Abstract
Background
Since its discovery 100 years ago, insulin, as the ‘cure’ for type 1 diabetes, has rescued the lives of countless individuals. As the century unfolded and the autoimmune nature of type 1 diabetes was recognised, a darker side of insulin emerged. Autoimmunity to insulin was found to be an early marker of risk for type 1 diabetes in young children. In humans, it remains unclear if autoimmunity to insulin is primarily due to a defect in the beta cell itself or to dysregulated immune activation. Conversely, it may be secondary to beta-cell damage from an environmental agent (e.g., virus). Nevertheless, direct, interventional studies in non-obese diabetic (NOD) mouse models of type 1 diabetes point to a critical role for (pro)insulin as a primary autoantigen that drives beta cell pathology.
Scope of review
Modelled on Koch's postulates for the pathogenicity of an infectious agent, evidence for a pathogenic role of (pro)insulin as an autoantigen in type 1 diabetes, particularly applicable to the NOD mouse model, is reviewed. Evidence in humans remains circumstantial. Additionally, as (pro)insulin is a target of autoimmunity in type 1 diabetes, its application as a therapeutic tool to elicit antigen-specific immune tolerance is assessed.
Major conclusions
Paradoxically, insulin is both a ‘cure’ and a potential ‘cause’ of type 1 diabetes, actively participating as an autoantigen to drive autoimmune destruction of beta cells - the instrument of its own destruction.
Keywords: Beta cell, Insulin, Autoantigen, Autoantibody, T cell, Immune tolerance, NOD mouse
Graphical abstract
1. Prologue
One hundred years ago in Toronto, Frederick Banting, Charles Best, John Macleod, and James Collip demonstrated that a partially purified extract of dog pancreas (which they later called insulin) decreased glycemia and prolonged the life of pancreatectomised dogs [1]. Within a year, insulin became the elixir of life for young people ravaged by type 1 diabetes. This remarkable bench-to-bedside story stands as one of the greatest life-saving achievements of medical science. As the 20th century unfolded, the amino acid sequence and structure of insulin were determined, and insulin deficiency in type 1 diabetes was confirmed by specific radioimmunoassay. In addition, though the inflammation of the islets of Langerhans in diabetes was described before the discovery of insulin [2], the infiltration of islets by immune cells in type 1 diabetes (the ‘insulitis’ lesion) was documented [3]. Evidence then emerged regarding the autoimmune nature of beta-cell destruction in the form of antibodies to the islets [4], which were present before the onset of the clinical disease [5]. Today, defined by their antigen specificity, islet autoantibodies to insulin (IAA), glutamic acid decarboxylase 65,000 mol. wt. isoform (GADA), insulinoma-like antigen-2 (IA-2A), or beta cell-specific zinc transporter-8 (ZTA) is present in at least 90% of Caucasian children months or years before the onset of clinical type 1 diabetes, and their number and titer correlate with the rate of progression to clinical diabetes [[6], [7], [8], [9], [10]].
My purpose here is not to generally elaborate on the autoimmune features of type 1 diabetes but to focus on autoimmunity to insulin/proinsulin and its role in the pathogenesis of the disease. George Eisenbarth first proposed that autoimmunity to insulin was the rate-limiting factor in the pathogenesis of type 1 diabetes [11], a view that has received considerable attention and support [[12], [13], [14]].
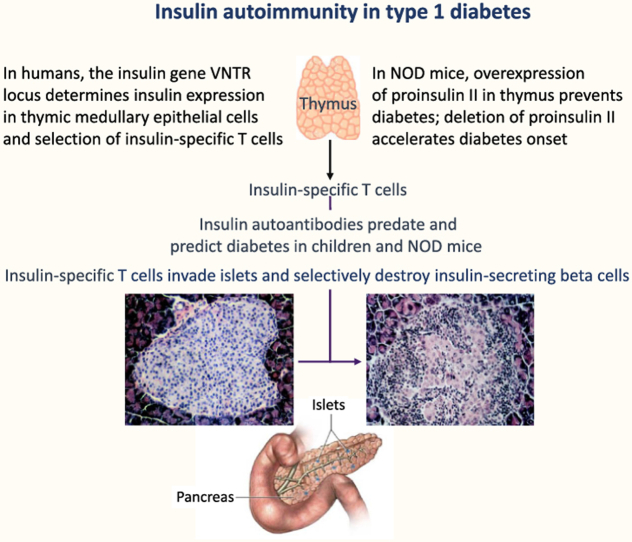
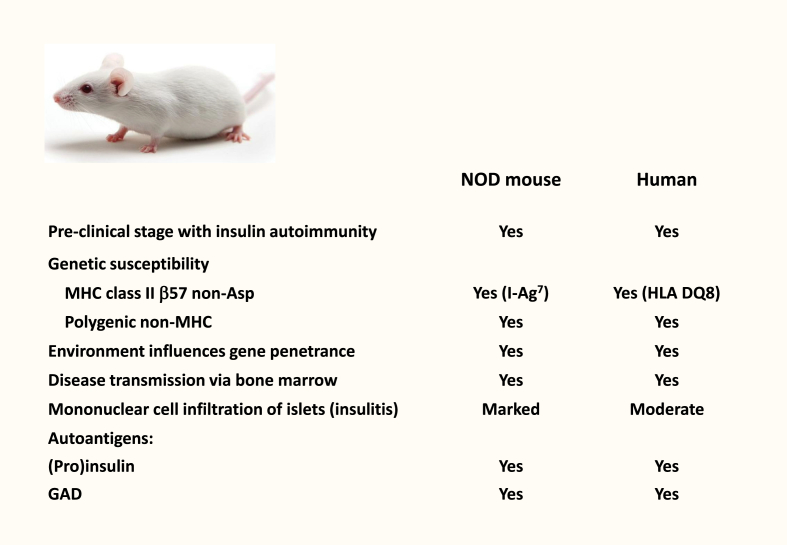
Insulin is an early target autoantigen in beta-cell autoimmunity in children who develop type 1 diabetes, but is it pathogenic - the cause of its own destruction? Ultimately, evidence for ‘causality’ is not possible to educe in humans. However, criteria for insulin as a primary, pathogenic autoantigen (analogous to those used by Robert Koch [15] to identify the pathogenicity of a microorganism) can be applied in the NOD mouse model of type 1 diabetes (Figure 1). Thus, if insulin/proinsulin is pathogenic and drives beta-cell destruction, it must be 1) expressed uniquely in the autoimmune target beta cell, 2) a target of immune responses that predate and predict clinical disease, 3) the target of antibodies or T cells that transfer disease to healthy recipients, and 4) able to prevent disease when presented in an immune ‘tolerogenic’ form.
