Abstract
Background
Insulin has been demonstrated to exert direct and indirect effects on vascular tissues. Its actions in vascular cells are mediated by two major pathways: the insulin receptor substrate 1/2-phosphoinositide-3 kinase/Akt (IRS1/2/PI3K/Akt) pathway and the Src/mitogen-activated protein kinase (MAPK) pathway, both of which contribute to the expression and distribution of metabolites, hormones, and cytokines.
Scope of review
In this review, we summarize the current understanding of insulin's physiological and pathophysiological actions and associated signaling pathways in vascular cells, mainly in endothelial cells (EC) and vascular smooth muscle cells (VSMC), and how these processes lead to selective insulin resistance. We also describe insulin's potential new signaling and biological effects derived from animal studies and cultured capillary and arterial EC, VSMC, and pericytes. We will not provide a detailed discussion of insulin's effects on the myocardium, insulin's structure, or its signaling pathways' various steps, since other articles in this issue discuss these areas in depth.
Major conclusions
Insulin mediates many important functions on vascular cells via its receptors and signaling cascades. Its direct actions on EC and VSMC are important for transporting and communicating nutrients, cytokines, hormones, and other signaling molecules. These vascular actions are also important for regulating systemic fuel metabolism and energetics. Inhibiting or enhancing these pathways leads to selective insulin resistance, exacerbating the development of endothelial dysfunction, atherosclerosis, restenosis, poor wound healing, and even myocardial dysfunction. Targeted therapies to improve selective insulin resistance in EC and VSMC are thus needed to specifically mitigate these pathological processes.
Keywords: Diabetes, CVD, Insulin resistance, Cardiovascular complications
Highlights
-
•
Insulin's actions in vascular cells have a significant influence on systemic metabolism.
-
•
Insulin exerts its vascular effects through its receptors and signaling cascades.
-
•
Inhibition or enhancement of different insulin signaling leads to selective insulin resistance.
-
•
Loss of insulin's actions causes endothelial dysfunction and vascular complications in diabetes.
1. Introduction
Since its discovery in 1921, insulin has been reported to exert actions in almost every type of cell and tissue. This is not surprising because insulin can affect cellular signaling pathways that regulate metabolism, survival, and replication. However, insulin's effects vary depending on cell types, including vascular cells. The vascular system includes arteries, veins, capillaries, and venules, often with addition of the myocardium. Its primary mission is to distribute nutrients, hormones, cytokines, and other signaling molecules among various tissues as well as remove their by-products. Equally important is the vascular system's ability to produce many of these signaling metabolites, hormones, and cytokines, with autocrine, paracrine, and hormonal actions, which have profound importance to many physiological processes [[1], [2], [3], [4], [5]]. As vascular cells are closely integrated with the tissues they nourish, it is not surprising that insulin exerts important direct and indirect actions on these cells. A few years after its discovery, Dr. Elliott Joslin noted that insulin has important indirect actions on the vascular system through its regulation of lipids and the development of hardening of the arteries. This review will focus on insulin's direct actions on vascular cells and briefly discuss the myocardium, because it is very different from vascular cells (sharing many similarities with skeletal muscle) and thus deserves to be reviewed separately. The vascular system is composed of endothelial cells (EC), vascular contractile smooth muscle cells (VSMC), and capillary pericytes. EC undergo intimate interactions with plasma and circulating cells, regulating cellular traffic, hemostasis, and permeability at the capillary level [[6], [7], [8], [9], [10]]. EC form various types of capillaries and have unique functions in different tissues. Continuous capillaries are those in which EC are joined by junction proteins with different levels of diffusion barriers, which occur in many organs such as the skeletal muscle, heart, adipose tissue, lung, skin, central nervous system, and retina. Fenestrated capillaries are those in which gaps or openings exist within or between EC such as in the renal glomeruli, pancreatic islets, intestines, and sinusoidal capillaries in the liver, spleen, and bone marrow and exhibit breaks between EC. VSMC are critical for maintaining blood pressure and flow [11,12]. However, there are many interactions between vascular cells and their substromal cells, including adipose cells, which may even cause progenitor cells in the body [13]. Insulin has been shown to possess receptors on all differentiated vascular cells and their progenitor cells. We will not provide a detailed discussion of insulin's structure or its signaling pathways' various steps, as other articles in this issue discuss these areas in depth. In the following sections, we provide a review of insulin's direct actions on vascular cells starting with its physiological and potential pathological contributions to diabetes and insulin-resistant states. We also describe insulin's potential new signaling and biological effects derived from animal studies, cultured capillary and arterial EC, VSMC, and pericytes. These studies from many laboratories have clearly established that insulin has important specialized physiological actions in vascular cells. When these actions are either diminished or chronically activated, they contribute to many of the vascular complications of diabetes.
2. Structure and signaling of insulin receptors in EC and VSMC
Vascular cells have insulin receptors (IR) composed of an α chain that binds insulin and a β chain containing tyrosine kinase, comparable to IR in other tissues. As shown in Figure 1, IR signaling in vascular cells occurs mainly via the IR substrate (IRS)1/2 and phosphoinositide (PI)-3 kinase/Akt (IRS1/2/PI3K/Akt) and Src/mitogen-activated protein kinase (MAPK) cascades, similar to other tissues. However, IR processing and actions are unusual and differ from other tissues. When IR were first studied in EC, we found that most of the internalized insulin was not degraded even after several hours at 37 °C, which was very different from hepatocytes, adipocytes, and even VSMC [[14], [15], [16]]. This lack of internalized insulin to be degraded in EC led to the discovery that receptor-mediated transport of insulin occurs from the apical to the luminal surfaces of EC, termed receptor-mediated transcytosis, which has been shown to occur in other hormones or growth factors such as epidermal growth factor (EGF) and transferrin [14,[17], [18], [19]]. However, the insulin transport process across the endothelium remains to be completely defined. It likely differs depending on the type of capillaries in the various organs. For example, in certain regions of the brain and retina where tight junctions are present in continuous capillaries, IR-mediated transport is likely to be important. In contrast, fenestrated capillaries in various endocrine glands and sinusoidal capillaries in the liver and spleen provide very few barriers to insulin. However, in tissues such as muscle and fat that have continuous capillaries but express few zona occludens, assessing the role of IR in EC transport has been difficult to document. A study from the Kahn lab at the Joslin Diabetes Center showed that specific knock-out of IR in EC delayed the activation of insulin signaling in skeletal muscle, fat, and several regions of the brain, but not in the liver or olfactory bulb, indicating that IR EC is important for insulin delivery in EC [20]. Other studies found that insulin transport across the interstitial skeletal muscle are saturable, which may be part of the caveolin cycling process [21,22]. However, another study showed that insulin transport across the blood–brain barrier may not be mediated by IR [23]. While the physiological significance of IR's role in mediating EC transport across the continuous capillaries is still being clarified, it is likely to be important in tissues such as the brain and retina, which have limited paracellular capillary permeability due to an endothelial barrier with tight junction protein complexes [24], although the regulation of this process needs to be further studied. A recent study showed that Notch signaling in EC was involved in regulating insulin transport across the endothelium to muscle cells. Inhibition of Notch signaling affected caveolae formation in EC and improved insulin sensitivity, glucose tolerance, and glucose uptake in muscle in a high-fat diet model [25]. In contrast to EC, internalized insulin is degraded in VSMC, and directional transport of intact insulin is not observed. Furthermore, downregulation of surface IR is observed in both EC and VSMC when exposed to elevated levels of insulin, which can contribute to insulin resistance in these cells [19]. It is also possible that downregulation of IR in EC may indirectly contribute to systemic insulin resistance by regulating various aspects of insulin transport to the tissues such as blood flow, permeability, or cytokine levels [1,24].
