Summary
This Scoping Review synthesises evidence of the impacts of European Union (EU) law, regulation, and policy on access to medicines in in non-EU low- and middle-income countries (LMICs), and the mechanisms and nature of those impacts. We searched eight scholarly databases and grey literature published between 1995-2021 in four languages. The EU exerts global influence on pharmaceuticals in LMICs in three ways: explicit agreements between EU-LMICs (ex. accession, trade, and economic agreements); LMICs' reliance on EU internal regulation, standards, or methods (ex. market authorisation); ‘soft’ forms of EU influence (ex. research funding, capacity building). This study illustrates that EU policy makers adopt measures with the potential to influence medicines in LMICs despite limited evidence of their positive and/or negative impact(s). The EU's fragmented internal and external actions in fields related to pharmaceuticals reveal the need for principles for global equitable access to medicines to guide EU policy.
Abstract
Esta revisión exploratoria sintetiza la evidencia disponible sobre el impacto que ejercen las leyes, las políticas y las regulaciones de la Unión Europea (UE) sobre el acceso a los medicamentos en países de bajo y mediano ingreso (PBMI) que no pertenecen a la UE. La búsqueda se realizó en ocho bases de datos académicas, incluyendo literatura gris. Se incluyeron publicaciones en cuatro idiomas entre 1995 y 2021. Como resultado principal se encontró que la UE ejerce su influencia sobre los productos farmacéuticos en los PBMI a través de tres mecanismos principales: i) acuerdos explícitos entre la UE y los PBMI, por ejemplo, acuerdos de ascensión a la UE o tratados comerciales, ii) utilización de la normativa, estándares o métodos de la UE por parte de los PBMI (reliance) para, por ejemplo, autorizar el ingreso de nuevos medicamentos a partir de la autorización previa por parte de la UE) y, iii) formas blandas de influencia de la UE, por ejemplo, a través de financiación a la investigación o al desarrollo de capacidades locales. Esta revisión revela que los tomadores de decisión de la UE adoptan medidas que, a pesar de la escasa evidencia que sustenta su impacto, positivo o negativo, tienen el potencial de influir en el acceso a los medicamentos de los PBMI. El accionar fragmentado de la UE respecto a los productos farmacéuticos, tanto a nivel interno como externo, son una clara muestra de la necesidad de crear principios que guíen las políticas de la UE frente al acceso equitativo a los medicamentos a nivel global.
Abstract
Это обзорное исследование обобщает данные о глобальном регуляторном влиянии Европейского Союза (ЕС) на доступ к лекарственным средствам в странах с низким и средним уровнем доходом (СНСД), которые не входят в ЕС. Был осуществлен поиск словосочетаний «развивающиеся страны», «Европейский Союз», «лекарственные средства» в восьми базах данных (OvidMedline, Scopus, Web of Science, Epistemonikos, HeinOnline, LILACS, E-library, Научный архив) и серой литературе. Были рассмотрены документы на английском, испанском, португальском и русском, опубликованные между 1995-2021, которые касались права ЕС, регуляторной политики относительно доступа к лекарственным средствам в СНСД. Было отобрано 3954 публикаций, из которых 109 включены (13 научных статей). ЕС оказывает глобальное влияние на лекарственные средства в СНСД тремя способами: определенные соглашения между ЕС-СНСД; использование СНСД внутренних норм, стандартов и методов ЕС; «мягкие» формы влияния ЕС. Данное исследование демонстрирует, что разработчики политики ЕС принимают меры с потенциальным влиянием на лекарственные средства в СНСД, несмотря на ограниченные данные об их положительном и/или негативном влиянии (- ях).
1. Introduction
An estimated 33-50% of the global population had coverage for essential health services, including medicines [1]. Barriers to access medicines disproportionately affect people in low- and middle-income countries (LMICs) due to unaffordable medicines prices, medicines shortages, and insufficient health coverage [2,3]. These challenges are compounded by insufficient research and drug development for neglected, poverty-related, and vaccine-preventable diseases [2]. These realities persist despite UN Resolutions and political commitments to achieve universal access to essential medicines, which is an integral part of the right to health enshrined in the International Convention on Social, Economic, and Cultural Rights, and the Sustainable Development Goal 3 for health [4,5].
The European Union (EU) can potentially have a major influence on access to medicines in non-EU LMICs (hereafter LMICs). The EU is a key pharmaceutical exporter to LMICs, a trade hub for medicines in transit, and a major global health donor for drug development and procurement by international agencies [6,7]. The 2010 Council Conclusions on the EU's Role in Global Health were adopted following earlier criticisms of the EU's incoherent trade and aid agendas for access to medicines [8,9]. The Council Conclusions established that EU action on health outside its borders should be guided by solidarity and ‘ensure that the main components of health systems - including access to medicines- are effective enough to deliver universal coverage of basic quality care, through a holistic and rights-based approach’ [10]. However, the policy influence of the Global Health Council Conclusions has remained limited, and its impact on access to medicines outside the EU is unknown [11,12].
The pharmaceutical markets of the EU and many LMICs are intertwined, and in some ways, interdependent. On one hand, the EU's internal pharmaceutical policy appears to impact medicines policy and access in LMICs [13,14]. For example, the EU's large-scale Covid-19 vaccine procurement and export bans stunted the available global supply, disproportionately impairing access for people in LMICs. On the other hand, LMICs are increasingly instrumental to guaranteeing the EU's pharmaceutical supply, e.g. as clinical testing sites for new medicines marketed in the EU, and a source of raw materials [15].
The precise mechanisms and impacts of the EU's external and internal action on LMICs’ access to medicines have not been investigated. Regulatory theories and international relations literature point to several mechanisms through which the EU could influence commodities in non-EU markets [16]. However, these mechanisms have not been explored in relation to pharmaceuticals, which, compared to other commodities, are a highly regulated market with many intermediaries. Empirical studies of pharmaceutical policy and regulation in LMICs, and legal analyses, often do not distinguish between the effects of EU action, and the related activities of other stringent regulatory authorities, individual EU member states, and/or the World Health Organization (WHO) [15,17,18]. A holistic understanding of how EU action influences pharmaceutical access in LMICs, as well as the gaps in that knowledge, will be a first step towards a more resilient EU approach for global health in all policies.
To address this need we conducted a Scoping Review to synthesise what is known about the impacts of EU law, regulation, and policy (hereafter called ‘EU action’) on access to medicines in LMICs, and the mechanisms and nature of those impacts. A more complete understanding of the external impacts of EU action on pharmaceuticals can support resilient global medicines supply chains and health systems, sustained disease eradication, poverty alleviation, and sustainable development, and expand the pandemic preparedness and response efforts [2,[19], [20], [21]].
