucagon gene transcription in intestinal endocrine cells via two elements related to cyclic AMP response element. Diabetologia 47 (5): 926–936, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Gilleron J, Gerdes JM, Zeigerer A. Metabolic regulation through the endosomal system. Traffic 20 (8): 552–570, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giralt M, Gavalda-Navarro A, Villarroya F. Fibroblast growth factor-21, energy balance and obesity. Mol Cell Endocrinol 418 (Pt 1): 66–73, 2015. [DOI] [PubMed] [Google Scholar]
- 130.Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ, Larsson O. {beta}-Arrestin is crucial for ubiquitination and downregulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem 280 (26): 24412–24419, 2005. [DOI] [PubMed] [Google Scholar]
- 131.Glick G, Parmley WW, Wechsler AS, Sonnenblick EH. Glucagon. Its enhancement of cardiac performance in the cat and dog and persistence of its inotropic action despite beta-receptor blockade with propranolol. Circ Res 22 (6): 789–799, 1968. [DOI] [PubMed] [Google Scholar]
- 132.Goldstein I. The Liver: Biology and Pathobiology (6th ed). Hoboken, NJ: Wiley Online Library, 2020. [Google Scholar]
- 133.Goldstein I, Yizhak K, Madar S, Goldfinger N, Ruppin E, Rotter V. p53 Promotes the expression of gluconeogenesis-related genes and enhances hepatic glucose production. Cancer Metab 1 (1): 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gosmain Y, Avril I, Mamin A, Philippe J. Pax-6 and c-Maf functionally interact with the alpha-cell-specific DNA element G1 in vivo to promote glucagon gene expression. J Biol Chem 282 (48): 35024–35034, 2007. [DOI] [PubMed] [Google Scholar]
- 135.Gosmain Y, Cheyssac C, Heddad Masson M, Dibner C, Philippe J. Glucagon gene expression in the endocrine pancreas: The role of the transcription factor Pax6 in alpha-cell differentiation, glucagon biosyn-thesis and secretion. Diabetes Obes Metab 13 (Suppl 1): 31–38, 2011. [DOI] [PubMed] [Google Scholar]
- 136.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28 (1): 84–116, 2007. [DOI] [PubMed] [Google Scholar]
- 137.Gromada J, Ma X, Høy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/−mouse alpha-cells. Diabetes 53: S181–S189, 2004. [DOI] [PubMed] [Google Scholar]
- 138.Gu W, Yan H, Winters KA, Komorowski R, Vonderfecht S, Atangan L, Sivits G, Hill D, Yang J, Bi V, Shen Y, Hu S, Boone T, Lindberg RA, Véniant MM. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 331 (3): 871–881, 2009. [DOI] [PubMed] [Google Scholar]
- 139.Guan HP, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, Wright M, Shah V, Herath K, Xie D, Szeto D, Forrest G, Xiao JC, Palyha O, Sun L-P, Andryuk PJ, Engel SS, Xiong Y, Lin S, Kelley DE, Erion MD, Davis HR, Wang L. Glucagon receptor antagonism induces increased cholesterol absorption. J Lipid Res 56 (11): 2183–2195, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guettet C, Mathe D, Riottot M, Lutton C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim Biophys Acta 963 (2): 215–223, 1988. [DOI] [PubMed] [Google Scholar]
- 141.Guettet C, Rostaqui N, Mathe D, Lecuyer B, Navarro N, Jacotot B. Effect of chronic glucagon administration on lipoprotein composition in normally fed. Fasted and cholesterol-fed rats. Lipids 26 (6): 451–458, 1991. [DOI] [PubMed] [Google Scholar]
- 142.Guzman CB, Zhang XM, Liu R, Regev A, Shankar S, Garhyan P, Pillai SG, Kazda C, Chalasani N, Hardy TA. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab 19 (11): 1521–1528, 2017. [DOI] [PubMed] [Google Scholar]
- 143.Gylfe E. Patrick Gilon Glucose regulation of glucagon secretion. Diabetes Res Clin Pract 103 (1): 1–10, 2014. [DOI] [PubMed] [Google Scholar]
- 144.Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 6 (12): 689–697, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschöp MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 62 (5): 1453–1463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan K-L, Denu JM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell 41 (2): 139–149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Han SAT, Previs SF, Herath K, Roddy TP, Jensen KK, Guan HP, Murphy BA, McNamara LA, Shen X, Strapps W, Hubbard BK, Pinto S, Li C, Li J. Effects of small interfering RNA-mediated hepatic glucagon receptor inhibition on lipid metabolism in Db/Db mice. J Lipid Res 54 (10): 2615–2622, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res 16 (1): 97–107, 1986. [DOI] [PubMed] [Google Scholar]
- 149.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 4 (8): 551–560, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hanyaloglu AC. Advances in membrane trafficking and endosomal signaling of G protein-coupled receptors. Int Rev Cell Mol Biol 339: 93–131, 2018. [DOI] [PubMed] [Google Scholar]
- 151.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48: 537–568, 2008. [DOI] [PubMed] [Google Scholar]
- 152.Harano Y, Kosugi K, Kashiwagi A, Nakano T, Hidaka H, Shigeta Y. Regulatory mechanism of ketogenesis by glucagon and insulin in isolated and cultured hepatocytes. J Biochem 91 (5): 1739–1748, 1982. [DOI] [PubMed] [Google Scholar]
- 153.Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 59 (7): 1765–1770, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He L, Cao J, Meng S, Ma A, Radovick S, Wondisford FE. Activation of basal gluconeogenesis by coactivator p300 maintains hepatic glycogen storage. Mol Endocrinol 27 (8): 1322–1332, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heckemeyer CM, Barker J, Duckworth WC, Solomon SS. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Endocrinology 113 (1): 270–276, 1983. [DOI] [PubMed] [Google Scholar]
- 156.Heibel SK, Lopez GY, Panglao M, Sodha S, Mariño-Ramírez L, Tuchman M, Caldovic L. Transcriptional regulation of N-acetylglutamate synthase. PloS One 7 (2): e29527, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heim T, Hull D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J Physiol 187 (2): 271–283, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Heimberg M, Weinstein I, Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3′,5′-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem 244 (19): 5131–5139, 1969. [PubMed] [Google Scholar]
- 159.Henderson SJ, Konkar A, Hornigold DC, Trevaskis JL, Jackson R, Fredin MF, Jansson-Löfmark R, Naylor J, Rossi A, Bednarek MA, Bhagroo N, Salari H, Wil S, Oldham S, Hansen G, Feigh M, Klein T, Grimsby J, Maguire LJ. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab 18 (12): 1176–1190, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 5 (9): a016766, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 116 (7): 1767–1775, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Herzig S, Fuzesi L, Knepel W. Heterodimeric Pbx-Prep1 homeodomain protein binding to the glucagon gene restricting transcription in a cell type-dependent manner. J Biol Chem 275 (36): 27989–27999, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413 (6852): 179–183, 2001. [DOI] [PubMed] [Google Scholar]
