Abstract
Background
The 100th anniversary of the discovery of insulin in Toronto in 1921 is an important moment in medical and scientific history. The demonstration that an extract of dog pancreas reproducibly lowered blood glucose, initially in diabetic dogs and then in humans with type 1 diabetes, was a medical breakthrough that changed the course of what was until then a largely fatal disease. The discovery of the “activity”, soon named “insulin”, was widely celebrated, garnering a Nobel Prize for Banting and McLeod in 1923. Over the ensuing 100 years, research on insulin has advanced on many fronts, producing insights that have transformed our understanding of diabetes and our approach to its treatment.
Scope of Review
This paper will review research on insulin that had another consequence of far broader scientific significance, by serving as a pacesetter and catalyst to bioscience research across many fields. Some of this was directly insulin-related and was also recognized by the Nobel Prize. Equally important, however, was research stimulated by the discovery of insulin that has profoundly influenced biomedical research, sometimes also recognized by the Nobel Prize and sometimes without this recognition.
Major Conclusions
By reviewing some of the most notable examples of both insulin-related and insulin-stimulated research, it becomes apparent that insulin had an enormous and frequently under-appreciated impact on the course of modern bioscience.
Keywords: Insulin, Discovery of insulin, Measurement of insulin, Sequence of insulin, Cloning of insulin, Insulin receptor
1. The discovery of insulin
The story of course begins with the discovery of insulin in Toronto in 1921. Since this epochal event has been so well researched and documented [for example, the excellent book “The Discovery of Insulin” by Michael Bliss [1]], our discussion of that discovery is limited. In fact, the “discovery” of insulin as a transformative therapeutic activity provided no information on the identity of “insulin” at a molecular level. Indeed, McLeod, who shared the Nobel Prize for insulin's discovery, did not think it was a protein. Over the ensuing decades, however, multiple researchers sought to determine the precise identity of insulin, concluded that it was indeed a protein, identified insulin's amino acid sequence, synthesized insulin using conventional protein synthesis, showed that this “man-made” version was actually biologically active, elucidated its crystal structure; developed a technique to measure its levels in biologic specimens, cloned its cDNA, and eventually – employing recombinant DNA technology – made insulin the first recombinant therapeutic protein. These, among many other advances, place insulin research at the center of modern bioscientific discovery.
Understanding the work leading to the discovery of insulin as an activity capable of reversing the metabolic dysfunction of diabetes involves consideration of two distinct but related periods. The first was between 1889 and 1920, involving the work of many international investigators; the second involved the activities of the Toronto group between 1920 and 1922.
In 1889, in Germany, Oskar Minkowski and Joseph von Mering at Strasbourg University reported that removal of the pancreas in experimental animals caused severe diabetes mellitus, allowing them to hypothesize that the pancreas contained a substance required for control of blood glucose. This powerful idea gained support from independent observations that diabetes was sometimes associated with pancreatic damage. In addition, the concept of internal endocrine secretions was beginning to develop, with the discovery of the first hormone, secretin, by Bayliss and Starling at University College London in 1902 [2]. As a result, the hypothesis that the pancreas produced an internal secretion that controlled carbohydrate metabolism became increasingly discussed.
Although the scientific community was vastly smaller and less connected than it is today, a number of investigators, starting with Oskar Minkowski, attempted to show that pancreatic extracts could reverse diabetes, mainly in animals, but some in humans. While there were many suggestions of activity in experiments carried out by Georg Zuelzer in Germany, Ernest Scott and Israel Kleiner in the US, and Nicolae Paulescu in Romania, these were accompanied by toxic reactions from contaminants in the crude extracts being tested, and none allowed a claim that the long-sought pancreatic activity had been found. In addition, the promising work of Paulescu was disrupted by World War I, and Kleiner, who was quite close to making the discovery, abandoned the work when he lost his position at Rockefeller University in 1919 and moved to what was to become New York Medical College, where he was unable to continue his research [3].
The discovery of insulin in Toronto in 1921 had an extremely unlikely provenance. It was not initiated by a researcher interested in or knowledgeable about metabolism or diabetes, but by Frederick Banting, who in 1920 was a 22-year-old orthopedic surgeon trying to establish a practice in London, Ontario, after returning from the war. The slow start to his practice led him to take on a part-time teaching role at London's Western University, where he was assigned to talk with students about carbohydrate metabolism. To learn about this subject, in which he had no expertise, he read the lead article in the November 1920 issue of the journal Surgery, Gynecology and Obstetrics, entitled “The Relation of the Islets of Langerhans to Diabetes with Special Reference to Cases of Pancreatic Lithiasis”. Reading this article led Banting to develop an experimental hypothesis that by ligating the pancreatic ducts of dogs (analogous to the obstruction caused by a ductal stone) and then waiting for the acinar tissue to degenerate, he might then be able to extract the pancreatic factor capable of reversing diabetes and glycosuria. He documented these thoughts in a notebook he eventually left to the University of Toronto.