2. Methods
A scoping review was selected because evidence in this field is fragmented across disciplines. This review concerns access to medicines, which we define broadly as the development, marketing, and use of medicinal products at all stages of the product lifecycle. Medicines are understood as medicinal products defined by the European Medicines Agency (EMA) as ‘A substance or combination of substances that is intended to treat, prevent or diagnose a disease, or to restore, correct or modify physiological functions by exerting a pharmacological, immunological or metabolic action.’ [22]
2.1. Search strategy and selection criteria
According to our protocol (unpublished, see Supplementary Annex), designed in line with the PRISMA-Sr guidelines [23], we searched eight databases (Ovid Medline, Scopus, Web of Science, Epistemonikos, HeinOnline, Latin American and Caribbean Health Sciences Literature, E-library, Scientific Archive of Russian Federation Ministry of Education and Science) and Google by combining three blocks of search terms in relevant MeSH terms and free text: “low- and middle-income countries”, “European Union”, and “pharmaceuticals”. All publications, regardless of language and article type, from 1995-2021 were retrieved. In addition, publications were collected from the reference lists of included articles and the authors’ records. The detailed search strategy is available in the Supplementary Annex.
Following a period of calibration among the authors, search results were screened by KP, CD, ID and PP according to the inclusion criteria that the publication concerned an EU law, regulation, or policy in relation to access to medicines in LMICs generally or in specific LMICs defined by the World Bank. Access to medicines was broadly understood as the development, marketing/provision, and/or use of pharmaceuticals. Relevant publications underwent full text screening for eligibility by KP (English), PP (English), CD (Spanish/Portuguese), and ID (Russian). Ineligible publications lacked a reference to EU action, to medicines access in LMICs, or to a connection of these two concepts; were in a language other than English, Spanish, Portuguese, or Russian; or did not have a full text. The results were validated in a cross-over arrangement by CD (English), ID (English), and VM (English/Spanish), who independently checked the full text of 25% of the publications. Discrepancies were discussed and resolved.
A data abstraction template was developed based on a structure-outcome-impact framework and tested by KP. The template included generic information (citation, article type, funding source, authors’ affiliations by sector) and study-specific data for original and review articles (LMIC(s) setting; study design; data sources; EU law, regulation or policy; outcome and impact of EU action). Data were extracted by KP (English), CD (Spanish), and ID (Russian). Abstraction was validated for 24% of the included English documents (including all original scientific articles) by CD, ID, and PP. Evidence was classified through iterative discussion among co-authors according to a framework of EU global regulatory influence (described elsewhere) [16]. A quality appraisal was not conducted because the included publications were too heterogeneous in scope and design. Many publications reviewed claimed (untested) associations (rather than causation) between EU action and pharmaceuticals in LMICs. The analysis below includes the most relevant evidence from this review. The Supplementary Annex provides a list of all included publications.
2.2. Role of the funding source
The funders had no role in the impetus, design, conduct, analysis or writing of this review. The corresponding author had full access to all data and final responsibility for the decision to submit this review for publication.
3. Results
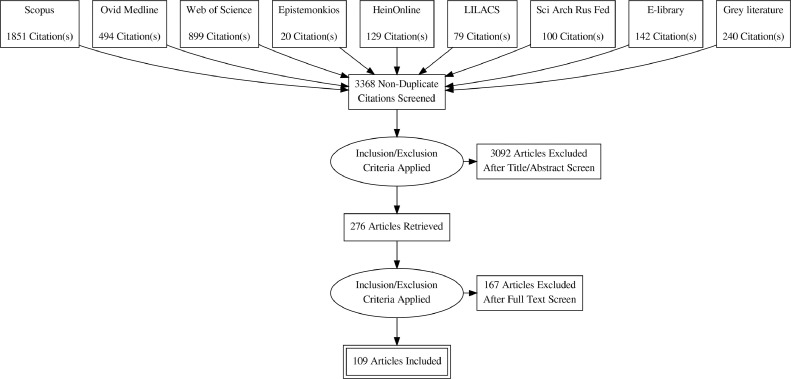
The initial search produced 3954 documents, which, after removing 586 duplicates, resulted in 3368 documents that underwent title/abstract screening. See Figure 1. Of these, 3092 documents were excluded, leaving 276 documents that underwent full-text review. Of these, 167 documents were excluded because there was no link between an EU action and LMICs (n=92), no EU action (n=34), no full text (n=27), pharmaceuticals for human use were not investigated (n=8), or the study was in a language besides English, Spanish, Portuguese, or Russian (n=6). 109 publications were included in the review. Table 1 shows the characteristics of the included publications.
Fig. 1.
Document selection process.
Table 1.
Characteristics of the included publications.
| All included publications | n=109 | % |
|---|---|---|
| Publication Type | ||
| Original articles | ||
| Scientific | 13 | 12% |
| Legal or social science | 16 | 15% |
| Reviews and reports | 27 | 25% |
| Opinions, news, and editorials | 33 | 30% |
| Grey literature | 20 | 18% |
| Language | ||
| English | 105 | 96% |
| Russian | 3 | 3% |
| Spanish | 1 | 1% |
| Original articles, reviews, and reports | n=56 | % |
| Source of funding (where declared) | ||
| EU entity, program, or project* | 4 | 7% |
| Pharmaceutical or medical device industry* | 5 | 9% |
| Author affiliation (at least one author) | ||
| EU entity, program, or project* | 3 | 5% |
| Pharmaceutical or medical device industry* | 9 | 16% |
=not mutually exclusive.
3.1. Current knowledge on the impact of EU action on access to medicines in LMICs
This section synthesises the evidence of three mechanisms through which the EU influences access to medicines in LMICs. See Table 2.
Table 2.
Three mechanisms of EU regulatory influence over access to medicines in LMICs and their known impacts.