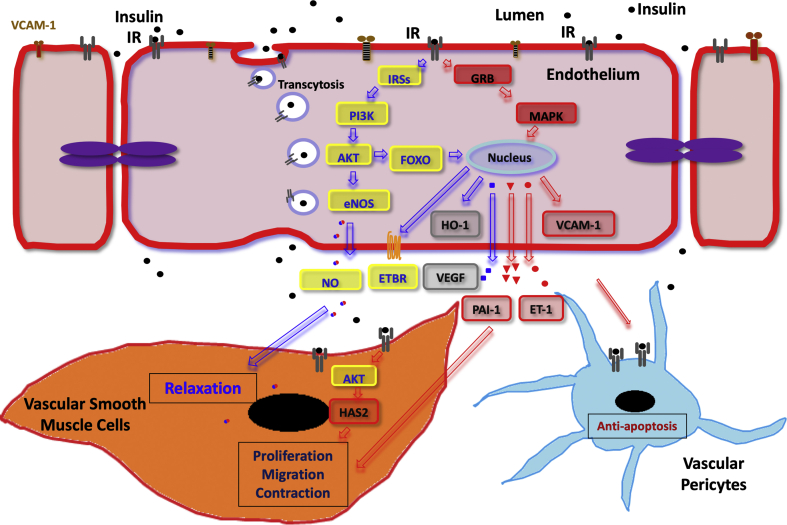
Figure 1.
An overview of insulin's various actions on the vascular wall. Insulin receptors mediate insulin transport across the endothelial barrier via receptor-mediated transcytosis. In the endothelium, insulin activates either the PI3K/Akt pathway (through IRS) or the MAPK/Erk pathway and regulates the expression of HO-1, VEGF, and VCAM-1. In vascular smooth muscle cells, the PI3K/Akt pathway eventually leads to NO activation and muscular relaxation, while the MAPK/Erk pathway leads to ET-1 activation and consequently muscular contraction as well as cellular proliferation and migration. In vascular pericytes, insulin can also exert anti-apoptotic actions by inhibiting caspases and FOXO and increasing HO-1. IRS, insulin receptor substrate; HO-1, heme oxygenase; VEGF, vascular endothelial growth factor; VCAM-1, vascular cell adhesion molecule-1; NO, nitric oxide; ET-1, endothelin-1; ETBR, endothelin receptor type B; HAS2, hyaluronan synthase 2.
Functionally, insulin's actions in EC and VSMC mostly focus on metabolism and are vascular-specific. For glucose metabolism, insulin does not have significant actions on glucose uptake since GLUT-1 is the predominant glucose transporter in vascular cells, with the exception of myocardial cells [26]. However, insulin can affect glucose and fatty acid metabolism via glycolytic and mitochondrial fluxes, which can indirectly alter many cellular functions [1]. Thus, the combination of hyperinsulinemia, hyperglycemia, and hyperlipidemia in diabetes can exert profound actions on vascular cells and lead to different vascular complications. For instance, hyperinsulinemia associated with insulin resistance can cause macrovascular complications. Hyperglycemia can cause endothelial dysfunction directly and accelerate vascular complications, especially when combined with other risk factors such as hyperlipidemia or insulin resistance. The presence of insulin resistance and the lack of autoimmunity to pancreatic beta cells in type 2 diabetes (T2D) are important factors to consider when evaluating the risk of various diabetes-related complications. The presence of autoimmunity, in particular, has been shown to be associated with cardiac failure in people with type 1 diabetes (T1D) [27]. Insulin's actions on vascular cells are also integrated with the cellular actions of insulin growth factor (IGF)-1. The admixture of insulin and IGF1 on vascular cells is due to the similarity of IGF1 receptors (IGF1R) in structure, metabolic, and growth-promoting activities as commonly reported in many other types of cells. In vascular cells, IGF1R are equally or more numerous than IR and hybrid IR/IGF1 receptors [28]. Their interactions will be discussed in more detail in the following sections, including the findings that insulin's actions mediated by the IRS1/2/PI3K/Akt pathway mediate many effects that are anti-atherogenic, whereas those via the MAPK pathways are often pro-atherogenic or proliferative (Figure 1).
3. Evidence supporting the association between insulin and cardiovascular disease
Cardiovascular disease (CVD) is a major cause of mortality and morbidity in T1D and T2D [29]. The increased CVD risk in T1D, which is characterized by insulin deficiency, has been attributed to suboptimal glycemic control and peripheral hyperinsulinemia from exogenous insulin therapy [30]. In T2D, CVD risk is also related to hyperinsulinemia, which arises as a result of insulin resistance [31]. However, while multiple early studies associated hyperinsulinemia and insulin resistance to increased CVD risk, recent major clinical trials such as ORIGIN (Outcome Reduction with Initial Glargine Intervention) did not show increased CVD risk with exogenous hyperinsulinemia, having established a neutral cardiovascular (CV) effect of long-acting insulin glargine compared with standard glycemic care in subjects with CV risk factors and either pre-diabetes or T2D [32]. More recently, a trial comparing cardiovascular safety of insulin degludec versus insulin glargine in patients with type 2 diabetes at a high risk of cardiovascular events (DEVOTE) studied patients with established CVD and chronic T2D and similarly did not detect differences in CV mortality between insulin glargine and a newer long-acting insulin, degludec, despite reduced hypoglycemia risks in the latter [33]. These trials suggest that CVD risk in diabetes is most likely related to endogenous rather than exogenous hyperinsulinemia [34]. The association between hyperinsulinemia and CVD has also been reported in observational studies involving pre- and non-diabetic populations [35] and was found to be independent of other traditional CVD risk factors [36]. Some clinical studies have shown that proinsulin and by-products of insulin synthesis may likewise contribute to CVD risk. In several population-based cohorts, for instance, proinsulin levels were found to be a strong independent predictor of CV death and morbidity regardless of glycemic tolerance [[37], [38], [39]]. Furthermore, fasting serum C-peptide levels have been shown to significantly increase CV death in non-diabetic adults [40]. It is important to note, however, that there is difficulty separating the effects of insulin resistance from those of elevated proinsulin and C-peptide, which are usually elevated in parallel.
On the opposite end of the spectrum, insulin-deficient states such as T1D are similarly tied to CVD risk. This is due not only to insulin deficiency and hyperglycemia, but also to a host of other metabolic abnormalities such as dyslipidemia, pathologic oxidation and glycation, and inflammation (similar to T2D). In the Diabetes Control and Complications Trial (DCCT), tight glycemic control in T1D patients, which included adequate provision of insulin therapy, resulted in a significant reduction in vascular complications including CVD [41]. The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study found that relative insulin deficiency can elevate levels of apolipoprotein C3, which prevents the clearance of triglyceride-rich lipoproteins and their remnants [42].
Several animal studies have characterized insulin's direct effect on the arterial wall and severity of atherosclerosis [[43], [44], [45], [46]]. When hyperinsulinemia was induced exogenously with implanted insulin pellets to mimic exogenous insulin treatment for diabetic patients, atherosclerosis decreased and lipid levels improved through actions on the liver, lowering inflammatory cytokines, inhibiting vascular cell adhesion molecule 1 (VCAM1), activating endothelial nitric oxide synthase (eNOS), and decreasing monocyte recruitment in the arterial wall in apolipoprotein E knock-out (ApoE−/−) mice [45]. However, endogenously induced hyperinsulinemia in the absence of systemic insulin resistance did not alter the development of atherosclerosis. Studies in single-allele insulin receptor-ablated (IR+/-) ApoE−/− mice, which were generated with elevated plasma insulin and comparable insulin sensitivity to control mice, did not exhibit any difference in atherosclerosis compared to control ApoE−/− mice even after 52 weeks of hyperinsulinemia [44]. These studies suggest that neither endogenous nor exogenous hyperinsulinemia alone has a direct effect to accelerate the development of atherosclerosis. Thus, the loss of insulin's direct actions on EC is related to endothelial dysfunction and may cause the worsening of CVD in insulin resistance and diabetes.
