Abstract
Background
The insulin-like growth factor family of ligands (IGF-I, IGF-II, and insulin), receptors (IGF-IR, M6P/IGF-IIR, and insulin receptor [IR]), and IGF-binding proteins (IGFBP-1-6) play critical roles in normal human physiology and disease states.
Scope of review
Insulin and insulin receptors are the focus of other chapters in this series and will therefore not be discussed further. Here we review the basic components of the IGF system, their role in normal physiology and in critical pathology's. While this review concentrates on the role of IGFs in human physiology, animal models have been essential in providing understanding of the IGF system, and its regulation, and are briefly described.
Major conclusions
IGF-I has effects via the circulation and locally within tissues to regulate cellular growth, differentiation, and survival, thereby controlling overall body growth. IGF-II levels are highest prenatally when it has important effects on growth. In adults, IGF-II plays important tissue-specific roles, including the maintenance of stem cell populations. Although the IGF-IR is closely related to the IR it has distinct physiological roles both on the cell surface and in the nucleus. The M6P/IGF-IIR, in contrast, is distinct and acts as a scavenger by mediating internalization and degradation of IGF-II. The IGFBPs bind IGF-I and IGF-II in the circulation to prolong their half-lives and modulate tissue access, thereby controlling IGF function. IGFBPs also have IGF ligand-independent cell effects.
Keywords: Insulin-like growth factors, Insulin-like growth factor receptors, Insulin-like growth factor binding proteins, Growth, Cancer, Metabolism
Highlights
-
•
The IGF system comprises a complex system of ligands, receptors, and binding proteins that play an essential role in all aspects of physiology.
-
•
In addition to its established essential role in somatic growth, other important roles continue to be discovered, including important roles in metabolism, aging, and stem cell maintenance.
-
•
The IGF system plays an important role in the progression of many cancers, although clinical therapies blocking components of the system have yet to adopted due to lack of a clear targeting strategy.
-
•
Along with insulin, the IGF system has been at the forefront in discoveries in relation to cell communication, from structural chemistry through cell biology and clinical medicine.
1. IGFs
IGF-I (70 amino acids) is produced under the control of growth hormone (GH), a phenomenon first described by Salmon and Daughaday in 1957 [1]. IGF-I was originally called “somatomedin C″ to describe its role in promoting sulfate incorporation into cartilage, leading to somatic growth. IGF-II (67 amino acids) was then discovered to have similar growth-promoting activities, but its expression is not under GH control. IGF-I and IGF-II share considerable amino acid sequence similarity with insulin. These three peptides adopt similar structures including 3 disulfide bonds that maintain the correct peptide fold. IGFs differ from insulin in that mature peptides are single chains with a linking domain (C domain) between the N-terminal B chain and C-terminal A chain. They are derived from post-translational processing of pre-pro-IGF peptides involving removal of the N-terminal signal peptide and C-terminal E peptide. IGF peptide folding and maturation is assisted by the chaperone GRP94 [2].
While humans express two IGF peptides and insulin, Caenorhabditis elegans express 38 insulin-like peptides (ILPs) and Drosophila express 8 insulin-like peptides (DILP 1–8) [3]. C. elegans ILPs act via a single receptor, which has high similarity to the human IR. Similarly, there is only a single IR-like receptor in Drosophila. In contrast, in humans, two related receptors (the IR and IGF-IR) independently mediate insulin and IGF actions. These receptors are believed to have developed through gene duplication. Comparison of insulin/IGF receptor signaling across these diverse species has highlighted the key functions of growth and aging regulation by the insulin/IGF system.
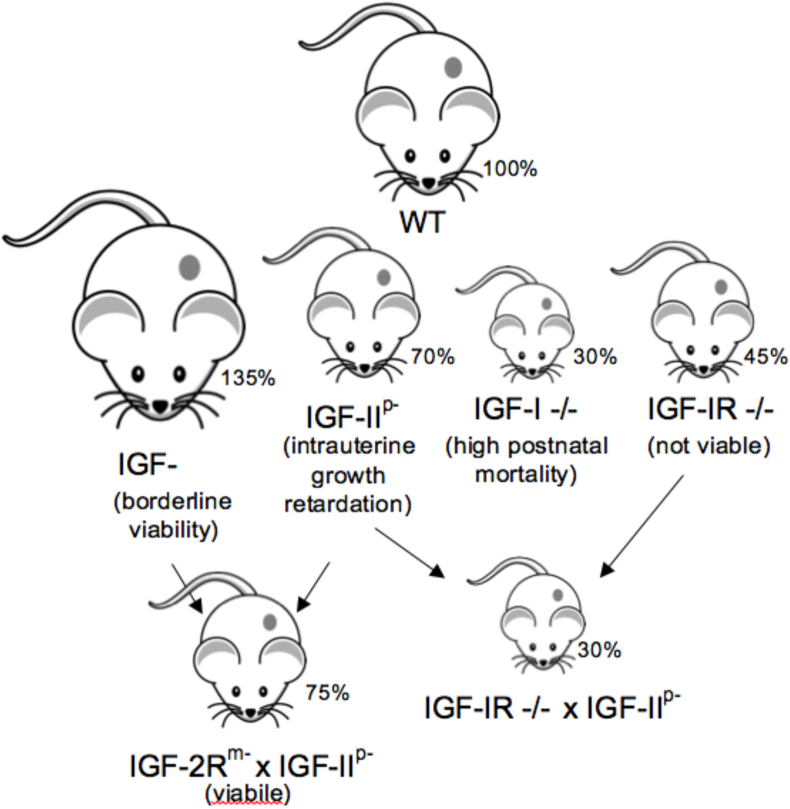
Further insights into the biological functions of mammalian IGFs have been reported in studies of knock-out and transgenic mice (Figure 1) [4]. Igf1-null mice are 30% the size and weight of wild-type mice and exhibit high post-natal mortality [5]. Those that survive also show post-natal growth retardation, particularly evident at the GH-dependent pubertal and post-pubertal growth stages. Their organs are proportionally smaller and bone growth is notably affected. Knock-out of Igf-1r also results in significant growth deficiency and is embryonically lethal [6].
Figure 1.
Summary of mouse studies investigating IGF system genes and their role in somatic growth. Percentages indicate body weight relative to age-matched normal mice. M indicates maternally disrupted allele, P indicates paternally disrupted allele and −/− indicates both alleles disrupted.
Igf2-null mice are intrauterine growth restricted and small at birth (70% of wild-type) (Figure 1) [7]. As Igf2 is an imprinted gene that is expressed from the paternal allele, knock-out is achieved through disrupting the paternal allele. Normally, Igf2 expression is downregulated after birth, and Igf2 knock-out mice catch up to wild-type size by adulthood. Overexpression of Igf2 in transgenic mice increases their overall size [8]. These mouse studies suggest that IGF-II plays a major role in prenatal growth and development. In contrast, loss-of-function mutation in human Igf2 affects both pre- and post-natal growth and, interestingly, circulating and tissue IGF-II levels normally remain high after birth in humans.
A further reduction in size results after crossing Igf2 −/− mice with Igf-1r −/− mice (Figure 1), possibly following the development of conditional knock-outs to overcome Igf-1r −/− lethality. This observation provided evidence of IGF-II's action through an alternate receptor, which is the alternate splice variant of the IR (IR-A) [9]. An additional receptor regulating IGF-II's action is the IGF-II receptor (cation-independent mannose-6-phosphate receptor, M6P/IGF-IIR). Normally, the M6P/IGF-IIR targets IGF-II for lysosomal degradation, thereby maintaining optimal IGF-II levels. Disruption of the maternal Igf-2r allele (also imprinted) leads to increased mouse size [10]. Lowering M6P/IGF-IIR expression results in elevated circulating IGF-II and increased growth.
While global knock-out studies have been instrumental for our understanding of the roles of IGF-I and IGF-II in normal growth and development, both IGF-I and IGF-II are expressed in different tissues at defined times, leading to tissue-specific actions. Tissue-specific knock-out and knock-in studies have provided an understanding of IGF-I and IGF-II's tissue-specific actions as has been reviewed previously [4]. We next consider the unique tissue-specific actions of each, highlighting their roles in normal human growth and development as well as disease.
1.1. IGF-I
IGF-I is produced under the control of GH through the action of signal transducer and activator of transcription 5b (STAT5b) [11], with the liver contributing to 75% of circulating IGF-I and 25% being derived from adipose and muscle [12]. In addition to GH, IGF-I production is very dependent on nutrition [13], which may be more important from an evolutionary perspective as the primary signaling pathways activated by IGF-I in metazoan were originally directly activated by nutrients [14]. Not only does IGF-I act in an endocrine manner to promote longitudinal growth, but it also acts in a paracrine and autocrine manner to promote cell proliferation and protein synthesis in most cells in the body. Circulating IGF-I's role in bone growth has been extensively investigated using mouse models as previously mentioned. IGF-I promotes bone growth by stimulating mesenchymal stem and progenitor cell differentiation into osteoblasts and chondrocytes [15]. It also promotes their proliferation. Even if IGF-I expression is ablated in the liver (LID mice), normal growth is achieved through the paracrine action of muscle-derived IGF-I [16]. However, a recent muscle-specific, inducible knock-out mouse model (MID) demonstrated muscle knock-out at birth leads to significantly decreased body weight mainly due to reduced muscle mass [17]. Clearly, skeletal muscle IGF-I also plays an important autocrine role. This study also interestingly pointed toward muscle IGF-I having significant paracrine influence over metabolic processes including glucose disposal.
Additional mouse models with targeted ablation of IGF-I production in the brain reveal roles in nervous system development and function (recently reviewed by Lewitt and Boyd, 2019 [18]). IGF-I is expressed in brain neurons and glial cells. It promotes neuron proliferation and protects against apoptosis as well as processes outgrowth and synaptogenesis. There is impaired neuronal somatic and dendritic growth in Igf1 −/− mice, whereas Igf1-overexpressing mice have increased post-natal brain growth. Lack of IGF signaling also affects cognition and mood [19]. Specific functions in the hippocampus and central amygdala of memory and temperature control, respectively, are evident after IGF-IR/IR knock-out in those tissues [20]. IGF-I is able to cross the blood–brain barrier such that circulating IGF-I also has endocrine roles in the brain. Negative feedback by circulating IGF-I inhibits GH secretion by the anterior pituitary.
Another role of IGF-I is to promote proliferation and survival of pancreatic islets. Somewhat counterintuitively, pancreatic-specific IGF-I knock-out results in enlarged islets and protects against islet destruction (streptozotocin-induced or high-fat diet-induced type 2 diabetes) through upregulation of regenerating islet proteins (Reg proteins) [21]. IGF-I appears to play an important role in maintaining stem cells in a number of tissues [22,23]. IGF-I also plays key roles in other tissues such as the kidney [24], lung [25], vascular endothelium [26], and eye [27].
1.2. IGF-II
Far fewer studies have been conducted on IGF-II's role in normal physiology and disease. In contrast to IGF-I, IGF-II is not expressed under the control of GH. However, like IGF-I, in adults IGF-II is expressed by most tissues, with most of the circulating IGF-II being derived from the liver. The IGF-II gene is located on chromosome 11p15.5 and is maternally imprinted, with expression arising from the paternally inherited allele except for in the liver, choroid plexus, and leptomeninges, where there is biallelic expression from the P1 promoter (one of 5 promoters) (reviewed recently by Holly et al. [28] and Baral and Rotwein [29]). The circulating levels of IGF-II in adults are approximately three-fold higher than IGF-I. Despite this, IGF-II is seen as playing its most important roles in fetal growth and development (perhaps a legacy from the fact that IGF-II is not detected in adult mice serum) and is abundant in fetal skeletal muscle. However, recent evidence derived by inducible knock-down of IGF-II in adult mice suggests that, although it may have little role as a circulating factor, locally produced IGF-II may have important post-natal roles, with IGF-II being essential for longitudinal and appositional murine post-natal bone development [30]. The fact that post-natal human IGF-II is abundantly expressed throughout life, in contrast to rodents, and is highly regulated at the level of Igf2 gene expression through its interaction with IGFBPs and the M6P/IGF-IIR all suggest that it has essential roles in adult humans. Indeed, evidence is accumulating suggesting an important role of IGF-II in human metabolism [28].
Other tissues in which IGF-II plays important roles include the placenta and brain. Knock-out of placental Igf2 leads to reduced placental growth and fetal growth restriction. In the fetal and adult brain, IGF-II is mainly produced by the choroid plexus, leptomeninges, and parenchymal vasculature. IGF-II is found in cerebral spinal fluid and promotes neurogenesis in the subventricular and subgranular zone of the adult brain. It appears to play an important role in neurogenesis in the hippocampus, thereby playing a role in memory consolidation [31].
Another IGF–II–specific function is to promote stem cell self-renewal through the activation of the IR-A. IGF-II:IR-A signaling supports neural stem cell maintenance and the expansion of neural progenitor cells [32]. This role in stem cell renewal extends to other tissues as identified using stem cell-specific knock-out of Igf2 in young adult mice in which growth of intestinal stem cells is also inhibited [33]. In addition, IGF-II stimulates the differentiation of stem cells into post-mitotic myotubes [34].