Even more remarkable, the young surgeon decided to attempt to experimentally prove his hypothesis. He returned to his alma mater, the University of Toronto, met with the professor of physiology, J.J. Macleod, and tried to convince Macleod to provide him with facilities and supplies to attempt the work. Despite understandable skepticism about the likelihood of success, Macleod recognized Banting's ability to conduct the surgical component of the work and his obvious passion for the effort. In the summer of 1921, Macleod agreed to provide the facilities, several dogs, and a medical student assistant, Charles Best, to pursue this study (Figure 1).
Figure 1.
Banting and Best and the experimental dog Marjorie on roof of medical building in Toronto, 1921.
Working with Best, under the supervision of Macleod, the work began in May 1921. Banting and Best performed duct ligations in one group of dogs and de-pancreatectomized others to create diabetes. By the end of July, with Macleod on vacation, they began to make saline extracts of chilled atrophied pancreas for injection into diabetic dogs. These produced variable effects, but on August 3, 1921, Banting and Best's crude extracts from the pancreas of a pancreatectomized dog named Marjorie showed reduced hyperglycemia. After reviewing their results, Macleod encouraged Banting and Best to add a visiting biochemist, J.B. Collip, to the team in an attempt to improve the approach to preparing the pancreatic extract. During this period, Banting also obtained beef pancreata from the slaughterhouse, to see if he could bypass the need to perform duct ligations in dogs, and when it became evident that this approach might also work, their progress accelerated.
On January 11, 1922, less than six months after the experiments in dogs, Leonard Thompson, a 14-year-old diabetic boy, became the first human to receive insulin when he was injected by doctors at Toronto General Hospital with 15 ml of a beef pancreatic extract prepared by Banting and Best. Unfortunately, there was only a minimal effect on blood and urine glucose, no effect on clinical status, and a sterile abscess resulted. However, on January 23, using an improved extract prepared by Collip, Thomson had an immediate response, with blood glucose becoming normal, ketones disappearing, and obvious clinical improvement. This was the first proof that a pancreatic extract could indeed reverse, at least briefly, the diabetic state. Success in other patients followed. The existence of an internal pancreatic secretion that regulated carbohydrate metabolism seemed secure [4] (Figure 2).
Figure 2.
Patient J.L, age 3, before and after insulin in 1923.
On May 3, 1922, armed with additional results, Macleod presented a summary of the Toronto research at a meeting of the Association of American Physicians in Washington, DC. The audience included many experts in the field, and he received a standing ovation for what was seen as one of the major achievements of medical history. Sadly, this achievement was accompanied by ongoing personal rivalries within the team. Banting and Best saw Collip and Macleod as wanting to take over the work and gain credit for it, refusing to recognize their important contributions. How this played out among the four individuals is well described in the historical work by Bliss [1].
A year and a half after this presentation, the 1923 Nobel Prize in Medicine or Physiology was awarded to Banting and Macleod. This enraged Banting, who had to be convinced to accept the Prize for the glory of Canada. He decided to share his half of the Prize with Best, prompting Macleod to share his with Collip, who Bliss thought should have been named a co-recipient by the Nobel Committee. Despite the Nobel going to Banting and Macleod, most people asked today who discovered insulin will say Banting and Best. Remarkably, none of the four conducted important insulin research again.
The University of Toronto, to which patents were assigned by Banting, Best, and Collip for $1, made rapid commercial arrangements for insulin production by extraction of animal pancreas, first with Eli Lilly, then with Connaught Labs in Canada and the Nordisk Insulinlaboratorium, the Danish company founded by August and Marie Krogh, which eventually became Novo-Nordisk. As 1923 drew to a close, commercially produced beef and pork insulin was being used safely in most Western countries, a truly remarkable achievement.
2. The molecular identity of insulin
The discovery of insulin in Toronto provided no insights into the identity of the molecule (or molecules) responsible for producing the activity they called insulin. The field of endocrinology was very young, and no hormone had yet had its molecular identity revealed. In the 1920s and 1930s, several studies employing a variety of approaches supported the idea that insulin was most likely a protein. These included the crystallization of insulin by Abel in 1926, and reports that its biologic activity was lost after exposure to proteases. The industrial mass-production of insulin as a therapeutic, even if only partially purified by modern standards, provided scientists with substantial quantities of the hormone with which to help solve this problem.