- 164.Heurich RO, Buggy JJ, Vandenberg MT, Rossomando AJ. Glucagon induces a rapid and sustained phosphorylation of the human glucagon receptor in Chinese hamster ovary cells. Biochem Biophys Res Commun 220 (3): 905–910, 1996. [DOI] [PubMed] [Google Scholar]
- 165.Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, Burke SJ, Collier JJ, Qualls-Creekmore E, Solon-Biet SM, Simpson SJ, Berthoud HR, Münzberg H, Morrison CD. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep 27 (10): 2934–2947, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kah CR. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464 (7285): 121–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong J, Abudula R, Chen J, Jeppesen PB, Dyrskog SE, Xiao J, Colombo M, Hermansen K. The short-term effect of fatty acids on glucagon secretion is influenced by their chain length, spatial configuration, and degree of unsaturation: Studies in vitro. Metabolism 54 (10): 1329–1336, 2005. [DOI] [PubMed] [Google Scholar]
- 168.Hoosein NM, Gurd RS. Identification of glucagon receptors in rat brain. Proc Natl Acad Sci U S A 81 (14): 4368–4372, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43 (8): 1012–1019, 2000. [DOI] [PubMed] [Google Scholar]
- 170.Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett 2009 (583): 17, 2009. [DOI] [PubMed] [Google Scholar]
- 171.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5 (6): 415–425, 2007. [DOI] [PubMed] [Google Scholar]
- 172.Ingram DL, Kaciuba-Uscilko H. Metabolic effects of glucagon in the young pig. Horm Metab Res 12 (9): 430–433, 1980. [DOI] [PubMed] [Google Scholar]
- 173.Janah L, Kjeldsen S, Galsgaard KD, Winther-Sørensen M, Stojanovska E, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and glucagon resistance. Int J Mol Sci 20 (13): 3314, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jelinek LJ, Lok S, Rosenberg GB, Grant FJ, Biggs S, Bensch PA, Kuijper JL, Sheppard PO, Sprecher CA. Expression cloning and signaling properties of the rat glucagon receptor. Science 259 (5101): 1614–1616, 1993. [DOI] [PubMed] [Google Scholar]
- 175.Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem 57 (9): 811–824, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol-Endocrinol Metabol 284 (4): E671–E678, 2003. [DOI] [PubMed] [Google Scholar]
- 177.Jimenez V, Jambrina C, Casana E, Sacristan V, Muñoz S, Darriba S, Rodó J, Mallol C, Garcia M, León X, Marcó S, Ribera A, Elias I, Casellas A, Grass I, Elias G, Ferré T, Motas S, Franckhauser S, Mulero F, Navarro M, Haurigot V, Ruberte J, Bosch F. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol Med 10 (8): e8791, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: An immunocytochemical study. J Comp Neurol 271 (4): 519–532, 1988. [DOI] [PubMed] [Google Scholar]
- 179.Jin T. Mechanisms underlying proglucagon gene expression. J Endocrinol 198 (1): 17–28, 2008. [DOI] [PubMed] [Google Scholar]
- 180.Jin T, Drucker DJ. Activation of proglucagon gene transcription through a novel promoter element by the caudal-related homeodomain protein cdx-2/3. Mol Cell Biol 16 (1): 19–28, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joel CD. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem 241 (4): 814–821, 1966. [PubMed] [Google Scholar]
- 182.Johanns M, Lai YC, Hsu MF, Jacobs R, Vertommen D, Van Sande J, Dumont JE, Woods A, Carling D, Hue L, Viollet B, Foretz M, Rider MH. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat Commun 7: 10856, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Juan-Fita MJ, Vargas ML, Kaumann AJ, Hernandez CJ. Rolipram reduces the inotropic tachyphylaxis of glucagon in rat ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol 370 (4): 324–329, 2004. [DOI] [PubMed] [Google Scholar]
- 184.Junker AE, Gluud LL, van Hall G, Holst JJ, Knop FK, Vilsbøll T. Effects of glucagon-like peptide-1 on glucagon secretion in patients with non-alcoholic fatty liver disease. J Hepatol 64 (4): 908–915, 2016. [DOI] [PubMed] [Google Scholar]
- 185.Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G. Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev 13 (4): 495–504, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaneko K, Shirotani T, Araki E, Matsumoto K, Taguchi T, Motoshima H, Yoshizato K, Kishikawa H, Shichiri M. Insulin inhibits glucagon secretion by the activation of PI3-kinase in In-R1-G9 cells. Diabetes Res Clin Pract 44 (2): 83–92, 1999. [DOI] [PubMed] [Google Scholar]
- 187.Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, Yan H, Ussher JR, Oudit GY, Lopaschuk GD. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol 18 (1): 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Katz RL, Hinds L, Mills CJ. Ability of glucagon to produce cardiac stimulation without arrhythmias in halothane-anaesthetized animals. Br J Anaesth 41 (7): 574–578, 1969. [DOI] [PubMed] [Google Scholar]
- 189.Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, Fu H, Watson DE, Lewin AJ, Landschulz WH, Deeg MA, Moller DE, Hardy TA. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care 39 (7): 1241–1249, 2016. [DOI] [PubMed] [Google Scholar]
- 190.Kedees MH, Grigoryan M, Guz Y, Teitelman G. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Mol Cell Endocrinol 311 (1–2): 69–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Keller U, Schnell H, Sonnenberg GE, Gerber PPG, Stauffacher W. Role of glucagon in enhancing ketone body production in ketotic diabetic man. Diabetes 32 (5): 387–391, 1983. [DOI] [PubMed] [Google Scholar]
- 192.Kelley D, Mokan M, Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism 43 (12): 1549–1557, 1994. [DOI] [PubMed] [Google Scholar]
- 193.Kelly RP, Garhyan P, Raddad E, Fu H, Lim CN, Prince MJ, Pinaire JA, Loh MT, Deeg MA. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes Metab 17 (4): 414–422, 2015. [DOI] [PubMed] [Google Scholar]
- 194.Kennedy JE, Marchese A. Regulation of GPCR trafficking by ubiquitin. Prog Mol Biol Transl Sci 132: 15–38, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115 (6): 1627–1635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kieffer TJ, Heller RS, Unson CG, Weir GC, Habener JF. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology 137 (11): 5119–5125, 1996. [DOI] [PubMed] [Google Scholar]
- 197.Kilberg MS, Barber EF, Handlogten ME. Characteristics and hormonal regulation of amino acid transport system A in isolated rat hepatocytes. Curr Top Cell Regul 25: 133–163, 1985. [DOI] [PubMed] [Google Scholar]
- 198.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic alpha-cells in mouse models of ss-cell regeneration. Islets 2 (3): 149–155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim H, Mendez R, Chen X, Fang D, Zhang K. Lysine acetylation of CREBH regulates fasting-induced hepatic lipid metabolism. Mol Cell Biol 35 (24): 4121–4134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R, Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology 155 (3): 769–782, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim T, Nason S, Holleman C, Pepin M, Wilson L, Berryhill TF, Wende AR, Steele C, Young ME, Barnes S, Drucker DJ, Finan B, DiMarchi R, Perez-Tilve D, Tschop M, Habegger KM. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes 67 (9): 1773–1782, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim WD, Lee YH, Kim MH, Jung S-Y, Son W-C, Yoon S-J, Lee B-W. Human monoclonal antibodies against glucagon receptor improve glucose homeostasis by suppression of hepatic glucose output in diet-induced obese mice. PLoS One 7 (12): e50954, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kimball C, Murlin JR. Aqueous extracts of pancreas III. Some precipitation reactions of insulin. J Biol Chem 58 (1): 337–348, 1923. [Google Scholar]