IGF-II plays a role in metabolic control through regulation of adipocyte differentiation and glucose uptake [35]. It also influences insulin secretion by maintaining β cell mass. Selective pancreatic silencing of Igf2 leads to suppression of insulin secretion [36]. The other major insulin-responsive metabolic tissue is skeletal muscle, and IGF-II promotes the development and maintenance of skeletal muscle mass. IGF-II signaling regulates MyoD function to stimulate muscle differentiation [37]. IGF-II also stimulates glucose uptake in mature skeletal muscle [38].
1.3. IGFs and disease
1.3.1. Growth disorders
In humans, mutations in the GH/IGF system components affect growth. Excessive production of GH (arising in situations such as pituitary adenomas) results in gigantism or acromegaly due to increased circulating IGF-I [39]. However, germline mutations that affect GH expression lead to growth deficiency, and mutations in the GHR result in short stature (Laron's syndrome) [40,41]. Many of these phenotypic changes are due to reduced serum IGF-I levels [40]. Reduced serum bioavailable IGF-I leading to growth deficiency has also been observed due to mutations in pregnancy-associated plasma protein A2 (PAPP-A2), an IGFBP protease [42]. There are multiple examples of severe growth failure of children resulting from reduced paracrine/autocrine IGF-I activity due to loss-of-function mutations in the Igf1 gene or IGF-IR mutations [43,44]. Clinical features of patients with Igf1 mutations include prenatal and post-natal growth failure, with severity proportional to the gene dose (homozygous vs heterozygous) and biological activity of the Igf1 variant. Microcephaly, retrognathia, poor feeding, sensorineural deafness, and severe global developmental delays are also associated with lack of IGF-I activity.
Overgrowth can also arise due to overexpression of IGF-II caused by loss of Igf2 imprinting, leading to Beckwith-Wiedemann syndrome [45]. This is characterized by developmental abnormalities and can lead to increased risk of childhood cancer. Both epimutation of the telomeric imprinting center region on chromosome 11p15 [46] and copy number variants of IGF-II [47] result in poor growth characteristics of Silver-Russell syndrome. Similarly, a paternally transmitted Igf2 loss-of-function variant [p.Ser64Ter] [48] and other similar variants (reviewed in Forbes, 2020 [43]) result in poor growth characterized by relative macrocephaly and short stature at preschool age, varying degrees of feeding problems, and neuropsychomotor developmental delays. Other phenotypic changes include cardiac and male genital anomalies, facial dimorphism, and finger deformities [43]. IGF-II variants are not only associated with poor growth, but may also have an effect on the risk of diabetes mellitus type 2 (T2D) [49]. In individuals of Latino descent with and without T2D, a novel genetic variant in Igf2 is associated with a ∼20% reduced risk of T2D. This variant prevents splicing between Igf2 exons 1 and 2 and is associated with a specific allele dosage-dependent reduction in the expression of Igf2 isoform 2. The mechanism underlying this association with diabetes risk is not yet clear as this isoform is minimally expressed in human tissues [50]. This again highlights the association of IGF-II with control of metabolism.
1.3.2. Cancer
Due to their potent pro-proliferative and survival activities, IGFs have been recognized for some time to be involved in cancer biology [51]. Elevated circulating levels of IGF-I have been associated with increased risk of developing certain cancers, including increasing risk of breast cancer in premenopausal women, prostate, and colorectal cancers [52,53]. In addition, through genetic changes, a wide range of tumors acquire altered expression of IGFs and IGF-IR. In some cases, this can have a driver effect to increase risk of malignancy. Examples of this include gastrointestinal stromal tumors, breast cancer, and osteosarcoma, where there is upregulation of IGF-IR or somatic mutations other than IGF system genes [54,55]. However, in the majority of solid tumors, the IGF system plays a secondary role in mutations in tumor suppressor genes and plays a role in augmenting the state of dysregulation by promoting tumor growth, survival, angiogenesis, and metastasis. In these situations, IGF signaling reduces the effectiveness of radiation therapy and chemotherapies [51].
IGF-II expression appears to be commonly upregulated in cancer [28]. In Wilms' tumor, colorectal, and hepatocellular cancer, there is a loss of imprinting, leading to biallelic Igf2 expression. In many tumors, including non-islet cell tumors, there is overexpression of “big IGF-II,” which has not post-translationally matured and still retains the IGF-II precursor E domain [56]. Big IGF-II is apparently readily bioavailable and effectively activates IGF-IR. Interestingly, there is also often a switch to the expression of IR-A by a wide range of cancer cell types (for example, thyroid, breast, and colon cancer) [57]. IGF-II has a relatively high affinity for IR-A, and signaling via IR-A promotes mitogenic processes, leading to cancer cell growth and survival [58]. Therefore, this IGF-II:IR-A autocrine loop represents an additional mechanism by which IGF-II can promote tumor growth. It also poses additional challenges to developing IGF inhibitors that block IGF-II's action without perturbing metabolic signaling.
Considerable effort has been applied to developing inhibitors of IGF's action to treat a wide range of cancers [51]. These have been developed to target IGFs (monoclonal antibodies or ligand traps) or IGF-IR (ligand binding blocking monoclonal antibodies or tyrosine kinase inhibitors). Approaches targeting IGF-IR have been intensely pursued with disappointing outcomes as will be discussed. Perhaps more promising are approaches targeting ligands. One potentially interesting molecule, a dual-specific anti-IGF-I and anti-IGF-II human monoclonal antibody, has been shown in preclinical studies to inhibit cancer growth. The molecule blocks binding of both ligands to IGF-IR and IGF-II to mitogenic IR-A [59]. Clinical efficacy with hopefully fewer side effects is awaited expectantly. An innovative technique involves the IGF-Trap, a novel anti-cancer therapeutic. The IGF-Trap is a heterotetramer consisting of the entire extracellular domain of IGF-IR fused to the Fc portion of human IgG1. It binds human IGF-I and IGF-II with higher affinity than insulin and could therefore inhibit IGF-IR and IR-A specifically and was shown to inhibit cellular functions such as survival, proliferation, and invasion in multiple in vitro carcinoma cell models [60]. Therefore, anti-ligand approaches are currently being pursued and are promising for treating IGF-responsive tumors. Developing suitable biomarkers to select target patients is a major area of investigation and will be essential for translating anti-IGF therapies into practice.
1.3.3. Cardiovascular disease
IGF-I promotes angiogenesis through its effects on vascular endothelial cells by promoting endothelial cell proliferation and survival. As the vascular endothelium regulates vascular permeability, angiogenesis, and inflammation, IGF-I signaling significantly impacts normal function and in cardiovascular disease (reviewed by Higashi et al., 2019 [61]). IGF-I plays a protective role in cardiovascular disease by supporting the proliferation and survival of smooth muscle cells. It promotes vascular smooth muscle migration and their production of plaque-stabilizing matrix components. Evidence of a protective effect comes from subcutaneous IGF-I treatment of Apoe-null mice, which prevented atherogenesis [62]. Importance of IGF-I signaling in inflammation associated with cardiovascular disease comes from a study of myeloid specific IGF-IR-deficient mice on a Apoe-null background (Lyz2-Cre/Igf1Rfl/fl/Apoe −/− mice) [63]. These mice have increased plaque instability and elevated macrophage numbers in atherosclerotic lesions, leading to the conclusion that IGF-I signaling is important for promoting plaque stability by anti-inflammatory signaling and reducing foam cell formation from macrophages.
Interestingly, PAPP-A matrix metalloproteinase regulates the bioavailability of IGFs to vascular smooth muscle cells and elevated levels of PAPP-A promotes atherosclerosis, suggesting that above a certain concentration, the protective effects of IGF-I are secondary to overgrowth effects that lead to atherosclerosis [64]. Furthermore, in diabetes, increased atherosclerosis has been attributed to altered IGF-IR signaling via the SHPS-1 and αVβ3 integrin-mediated pathway, leading to enhanced proliferation, migration, and dedifferentiation of vascular smooth muscle cells under hyperglycemic conditions [65]. Targeting of both the PAPP-A and SHPS-1/αVβ3 pathways with specific inhibitors has proven effective at reducing atherosclerosis in animal models and both may represent promising approaches for treating atherosclerosis in the future.
1.3.4. Neurodegenerative disorders
Dysregulation of the IGF system is involved in neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's diseases (as reviewed by Lewitt and Boyd [18]). Plaque formation (associated with β-amyloid aggregation) and neurofibrillary tangle formation (associated with tau hyperphosphorylation) are the two underlying features of Alzheimer's disease. In Alzheimer's disease, cognitive decline is associated with IGF-IR signaling resistance. In humans and mouse models of Alzheimer's disease, there is lower expression of IGFs and IGF signaling molecules with increased IGF-IR expression [66]. Reduced IGF-IR signaling impacts both β-amyloid aggregation and tau phosphorylation status. However, Alzheimer's disease mouse models crossed with Igf-1r knock-out mice demonstrate that IGF-IR signaling is protective as it reduces β-amyloid accumulation and promotes its clearance [67]. Interestingly, peripheral IGF-I levels do not appear to impact β-amyloid accumulation or the status of tau phosphorylation.
Parkinson's disease is characterized by the aggregation of misfolded α-synuclein protein, which forms insoluble cytoplasmic inclusions (Lewy bodies). There is a gradual selective loss of dopaminergic neurons of the substantia nigra pars compacta region. Lower circulating IGF-I levels are associated with poor cognition in patients with Parkinson's disease [68]. However, while this can be predictive of decline, the confounding factors of age and obesity make it a poor biomarker [69]. Expression of IGF system components in postmortem brain tissue from Parkinson's disease patients showed increased IGF-I expression in the frontal cortex and decreased expression of IGF-II, IGF-IR, IR, and M6P/IGF-IIR in the white matter and amygdala [70].
In Huntington's disease, higher circulating levels were associated with cognitive impairment and predicted further decline [18]. As seen with other neurodegenerative diseases, IGF-I is protective in cell models of Huntington's disease. This highlights the apparent need for tightly regulated IGF-IR signaling for normal brain function. The neuroprotective properties of IGFs have also sparked investigation of IGF-I as a therapy for several neurodegenerative disorders including amyotrophic lateral sclerosis and multiple sclerosis. These have not proven successful as yet but as the IGF system biology associated with these diseases is better understood, there may be a role for IGF therapy in the future. Application to situations of ischemic stroke and acute brain injury is also being considered.
Some of the aspects of these neurodegenerative diseases are linked to vascular remodeling and this is particularly relevant to the highly vascular areas of the brain that are regions of neurogenic capacity. In rodent studies, IGF-I has been shown to be important for vascular remodeling in the adult brain. Interestingly, aging can also contribute to neurodegeneration through cerebrovascular changes.
1.3.5. Aging
Not only does the highly conserved insulin-like signaling pathway control growth, development, and metabolism, but it has also been shown to regulate lifespan in organisms from unicellular yeast to worms, flies, and humans [[71], [72], [73]]. In mice, longevity is associated with reduced GH [74] or IGF-I [75] signaling. The data in humans are less clear. As indicated in the prior discussion of neurodegenerative disorders, it is evident that there is apparently a “sweet spot” of IGF-I levels that promote longevity and healthy aging above which IGF-I promotes diseases such as cancer, cardiovascular, and neurodegenerative diseases that negatively impact health and aging. A better understanding of the optimal IGF-I levels and how factors such as diet and exercise impact IGF levels will ultimately allow strategies to improve healthy aging in the future.
2. IGFBPs
The physiology of IGFs is very different from that of insulin, which is produced and stored in an endocrine gland. From early on, it was apparent that the vast majority of IGFs present in the circulation were of considerably larger size than the peptides, which was due to them being bound by binding proteins that were specific to binding IGFs but did not bind to insulin [76]. In placental mammals including humans, there are 6 closely related high-affinity IGF-binding proteins (IGFBP-1 to IGFBP-6). (Figure 2) These proteins have been conserved from a single early ancestral chordate IGFBP that initially underwent gene duplication, resulting in two IGFBP genes. Two episodes of whole genome duplications throughout evolution subsequently led to eight IGFBPs, two of which were then lost, resulting in the six IGFBPs found in placental mammals [77]. In humans, IGFBP-1 and IGFBP-3 are found together on chromosome 7 and IGFBP-2 and IGFBP-5 are together on chromosome 2, whereas IGFBP-4 is on chromosome 17 and IGFBP-6 is found on chromosome 12. IGFBPs are pluripotential, serving multiple physiological functions; their general structure and physiology were previously reviewed [78]. They all share the same conserved modular structure with three subdomains: highly conserved cysteine-rich N- and C-terminal domains with the cysteines forming intradomain disulphide bonds that hold these terminal regions in rigid globular structures with both contributing to high-affinity IGF binding. In contrast, the central linker domain is unstructured, is the least conserved among the different IGFBPs, and appears to provide each with their individual functions. Additional specificity is conferred by a number of post-translational modifications including glycosylation, phosphorylation, and proteolytic cleavage. The functions of IGFBPs can be broadly divided into those dependent on their ability to bind and modulate the activity of IGFs and functions that appear to be independent of direct IGF binding, although this division may not be definitive.