Figure 1.
The NOD mouse shares features with human childhood type 1 diabetes.
2. Insulin is expressed uniquely in the autoimmune target beta cell
Except for self-antigen expressing cells in lymphoid tissues that determine the selection of T cells during development [16] (see below), insulin is the only known type 1 diabetes autoantigen uniquely expressed in beta cells.
3. Insulin is a target of immune responses that predate and predict clinical type 1 diabetes
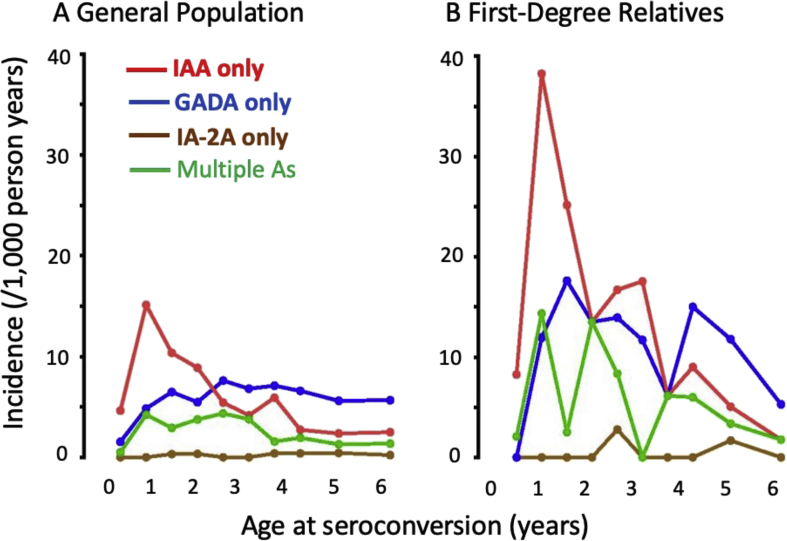
Circulating antibodies to insulin was first documented in the 1980s in a small proportion of newly diagnosed individuals who had not yet been treated with insulin [17]. Antibodies to insulin are known to develop in response to insulin therapy, but their prior presence suggests autoimmunity to endogenous insulin, which could be primarily due to a defect in the beta cell or the dysregulated immune activation. Conversely, it may be secondary to beta-cell damage from an environmental agent (e.g., virus). In studies of children at an increased genetic risk of type 1 diabetes (with a first-degree relative with type 1 diabetes) followed from birth, high-affinity IgG IAA was associated with young age, when they are more often the first sign of islet autoimmunity, especially in HLA-DR 4 individuals [8]. Moreover, they frequently antedate autoantibodies to other islet antigens (e.g., GADA or IA-2A; Figure 2) and, in combination, denote the highest risk for progression to clinical type 1 diabetes [[6], [7], [8], [9], [10]]. In NOD mice, IAA also predates and predicts clinical disease [18].
Figure 2.
Incidence of first-appearing islet autoantibodies in A) general population and B) first-degree relatives of a proband with type 1 diabetes. adapted from krischer JP et al. Diabetes care 2017;40:1194-1202.
Insulin-specific T cells are inferred by the presence of IgG antibodies to insulin, given that T cells are required for immunoglobulin class switching. Peripheral blood CD4+ and CD8+ islet antigen-specific T cells (including insulin, proinsulin, and C-peptide) have been identified by different assay methods [19,20], including studies of islet autoantibody-positive at-risk individuals before the onset of clinical type 1 diabetes [[21], [22], [23], [24], [25], [26], [27]]. These studies have shown that T cell reactivity to (pro)insulin protein or peptide epitopes is specific for and predates clinical type 1 diabetes, though the predictive value of insulin-reactive T cells has not yet been established due to the paucity of longitudinal studies to quantify their number and function.