4. Physiologic effects of insulin on the vascular system
The endothelium can regulate insulin transport into peripheral organs that possess continuous vascular connections such as the central nervous system (CNS), adipose tissue, and skeletal muscle, in contrast to organs with fenestrated capillaries such as the liver, renal glomeruli, and pituitary [14,47]. A specific vascular effect of insulin is its regulation of blood flow, with multiple studies having shown that insulin can mediate vasodilation by directly stimulating nitric oxide (NO) release from the endothelium. This has been demonstrated via a dose-dependent physiologic increase in skeletal muscle blood flow, which could be impaired very early as a result of marked endothelial dysfunction in insulin-resistant states, diabetes, and metabolic syndrome [48]. In vivo studies have also shown that physiological hyperinsulinemia can increase total skeletal muscle blood flow and rapidly recruit muscle capillaries (by relaxing resistance and terminal arterioles, respectively) in a time-, dose-, and NO-dependent manner, which precedes insulin's effects of increasing muscle glucose uptake or activating downstream kinase pathways [9,10,49]. Mice with IRS2 deletion in their endothelial cells (ETIRS2KO mice) are also insulin resistant, with reductions in insulin-induced eNOS phosphorylation and capillary recruitment and impaired glucose uptake by skeletal muscle [50]. In people with diabetes, Rask-Madsen et al. reported that insulin treatment can improve insulin-stimulated forearm blood flow [51]. They also reported that infusion of tumor necrosis factor (TNF)-α inhibited insulin-induced glucose uptake and endothelium-dependent vasodilation in the forearm, but did not inhibit sodium nitroprusside-induced blood flow, indicating that inflammatory cytokines can inhibit insulin-induced NO production and vasodilation to cause endothelial dysfunction as observed in insulin resistance and diabetes [9,10,52]. Activation of the renin-angiotensin-aldosterone system in insulin resistance may also contribute to inhibit insulin-induced formation of reactive oxygen species, leading to insulin resistance, endothelial dysfunction, hypertension, and CVD [6,7,53].
At the cellular level, we characterized the IR dimeric structure in EC, VSMC, and capillary pericytes [54]. For insulin-induced arterial vasodilation, the role of NO has been clearly demonstrated by many conclusive studies, including findings that mice with eNOS deletion developed endothelial dysfunction and insulin resistance [46]. The best evidence was reported by Quon et al. that insulin can activate eNOS through the PI3K/AKT pathway [55]. This provided the first identification of insulin's unique action in the vascular system, leading to other discoveries further showing insulin's actions on vascular flow, angiogenesis, monocyte adhesion, thrombosis, pericyte apoptosis, and cytokine and oxidant production [1,3] (Figure 1).
5. Selective insulin resistance
We previously discussed that IR can mediate insulin transport across the endothelial barrier via transcytosis without significant insulin degradation. This process has been shown to decrease in insulin resistance, as the number of IR on plasma membranes can decrease in the presence of hyperinsulinemia [19]. We also discussed that insulin exerts its vascular effects through at least two signaling pathways: the IRS1/2/PI3K/Akt and Src/MAPK cascade pathways. Insulin's activation of the IRS1/2/PI3K/Akt pathway at low physiologic levels can increase the expression of eNOS, vascular endothelial growth factor (VEGF), endothelin (ET)-1, heme oxygenase (HO)-1, plasminogen activator inhibitor (PAI)-1, and endothelin-B receptor (ETBR), as well as lower levels of VCAM1. These findings suggest that insulin's actions via the IRS1/2/PI3K/Akt pathway may enhance angiogenesis through VEGF, promote vasodilation and inhibit atherosclerosis (eNOS and ETBR), and decrease oxidation and inflammation (HO-1 and VCAM1) [43,46,56,57] (Figure 1). The IRS1/2/PI3K/Akt pathway also enables insulin to enhance cardiac contractility and increase cardiomyocyte filament sensitivity to calcium [58]. Insulin can additionally exert anti-atherogenic actions through several anti-apoptotic mechanisms such as repressing the transcription factor FoxO and inhibiting the pro-apoptotic molecule caspase-9 [59,60].
In contrast, insulin's actions via the Src/MAPK pathway facilitate vasoconstriction through ET-1, cause inflammation through plasminogen activator inhibitor (PAI-1), and promote VSMC proliferation and migration. Increases in ET-1 expression and the resultant migration and proliferation of vascular contractile cells cause vasoconstriction and an eventual atherogenic cascade [61]. Furthermore, in obese individuals, insulin has been reported to increase sympathetic nerve activity under basal conditions, but not during a euglycemic hyperinsulinemic clamp as seen in insulin-sensitive controls [62].
From this spectrum of insulin's actions on the vasculature, impairment of the IRS1/2/PI3K/Akt pathway will lead to loss of insulin's pro-angiogenic, anti-oxidative, and anti-inflammatory actions, whereas enhancements in the Src/MAPK cascade will accelerate the opposite effects, resulting in selective insulin resistance (Figure 2). We initially reported in 1999 that selective insulin resistance occurred in the aortas of obese Zucker rats [57], in which the IRS/1/2/PI3K/Akt pathway was impaired with decreased activation, but activation of the Src/MAPK pathway was not altered [1]. This selective insulin resistance has also been reported in other tissues including the myocardium, liver, renal glomeruli, wound fibroblasts, gingivae, and angioblasts. The potential loss of insulin's protective actions and selective insulin resistance are likely caused by inflammation and dysmetabolites of insulin resistance, diabetes, and metabolic syndrome. Kanter et al. further supported this concept of Akt-mediated selective insulin protection on atherosclerosis. In an insulin-resistant atherosclerosis model, Ldlr−/− mice treated with mimetic peptide S597, which preferentially activates the IRS/1/2/PI3K/Akt pathway over the Src/MAPK pathway, were shown to be protected against advanced atherosclerosis, with reductions in inflammatory monocyte accumulation in atherosclerosis lesions [63].
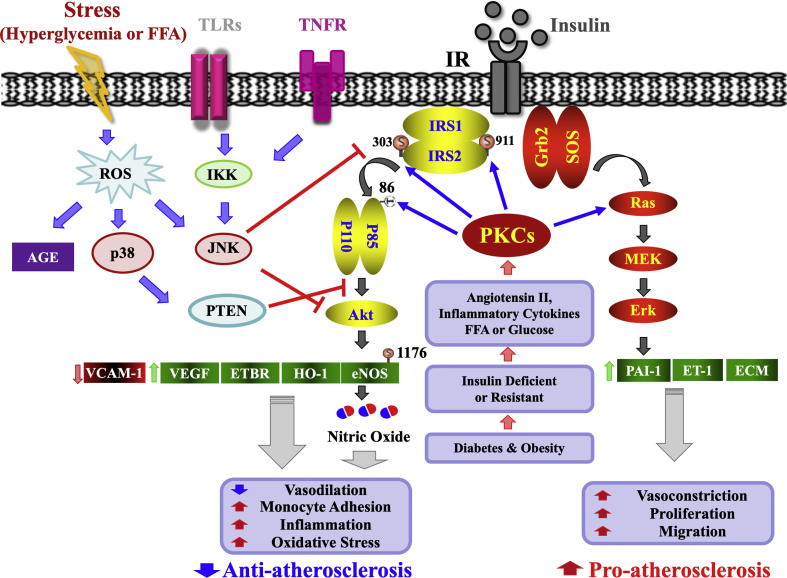
Figure 2.
Selective insulin resistance in vascular endothelial cells (EC). Selective insulin resistance in EC is induced by angiotensin II, elevated FFA, high glucose levels, and proinflammatory cytokines from diabetes and insulin resistance. These stimulate PKC isoforms and other stress kinases to inhibit only the IRS/PI3K/Akt pathway through phosphorylation of IRS1/2. In contrast, insulin's stimulation of the SOS/Grb2/MAPK pathway is enhanced in diabetes and insulin resistance. The selective loss of insulin's actions via the IRS/PI3K/Akt pathway causes the reduction in insulin's anti-atherosclerotic action and contributes to the acceleration of atherosclerosis and other cardiovascular pathologies in diabetes and insulin resistance. FFA, free fatty acid; PKC, protein kinase C; IRS1/2, insulin receptor substrate 1/2; PTEN, phosphatase and tensin homolog; ETBR, endothelin receptor type B; ROS, reactive oxygen species.
6. Mechanisms of selective insulin resistance
One of the molecular mechanisms identified to cause selective insulin resistance is the activation of stress kinases, including p38/MAPK and protein kinase C (PKC). Multiple studies have shown that elevations of glucose and lipid metabolites combined with increases in oxidants and inflammatory cytokines will increase the de novo synthesis of diacylglycerol (DAG), an activator of many PKC isoforms. We previously reported that PKC isoforms β and δ can inhibit insulin's anti-atherosclerotic actions through the IRS/1/2/PI3K/Akt pathway [64] while enhancing stress kinases such as MAPK to accelerate pro-atherosclerotic actions [65]. Among PKC isoforms, β and δ isoforms have been shown to be activated in many vascular tissues in insulin-resistant and diabetic states, including the retina, capillaries, renal glomeruli, chronic wounds, gingiva, aorta, and heart [[66], [67], [68], [69], [70], [71], [72], [73], [74]]. Cell-based studies showed that the PKC-β isoform induced selective insulin resistance in EC by phosphorylation of threonine-86 of the P85/PI3K subunit and serine of IRS2, blunting pAkt and subsequently eNOS activation and VEGF expression [64] (Figure 2).