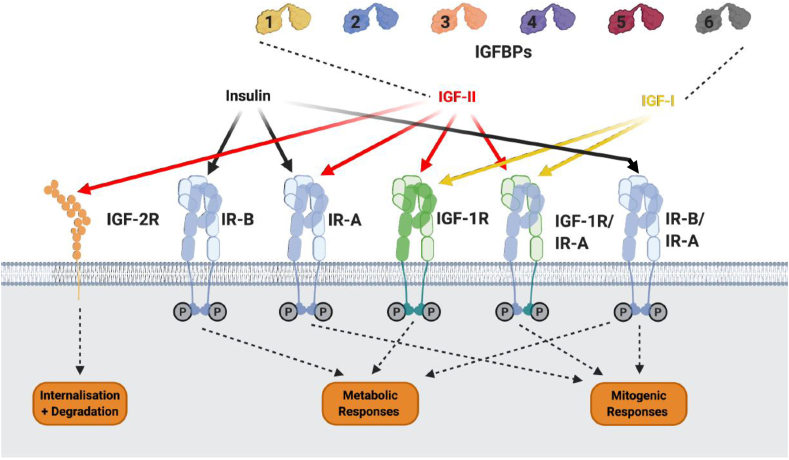
Figure 2.
Major components of the IGF system. The three insulin peptides, IGF-I, and IGF-II act via a series of cell surface receptors. The availability of IGF-I and IGF-II but not insulin to bind to these receptors is modulated via binding to six soluble high-affinity binding proteins, IGFBP-1 to −6. IGF-2R also modulates the availability of IGF-II by binding, internalizing, and targeting IGF-II for degradation. Insulin binds with the highest affinity to the two insulin receptor isoforms IR-A and IR-B. IR-A and IR-B are splice variants of the IR, with IR-A having a modified ligand-binding domain due to exon 11 being spliced out. Insulin has a low affinity for the IGF-IR. Each “hemireceptor” of IR-A, IR-B, and IGF-IR are comprised of an α- and β-subunit; each of these hemireceptors can either homodimerize or heterodimerize, forming hybrid receptors. IGF-I binds with a high affinity to the IGF-IR, IGF-IR/IR-A, and IGF-IR/IR-B hybrid receptors. IGF-II binds with high affinity to the IGF-IR, IR-A, and IGF-IR/IR-A hybrid receptors. Insulin only binds to hybrid receptors with low affinity. Activation of IR-B, IGF-1R, and IR-A/IR-B hybrids elicits metabolic responses. Activation of IR-A, IGF-IR, IGF-IR/IR-A, and IR-A/IR-B hybrids elicits mitogenic responses.
2.1. IGF-dependent functions of IGFBPs
IGFBPs bind to both IGF-I and IGF-II with higher affinity than the cell surface IGF receptors and therefore limit the availability of IGFs for receptor activation. There are two broad actions of IGFBPs that are fundamental to the physiology of IGFs. Although IGFs are widely expressed throughout the body, there are no intracellular stores in any tissues as they are produced and then immediately secreted via the constitutive secretory pathway. Following secretion, IGFs are immediately bound by high-affinity IGFBPs that form binary complexes of around 30–40 kDa (kDa). Unbound IGFs are cleared very rapidly, with a half-life of approximately 10 min; however, when bound to an IGFBP in a binary complex, their half-life is extended to around 30–90 min [79]. Two IGFBPs, IGFBP-3 and -5, when bound to IGFs can then bind to a further glycoprotein, the acid-labile subunit (ALS), forming a ternary complex of approximately 150 kDa. This ternary complex is too large to cross capillary barriers and hence is retained and accumulates in the circulation, extending the half-life of IGFs to between 12 and 20 h [79]. In the absence of tissue stores, IGFBPs therefore facilitate the accumulation of large reservoirs of IGFs within the body; in humans, the total concentration of IGFs in the circulation is around 100 nM, with 80–90% present in the ternary complex with IGFBP-3. In addition to providing a store, this also enables IGFs to act in an endocrine manner, having systemic effects in addition to acting as a conventional local tissue-growth factor. IGFs, IGFBP-3, and ALS are all produced in the liver but under different regulation; IGF-I and ALS are produced by hepatocytes, and their expression is GH regulated [80]. In contrast, IGFBP-3 is produced by Kupffer cells, where its expression in not directly regulated by GH, IGF-I, or insulin, but when Kupffer cells are cocultured with hepatocytes, its expression is stimulated by IGF-I and insulin [80]. However, because the formation of the ternary complex has such an impact on the clearance rate and with both IGF-I and ALS production being GH regulated, the circulating levels of IGFBP-3 and the ternary complex are closely related to GH status, which ensures that the size of the circulating IGF reservoir is GH-dependent. Genetic targeting generating ALS knock-out mice (ALSKO) resulted in a 65% reduction in circulating IGF-I levels, indicating the effect of ternary complex formation [81]. This was almost as low as the 75% reduction in mice with targeted deletion of IGF-I expression in the liver, LID mice [82]. Deficiency of ALS resulting in short stature and an increased risk of type 2 diabetes was the first monogenic defect described involving the IGFBP system in humans [83]. Subjects with complete absence of ALS have very low circulating levels of IGF-I and IGF-II and even lower levels of IGFBP-3, all of which fail to respond to administration of recombinant GH [83]. This confirms the critical role of the IGF/IGFBP-3/ALS ternary complex in the functioning of the GH/IGF endocrine axis.
The other critical role that IGFBPs may play is to provide additional mechanisms for conferring specificity to IGFs. IGFs are produced in most tissues and can regulate most cell functions. IGFBPs are produced in different amounts, at different times, and in different combinations in any tissue to confer some specificity to IGF activity. In addition, tissue exposure to IGFs is contributed by a combination of IGFs produced by the various cell types within the tissue and also IGFs perfusing into the tissue from the circulation. IGFBPs can modulate these different components. IGFs held in ternary complexes are restricted from crossing the capillary barrier. Some IGFBPs, however, can actually enhance the sequestration of IGFs into specific tissue compartments from the circulation. In an isolated beating heart perfusion system, it was demonstrated that if the perfusate contained IGFs in association with IGFBP-1 or IGFBP-2, then more IGFs accumulated in the tissue than if perfused by IGFs alone and the location within the tissue was altered [84]. In addition to binding IGFs, IGFBPs contain specific sequences for binding to proteoglycans, which are present on the extracellular matrix (ECM) and cell surfaces, providing a number of potential mechanisms for sequestration of IGFs in specific tissue compartments. It was also shown that this compartmentalization of IGFs into tissue was hormonally regulated, with insulin promoting the efflux of IGFBP-1-associated IGFs into the heart tissue [85].
As with IGFBP-3, the majority of circulating IGFBP-1 is derived from the liver, where its production in hepatocytes is inversely regulated by insulin such that its secretion is dynamically suppressed by insulin according to metabolic conditions [86]. This results in a marked circadian variation in the concentration of IGFBP-1 in the circulation [87]. This appears to facilitate an additional “endocrine” mechanism for acutely controlling IGF activity and entraining it to metabolic conditions. When nutritional intake falls, there is a reduction in insulin levels, and this releases the suppression of IGFBP-1 production, resulting in increasing circulating IGFBP-1 levels, which then restricts IGF activity [88]. The circulating levels of IGFBP-2 are also metabolically regulated, although with less acute fluctuations, and reflecting long-term insulin sensitivity with levels reduced in obese subjects [89]. Mice with specific knock-out of the IGFBP-2 gene had reduced bone mineral density [90] and high fat mass [91]. In addition, when IGFBP-2 was overexpressed in mice under the control of its native promoter, to maintain its normal tissue expression pattern, interesting metabolic effects were revealed [92]. Transgenic mice did not develop normal age-related glucose intolerance, insulin resistance, and high blood pressure and were protected from developing obesity on a high-energy or high-fat diet [92]. Both IGFBP-1 and IGFBP-2 therefore confer some metabolic endocrine regulation of systemic IGF activity. In contrast to the regulation of hepatic production of IGFBP-1, IGFBP-2, and IGFBP-3 by insulin and GH determining their systemic roles, all six IGFBPs are produced in varying amounts in other tissues in a very tissue-specific manner regulated by a variety of hormones and cytokines in a context-dependent manner [93,94]. This enables IGFBPs to confer some local specificity both in terms of their own IGF-independent actions and to IGFs via their IGF-dependent actions.
A large reservoir of IGFs is bound with higher affinity to IGFBPs than to cell surface IGF receptors: to activate this latent reservoir, there has to be mechanisms for liberating IGFs for receptor activation. A potential mechanism for mobilizing the circulating IGF reservoir arises due to the binding site for ALS on IGFBP-3 being in the C-terminal domain in the same region as a proteoglycan-binding site. This raises the possibility that proteoglycans on the surface of the capillary endothelium may compete for binding to IGFBP-3 and displace the ALS, converting the ternary complex into a binary complex that could then pass from the circulation into the tissues [95,96].
Another mechanism for accessing IGFBP-bound IGF was suggested by the demonstration that proteolysis of IGFBPs could lower the affinity with which they bind to IGFs [97,98]. That this actually occurred in vivo was confirmed with observations that the majority of IGFBP-3 in the circulation during human pregnancy was in a proteolytically cleaved state [99,100]. It was soon realized that this was a general phenomenon, with circulating IGFBP-3 being proteolytically modified in many situations, particularly those associated with increased metabolic demands [101]. The proteolysis of IGFBP-3 in the circulation is specific for just IGFBP-3 and only results in limited cleavage of the protein, which still retains IGF in the ternary complex. IGFBPs bind IGFs with high affinity and a slow dissociation rate, and a large IGFBP-3 fragment isolated from late pregnancy serum was found to have an 11-fold reduced affinity for IGF-I and a 4.5-fold reduced affinity for IGF-II compared to intact IGFBP-3 almost entirely due to a faster off rate [102]. This decrease in affinity could shift the complex equilibrium, with IGF being redistributed from proteolyzed IGFBP-3 to other IGFBPs that do not bind to the ALS and only form binary complexes, which can then cross the capillary barrier and transport IGFs into target tissues [103]. In extravascular fluids, IGFBP-3 proteolysis appears to be even more evident, even in normal healthy individuals in which there is limited detectable proteolysis in the circulation due to the presence of serum protease inhibitors [104]. The increased proteolysis of IGFBP-3 in the tissues reduces its inhibitory effects on IGF activity [105], consistent with a mechanism for delivering IGFs to target tissues.
It has subsequently become clear that all six IGFBPs are subject to proteolysis, which is generally being specific to an individual IGFBP [106]. This is due to proteolysis generally occurring in the non-conserved central linker domain [107], although the large circulating IGFBP-3 fragment appears to be due to cleavage in the C-terminal domain [102]. Although many extracellular proteases are capable of IGFBP proteolysis, the protease responsible for cleavage of circulating IGFBP-3 remains unclear despite over 30 years of studies [102]. The most well-characterized IGFBP protease is pregnancy-associated plasma protein-A (PAPP-A), which in vivo appears to mainly cleave IGFBP-4 [108]. PAPP-A is a metalloproteinase that binds to glycosaminoglycans present on cell surfaces, where it cleaves IGFBP-4 to increase IGF activity and has been shown to play an important role in several tissues such as bone, the cardiovascular system, and some cancers [108,109].
At the cellular level, IGFBPs can modulate IGF activity in many different ways depending on the specific IGFBP, the cell model, and the local conditions. In addition to inhibiting IGF activity via competitive binding, IGFBPs can also enhance IGF activity via a number of mechanisms. This can occur via IGFBP interacting with the ECM or cell surfaces to localize and enhance the IGF concentration in the vicinity of cell receptors or by altering the kinetics of IGF/receptor interactions and reducing receptor downregulation [78]. For example, IGFBP-5 binds to components of the ECM in fibroblast cultures, which reduces its affinity for binding IGF-I and increasing IGF-I activity [110]. In models of wound healing, the activity of IGF-I can be enhanced by IGFBPs [111], which can be mediated by interactions with vitronectin [112].
2.2. Functions of IGFBPs not dependent on binding to IGFs
The ancestry of IGFBPs can be traced back 520 million years to the amphioxus chordate whose single IGFBP lacks the ability to bind contemporary IGFs, although it does have a nuclear localization sequence in common with mammalian IGFBPs [113]. This implies that IGFBPs initially had function(s) that did not involve binding to IGFs and subsequently in evolution acquired this characteristic. This is a common feature of other hormone-binding proteins such as thyroxine-binding globulin (TBG) and corticosteroid-binding globulin (CBG), which evolved as serine protease inhibitors and later acquired the ability to bind hormones [114]. Although TBG is no longer a proteinase inhibitor, it still interacts with and is cleaved by neutrophil elastase, which lowers the affinity with which it binds to cortisol and hence acts to deliver cortisol to sites of inflammation. Therefore, a protein that evolved to control protease activity at sites of tissue damage acquired a further advantage by modulating the delivery of an anti-inflammatory modulator [115]. IGFBPs share a number of structural features with a larger group of cysteine-rich modular proteins, the CCN proteins, which possess pleiotropic cell functions that are similar to those described for IGFBPs [116]. It seems likely that IGFBPs similarly evolved as intrinsic cell regulators that subsequently acquired an ability to affect cell metabolism via binding and modulating the activity of IGFs, which then proved advantageous and complemented their original function.