| Mechanisms of EU influence | Area of EU law, regulation, or policy | Nature of impacts in LMICs | Illustrative publications |
|---|---|---|---|
| Explicit agreements between EU-LMICs | |||
| EU enlargement | Candidate EU member states harmonize local standards with EU regulatory data protection | (no data) | [25] |
| Candidate EU member state recognises on its local market approvals granted through EU procedures | At the time of accession about 1/3 of market approvals in the candidate member state were granted through the EU recognition procedure, and 2/3 through national authorization After accession the majority of new market approvals in the new EU member state were granted through EU procedures |
[26] | |
| EU-LMIC trade agreements and economic partnerships | LMIC trade partners adopt longer periods of regulatory data protection and patent extensions | Lost savings on pharmaceuticals Potential for reduced availability and market shares of generic medicines launched on LMIC markets post-EU trade agreement |
[29] |
| LMIC trade partners remove tariffs on EU pharmaceutical imports | Minor welfare benefits for LMICs due to ‘cheaper’ imported medicines from EU Possible negative effects for LMICs’ trade relations with other medicines exporting countries |
[7] | |
| EU internal market action affects LMICs | |||
| EU exports and shipments transiting through EU | Waiver for EU patent term extensions allow generic and biosimilar medicines to be produced in the EU for export (patent term extensions are also called supplementary protection certificates) |
(no data) | [32] |
| Measures to prevent the re-importation into the EU of low-priced medicines for HIV/AIDS, TB, malaria intended for LMICs | List prices of medicines registered under this system are not significantly lower than for products supplied outside of this regulation nor the same products sold in other countries | [35] | |
| EU customs enforcement measures | Detention of shipments of generic medicines in transit through the EU and destined for LMICs. Shipments are delayed reaching LMICs or are returned to their country of origin. | [30] | |
| EU internal regulation influences actors in LMICs to adhere to EU standards | In order to access the EU market, medicines manufacturers and their affiliates must declare to have followed good clinical practices and ethical standards during clinical development | (no data) |
[41] |
| LMIC regulatory reliance and mirroring of EU internal market regulation | LMIC regulators recognize or abbreviate the EC's market authorization decisions for new chemical entities on local markets | May result in expedited regulatory approval of essential medicines in LMICs. May reduce LMICs’ autonomy over their regulatory decisions, which is problematic when the EU's decisions being mirrored have a weak evidence base. |
[43] [45] |
| ‘Soft’ forms of EU influence on LMICs | |||
| EU technical assistance, resource mobilization and ‘capacity building’ | Through the EU-Medicines4all (Article 58 procedure) the EMA assesses and provides scientific opinions of new medicines solely intended for third countries (usually LMICs) | Six medicines with positive EMA opinions were granted 138 regulatory approvals in 90 countries. | [60] |
| EU funding for research and clinical development of pharmaceuticals for neglected infectious diseases (e.g., EDCTP) | Development and market approval of new medical products (e.g., first malaria vaccine, RTS,S) |
[66] | |
| EU coordinates development funding for harmonizing LMICs’ pharmaceutical regulation and establishing pooled procurement | (no data) | [68] | |
| EU-funded humanitarian aid organizations must respect internationally recognized principles of good practice for medical products for procuring medicines | (no data) |
[18] |
|
3.1.1. Explicit agreements between EU-LMICs
3.1.1.1. EU enlargement
Enlarging the EU to include its neighbouring countries is a means to motivate potential candidate and candidate countries (mostly LMICs in the European region) to align aspects of their internal market policies related to medicines with those of the EU, which is a condition for EU membership. As part of this process EU candidate and potential candidate countries may receive technical and other support from the European Commission (EC, the executive branch of the EU) in the field of pharmaceuticals [24]. Accession countries need to reform their legislation to adopt regulatory data protection of pharmaceuticals conform with EU levels, which is higher than the minimum required in global trade law (Agreement on Trade Related Aspects of Intellectual Property, TRIPS Agreement). Regulatory data protection can delay generic competition, resulting in lost savings on medicines purchases. Survival analysis demonstrates that EU applicants (mostly LMICs) are 7.47 times more likely to adopt higher (TRIPS+) standards of regulatory data protection than non-applicant countries [25]. EU accession was the primary motivation for these regulatory data protection reforms. The process of EU accession has been called the ‘coercive diffusion’ of EU norms because LMICs applicants lacked other national interests in stronger data protection standards and resisted and delayed their adoption [25].
Prior to accession candidate EU Member States receive technical support and capacity building in order to harmonise their drug regulation standards with those of the EU. In addition, in the case of Croatia, it was permitted to exchange confidential and safety information with EU regulators and to adopt a simplified recognition procedure for EU market approvals previously granted [26,27]. These measures are described as improving access to medicines in Croatia by increasing the number of medicinal products with market approval in the country “by nearly one-third when Croatia acceded to the EU”; however, it was unclear whether these products were available on the Croatian market [26]. In the four years after accession an average of 88% of 'recently licensed medicinal products in Croatia were approved through an EU (not a Croatian) procedure [26]. The precise role of the EU accession agreement was not robustly tested.
3.1.1.2. EU trade agreements and economic partnerships
Bilateral and multilateral trade agreements and economic partnerships between the EU and LMICs often require trade partners seeking access to the EU market to adopt the same or similar standards into the EU in their domestic law. The IP standards and tariffs in these agreements could affect LMIC trade partners’ capacity to trade pharmaceuticals globally and to regulate prices domestically. For example, the EC negotiated five years of regulatory data protection and five-year patent term extensions in the 2013 EU-Peru/Colombia free trade agreement (FTA, which Ecuador joined in 2017) [28]. After concluding the FTA these measures were introduced into LMICs’ domestic law despite exceeding the minimum levels of protection required by the TRIPS Agreement.
No peer-reviewed studies assessed the impact of EU trade agreements on LMICs’ medicines supply. IQVIA, a company collecting data on pharmaceuticals, reported that regulatory data protection and patent extensions in the EU-Peru/Colombia FTA caused lost savings on retail pharmaceuticals in Peru and Colombia (between 2013-2018), and Ecuador (2017-2018) [29]. A pre-/post-analysis by IQVIA in Ecuador found a moderate reduction the number and proportion of generic manufacturers marketing medicines in Ecuador, and a substantial reduction in the market shares of generic medicines for hypertension [29]. This means that the reduced availability and success of generic medicines launched in Ecuador after 2017 may be attributable to the FTA; it was not reported whether the stronger data protection standards contributed to these pre/post changes.
Reduced tariffs on pharmaceuticals (among other products) are commonly pursued in trade agreements. An ex-ante economic analysis found the reduced tariffs proposed in the EU-Vietnam Trade Agreement (EVFTA) would lead to increases in EU medicines imports to Vietnam, particularly of analgesics, antipyretics, anaesthetics, antibiotics and antiretrovirals for HIV/AIDS, cancer, antiparkinsonians, and cardiovascular medicines [7]. As a result, EU medicines imports would be ‘cheaper’ than before the EVFTA, leading them to compete with domestic manufacturers and imports from other low-priced Asian markets (i.e. India, China, Thailand) where Vietnam also has trade agreements [7]. Reduced tariffs on pharmaceuticals would ultimately yield minor welfare benefits for Vietnam, and possible negative effects for the country's integration in the Asian region [7].
Another IP standard requested by the EC and adopted in EU-LMIC trade agreements is customs enforcement measures, for which no evidence of impact was retrieved [30].
3.1.2. EU internal market action affects LMICs
3.1.2.1. Medicines exports and shipments to LMICs
A major objective of the EU is to regulate the smooth functioning of its single market consisting of 27 EU Member States, Iceland, Liechtenstein, and Norway. The EU achieves this through its internal decisions in a range of policy areas that can affect the export and/or transit of pharmaceuticals through the EU to LMICs.
One policy area is the adoption of EU patent term extensions (called supplementary protection certificates, SPCs) that, when first introduced, prevented EU generics and biosimilar producers from manufacturing medicines for export while they were still protected by the SPC in the EU. In 2019 the EU introduced a waiver allowing EU generics and biosimilar producers to manufacture medicines for export [31]. No medicinal product has been produced for export under this waiver system [32].