Activation of the PKC-β isoform has been shown to mediate many of the adverse effects of hyperglycemia by selectively inhibiting insulin activation of the IRS/1/2/PI3K/Akt pathway in the endothelium. Specific inhibition of the PKC-β isoform has been shown to decrease the severity of atherosclerosis, retinopathy (DR), and nephropathy (DN) in diabetic animals, suggesting that its inhibition is an attractive therapeutic option. Nevertheless, clinical trials have provided mixed results. Ruboxistaurin, an orally available PKC-β inhibitor, has been extensively studied in DR and appears to be well-tolerated. In the PKC-DRS1 and DRS2 trials, ruboxistaurin produced a reduction in sustained moderate visual loss (SMVL) and laser treatment initiation in participants with macular edema and non-proliferative DR compared to placebo; however, it had no effect on DR progression [75,76]. For diabetic nephropathy (DN), ruboxistaurin was also able to reduce the albumin-creatinine ratio (ACR), but failed to improve eGFR in participants with persistent albuminuria. However, the intergroup differences between the ruboxistaurin and placebo groups were not statistically significant and there was similarly no difference in kidney outcomes between the two groups [77]. In diabetic neuropathy, two studies showed evidence of neuropathic symptom reduction over six and 12 months of ruboxistaurin treatment, respectively. Larger studies are needed to better elucidate the role of ruboxistaurin in neuropathy treatment [78,79].
A clear example of the functional significance of selective insulin resistance is in the myocardium. Post-ischemia collateral angiogenesis is important for retaining myocardial tissue and is related to VEGF expression [80]. In diabetes or insulin resistance, poor collateral vessel formation is commonly observed in many peripheral tissues and is related to poor survival of the myocardium [80,81]. We also reported that VEGF expression in the myocardium is likewise decreased in insulin resistance and diabetes [82]. The targeted deletion of IR in the myocardium caused parallel reductions in insulin-induced activation of IRS1/2/PI3K/Akt and VEGF and exacerbated myocardial damage with induced ischemia [80], similar to that induced by diabetes. Similar effects are seen with PKC activation, especially the β isoform, which is found in myocardia with diabetes or insulin resistance [80,82]. In the following sections, the critical roles of IR in EC and VSMC will be described.
7. Insulin signaling in endothelial dysfunction
As previously described, IR mediates several specific functions, such as the activation of eNOS and expression of VEGF, HO-1, VCAM1, and ETBR. As also mentioned, insulin can affect glucose metabolism in the EC, although glucose uptake in vascular cells is mainly mediated by GLUT-1. Of note, GLUT-1 in EC was not downregulated by high extracellular glucose concentration, which differs from VSMC [26]. This lack of GLUT1 reduction in hyperglycemia has often been cited as a reason for the induction of many functional and apoptotic changes exhibited by EC with diabetes. Deletion of IR specific to EC has provided a great deal of insulin's selective vascular effects. Kondo et al. reported that deletion of IR in EC reduced ischemia-induced retinal neovascularization in parallel with reductions in VEGF, eNOS, and ET-1 expression. Interestingly, deletion of IGF1R in EC also had similar but more modest effects than IR deletion [83]. However, this laboratory did not find any effects of IR deletion on blood–brain permeability or systemic insulin sensitivity on regular diets [84].
The importance of endothelial insulin's action on atherosclerosis was also demonstrated by deletion of endothelial insulin receptor on ApoE−/− (VEIRKO) mice, in which the IR gene was intact or conditionally deleted in vascular endothelial cells. Systemic insulin sensitivity, glucose tolerance, plasma lipids, and blood pressure were similar between the two groups, but atherosclerotic lesion size was more than 2-fold higher in VEIRKO mice that lacked endothelial insulin signaling [43]. Endothelium-dependent vasodilation was also impaired. However, endothelial cell VCAM-1 expression increased in VEIRKO mice in parallel with increased adhesion of mononuclear cells to the endothelium in vivo by 4-fold, which was reduced to below control values by a VCAM-1-blocking antibody. These findings provided very strong evidence that loss of insulin signaling in the endothelium accelerated atherosclerosis [43,[85], [86], [87]].
In contrast, when insulin signaling was enhanced specifically in EC, NO production greatly increased and atherosclerosis decreased. Overexpression of IRS1 in the endothelia of ApoE−/− mice (IRS1/ApoE−/−) improved insulin signaling in the aorta. Complex lesions of atherosclerosis were minimized in IRS1/ApoE−/− mice on high-fat diet with hyperinsulinemia and hyperglycemia. This enhanced anti-atherogenic action in EC was due to a dramatic induction of NO action, which increased ETRB expression and intracellular Ca2+. Interestingly, mice with knock-in mutation of eNOS, which had Ser1176 mutated to alanine (AKI), deleting the only known mechanism for insulin to activate eNOS (Aki/ApoE−/− mice), still exhibited significantly decreased atherosclerosis. Endothelial ETRB expression was selectively reduced in the arterial intima of diabetic patients and rodents, but upregulated by insulin via the IRS1/2/P13K/Akt pathway. However, ETRB deletion in EC of Ldlr−/− and IRS1/Ldlr−/− mice decreased NO production and accelerated atherosclerosis compared with Ldlr−/− mice. Accelerated atherosclerosis in diabetes may be reduced by selectively improving insulin signaling via the IRS1/2/PI3K/Akt pathway in EC by inducing ETRB expression and NO production (Figure 2) [46]. In addition, a recent study showed that deletion of IGF1R in EC in apoE−/− background (En-IGF1R-KD mice) also increased atherosclerosis without altering leukocyte adhesion, but rather by the loss of endothelial barrier function [88].
Enhancing IR actions in the endothelium also improved wound healing in insulin resistance and diabetes. We reported that epithelization and angiogenesis are delayed or reduced in diet-induced diabetic mice. However, overexpression of IRS1 targeted to EC improved angiogenesis and wound healing in both diet-induced insulin-resistant and insulin-deficient mice by improving the differentiation of endogenous angioblasts in the wound to EC [89], which is also related to NO production and VEGF expression. These results strongly demonstrated that IR-mediated signaling is important for EC function, specifically via the IRS1/2/PI3K/Akt pathway.
As previously mentioned, one mechanism by which metabolic changes lead to selective insulin resistance is activation of stress kinases including p38/MAPK and PKC, which inhibit the anti-atherosclerotic IRS1/2/PI3K/Akt pathway [64] and enhance the pro-atherosclerotic MAPK pathway [65]. Among PKC isoforms, PKC-β isoform activation has been documented to induce selective insulin resistance in EC by phosphorylation of threonine-86 of the P85/PI3K subunit and serine of IRS2, further blunting pAkt, eNOS, and VEGF expression. Overexpressing PKC-β2 in EC (Tg (Prkcb) ApoE−/− mice) induced selective insulin resistance through loss of Akt/eNOS signaling and elevated angiotensin-induced ET-1 expression, which aggravated endothelial dysfunction and accelerated atherosclerosis [90,91], while deletion of PKC-β or using its chemical inhibitor, ruboxistaurin (RBX), in ApoE−/− mice led to a large reduction in atherosclerosis [92,93]. Activation of PKC-δ in diabetes has also been reported in many vascular tissues such as the aorta, retina, heart, renal glomeruli, and chronic wounds. We reported that PKC-δ levels were elevated and activated in chronic wounds and fibroblasts from diabetic people and rodents. Introducing PKC-δ inhibitors improved insulin signaling in the wounds and their fibroblasts, with parallel elevation of VEGF expression, pAkt, and wound-healing rates. These findings provide strong evidence that selective insulin resistance, with the loss of signaling via the IRS1/2/PI3K/Akt cascade, is a major contributor to endothelial dysfunction and many of the vascular complications of diabetes (Figure 2) [94].