IGFBPs are multifunctional proteins with a large number of different intrinsic actions being reported that are not dependent on binding to IGFs. There have been sporadic reports of specific cell surface receptors for IGFBP-5 and IGFBP-3, although these remain to be characterized or verified [117,118]. The most straightforward actions are those of IGFBP-1 and IGFBP-2 via cell integrin receptors as both of these IGFBPs contain classical integrin-binding Arg-Gly-Asp sequences that mediate a variety of actions on many different cells [94,119,120]. The related CCN proteins also have many actions mediated via integrin receptors; however, they interact with integrins via non-classical recognition sequences [121]. Similarly, other IGFBPs may act via integrin receptors despite not possessing classical integrin-recognition sequences; for example, the effects of IGFBP-3 and IGFBP-5 on apoptosis and cell attachment can be blocked by low doses of a disintegrin [122]. IGFBPs also bind to many other proteins that may affect cell functions; for example, some actions of IGFBP-3 appear to relate to its binding to type V transforming growth factor β (TFGβ) receptor, which also acts as the low-density lipoprotein receptor-related protein-1 (LRP-1) [123,124]. This receptor also binds to IGFBP-4 and -5 but not IGFBP-1, -2, or -6 [125]. Some actions of IGFBP-2 appear to be induced via binding to receptor protein tyrosine phosphatase β (RPTPβ), resulting in a reduction in its phosphatase activity [126].
Consistent reports have also indicated that IGFBPs can be internalized and translocated to the nucleus within cells, interact with nuclear receptors, and have transcriptional activity. These properties were present in the ancestral amphioxus IGFBP [113], suggesting that these were some of their original functions. The most studied has been IGFBP-3, which has been reported to interact with several nuclear receptors, most notably the retinoid X receptor (RXR-α), which functions as a ligand-dependent transcription factor by dimerizing with itself or other nuclear receptors such as the retinoic acid receptor (RAR), peroxisome proliferator-activated receptor-γ (PPAR-γ), vitamin D receptor, and Nur77, interactions that contribute to some of its activities [127].
Similar to the actions dependent on binding to IGFs, the actions independent of this interaction have also been described to be either stimulatory or inhibitory to cell functions [78,94]. Even in the same cell model, some IGFBPs can either stimulate or inhibit cell functions depending on the conditions and context. Changes in the cellular microenvironment, particularly the ECM, can switch actions of IGFBPs from stimulatory to inhibitory [122]. In addition, the actions of some IGFBPs may be determined by the effects on the sphingolipid rheostat in the plasma membrane with interconversion between sphingosine-1-phosphate and ceramides that, respectively, activate positive and negative signaling pathways [128,129].
There have also been many reports that IGFBPs may act directly on DNA repair mechanisms [130]. Damage to DNA results in activation of p53, all 6 IGFBP genes contain p53 response elements, and activation of p53 results in induction of a number of them [130]. IGFBP-3 has been shown to enhance the p53 response to DNA damage [131]. Within cell nuclei, both IGFBP-2 and IGFBP-3 have been shown to promote DNA repair by interacting in a complex with the epidermal growth factor receptor (EGFR) and DNA-dependent protein kinase (DNA-PK) [130,132].
Although these intrinsic actions of IGFBPs are frequently referred to as IGF independent, the relationship between these intrinsic actions and those mediated by modulating IGFs are still far from completely understood. At least in some systems, there appears to be a close integration of these actions: in breast cancer cells, it was shown that IGFBP-2 could modulate the activity of IGF-II via IGF-IR, but in addition, IGF-II could modulate the availability of IGFBP-2 to interact with integrin receptors [133]. These interactions were not just extracellular, as IGF-II could induce the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a key phosphatase that returns the PI3K/AKT/mTOR pathway to its inactivated state, and IGFBP-2 via integrin receptors could suppress the abundance of PTEN [133]. This action of IGFBP-2 on PTEN has been shown to operate in many different cells, suggesting that with IGFs activating PI3K and IGFBP-2 suppressing PTEN, there is coordinated regulation of both the “accelerator” and “brake” on this signaling pathway [134]. In vascular smooth muscle cells, a similar effect is achieved by IGF-I stimulating the phosphorylation and inactivation of PTEN, taking the brake off from the PI3K pathway, and IGFBP-2 binds to RPTPβ on the cell surface, inhibiting it from dephosphorylating PTEN, thereby preventing PTEN from applying the brake [135]. Although this does not depend on IGFBP-2 binding to IGF-I, the two act together in concert.
3. IGF receptors
The insulin receptor (IR) and insulin-like growth factor receptor (IGF-IR) are closely related tyrosine kinase receptors and share 57% sequence identity and high structural similarity; in particular, their tyrosine kinase domains have almost 85% identity. In contrast, the IGF-II receptor (M6P/IGF-IIR) is distinct from the IR and IGF-IR, both structurally and lacking intrinsic tyrosine kinase or any enzyme activity [136].
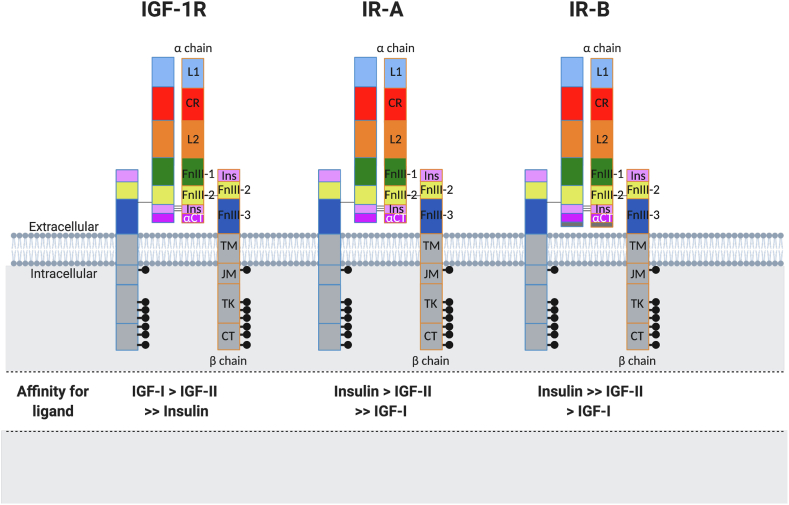
The IGF-IR, like the IR, is synthesized as a polypeptide precursor, followed by post-translational modifications. Cleavage of the precursor molecule leads to separate α and β subunits. The heterotetramer receptor is composed of two α and two β subunits joined by disulfide bridges (Figure 2). Detailed structural/functional analyses have shown that the α subunit contains conserved regions involved in ligand binding that include the leucine-rich 1 domain (L1) and an α-helical motif (αCT peptide) located at the end of the insert domain and residues in the first fibronectin–III–like domain (FnIII-1) (Figure 3). The IGF-1R β-subunit comprises 627 amino acids, with 196 residues incorporated in the extracellular domain. A short transmembrane domain (TM) connects the extracellular domain with the intracellular portion. The intracellular domain of the β-subunit is subdivided into three domains: the juxtamembrane domain (JM), enzymatic tyrosine kinase (TK) domain, and C-terminal domain. The TK ATP-binding motif (GXGXXG) resides at positions 976–981 and there is a catalytic lysine in position 1003, which is critical for Mg–ATP binding. A cluster of three tyrosines located at positions 1131, 1135, and 1136 within the activation loop of the TK domain are critical for receptor autophosphorylation. An NPEY motif resides in the JM portion and, once phosphorylated, acts as a docking site for insulin receptor substrates (IRS) and Shc, recruitment of which represents the first steps in downstream signaling. The NPEY motif is important for receptor internalization that regulates signaling (Figure 3). Other substrate binding sites also reside in the C-terminus.
Figure 3.
The IGF-1R and IR structural domains and affinities. L1 and L2: leucine-rich repeat domains; CR: cysteine-rich region; FnIII-1, FnIII-2, and FnIII-3: fibronectin-type III domains; Ins: insertion domains (α-chain C-terminal component and β-chain component); TM: transmembrane region; JM: juxtamembrane region; TK: tyrosine kinase catalytic domain; CT: C-terminal region.
The receptor-formation process begins in the endoplasmic reticulum. It is then transported through the Golgi apparatus and reaches the plasma membrane. Throughout this process, the pre-pro-IGF-IR peptide is proteolytically processed, the αβ subunits of a monomer are joined by disulfide bridges, and then two of these αβ subunits are joined by disulfide bridges extracellularly between the α subunits to form an αβ homodimer (Figure 3).
Our understanding of IGF-IR structure and function studies has been advanced through mutational analyses [137,138], X-ray crystallography [139], and most recently by high-resolution cryo-electron microscopy [140,141]. The ectodomain structures have a Λ-shaped conformation. The FnIII-2 and FnIII-3 domains forming the ectodomain “legs” are far apart in the apo (unbound) IGF-IR and hold the receptor in an inactive conformation. When IGF-I binds, there is a significant conformational rearrangement that reduces the distance between the two legs, thereby bringing the TM and intracellular TK domains in close proximity and alleviating the inhibition. This facilitates trans-autophosphorylation of the intracellular TK domains and subsequent activation of the intracellular-signaling process. This process is very similar to those of insulin/IR interactions [142,143].
Classically, the IGF-IR, which is expressed on most cells, is involved in cellular proliferation and anti-apoptosis during growth and development and has specific functions in well-differentiated cells such as neurons. However, as described for IGF-I (Section 1.1), the pluripotential roles of the IGF-IR continue to be described; for example, a new role of the IGF-IR in viral entry to cells has just been described [144]. The IR on the other hand classically supports metabolic functions of cells and tissues. The mitogenic IGF-IR is expressed at high levels in the fetus and then decreases post-natally, whereas the metabolic IR (IR-B) is mainly expressed in the liver, muscle, and adipose tissues [145]. While the most common expression of the IGF-IR (and IR) is as holoreceptors containing αβ chains transcribed from a single gene, hybrid receptors may form from two separate gene products, namely the IGF-IR and closely related IR [146] (Figure 2). The IR is furthermore differentially expressed as either the more mitogenic IR-A subtype or more metabolic IR-B subtype depending on the presence or absence of exon 11. These arise due to splicing factors that vary in different tissues and whose expression varies according to physiological and pathological conditions. Thus, hybrid receptors may result in IGF-IR/IR-A or IGF-IR/IRB receptors [147]. These hybrid receptors may alter ligand binding and post-receptor signaling compared with holoreceptors. Thus, the differences between IGF and insulin activities reflects the expression of each receptor and/or hybrid receptor in each cell and tissue.
Consistent with a role in cell proliferation and anti-apoptosis, the IGF-IR is upregulated by a number of transcription factors associated with tissue growth, including the androgen receptor, estrogen receptor, high-mobility group A1 (HMBA1), Krüppel-like factor 6 (KLF6), E2F1, and c-Jun, and downregulated by several tumor-suppressor genes, including tumor protein 53 (TP53), Wilms’ tumor protein-1 (WT1), Von Hippel-Lindau tumor suppressor (vHL), and breast cancer type 1 susceptibility protein (BRCA1) [148].
3.1. Post-receptor signaling
The IGF-IR possesses high binding affinity for IGF-I and IGF-II but also binds to insulin albeit with 50- to 100-fold lower affinity. IR-B binds to insulin with high affinity and binds to IGFs with low affinity. This difference plays important roles in mediation of distinct bioactivities of IGFs and insulin [58,149,150]. However, IR-A has a high affinity for not only insulin but also IGF-II and less so for IGF-I, suggesting that alternative splicing of insulin receptor RNA causes changes in cellular responsiveness to insulin-like peptides. Interestingly, hybrids of IGF-I receptor and IR bind to IGFs with higher affinity than insulin [151,152].
Ligand binding of IGF-I, IGF-II, or insulin to the IGF-IR sets up a cascade of events. The juxtaposition of the TK domains leads to tyrosine autophosphorylation of each β subunit mediated by the TK of the opposite β subunit. Phosphorylation of tyrosine residue 950 of the NPXY motif in the juxta membrane leads to recruitment of docking proteins. These docking proteins, which are common to both the IR and IGF-IR, called insulin receptor substrates (IRS1 and 2) and Shc, are then phosphorylated [[153], [154], [155]]. This further activates the phosphoinositide 3-kinase-protein kinase B (PI3K-AKT) and RAS-mitogen-activated protein kinase (MAPK) [156] signaling pathways (see Figure 4). In most cellular contexts, the IGF-IR activation of the PI3-AKT and MAPK pathways leads to downstream cellular proliferation, anti-apoptosis, and differentiation functions via other downstream intracellular molecules (Figure 4). The C-terminal region of the IGF-IR may also be associated with various proteins such as receptor for activated C kinase 1 (RACK1) [157], Vav [158], focal adhesion kinase (FAK) [159], Jun N-terminal kinase (JNK) [160], tissue inhibitor of metalloproteinase 2 (TIMP2) [161], Janus kinase 1/2 (JAK1/2), and suppressor of cytokine signaling 1/2/3 (SOCS1/2/3) [162], which may mediate or modulate IGF-IR signaling [163]. Girnita et al. studied the cross-talk between the IGF-IR and G-protein couple receptors (GPCRs) that opens up new avenues for research in cancer as well as potential new therapeutic opportunities [163]. For example, β-arrestins can bind the IGF-IR intracellularly and one isoform maintains IGF-IR-activated signaling, whereas another isoform may inhibit signaling [164]. As with other receptor-initiated intracellular signaling, it has become clear that most IGF signaling is not mediated via simple pathways but occurs via the activation of complex signaling networks involving many components, and the reader is referred to more specialized reviews for a more detailed description of those activated by IGFs [163,165,166].