Enter Fred Sanger. His interest in insulin and its commercial availability convinced Sanger to launch a major effort to determine whether insulin was a protein and what its amino acid sequence might be. By 1945, Sanger had developed a method for identifying and quantitatively measuring the terminal amino acids in insulin, making it possible to estimate the number and length of peptide chains in the protein. Using this, Sanger showed that bovine insulin contained two end-group amino acids, establishing that insulin was composed of two chains (A and B chains) linked together by cystine rather than 18 chains as had been hypothesized by others. Ultimately, sequences of these chains were published in 1951 and 1952, respectively [5,6].
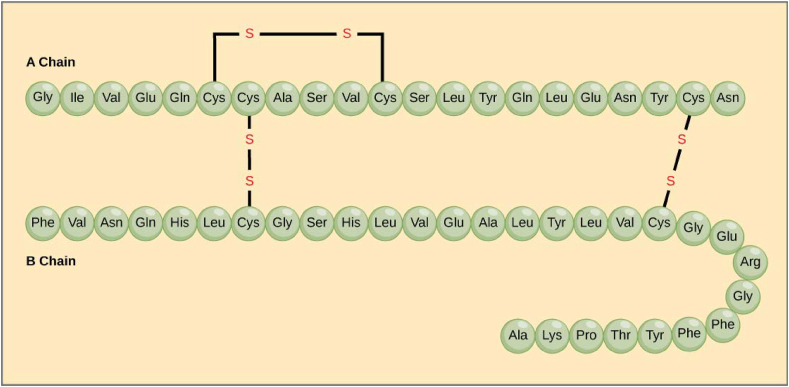
To reach this conclusion, Sanger had to develop multiple new techniques. To determine insulin's amino acid composition, he employed a partition chromatography method initially used to study the amino acid composition of wool. The key to his novel approach was to develop a chemical reagent, 1-fluoro-2,4-dinitrobenzene (DNFB), now called Sanger's reagent, to label and then identify the N-terminal amino acids at one end of polypeptide chains. He hydrolyzed insulin into many short peptides using hydrochloric acid or trypsin, then fractionated these in two dimensions on filter paper. The fragments were identified by ninhydrin, revealing what he termed peptide “fingerprints”. After hydrolysis, the N-termini were identified as the dinitrophenyl-amino acids. By repeating these procedures multiple times with different hydrolysates, Sanger could deduce the complete sequence of the insulin molecule. This involved sequencing both the individual A and B chains, and then eventually identifying the two interchain and one intra-chain disulfide bonds. In this way, insulin was the first protein to have its amino acid sequence and chain composition identified, a truly profound achievement, considering that prior to this work, most assumed that proteins were somewhat amorphous in structure (Figure 3).
Figure 3.
The amino acid sequence of insulin as determined by Sanger.
For this profound insight, in 1958, Sanger was awarded the first of his two Nobel Prizes in Chemistry. In 1980, he was recognized with his second for discovery of the “dideoxy” chain termination method for sequencing DNA, a prize he shared with Walter Gilbert and Paul Berg. The dideoxy method was eventually employed to sequence the human genome.
3. Synthesis of insulin
Although Sanger was awarded the Nobel Prize for developing the general technique for sequencing a protein, applied first to insulin, this work left many questions unanswered. How could we know whether Sanger had arrived at the right amino acid sequence, and would the molecule identified by Sanger actually evoke the biologic actions of insulin? There was then no independent standard against which the proposed amino acid sequence for insulin could be checked (cloning of the gene would not occur until many years later – see below). Furthermore, it was possible that some further modification of the primary structure might be required to render the hormone biologically active.
For these reasons, several groups in the emerging field of synthetic protein chemistry began work in the 1950s to synthesize insulin from its component amino acids. Their goal was to test the fully synthetic insulin for biological activity, thereby confirming that the sequence “proposed” by Sanger was indeed correct. If their synthetic molecule was inactive in insulin bioassays, this could have several explanations: their synthesis might be in error, the sequence proposed by Sanger might be in error, or some unknown further modifications might be required for acquisition of biological activity. However, if the “Sanger insulin molecule” they synthesized was fully active, they would have confirmed that the proposed sequence was correct and demonstrated the capacity of a fully synthetic protein to act as insulin.
Three scientific teams pursued this work: one led by PG Katsoyannis in the US, one led by Helmut Zahn in Germany, and a third group in China. At that time, synthetic techniques had already produced several small peptide hormones, specifically the posterior pituitary hormones oxytocin and vasopressin. This work was viewed as important, and Vincent Du Vigneaud, an American biochemist, was awarded the Nobel Prize for Chemistry in 1955 for that pathbreaking research. However, a protein as large and complex as insulin, with two chains and a total of 51 amino acids, was an enormous challenge.
