Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) is designated as a potentially lethal adverse drug effect with characteristic signs and symptoms such as skin rash, fever, leucocytosis with eosinophilia or atypical lymphocytes, lymphadenopathy and liver or renal dysfunction. In addition to most commonly implicated drug category (aromatic anticonvulsants), lamotrigine, sulfonamides, dapsone and abacavir may also induce this syndrome. We describe here a case a sulfasalazine-induced DRESS with coexisting chikungunya fever. The shared presentation of fever with rash in both conditions made it a challenging diagnosis. Sulfasalazine hypersensitivity manifesting as DRESS has rarely been reported. Furthermore, we document chikungunya virus (CV) as a possible triggering agent for DRESS. To the best of our knowledge, CV as a viral aetiology in DRESS has not been reported previously in the literature.
Keywords: dermatology, drugs and medicines, tropical medicine (infectious disease), unwanted effects / adverse reactions
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) is an uncommon, potentially life-threatening multiorgan adverse drug reaction. This severe adverse cutaneous reaction is a type IV hypersensitivity syndrome (HSS) that presents with severe cutaneous eruption, fever, lymphadenopathy, hepatitis, haematologic abnormalities (eosinophilia, atypical lymphocytes) and may involve other organs. In the past, many acronyms such as ‘anticonvulsant HSS’, ‘drug-induced delayed multiorgan HSS’ and ‘drug-induced HSS’ (DIHS) had been suggested by authors due to its varied clinical presentations.1 2 The incidence of DRESS is estimated to be 1 per 1000 to 10 000 drug exposures and is associated with a substantially high mortality rate of 10%.2 3 The latency between drug exposure to disease manifestation is often long (2–6 weeks). The suggested pathogenesis relates to an abnormal immune response in a genetically vulnerable individual—presence of human leucocyte antigen (HLA)*5801 (allopurinol), HLA-B* 5701 (abacavir) genotype. The formation of various reactive drug metabolites and its accumulation in the body have been attributed to slow acetylation metabolic pathways and reactivation of various human herpes viruses (HHV).2 4 5 In this report, we describe a case of sulfasalazine-induced DRESS with concomitant chikungunya virus (CV) infection. We further explore the role of CV as a possible instigating factor in DRESS.
Case presentation
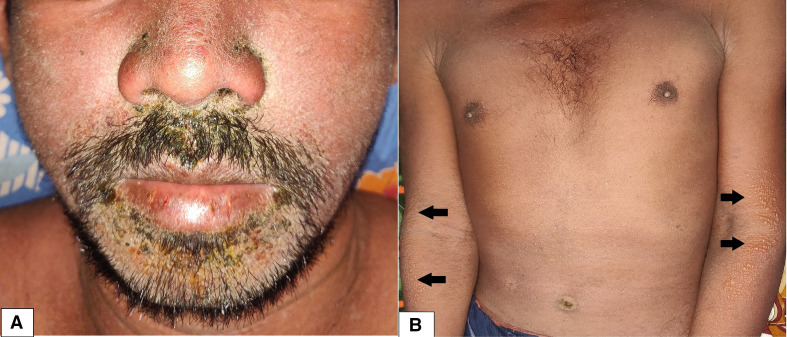
A 30-year-old man, without any known comorbidities, presented with a history of fever, pruritic skin eruption and arthralgia of small joints of both hands and feet (symmetrical involvement) for last 14 days. On initial diagnostic workup by his primary care physician, he was diagnosed with chikungunya fever (chikungunya IgM-positive at titre 1:80 with reference range of 1:10). However, in spite of conservative symptomatic management with oral antipyretic, antihistaminic, topical emollient and mid-potent corticosteroid lotion, there was persistence of low-grade fever and skin rash. On examination, the patient was febrile (100.8 F). Bilateral cervical (mainly posterior triangle) and axillary lymphadenopathy were noted along with hepatosplenomegaly. Cutaneous examination revealed facial puffiness with generalised morbilliform eruption. Oedema with vesiculation, follicular and non-follicular pustules predominated the flexor aspect of the extremities along with involvement of trunk, palms, soles, and mucosal surfaces were spared (Figure 1). Nikolsky sign could not be elicited. Examination of other organ systems was unremarkable. Suspecting an adverse drug reaction, further enquiry into past drug history revealed that he had taken sulfasalazine, advised by a local physician for non-specific joint pain, for a duration of 2 months (stopped about 12 days prior to present consultation).