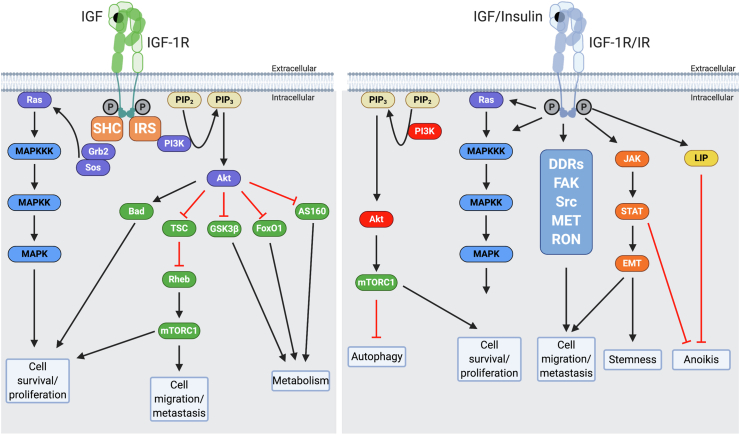
Figure 4.
Left panel: Canonical IGF-I and IGF-II signaling through the IGF-IR. IGF-I and IGF-II bind the IGF-IR at the extracellular α subunit, leading to autophosphorylation of β subunit residues, which then act as docking sites for insulin receptor substrates (IRS) 1–4 and other signaling proteins, such Shc. IRS-1 recruits the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K), which through the catalytic effects of its p110 subunit recruits protein kinase B (Akt) to the cell membrane, where it is phosphorylated and activated. Activated Akt has many substrates, including Bcl2 antagonist of cell death (Bad), glycogen synthase kinase 3β (GSK3β), forkhead transcription factors (such as FoxO1), and Akt substrate of 160 kDa (AS160). These factors are chiefly involved in regulating apoptosis and cell metabolism. Akt also regulates protein synthesis by phosphorylating tuberous sclerosis protein (TSC2), releasing its inhibition of Rheb to activate the mammalian target of rapamycin (mTORC1). The phosphorylation of IRS-1 and Shc can also lead to recruitment of growth factor receptor-bound protein 2 (Grb2), Son of Sevenless (SOS), and Ras, with subsequent activation via a phosphorylation series of the mitogen-activated protein kinase (MAPKKK, MAPKK, and MAPK) pathways, leading to cell proliferation, migration, and survival. Right panel: More novel signaling through the IGF-IR and IR mediated via additional interactions of the C-terminal intracellular region of the activated receptors with factors such as discoidin domain receptors (DDRs), focal adhesion kinase (FAK), receptor tyrosine-protein kinases such as Src, mesenchymal epithelial transition factor (MET), and recepteur d'origine nantais (RON). The activated receptor can also interact with Janus kinase (JAK), signal transducer, and activator of transcription (STAT). These interactions contribute to receptor actions on autophagy, epithelial–mesenchymal transition (EMT), stemness, and anoikis.
3.2. Internalization
Recent studies demonstrated that the IGF-IR (and IR) can migrate to the nucleus in both normal and malignant cells by as yet to be fully discovered mechanisms, although sumoylation is believed to be one mechanism. The IGF-IR can bind DNA and regulate gene transcription involved in apoptosis and the cell cycle as well as its own receptor gene [[167], [168], [169]]. The full range of IGF-IR actions within the cell are still being described and were recently reviewed in more detail [170].
Internalization of the IGF-IR is a complex process that involves partial degradation of the receptor as well as subcellular trafficking, intracellular signaling, and recycling the receptor to the surface. Increased internalization (endocytosis) of the IGF-IR is initiated by ligand binding [170]. Tyrosine residues 1250 and 1251 in the C-terminus play a role in internalization and degradation through substrates that specifically bind to these residues [171]. Internalization occurs via the acidic endosomal pathway and dissociates the ligand from the receptor [166]. Internalization may be via clathrin-dependent processes or caveolin-dependent pathways. The receptor may be degraded via the lysosomal or proteosomal pathways. Ligand binding induces receptor ubiquitination, a process that leads to degradation of the receptor via the proteosomal route. Thus, ligand binding and internalization may result in downregulation of the receptor concentration on the surface, although the levels may be normalized by increased IGF-IR gene expression. Subcellular trafficking maybe controlled by adhesion-associated protein complexes. These include the non-integrin collagen RTK, discoidin domain receptor 1 (DDR1) [172], and non-receptor tyrosine adhesion kinase FES-related (FER) [173]. In addition to being targeted for degradation, the IGF-IR can be translocated to the intracellular membrane compartments of the Golgi apparatus or nucleus [170]. In the Golgi, the IGF-IR can initiate intracellular signaling, which appears to be involved in the migratory behavior of cancer cells [166]. The translocation of the IGF-IR to the nucleus is induced by IGF-I and in the nucleus IGF-IR may associate with DNA to enhance transcription with effects that appear to facilitate an aggressive cancer phenotype [170].
3.3. IGF-IR and cancer
Given its effects on cell proliferation, the IGF-IR has long been recognized as contributing to cancer growth and metastases. Many cancers express high levels of the IGF-IR and IGF-II ligand. The upregulation of the receptor that is frequently seen in solid tumors is often due to loss of the regulatory influence on transcription of tumor-suppressor genes including TP53, WT1, vHL, and BRCA1 as previously described [148]. While the IGF-IR is not by itself oncogenic, it is important for tumor progression, enhancing proliferation, and inhibiting apoptosis, with resultant resistance to therapeutic modalities resulting in a poorer outcome [174]. Nuclear IGF-IR binds to promoters for genes such as JUN and FAM21A promoters, and enhanced gene expression results in chemo-resistance [168]. Drug-resistant cancers were associated with upregulation of the IGF-IR, suggesting that cancer used the IGF-IR pathway to escape the effects of therapy and therefore the IGF-IR could be a potential candidate for anti-cancer therapeutic regimes [156,175]. Compensatory upregulation was observed with various therapies including TKI-targeting EGFR, HER2, chemotherapy, and hormonal therapies [[175], [176], [177]].
Given its potential importance in cancer progression, in vitro cell and preclinical studies have demonstrated the effects of inhibiting the IGF-IR resulting in the inhibition of cancer growth [[178], [179], [180]]. Several strategies have been tested to block IGF-IR signaling to inhibit cancer growth in patients and many clinical trials have been instituted to determine their efficacy. These include IGF-binding proteins, monoclonal antibodies (mAb), small molecule tyrosine kinase inhibitors (TKIs) of the IGF-IR and IRs, and ligand-neutralizing strategies [181,182]. Using monoclonal antibodies, while initially showing promise, clinical trials were discontinued either due to lack of efficacy, complications, or the development of insulin resistance, hyperinsulinemia, and even hyperglycemia. Hyperglycemia was probably due to elevation of GH levels secondary to blocking the IGF-IR and removal of IGF-I feedback and the GH then causing insulin resistance through multiple mechanisms, although this has yet to be formally established by co-administration of a GH blocker. One involves lipolysis in adipocytes and the release of free fatty acids and other compounds that interfere with insulin action, for example at the liver. Another mechanism involves GH action at the liver directly [183]. Furthermore, blocking the IGF-IR facilitates a compensatory increased IR expression by cancer cells and led to the investigation and development of combined IR and IGF-IR TKIs. While preclinical studies demonstrated a high level of efficacy in inhibiting cancer growth, these TKIs also led to worsening of insulin resistance as have many inhibitors of downstream signaling molecules. Other potentially interesting approaches have been developed including dual-specific anti-ligand human monoclonal antibodies and an IGF-Trap based on the extracellular domain of the IGF-IR as previously described.
3.4. IGF-IR and thyroid eye disease
The autoimmunity of Graves' disease also involves the eye. Immunoglobulins in this disorder also activate the IGF-IR, and conversely IGF-I is known to enhance the actions of thyrotropin. Orbital fibroblasts, T and B cells, overexpress the IGF-IR in patients with Graves' disease. Teprotumumab, a humanized IGF-IR-inhibitory monoclonal antibody, after appropriate clinical trials is now used to treat Graves’ disease ophthalmopathy and has been shown to successfully reduce proptosis [27].
3.5. The IGF-II receptor
The IGF-II receptor (cation-independent mannose-6-phosphate receptor, M6P/IGF-IIR) is a single-chain transmembrane protein that binds IGF-II and mannose-6-phosphate-containing proteins. Unlike the IR and IGF-IR, the M6P/IGF-IIR has no catalytic function and therefore does not activate intracellular signaling processes. It is involved in both intracellular trafficking and cellular uptake of lysosomal enzymes and circulating IGF-II. Regarding IGF-II, the ligand has high affinity for this receptor, and the receptor acts as a scavenger by mediating internalization and degradation of IGF-II, removing the mitogenic effects of IGF-II that are mediated by the IGF-IR and mitogenic IR-A. Loss of expression and function of the M6P/IGF-IIR has frequently been associated with carcinogenesis, consistent with this implied role as a scavenger reducing the action of IGF-II at the cell surface [184]. Examples include altered expression of M6P/IGF-IIR in human pancreatic cancer and frequent loss of heterozygosity and/or mutations at the receptor locus in human hepatocellular tumor [185,186].
4. Concluding remarks
The IGF system is ubiquitous both in the circulation as an endocrine system and at the target tissue as a paracrine/autocrine system. The system affects normal physiology in all systems and has important effects on pathology affecting multiple disease states. Given this extensive interaction at each level, it supports the importance of intensive ongoing studies of the IGF system to understand its normal role(s) and regulate the effects when normal physiology becomes deranged. To date, most studies have investigated individual components of the system or how IGFs interact with IGFBPs or IGF receptors; however, it is clear that in vivo IGFs, IGFBPs, and IGF receptors form a fully integrated system. This has just started to be investigated. Studies have indicated that IGFs interact with IGFBP-2 outside the cell to each modulate the bioavailability of the other, with IGFs initiating cell signaling via IGF-IR-activating intracellular kinases and IGFBP-2 potentiating this by interacting on the cell surface with RPTPβ [135] or integrins [133] to inactivate phosphatases within the cell. The challenge for the future is to elucidate how all of the system's components are integrated and interact to regulate cell functions.
Conflict of interest
None declared.