- 204.Klaff LJ, Taborsky GJ. Pancreatic somatostatin is a mediator of glucagon inhibition by hyperglycemia. Diabetes 36 (5): 592–596, 1987. [DOI] [PubMed] [Google Scholar]
- 205.Kleinert M, Sachs S, Habegger KM, Hofmann SM, Muller TD. Glucagon regulation of energy expenditure. Int J Mol Sci 20 (21): 5407, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Knepel W, Jepeal L, Habener JF. A pancreatic islet cell-specific enhancer-like element in the glucagon gene contains two domains binding distinct cellular proteins. J Biol Chem 265 (15): 8725–8735, 1990. [PubMed] [Google Scholar]
- 207.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5 (7): e11707, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437 (7062): 1109–1111, 2005. [DOI] [PubMed] [Google Scholar]
- 209.Kostic A, King TA, Yang F, Chan K-C, Yancopoulos GD, Gromada J, Harp JB. A first-in-human pharmacodynamic and pharmacokinetic study of a fully human anti-glucagon receptor monoclonal antibody in normal healthy volunteers. Diabetes Obes Metab 20 (2): 283–291, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kotowski Sarah J, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron 71 (2): 278–290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Krilov L, Nguyen A, Miyazaki T, Unson CG, Bouscarel B. Glucagon receptor recycling: Role of carboxyl terminus, β-arrestins, and cytoskeleton. Am J Physiol-Cell Physiol 295 (5): C1230–C1237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Krilov L, Nguyen A, Miyazaki T, Unson CG, Bouscarel B. Glucagon receptor recycling: Role of carboxyl terminus, beta-arrestins, and cytoskeleton. Am J Physiol Cell Physiol 295 (5): C1230–C1237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krones A, Kietzmann T, Jungermann K. Periportal localization of glucagon receptor mRNA in rat liver and regulation of its expression by glucose and oxygen in hepatocyte cultures. FEBS Lett 421 (2): 136–140, 1998. [DOI] [PubMed] [Google Scholar]
- 214.Kurose TSY, Nishi S, Tsuji K, Taminato T, Tsuda K, Imura H. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. Am J Physiol 258: 220–227, 1990. [DOI] [PubMed] [Google Scholar]
- 215.Kurose YKH, Honda K, Azuma Y, Sugahara K, Hasegawa S, Kobayashi S. Effects of central administration of glucagon on feed intake and endocrine responses in sheep. Anim Sci J 80 (6): 868–890, 2009. [DOI] [PubMed] [Google Scholar]
- 216.Kuroshima A, Yahata T. Thermogenic responses of brown adipocytes to noradrenaline and glucagon in heat-acclimated and cold-acclimated rats. Jpn J Physiol 29 (6): 683–690, 1979. [DOI] [PubMed] [Google Scholar]
- 217.Lacey JH, Bradford NM, Joseph SK, McGivan JD. Increased activity of phosphate-dependent glutaminase in liver mitochondria as a result of glucagon treatment of rats. Biochem J 194 (1): 29–33, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Münzberg H, Morrison CD. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep 16 (3): 707–716, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest 124 (9): 3913–3922, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Laffel L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diab/Metab Res Rev 15 (6): 412–426, 1999. [DOI] [PubMed] [Google Scholar]
- 221.Langhans W, Pantel K, Muller-Schell W, Eggenberger E, Scharrer E. Hepatic handling of pancreatic glucagon and glucose during meals in rats. Am J Physiol 247 (5 Pt 2): R827–R832, 1984. [DOI] [PubMed] [Google Scholar]
- 222.Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science 218 (4575): 894–896, 1982. [DOI] [PubMed] [Google Scholar]
- 223.LaPierre MP, Abraham MA, Yue JT, Filippi BM, Lam TK. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep 16 (10): 1299–1307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Laraia PJ, Craig RJ, Reddy WJ. Glucagon: Effect on adenosine 3′,5′-monophosphate in the rat heart. Am J Physiol 215 (4): 968–970, 1968. [DOI] [PubMed] [Google Scholar]
- 225.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77 (1): 257–270, 1997. [DOI] [PubMed] [Google Scholar]
- 226.Lau J, Behrens C, Sidelmann UG, Lundt B, Sams C, Ynddal L, Brand CL, Pridal L, Ling A, Kiel D, Plewe M, Shi S, Madsen P. New beta-alanine derivatives are orally available glucagon receptor antagonists. J Med Chem 50 (1): 113–128, 2007. [DOI] [PubMed] [Google Scholar]
- 227.Le Sauter J, Noh U, Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Am J Physiol 261 (1 Pt 2): R162–R165, 1991. [DOI] [PubMed] [Google Scholar]
- 228.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60 (2): 391–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Sanchez PV, Leibiger IB, Berggren P-O. Glucagon regulates its own synthesis by autocrine signaling. Proc Natl Acad Sci U S A 109 (51): 20925–20930, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li K, Qiu C, Sun P, Liu D-C, Wu T-J, Wang K, Zhou Y-C, Chang X-A, Yin Y, Chen F, Zhu Y-X, Han X. Ets1-mediated acetylation of FoxO1 is critical for gluconeogenesis regulation during feed-fast cycles. Cell Rep 26 (11): 2998–3010.e2995, 2019. [DOI] [PubMed] [Google Scholar]
- 231.Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine 45 (2): 178–189, 2014. [DOI] [PubMed] [Google Scholar]
- 232.Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53 (2): 410–417, 2004. [DOI] [PubMed] [Google Scholar]
- 233.Lim SK, Cynober L, De Bandt JP, Aussel C. A Na(+)-dependent system A and ASC-independent amino acid transport system stimulated by glucagon in rat hepatocytes. Cell Biol Int 23 (1): 7–12, 1999. [DOI] [PubMed] [Google Scholar]
- 234.Lin HV, Wang J, Wang J, Li W, Wang X, Alston JT, Thomas MK, Briere DA, Syed SK, Efanov AM. GPR142 prompts glucagon-like Peptide-1 release from islets to improve beta cell function. Mol Metab 11: 205–211, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lin MC, Nicosia S, Rodbell M. Effects of iodination of tyrosyl residues on the binding and action of glucagon at its receptor. Biochemistry 15 (20): 4537–4540, 1976. [DOI] [PubMed] [Google Scholar]
- 236.Linn T, Santosa B, Grönemeyer D, Aygen S, Scholz N, Busch M, Bretzel RG. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 43 (10): 1257–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 237.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, John Yates JO 3rd, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456 (7219): 269–273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Livingston JN, Einarsson K, Backman L, Ewerth S, Arner P. Glucagon receptor of human liver. Studies of its molecular weight and binding properties, and its ability to activate hepatic adenylyl cyclase of nonobese and obese subjects. J Clin Investigat 75 (2): 397–403, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, Drucker DJ. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: Evidence for a circulating alpha-cell growth factor. Diabetes 62 (4): 1196–1205, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Longuet CSE, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 8 (5): 359–371, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lucchesi BR. Cardiac actions of glucagon. Circ Res 22 (6): 777–787, 1968. [DOI] [PubMed] [Google Scholar]