Figure 1.
Facial oedema with focal purulence and crusting (A) and generalised morbilliform eruption with vesiculation over upper extremities and multiple small follicular and non-follicular pustules over trunk (B).
Investigations
Laboratory investigations revealed marked leucocytosis (white cell count—26.1×109/L; neutrophils 54%, eosinophils 21%, lymphocytes 17%, monocytes 8%) with eosinophilia (1.8×109/L), significant proportion of atypical lymphocytes (14% on peripheral blood smear), mildly elevated erythrocyte sedimentation rate of 25 mm/hour (normal range 0–20 mm/hour), elevated hepatic transaminases (alanine transaminase 190 IU/L; aspartate aminotransferase 212 IU/L), elevated amylase (200 U/L; reference range 30–110 U/L) and lipase (210 U/L; reference range 0–160 U/L). Urea, electrolytes, thyroid profile, blood and urine culture, serology for antinuclear antibodies, rheumatoid factor, viral hepatitis markers, HIV, HHV-6, chlamydia, cytomegalovirus, mycoplasma and Ebstein-Barr virus were negative. Reverse transcription-PCR (RT-PCR) for SARS-CoV-2 from nasopharyngeal and oropharyngeal swab was negative. A 4 mm punch biopsy taken from his right arm revealed epidermal spongiosis with superficial perivascular lymphohistiocytic infiltration.
Differential diagnosis
The differential diagnoses of DRESS include acute viral infections, hypereosinophilic syndrome, Kawasaki disease, Still’s disease, pseudolymphoma, serum sickness-like reaction and other cutaneous drug eruptions. Amidst the present ongoing pandemic, similar presentation can arise due to infection with COVID-19, medication for the disease and postvaccination.6–10 Attributes like delayed onset, longer resolution time, facial oedema, eosinophilia and involvement of multiple internal organs helped differentiate DRESS from its simulating entities. The patient was found to score 8 (‘definite case’) on the RegiSCAR11 and ‘7 (typical DIHS)’ on J-SCAR (Japanese Research Committee on SCAR)12 diagnostic criteria of DRESS, which established the diagnosis of sulfasalazine-induced DRESS (table 1). The Naranjo probability scale13 indicated a ‘probable’ causality between sulfasalazine and DRESS syndrome in this patient.
Table 1.
RegiSCAR scoring system in DRESS for the patient
| Criteria | No | Yes | Unknown/unclassified | Current case |
| Fever ≥38.5°C | −1 | 0 | −1 | 1 |
| Lymphadenopathy (≥2 sites; >1 cm) | 0 | 1 | 0 | 1 |
| Circulating atypical lymphocytes | 0 | 1 | 0 | 1 |
| Peripheral eosinophilia | 0 | 0 | 2 | |
| 0.7–1.499 × 109 /L or 10%–19.9% | 1 | |||
| ≥1.5 × 109 /L or ≥20% | 2 | |||
| Skin involvement: | 1 | |||
| Extent of cutaneous eruption >50% BSA | 0 | 1 | 0 | |
| Cutaneous eruption suggestive of DRESS | −1 | 1 | 0 | |
| Biopsy suggests DRESS | −1 | 0 | 0 | |
| Internal organ involved: | 0 | 0 | 1 | |
| One | 1 | |||
| Two | 2 | |||
| Resolution in ≥15 days | −1 | 0 | 1 | 0 |
| Laboratory results negative for at least three of the following (and none positive): | 0 | 1 | 0 | 1* |
| ANA | 1 | |||
| Blood cultures | 1 | |||
| HAV/HBV/HCV serology | 1 | |||
| Chlamydia and mycoplasma serology | 1 | |||
| Final score† | 8 | |||
*All laboratory tests mentioned in the list were negative, which led to score=1 in this category.