Recently, novel insulin neo-epitopes called hybrid insulin peptides (HIPs), formed within secretory granules of beta cells by covalent bonding between (pro)insulin peptides and other beta-cell peptides, have been described in humans with type 1 diabetes and NOD mice [28]. In NOD mice, HIP-specific T cells secrete pro-inflammatory cytokines and transfer diseases, reflecting the disease activity of pre-diabetes. Moreover, the epitope for the prototypical diabetogenic BDC-2.5 T-cell clone from NOD mice is an HIP neoepitope formed from a fragment of insulin C-peptide bound to the N-terminal sequence of the WE14 peptide of secretory granule chromogranin A. Importantly, in a recent longitudinal study of humans genetically at-risk for type 1 diabetes, T-cell responses to insulin-based HIPs were stronger than the responses to insulin peptides and associated with the development of insulin autoantibodies or progression to clinical type 1 diabetes [29]. The post-translational generation of HIPs may explain how immune tolerance to insulin (ordinarily expressed in the thymus to induce self-tolerance) is broken, resulting in autoimmunity to insulin. Immune tolerance to insulin could be bypassed by other post-translational modifications of insulin [e.g., [30]], but HIPs have emerged as a compelling mechanism.
4. Insulin is the target of antibodies or T cells that transfer type 1 diabetes to healthy recipients
There is no evidence that IAA can transfer diabetes in humans or NOD mice. However, immune cells can transfer disease and are indicated by observing that type 1 diabetes has developed in recipients of bone marrow transplanted from type 1 diabetes donors [31]. Adoptive transfers in animal models have shown that insulin-specific T cells transfer autoimmune diabetes. In NOD mice, most T-cell lines isolated from islets react to the B-chain of insulin [32], and insulin B9–B23 peptide-specific T-cell clones transfer diabetes to healthy syngeneic recipients [33]. Similarly, in Lewis rats, CD4+ T-cell clones to MHC class II-binding proinsulin B23–C39, but not several GAD peptides, were able to transfer diabetes [34]. Thus, in animal models, adoptive transfer demonstrates that insulin-specific T cells mediate beta-cell destruction.
5. Insulin prevents type 1 diabetes when presented in an immune ‘tolerogenic’ form
5.1. Pre-natal immune tolerance to insulin
Many chromosomal loci have been associated with the risk for type 1 diabetes [35,36], but most are weak associations, defined only by single nucleotide polymorphisms (SNPs) without known functional contributions to pathogenesis. However, it is clear that the HLA locus (IDDM1) is the single most crucial genetic contributor [37], accounting for about half the lifetime risk of disease. The second most important contribution is from the insulin gene (INS) locus (IDDM2), which maps 5′ of the coding sequence to a variable number of tandem repeats (VNTR). This effect may explain why autoimmunity targets insulin-producing beta cells in an organ-specific manner in type 1 diabetes. Among many different VNTR alleles, short class I alleles (26–63 repeats, present in ~80% population frequency) confer susceptibility to type 1 diabetes, and long class III alleles (141–209 repeats, ~20% population frequency) confer dominant protection (3- to 5-fold decrease in risk compared with class I/I homozygosity) [38]. Short (class I) and long (class III) VNTR alleles are associated, respectively, with a lower and higher transcription of proinsulin messenger RNA in medullary thymic epithelial cells under the control of the autoimmune regulator (AIRE) gene [39]. In addition, blood myeloid cells express a proinsulin RNA splice variant, the abundance of which also correlates with the length of the VNTR [40]. Proinsulin expression in the immune system, regulated by the IDDM2 VNTR, may determine the extent of the deletion of proinsulin-specific T cells during development. The short VNTR associated with lower proinsulin expression results in weak avidity for signalling proinsulin-specific T cells, leading to their positive selection and survival. However, the long VNTR associated with higher proinsulin expression results in strong avidity for signalling, leading to the negative selection and death of proinsulin-specific T cells. This mechanism predicts differences in the frequency and function of proinsulin-reactive T cells in the periphery according to the VNTR, but evidence for this effect [41] requires confirmation. In summary, the INS VNTR (IDDM2) provides a genetic mechanism for autoimmunity that targets proinsulin and the β cell in humans.
Mice do not have an INS VNTR; they use a different mechanism to regulate immunity to insulin. Unlike humans, they have two non-allelic insulin genes, InsI and InsII, located on different chromosomes. Both are expressed in β cells but encode slightly different proteins.
In the thymus, proinsulin is expressed by InsII. Based on a preliminary finding of low or absent InsII expression in the thymus of NOD mice [42], subsequently confirmed [43], we determined that the overexpression of InsII in NOD mice prevents diabetes by transgenically expressing InsII on a major histocompatibility complex (MHC) class II promoter in antigen-presenting cells (APCs), including those in the thymus [42]. Transgenic mice did not develop insulitis or diabetes and were resistant to the induction of diabetes by cyclophosphamide, which accelerates diabetes onset by selectively inhibiting regulatory T cells [44]. Thus, proinsulin-specific effector T cells appeared to have a pivotal role in effecting islet autoimmunity.
The total prevention of diabetes in NOD mice was also observed after syngeneic transplantation of minimal numbers of hematopoietic stem cells encoding proinsulin transgenically expressed in APCs [45]. Moreover, the transgenic model was extended to show that islet autoimmunity is prevented by tolerance to proinsulin but not the islet autoantigen IGRP [46]. Mice rendered tolerant to insulin by transgenic InsII overexpression in APCs did not develop responses to the immunodominant IGRP206-214 epitope and were protected from diabetes. Conversely, mice transgenically tolerised to IGRP expressed were not protected from diabetes, indicating that IGRP-specific immunity is downstream of insulin-specific immunity. Furthermore, the prerequisite for insulin-specific immunity was observed in NOD8.3 mice, transgenic for islet-reactive T cells bearing a receptor for the IGRP206-214 epitope, present in abundance in the early islet lesion [47].
