Abstract
Aim
Growth hormone secretagogue receptor 1a (GHS-R1a) is widely distributed in brain including the hippocampus. Studies have demonstrated the critical role of hippocampal ghrelin/GHS-R1a signaling in synaptic physiology, memory and cognitive dysfunction associated with Alzheimer’s disease (AD). However, current reports are inconsistent, and the mechanism underlying memory modulation of GHS-R1a signaling is uncertain. In this study, we aim to investigate the direct impact of selective increase of GHS-R1a expression in dCA1 excitatory/inhibitory neurons on learning and memory.
Methods
Endogenous GHS-R1a distribution in dCA1 excitatory/inhibitory neurons was assessed by fluorescence in situ hybridization. Cre-dependent GHS-R1a overexpression in excitatory or inhibitory neurons was done by stereotaxic injection of aav-hSyn-DIO-hGhsr1a-2A-eGFP virus in dCA1 region of vGlut1-Cre or Dlx5/6-Cre mice respectively. Virus-mediated GHS-R1a upregulation in dCA1 neurons was confirmed by quantitative RT-PCR. Different behavioral paradigms were used to evaluate long-term memory performance.
Results
GHS-R1a is distributed both in dCA1 excitatory pyramidal neurons (αCaMKII+) and in inhibitory interneurons (GAD67+). Selective increase of GHS-R1a expression in dCA1 pyramidal neurons impaired spatial memory and object-place recognition memory. In contrast, selective increase of GHS-R1a expression in dCA1 interneurons enhanced long-term memory performance. Our findings reveal, for the first time, a neuronal type-specific role that hippocampal GHS-R1a signaling plays in regulating memory. Therefore, manipulating GHS-R1a expression/activity in different subpopulation of neurons may help to clarify current contradictory findings and to elucidate mechanism of memory control by ghrelin/GHS-R1a signaling, under both physiological and pathological conditions such as AD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13041-021-00866-8.
Keywords: GHS-R1a, Ghrelin, Memory, Hippocampus, Interneuron, Alzheimer’s disease
Ghrelin is the only identified orexigenic gastric hormone that promotes feeding, and is critical for metabolism regulation in both human and rodents [1]. It has been reported that only acylated ghrelin (AG) in circulation is capable of binding to ghrelin receptor, the growth hormone secretagogue receptor 1a (GHS-R1a), which is widely distributed in multiple brain regions including the hippocampus [2]. In contrast, unacylated ghrelin (UAG), the most abundant form of circulating ghrelin, is unable to activate GHS-R1a [3, 4].
Studies have highlighted intriguing contradictory roles that ghrelin and GHS-R1a play in regulating multiple neuronal functions such as learning and memory, other than nutrient sensing and metabolic control [5]. For instance, pharmacological studies have reported that ghrelin activating GHS-R1a either facilitates or impairs memory processes [6, 7]. Genetic GHS-R1a null mutation also gave rise to opposite effects on hippocampus-dependent memory encoding [8, 9]. To date, the reason for those conflicting findings remains unclear, and the mechanism underlying memory modulation by GHS-R1a signaling is not well explored.
It is important to note that GHS-R1a displays two uncommon features that may greatly contribute to its functional complexity, extremely high constitutive activity [10] and multiple downstream signaling pathways involved under different experimental conditions [11]. In particular, recent studies have illustrated physiological importance of constitutive activity of GHS-R1a in regulating food intake, growth hormone release, and memory processes [12, 13]. Therefore, altered GHS-R1a expression might lead to distinct biological outcomes from that of ghrelin-dependent activation, under both physiological and pathological conditions like AD. Therefore, in this study, we sought to investigate the direct effect of increasing GHS-R1a expression in specific populations of dCA1 neurons on hippocampus-dependent learning and memory.