Another policy area is EU regulation to prevent trade diversion (re-importation into the EU) of lower-priced medicines for HIV/AIDS, TB, and malaria medicines destined for LMICs [33]. In 2003, the EU introduced a database and visual recognition for registered EU exports to 76 LMICs to encourage manufacturers to supply to LMICs at reduced prices and large quantities without fear of trade diversion [34]. No peer-reviewed studies investigated the impact of this regulation. An evaluation commissioned by the EC found that from 2003-2013 only one company registered nine antiretrovirals to treat HIV/AIDS under the regulation [34,35]. Those medicines were supplied at higher initial volumes in the 76 countries (2005-2008) than before being covered by the regulation [35]. The medicines list prices were not significantly lower than products supplied outside of this regulation nor compared to these products sold in other countries [35].
A third area is the enforcement of the EU's IP standards on shipments of generic medicines transiting through the EU and destined for LMICs. Although the shipments are not protected by IP in their countries of manufacture and destination (often LMICs), they may be detained when passing through the EU if they are suspected of infringing on IP rights protected in the EU [29,36,37]. The EU's IP enforcement measures aim to protect against the illegitimate trade in counterfeit or pirated goods, and risks to EU consumer health and safety; these rules apply to products both imported into and transiting through the EU. Numerous shipments of legitimate generics transiting to LMICs were detained at EU borders between 2008-2010; however, no peer-reviewed impact analysis was retrieved [29,37]. One example is of a shipment of losartan, equivalent to one-month hypertension treatment for 300,000 Brazilian patients, that was detained by the Dutch customs authorities for 36 days. After being released to return to India, the shipment never reached Brazil [37,38]. In 2013 the EU adopted legislation intending to safeguard against such seizures, however, subsequent EU regulation (adopted in 2015 and 2017) could allow for detentions of medicines in transit suspected of infringing on protected trademarks in the EU. No studies report the impact of these EU laws.
3.1.2.2. EActors adhere to EU standards in LMICs
The EU regulates the safety, efficacy, and quality of pharmaceuticals on the internal market. In doing so it imposes certain obligations on manufacturers commercialising their medicines in the EU. Those obligations require manufacturers and their affiliates (involved in researching, producing, or marketing medicines in the EU) to adhere to certain EU norms, even if these entities or their activities are located outside the EU. In this way, norms codified in EU law for good clinical practices (including pre-marketing safety), good manufacturing practices, and post-marketing pharmacovigilance and reporting may be externalised and respected by manufacturers active in LMICs [39]. For example, medicines manufacturers seeking access to the EU market must declare that all clinical testing adhered to the EU's ethical standards and good clinical practice, including at testing sites outside the EU (i.e. in LMICs) [40,41]. Commentators from the pharmaceutical industry suggest companies are key intermediaries with the experience, competence, and obligations from highly regulated markets, as well as the resources, to perform pre- and post-market safety reporting well in LMICs [42]. No peer-reviewed study examined the impact of such EU regulation on company behaviour in LMICs, nor on the decisions of LMIC regulators.
3.1.2.3. LMIC regulatory reliance and mirroring of EU internal market regulation
LMIC authorities rely on, mirror, and/or adapt EU decisions and technical standards for local regulatory approval, and transpose EU methods and tools for monitoring medicines use in LMIC markets. Regulatory reliance on foreign decisions, standards, and methods occurs on a spectrum ranging from inspiration for local adaptation to direct local recognition without further adjustments [43,44].
Regulatory reliance by LMICs is most frequently reported in relation to the EC's market authorisation decisions, notably in non-EU Eastern European countries and Latin America [43,[45], [46], [47]]. Thirteen Latin American regulatory bodies responsible for 34 countries have adopted domestic legislation that recognises or abbreviates the EC's market authorisation decisions (among other reference regulators) for new chemical entities [43]. Twelve of these regulators also recognise the EC's market approval without an additional local assessment [43]. Latin American regulators rely on the EC's decisions about cancer medicines based on poorly performed clinical trials using surrogate endpoints [43]. Between 2009-2013 six Latin American regulators approved more than 70% of the 17 EC-authorised cancer medicines that were lacking evidence of overall survival (primary endpoint) [45]. From 2014-2016 Latin American regulators approved between 62% (Panama) to 85% (Brazil) of the 13 EC-authorised cancer medicines despite the fact that no clinical trial was submitted in support of the decision, or all submitted trials were judged to be at a high risk of bias [45].
Regulatory reliance was reported in other areas of pharmaceutical policy. LMIC regulators reportedly use an iteration of the EMA guidelines for evaluating biosimilar products as a model for standards adopted in China [48], Malaysia [48], South Africa [49], and Ukraine [49,50]. LMIC regulators may also draw from the EMA's post-marketing updates to market authorisations. For example, LMIC regulators suspended rosiglitazone-containing antidiabetics from their markets after the EMA announced the same measure on September 23, 2010 due to safety concerns [51]. A causal link between EMA's and LMICs’ decisions were not evaluated. Other LMIC authorities have adopted the definitions, methods, and tools from the European Centre for Disease Prevention and Control or Eurobarometer to monitor local antibiotic use and generate cross-national comparisons [52,53]. National health ministries adapted these EU tools to generate data supporting the implementation of Serbia's national antibiotic guidelines and informing Thailand's public communication and strategy on antimicrobial resistance [52,53].
Reliance on EU regulation appears to be the decision of LMIC regulators and/or legislators. Their decisions may be modulated by the EU's status as one of the few ‘stringent regulatory authorities’ worldwide, and as such a potential model for other regulators. Additionally, the regulations, policies, and actions of other ‘stringent regulatory authorities’ (e.g., United States Food and Drug Administration) may impact on LMICs’ decisions to rely on the EC/EMA instead of or in addition to other regulators [43,54]. LMICs’ market size may also modulate their reliance on the EC/EMA. One trend suggests that in order to assess the bioequivalence of local generics regulators, small and some mid-sized markets (e.g., South Africa) accept studies with foreign comparators that have been approved in founding members of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (e.g., EU), whereas large-market regulators (e.g. Brazil, Mexico) do not [55].
Manufacturers opting to first seek EC approval for their product (before seeking approval in a LMIC) may also do so to enjoy the accompanying advantages, such as gaining access to the EU market and benefiting from EU incentives for orphan medicines [56,57]. EU regulatory incentives for designated orphan medicines include advice on clinical development, expedited evaluation, and, if authorised, market exclusivity blocking competitors on the EU market for ten years [57]. Several medicines that were primarily intended for LMICs have received an EU orphan designation and subsequent EU market authorisation, such as (non-exhaustive list): dihydroartemisinin / piperaquine phosphate (malaria) [56,57], a TB vaccine [58], paromomycin (visceral leishmaniasis) [55,58] (the product developers requested to withdraw the EC orphan status after the designation was granted[56]). It is unclear what role, if any, the EU orphan designation played in follow-on impacts in LMICs, such as market approval.