References
- 1.Salmon W.D., Jr., Daughaday W.H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. The Journal of Laboratory and Clinical Medicine. 1957;49(6):825–836. [PubMed] [Google Scholar]
- 2.Argon Y., Bresson S.E., Marzec M.T., Grimberg A. Glucose-regulated protein 94 (GRP94): a novel regulator of insulin-like growth factor production. Cells. 2020;9(8):1844. doi: 10.3390/cells9081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roed N.K., Viola C.M., Kristensen O., Schluckebier G., Norrman M., Sajid W. Structures of insect Imp-L2 suggest an alternative strategy for regulating the bioavailability of insulin-like hormones. Nature Communications. 2018;9(1):3860. doi: 10.1038/s41467-018-06192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yakar S., Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Hormone & IGF Research. 2016;28:26–42. doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D. IGF-I is required for normal embryonic growth in mice. Genes & Development. 1993;7(12B):2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 6.Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 7.DeChiara T.M., Efstratiadis A., Robertson E.J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 8.Ward A., Bates P., Fisher R., Richardson L., Graham C.F. Disproportionate growth in mice with Igf-2 transgenes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(22):10365–10369. doi: 10.1073/pnas.91.22.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 10.DeChiara T.M., Robertson E.J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A., Alzhanov D., Rotwein P. Defining human insulin-like growth factor I gene regulation. American Journal of Physiology, Endocrinology and Metabolism. 2016;311(2):E519–E529. doi: 10.1152/ajpendo.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J.L., Yakar S., LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141(12):4436–4441. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 13.Rabkin R. Nutrient regulation of insulin-like growth factor-I. Mineral and Electrolyte Metabolism. 1997;23(3–6):157–160. [PubMed] [Google Scholar]
- 14.Byfield M.P., Murray J.T., Backer J.M. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. Journal of Biological Chemistry. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 15.Dixit M., Poudel S.B., Yakar S. Effects of GH/IGF axis on bone and cartilage. Molecular and Cellular Endocrinology. 2020;519:111052. doi: 10.1016/j.mce.2020.111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yakar S., Liu J.L., Stannard B., Butler A., Accili D., Sauer B. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassilakos G., Lei H., Yang Y., Puglise J., Matheny M., Durzynska J. Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice. Federation of American Societies for Experimental Biology Journal. 2019;33(1):181–194. doi: 10.1096/fj.201800459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewitt M.S., Boyd G.W. The role of insulin-like growth factors and insulin-like growth factor-binding proteins in the nervous system. Biochemistry Insights. 2019;12 doi: 10.1177/1178626419842176. 1178626419842176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez A.M., de la Vega A.G., Torres-Aleman I. Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1253–1258. doi: 10.1073/pnas.95.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto M., Cai W., Konishi M., Kahn C.R. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(13):6379–6384. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y., Ponton A., Okamoto H., Takasawa S., Herrera P.L., Liu J.L. Activation of the Reg family genes by pancreatic-specific IGF-I gene deficiency and after streptozotocin-induced diabetes in mouse pancreas. American Journal of Physiology. Endocrinology and Metabolism. 2006;291(1):E50–E58. doi: 10.1152/ajpendo.00596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wamaitha S.E., Grybel K.J., Alanis-Lobato G., Gerri C., Ogushi S., McCarthy A. IGF1-mediated human embryonic stem cell self-renewal recapitulates the embryonic niche. Nature Communications. 2020;11(1):764. doi: 10.1038/s41467-020-14629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oherle K., Acker E., Bonfield M., Wang T., Gray J., Lang I. Insulin-like growth factor 1 supports a pulmonary niche that promotes type 3 innate lymphoid cell development in newborn lungs. Immunity. 2020;52(2):275–294. doi: 10.1016/j.immuni.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach L.A., Hale L.J. Insulin-like growth factors and kidney disease. American Journal of Kidney Diseases. 2015;65(2):327–336. doi: 10.1053/j.ajkd.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Seedorf G., Kim C., Wallace B., Mandell E.W., Nowlin T., Shepherd D. rhIGF-1/BP3 preserves lung growth and prevents pulmonary hypertension in experimental bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine. 2020;201(9):1120–1134. doi: 10.1164/rccm.201910-1975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach L.A. Endothelial cells and the IGF system. Journal of Molecular Endocrinology. 2015;54(1):R1–R13. doi: 10.1530/JME-14-0215. [DOI] [PubMed] [Google Scholar]
- 27.Smith T.J., Janssen J.A.M.J.L. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocrine Reviews. 2019;40(1):236–267. doi: 10.1210/er.2018-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holly J.M.P., Biernacka K., Perks C.M. The neglected insulin: IGF-II, a metabolic regulator with implications for diabetes, obesity, and cancer. Cells. 2019;8(10):1207. doi: 10.3390/cells8101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baral K., Rotwein P. The insulin-like growth factor 2 gene in mammals: organizational complexity within a conserved locus. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0219155. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchimura T., Hollander J.M., Nakamura D.S., Liu Z., Rosen C.J., Georgakoudi I. An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development. 2017;144(19):3533–3546. doi: 10.1242/dev.155598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberini C.M., Chen D.Y. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends in Neurosciences. 2012;35(5):274–283. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler A.N., Schneider J.S., Qin M., Tyler W.A., Pintar J.E., Fraidenraich D. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30(6):1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler A.N., Feng Q., Chidambaram S., Testai J.M., Kumari E., Rothbard D.E. Insulin-like growth factor II: an essential adult stem cell niche constituent in brain and intestine. Stem Cell Reports. 2019;12(4):816–830. doi: 10.1016/j.stemcr.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florini J.R., Magri K.A., Ewton D.Z., James P.L., Grindstaff K., Rotwein P.S. Spontaneous" differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. Journal of Biological Chemistry. 1991;266(24):15917–15923. [PubMed] [Google Scholar]
- 35.Alfares M.N., Perks C.M., Hamilton-Shield J.P., Holly J.M.P. Insulin-like growth factor-II in adipocyte regulation: depot-specific actions suggest a potential role limiting excess visceral adiposity. American Journal of Physiology, Endocrinology and Metabolism. 2018;315(6):E1098–E1107. doi: 10.1152/ajpendo.00409.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song L., Sun Z., Kim D.S., Gou W., Strange C., Dong H. Adipose stem cells from chronic pancreatitis patients improve mouse and human islet survival and function. Stem Cell Research & Therapy. 2017;8(1):192. doi: 10.1186/s13287-017-0627-x. 2017 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson E.M., Rotwein P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. Journal of Biological Chemistry. 2006;281(40):29962–29971. doi: 10.1074/jbc.M605445200. [DOI] [PubMed] [Google Scholar]
- 38.Zierath J.R., Bang P., Galuska D., Hall K., Wallberg-Henriksson H. Insulin-like growth factor II stimulates glucose transport in human skeletal muscle. Federation of European Biochemical Societies Letters. 1992;307(3):379–382. doi: 10.1016/0014-5793(92)80717-u. [DOI] [PubMed] [Google Scholar]
- 39.Janssen J.A.M.J.L. Mechanisms of putative IGF-I receptor resistance in active acromegaly. Growth Hormone & IGF Research. 2020;52:101319. doi: 10.1016/j.ghir.2020.101319. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld R.G., Rosenbloom A.L., Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocrine Reviews. 1994;15(3):369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 41.Laron Z. Lessons from 50 years of study of Laron syndrome. Endocrine Practice. 2015;21(12):1395–1402. doi: 10.4158/EP15939.RA. [DOI] [PubMed] [Google Scholar]
- 42.Fujimoto M., Andrew M., Dauber A. Disorders caused by genetic defects associated with GH-dependent genes: PAPPA2 defects. Molecular and Cellular Endocrinology. 2020;518:110967. doi: 10.1016/j.mce.2020.110967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes B.E., Blyth A.J., Wit J.M. Disorders of IGFs and IGF-1R signaling pathways. Molecular and Cellular Endocrinology. 2020;518:111035. doi: 10.1016/j.mce.2020.111035. [DOI] [PubMed] [Google Scholar]
- 44.Storr H.L., Chatterjee S., Metherell L.A., Foley C., Rosenfeld R.G., Backeljauw P.F. Nonclassical GH insensitivity: characterization of mild abnormalities of GH action. Endocrine Reviews. 2019;40(2):476–505. doi: 10.1210/er.2018-00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azzi S., Abi Habib W., Netchine I. Beckwith-Wiedemann and Russell-Silver Syndromes: from new molecular insights to the comprehension of imprinting regulation. Current Opinion in Endocrinology, Diabetes and Obesity. 2014;21(1):30–38. doi: 10.1097/MED.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 46.Demars J., Le Bouc Y., El-Osta A., Gicquel C. Epigenetic and genetic mechanisms of abnormal 11p15 genomic imprinting in Silver-Russell and Beckwith-Wiedemann syndromes. Current Medicinal Chemistry. 2011;18(12):1740–1750. doi: 10.2174/092986711795496764. [DOI] [PubMed] [Google Scholar]
- 47.Begemann M., Spengler S., Gogiel M., Grasshoff U., Bonin M., Betz R.C. Clinical significance of copy number variations in the 11p15.5 imprinting control regions: new cases and review of the literature. Journal of Medical Genetics. 2012;49(9):547–553. doi: 10.1136/jmedgenet-2012-100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Begemann M., Zirn B., Santen G., Wirthgen E., Soellner L., Büttel H.M. Paternally Inherited IGF2 Mutation and Growth Restriction. New England Journal of Medicine. 2015;373(4):349–356. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- 49.Mercader J.M., Liao R.G., Bell A.D., Dymek Z., Estrada K., Tukiainen T. A loss-of-function splice acceptor variant in IGF2 is protective for type 2 diabetes. Diabetes. 2017;66(11):2903–2914. doi: 10.2337/db17-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rotwein P. The complex genetics of human insulin-like growth factor 2 are not reflected in public databases. Journal of Biological Chemistry. 2018;293:4324–4333. doi: 10.1074/jbc.RA117.001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osher E., Macaulay V.M. Therapeutic targeting of the IGF Axis. Cells. 2019;8(8):895. doi: 10.3390/cells8080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renehan A.G., Zwahlen M., Minder C., O'Dwyer S.T., Shalet S.M., Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 53.Knuppel A., Fensom G.K., Watts E.L., Gunter M.J., Murphy N., Papier K. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK biobank. Cancer Research. 2020;80(18):4014–4021. doi: 10.1158/0008-5472.CAN-20-1281. [DOI] [PubMed] [Google Scholar]
- 54.Nik-Zainal S., Davies H., Staaf J., Ramakrishna M., Glodzik D., Zou X. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarn C., Rink L., Merkel E., Flieder D., Pathak H., Koumbi D. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaap van Doorn J. Insulin-like growth factor-II and bioactive proteins containing a part of the E-domain of pro-insulin-like growth factor-II. BioFactors. 2020;46(4):563–578. doi: 10.1002/biof.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denley A., Wallace J.C., Cosgrove L.J., Forbes B.E. The insulin receptor isoform exon 11- (IR-A) in cancer and other diseases: a review. Hormone and Metabolic Research. 2003;35(11-12):778–785. doi: 10.1055/s-2004-814157. [DOI] [PubMed] [Google Scholar]
- 58.Belfiore A., Malaguarnera R., Vella V., Lawrence M.C., Sciacca L., Frasca F. Insulin receptor isoforms in physiology and disease: an updated view. Endocrine Reviews. 2017;38(5):379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Q., Tran H., Dimitrov D.S., Cheung N.K. A dual-specific anti-IGF-1/IGF-2 human monoclonal antibody alone and in combination with temsirolimus for therapy of neuroblastoma. International Journal of Cancer. 2015;137(9):2243–2252. doi: 10.1002/ijc.29588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.M., Qi S., Perrino S., Hashimoto M., Brodt P. Targeting the IGF-Axis for cancer therapy: development and validation of an IGF-trap as a potential drug. Cells. 2020;9(5):1098. doi: 10.3390/cells9051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higashi Y., Gautam S., Delafontaine P., Sukhanov S. IGF-1 and cardiovascular disease. Growth Hormone & IGF Research. 2019;45:6–16. doi: 10.1016/j.ghir.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sukhanov S., Higashi Y., Shai S.-Y., Vaughn C., Mohler J., Li Y. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(12):2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 63.Higashi Y., Sukhanov S., Shai S.-Y., Danchuk S., Tang R., Snarski P. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. 2016;133(23):2263–2278. doi: 10.1161/CIRCULATIONAHA.116.021805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steffensen L.B., Conover C.A., Oxvig C. PAPP-A and the IGF system in atherosclerosis: what's up, what's down? American Journal of Physiology - Heart and Circulatory Physiology. 2019;317(5):H1039–H1049. doi: 10.1152/ajpheart.00395.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clemmons D.R., Maile L.A., Ling Y., Yarber J., Busby W.H. Role of the integrin alphaVbeta3 in mediating increased smooth muscle cell responsiveness to IGF-I in response to hyperglycemic stress. Growth Hormone & IGF Research. 2007;17(4):265–270. doi: 10.1016/j.ghir.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moloney A.M., Griffin R.J., Timmons S., O'Connor R., Ravid R., O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signaling. Neurobiology of Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Cohen E., Paulsson J.F., Blinder P., Burstyn-Cohen T., Du D., Estepa G. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139(6):1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma J., Jiang Q., Xu J., Sun Q., Qiao Y., Chen W. Plasma insulin-like growth factor 1 is associated with cognitive impairment in Parkinson's disease. Dementia and Geriatric Cognitive Disorders. 2015;39(5–6):251–256. doi: 10.1159/000371510. [DOI] [PubMed] [Google Scholar]