A series of elegant knockout studies corroborated the role of proinsulin as a primary driver of autoimmune beta cell destruction in NOD mice. A deficiency of InsI or InsII did not affect insulin production due to compensation, but a deficiency of InsII (leading to lack of proinsulin expression in the thymus) resulted in peripheral T-cell reactivity to insulin and C-peptide [48]. InsII−/− NOD mice had accelerated insulitis and diabetes onset with enhanced autoimmunity to insulin [49]. Conversely, insulitis and diabetes onset were decreased in InsI−/− NOD mice [50]. Subsequently, the tolerogenic effect of thymus-specific proinsulin expression was shown directly by InsII−/− knockout in Aire-expressing medullary thymic epithelial cells, without affecting its expression in the β cells [51]. Providing convincing evidence that the insulin B chain contains the initiating autoepitope when Ins1 and Ins2 were deleted and replaced by a hormonally active Ins transgene carrying a single amino acid mutation at B16, NOD mice were completely protected from insulitis and diabetes [52]. The B16 mutation impaired recognition by both CD4+ and CD8+ T cells of overlapping epitopes B9-23 and B15-23, respectively.
5.2. Post-natal immune tolerance to insulin
The application of insulin or proinsulin as a therapeutic tool for inducing autoantigen-specific tolerance, which translated into the prevention of diabetes, would reinforce the evidence for (pro)insulin as a primary driver of beta-cell destruction. To this end, proof-of-concept studies were initiated in NOD mice beginning in the 1980s. Insulin or proinsulin was administered via a tolerogenic route (mucosal, dermal), cell type (resting dendritic cell), mode (with blockade of co-stimulation molecules), or form (as an ‘altered peptide ligand’). The aim was to determine whether autoantigen-specific immunotherapy would boost or restore immune regulatory mechanisms (e.g., induce regulatory T cells [iTreg]) and/or delete or anergize effector T cells, preventing diabetes. The many studies in this area are reviewed in detail elsewhere [13,[53], [54], [55], [56], [57]]. In summary, insulin protein, proinsulin peptides, or proinsulin DNA administered to the mucosa (via oral or nasal routes; or by subcutaneous [s.c.] or intraperitoneal injection), in which it is not absorbed but locally acts as an antigen to induce Treg, decreased the incidence or delayed the onset of diabetes in NOD mice. However, the doses and schedules used did not completely prevent the disease.
On this basis and despite many other interventions having similar partial benefits in NOD mice, and as safety and regulatory issues with insulin administration were minor, randomised control trials of s.c., oral, and nasal insulin and s.c. proinsulin peptides were initially implemented in individuals with recent-onset type 1 diabetes (tertiary prevention). It was then performed in first-degree relatives with islet autoantibodies at high risk for clinical disease (secondary prevention). In general, these trials yielded important insights, though the outcomes of beta-cell preservation and delayed progression to clinical diseases were disappointing. Several factors may account for the apparent failure of insulin-specific immunotherapy trials, including the challenge of finding an optimum dose/schedule to achieve measurable bioeffects and this approach likely being more effective for primary prevention before the onset of disease (as shown in NOD mice). The reader is referred to other sources [13,[53], [54], [55], [56], [57]] for details of these trials.
The Diabetes Prevention Trial (DPT)-1 in the USA was initiated in 1994 to determine whether antigen-specific therapy with either systemic or oral insulin would delay or prevent diabetes onset in asymptomatic high-risk first-degree relatives with islet autoantibodies. Intensive systemic insulin therapy had been reported to prolong the “honeymoon phase” after clinical diagnosis [58], and a pilot study of systemic insulin had suggested that this approach might be beneficial in high-risk relatives [59]. Systemic insulin might act as a hormone to control blood glucose and “rest” beta cells, making them less sensitive to immune attacks. It might also act as an antigen to induce immune tolerance, though read-outs to identify immune mechanisms were not employed. Low-dose systemic insulin (annual intravenous insulin infusion and daily subcutaneous insulin injection) in high-risk relatives matched with untreated but closely monitored controls did not affect diabetes incidence [60].
Subsequently, in a randomised controlled trial of oral insulin (DPT-1), high-risk relatives with a 25%–50% 5-year risk of diabetes were given 7.5 mg human insulin or a placebo daily for a median of 4.3 years. Overall, there was no effect, but post-hoc hypothesis testing revealed a significant delay of approximately 4 years in diabetes onset in participants who were unequivocally positive for insulin autoantibodies at the beginning of the study [61]. It is possible that allelism at the insulin VNTR (IDDM2) could shape not only the immune response to endogenous insulin as a target autoantigen but also to oral insulin. Prompted by the post-hoc DPT-1 finding, a follow-up international trial of 7.5 mg/day oral insulin was performed by TrialNet between 2007 and 2016 [62], though no effect to delay or prevent diabetes was found.