8. Insulin signaling in vascular smooth muscle cells
VSMC, which surround EC, have been reported to be regulated by insulin indirectly through NO/cGMP activation (leading to vasodilation) and ET-1 activation (leading to vasocontraction) [95,96]. To date, however, there has been little discussion about insulin's actions directly on VSMC and its role on restenosis and atherosclerosis. Previous studies on insulin's action have reported its importance in maintaining α-smooth muscle actin (α-SMA) expression for the contractile phenotype and VSMC migration [97]. In addition, insulin and IGF1 can promote the proliferation of VSMC and have been postulated to accelerate atherosclerosis in hyperinsulinemic and insulin-resistant states [26,28,[97], [98], [99], [100], [101], [102], [103]].
VSMC, the predominant cell type within the arterial wall, regulate artery tone under normal physiological conditions. Under pathophysiological conditions, such as hypertension, atherosclerosis, restenosis, and aneurysms, VSMC undergo phenotype switching from a contractile phenotype to synthetic phenotype, with increased proliferation and secretion of the extracellular matrix (ECM). The important role of VSMC in atherosclerotic plaques was revealed by lineage-tracing studies that showed that 30% of plaque cells were derived from VSMC [104]. Most VSMC in the atherosclerotic plaque lose classic VSMC markers, such as Myh11 and SM22, making it difficult to study the function of VSMC in atherosclerosis. Single-cell RNA sequencing studies revealed that there were inflammatory VSMC, osteogenic VSMC, and fibrotic VSMC in the plaque, each playing different roles [105,106]. Increased fibrotic VSMC might enhance plaque stability, inflammatory VSMC might promote atherosclerosis, while osteogenic VSMC might mediate arterial calcification in diabetes. The differentiation of different groups of VSMC is regulated by different transcription factors.
VSMC proliferation and migration and the effects on the extracellular matrix are critical to promote the formation and development of atherosclerosis and restenosis, which are both accelerated in people with T1D or T2D [11,107,108]. Paradoxically, VSMC proliferation may be also beneficial for plaque stability, with proliferation of different VSMC groups resulting in different outcomes. Proliferation of inflammatory or osteogenic VSMC might contribute to atherosclerosis, whereas proliferation of fibrotic VSMC might increase the cap thickness and ECM content in the plaque, enhancing plaque stability [107,109].
Insulin was reported to promote VSMC proliferation in vitro many decades ago [26]. High concentrations of insulin, such as 10–100 nM, were usually used in these studies, which raised doubts about their physiologic significance. Insulin can activate both IR and IGF1R at elevated concentrations, making it difficult to interpret whether its effects were mediated by IR or IGF1R. Both IR and IGF1R are expressed on VSMC. Structurally and functionally, both are dimers formed by two monomers conjugated by disulfide bonds (Figure 3). In addition to forming homozygous IR and IGF1R, IR and IGF1R can also form a hybrid insulin/IGF1 receptor. The abundance of IGF1R is approximately 8- to 10-fold higher than IR in most vascular cells including VSMC, resulting in about 80% IR forming a hybrid receptor with IGF1R and only 20% IR forming homozygous IR (homo IR). Physiological levels of insulin (1–10 nM) can only bind to homo IR, whereas physiological levels of IGF1 can activate both homo IGF1R and hybrid insulin/IGF1R. Therefore, we reported that IGF1R can inhibit insulin effects by binding with IR to form a hybrid insulin/IGF1 receptor, reducing the amount of homo IR. Knock-out of IR targeted to VSMC decreased insulin-stimulated VSMC proliferation and reduced wire injury-induced intimal hyperplasia of femoral arteries in a rodent model of restenosis. In contrast, deletion of IGF1R enhanced insulin-stimulated VSMC proliferation and exacerbated injury-induced intimal hyperplasia by enhancing insulin signaling and upregulation of Has2 [28], an enzyme that has important biological effects on VSMC migration and proliferation (Figure 3).
Figure 3.
Signaling of homozygous insulin receptor (IR/IR), IGF-1 receptor (IGF1R/IGF1R), and hybrid IR/IGF1R in vascular smooth muscle cells (VSMC). Physiological levels of insulin only bind to homo IR/IR, which enhance VSMC proliferation and exacerbate injury-induced intimal hyperplasia and atherosclerotic plaque stability by activating insulin signaling and upregulating Has2. In hyperinsulinemic conditions, insulin can bind to IR/IGF1R and homo IGF1R/IGF1R, enhance proliferation and migration, and accelerate restenosis. However, insulin resistance or hypoglycemia decreases restenosis and plaque stability. IGF-1, which mainly binds to IGF1R/IGF1R, does not alter restenosis but can increase plaque stability by enhancing VSMC proliferation, migration, and secretion of the extracellular matrix. HAS2, hyaluronan synthase 2.
Insulin also plays a role in VSMC apoptosis, an important process that can promote atherosclerosis by inducing inflammation [110]. In addition, VSMC apoptosis also results in the thinning of the atherosclerotic plaque cap and decreased content of ECM in the plaque, leading to plaque instability. Insulin inhibited VSMC apoptosis induced by cytokines in vitro and LPS in vivo in diabetic rats [111]. The role of IR in VSMC and atherosclerotic plaque stability has not yet been reported. The atherosclerotic plaque of ApoE−/−/IRS2+/− mice, which had systemic insulin resistance, demonstrated unstable features, including increased VSMC apoptosis, necrotic areas, elastic lamina breaks, and reduced plaque cap thickness [112].
Insulin's effects on VSMC contraction and relaxation are controversial, with both vasodilatory and vasoconstrictive effects of insulin being reported [95,96]. VSMC contraction is regulated by MLCK, which phosphorylates myosin, and MBP, a phosphatase that dephosphorylates myosin. Contractile agonists increase MLCK activity while inhibiting MBP activity, resulting in overall increased phosphorylation of myosin. Insulin has been reported to increase the activity of MBP through inhibiting Rho kinase-induced MBP phosphorylation, inducing VSMC relaxation [113]. However, insulin has also been reported to increase the expression of endothelin-A (ETA) [114] or the α1-adrenergic receptor [115] to enhance ET-1 expression or catecholamine-induced VSMC contraction. The upregulation of α1-adrenergic receptors was induced by insulin at 100 nM, which could activate both IR and IGF1R. Future studies using VSMC-specific IR knock-out mice will help clarify insulin's effects on artery contraction and relaxation.
9. Macrophages
Monocytes are recruited into apolipoprotein B (apoB)-containing lipoproteins and infiltrate into the subendothelial area where they differentiate into macrophages, become cholesterol-loaded foam cells, and form atherosclerotic plaque. Macrophages in the plaque play a pro-atherosclerotic role by increasing necrosis with enhanced inflammation, protease secretion, coagulation, and thrombosis [116]. However, there is conflicting evidence on the atherosclerotic-related effects of insulin's action on macrophages. In a recent study, lysozyme M-driven Cre recombinase-mediated myeloid lineage-specific IR-knock-out mice (MphIRKO mice) on an apoE−/− background showed decreased atherosclerotic lesions. Fetal liver cell transplantation of IRS2−/−/apoE−/− mice into lethally irradiated apoE−/− recipients consistently displayed ameliorated atherosclerosis, suggesting that defective insulin signaling via the IRS/PI3K pathway in macrophages promotes atherosclerosis. An in vitro study showed that IR-deficient macrophages retarded lipopolysaccharide (LPS)-stimulated interleukin-6 (IL-6) and interleukin 1-beta (IL-1β) inflammation responses [117], while in another study, transplantation of IR−/− bone marrow into low-density lipoprotein receptor knock-out (ldlr−/−) mice led to the development of severe atherosclerotic lesions with increased necrotic and endoplasmic reticulum stress-mediated apoptosis, which was partially related to impairment of the IRS/PI3K/Akt pathway in later stages of atherosclerosis [118]. These studies indicated that selective insulin resistance through the IRS/PI3K/Akt pathway contributed to different stages of atherosclerosis. In addition, deletion of IR in the bone marrow caused skewed differentiation of multipotent progenitors, with an expansion of myeloid cells, including macrophages, but a reduction of lymphoid cells by a mammalian target of rapamycin (mTOR)-activated signal transducer and activator of the transcription 3 (STAT3) pathway [119].