- 69.Pellecchia M.T., Santangelo G., Picillo M., Pivonello R., Longo K., Pivonello C. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naïve Parkinson's disease. European Journal of Neurology. 2014;21(5):802–807. doi: 10.1111/ene.12137. [DOI] [PubMed] [Google Scholar]
- 70.Tong M., Dong M., de la Monte S.M. Brain insulin-like growth factor and neurotrophin resistance in Parkinson's disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. Alzheimers Disease. 2009;16(3):585–599. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartke A., List E.O., Kopchick J.J. The somatotropic axis and aging: benefits of endocrine defects. Growth Hormone & IGF Research. 2016;27:41–45. doi: 10.1016/j.ghir.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vitale G., Pellegrino G., Vollery M., Hofland L.J. Role of IGF-1 system in the modulation of longevity: controversies and new insights from a centenarians' perspective. Frontiers in Endocrinol (Lausanne) 2019;10:27. doi: 10.3389/fendo.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milman S., Huffman D.M., Barzilai N. The somatotropic Axis in human aging: framework for the current state of knowledge and future research. Cell Metabolism. 2016;23(6):980–989. doi: 10.1016/j.cmet.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown-Borg H.M., Borg K.E., Meliska C.J., Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 75.Holzenberger M., Dupont J., Ducos B., Leneuve P., Géloën A., Even P.C. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 76.Hintz R.L., Liu F. Demonstration of specific plasma protein binding sites for somatomedin. Journal of Clininical Endocrinology and Metabolism. 1977;45(5):988–995. doi: 10.1210/jcem-45-5-988. [DOI] [PubMed] [Google Scholar]
- 77.Daza D.O., Sundström G., Bergqvist C.A., Duan C., Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152(6):2278–2289. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- 78.Firth S.M., Baxter R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocrine Reviews. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 79.Guler H.-P., Zapf J., Schmid C., Froesch E.R. Insulin-like growth factors-I and -II in healthy man: estimation of half-lives and production rates. Acta Endocrinologica. 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- 80.Scharf J., Ramadori G., Braulke T., Hartmann H. Synthesis of insulinlike growth factor binding proteins and of the acid-labile subunit in primary cultures of rat hepatocytes, of Kupffer cells, and in cocultures: regulation by insulin, insulinlike growth factor, and growth hormone. Hepatology. 1996;23(4):818–827. doi: 10.1053/jhep.1996.v23.pm0008666337. [DOI] [PubMed] [Google Scholar]
- 81.Ueki I., Ooi G.T., Tremblay M.L., Hurst K.R., Bach L.A., Boisclair Y.R. Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6868–6873. doi: 10.1073/pnas.120172697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J.L., Yakar S., LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141(12):4436. doi: 10.1210/endo.141.12.7825. 41. [DOI] [PubMed] [Google Scholar]
- 83.Domené S., Domené H.M. The role of acid-labile subunit (ALS) in the modulation of GH-IGF-I action. Molecular and Cellular Endocrinolology. 2020;518:111006. doi: 10.1016/j.mce.2020.111006. 2020. [DOI] [PubMed] [Google Scholar]
- 84.Bar R.S., Clemmons D.R., Boes M., Busby W.H., Booth B.A., Dake B.L. Transcapillary permeability and subendothelial distribution of endothelial and amniotic fluid insulin-like growth factor binding proteins in the rat heart. Endocrinology. 1990;127(3):1078–1086. doi: 10.1210/endo-127-3-1078. [DOI] [PubMed] [Google Scholar]
- 85.Bar R.S., Boes M., Clemmons D.R., Busby W.H., Sandra A., Dake B.L. Insulin differentially alters transcapillary movement of intravascular IGFBP-1, IGFBP-2 and endothelial cell IGF-binding proteins in the rat heart. Endocrinology. 1990;127(1):497–499. doi: 10.1210/endo-127-1-497. [DOI] [PubMed] [Google Scholar]
- 86.Unterman T.G. Insulin-like growth factor binding protein-1: identification, purification, and regulation in fetal and adult life. Advances in Experimental Medicine & Biology. 1993;343:215–226. doi: 10.1007/978-1-4615-2988-0_21. [DOI] [PubMed] [Google Scholar]
- 87.Holly J.M., Biddlecombe R.A., Dunger D.B., Edge J.A., Amiel S.A., Howell R. Circadian variation of GH-independent IGF-binding protein in diabetes mellitus and its relationship to insulin. A new role for insulin? Clinical Endocrinology. 1988;29:667–675. doi: 10.1111/j.1365-2265.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 88.Taylor A.M., Dunger D.B., Preece M.A., Holly J.M., Smith C.P., Wass J.A. The growth hormone independent insulin-like growth factor–I binding protein BP-28 is associated with serum insulin-like growth factor-I inhibitory bioactivity in adolescent insulin-dependent diabetics. Clinical Endocrinology (Oxford) 1990;32:229–239. doi: 10.1111/j.1365-2265.1990.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 89.Wheatcroft S.B., Kearney M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends in Endocrinology and Metabolism. 2009;20(4):153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Kawai M., Breggia A.C., DeMambro V.E., Shen X., Canalis E., Bouxsein M.L. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. Journal of Biological Chemistry. 2011;286(16):14670–14680. doi: 10.1074/jbc.M110.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xi G., Solum M.A., Wai C., Maile L.A., Rosen C.J., Clemmons D.R. The heparin-binding domains of IGFBP-2 mediate its inhibitory effect on preadipocyte differentiation and fat development in male mice. Endocrinology. 2013;154(11):4146–4157. doi: 10.1210/en.2013-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wheatcroft S.B., Kearney M.T., Shah A.M., Ezzat V.A., Miell J.R., Modo M. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bach L.A. IGF-binding proteins. Journal of Molecular Endocrinology. 2018;61(1):T11–T28. doi: 10.1530/JME-17-0254. [DOI] [PubMed] [Google Scholar]
- 94.Holly J., Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83(3–4):154–160. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- 95.Baxter R.C. Inhibition of the insulin-like growth factor (IGF)-IGF-binding protein interaction. Hormone Research. 2001;55(Suppl 2):68–72. doi: 10.1159/000063479. [DOI] [PubMed] [Google Scholar]
- 96.Payet L.D., Firth S.M., Baxter R.C. The role of the acid-labile subunit in regulating insulin-like growth factor transport across human umbilical vein endothelial cell monolayers. Journal of Clinical Endocrinology & Metabolism. 2004;89(5):2382–2389. doi: 10.1210/jc.2003-031880. [DOI] [PubMed] [Google Scholar]
- 97.Clemmons D.R., Underwood L.E., Chatelain P.G., Van-Wyk J.J. Liberation of immunoreactive somatomedin-C from its binding proteins by proteolytic enzymes and heparin. Journal of Clinical Endocrinology & Metabolism. 1983;56:384–389. doi: 10.1210/jcem-56-2-384. [DOI] [PubMed] [Google Scholar]
- 98.Chatelain P.G., Van-Wyk J.J., Copeland K.C., Blethen S.L., Underwood L.E. Effect of in vitro action of serum proteases or exposure to acid on measurable immunoreactive somatomedin-C in serum. Journal of Clinical Endocrinology & Metabolism. 1983;56:376–383. doi: 10.1210/jcem-56-2-376. [DOI] [PubMed] [Google Scholar]
- 99.Giudice L.C., Farrell E.M., Pham H., Lamson G., Rosenfeld R.G. Insulin-like growth factor binding proteins in maternal serum through-out gestation and in the puerperium, effects of a pregnancy associated serum protease activity. Journal of Clinical Endocrinology & Metabolism. 1990;71:806–816. doi: 10.1210/jcem-71-4-806. [DOI] [PubMed] [Google Scholar]
- 100.Hossenlopp P., Segovia B., Lassarre C., Roghani M., Bredon M., Binoux M. Evidence of enzymatic degradation of insulin-like growth factor-binding proteins in the 150K complex during pregnancy. Journal of Clinical Endocrinology & Metabolism. 1990;71:797–805. doi: 10.1210/jcem-71-4-797. [DOI] [PubMed] [Google Scholar]
- 101.Maile L.A., Holly J.M. Insulin-like growth factor binding protein (IGFBP) proteolysis: occurrence, identification, role and regulation. Growth Hormone & IGF Research. 1999;9(2):85–95. doi: 10.1054/ghir.1999.0096. [DOI] [PubMed] [Google Scholar]
- 102.Ahlsén M., Carlsson-Skwirut C., Jonsson A.P., Cederlund E., Bergman T., Bang P. A 30-kDa fragment of insulin-like growth factor (IGF) binding protein-3 in human pregnancy serum with strongly reduced IGF-I binding. Cellular and Molecular Life Sciences. 2007;64(14):1870–1880. doi: 10.1007/s00018-007-7201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blat C., Villaudy J., Binoux M. In vivo proteolysis of serum insulin-like growth factor (IGF) binding protein-3 results in increased availability of IGF to target cells. Journal of Clinical Investigation. 1994;93:2286–2290. doi: 10.1172/JCI117229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maile L.A., Xu S., Cwyfan-Hughes S.C., Fernihough J.K., Pell J.M., Holly J.M. Active and inhibitory components of the insulin-like growth factor binding protein-3 protease system in adult serum, interstitial, and synovial fluid. Endocrinology. 1998;139(12):4772–4781. doi: 10.1210/endo.139.12.6359. [DOI] [PubMed] [Google Scholar]
- 105.Xu S., Savage P., Burton J.L., Sansom J., Holly J.M. Proteolysis of insulin-like growth factor-binding protein-3 by human skin keratinocytes in culture in comparison to that in skin interstitial fluid: the role and regulation of components of the plasmin system. Journal of Clinical Endocrinology and Metabolism. 1997;82(6):1863–1868. doi: 10.1210/jcem.82.6.4025. 1997 Jun. [DOI] [PubMed] [Google Scholar]
- 106.Bunn R.C., Fowlkes J.L. Insulin-like growth factor binding protein proteolysis. Trends in Endocrinology and Metabolism. 2003;14(4):176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 107.Berg U., Bang P., Carlsson-Skwirut C. Calpain proteolysis of insulin-like growth factor binding protein (IGFBP) -2 and -3, but not of IGFBP-1. Biological Chemistry. 2007;388(8):859–863. doi: 10.1515/BC.2007.098. [DOI] [PubMed] [Google Scholar]
- 108.Monget P., Oxvig C. PAPP-A and the IGF system. Annales d’Endocrinolie (Paris) 2016;77(2):90–96. doi: 10.1016/j.ando.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Conover C.A., Oxvig C. PAPP-A and cancer. Journal of Molecular Endocrinology. 2018;61(1):T1–T10. doi: 10.1530/JME-17-0236. [DOI] [PubMed] [Google Scholar]
- 110.Jones J.I., Gockerman A., Busby W.H., Camacho-Hubner C., Clemmons D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. Journal of Cell Biology. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamon G.A., Hunt T.K., Spencer E.M. In vivo effects of systemic insulin-like growth factor-I alone and complexed with insulin-like growth factor binding protein-3 on corticosteroid suppressed wounds. Growth Regulation. 1993;3:53–56. [PubMed] [Google Scholar]
- 112.Upton Z., Cuttle L., Noble A., Kempf M., Topping G., Malda J. Vitronectin: growth factor complexes hold potential as a wound therapy approach. Journal of Investigative Dermatology. 2008;128(6):1535–1544. doi: 10.1038/sj.jid.5701148. [DOI] [PubMed] [Google Scholar]
- 113.Zhou J., Xiang J., Zhang S., Duan C. Structural and functional analysis of the amphioxus IGFBP gene uncovers ancient origin of IGF-independent functions. Endocrinology. 2013;154(10):3753–3763. doi: 10.1210/en.2013-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carrell R.W., Read R.J. How serpins transport hormones and regulate their release. Seminars in Cell & Developmental Biology. 2017;62:133–141. doi: 10.1016/j.semcdb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Hammond G.L. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. Journal of Endocrinology. 2016;230(1):R13–R25. doi: 10.1530/JOE-16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen C.C., Lau L.F. Functions and mechanisms of action of CCN matricellular proteins. The International Journal of Biochemistry & Cell Biology. 2009;41(4):771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andress D.L. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates phosphorylation of the IGFBP-5 receptor. American Journal of Physiology. 1998;274(4 Pt 1):E744–E750. doi: 10.1152/ajpendo.1998.274.4.E744. [DOI] [PubMed] [Google Scholar]
- 118.Yamanaka Y., Fowlkes J.L., Wilson E.M., Rosenfeld R.G., Oh Y. Characterization of insulin like growth factor binding protein-3 (IGFBP-3) binding to human breast cancer cells: kinetics of IGFBP-3 binding and identification of receptor binding domain on the IGFBP-3 molecule. Endocrinology. 1999;140:1319–1328. doi: 10.1210/endo.140.3.6566. [DOI] [PubMed] [Google Scholar]
- 119.Jones J.I., Gockerman A., Busby W.H., Jr., Wright G., Clemmons D.R. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg-Gly-Asp sequence. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schutt B.S., Langkamp M., Rauschnabel U., Ranke M.B., Elmlinger M.W. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. Journal of Molecular Endocrinology. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 121.Lau L.F., Lam S.C. The CCN family of angiogenic regulators: the integrin connection. Experimental Cell Research. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 122.McCaig C., Perks C.M., Holly J.M. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. Journal of Cell Science. 2002;115:4293–4303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- 123.Leal S., Liu Q., Huang S., Huang J. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. Journal of Biological Chemistry. 1997;272:20572–20576. doi: 10.1074/jbc.272.33.20572. [DOI] [PubMed] [Google Scholar]
- 124.Huang S.S., Ling T.Y., Tseng W.F., Huang Y.H., Tang F.M., Leal S.M. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. Federation of American Societies for Experimental Biology Journal. 2003;17(14):2068–2081. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- 125.Leal S.M., Huang S.S., Huang J.S. Interactions of high affinity insulin-like growth factor-binding proteins with the type V transforming growth factor-b receptor in mink lung epithelial cells. Journal of Biological Chemistry. 1999;274:6711–6717. doi: 10.1074/jbc.274.10.6711. [DOI] [PubMed] [Google Scholar]