- 242.Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grøndahl M, Hartmann B, Mathiesen ER, Hansen CP, Storkholm JH, van Hall G, Rehfeld JF, Hornburg D, Meissner F, Mann M, Larsen S. Evidence of extrapancreatic glucagon secretion in man. Diabetes 65 (3): 585–597, 2016. [DOI] [PubMed] [Google Scholar]
- 243.Lund A, Vilsboll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab 300 (6): E1038–E1046, 2011. [DOI] [PubMed] [Google Scholar]
- 244.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115 (Pt 3): 455–465, 2002. [DOI] [PubMed] [Google Scholar]
- 245.MacDonald PE, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P. ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLos Biol 5 (6): e143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Madison LL, Seyffert WA, Unger RH, Barker B. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17 (4): 301–304, 1968. [DOI] [PubMed] [Google Scholar]
- 247.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63 (12): 4057–4063, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Markova M, Hornemann S, Sucher S, Wegner K, Pivovarova O, Rudovich N, Thomann R, Schneeweiss R, Rohn S, Pfeiffer AFH. Rate of appearance of amino acids after a meal regulates insulin and glucagon secretion in patients with type 2 diabetes: A randomized clinical trial. Am J Clin Nutr 108 (2): 279–291, 2018. [DOI] [PubMed] [Google Scholar]
- 249.Marliss EBGL, Seydoux J, Wollheim CB, Kanazawa Y, Orci L, Renold AE, Porte D. Glucagon release induced by pancreatic nerve stimulation in the dog. J Clin Invest 52 (5): 1246–1259, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. Direct effects of FGF21 on glucose uptake in human skeletal muscle: Implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 27 (3): 286–297, 2011. [DOI] [PubMed] [Google Scholar]
- 251.McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, Dhanvantari S. Glucose dependence of the regulated secretory pathway in alphaTC1–6 cells. Endocrinology 146 (10): 4514–4523, 2005. [DOI] [PubMed] [Google Scholar]
- 252.McNally KE, Cullen PJ. Endosomal retrieval of cargo: Retromer is not alone. Trends Cell Biol 28 (10): 807–822, 2018. [DOI] [PubMed] [Google Scholar]
- 253.Meek TH, Dorfman MD, Matsen ME, Fischer JD, Cubelo A, Kumar MR, Taborsky GJ, Morton GJ. Evidence that in uncontrolled diabetes, hyperglucagonemia is required for ketosis but not for increased hepatic glucose production or hyperglycemia. Diabetes 64 (7): 2376–2387, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 46 (6): 798–801, 2003. [DOI] [PubMed] [Google Scholar]
- 255.Merlen C, Fabrega S, Desbuquois B, Unson CG, Authier F. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsalpha in rat liver. FEBS Lett 580 (24): 5697–5704, 2006. [DOI] [PubMed] [Google Scholar]
- 256.Mighiu PI, Yue JT, Filippi BM, Abraham MA, Chari M, Lam CKL, Yang CS, Christian NR, Charron MJ, Lam TKT. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med 19 (6): 766–772, 2013. [DOI] [PubMed] [Google Scholar]
- 257.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud P-D, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145 (4): 607–621, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Miller RA, Birnbaum MJ. Glucagon: Acute actions on hepatic metabolism. Diabetologia 59 (7): 1376–1381, 2016. [DOI] [PubMed] [Google Scholar]
- 259.Miller RA, Shi Y, Lu W, Pirman DA, Jatkar A, Blatnik M, Wu H, Cárdenas C, Wan M, Foskett JK, Park JO, Zhang Y, Holland WL, Rabinowitz JD, Birnbaum MJ. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat. Med 24 (4): 518–524, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Milligan G. G protein-coupled receptor dimerisation: Molecular basis and relevance to function. Biochim Biophys Acta 1768 (4): 825–835, 2007. [DOI] [PubMed] [Google Scholar]
- 261.Mitrakou A, Kelley D, Veneman T, Jenssen T, Pangburn T, Reilly J, Gerich J. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes 39 (11): 1381–1390, 1990. [DOI] [PubMed] [Google Scholar]
- 262.Mochiki E, Suzuki H, Takenoshita S, Nagamachi Y, Kuwano H, Mizumoto A, Itoh Z. Mechanism of inhibitory effect of glucagon on gastrointestinal motility and cause of side effects of glucagon. J Gastroenterol 33 (6): 835–841, 1998. [DOI] [PubMed] [Google Scholar]
- 263.Moede T, Leibiger IB, Berggren PO. Alpha cell regulation of beta cell function. Diabetologia 63 (10): 2064–2075, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moffett RC, Vasu S, Thorens B, Drucker DJ, Flatt PR. Incretin receptor null mice reveal key role of GLP-1 but not GIP in pancreatic beta cell adaptation to pregnancy. PLoS One 9 (6): e96863, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 261 (25): 11880–11889, 1986. [PubMed] [Google Scholar]
- 266.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482, 2007. [DOI] [PubMed] [Google Scholar]
- 267.Mu J, Qureshi SA, Brady EJ, Muise ES, Candelore MR, Jiang G, Li Z, Wu MS, Yang X, Dallas-Yang Q, Miller C, Xiong Y, Langdon RB, Parmee ER, Zhang BB. Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PLoS One 7 (11): e49572, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mukharji A, Drucker DJ, Charron MJ, Swoap SJ. Oxyntomodulin increases intrinsic heart rate through the glucagon receptor. Physiol Rep 1 (5): e00112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Muller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ. Glucagon-like peptide 1 (GLP-1). Mol Metab 30: 72–130, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Muller TD, Finan B, Clemmensen C, DiMarchi RD, Tschop MH. The new biology and pharmacology of glucagon. Physiol Rev 97 (2): 721–766, 2017. [DOI] [PubMed] [Google Scholar]
- 271.Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283 (3): 109–115, 1970. [DOI] [PubMed] [Google Scholar]
- 272.Muller WA, Faloona GR, Unger RH. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med 54 (1): 52–57, 1973. [DOI] [PubMed] [Google Scholar]
- 273.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137 (3): 560–570, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nason SR, Kim T, Antipenko JP, Finan B, DiMarchi R, Hunter CS, Habegger KM. Glucagon-receptor signaling reverses hepatic steatosis independent of leptin receptor expression. Endocrinology 161 (1): bqz013, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ni Z, Anini Y, Fang X, Mills G, Brubaker PL, Jin T. Transcriptional activation of the proglucagon gene by lithium and beta-catenin in intestinal endocrine L cells. J Biol Chem 278 (2): 1380–1387, 2003. [DOI] [PubMed] [Google Scholar]