†Interpretation of score:<2 = no case, 2–3=possible case, 4–5=probable case,>5 = definite case.
ANA, antinuclear antibody; BSA, Body surface area; DRESS, drug reaction with eosinophilia and systemic symptoms; HAV, Hepatitis A virus; HBV, Hepatitis B virus; HCV, Hepatitis C virus; RegiSCAR, registry of severe cutaneous adverse reaction.
Treatment
All non-essential drugs were promptly discontinued and he was started on oral prednisolone (1 mg/day) along with adequate supportive care. On fourth day of therapy, fever subsided while the rash resolved with desquamation after further 10 days. Normalisation of biochemical parameters was observed after a fortnight. Repeat blood test for chikungunya serology revealed positive chikungunya virus IgG. Systemic steroid was tapered slowly over 8 weeks without any relapse.
Outcome and follow-up
He continues to be on regular follow-up, although symptom free.
Discussion
Sulfasalazine is an orally administered compound comprising of two main constituents—sulphapyridine and 5-aminosalicylic acid.14 The drug has proved to be extremely useful in many inflammatory diseases due to its potential nuclear factor-kappa B and tumour necrosis factor-alpha inhibiting activity.15 The varied presentations of sulfasalazine allergy include photosensitivity and fixed drug eruption, but sulfasalazine hypersensitivity manifesting as DRESS has rarely been documented.14 16 Other implicated drugs in severe DRESS include allopurinol, aspirin, carbamazepine, hydroxychloroquine, lamotrigine, minocycline, nevirapine, olanzapine, oxcarbazepine, phenylbutazone, spironolactone, streptomycin and vancomycin among others.17
The accountability for the adverse cutaneous reaction in this patient in all probability lies with the sulphonamide (sulphapyridine) component of sulfasalazine, as suggested by Teo and Tan.14 The pathomechanism in DRESS appears to be a complex interplay factors involving the culprit drug, genetic predisposition, comorbidities affecting drug metabolism, and transient hypogammaglobinaemia have some part to play. Current research have also identified reactivation of the HHV-6, 7, Ebstein-Barr virus and cytomegalovirus as aetiological factors for DRESS.4 5 14 CV might have trigged the condition by interfering with drug-detoxifying enzymes or through a yet unknown immune mechanism. Further studies are needed in this regard to establish or refute CV as an instigating agent in DRESS.
Chikungunya fever is an acute febrile illness presenting with fever, joint pain, other constitutional symptoms and a myriad of mucocutaneous changes and may mimic the clinical presentation of DRESS.18 19
The European registry of severe cutaneous adverse reaction (RegiSCAR) scoring system had been established to delineate the diagnosis of DRESS.11 Although guidelines for the treatment of DRESS are still lacking, systemic corticosteroids typically represent first-line therapy. Intravenous immunoglobulins may also provide therapeutic benefit.20
Proper drug history should be elicited in patients presenting with fever and rash. Increased awareness among physicians regarding this uncommon diagnosis will enable improved therapeutic outcomes in these patients. We also suggest the role of CV as triggering agent in DRESS. This association, however, needs further in-depth scientific exploration.
Learning points.
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a rare type of drug reaction commonly mistaken for a viral infection. It must be recognised promptly due to its high morbidity and 10% mortality rate.
Facial oedema, eosinophilia and multiorgan involvement differentiate this entity from other common drug reactions.
Chikungunya virus may have a possible triggering effect in DRESS.