3.1.3. ‘Soft’ forms of EU influence on LMICs: technical assistance, resource mobilisation, and ‘capacity building
The notion of ‘soft’ forms of EU influence is inspired by the description of the EC's 2010 Globalisation and Health conference and its framing around EU policies as a means of “spreading European influence by 'soft' means of discussion, exchange and funding, rather than 'hard' means of trade and war”[59].
The EU provides technical assistance, mobilises global financing, and engages in capacity building on pharmaceutical issues towards LMICs in different policy areas.
Through EU-Medicines4all (also called the Article 58 procedure) the EMA provides scientific opinions on medicines intended for use in third countries (usually LMICs) in collaboration with WHO. The EMA's assessment involves WHO and LMIC regulators as observers; it does not lead to EU market authorisation [54]. LMIC regulators may rely on EMA opinions alone or in combination with decisions by WHO or other regulators when assessing the same medicine [17]. Between 2004-2019 EMA issued 10 positive opinions for new medicines, of which four were withdrawn by the opinion holder (company). For the remaining six medicines with a positive opinion, 138 regulatory approvals were granted in 90 countries [60]. The exact role of EMA's positive opinion in the LMIC approval processes was not investigated.
The EC's Directorate General (DG) for Research and Innovation financially supports the development and testing of pharmaceuticals and tools to improve the use of medicines primarily intended for priority needs in LMICs [60]. The European Commission's Framework Programmes (FP) 4 to FP6 (1997-2006) funded research on neglected infectious diseases (including HIV/AIDS, TB, and malaria) including vaccine research (FP5-FP7) leading to the development of several candidates for Buruli ulcer, leishmaniasis, and TB [62,63]. Within the EC's FPs is the ‘European and Developing Countries Clinical Trials Partnership’ (EDCTP), an EC initiative that catalysed global contributions towards the clinical development of priority HIV/AIDS, TB, and malaria medicines for Sub-Saharan Africa [63,64]. The EDCTP leverages EC funding to pool contributions from EU Member States and non-EU governments, industry, and philanthropies for the clinical development of medicines and capacity building of Sub-Saharan partners on ethical review standards and the conduct of clinical trials [64]. Every Euro invested by the EC in the EDCTP attracted an additional 1.50 Euro (2003-2011) [65] and 2.60 Euro (projected 2014-2020) from other contributors [64]. By 2018 two major achievements of the EDCTP were the development and market approval of the first malaria vaccine for humans (RTS,S) and the rapid development of the candidate Ebola vaccine [66].
DG International Partnerships coordinates the EU's development policy, including the European Development Fund (EDF) that mobilises funding directly from EU Member States and is managed outside of the EU budget. EDF supports collaboration between the EU, the African, Caribbean, and Pacific States (ACP), and WHO for harmonising pharmaceutical regulation and establishing pooled procurement aligned with WHO regulatory standards [67,68].
DG European Civil Protection and Humanitarian Aid Operations requires entities providing aid (often in LMICs) financed by the EU to respect internationally recognised principles of good practice for medical products, particularly for pharmaceutical procurement (ex. good manufacturing, storage, laboratory, clinical and distribution practices; WHO's quality assurance standards) [18]. In this way the EC promotes the diffusion of pharmaceutical standards that include but are not limited to the EU's own standards.
No peer-reviewed studies examined the follow-on impacts of the EC's direct investments nor coordination activities on, for example, LMICs’ pharmaceutical policy reform, or the approval, availability, or use of medicines in LMICs that were developed or provided through the above programs.
4. Discussion
Our review coalesces evidence from different disciplines to illustrate three mechanisms through which EU action impacts on medicines in LMICs. One, the EU's external, treaty-based actions establish explicit agreements with LMICs and can affect their pharmaceutical trade, sales, and use. Two, EU's internal market regulation, standards, and methods are used as bases, models or sources of inspiration for pharmaceutical governance in LMICs. Three, ‘soft’ forms of EU influence manifest through the EU's technical assistance, its mobilisation of research and development (aid) funding, and its ‘capacity building’ activities towards LMIC actors in the field of pharmaceuticals. For each mechanism several examples from pharmaceutical policy were retrieved. The impacts of EU action ranged from the development of new medicines primarily for LMICs, to changes in the availability of generics and on medicines spending in LMICs, and the potential for a more efficient yet less autonomous local market approval process. Most evidence of impact was not peer reviewed. Overall, this study points to the question of how to support resilient global pathways for drug development and regulation that generate efficiencies while still being responsive and accountable to the local public interest.
Although the EU can influence access to medicines and the sustainability and resilience of health systems in LMICs, peer-reviewed evidence of the EU's impact is limited in scope, volume, and scientific rigour [69]. This may be due to a lag in the international diffusion of EU action and/or delays in local implementation, which means the follow-on effects manifest in LMICs years or decades later [17]. Another reason may be the publication bias towards studies in LMICs. Additionally, some areas of EU policy (with the potential to impact LMICs’ pharmaceutical policies) were scarcely addressed in the literature, such as the EU's external aid agenda, the EU network for health technology assessment (which includes one LMIC, Ukraine [70]), and the EU joint Covid-19 vaccine procurements. Despite numerous claims made in the literature, there is little investigation of the full range of both potential positive and negative impacts for LMICs’ pharmaceuticals. Moreover, the literature heavily focuses on impacts in the field of HIV/AIDS, TB, malaria, and other infectious diseases while neglecting impacts on access to new medicines for non-communicable diseases (ex. cancer, diabetes, and cardiovascular disease) and rare diseases, which are often high-priced in LMICs. Finally, some study designs did not report the EU's role independent of EU Member States, comparable global powers (e.g., US) and/or international bodies (e.g., WHO, World Trade Organization (WTO)) that promote similar policy measures [17,71].
This research has important implications for EU law and policymaking. This study illustrates that EU policy makers adopt measures with the potential to influence medicines in LMICs despite limited evidence of their impact. For example, this review reveals that the EU's market authorisations have the potential to influence regulatory decisions affecting at least 380 million people in Latin America (among other world regions). This review also demonstrates the key role of EMA in influencing LMIC pharmaceutical policy, both intentionally through the EUMeds4All/Article 58 procedure, and unintentionally when LMICs rely on EMA's regulatory actions. LMICs appear to rely on the EU's regulatory decisions (instead of WHO recommendations, for example) because companies marketing a new medicine may first seek the approval of a ‘stringent regulatory authority’ such as the EU, which serves as a basis for WHO pre-qualification (PQP) [17], and because some LMIC regulators and WHO PQP require a foreign comparator for generic and/or biosimilar products [55].