What explains the lack of efficacy of oral insulin in humans compared to NOD mice? First, treatment in humans was initiated after the onset of beta-cell autoimmunity, whereas it was ineffective in the mice. Second, the dose of insulin (i.e., 7.5 mg daily) equated to only a few micrograms in mice on a bodyweight basis, in which milligrams of gavaged insulin were required to induce anti-diabetogenic CD4+ Treg. Unfortunately, the opportunity to test a higher dose was not taken, nor was the evaluation of bioavailability (e.g., determining if the 7.5 mg dose had any immune effects). Other reasons for failure could include the degradation of insulin before reaching the upper small intestine, genetic heterogeneity of responsiveness, and co-induction of pathogenic T cells. Regarding the latter, antigen presentation in the mucosa may be a ‘two-edged sword’ that simultaneously induced both iTreg and pathogenic cytotoxic CD8+ T cells, and a clinical effect may not be seen without suppression of the latter (e.g., by co-stimulation blockade with anti-CD40 ligand antibody) [63]. Insulin contains potentially pathogenic cytotoxic T-cell epitopes, though whether mucosal insulin induces cytotoxic CD8+ T cells in addition to protective Treg is unknown. In NOD mice, a proinsulin B–C chain peptide containing both CD4+ (I–Ag7-restricted) and CD8+ (Kd-restricted) T-cell epitopes induced CD4+ Treg after nasal administration but was significantly more protective when the C-terminal p9 anchor residue for binding to Kd was deleted or mutated [64]. This effect suggests that the nature of T-cells epitopes in proinsulin is critical in mucosa-mediated immunotherapy.
We considered that insulin administered nasally might be more effective in inducing mucosal tolerance. In a small peptide, responses occurred after naso-respiratory (but not oral) administration [65]. Moreover, the nasal administration of the model antigen, ovalbumin, elicited ovalbumin-specific T-cell responses in cervical, mediastinal, and mesenteric lymph nodes, whereas the oral administration only elicited a response in the mesenteric nodes [66]. We observed that aerosol insulin, which decreased diabetes incidence when administered to young NOD mice [67] and nasal insulin when administered to humans with multiple islet autoantibodies [[68], [69], [70]], elicited an insulin antibody response. Insulin autoantibodies are a risk marker for type 1 diabetes, and an increase in insulin antibodies in response to naso-respiratory insulin appears to be counterintuitive. However, naso-respiratory insulin in both NOD mice and humans was also associated with a decrease in T-cell responses to insulin. These findings are consistent with the earliest descriptions of mucosal tolerance and later landmark studies in humans using keyhole limpet hemocyanin (KLH) as a model antigen. When KLH was administered nasally to human volunteers, it elicited a modest antibody response. However, after the challenge with subcutaneous KLH, both antibody and T-cell responses were suppressed [71]. We observed this effect in a randomised trial of nasal insulin in individuals with recent-onset type 1 diabetes who did not initially require insulin treatment. Those who received nasal insulin had a slight increase in insulin antibodies, but the insulin antibody response to subsequent subcutaneous insulin injection was suppressed [69]. This evidence for nasal insulin-induced immune tolerance cannot only be extrapolated to endogenous “autoantigenic” insulin. Nevertheless, it provides a mechanistic rationale for randomised trials of nasal insulin vaccination. Two such trials have been performed in islet autoantibody-positive first-degree relatives at-risk for type 1 diabetes. In the Type 1 Diabetes Prediction and Prevention Project (DIPP) trial in Finland [72], nasal insulin (1 U/kg daily) did not affect the progression to diabetes in children less than 3 years of age who were at very high risk. In the Australian Intranasal Insulin Trial II (INIT II) [70], nasal insulin at two doses (40 or 440 U) or a nasal placebo was administered daily for 7 days and then weekly for a year, with a further 4 years’ follow-up, in first-degree relatives aged 4–30 years (25–50% risk of diabetes over 5 years). The insulin dose in INIT II was substantially higher than in the DIPP trial, and the participants were older and had a less advanced preclinical disease. Nasal insulin induced a significant dose-dependent increase in serum insulin antibody concentration, which peaked after several months then dropped to pre-treatment concentrations within the treatment year, consistent with immune tolerance to exogenous insulin. However, this bioeffect did not translate into protection against diabetes. From these trials, we learned that oral and nasal insulin are safe, and simple biomarkers demonstrate that nasal insulin is bioactive and induces tolerance to exogenous insulin.
As reasoned above, antigen-specific vaccination is most likely to be effective before the onset of the disease. The oral and nasal insulin trials are a foundation for the primary prevention trials of mucosal insulin in genetically at-risk, islet autoantibody-negative children. A pilot study [73] found that high-dose oral insulin (67.5 mg daily for 3–12 months) was associated with antibody and T-cell responses in five of six children. Subsequently, a phase I/II randomised controlled trial was conducted in 44 islet autoantibody-negative children aged from 6 months with a susceptible HLA DR4-DQ8 genotype [74]. Oral insulin (7.5 mg with dose escalation to 67.5 mg) or placebo was administered daily for 12 months. The primary outcome was immune efficacy, pre-specified as the induction of antibody or T cell responses to insulin. Oral insulin was well-tolerated, but no effect on primary outcomes was demonstrated. Exploratory analyses suggested that antibody responses to oral insulin may occur in children with a susceptible IDDM2 genotype, and inflammatory episodes may promote the activation of insulin-responsive T cells. These factors may have impacted the trial's outcome and are important to consider in designing future trials. The case for insulin as a primary autoantigen in humans does not rely on, but would be strengthened by, the successful application of insulin as a therapeutic tool to prevent type 1 diabetes.