We previously reported that diabetes can activate the PKCδ isoform in monocytes in rodents with diabetes from insulin resistance or deficiency. However, we surprisingly found opposite results after deleting the PKCδ isoform. Macrophages in PKCδ knock-out mice (MPKCδKO/apoE−/− mice) exhibited accelerated aortic atherosclerotic lesions with decreased apoptosis and increased proliferation in macrophages, which were associated with elevated phosphorylation levels of the pro-survival cell-signaling proteins Akt and FoxO3a, and reduction of the pro-apoptotic protein Bim [120]. These data suggested that inhibition of PKCδ to improve insulin resistance in different cell types may have contradicting effects on the progression of atherosclerosis in diabetic and insulin-resistant states.
10. Summary
In summary, insulin mediates many important functions in vascular cells via its receptors and signaling cascades. Its direct actions on EC and VSMC are important for transporting and communicating nutrients, cytokines, hormones, and other signaling molecules. These vascular actions are also important for insulin's actions on the regulation of systemic fuel metabolism and energetics. Abnormalities in insulin's action on the vessel wall are major contributors to many macro- and microvascular diseases. Targeted therapies to improve selective insulin resistance in EC and VSMC are needed to specifically mitigate pathological processes such as endothelial dysfunction, atherosclerosis, restenosis, wound healing, myocardial dysfunction, and microvascular/capillary closures that are major complications related to diabetes and insulin resistance.
Acknowledgments
The authors are grateful for the support of the National Institutes of Health (NIH) grant 5P30-DK-036836. G.L.K. is supported by NIH grant R01-DK- 053105 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 1DP3-DK-094333-01. J.F. is supported by a Mary K. Iacocca Research Fellowship Award and an American Diabetes Association Scientific Sessions Young Investigator Award. M.G.Y. is supported by the American Diabetes Association (ADA, 9-18-CVD1-005). Q.L. is supported by the American Diabetes Association Mentor-Based Postdoctoral Fellowship Award.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of interest
None declared.
References
- 1.King G.L., Park K., Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman award Lecture. Diabetes. 2016;65(6):1462–1471. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King G.L. The role of hyperglycaemia and hyperinsulinaemia in causing vascular dysfunction in diabetes. Annals of Medicine. 1996;28(5):427–432. doi: 10.3109/07853899608999103. [DOI] [PubMed] [Google Scholar]
- 3.Muniyappa R., Montagnani M., Koh K.K., Quon M.J. Cardiovascular actions of insulin. Endocrine Reviews. 2007;28(5):463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 4.Muniyappa R., Quon M.J. Insulin action and insulin resistance in vascular endothelium. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(4):523–530. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- 5.Mather K.J., Steinberg H.O., Baron A.D. Insulin resistance in the vasculature. Journal of Clinical Investigation. 2013;123(3):1003–1004. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Reviews Drug Discovery. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel R., Folkman J. Clinical translation of angiogenesis inhibitors. Nature Reviews Cancer. 2002;2(10):727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 8.Gimbrone M.A., Jr., Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eelen G., de Zeeuw P., Treps L., Harjes U., Wong B.W., Carmeliet P. Endothelial cell metabolism. Physiological Reviews. 2018;98(1):3–58. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett M.R., Sinha S., Owens G.K. Vascular smooth muscle cells in atherosclerosis. Circulation Research. 2016;118(4):692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacolley P., Regnault V., Segers P., Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiological Reviews. 2017;97(4):1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 13.Knights A.J., Wu J., Tseng Y.H. The heating microenvironment: intercellular cross talk within thermogenic adipose tissue. Diabetes. 2020;69(8):1599–1604. doi: 10.2337/db20-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King G.L., Johnson S.M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- 15.Hachiya H.L., Halban P.A., King G.L. Intracellular pathways of insulin transport across vascular endothelial cells. American Journal of Physiology. 1988;255(4 Pt 1):C459–C464. doi: 10.1152/ajpcell.1988.255.4.C459. [DOI] [PubMed] [Google Scholar]
- 16.Rask-Madsen C., Kahn C.R. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jialal I., King G.L., Buchwald S., Kahn C.R., Crettaz M. Processing of insulin by bovine endothelial cells in culture. Internalization without degradation. Diabetes. 1984;33(8):794–800. doi: 10.2337/diab.33.8.794. [DOI] [PubMed] [Google Scholar]
- 18.Hachiya H.L., Takayama S., White M.F., King G.L. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. Journal of Biological Chemistry. 1987;262(13):6417–6424. [PubMed] [Google Scholar]
- 19.Barrett E.J., Liu Z. The endothelial cell: an "early responder" in the development of insulin resistance. Reviews in Endocrine & Metabolic Disorders. 2013;14(1):21–27. doi: 10.1007/s11154-012-9232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konishi M., Sakaguchi M., Lockhart S.M., Cai W., Li M.E., Homan E.P. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(40):E8478–E8487. doi: 10.1073/pnas.1710625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Wang A.X., Barrett E.J. Caveolin-1 is required for vascular endothelial insulin uptake. American Journal of Physiology. Endocrinology and Metabolism. 2011;300(1):E134–E144. doi: 10.1152/ajpendo.00498.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Wang A.X., Aylor K., Barrett E.J. Caveolin-1 phosphorylation regulates vascular endothelial insulin uptake and is impaired by insulin resistance in rats. Diabetologia. 2015;58(6):1344–1353. doi: 10.1007/s00125-015-3546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhea E.M., Rask-Madsen C., Banks W.A. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. Journal of Physiology. 2018;596(19):4753–4765. doi: 10.1113/JP276149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray S.M., Barrett E.J. Insulin transport into the brain. American Journal of Physiology - Cell Physiology. 2018;315(2):C125–C136. doi: 10.1152/ajpcell.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan S.S., Jabs M., Taylor J., Wiedmann L., Leibing T., Nordstrom V. Endothelial Notch signaling controls insulin transport in muscle. EMBO Molecular Medicine. 2020;12(4) doi: 10.15252/emmm.201809271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King G.L., Buzney S.M., Kahn C.R., Hetu N., Buchwald S., Macdonald S.G. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. Journal of Clinical Investigation. 1983;71(4):974–979. doi: 10.1172/JCI110852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sousa G.R., Pober D., Galderisi A., Lv H., Yu L., Pereira A.C. Glycemic control, cardiac autoimmunity, and long-term risk of cardiovascular disease in type 1 diabetes mellitus. Circulation. 2019;139(6):730–743. doi: 10.1161/CIRCULATIONAHA.118.036068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Fu J., Xia Y., Qi W., Ishikado A., Park K. Homozygous receptors for insulin and not IGF-1 accelerate intimal hyperplasia in insulin resistance and diabetes. Nature Communications. 2019;10(1):4427. doi: 10.1038/s41467-019-12368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawshani A., Rawshani A., Franzen S., Eliasson B., Svensson A.M., Miftaraj M. Mortality and cardiovascular disease in type 1 and type 2 diabetes. New England Journal of Medicine. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 30.de Ferranti S.D., de Boer I.H., Fonseca V., Fox C.S., Golden S.H., Lavie C.J. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130(13):1110–1130. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 31.Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care. 2010;33(2):442–449. doi: 10.2337/dc09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Investigators O.T., Gerstein H.C., Bosch J., Dagenais G.R., Diaz R., Jung H. Basal insulin and cardiovascular and other outcomes in dysglycemia. New England Journal of Medicine. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 33.Marso S.P., Buse J.B. Safety of degludec versus glargine in type 2 diabetes. New England Journal of Medicine. 2017;377(20):1995–1996. doi: 10.1056/NEJMc1712575. [DOI] [PubMed] [Google Scholar]
- 34.Livingstone S.J., Looker H.C., Hothersall E.J., Wild S.H., Lindsay R.S., Chalmers J. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Medicine. 2012;9(10) doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baltali M., Korkmaz M.E., Kiziltan H.T., Muderris I.H., Ozin B., Anarat R. Association between postprandial hyperinsulinemia and coronary artery disease among non-diabetic women: a case control study. International Journal of Cardiology. 2003;88(2–3):215–221. doi: 10.1016/s0167-5273(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 36.Despres J.P., Lamarche B., Mauriege P., Cantin B., Dagenais G.R., Moorjani S. Hyperinsulinemia as an independent risk factor for ischemic heart disease. New England Journal of Medicine. 1996;334(15):952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 37.Zethelius B., Byberg L., Hales C.N., Lithell H., Berne C. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation. 2002;105(18):2153–2158. doi: 10.1161/01.cir.0000015855.04844.e7. [DOI] [PubMed] [Google Scholar]