Notably, the impacts of other EC policy responses for the development and deployment of pharmaceuticals needed globally in the Covid-19 pandemic were not examined in the literature. In practice, these measures appeared to take an insular, EU-centric approach without considering the external-EU policy impacts. For example, the EC's advanced purchase agreements for Covid-19 vaccines, and the EC's plans to upscale vaccine production (foreseen in the European Health Emergency Preparedness and Response Authority Incubator) do not address how these EU actions could support greater vaccine production globally, such as through IP licensing and technology transfer [72]. One step in this direction would be for the EC to require its funding recipients to share the resulting IP, know-how and knowledge for Covid-19 medical products with the WHO Covid-19 Technology Access Pool.
The fragmentation of EU internal and external actions in fields related to pharmaceuticals illustrate the need for a series of principles for global equitable access to medicines to guide EU policy. These principles should be developed based on EU legal principles and values, and the Global Health Council Conclusions. Moreover, the EU's impact assessments (prospective and retrospective) of its own legal and policy interventions should consider unintended impacts on pharmaceuticals in third countries, which requires the development and testing of new methodologies.
Further research is needed to categorically unpack the full spectrum of EU influence on pharmaceuticals in LMICs, and inform policy makers in Europe and abroad. Transdisciplinary research approaches should combine theories and methods from different disciplines to elucidate the normative role of the EU in global access to medicines, the power relations between the EU and LMICs, and the intentional and unintentional impacts of EU action on pharmaceuticals, and how these impacts can be compared, monitored, and optimised. The potential differential effects of EU action in LMICs should be disaggregated by their level of economic development, geographic scope, and/or EU candidate/partnership status. Priority policy areas for future study are those in the EU's 2021-2027 Pharmaceutical Strategy [21]. Robust empirical designs, such as longitudinal and/or quasi-experimental designs, should be used to permit conclusions about causality. A range of dependent variables should be applied to capture the intermediate outcomes and end impacts on LMICs’ legal, policy and institutional structures (e.g., regulatory reform, medicines litigation), health systems processes (e.g., market launch, and the selection, availability, pricing, and affordability of medicines), and individual and population health outcomes. Establishing advisory panels in LMICs can help identify and capture meaningful indicators for local populations. Future research should consider intermediaries’ (e.g., multinational and domestic pharmaceutical manufacturers, and third-party procurers supported by EU funding) practices for product development, marketing, and/or distribution, and how these practices modulate the dissemination and impact of EU action in LMICs [17].
This Scoping Review coalesces literature from a range of policy areas in four UN languages and across the interrelated, yet often siloed, disciplines of public health policy, international relations, and EU law. It synthesises the current knowledge about the mechanisms and impact of EU action on pharmaceuticals in LMICs through the lens of EU global regulatory influence. Our snowball reference search cross-referenced many publications that were already retrieved through our database search, which suggests our database search is complete. Nevertheless, this study does not capture the EC's early international cooperation actions about pharmaceuticals (ongoing since 1983), and may have missed literature in other languages. The selection of Russian articles was not validated by a second researcher. This study does not investigate the impacts of individual or groups of EU Member States, the EU's global political activities within multilateral bodies (ex. WHO, WTO), nor the EU's financial support of international initiatives for access to medicines (ex. Global Fund, Covax).
Contributors
KP and AdR conceived of the study. KP designed the protocol and analysis, that was reviewed by all authors. KP, CD, and ID conducted the search. KP, CD, ID, VM, and PP reviewed the literature. KP and CD conducted the analysis. KP drafted the manuscript that all authors revised. KP, CD, ID, FS and AdR participated in the interpretation. All authors critically revised the manuscript for intellectual content.
Declaration of interests
AdR and PP report grants from European Commission's Horizon 2020 programme (Grant no. 874653); AdR reports a grant from Dutch Research Council; KP reports grants from Unitaid, grants from Flemish Interuniversity Council (VLIR-UOS) and the Belgian Directorate-General for Development Cooperation during the conduct of the study. ID is a private attorney. His clients (including pharmaceutical companies) have no direct interest in the topic of this article. CD, VM, and FS have nothing to disclose.
The funders had no role in the impetus, design, conduct, analysis or writing of this review. The corresponding author had full access to all data and the final responsibility for the decision to submit this review for publication.
Acknowledgments
The authors acknowledge the helpful feedback from the anonymous peer reviewers.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100219.
Appendix. Supplementary materials
References
- 1.WHO . World Health Organization; Geneva: 2019. Primary Health Care on the Road to Universal Health Coverage: 2019 Monitoring Report: Executive Summary.https://apps.who.int/iris/handle/10665/328913 [Google Scholar]
- 2.Wirtz VJ, Hogerzeil H v, Gray AL. Essential medicines for universal health coverage. Lancet. 2017;389 doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PubMed] [Google Scholar]
- 3.Khatib R, McKee M, Shannon H. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387 doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 4.Perehudoff K, Toebes B, Hogerzeil H v. Essential Medicines in National Constitutions: Progress Since 2008. Health Hum Rights. 2016;18:141–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Perehudoff K. Universal access to essential medicines as part of the right to health: a cross-national comparison of national laws, medicines policies, and health system indicators. Glob Health Action. 2020;13 doi: 10.1080/16549716.2019.1699342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin C, Cloez S, Dujardin C, Ravinetto R. Europe should lead in coordinated procurement of quality-assured medicines for programmes in low-income and middle-income countries. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vu HT. Assessing potential impacts of the EVFTA on Vietnam's pharmaceutical imports from the EU: an application of SMART analysis. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-3200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wouters J, Goddeeris I, Natens B, Ciortuz F. Some Critical Issues in the EU-India Free Trade Agreement Negotiations. 2014 http://business.gov.in/trade/trade_agreements.php.
- 9.Chatterjee P. India–EU free-trade pact could stifle generics industry. Lancet. 2011:377. doi: 10.1016/S0140-6736(11)60524-2. [DOI] [PubMed] [Google Scholar]
- 10.Council of the European Union. Council conclusions on the EU role in Global Health. 3011th FOREIGN AFFAIRS Council meeting, 2010.