- 276.Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, Zambre Y, Pipeleers D, Friedman TC. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J Clin Invest 105 (7): 955–965, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophy Acta 1492 (1): 203–206, 2000. [DOI] [PubMed] [Google Scholar]
- 278.Oh K-J, Han H-S, Kim M-J, Koo S-H. CREB and FoxO1: Two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep 46 (12): 567–574, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell 12 (3): 685–698, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Okamoto H, Kim J, Aglione J, Lee J, Cavino K, Na E, Rafique A, Kim JH, Harp J, Valenzuela DM, Yancopoulos GD, Murphy AJ, Gromada J. Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 156 (8): 2781–2794, 2015. [DOI] [PubMed] [Google Scholar]
- 281.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 87 (2): 415–423, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Osei K, Falko JM, O’Dorisio TM, Fields PG, Bossetti B. Gastric inhibitory polypeptide responses and glucose turnover rates after natural meals in type II diabetic patients. J Clin Endocrinol Metab 62 (2): 325–330, 1986. [DOI] [PubMed] [Google Scholar]
- 283.Osundiji MA, Evans ML. Brain control of insulin and glucagon secretion. Endocrinol Metab Clin North Am 42 (1): 1–14, 2013. [DOI] [PubMed] [Google Scholar]
- 284.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20 (4): 670–677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pal K, Melcher K, Xu HE. Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors. Acta Pharmacol Sin 33 (3): 300–311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Paloyan E, Harper PV. Glucagon as a regulating factor of plasma lipids. Metabolism 10: 315–323, 1961. [PubMed] [Google Scholar]
- 287.Parmley WW, Glick G, Sonnenblick EH. Cardiovascular effects of glucagon in man. N Engl J Med 279 (1): 12–17, 1968. [DOI] [PubMed] [Google Scholar]
- 288.Pederson RA, Brown JC. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology 103 (2): 610–615, 1978. [DOI] [PubMed] [Google Scholar]
- 289.Penick SB, Hinkle LE Jr. Depression of food intake induced in healthy subjects by glucagon. N Engl J Med 264: 893–897, 1961. [DOI] [PubMed] [Google Scholar]
- 290.Perry RJ, Zhang D, Guerra MT, Brill A. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 579 (7798): 279–283, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Petersen KM, Bogevig S, Holst JJ, Knop FK, Christensen MB. Hemo-dynamic effects of glucagon: A literature review. J Clin Endocrinol Metab 103 (5): 1804–1812, 2018. [DOI] [PubMed] [Google Scholar]
- 292.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13 (10): 572, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Philippe J. Glucagon gene transcription is negatively regulated by insulin in a hamster islet cell line. J Clin Invest 84 (2): 672–677, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Philippe J. Structure and pancreatic expression of the insulin and glucagon genes. Endocr Rev 12 (3): 252–271, 1991. [DOI] [PubMed] [Google Scholar]
- 295.Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci U S A 88 (16): 7224–7227, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Philippe J, Drucker DJ, Habener JF. Glucagon gene transcription in an islet cell line is regulated via a protein kinase C-activated pathway. J Biol Chem 262 (4): 1823–1828, 1987. [PubMed] [Google Scholar]
- 297.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2 (10): 727–733, 2001. [DOI] [PubMed] [Google Scholar]
- 298.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest 116 (7): 1761–1766, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 58 (10): 2258–2266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A 106 (26): 10853–10858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Prip-Buus C, Pegorier JP, Duee PH, Kohl C, Girard J. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Biochem J 269 (2): 409–415, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pryor HJ, Smyth JE, Quinlan PT, Halestrap AP. Evidence that the flux control coefficient of the respiratory chain is high during gluconeogenesis from lactate in hepatocytes from starved rats. Implications for the hormonal control of gluconeogenesis and action of hypoglycaemic agents. Biochem J 247 (2): 449–457, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Qiang L, Banks AS, Accili D. Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J Biol Chem 285 (35): 27396–27401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Quant PA, Tubbs PK, Brand MD. Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme. Eur J Biochem 187 (1): 169–174, 1990. [DOI] [PubMed] [Google Scholar]
- 305.Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: Role in glucose homeostasis and diabetes. J Endocrinol 199 (1): 5–19, 2008. [DOI] [PubMed] [Google Scholar]
- 306.Quinn PG, Granner DK. Cyclic AMP-dependent protein kinase regulates transcription of the phosphoenolpyruvate carboxykinase gene but not binding of nuclear factors to the cyclic AMP regulatory element. Mol Cell Biol 10 (7): 3357–3364, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Quiñones MA, Al-Massadi O, Gallego R, Fernø J, Diéguez C, López M, Nogueiras R. Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Mol Metab 4 (12): 961–970, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ramnanan C, Edgerton D, Kraft G, Cherrington A. Physiologic action of glucagon on liver glucose metabolism. Diabetes, Obes Metabol 13: 118–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Raskin P, Aydin I, Yamamoto T, Unger RH. Abnormal alpha cell function in human diabetes: The response to oral protein. Am J Med 65 (6): 988–997, 1978. [DOI] [PubMed] [Google Scholar]
- 310.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 54 (6): 1789–1797, 2005. [DOI] [PubMed] [Google Scholar]
- 311.Ravnskjaer K, Hogan MF, Lackey D, Tora L, Dent SYR, Olefsky J, Montminy M. Glucagon regulates gluconeogenesis through KAT2B- and WDR5-mediated epigenetic effects. J Clin Investigat 123 (10): 4318–4328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, Montminy M. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J 26 (12): 2880–2889, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64 (1): 106–110, 1987. [DOI] [PubMed] [Google Scholar]
- 314.Reed SM, Quelle DE. p53 acetylation: Regulation and consequences. Cancers (Basel) 7 (1): 30–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: An update. Cell Death Dis 9 (3): 388, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ritz-Laser B, Estreicher A, Gauthier BR, Mamin A, Edlund H, Philippe J. The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia 45 (1): 97–107, 2002. [DOI] [PubMed] [Google Scholar]
- 317.Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest 5 (9): 2346–2351, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med 48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rouille Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem 270 (44): 26488–26496, 1995. [DOI] [PubMed] [Google Scholar]