The chemistry required to build the insulin A and B chains from individual amino acids was laborious, requiring multiple syntheses each with steps to protect amino and carboxyl groups then unblocking these protections, and finally condensing fragments to build the full chains. As this work proceeded, chemical techniques also needed to be developed to separate and then recombine A and B chains derived from animal insulin with sufficient yield and proper disulfide bond formation to create a bioactive molecule so that these techniques could be used to recombine the synthetic insulin chains. The demonstration that synthetic insulin built according to the Sanger model had full insulin biologic activity allowed the conclusion that the structure proposed by Sanger was indeed correct. The groups of Katsoyannis and Zahn reported their successes almost simultaneously in 1963/4, and the Chinese group published its findings the following year [7,8].
These results were a major advance in the study of insulin and were simultaneously an advance for the broader field of protein biology. Katsoyannis listed three reasons he considered the work to be important. First, as already stated, it confirmed that the Nobel Prize-winning insulin sequence published by Sanger was correct. Second, he speculated that, as diabetes increased in prevalence, it might be necessary to supplement animal-derived insulins with synthetic human insulin as a therapeutic. This did not come to pass through protein chemistry, largely because recombinant human insulin eventually became the preferred option, as discussed below. Third, he correctly surmised that synthesis of insulin would enable creation of modified insulin analogs of known amino acid composition to examine the precise structural determinants of insulin action. The work Katsoyannis, Zahn and their colleagues produced many such insulin analogs, providing new insights into insulin action and opening the door to future design of insulin analogs with improved characteristics, therapeutics that now dominate the insulin market. While some observers thought the synthesis of biologically active insulin might garner Katsoyannis and Zahn another insulin-related Nobel Prize, that was not to be.
4. The three-dimensional structure of the insulin molecule
While Sanger was working to deduce the amino acid sequence of insulin, Dorothy Hodgkin was working to advance the field of X-ray crystallography as a means to determine the three-dimensional structure of biological molecules. Her initial success revealed the structures of penicillin and vitamin B12. The latter, published in 1955, brought her the Nobel Prize in Chemistry in 1964, but her work also added an important chapter in the insulin story, which began even before her award-winning work.
Hodgkin graduated from Oxford with a degree in chemistry in 1932 and was awarded a PhD from the University of Cambridge for pioneering studies of X-ray crystallography. Her PhD work was conducted with J.D. Bernal, a pioneer in the use of X-ray crystallography in molecular biology, including determining the x-ray structure of pepsin, the first biological substance successfully studied in this way. Her interest in insulin began very early, in 1934, when she was given a crystal of insulin. She used this to make an image of the insulin crystal with techniques available at the time, which were inadequate to assess a protein of insulin's size and complexity. However, she remained fascinated by the biology of insulin, and after a career of pioneering technical advances and winning the Nobel Prize, she eventually returned to solving the structure of insulin. Working with a team at Oxford University that included Thomas Blundell and Guy Dodson, they solved the structure of insulin and published the results in 1969 [9,10]. In the 1950s and 1960s, Dorothy Hodgkin also helped train Chinese scientists, who returned to China and almost simultaneously determined the structure of insulin. By 1971 and 1972, they obtained crystal structures of porcine insulin at resolutions of 2.5 and 1.8 Å, respectively, demonstrating that in this area of science, China had reached a first-class international level.
Thus, insulin was the first important protein to have its structure solved. Scientists wanted to work on insulin because it was a molecule known to have powerful biological effects, and their hope was that understanding its structure would facilitate deeper understanding of its mechanism of action.
5. The measurement of insulin in blood
Endocrinology is the study of hormones, signaling molecules produced in a regulated fashion in endocrine cells in one tissue, then released into the blood, where they act by binding to receptors on or in one or more target tissues, often at a distance. A major step in understanding the biology of any hormone and its role in disease is, therefore, to measure its level in the blood and determine what makes its levels go up or down. In the case of insulin, understanding its levels in blood in response to glucose and in diseases, such as diabetes, was critical in understanding its physiology and pathophysiology. It was not until 1959, 38 years after its discovery, that insulin could be unambiguously detected and quantified in human serum. This was made possible by a technique first applied to insulin (though broadly applicable) called radioimmunoassay (RIA). RIA was invented by Berson and Yalow. Yalow won the Nobel Prize for this work in 1977, after Berson's death in 1972.
The first attempts to measuring insulin in plasma utilized a wide variety of bioassays. In these, extracts of plasma were administered to animals in which insulin could lower glucose levels or were added to in vitro preparations of fat or muscle in which insulin stimulated glucose uptake or metabolism. Such assays had some utility, but they were technically very challenging and provided widely variable results. Furthermore, their interpretation and specificity were limited both by the poor sensitivity of these approaches and the uncertainty as to whether actions were caused by insulin itself or by other molecules in the samples with insulin-like activity. Indeed, when insulin was added to plasma, it was often difficult to recover its full activity, suggesting that blood might also contain circulating antagonists which could modify insulin action, thus confounding interpretations.