- 11.Steurs L, van de Pas R, Decoster K, Delputte S, Orbie J. Role of the European Union in global health. Lancet Glob Health. 2017;5 doi: 10.1016/S2214-109X(17)30212-7. [DOI] [PubMed] [Google Scholar]
- 12.Steurs L, van de Pas R, Delputte S, Orbie J. The global health policies of the EU and its member states: a common vision? Int J Health Policy Manag. 2017;7 doi: 10.15171/ijhpm.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kickbusch I, de Ruijter A. How a European health union can strengthen global health. Lancet Reg Health - Europe. 2021;1 doi: 10.1016/j.lanepe.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durán CE, Cañás M, Christiaens T. EMA and FDA decisions based on flawed evidence to approve new cancer drugs negatively affect Latin American patients. BMJ. 2019 doi: 10.1136/bmj.l6017. published online Oct 15. [DOI] [PubMed] [Google Scholar]
- 15.Limaye D, Langer JM, Rühling T, Fortwengel G. A critical appraisal of clinical trials conducted and subsequent drug approvals in India and South Africa. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford A. Oxford University Press; 2020. The Brussels Effect. [DOI] [Google Scholar]
- 17.Ahonkhai V, Martins SF, Portet A, Lumpkin M, Hartman D. Speeding access to vaccines and medicines in low- and middle-income countries: a case for change and a framework for optimized product market authorization. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrin Christophe, Cloëz Sandrine. Analysis of the Quality Assurance and Pharmaceutical Procurement Policies of a Sample of European donors. Antwerp, 2021.
- 19.Guerin PJ, Singh-Phulgenda S, Strub-Wourgaft N. The consequence of COVID-19 on the global supply of medical products: Why Indian generics matter for the world? F1000Research. 2020;9 doi: 10.12688/f1000research.23057.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow P, van Schalkwyk MC, McKee M, Labonté R, Stuckler D. COVID-19 and the collapse of global trade: building an effective public health response. Lancet Planetary Health. 2021;5 doi: 10.1016/S2542-5196(20)30291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa S, van Leeuwen R, Gray CC. HERA: a new era for health emergency preparedness in Europe? Lancet. 2021;397 doi: 10.1016/S0140-6736(21)01107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. Medicinal product. https://www.ema.europa.eu/en/glossary/medicinal-product (accessed July 8, 2021).
- 23.Tricco AC, Lillie E, Zarin W. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 24.Robinson D, Gibson C. Governing knowledge: Discourses and tactics of the european union in trade-related intellectual property negotiations. Antipode. 2011;43:1883–1910. [Google Scholar]
- 25.Michael GJ. International Coercion and the diffusion of regulatory data protection. J World Intellect Prop. 2016;19:2–27. [Google Scholar]
- 26.Škrnjug I, Uzeirbegović S, Romčević ML, Tomić S, Meyer H, Conrad C. Mutual recognition in the European system: A blueprint for increasing access to medicines? Regul Toxicol Pharmacol. 2019;106:270–277. doi: 10.1016/j.yrtph.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Tomić S, Sucić AF, Martinac AI. Granting marketing authorisation for medicines in South East European countries: the point of view of the authority. Regul Toxicol Pharmacol. 2010;57:325—332. doi: 10.1016/j.yrtph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 28.El-Said MK. TRIPS-Plus, Public Health and Performance-Based Rewards Schemes Options and Supplements for Policy Formation in Developing and Least Developed Countries Performance-Based Rewards Schemes Options And Supplements For Policy Formation In Developing And Least Developed Countries. 2016 http://digitalcommons.wcl.american.edu/auilrhttp://digitalcommons.wcl.american.edu/auilr/vol31/iss3/2.
- 29.IQVIA. Impact of free trade agreements (FTAs) on generic & biosimilar medicines markets. 2020.
- 30.Mercurio B. “Seizing” pharmaceuticals in transit: Analysing the wto dispute that wasn't. Int Comp Law Q. 2012;61:389–426. [Google Scholar]
- 31.Vidal-Quadras M. Analysis of EU Regulation 2019/933 on the SPC Manufacturing Waiver Exception. IIC Int Rev Intellect Property Comp Law. 2019;50:971–1005. [Google Scholar]
- 32.WTO. Notifications by exporting WTO members. https://www.wto.org/english/tratop_e/trips_e/public_health_notif_export_e.htm.
- 33.Outterson K. 8th ed. MLA; Outterson, Kevin: 2005. Pharmaceutical Arbitrage: Balancing Access and Innovation in International Prescription Drug Markets’ (2005) 5(1) Yale Journal of Health Policy, Law, and Ethics 193.http://www.gphaonline.com [PubMed] [Google Scholar]
- 34.Atik J, Lidgard HH. Embracing price discrimination: Trips and the suppression of parallel trade in pharmaceuticals. Univ Pennsylvania J Int Econ Law. 2006;27:1043–1076. [Google Scholar]
- 35.Wilsdon T, Attridge J, Barron A, Ginoza S, Li L, Associates CR. 2015 Evaluation of Council Regulation (EC) No 953/2003 to avoid trade diversion into the EU of certain key medicines. 2015.
- 36.Arkinstall J, Childs M, Menghaney L, Ford N, von Schoen-Angerer T. The reality behind the rhetoric: How European policies risk harming access to generic medicines in developing countries. J Generic Med. 2011;8:14–22. [Google Scholar]
- 37.Große Ruse-Khan H, Jaeger T. Policing Patents Worldwide?-EC Border Measures Against Transiting Generic Drugs Under EC and WTO Intellectual Property Regimes. https://beck-online-beck-de.proxy.uba.uva.nl/Print/CurrentMagazine?vpath=bibdata%5Czeits%5Ciic%5C2009%5Ccont%5Ciic.2009.502.1.htm&hlwords=on.
- 38.Government of Brazil . WTO General Council (February 03 and 04, 2008) 2008. Intervention by Brazil; pp. 1–4. [Google Scholar]
- 39.Tomić S, Sučić A, Martinac A. Good manufacturing practice: The role of local manufacturers and competent authorities. Arh Hig Rada Toksikol. 2010;61:425–436. doi: 10.2478/10004-1254-61-2010-2035. [DOI] [PubMed] [Google Scholar]
- 40.Schipper I, Weyzig F. SO M O Ethics for Drug Testing in Low and Middle Income Countries Considerations for European Market Authorisation. 2008 http://ssrn.com/abstract=1660433.