- 320.Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel) 10 (10): 9002–9025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rui L. Energy metabolism in the liver. Compr Physiol 4 (1): 177–197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Rutter GA. Regulating glucagon secretion: Somatostatin in the spotlight. Diabetes 58 (2): 299–301, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Saberi M, Bjelica D, Schenk S, Imamura T, Bandyopadhyay G, Li P, Jadhar V, Vargeese C, Wang W, Bowman K, Zhang Y, Polisky B, Olefsky JM. Novel liver-specific TORC2 siRNA corrects hyperglycemia in rodent models of type 2 diabetes. Am J Physiol Endocrinol Metab 297 (5): E1137–E1146, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sakurai H, Dobbs RE, Unger RH. The role of glucagon in the pathogenesis of the endogenous hyperglycemia of diabetes mellitus. Metabolism 24 (11): 1287–1297, 1975. [DOI] [PubMed] [Google Scholar]
- 325.Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES, Comninos AN, Buckley A, Win Z, Al-Nahhas A, Rabiner EA, Gunn RN, Budge H, Symonds ME, Bloom SR, Tan TM, Dhillo WS. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab 18 (1): 72–81, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Salter JM. Metabolic effects of glucagon in the wistar rat. Am J Clin Nutr 8 (5): 535–539, 1960. [Google Scholar]
- 327.Salter JM, Ezrin C, Laidlaw JC, Gornall AG. Metabolic effects of glucagon in human subjects. Metabolism 9: 753–768, 1960. [PubMed] [Google Scholar]
- 328.Sammons MF, Lee EC. Recent progress in the development of small-molecule glucagon receptor antagonists. Bioorg Med Chem Lett 25 (19): 4057–4064, 2015. [DOI] [PubMed] [Google Scholar]
- 329.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 2 (7409): 415–416, 1965. [DOI] [PubMed] [Google Scholar]
- 330.Sánchez J, Palou A, Picó C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: Striking effects on fibroblast growth factor-21 induction. Endocrinology 150 (12): 5341–5350, 2009. [DOI] [PubMed] [Google Scholar]
- 331.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59 (7): 1817–1824, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sasaki H, Ebitani I, Tominaga M, Yamatani K, Yawata Y, Hara M. Glucagon-like substance in the canine brain. Endocrinol Jpn 27 (Suppl 1): 135–140, 1980. [DOI] [PubMed] [Google Scholar]
- 333.Schulman JL, Carleton JL, Whitney G, Whitehorn JC. Effect of glucagon on food intake and body weight in man. J Appl Physiol 11 (3): 419–421, 1957. [DOI] [PubMed] [Google Scholar]
- 334.Schutz Y. Protein turnover, ureagenesis and gluconeogenesis. Int J Vitam Nutr Res 81 (2–3): 101–107, 2011. [DOI] [PubMed] [Google Scholar]
- 335.Secchi ACR, Caumo A, Monti LD, Bonfatti D, Di Carlo V, Pozza G. Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism 44 (9): 1153–1158, 1995. [DOI] [PubMed] [Google Scholar]
- 336.Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsbøll T. Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Front Endocrinol 9: 649, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sengupta P, Philip F, Scarlata S. Caveolin-1 alters Ca(2+) signal duration through specific interaction with the G alpha q family of G proteins. J Cell Sci 121 (Pt 9): 1363–1372, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Seyffert WA, Madison LL. Physiologic effects of metabolic fuels on carbohydrate metabolism. I. Acute effect of elevation of plasma free fatty acids on hepatic glucose output, peripheral glucose utilization, serum insulin, and plasma glucagon levels. Diabetes 16 (11): 765–776, 1967. [DOI] [PubMed] [Google Scholar]
- 339.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85 (11): 4053–4059, 2000. [DOI] [PubMed] [Google Scholar]
- 340.Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med 215 (2): 383–396, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Sharma AX, Quittner-Strom EB, Lee Y, Johnson JA, Martin SA, Yu X, Li J, Lu J, Cai Z, Chen S, Wang M-Y, Zhang Y, Pearson MJ, Dorn AC, McDonald JG, Gordillo R, Yan H. Glucagon receptor antagonism improves glucose metabolism and cardiac function by promoting AMP-mediated protein kinase in diabetic mice. Cell Rep 22 (7): 1760–1773, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Shenoy SK. Arrestin interaction with E3 ubiquitin ligases and deubiquitinases: Functional and therapeutic implications. Handb Exp Pharmacol 219: 187–203, 2014. [DOI] [PubMed] [Google Scholar]
- 343.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A 106 (16): 6650–6655, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sherwin RS. Role of the liver in glucose homeostasis. Diabetes Care 3 (2): 261–265, 1980. [DOI] [PubMed] [Google Scholar]
- 345.Shibata C, Naito H, Jin XL, Ueno T, Funayama Y, Fukushima K, Hashimoto A, Matsuno S, Sasaki I. Effect of glucagon, glicentin, glucagon-like peptide-1 and −2 on interdigestive gastroduodenal motility in dogs with a vagally denervated gastric pouch. Scand J Gastroenterol 36 (10): 1049–1055, 2001. [DOI] [PubMed] [Google Scholar]
- 346.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature 210 (5041): 1178–1179, 1966. [DOI] [PubMed] [Google Scholar]
- 347.Shimazu T, Minokoshi Y. Systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus (VMH). J Endocr Soc 1 (5): 449–459, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res 35 (9): 1535–1541, 1994. [PubMed] [Google Scholar]
- 349.Snodgrass PJ, Lin RC, Muller WA, Aoki TT. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem 253 (8): 2748–2753, 1978. [PubMed] [Google Scholar]
- 350.Solloway MJ, Madjidi A, Gu C, Eastham-Anderson J, Clarke HJ, Kljavin N, Zavala-Solorio J, Kates L, Friedman B, Brauer M, Wang J, Fiehn O, Kolumam G, Stern H, Lowe JB, Peterson AS. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Cell Rep 12 (3): 495–510, 2015. [DOI] [PubMed] [Google Scholar]
- 351.Song K-H, Chiang JYL. Glucagon and cAMP inhibit cholesterol 7α-hydroxylase (CYP7a1) gene expression in human hepatocytes: Discordant regulation of bile acid synthesis and gluconeogenesis. Hepatology 43 (1): 117–125, 2006. [DOI] [PubMed] [Google Scholar]
- 352.Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev 9 (1): 463–475, 2011. [PMC free article] [PubMed] [Google Scholar]
- 353.Starke A, Imamura T, Unger RH. Relationship of glucagon suppression by insulin and somatostatin to the ambient glucose concentration. J Clin Invest 79 (1): 20–24, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Stasevich TJ, Hayashi-Takanaka Y, Sato Y, Maehara K, Ohkawa Y, Sakata-Sogawa K, Tokunaga M, Nagase T, Nozaki N, McNally JG, Kimura H. Regulation of RNA polymerase II activation by histone acetylation in single living cells. Nature 516 (7530): 272–275, 2014. [DOI] [PubMed] [Google Scholar]
- 355.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581 (Pt 2): 431–444, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 387 (6631): 406–409, 1997. [DOI] [PubMed] [Google Scholar]
- 357.Struck E, Ashmore J, Wieland O. Stimulation of gluconeogenesis by long chain fatty acids and glucagon. Biochem Z 343 (1): 107–110, 1965. [PubMed] [Google Scholar]
- 358.Strzelecki T, Strzelecka D, Koch CD, LaNoue KF. Sites of action of glucagon and other Ca2+ mobilizing hormones on the malate aspartate cycle. Arch Biochem Biophys 264 (1): 310–320, 1988. [DOI] [PubMed] [Google Scholar]