Enter Berson and Yalow, a physician and a physicist, respectively, who worked together starting in 1950 to use radioisotopes to probe biological systems at the Bronx Veterans Hospital. Their research on insulin did not initially arise from a desire to measure the hormone, but a desire to measure its degradation. They became aware of research by I. Arthur Mirsky, who had hypothesized that maturity-onset diabetes was the consequence of insulin being rapidly degraded by a hepatic insulin-degrading enzyme or insulinase. They decided to use their facility with radioisotopes to test this hypothesis in quite a remarkable way. They iodinated (beef) insulin with I131 and administered the tracer insulin to healthy humans and patients treated with insulin, following the course of radioactivity in blood after injection. If Mirsky was correct, they surmised that labeled insulin would disappear more rapidly. Instead, they observed that labeled insulin disappeared more slowly in insulin-treated subjects. Although there were many possible explanations, they hypothesized that these insulin-treated subjects had developed antibodies to administered insulin that delayed its clearance.
To further explore this hypothesis, they assessed the interaction of labeled insulin with plasma protein using chromatoelectrophoresis and other techniques. This demonstrated that labeled insulin bound to a globulin fraction in these patients. Further, they found that the binding was of high affinity and could be blocked by addition of unlabeled insulin. With this observation, they made the critical conceptual leap by realizing that the system could be used to detect and quantify the amount of insulin in a sample by comparing inhibition caused by the unknown sample to a standard curve using known insulin concentrations.
Initial efforts to measure insulin in human plasma using antibodies were unsuccessful, due to relatively low affinity of human insulin as a competitor, since the antibodies in human blood had been evoked to beef and pork insulin used as therapy, which differ from human insulin by three and one amino acid, respectively. However, they persevered and discovered that antibodies elicited by immunizing guinea pigs with beef insulin did cross-react with human insulin with sufficient affinity to permit measurement of human insulin in plasma. In a definitive paper in the Journal of Clinical Investigation in 1960, they reported that insulin measurements in human plasma were sensitive and reproducible, that insulin levels rose after glucose ingestion, and rose to higher levels in subjects with maturity onset diabetes who had never been treated with insulin [11] (Figure 4). They also reported increased insulin levels in patients with hypoglycemia due to islet cell tumors and patients with leucine-sensitive hypoglycemia.
Figure 4.
The measurement of insulin by radioimmunoassay, as reported by Berson and Yalow in the Journal of Clinical Investigation in 1960.
Although it took several years from their initial publication, by the mid-1960s, the RIA approach was widely applied to the study of insulin physiology and pathophysiology, ushering in an era of many in vitro and in vivo investigations of insulin secretion and insulin action, none of which would have been possible without the ability to measure insulin with precision and sensitivity. The concept of RIA was not limited to insulin, of course. The technique was rapidly applied to the measurement of innumerable molecules of biologic interest, hormones and many others, essentially any molecule to which high affinity antibodies could be developed, transforming many fields of study. The principles of the RIA would also be eventually be applied to quantitating interactions between a labeled ligand (hormone, drug, etc.) and its receptor on cells or other bimolecular interactions of interest. Eventually, RIAs employing radioactively-labeled insulin were superseded by enzyme-linked immunosorbant assays (ELISAs) and related techniques, in which an analyte is quantitated by its binding to an antibody linked to an enzyme, which could be measured by its activity, thus eliminating the need for radioactivity. However, the place of RIA in the history of modern biomedical research is assured.
An interesting and initially undesired “side-effect” of RIA was to give no credence to the insulin-like bioactivity in blood which RIA did not measure and which could not be suppressed in bioassays by the addition of insulin antibodies. This non-suppressible insulin-like activity (NSILA), also called “atypical insulin”, was initially discounted as being unimportant by Berson, Yalow, and many in the field, since it was not measured in the RIA for insulin. Thus, it was more than a decade later before this was recognized as being the activity of the insulin-like growth factors IGF-1 and IGF-2, which were also present in serum at even higher concentrations than insulin and circulated as high molecular weight complexes since they were bound to their many IGF binding proteins [12]. The purification, sequencing, and ultimate cloning of these insulin-like growth factors (IGFs) demonstrated that insulin was part of a larger family of hormones, the insulin-IGF family, with the IGFs having up to 50% sequence homology with insulin, but with their own receptors, very low affinities for insulin antibodies, and a very different balance between effects on metabolism, where insulin is dominant, and effects on cell growth, where the IGF's are dominant. Indeed, in higher organisms, IGF-1 is the primary mediator of the action of growth hormone [13].