- 126.Shen X., Xi G., Maile L.A., Wai C., Rosen C.J., Clemmons D.R. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Molecular and Cellular Biology. 2012;32(20):4116–4130. doi: 10.1128/MCB.01011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baxter R.C. Nuclear actions of insulin-like growth factor binding protein-3. Gene. 2015;569:7–13. doi: 10.1016/j.gene.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Granata R., Trovato L., Garbarino G., Taliano M., Ponti R., Sala G. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. Federation of American Societies for Experimental Biology Journal. 2004;18(12):1456–1458. doi: 10.1096/fj.04-1618fje. [DOI] [PubMed] [Google Scholar]
- 129.McCaig C., Perks C.A., Holly J.M.P. Signaling pathways involved in the direct effects of IGFBP-5 on breast epithelial cell attachment and survival. Journal of Cellular Biochemistry. 2002;84:784–794. doi: 10.1002/jcb.10093. [DOI] [PubMed] [Google Scholar]
- 130.Chua M.W., Lin M.Z., Martin J.L., Baxter R.C. Involvement of the insulin-like growth factor binding proteins in the cancer cell response to DNA damage. Journal of Cell Communication and Signaling. 2015;9(2):167–176. doi: 10.1007/s12079-015-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hollowood A.D., Lai T., Perks C.M., Newcomb P.V., Alderson D., Holly J.M. IGFBP-3 prolongs the p53 response and enhances apoptosis following UV irradiation. International Journal of Cancer. 2000;88(3):336–341. [PubMed] [Google Scholar]
- 132.Zhou Z., Lu H., Zhu S., Gomaa A., Chen Z., Yan J. Activation of EGFR-DNA-PKcs pathway by IGFBP2 protects esophageal adenocarcinoma cells from acidic bile salts-induced DNA damage. Journal of Experimental & Clinical Cancer Research. 2019;38(1):13. doi: 10.1186/s13046-018-1021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perks C.M., Vernon E.G., Rosendahl A.H., Tonge D., Holly J.M. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26(40):5966–5972. doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- 134.Zeng L., Perks C.M., Holly J.M. IGFBP-2/PTEN: a critical interaction for tumours and for general physiology? Growth Hormone & IGF Research. 2015;25(3):103–107. doi: 10.1016/j.ghir.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 135.Shen X., Xi G., Wai C., Clemmons D.R. The coordinate cellular response to insulin-like growth factor-I (IGF-I) and insulin-like growth factor-binding protein-2 (IGFBP-2) is regulated through vimentin binding to receptor tyrosine phosphatase beta (RPTPbeta) Journal of Biological Chemistry. 2015;290(18):11578–11590. doi: 10.1074/jbc.M114.620237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y., MacDonald R.G., Thinakaran G., Kar S. Insulin-Like Growth Factor-II/Cation-Independent Mannose 6-Phosphate Receptor in Neurodegenerative Diseases. Molecular Neurobiology. 2017;54(4):2636–2658. doi: 10.1007/s12035-016-9849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ward C.W., Menting J.G., Lawrence M.C. The insulin receptor changes conformation in unforeseen ways on ligand binding: sharpening the picture of insulin receptor activation. BioEssays. 2013;35(11):945–954. doi: 10.1002/bies.201300065. [DOI] [PubMed] [Google Scholar]
- 138.Pierre De Meyts P. Insulin/receptor binding: the last piece of the puzzle? What recent progress on the structure of the insulin/receptor complex tells us (or not) about negative cooperativity and activation. BioEssays. 2015;37(4):389–397. doi: 10.1002/bies.201400190. [DOI] [PubMed] [Google Scholar]
- 139.Xu Y., Kong G.K.-W., Menting J.G., Margetts M.B., Delaine C.A., Jenkin L.M. How ligand binds to the type 1 insulin-like growth factor receptor. Nature Communications. 2018;9(1):821. doi: 10.1038/s41467-018-03219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li J., Choi E., Yu H., Bai X.C. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nature Communications. 2019;10(1):4567. doi: 10.1038/s41467-019-12564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu Y., Kirk N.S., Venugopal H., Margetts M.B., Croll T.I., Sandow J.J. How IGF-II binds to the human type 1 insulin-like growth factor receptor. Structure. 2020;28(7):786–798. doi: 10.1016/j.str.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weis F., Menting J.G., Margetts M.B., Chan S.J., Xu Y., Tennagels N. The signaling conformation of the insulin receptor ectodomain. Nature Communications. 2018;9(1):4420. doi: 10.1038/s41467-018-06826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gutmann T., Kim K.H., Grzybek M., Walz T., Coskun Ü. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. Journal of Cell Biology. 2018;217(5):1643–1649. doi: 10.1083/jcb.201711047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Griffiths C.D., Bilawchuk L.M., McDonough J.E., Jamieson K.C., Elawar F., Cen Y. IGF1R is an entry receptor for respiratory syncytial virus. Nature. 2020;583:615–619. doi: 10.1038/s41586-020-2369-7. [DOI] [PubMed] [Google Scholar]
- 145.LeRoith D., Werner H., Beitner-Johnson D., Roberts C.T. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocrine Reviews. 1995;16(2):143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 146.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? Journal of the National Cancer Institute. 2012;104(13):975–981. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Denley A., Bonython E.R., Booker G.W., Cosgrove L.J., Forbes B.E., Ward C.W. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Molecular Endocrinology. 2004;18(10):2502–2512. doi: 10.1210/me.2004-0183. [DOI] [PubMed] [Google Scholar]
- 148.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31(22):2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 149.Rechler M.M., Schilling E.E., King G.S., Fraioli F., Rosenberg A.M., Higa O.Z. Receptors for insulin and insulin-like growth factors in disease. Advances in Biochemical Psychopharmacology. 1980;21:489–497. [PubMed] [Google Scholar]
- 150.Bayne M.L., Applebaum J., Underwood D., Chicchi G.G., Green B.G., Hayes N.S. The C region of human insulin-like growth factor (IGF) I is required for high affinity binding to the type 1 IGF receptor. Journal of Biological Chemistry. 1989;264(19):11004–11008. [PubMed] [Google Scholar]
- 151.Slaaby R., Schäffer L., Lautrup-Larsen I., Andersen A.S., Shaw A.C., Mathiasen I.S. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. Journal of Biological Chemistry. 2006;281(36):25869–25874. doi: 10.1074/jbc.M605189200. [DOI] [PubMed] [Google Scholar]
- 152.Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine Reviews. 2009;30(6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 153.Craparo A., O'Neill T.J., Gustafson T.A. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. Journal of Biological Chemistry. 1995;270(26):15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- 154.Dey B.R., Frick K., Lopaczynski W., Nissley S.P., Furlanetto R.W. Evidence for the direct interaction of the insulin-like growth factor I receptor with IRS-1, Shc, and Grb10. Molecular Endocrinology. 1996;10(6):631–641. doi: 10.1210/mend.10.6.8776723. [DOI] [PubMed] [Google Scholar]
- 155.Xu P., Jacobs A.R., Taylor S.I. Interaction of insulin receptor substrate 3 with insulin receptor, insulin receptor-related receptor, insulin-like growth factor-1 receptor, and downstream signaling proteins. Journal of Biological Chemistry. 1999;274(21):15262–15270. doi: 10.1074/jbc.274.21.15262. [DOI] [PubMed] [Google Scholar]
- 156.Chitnis M.M., Yuen J.S., Protheroe A.S., Pollak M., Macaulay V.M. The type 1 insulin-like growth factor receptor pathway. Clinical Cancer Research. 2008;14(20):6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 157.Hermanto U., Zong C.S., Li W., Wang L.H. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Molecular and Cellular Biology. 2002;22(7):2345–2365. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Uddin S., Yetter A., Katzav S., Hofmann C., White M.F., Platanias L.C. Insulin-like growth factor-1 induces rapid tyrosine phosphorylation of the vav proto-oncogene product. Experimental Hematology. 1996;24(5):622–627. [PubMed] [Google Scholar]
- 159.Baron V., Calléja V., Ferrari P., Alengrin F., Van Obberghen E. p125Fak focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tyrosine kinase receptors. Journal of Biological Chemistry. 1998;273(12):7162–7168. doi: 10.1074/jbc.273.12.7162. [DOI] [PubMed] [Google Scholar]
- 160.O'Connor R. Regulation of IGF-I receptor signaling in tumor cells. Hormone and Metabolic Research. 2003;35(11–12):771–777. doi: 10.1055/s-2004-814166. [DOI] [PubMed] [Google Scholar]
- 161.Fernandez C.A., Roy R., Lee S., Yang J., Panigrahy D., Van Vliet K.J. The anti-angiogenic peptide, loop 6, binds insulin-like growth factor-1 receptor. Journal of Biological Chemistry. 2010;285(53):41886–41895. doi: 10.1074/jbc.M110.166439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dey B.R., Furlanetto R.W., Nissley P. Suppressor of cytokine signaling (SOCS)-3 protein interacts with the insulin-like growth factor-I receptor. Biochemical and Biophysical Research Communications. 2000;278(1):38–43. doi: 10.1006/bbrc.2000.3762. [DOI] [PubMed] [Google Scholar]
- 163.Girnita L., Worrall C., Takahashi S., Seregard S., Girnita A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cellular and Molecular Life Sciences. 2014;71(13):2403–2427. doi: 10.1007/s00018-013-1514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Girnita L., Shenoy S.K., Sehat B., Vasilcanu R., Vasilcanu D., Girnita A. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. Journal of Biological Chemistry. 2007;282(15):11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- 165.Siddle K. Signaling by insulin and IGF receptors: supporting acts and new players. Journal of Molecular Endocrinology. 2011;47(1):R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 166.Hakuno F., Takahashi S.-I. IGF1 receptor signaling pathways. Journal of Molecular Endocrinology. 2018;61(1):T69–T86. doi: 10.1530/JME-17-0311. [DOI] [PubMed] [Google Scholar]
- 167.Aleksic T., Chitnis M.M., Perestenko O.V., Gao S., Thomas P.H., Turner G.D. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Research. 2010;70(16):6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aleksic T., Gray N., Wu X., Rieunier G., Osher E., Mills J. Nuclear IGF1R interacts with regulatory regions of chromatin to promote RNA polymerase II recruitment and gene expression associated with advanced tumor stage. Cancer Research. 2018;78(13):3497–3509. doi: 10.1158/0008-5472.CAN-17-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sehat B., Tofigh A., Lin Y., Trocmé E., Liljedahl U., Lagergren J. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Science Signaling. 2010;3(108):ra10. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- 170.Rieger L., O'Connor R. Controlled signaling-insulin-like growth factor receptor endocytosis and presence at intracellular compartments. Frontiers in Endocrinology. 2021;11:620013. doi: 10.3389/fendo.2020.620013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miura M., Surmacz E., Burgaud J.L., Baserga R. Different effects on mitogenesis and transformation of a mutation at tyrosine 1251 of the insulin-like growth factor I receptor. Journal of Biological Chemistry. 1995;270(38):22639–22644. doi: 10.1074/jbc.270.38.22639. [DOI] [PubMed] [Google Scholar]
- 172.Lappano R., Ragusa M., Morrione A., Vella V. A novel functional crosstalk between DDR1 and the IGF axis and its relevance for breast cancer. Cell Adhesion & Migration. 2018;12(4):305–314. doi: 10.1080/19336918.2018.1445953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stanicka J., Rieger L., O'Shea S., Cox O., Coleman M., O'Flanagan C. FES-related tyrosine kinase activates the insulin-like growth factor-1 receptor at sites of cell adhesion. Oncogene. 2018;37(23):3131–3150. doi: 10.1038/s41388-017-0113-z. [DOI] [PubMed] [Google Scholar]
- 174.Aleksic T., Verrill C., Bryant R.J., Han C., Worrall A.R., Brureau L. IGF-1R associates with adverse outcomes after radical radiotherapy for prostate cancer. British Journal of Cancer. 2017;117(11):1600–1606. doi: 10.1038/bjc.2017.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holohan C., Van Schaeybroeck S., Longley D.B., Patrick G., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 176.Ye X.M., Zhu H.Y., Bai W.D., Wang T., Wang L., Chen Y. Epigenetic silencing of miR-375 induces trastuzumab resistance in HER2-positive breast cancer by targeting IGF1R. BMC Cancer. 2014;14:134. doi: 10.1186/1471-2407-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Heskamp S., Boerman O.C., Molkenboer-Kuenen J.D., Wauters C.A., Strobbe L.J., Mandigers C.M. Upregulation of IGF-1R expression during neoadjuvant therapy predicts poor outcome in breast cancer patients. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reinmuth N., Liu W., Fan F., Jung Y.D., Ahmad S.A., Stoeltzing O. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clinical Cancer Research. 2002;8(10):3259–3269. [PubMed] [Google Scholar]
- 179.Pietrzkowski Z., Mulholland G., Gomella L., Jameson B.A., Wernicke D., Baserga R. Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Research. 1993;53(5):1102–1106. [PubMed] [Google Scholar]
- 180.D'Ambrosio C., Ferber A., Resnicoff M., Baserga R. A soluble insulin-like growth factor I receptor that induces apoptosis of tumor cells in vivo and inhibits tumorigenesis. Cancer Research. 1996;56(17):4013–4020. [PubMed] [Google Scholar]
- 181.Langer C.J., Novello S., Park K., Krzakowski M., Karp D.D., Mok T. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2014;32(19):2059–2066. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buck E., Gokhale P.C., Koujak S., Brown E., Eyzaguirre A., Tao N. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Molecular Cancer Therapeutics. 2010;9(10):2652–2664. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 183.Ekyalongo R.C., Yee D. Revisiting the IGF-1R as a breast cancer target. NPJ Precision Oncology. 2017;1:14. doi: 10.1038/s41698-017-0017-y. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ishiwata T., Bergmann U., Kornmann M., Lopez M., Beger H.G., Korc M. Altered expression of insulin-like growth factor II receptor in human pancreatic cancer. Pancreas. 1997;15(4):367. doi: 10.1097/00006676-199711000-00006. 73. [DOI] [PubMed] [Google Scholar]
- 185.De Souza A.T., Hankins G.R., Washington M.K., Fine R.L., Orton T.C., Jirtle R.L. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10(9):1725–1729. [PubMed] [Google Scholar]
- 186.De Souza A.T., Hankins G.R., Washington M.K., Orton T.C., Jirtle R.L. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nature Genetics. 1995;11(4):447–449. doi: 10.1038/ng1295-447. [DOI] [PubMed] [Google Scholar]