- 38.Alssema M., Dekker J.M., Nijpels G., Stehouwer C.D., Bouter L.M., Heine R.J. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28(4):860–865. doi: 10.2337/diacare.28.4.860. [DOI] [PubMed] [Google Scholar]
- 39.Haffner S.M., Mykkanen L., Stern M.P., Valdez R.A., Heisserman J.A., Bowsher R.R. Relationship of proinsulin and insulin to cardiovascular risk factors in nondiabetic subjects. Diabetes. 1993;42(9):1297–1302. doi: 10.2337/diab.42.9.1297. [DOI] [PubMed] [Google Scholar]
- 40.Patel N., Taveira T.H., Choudhary G., Whitlatch H., Wu W.C. Fasting serum C-peptide levels predict cardiovascular and overall death in nondiabetic adults. American Heart Journal Association. 2012;1(6) doi: 10.1161/JAHA.112.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diabetes C., Complications Trial Research G., Nathan D.M., Genuth S., Lachin J., Cleary P. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 42.Kanter J.E., Shao B., Kramer F., Barnhart S., Shimizu-Albergine M., Vaisar T. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. Journal of Clinical Investigation. 2019;129(10):4165–4179. doi: 10.1172/JCI127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rask-Madsen C., Li Q., Freund B., Feather D., Abramov R., Wu I.H. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metabolism. 2010;11(5):379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rask-Madsen C., Buonomo E., Li Q., Park K., Clermont A.C., Yerokun O. Hyperinsulinemia does not change atherosclerosis development in apolipoprotein E null mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(5):1124–1131. doi: 10.1161/ATVBAHA.111.239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park K., Li Q., Evcimen N.D., Rask-Madsen C., Maeda Y., Maddaloni E. Exogenous insulin infusion can decrease atherosclerosis in diabetic rodents by improving lipids, inflammation, and endothelial function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(1):92–101. doi: 10.1161/ATVBAHA.117.310291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park K., Mima A., Li Q., Rask-Madsen C., He P., Mizutani K. Insulin decreases atherosclerosis by inducing endothelin receptor B expression. JCI Insight. 2016;1(6) doi: 10.1172/jci.insight.86574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yazdani S., Jaldin-Fincati J.R., Pereira R.V.S., Klip A. Endothelial cell barriers: transport of molecules between blood and tissues. Traffic. 2019;20(6):390–403. doi: 10.1111/tra.12645. [DOI] [PubMed] [Google Scholar]
- 48.Baron A.D. Vascular reactivity. The American Journal of Cardiology. 1999;84(1A):25J–27J. doi: 10.1016/s0002-9149(99)00354-9. [DOI] [PubMed] [Google Scholar]
- 49.Vincent M.A., Clerk L.H., Lindner J.R., Klibanov A.L., Clark M.G., Rattigan S. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53(6):1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 50.Kubota T., Kubota N., Kumagai H., Yamaguchi S., Kozono H., Takahashi T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metabolism. 2011;13(3):294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Rask-Madsen C., Ihlemann N., Krarup T., Christiansen E., Kober L., Nervil Kistorp C. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50(11):2611–2618. doi: 10.2337/diabetes.50.11.2611. [DOI] [PubMed] [Google Scholar]
- 52.Rask-Madsen C., Dominguez H., Ihlemann N., Hermann T., Kober L., Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108(15):1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 53.Cooper S.A., Whaley-Connell A., Habibi J., Wei Y., Lastra G., Manrique C. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293(4):H2009–H2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 54.Shankar R.R., Wu Y., Shen H.Q., Zhu J.S., Baron A.D. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49(5):684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 55.Montagnani M., Chen H., Barr V.A., Quon M.J. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) Journal of Biological Chemistry. 2001;276(32):30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 56.Geraldes P., Yagi K., Ohshiro Y., He Z., Maeno Y., Yamamoto-Hiraoka J. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. Journal of Biological Chemistry. 2008;283(49):34327–34336. doi: 10.1074/jbc.M807036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Z.Y., Lin Y.W., Clemont A., Feener E.P., Hein K.D., Igarashi M. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. Journal of Clinical Investigation. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sowers J.R. Insulin resistance and hypertension. American Journal of Physiology - Heart and Circulatory Physiology. 2004;286(5):H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 59.Hermann C., Assmus B., Urbich C., Zeiher A.M., Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: a potent survival signaling cascade for endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(2):402–409. doi: 10.1161/01.atv.20.2.402. [DOI] [PubMed] [Google Scholar]
- 60.Geraldes P., King G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation Research. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rask-Madsen C., King G.L. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metabolism. 2013;17(1):20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vollenweider P., Randin D., Tappy L., Jequier E., Nicod P., Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. Journal of Clinical Investigation. 1994;93(6):2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanter J.E., Kramer F., Barnhart S., Duggan J.M., Shimizu-Albergine M., Kothari V. A novel strategy to prevent advanced atherosclerosis and lower blood glucose in a mouse model of metabolic syndrome. Diabetes. 2018;67(5):946–959. doi: 10.2337/db17-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeno Y., Li Q., Park K., Rask-Madsen C., Gao B., Matsumoto M. Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K) Journal of Biological Chemistry. 2012;287(7):4518–4530. doi: 10.1074/jbc.M111.286591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park K., Li Q., Rask-Madsen C., Mima A., Mizutani K., Winnay J. Serine phosphorylation sites on IRS2 activated by angiotensin II and protein kinase C to induce selective insulin resistance in endothelial cells. Molecular and Cellular Biology. 2013;33(16):3227–3241. doi: 10.1128/MCB.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaiser N., Sasson S., Feener E.P., Boukobza-Vardi N., Higashi S., Moller D.E. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42(1):80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 67.Greene D.A., Lattimer S.A., Sima A.A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. New England Journal of Medicine. 1987;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 68.Derubertis F.R., Craven P.A. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43(1):1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 69.Shiba T., Inoguchi T., Sportsman J.R., Heath W.F., Bursell S., King G.L. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. American Journal of Physiology. 1993;265(5 Pt 1):E783–E793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- 70.Craven P.A., Davidson C.M., DeRubertis F.R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- 71.Ishii H., Jirousek M.R., Koya D., Takagi C., Xia P., Clermont A. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272(5262):728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 72.Inoguchi T., Battan R., Handler E., Sportsman J.R., Heath W., King G.L. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(22):11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizutani K., Park K., Mima A., Katagiri S., King G.L. Obesity-associated gingival vascular inflammation and insulin resistance. Journal of Dental Research. 2014;93(6):596–601. doi: 10.1177/0022034514532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khamaisi M., Katagiri S., Keenan H., Park K., Maeda Y., Li Q. PKCdelta inhibition normalizes the wound-healing capacity of diabetic human fibroblasts. Journal of Clinical Investigation. 2016;126(3):837–853. doi: 10.1172/JCI82788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Group P.-D.S. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54(7):2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 76.Group P.-D., Aiello L.P., Davis M.D., Girach A., Kles K.A., Milton R.C. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 77.Tuttle K.R., Bakris G.L., Toto R.D., McGill J.B., Hu K., Anderson P.W. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28(11):2686–2690. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- 78.Casellini C.M., Barlow P.M., Rice A.L., Casey M., Simmons K., Pittenger G. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30(4):896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 79.Vinik A.I., Bril V., Kempler P., Litchy W.J., Tesfaye S., Price K.L. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clinical Therapeutics. 2005;27(8):1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 80.He Z., Opland D.M., Way K.J., Ueki K., Bodyak N., Kang P.M. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(4):787–793. doi: 10.1161/01.ATV.0000209500.15801.4e. [DOI] [PubMed] [Google Scholar]