6. Prohormone processing – another model paradigm with insulin as a leader
Application of the RIA to plasma also revealed that circulating insulin itself occurred in at least two forms, which differed in size and were simply identified as “big” and “little” insulin, with the latter of the appropriate molecular weight to match the hormone [14]. In patients with islet cell tumors, the fraction of big insulin was increased. Using cell labeling and protein chemistry techniques, in 1967, Donald Steiner discovered that this heterogeneity was due to the fact that human insulin was produced as a single-chain precursor with the A and B chains connected by a 33- to 35-amino acid C-peptide. He termed this precursor proinsulin [15] and showed that proinsulin was cleaved in the beta-cell to release the two-chain insulin molecule and equimolar amounts of C-peptide (Figure 5). This insight solved the mystery of how the A and B chains of insulin could relatively easily become associated to form the insulin molecule and served as the first unambiguous demonstration of the post-translational processing of a polypeptide precursor into the mature functional form of the hormone by specialized proteolytic enzymes, a paradigm which is now well appreciated for many hormones.
Figure 5.
The processing of preproinsulin to proinsulin, and then to insulin and C-peptide, as determined by Steiner.
Reduced efficiency of proinsulin processing in islet cell tumors, which led to the increase in “big” insulin in plasma, was the precursor for modern measurements of proinsulin in serum of patients with islet cell tumors, and the release of free C-peptide from the β-cell now serves as an independent marker for insulin secretion, especially in patients in whom insulin levels may be difficult to measure due to endogenous insulin antibodies. Steiner and his colleagues later discovered an even larger precursor of proinsulin, which they termed preproinsulin, i.e., proinsulin including its signal peptide targeting it for secretion [16]. The discovery of proinsulin and preproinsulin established the field of protein-precursor processing, paving the way to understanding how many other peptide hormones and neuropeptides are made and processed and was often discussed as another insulin-related discovery worthy of consideration for a Nobel Prize.
7. Cloning and expression of the insulin gene, and insulin as the first recombinant human therapeutic
During the decades that insulin biology advanced through the application of techniques of protein chemistry, protein synthesis, X-ray crystallography, and assay development, another biologic revolution was taking place that would also leave insulin's mark on modern bioscience. That revolution was the understanding of DNA biochemistry, function, and techniques to manipulate it and created an ability to synthesize insulin and other proteins in a simpler way, thereby unlocking enormous therapeutic possibilities. In 1962, the Nobel Prize for Physiology or Medicine was awarded to Francis Crick, James Watson, and Maurice Wilkins “for their discoveries concerning the molecular structure of nucleic acids and its significance for information transfer in living material”. Over the ensuing years, many scientists focused their attention on understanding the function and regulation of DNA and RNA, and by the early 1970s, recombinant DNA technology emerged as a powerful tool to combine, amplify and express DNA from different species.
In 1973/4, Stanley Cohen of Stanford and Herbert Boyer of UCSF collaborated to develop a process for joining and replicating DNA from different species, and a patent based on this work submitted by Stanford and UCSF was granted by the US Patent Office in 1980 [17]. Many scientists saw the implications of recombinant DNA for development of recombinant therapeutic proteins. It soon became evident that insulin was the first and most important molecule on which to focus, since by that time the Eli Lilly Company was using pancreata from 56 million pigs and cattle each year just to supply the growing US insulin market, and even with only 1 and 3 amino acid differences, respectively, some patients developed significant antibodies to insulin, reducing its potency in vivo.
At least three major groups set out to win the scientific and commercial race to make recombinant human insulin in a commercially viable manner, seeking to replace the animal-derived insulins then available. The dominant groups in the race were at UCSF (led by Bill Rutter and Howard Goodman), Harvard (led by Wally Gilbert), and the newly formed company, Genentech, which had licensed the earlier Cohen and Boyer patents from Stanford and UCSF and which bet its future on their ability to win the race. In 1977, the UCSF and Harvard groups published results showing their distinct abilities to clone the rat insulin cDNAs, the former using mRNA of pancreatic islets, the latter using mRNA from a rat insulinoma [18,19]. The Harvard effort was further complicated by the fact that Cambridge, Massachusetts where Harvard is located had passed a city ordinance banning work on recombinant DNA, thus requiring Gilbert and his team to go to England's Porton Down facility to try and isolate human insulin. Rather than cDNA cloning, Genentech took the alternative approach of creating the human insulin-encoding sequence with synthetic DNA using technology developed by Riggs and Itakura at City of Hope Medical Center. Their approach was to separately clone and express the human insulin A and B chains and then recombine them to make intact insulin, as done earlier by insulin chemists like Katsoyannis and Zahn [20]. The Genentech group was the first to reach a commercially viable level of human insulin production, enabling them to sign an agreement with Lilly to perform clinical trials of recombinant human insulin, which proved to be highly effective and less immunogenic. Humulin was approved by the FDA in 1982 and marketed as the first recombinant human therapeutic in 1983, a landmark event.