Footnotes
Contributors: AS and AC prepared the manuscript with adequate planning and execution; they also collected data regarding the patient. AC and MSB were the direct care givers to the patient, who managed the case actively and collected relevant data on investigations with equal contributorship. AC helped in detailed supervision, final output and the review of literature regarding the manuscript. JDP supervised the entire management of the patient and has actively contributed in editing the manuscript. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a dress syndrome really exist? Br J Dermatol 2007;156:609–11. 10.1111/j.1365-2133.2006.07704.x [DOI] [PubMed] [Google Scholar]
- 2.Krishan P, Varma S, Kalra H. Sulfasalazine induced dress syndrome: a review of case reports. BJMMR 2016;11:1–11. [Google Scholar]
- 3.Descamps V, Ranger-Rogez S. Dress syndrome. Joint Bone Spine 2014;81:15–21. 10.1016/j.jbspin.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Descamps V, Mahe E, Houhou N, et al. Drug-Induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol 2003;148:1032–4. 10.1046/j.1365-2133.2003.05330.x [DOI] [PubMed] [Google Scholar]
- 5.Criado PR, Avancini J, Santi CG, et al. Drug reaction with eosinophilia and systemic symptoms (dress): a complex interaction of drugs, viruses and the immune system. Isr Med Assoc J 2012;14:577–82. [PubMed] [Google Scholar]
- 6.Bhanja DB, Sil A, Panigrahi A, et al. Ibuprofen-Induced generalised bullous fixed drug eruption. Postgrad Med J 2020;96:706–7. 10.1136/postgradmedj-2020-137486 [DOI] [PubMed] [Google Scholar]
- 7.Banik B, Bhar D, Sil A. Terbinafine-induced Steven-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) overlap. Postgrad Med J 2020. 10.1136/postgradmedj-2020-138326. [Epub ahead of print: 14 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Sil A, Das A. Hydroxychloroquine for COVID-19: myths vs facts. Dermatol Ther 2020;33:e13857. 10.1111/dth.13857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das A, Kumar S, Sil A, et al. Skin changes attributed to protective measures against COVID-19: a compilation. Dermatol Ther 2020;33:e13796. 10.1111/dth.13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda M, Dash S, Behera B, et al. Dermatological manifestations associated with COVID-19 infection. Indian J Dermatol 2021;66:237–45. 10.4103/ijd.ijd_464_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (dress): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071–80. 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 12.Shiohara T, Inaoka M, Kano Y. Drug-Induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int 2006;55:1–8. 10.2332/allergolint.55.1 [DOI] [PubMed] [Google Scholar]
- 13.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 14.Teo L, Tan E. Sulphasalazine-induced dress. Singapore Med J 2006;47:237–9. [PubMed] [Google Scholar]
- 15.Tabit CE, Holbrook M, Shenouda SM, et al. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med 2012;17:101–7. 10.1177/1358863X12440117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahat G, Celik HG, Tufan F, et al. Drug rash with eosinophilia and systemic symptoms syndrome induced by sulfasalazine. Joint Bone Spine 2010;77:87–8. 10.1016/j.jbspin.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Adwan MH. Drug reaction with eosinophilia and systemic symptoms (dress) syndrome and the rheumatologist. Curr Rheumatol Rep 2017;19:3. 10.1007/s11926-017-0626-z [DOI] [PubMed] [Google Scholar]
- 18.Sil A, Biswas SK, Bhanja DB, et al. Post-chikungunya hyperpigmentation. Postgrad Med J 2021;97:60. 10.1136/postgradmedj-2020-137504 [DOI] [PubMed] [Google Scholar]
- 19.Panigrahi A, Chakraborty S, Sil A. Chik sign in Chikungunya fever. Infection 2021;49:1075–6. 10.1007/s15010-020-01472-x [DOI] [PubMed] [Google Scholar]
- 20.Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (dress syndrome): an update. Dermatology 2003;206:353–6. 10.1159/000069956 [DOI] [PubMed] [Google Scholar]