- 359.Stunkard AJ, Van Itallie TB, Reis BB. The mechanism of satiety: Effect of glucagon on gastric hunger contractions in man. Proc Soc Exp Biol Med 89 (2): 258–261, 1955. [DOI] [PubMed] [Google Scholar]
- 360.Svendsen B, Larsen O, Gabe MBN, Christiansen CB, Rosenkilde MM, Drucker DJ, Holst JJ. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 25 (5): 1127–1134, e1122, 2018. [DOI] [PubMed] [Google Scholar]
- 361.Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol Cell Endocrinol 105 (2): 131–137, 1994. [DOI] [PubMed] [Google Scholar]
- 362.Szymanska E, Budick-Harmelin N, Miaczynska M. Endosomal “sort” of signaling control: The role of ESCRT machinery in regulation of receptor-mediated signaling pathways. Semin Cell Dev Biol 74: 11–20, 2018. [DOI] [PubMed] [Google Scholar]
- 363.Taborsky GJ, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: Implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 47 (7): 995–1005, 1998. [DOI] [PubMed] [Google Scholar]
- 364.Taborsky GJ. The physiology of glucagon. J Diabetes Sci Technol 4 (6): 1338–1344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Taborsky GJ Jr. The Physiology of Glucagon. Newbury Park, CA: SAGE Publications, 2010. [Google Scholar]
- 366.Tan TM, Field BC, McCullough KA, Troke RC, Chambers ES, Salem V, Maffe JG, Baynes KCR, De Silva A, Viardot A, Alsafi A, Frost GS, Ghatei MA, Bloom SR. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes 62 (4): 1131–1138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Taneera J, Jin Z, Jin Y, Muhammed SJ, Zhang E, Lang S, Salehi A, Korsgren O, Renström E, Groop L, Birnir B. γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia 55 (7): 1985–1994, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Taylor CW. Regulation of IP3 receptors by cyclic AMP. Cell Calcium 63: 48–52, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance in humans: Effects on insulin, C-peptide, and glucagon. Am J Physiol 270 (6): 1371–1379, 1996. [DOI] [PubMed] [Google Scholar]
- 370.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: Key regulator of whole-body energy metabolism. Trends Endocrinol Metab 22 (11): 458–466, 2011. [DOI] [PubMed] [Google Scholar]
- 371.Thorburn A, Litchfield A, Fabris S, Proietto J. Abnormal transient rise in hepatic glucose production after oral glucose in non-insulin-dependent diabetic subjects. Diabetes Res Clin Pract 28 (2): 127–135, 1995. [DOI] [PubMed] [Google Scholar]
- 372.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology 147 (5): 2346–2356, 2006. [DOI] [PubMed] [Google Scholar]
- 373.Tovey SC, Dedos SG, Rahman T, Taylor EJ, Pantazaka E, Taylor CW. Regulation of inositol 1, 4, 5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem 285 (17): 12979–12989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Traub S, Meier DT, Schulze F, Dror E, Nordmann TM, Goetz N, Koch N, Dalmas E, Stawiski M, Makshana V, Thorel F, Herrera PL, Böni-Schnetzler M, Donath MY. Pancreatic alpha cell-derived glucagon-related peptides are required for beta cell adaptation and glucose homeostasis. Cell Rep 18 (13): 3192–3203, 2017. [DOI] [PubMed] [Google Scholar]
- 375.Trejo J. Internal PDZ ligands: Novel endocytic recycling motifs for G protein-coupled receptors. Mol Pharmacol 67 (5): 1388–1390, 2005. [DOI] [PubMed] [Google Scholar]
- 376.Tsai WW, Niessen S, Goebel N, Yates JR 3rd, Guccione E, Montminy M. PRMT5 modulates the metabolic response to fasting signals. Proc Natl Acad Sci U S A 110 (22): 8870–8875, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tschop MH, Finan B, Clemmensen C, Gelfanov V, Perez-Tilve D, Müller TD, DiMarchi RD. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab 24 (1): 51–62, 2016. [DOI] [PubMed] [Google Scholar]
- 378.Tsvetanova NG, Irannejad R, von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J Biol Chem 290 (11): 6689–6696, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tucker JD, Dhanvantari S, Brubaker PL. Proglucagon processing in islet and intestinal cell lines. Regul Pept 62 (1): 29–35, 1996. [DOI] [PubMed] [Google Scholar]
- 380.Tups A, Benzler J, Sergi D, Ladyman SR, Williams LM. Central regulation of glucose homeostasis. Compr Physiol 7 (2): 741–764, 2017. [DOI] [PubMed] [Google Scholar]
- 381.Uebi T, Tamura M, Horike N, Hashimoto YK, Takemori H. Phosphorylation of the CREB-specific coactivator TORC2 at Ser(307) regulates its intracellular localization in COS-7 cells and in the mouse liver. Am J Physiol Endocrinol Metab 299 (3): E413–E425, 2010. [DOI] [PubMed] [Google Scholar]
- 382.Ui M, Claus TH, Exton JH, Park CR. Studies on the mechanism of action of glucagon on gluconeogenesis. J Biol Chem 248 (15): 5344–5349, 1973. [PubMed] [Google Scholar]
- 383.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J Clin Invest 122 (1): 4–12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1 (7897): 14–16, 1975. [DOI] [PubMed] [Google Scholar]
- 385.Unger RH, Orci L. Physiology and pathophysiology of glucagon. Physiol Rev 56 (4): 778–826, 1976. [DOI] [PubMed] [Google Scholar]
- 386.Unger RHA-PE, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 49 (4): 837–848, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Unson CG, Cypess AM, Wu CR, Goldsmith PK, Merrifield RB, Sakmar TP. Antibodies against specific extracellular epitopes of the glucagon receptor block glucagon binding. Proc Natl Acad Sci U S A 93 (1): 310–315, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Vajda EG, Logan D, Lasseter K, Armas D, Plotkin DJ, Pipkin JD, Li Y-X, Zhou R, Klein D, Wei X, Dilzer S, Zhi L, Marschke KB. Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD-6972 in healthy subjects and subjects with type 2 diabetes mellitus. Diabetes Obes Metab 19 (1): 24–32, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.van Dongen MG, Geerts BF, Morgan ES, Brandt TA, de Kam ML, Romijn JA, Cohen AF, Bhanot S, Burggraaf J. First proof of pharmacology in humans of a novel glucagon receptor antisense drug. J Clin Pharmacol 55 (3): 298–306, 2015. [DOI] [PubMed] [Google Scholar]
- 390.Van Dyke RW. Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiol 4: 1, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Vasu S, Moffett RC, Thorens B, Flatt PR. Role of endogenous GLP-1 and GIP in beta cell compensatory responses to insulin resistance and cellular stress. PLoS One 9 (6): e101005, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Verma R, Marchese A. The endosomal sorting complex required for transport pathway mediates chemokine receptor CXCR4-promoted lysosomal degradation of the mammalian target of rapamycin antagonist DEPTOR. J Biol Chem 290 (11): 6810–6824, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50 (2): 370–379, 2007. [DOI] [PubMed] [Google Scholar]
- 394.von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, Aeber-sold R, Stoffel M. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab 17 (3): 436–447, 2013. [DOI] [PubMed] [Google Scholar]
- 395.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149: 118–126, 2007. [DOI] [PubMed] [Google Scholar]
- 396.Vuylsteke C, Cornelis G, Duve CD. Influence du traitment au cobalt sur le contenu en facteur HG du pancreas de cobaye. Arch Int Physiol 60: 128, 1952. [Google Scholar]