The 1980 Nobel Prize in Chemistry was split, with half of the Prize awarded to Paul Berg for his contributions to recombinant DNA technology and the other half shared by Wally Gilbert and Fred Sanger for DNA sequencing. Although this Prize was not directly for cloning the insulin gene, it was certainly linked to insulin, since during the several years before his Nobel Prize, Gilbert's primary focus was on the cloning and expression of the insulin gene, and ultimately, insulin was the first human therapeutic derived using recombinant DNA technology.
8. How insulin brings about actions on target cells
In the 1960s, understanding of insulin's actions in target cells was extremely limited. The ability of insulin to increase glucose uptake and metabolism in fat and muscle was established, as was the ability of insulin to alter activity of several intracellular enzymes, but how this was accomplished was unknown and highly debated. Some scientists thought insulin, and other peptide hormones, entered cells and bound directly to enzymes it regulated (perhaps through formation of disulfide bonds), while others thought a membrane receptor binding step coupled to signal generation might be involved. The term “signal transduction”, which is widely used today, was 20 years in the future. However, this concept was greatly aided by studies of the mechanism of action of the hormone epinephrine by the group of Earl Sutherland, Jr. who showed that this beta-adrenergic agonist generated an intracellular mediator or second messenger named cyclic AMP, for which Sutherland was awarded the 1971 Nobel Prize in Physiology or Medicine [21]. The first step by which epinephrine interacted with cells to generate cAMP, however, was unknown.
Enter Jesse Roth, a physician scientist and Solomon Berson's first research fellow, and Pedro Cuatrecasas, a Spanish born and U.S. educated MD, both of whom came to the National Institutes of Health (NIH) in the early 1960s. This was a golden era at NIH, with both of these investigators doing truly ground-breaking work. Cuatrecasas, who was pioneering affinity chromatography, showed that insulin bound to sepharose beads was biologically active, suggesting it must work through a receptor on the surface of the cell. Likewise, Roth and his collaborators, showed that thyroid-stimulating hormone (TSH) also likely worked through a surface receptor on thyroid cells, since its activity could be reversed by addition of anti-TSH antibodies. Both aimed to devise methods to identify plasma membrane receptors for peptide hormones, using I131-labeled hormones. Roth's first effort involved ACTH, and a 1970 paper first authored by Robert Lefkowitz (who also went on to win a Nobel Prize for his work on adrenergic receptors) demonstrated high affinity and specific binding sites for labeled ACTH in adrenal membranes [22]. Roth soon turned his attention to insulin, and the 1971 paper from his lab characterizing high affinity and specific insulin binding to sites in rat liver membranes was the first demonstration of what soon became known as “insulin receptors” [23] (Figure 6). Cuatrecasas showed similar insulin receptor binding on adipocyte membranes, another major tissue of insulin action [24]. Over the next decade, both labs, but especially that of Jesse Roth, went on to further characterize insulin receptor binding kinetics, provide the evidence for physiologic regulation of their expression and their role in disorders of insulin resistance, and provide initial insights into their structure [25,26]. Similar methodologies were soon applied to studies of many other peptide hormones, thus creating a whole new field of membrane receptor biology. All of these insights occurred long before the receptor was purified, or its cDNA cloned [27]. One final link to Nobel history was that Stanley Cohen received the Nobel Prize for Physiology or Medicine in 1986 for his discovery of epidermal growth factor, which he showed to be the first receptor tyrosine kinase in 1982 [28], the year before Kahn and co-workers showed that the insulin receptor was a tyrosine kinase [29].
Figure 6.
First report of specific high-affinity insulin receptor binding by Freychet, Roth, and Neville in 1971.
Additional biochemical studies of what is now known as “signal transduction” and the additional analysis that became possible following the cloning of the receptor cDNA's have transformed both our understanding of insulin actions and provided new insights into parallel signaling systems used by insulin and the IGFs and the truly massive field of signal transduction from membrane receptors to numerous cellular pathways. Once again, insulin was at the heart of this burgeoning field of study.
This also opened the field to many other important aspects of insulin and its actions, many of which are covered in more detail in other articles in this issue. These include understanding the insulin molecule in evolution, defining the complimentary relationships between insulin and IGFs, understanding insulin and IGF-1 receptor signaling systems, including the role of insulin action in tissues not previously thought to be insulin sensitive, to the development of new insulin analogs to improve treatment of type 1 diabetes, and understanding the role of insulin resistance in type 2 diabetes and metabolic syndrome.
From the breadth of these topics, it should be clear that since its discovery 100 years ago, understanding the insulin molecule, how it works, and what goes wrong in diabetes have been important drivers in defining many paradigms underlying contemporary biology, biochemistry, physiology and medicine and many discoveries recognized by the Nobel Prize or worthy of that recognition.
Conflict of interest
None declared.