- 41.Altavilla A. News & views ethical standards for clinical trials conducted in third countries: The new strategy of the European medicines agency. Eur J Health Law. 2011;18:65–75. doi: 10.1163/157180911x546129. [DOI] [PubMed] [Google Scholar]
- 42.Ginsberg G, Shani S, Lev B. Cost-benefit analysis of risperidone and clozapine in the treatment of schizophrenia in Israel. Pharmacoeconomics. 1998;13:231–241. doi: 10.2165/00019053-199813020-00006. [DOI] [PubMed] [Google Scholar]
- 43.Durán CE, Cañás M, Urtasun MA. Regulatory reliance to approve new medicinal products in Latin American and Caribbean countries. Revista Panamericana de Salud Pública. 2021:1–10. doi: 10.26633/RPSP.2021.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luigetti R, Bachmann P, Cooke E, Salmonson T. Regulatory collaboration, not competition: developing new reliance models. WHO Drug Inf. 2016;30:558–566. [Google Scholar]
- 45.Durán CE, Cañás M, Urtasun M, Elseviers M, Vander Stichele R, Christiaens T. Potential negative impact of reputed regulators’ decisions on the approval status of new cancer drugs in Latin American countries: a descriptive analysis. PLoS One 16(7): e0254585. https://doi.org/10.1371/journal.pone.0254585 [DOI] [PMC free article] [PubMed]
- 46.Foteeva A v., Barshadskaya OS, Rostova NB. Description of the system of state registration of medicines in the Republic of Georgia as a development potential of domestic manufacturers. Drug Dev Reg. 2021;10 doi: 10.33380/2305-2066-2021-10-2-155-161. [DOI] [Google Scholar]
- 47.Anna Panfilova, Olesia Nemchenko, Liusine Simonian, Oksana Tsurikova. The analysis of organizational approaches in drug registration in the EU, Ukraine, Tajikistan, Turkmenistan and Uzbekistan. Res J Pharm Technol. 2018:2–8. [Google Scholar]
- 48.Rath PD, Chen DY, Gu J. Anti-tumor necrosis factor biosimilars and intended copies in rheumatology: Perspective from the Asia Pacific region. Int J Rheum Dis. 2019;22:9–24. doi: 10.1111/1756-185X.13371. [DOI] [PubMed] [Google Scholar]
- 49.Mounho B, Phillips A, Holcombe K, Grampp G. Chicago 17th ed. Barbara Mounho; Audrey Phillips; Kay Holcombe; Gustavo Grampp. 2010 https://heinonline.org/HOL/License. [PubMed]
- 50.Castañeda-Hernández G, Szekanecz Z, Mysler E. Biopharmaceuticals for rheumatic diseases in Latin America, Europe, Russia, and India: Innovators, biosimilars, and intended copies. Joint Bone Spine. 2014;81:471–477. doi: 10.1016/j.jbspin.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Nwokike J, Kabore L, Stergachis A. Actions of the national regulatory authorities in 10 low- and middle-income countries following stringent regulatory authority safety alerts on rosiglitazone. Ther Innov Regul Sci. 2015;49:279–283. doi: 10.1177/2168479014551644. [DOI] [PubMed] [Google Scholar]
- 52.Šuljagić V, Bajčetić M, Mioljević V. A nationwide assessment of the burden of healthcare-associated infections and antimicrobial use among surgical patients: results from Serbian point prevalence survey, 2017. Antimicrob Resist Infect Control. 2021;10 doi: 10.1186/s13756-021-00889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tangcharoensathien V, Sommanustweechai A, Chanvatik S, Kosiyaporn H, Tisocki K. Addressing the threat of antibiotic resistance in Thailand: monitoring population knowledge and awareness. 2018. [DOI] [PubMed]
- 54.Moran M, Strub-Wourgaft N, Guzman J, Boulet P, Wu L, Pecoul B. Registering new drugs for low-income countries: The African challenge. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Arieta A, Simon C, Santos GML. A survey of the regulatory requirements for the acceptance of foreign comparator products by participating regulators and organizations of the international generic drug regulators programme. J Phar Pharm Sci. 2019;22:28–36. doi: 10.18433/jpps30215. [DOI] [PubMed] [Google Scholar]
- 56.Doua JY, Geertruyden JP van. Registering medicines for low-income countries: How suitable are the stringent review procedures of the world health organisation, the us food and drug administration and the european medicines agency? Tro Med Int Health. 2014;19:23–36. doi: 10.1111/tmi.12201. [DOI] [PubMed] [Google Scholar]
- 57.Ubben D, Poll EM. MMV in partnership: The Eurartesim ® experience. Malar J. 2013;12 doi: 10.1186/1475-2875-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villa S, Compagni A, Reich MR. Orphan drug legislation: Lessons for neglected tropical diseases. Int J Health Plann Manage. 2009;24:27–42. doi: 10.1002/hpm.930. [DOI] [PubMed] [Google Scholar]
- 59.McCarthy M. European health research and globalisation: is the public-private balance right? Globalization Health. 2011;7:5. doi: 10.1186/1744-8603-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavaller Bellaubi M, Harvey Allchurch M, Lagalice C, Saint-Raymond A. The European Medicines Agency facilitates access to medicines in low- and middle-income countries. Expert Rev Clin Pharmacol. 2020;13:321–325. doi: 10.1080/17512433.2020.1724782. [DOI] [PubMed] [Google Scholar]
- 61.Pal SN, Olsson S, Brown EG. The monitoring medicines project: a multinational pharmacovigilance and public health project. Drug Saf. 2015;38:319–328. doi: 10.1007/s40264-015-0283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medaglini D, Hoeveler A. The European research effort for HIV/AIDS, malaria and tuberculosis. Vaccine. 2003;21 doi: 10.1016/S0264-410X(03)00212-3. [DOI] [PubMed] [Google Scholar]
- 63.Olesen OF, Ackermann M. Increasing European support for neglected infectious disease research. Comput Struct Biotechnol J. 2017;15:180–184. doi: 10.1016/j.csbj.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpkin VL, Renwick MJ, Kelly R, Mossialos E. Incentivising innovation in antibiotic drug discovery and development: Progress, challenges and next steps. J Antibiot. 2017;70:1087–1096. doi: 10.1038/ja.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PolicyCures. Savings lives and creating impact: EU investment in poverty-related neglected diseases. .
- 66.Olesen OF. European & Developing Countries Clinical Trials Partnership (EDCTP) Human Vaccines Immunotherapeutics. 2018;14:14–16. doi: 10.1080/21645515.2017.1356649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.PATH and Deutsche Stiftung Weltbevölkerung . 2017. Driving Health Impact through Regulatory Harmonization: How the EU Can Support Regulatory Harmonization in Africa to Accelerate Access to Essential Health Products. Seattle. [Google Scholar]
- 68.WHO . 2010. EC/ACP/WHO partnership on pharmaceutical policies. Geneva. [Google Scholar]
- 69.Catalá-López F, García-Altés A, Álvarez-Martín E, Gènova-Maleras R, Morant-Ginestar C. Does the development of new medicinal products in the European Union address global and regional health concerns? Popul Health Metrics. 2010;8 doi: 10.1186/1478-7954-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piniazhko OB, Kovtun LI, Zaliska OM, Oleshchuk OM, Leleka M v., Topachevskyi OA. Implementation of health technology assessment at the stage of market access for pharmaceuticals in Ukraine. Farm Zh. 2020 doi: 10.32352/0367-3057.3.20.05. published online July 9. [DOI] [Google Scholar]
- 71.Peters AJTP, Scharf MM, van Driel FTM, Jansen WHM. Where does public funding for HIV prevention go to? The case of condoms versus microbicides and vaccines. Globalization Health. 2010;6 doi: 10.1186/1744-8603-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boulet P, ‘t Hoen E, Perehudoff K, Mara K. 2021. Advanced purchase agreements for Covid-19 vaccines: Analysis and comments. Brussels. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.