- 397.Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: Paracrine, intrinsic or both? Diabetes Obes Metab 13: 95–105, 2011. [DOI] [PubMed] [Google Scholar]
- 398.Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes 66 (4): 858–867, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6 (11): a022616, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Wang LMM, Pan C, Yang S, Brandl K, Liu J, Reilly SM, Wang Y, Miao Z, Loomba R, Lu N, Guo Q, Liu J, Yu RT, Downes M, Evans RM, Brenner DA, Saltiel AR, Beutler B, Schnabl B. YIPF6 controls sorting of FGF21 into COPII vesicles and promotes obesity. Proc Natl Acad Sci U S A 116 (30): 15184–15193, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Watanabe O, Atobe Y, Akagi M, Nishi K. Effects of glucagon on myo-electrical activity of the stomach of conscious and anesthetized dogs. Eur J Pharmacol 79 (1–2): 31–41, 1982. [DOI] [PubMed] [Google Scholar]
- 402.Webb GC, Akbar MS, Zhao C, Swift HH, Steiner DF. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes 51 (2): 398–405, 2002. [DOI] [PubMed] [Google Scholar]
- 403.Weiser PC, Grande F. Calorigenic effects of glucagon and epinephrine in anesthetized dogs. Proc Soc Exp Biol Med 145 (3): 912–917, 1974. [DOI] [PubMed] [Google Scholar]
- 404.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes 53 (4): 1038–1045, 2004. [DOI] [PubMed] [Google Scholar]
- 405.West C, Hanyaloglu AC. Minireview: Spatial programming of G protein-coupled receptor activity: Decoding signaling in health and disease. Mol Endocrinol 29 (8): 1095–1106, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wewer Albrechtsen NJ, Faerch K, Jensen TM, Witte DR, Pedersen J, Mahendran Y, Jonsson AE, Galsgaard KD, Winther-Sørensen M, Torekov SS, Lauritzen T, Pedersen O, Knop FK, Hans T. Evidence of a liver-alpha cell axis in humans: Hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 61 (3): 671–680, 2018. [DOI] [PubMed] [Google Scholar]
- 407.Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windeløv JA, Plamboeck A, Bojsen-Møller KN, Idorn T, Feldt-Rasmussen B, Knop FK, Vilsbøll T, Madsbad S, Deacon CF, Holst JJ. Hyperglucagonaemia analysed by glucagon sandwich ELISA: Nonspecific interference or truly elevated levels? Diabetologia 57 (9): 1919–1926, 2014. [DOI] [PubMed] [Google Scholar]
- 408.Wewer Albrechtsen NJ, Junker AE, Christensen M, Hædersdal S, Wibrand F, Lund AM, Galsgaard KD, Holst JJ, Knop FK, Vilsbøll T. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am J Physiol Gastrointest Liver Physiol 314 (1): G91–g96, 2018. [DOI] [PubMed] [Google Scholar]
- 409.Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomark Med 10 (11): 1141–1151, 2016. [DOI] [PubMed] [Google Scholar]
- 410.Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, Gromada J, Vilstrup H, Knop FK, Holst JJ. The liver-alpha-cell axis and type 2 diabetes. Endocr Rev 40 (5): 1353–1366, 2019. [DOI] [PubMed] [Google Scholar]
- 411.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 17 (3): 126–139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic alpha-cells: Is this a paracrine mechanism enabling GLP-1 to act on beta-cells? J Endocrinol 211 (1): 99–106, 2011. [DOI] [PubMed] [Google Scholar]
- 413.Whitehouse FW, James TN. Chronotropic action of glucagon on the sinus node. Proc Soc Exp Biol Med 122 (3): 823–826, 1966. [DOI] [PubMed] [Google Scholar]
- 414.Wideman RD, Yu IL, Webber TD, Verchere CB, Johnson JD, Cheung AT, Kieffer TJ. Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1). Proc Natl Acad Sci U S A 103 (36): 13468–13473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wilson ME, Kalamaras JA, German MS. Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev 115 (1–2): 171–176, 2002. [DOI] [PubMed] [Google Scholar]
- 416.Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51 (10): 1843–1852, 2008. [DOI] [PubMed] [Google Scholar]
- 417.Wu MS, Jeng CY, Hollenback CB, Chen YD, Jaspan J, Reaven GM. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? J Clin Endocrinol Metab 70 (2): 410–416, 1990. [DOI] [PubMed] [Google Scholar]
- 418.Wynshaw-Boris A, Short JM, Loose DS, Hanson RW. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. I. Multiple hormone regulatory elements and the effects of enhancers. J Biol Chem 261 (21): 9714–9720, 1986. [PubMed] [Google Scholar]
- 419.Xiao C, Pavlic M, Szeto L, Patterson BW, Lewis GF. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60 (2): 383–390, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3 (1): 47–58, 2006. [DOI] [PubMed] [Google Scholar]
- 421.Yahata T, Habara Y, Kuroshima A. Effects of glucagon and noradrenaline on the blood flow through brown adipose tissue in temperature-acclimated rats. Jpn J Physiol 33 (3): 367–376, 1983. [DOI] [PubMed] [Google Scholar]
- 422.Yamazaki RK. Glucagon stimulation of mitochondrial respiration. J Biol Chem 250 (19): 7924–7930, 1975. [PubMed] [Google Scholar]
- 423.Yan H, Gu W, Yang J, Bi V, Shen Y, Lee E, Winters KA, Komorowski R, Zhang C, Patel JJ, Caughey D, Elliott GS, Lau YY, Wang J, Li Y-S, Boone T, Lindberg RA, Hu S, Véniant MM. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 329 (1): 102–111, 2009. [DOI] [PubMed] [Google Scholar]
- 424.Yao LF, MacLeod KM, McNeill JH. Glucagon-induced densensitization: Correlation between cyclic AMP levels and contractile force. Eur J Pharmacol 79 (1–2): 147–150, 1982. [DOI] [PubMed] [Google Scholar]
- 425.Ye J. Mechanisms of insulin resistance in obesity. Front Med 7 (1): 14–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 280 (2): 1457–1464, 2005. [DOI] [PubMed] [Google Scholar]
- 427.Yi F, Sun J, Lim GE, Fantus IG, Brubaker PL, Jin T. Cross talk between the insulin and Wnt signaling pathways: Evidence from intestinal endocrine L cells. Endocrinology 149 (5): 2341–2351, 2008. [DOI] [PubMed] [Google Scholar]
- 428.Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 68 (1): 361–371, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Zhang L, Yao W, Xia J, Wang T, Huang F. Glucagon-induced acetylation of energy-sensing factors in control of hepatic metabolism. Int J Mol Sci 20 (8): 1885, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Zhang T, Wang S, Lin Y, Xu W, Ye D, Xiong Y, Zhao S, Guan K-L. Acetylation negatively regulates glycogen phosphorylase by recruiting protein phosphatase 1. Cell Metabol 15 (1): 75–87, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Zhang W-S, Pan A, Zhang X, Ying A, Ma G, Liu B-L, Qi L-W, Liu Q, Li P. Inactivation of NF-κB2 (p52) restrains hepatic glucagon response via preserving PDE4B induction. Nat Commun 10 (1): 4303, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Zhao GN, Zhang P, Gong J, Zhang X-J, Wang P-X, Yin M, Jiang Z, Shen L-J, Ji Y-X, Tong J, Wang Y, Wei Q-F. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med 23 (6): 742–752, 2017. [DOI] [PubMed] [Google Scholar]
- 433.Zhu L, Rossi M, Cui Y, Lee RJ, Sakamoto W, Perry NA, Urs NM, Caron MG, Gurevich VV, Godlewski G, Kunos G, Chen M, Chen W, Wess J. Hepatic β-arrestin 2 is essential for maintaining euglycemia. J Clin Invest 127 (8): 2941–2945, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