References
- 1.Bliss M. University of Chicago Press; Chicago: 1982. The discovery of insulin; p. 304. 16 p. of plates. [Google Scholar]
- 2.Bayliss W.M., Starling E.H. The mechanism of pancreatic secretion. The Journal of Physiology. 1902;28(5):325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman J. 2015. Discovery, interrupted. (Harpers). [Google Scholar]
- 4.Banting F.G., Best C.H., Collip J.B., Campbell W.R., Fletcher A.A. Pancreatic extracts in the treatment of diabetes mellitus. Canadian Medical Association Journal. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 5.Sanger F., Tuppy H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochemical Journal. 1951;49(4):481–490. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanger F., Thompson E.O. The amino-acid sequence in the glycyl chain of insulin. Biochemical Journal. 1952;52(1):iii. [PubMed] [Google Scholar]
- 7.Meienhofer J., Schnabel E., Bremer H., Brinkhoff O., Zabel R., Sroka W. [Synthesis of insulin chains and their combination to insulin-active preparations] Zeitschrift für Naturforschung B. 1963;18:1120–1121. [PubMed] [Google Scholar]
- 8.Katsoyannis P.G. The synthesis of the insulin chains and their combination to biologically active material. Diabetes. 1964;13:339–348. doi: 10.2337/diab.13.4.339. [DOI] [PubMed] [Google Scholar]
- 9.Adam M.G., Coller L., Hodgkin D.C., Dodson G.G. X-ray crystallographic studies on zinc insulin crystals. The American Journal of Medicine. 1966;40(5):667–671. doi: 10.1016/0002-9343(66)90146-x. [DOI] [PubMed] [Google Scholar]
- 10.Blundell T.L., Dodson G.G., Dodson E., Hodgkin D.C., Vijayan M. X-ray analysis and the structure of insulin. Recent Progress in Hormone Research. 1971;27:1–40. doi: 10.1016/b978-0-12-571127-2.50025-0. [DOI] [PubMed] [Google Scholar]
- 11.Yalow R.S., Berson S.A. Immunoassay of endogenous plasma insulin in man. Journal of Clinical Investigation. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinderknecht E., Humbel R.E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. Journal of Biological Chemistry. 1978;253(8):2769–2776. [PubMed] [Google Scholar]
- 13.Roberts C.T., Jr., Leroith D. Molecular aspects of insulin-like growth factors, their binding proteins and receptors. Bailliere's Clinical Endocrinology and Metabolism. 1988;2(4):1069–1085. doi: 10.1016/s0950-351x(88)80030-2. [DOI] [PubMed] [Google Scholar]
- 14.Roth J., Gorden P., Pastan I. “Big insulin”: a new component of plasma insulin detected by immunoassay. Proceedings of the National Academy of Sciences of the United States of America. 1968;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner D.F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- 16.Lomedico P.T., Chan S.J., Steiner D.F., Saunders G.F. Immunological and chemical characterization of bovine preproinsulin. Journal of Biological Chemistry. 1977;252(22):7971–7978. [PubMed] [Google Scholar]
- 17.Cohen S.N., Chang A.C., Boyer H.W., Helling R.B. Construction of biologically functional bacterial plasmids in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1973;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W.J. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- 19.Villa-Komaroff L., Broome S., Naber S.P., Efstratiadis A., Lomedico P., Tizard R. The synthesis of insulin in bacteria: a model for the production of medically useful proteins in prokaryotic cells. Birth Defects: Original Article Series. 1980;16(1):53–68. [PubMed] [Google Scholar]
- 20.Goeddel D.V., Kleid D.G., Bolivar F., Heyneker H.L., Yansura D.G., Crea R. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland E.W., Robison G.A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacological Reviews. 1966;18(1):145–161. [PubMed] [Google Scholar]
- 22.Lefkowitz R.J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1970;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freychet P., Roth J., Neville D.M., Jr. Insulin receptors in the liver: specific binding of (125I)insulin to the plasma membrane and its relation to insulin bioactivity. Proceedings of the National Academy of Sciences of the United States of America. 1971;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocrine Reviews. 1981;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- 25.Flier J.S., Kahn C.R., Roth J. Receptors, antireceptor antibodies and mechanisms of insulin resistance. New England Journal of Medicine. 1979;300(8):413–419. doi: 10.1056/NEJM197902223000808. [DOI] [PubMed] [Google Scholar]
- 26.Kahn C.R., Baird K.L., Flier J.S., Grunfeld C., Harmon J.T., Harrison L.C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Progress in Hormone Research. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- 27.Ullrich A., Bell J.R., Chen E.Y., Herrera R., Petruzzelli L.M., Dull T.J. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S., Fava R.A., Sawyer S.T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasuga M., Fujita-Yamaguchi Y., Blithe D.L., Kahn C.R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]