6. Epilogue
When insulin was discovered, it was hailed as the cure for a disease that came to be known as type 1 diabetes. No one could have imagined that 100 years later, the cure would be viewed as contributing to its own destruction. The evidence that insulin is a primary autoantigen driving autoimmune beta-cell destruction (Table 1) relies heavily on intervention studies in the NOD mouse model of type 1 diabetes, as it is not possible to test some postulates for causality in humans directly. Nevertheless, the genetic regulation of insulin expression in humans, the early appearance of insulin autoimmunity and its high predictive value in classic childhood type 1 diabetes, and other parallels with autoimmune diabetes in NOD mice, suggest that insulin is a primary pathogenic autoantigen in type 1 diabetes.
Table 1.
Evidence for (pro)insulin as primary autoantigen in type 1 diabetes.
|
|
|
|
|
Acknowledgements
The author was funded by an Investigator Grant (APP1173945) and Program Grant (APP1037321) from the National Health and Medical Research Council of Australia, but the funder had no other involvement in the preparation of this article.
Conflict of interest
None declared.
References
- 1.Banting F.G., Best C.H., Collip J.B., Campbell W.R., Fletcher A.A. Pancreatic extracts in the treatment of diabetes mellitus. Preliminary report. Canadian Medical Association Journal. 1922;12:141–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Opie E.L. On the relation of chronic interstitial pancreatitis to the islands of Langerhans and to diabetes mellitus. Journal of Experimental Medicine. 1901;5:397–428. doi: 10.1084/jem.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gepts W., Lecompte P.M. The pancreatic islets in diabetes. American Journal of Medical Jurisprudence. 1981;70:105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- 4.Lendrum R., Walker G., Cudworth A.G., Theophanides C., Pyke D.A., Bloom A. Islet-cell antibodies in diabetes mellitus. Lancet. 1976;2:1273–1276. doi: 10.1016/s0140-6736(76)92033-x. [DOI] [PubMed] [Google Scholar]
- 5.Gorsuch A.N., Spencer K.M., Lister J., McNally J.M., Dean B.M., Bottazzo G.F. Evidence for a long prediabetic period in Type 1 (insulin-dependent) diabetes mellitus. Lancet. 1981;2:1363–1365. doi: 10.1016/s0140-6736(81)92795-1. [DOI] [PubMed] [Google Scholar]
- 6.Verge C.F., Gianani R., Kawasaki E., Yu L., Pietropaolo M., Jackson R.A. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach P., Koczwara K., Knopff A., Naserke H., Ziegler A.G., Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. Journal of Clinical Investigation. 2004;114:589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler A.G., Rewers M., Simell O., Simell T., Lempainen J., Steck A. Seroconversion to multiple islet antibodies and risk of progression to diabetes in children. Journal of the American Medical Association. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krischer J.P., Lynch K.F., Lernmark Å., Hagopian W.A., Rewers M.J., She J.X. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY Study. Diabetes Care. 2017;40:1194–1202. doi: 10.2337/dc17-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vehik K., Bonifacio E., Lernmark Å., Yu L., Williams A., Schatz D. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY study. Diabetes Care. 2020;43:2066–2073. doi: 10.2337/dc19-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbarth G.S., Jackson R.A., Pugliese A. Insulin autoimmunity: the rate limiting factor in pre-type I diabetes. Journal of Autoimmunity. 1992;5(Suppl A):241–246. doi: 10.1016/0896-8411(92)90039-s. [DOI] [PubMed] [Google Scholar]
- 12.Narendran P., Mannering S.I., Harrison L.C. Proinsulin-a pathogenic autoantigen in type 1 diabetes. Autoimmunity Reviews. 2003;2:204–210. doi: 10.1016/s1568-9972(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Nakayama M., Eisenbarth G.S. Insulin as an autoantigen in NOD/human diabetes. Current Opinion in Immunology. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brezar V., Carel J.-C., Boitard C., Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocrine Reviews. 2011;2:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 15.Koch R. Die aetiologie der tuberculose. Berlin Klinische Wochenschrift. 1882;19:221. [Google Scholar]
- 16.Pugliese A., Brown D., Garza D., Murchison D., Zeller M., Redondo M.J. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. Journal of Clinical Investigation. 2001;107:555–564. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer J.P., Asplin C.M., Clemons P., Lyen K., Tatpati O., Raghu P.K. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 18.Yu L., Robles D.T., Abiru N., Kaur P., Rewers M., Kelemen K. Early expression of anti-insulin autoantibodies in humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugliese A. Autoreactive T cells in type 1 diabetes. Journal of Clinical Investigation. 2017;127:2881–2891. doi: 10.1172/JCI94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S., Cerosaletti K., James E., Long S.A., Mannering S., Speake C. Standardizing T-cell biomarkers in type 1 diabetes: challenges and recent advances. Diabetes. 2019;68:1366–1379. doi: 10.2337/db19-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller R.J. Cellular immunity to human insulin in individuals at high risk for the development of type I diabetes mellitus. Journal of Autoimmunity. 1990;3:321–327. doi: 10.1016/0896-8411(90)90150-q. [DOI] [PubMed] [Google Scholar]
- 22.Harrison L.C., De Aizpurua H., Loudovaris T., Campbell I.L., Cebon J.S., Tait B.D. Reactivity to human islets and fetal pig proislets by peripheral blood mononuclear cells from subjects with preclinical and clinical insulin-dependent diabetes. Diabetes. 1991;40:1128–1133. doi: 10.2337/diab.40.9.1128. [DOI] [PubMed] [Google Scholar]