- 81.Veves A., King G.L. Can VEGF reverse diabetic neuropathy in human subjects? Journal of Clinical Investigation. 2001;107(10):1215–1218. doi: 10.1172/JCI13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou E., Suzuma I., Way K.J., Opland D., Clermont A.C., Naruse K. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 83.Kondo T., Vicent D., Suzuma K., Yanagisawa M., King G.L., Holzenberger M. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. Journal of Clinical Investigation. 2003;111(12):1835–1842. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kondo T., Hafezi-Moghadam A., Thomas K., Wagner D.D., Kahn C.R. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochemical and Biophysical Research Communications. 2004;317(2):315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez-Hernando C., Ackah E., Yu J., Suarez Y., Murata T., Iwakiri Y. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metabolism. 2007;6(6):446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuhlencordt P.J., Gyurko R., Han F., Scherrer-Crosbie M., Aretz T.H., Hajjar R. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104(4):448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 87.Tsuchiya K., Tanaka J., Shuiqing Y., Welch C.L., DePinho R.A., Tabas I. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metabolism. 2012;15(3):372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higashi Y., Sukhanov S., Shai S.Y., Danchuk S., Snarski P., Li Z. Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in Apoe-deficient mice. American Journal of Physiology - Heart and Circulatory Physiology. 2020;319(4):H730–H743. doi: 10.1152/ajpheart.00064.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katagiri S., Park K., Maeda Y., Rao T.N., Khamaisi M., Li Q. Overexpressing IRS1 in endothelial cells enhances angioblast differentiation and wound healing in diabetes and insulin resistance. Diabetes. 2016;65(9):2760–2771. doi: 10.2337/db15-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Q., Park K., Li C., Rask-Madsen C., Mima A., Qi W. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circulation Research. 2013;113(4):418–427. doi: 10.1161/CIRCRESAHA.113.301074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hennige A.M., Stefan N., Kapp K., Lehmann R., Weigert C., Beck A. Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. The FASEB Journal. 2006;20(8):1206–1208. doi: 10.1096/fj.05-4635fje. [DOI] [PubMed] [Google Scholar]
- 92.Harja E., Chang J.S., Lu Y., Leitges M., Zou Y.S., Schmidt A.M. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. The FASEB Journal. 2009;23(4):1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naruse K., Rask-Madsen C., Takahara N., Ha S.W., Suzuma K., Way K.J. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 94.Qi W., Li Q., Liew C.W., Rask-Madsen C., Lockhart S.M., Rasmussen L.M. SHP-1 activation inhibits vascular smooth muscle cell proliferation and intimal hyperplasia in a rodent model of insulin resistance and diabetes. Diabetologia. 2017;60(3):585–596. doi: 10.1007/s00125-016-4159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S., Yang Y., Kone B.C., Allen J.C., Kahn A.M. Insulin-stimulated cyclic guanosine monophosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmodulin-dependent protein kinase II. Circulation. 2003;107(11):1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
- 96.Doronzo G., Viretto M., Russo I., Mattiello L., Di Martino L., Cavalot F. Nitric oxide activates PI3-K and MAPK signalling pathways in human and rat vascular smooth muscle cells: influence of insulin resistance and oxidative stress. Atherosclerosis. 2011;216(1):44–53. doi: 10.1016/j.atherosclerosis.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Wang C.C., Gurevich I., Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52(10):2562–2569. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- 98.Shai S.Y., Sukhanov S., Higashi Y., Vaughn C., Kelly J., Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(10):1916–1924. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King G.L., Goodman A.D., Buzney S., Moses A., Kahn C.R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. Journal of Clinical Investigation. 1985;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du J., Delafontaine P. Inhibition of vascular smooth muscle cell growth through antisense transcription of a rat insulin-like growth factor I receptor cDNA. Circulation Research. 1995;76(6):963–972. doi: 10.1161/01.res.76.6.963. [DOI] [PubMed] [Google Scholar]
- 101.Pfeifle B., Ditschuneit H. Effect of insulin on growth of cultured human arterial smooth muscle cells. Diabetologia. 1981;20(2):155–158. doi: 10.1007/BF00262020. [DOI] [PubMed] [Google Scholar]
- 102.Bornfeldt K.E., Raines E.W., Nakano T., Graves L.M., Krebs E.G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. Journal of Clinical Investigation. 1994;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bornfeldt K.E., Arnqvist H.J., Capron L. In vivo proliferation of rat vascular smooth muscle in relation to diabetes mellitus insulin-like growth factor I and insulin. Diabetologia. 1992;35(2):104–108. doi: 10.1007/BF00402540. [DOI] [PubMed] [Google Scholar]
- 104.Shankman L.S., Gomez D., Cherepanova O.A., Salmon M., Alencar G.F., Haskins R.M. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nature Medicine. 2015;21(6):628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alencar G.F., Owsiany K.M., Karnewar S., Sukhavasi K., Mocci G., Nguyen A.T. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation. 2020;142(21):2045–2059. doi: 10.1161/CIRCULATIONAHA.120.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wirka R.C., Wagh D., Paik D.T., Pjanic M., Nguyen T., Miller C.L. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nature Medicine. 2019;25(8):1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernandez-Hernando C., Jozsef L., Jenkins D., Di Lorenzo A., Sessa W.C. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(12):2033–2040. doi: 10.1161/ATVBAHA.109.196394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bornfeldt K.E., Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metabolism. 2011;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maddaloni E., Xia Y., Park K., D'Eon S., Tinsley L.J., St-Louis R. High density lipoprotein modulates osteocalcin expression in circulating monocytes: a potential protective mechanism for cardiovascular disease in type 1 diabetes. Cardiovascular Diabetology. 2017;16(1):116. doi: 10.1186/s12933-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clarke M.C., Littlewood T.D., Figg N., Maguire J.J., Davenport A.P., Goddard M. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circulation Research. 2008;102(12):1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 111.Nakazawa T., Chiba T., Kaneko E., Yui K., Yoshida M., Shimokado K. Insulin signaling in arteries prevents smooth muscle apoptosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(4):760–765. doi: 10.1161/01.ATV.0000158307.66945.b4. [DOI] [PubMed] [Google Scholar]
- 112.Martinez-Hervas S., Vinue A., Nunez L., Andres-Blasco I., Piqueras L., Real J.T. Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovascular Research. 2014;103(2):324–336. doi: 10.1093/cvr/cvu115. [DOI] [PubMed] [Google Scholar]
- 113.Begum N., Sandu O.A., Duddy N. Negative regulation of rho signaling by insulin and its impact on actin cytoskeleton organization in vascular smooth muscle cells: role of nitric oxide and cyclic guanosine monophosphate signaling pathways. Diabetes. 2002;51(7):2256–2263. doi: 10.2337/diabetes.51.7.2256. [DOI] [PubMed] [Google Scholar]
- 114.Hopfner R.L., Hasnadka R.V., Wilson T.W., McNeill J.R., Gopalakrishnan V. Insulin increases endothelin-1-evoked intracellular free calcium responses by increased ET(A) receptor expression in rat aortic smooth muscle cells. Diabetes. 1998;47(6):937–944. doi: 10.2337/diabetes.47.6.937. [DOI] [PubMed] [Google Scholar]
- 115.Hu Z.W., Shi X.Y., Hoffman B.B. Insulin and insulin-like growth factor I differentially induce alpha1-adrenergic receptor subtype expression in rat vascular smooth muscle cells. Journal of Clinical Investigation. 1996;98(8):1826–1834. doi: 10.1172/JCI118983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tabas I., Tall A., Accili D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circulation Research. 2010;106(1):58–67. doi: 10.1161/CIRCRESAHA.109.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baumgartl J., Baudler S., Scherner M., Babaev V., Makowski L., Suttles J. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metabolism. 2006;3(4):247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han S., Liang C.P., DeVries-Seimon T., Ranalletta M., Welch C.L., Collins-Fletcher K. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metabolism. 2006;3(4):257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 119.Xia P., Wang S., Du Y., Huang G., Satoh T., Akira S. Insulin-InsR signaling drives multipotent progenitor differentiation toward lymphoid lineages. Journal of Experimental Medicine. 2015;212(13):2305–2321. doi: 10.1084/jem.20150618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Q., Park K., Xia Y., Matsumoto M., Qi W., Fu J. Regulation of macrophage apoptosis and atherosclerosis by lipid-induced PKCdelta isoform activation. Circulation Research. 2017;121(10):1153–1167. doi: 10.1161/CIRCRESAHA.117.311606. [DOI] [PMC free article] [PubMed] [Google Scholar]