- 23.Harrison L.C., Chu S.X., DeAizpurua H.J., Graham M., Honeyman M.C., Colman P.G. Islet-reactive T cells are a marker of preclinical insulin-dependent diabetes. Journal of Clinical Investigation. 1992;89:1161–1165. doi: 10.1172/JCI115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudy G., Stone N., Harrison L.C., Colman P.G., McNair P., Brusic V. Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Molecular Medicine. 1995;6:625–633. [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois-LaForgue D., Carel J.C., Bougnères P.F., Guillet J.-G., Boitard C. T-cell response to proinsulin and insulin in type 1 and pretype 1 diabetes. Journal of Clinical Immunology. 1999;19:127–134. doi: 10.1023/a:1020558601175. [DOI] [PubMed] [Google Scholar]
- 26.Mannering S.I., Morris J.S., Stone N.L., Jensen K.P., Van Endert P.M., Harrison L.C. CD4+ T cell proliferation in response to GAD and proinsulin in healthy, pre-diabetic, and diabetic donors. Annals of the New York Academy of Sciences. 2004;1037:16–21. doi: 10.1196/annals.1337.003. [DOI] [PubMed] [Google Scholar]
- 27.Oling V., Marttila J., Ilonen J., Kwok W.W., Nepom G., Knip M. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. Journal of Autoimmunity. 2005;25:235–243. doi: 10.1016/j.jaut.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Baker R.L., Jamison B.L., Haskins K. Hybrid insulin peptides are neo-epitopes for CD4 T cells in autoimmune diabetes. Current Opinion in Endocrinology Diabetes and Obesity. 2019;26:195–200. doi: 10.1097/MED.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell A.M., Alkanani A.A., McDaniel K.A., Pyle L., Waugh K., Steck A.K. T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proceedings of the National Academy of Sciences of the U S A. 2021;118 doi: 10.1073/pnas.2019129118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannering S.I., Harrison L.C., Williamson N.A., Morris J.S., Thearle D.J., Jensen K.P. The insulin A-chain epitope recognized by human T cells is post-translationally modified. Journal of Experimental Medicine. 2005;202:1191–1197. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampeter E.F., McCann S.R., Kolb H. Transfer of diabetes type 1 by bone marrow transplantation. Lancet. 1998;351:568–569. doi: 10.1016/S0140-6736(05)78555-X. [DOI] [PubMed] [Google Scholar]
- 32.Wegmann D.R., Norbury-Glaser M., Daniel D. Insulin-specific T cells are a pre-dominant component of islet infiltrates in pre-diabetic NOD mice. European Journal of Immunology. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 33.Daniel D., Gill R.G., Schloot N., Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. European Journal of Immunology. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 34.Griffin A.C., Zhao W., Wegmann K.W., Hickley W.F. Experimental autoimmune insulitis. Induction by T lymphocytes specific for a peptide of proinsulin. American Journal Of Pathology. 1995;147:845–857. [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature Genetics. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pociot F., Akolkar B., Concannon P., Erlich H.A., Julier C., Morahan G. Genetics of type 1 diabetes: what's next? Diabetes. 2010;59:1561–1571. doi: 10.2337/db10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble J.A., Erlich H.A. Genetics of type 1 diabetes. Cold Spring Harbour Perspectives Medical. 2012;2 doi: 10.1101/cshperspect.a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett S.T., Lucassen A.M., Gough S.C., Powell E.E., Undlien D.E., Pritchard L.E. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nature Genetics. 1995;9:284–292. doi: 10.1038/ng0395-284. [DOI] [PubMed] [Google Scholar]
- 39.Pugliese A., Zeller M., Fernandez A., Jr., Zalcberg L.J., Bartlett R.J., Ricordi C. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature Genetics. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 40.Narendran P., Neale A.M., Lee B.H., Ngui K., Steptoe R.J., Morahan G. Proinsulin is encoded by an RNA splice variant in human blood myeloid cells. Proceedings of the National Academy of Sciences of the U S A. 2006;103:16430–16435. doi: 10.1073/pnas.0607380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durinovic-Belló I., Wu R.P., Gersuk V.H., Sanda S., Shilling H.G., Nepom G.T. Insulin gene VNTR genotype associates with frequency and phenotype of the autoimmune response to proinsulin. Genes and Immunity. 2010;11:188–193. doi: 10.1038/gene.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.French M.B., Allison J., Cram D.S., Thomas H.E., Dempsey-Collier M., Silva A. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 43.Brimnes M.K., Jensen T., Jørgensen T.N., Michelsen B.K., Troelsen J., Werdelin O. Low expression of insulin in the thymus of non-obese diabetic mice. Journal of Autoimmunity. 2002;19:203–213. doi: 10.1006/jaut.2002.0616. [DOI] [PubMed] [Google Scholar]
- 44.Charlton B., Bacelj A., Slattery R.M., Mandel T.E. Cyclophosphamide-induced diabetes in NOD/WEHI mice: evidence for suppression in spontaneous autoimmune diabetes mellitus. Diabetes. 1989;38:441–447. doi: 10.2337/diab.38.4.441. [DOI] [PubMed] [Google Scholar]
- 45.Steptoe R.J., Ritchie J.M., Harrison L.C. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. Journal of Clinical Investigation. 2003;111:1357–1363. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamurthy B., Dudek N.L., McKenzie M.D., Purcell A.W., Brooks A.G., Gellert S. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. Journal of Clinical Investigation. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamurthy B., Mariana L., Gellert S.A., Colman P.G., Harrison L.C., Lew A.M. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. The Journal of Immunology. 2008;180:4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 48.Chentoufi A.A., Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 49.Thébault-baumont K., Dubois-laforgue D., Krief P., Briand J., Halbout P., Vallon-Geoffroy K. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. Journal of Clinical Investigation. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moriyama H., Abiru N., Paronen J., Sikora K., Liu E., Miao D. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y., Rudert W.A., Grupillo M., He J., Sisino G., Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. The EMBO Journal. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakayama M., Abiru N., Moriyama H., Babaya N., Liu E., Miao D. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison L.C. Vaccination against self to prevent autoimmune disease: the type 1 diabetes model. Immunology & Cell Biology. 2008;89:139–145. doi: 10.1038/sj.icb.7100151. [DOI] [PubMed] [Google Scholar]
- 54.Peakman M., von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes. 2010;59:2087–2093. doi: 10.2337/db10-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison L.C. Insulin-specific vaccination for type 1 diabetes: a step closer? Human Vaccines & Immunotherapeutics. 2012;8:834–837. doi: 10.4161/hv.19673. [DOI] [PubMed] [Google Scholar]
- 56.Harrison L.C., Wentworth J.M., Zhang Y., Bandala-Sanchez E., Böhmer R.M., Neale A.M. Antigen-based vaccination and prevention of type 1 diabetes. Current Diabetes Reports. 2013;13:616–623. doi: 10.1007/s11892-013-0415-7. [DOI] [PubMed] [Google Scholar]
- 57.Harrison L.C., Wentworth J.M. In: The autoimmune diseases. 6th ed. Rose N., Mackay I., editors. Elsevier; London: 2019. Prevention of autoimmune disease: the type 1 diabetes paradigm; pp. 1191–1207. [Google Scholar]
- 58.Shah S.C., Malone J.I., Simpson N.E. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. New England Journal of Medicine. 1989;320:550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- 59.Keller R.J., Eisenbarth G.S., Jackson R.A. Insulin prophylaxis in individuals at high risk of type 1 diabetes. Lancet. 1993;341:927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 60.Diabetes Prevention Trial-Type 1 diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. New England Journal of Medicine. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 61.Skyler J.S., Krischer J.P., Wolfsdorf J., Cowie C., Palmer J.P., Greenbaum C. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 62.Writing Committee for the Type 1 diabetes TrialNet Oral Insulin Study Group. Krischer J.P., Schatz D.A., Bundy B., Skyler J.S., Greenbaum C.J. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. Journal of the American Medical Association. 2017;318:1891–1902. doi: 10.1001/jama.2017.17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanninen A., Martinez N.R., Davey G.M., Heath W.R., Harrison L.C. Transient blockade of CD40 ligand dissociates pathogenic from protective mucosal immunity. Journal of Clinical Investigation. 2002;109:261–267. doi: 10.1172/JCI13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez N.R., Augstein P., Moustakas A.K., Papadopoulos G.K., Gregori S., Adorini L. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. Journal of Clinical Investigation. 2003;111:1365–1371. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Metzler B., Wraith D.C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. International Immunology. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 66.Hanninen A., Braakhuis A., Heath W.R., Harrison L.C. Mucosal antigen primes diabetogenic cytotoxic T-lymphocytes regardless of dose or delivery route. Diabetes. 2001;50:771–775. doi: 10.2337/diabetes.50.4.771. [DOI] [PubMed] [Google Scholar]
- 67.Harrison L.C., Dempsey-Collier M., Kramer D.R., Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. Journal of Experimental Medicine. 1996;184:2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison L.C., Honeyman M.C., Steele C.E., Stone N.L., Sarugeri E., Bonifacio E. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348–2355. doi: 10.2337/diacare.27.10.2348. [DOI] [PubMed] [Google Scholar]
- 69.Fourlanos S., Perry C., Gellert S.A., Martinuzzi E., Mallone R., Butler J. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60:1237–1245. doi: 10.2337/db10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison L.C., Hall C.R., Couper J.J., Donaghue K.C., Davis E.A., Cotterill A.M. Proc. Aust. Diab. Congr. Adelaide. 2018. A randomised controlled trial of intranasal insulin to prevent type 1 diabetes: intranasal insulin trial II (INIT II) [Google Scholar]
- 71.Waldo F.B., van den Wall Bake A.W., Mestecky J., Husby S. Suppression of the immune response by nasal immunization. Clinical Immunology and Immunopathology. 1994;72:30–34. doi: 10.1006/clin.1994.1103. [DOI] [PubMed] [Google Scholar]
- 72.Nanto-Salonen K., Kupila A., Simell S., Siljander H., Salonsaari T., Hekkala A. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 73.Bonifacio E., Ziegler A.G., Klingensmith G., Schober E., Bingley P.J., Rottenkolber M. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. Journal of the American Medical Association. 2015;313:1541–1549. doi: 10.1001/jama.2015.2928. [DOI] [PubMed] [Google Scholar]
- 74.Assfalg R., Knoop J., Hoffman K.L., Pfirrmann M., Zapardiel-Gonzalo J.M., Hofelich A. Oral insulin immunotherapy in children at risk for type 1 diabetes in a randomised controlled trial. Diabetologia. 2021;64:1079–1092. doi: 10.1007/s00125-020-05376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]