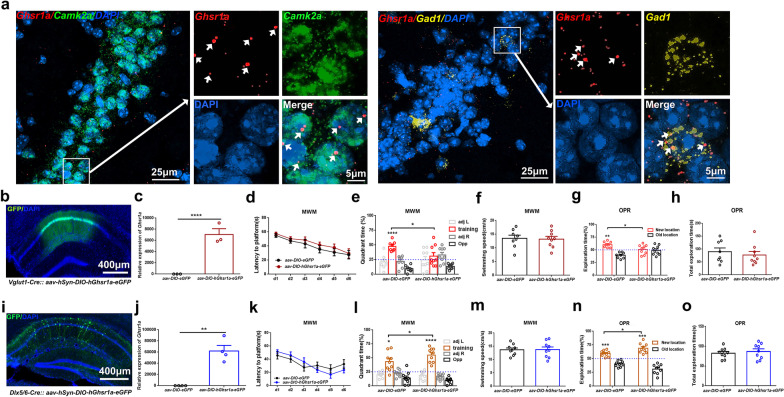
Endogenous GHS-R1a distribution in both excitatory and inhibitory dCA1 neurons was confirmed by fluorescent in situ hybridization assays (Fig. 1a). Cre-dependent GHS-R1a-expressing virus (aav-hSyn-DIO-hGhsr1a-2A-eGFP) or control virus (aav-hSyn-DIO-eGFP) was delivered in dCA1 of Vglut1-Cre or Dlx5/6-Cre male mice (3–4 month old) respectively to selectively increase GHS-R1a expression in excitatory or inhibitory neurons in dorsal hippocampus. GFP fluorescence in dCA1 region indicated successful viral transfection and virus-mediated GHS-R1a expression in pyramidal neurons or interneurons 3 weeks after injection (Fig. 1b, i). Virus-mediated GHS-R1a expression in dorsal hippocampus was quantified by RT-qPCR analyses (Fig. 1c, j). The detailed methods were described in Additional file 1.
Fig. 1.
Selective GHS-R1a upregulation in dCA1 pyramidal neurons or interneurons has opposite effect on hippocampus-dependent memory encoding. a Representative fluorescent in situ hybridization images showing endogenous Ghsr1a expression in both excitatory and inhibitory dCA1 neurons of C57BL/6J mice. Ghsr1a (red), Camk2a (green), Gad1 (yellow), DAPI (blue). Arrowheads (white) indicate Ghsr1a signals within Camk2a- or Gad1-expressing neurons. b, i Representative fluorescent images of dorsal hippocampus taken 4 weeks after virus injection. Vglut1-Cre mice (b), Dlx5/6-Cre mice (i). GFP (green), DAPI (blue). c, j RT-qPCR analyses showing increased hGhsr1a expression in dorsal hippocampus 4 weeks after delivery of hGhsr1a-expressing virus. Vglut1-Cre mice (c), n = 3 per group; Dlx5/6-Cre mice (j), n = 4 per group. d–h, k–o Learning and memory performance. Vglut1-Cre mice (d–h), Dlx5/6-Cre mice (k–o). d–f, k–m Morris water maze assays. d, k GHS-R1a upregulation does not affect spatial learning. e, l Spatial memory tested 24 h after the 6th day training. Elevated GHS-R1a in excitatory neurons impairs spatial memory (e), while increased GHS-R1a expression in inhibitory neurons enhances spatial memory (l). f, m Averaged swimming speed during probe test. g, h, n–o Object-place recognition (OPR) assays. g Cre-dependent GHS-R1a upregulation in excitatory neurons impairs OPR memory. n Cre-dependent GHS-R1a upregulation in inhibitory neurons improves OPR memory. h, o Total object exploration time during OPR test. Vglut1-Cre mice with GHS-R1a-expressing virus (n = 9), Vglut1-Cre mice with control virus (n = 8), Dlx5/6-Cre mice, n = 9 per group. All data is shown as means ± SEM. Two-way repeated-measure ANOVA with Sidak’s multiple comparisons test for (d, e, g, k, l, n), unpaired t test for (c, f, h, j, m, o), ****P < 0.0001, ***P < 0.001, ** P < 0.01 or *P < 0.05 means significant difference, n.s. means no significance
The effect of increasing GHS-R1a expression on hippocampus-dependent learning and memory performance was evaluated 4 weeks after viral injection. We found that selective GHS-R1a upregulation in dCA1 excitatory pyramidal neurons impairs hippocampus-dependent memory processes. Specifically, Vglut1-Cre mice transfected with aav-hSyn-DIO-hGhsr1a-2A-eGFP virus exhibited poor spatial memory (Fig. 1d, f), impaired object-place recognition (OPR) memory (Fig. 1g, h), in comparison to Vglut1-Cre mice receiving control aav-hSyn-DIO-eGFP virus injection. In contrast, we found that Dlx5/6-Cre mice receiving aav-hSyn-DIO-hGhsr1a-2A-eGFP virus displayed better spatial memory (Fig. 1k, m) and OPR memory (Fig. 1n, o) than control Dlx5/6-Cre mice, indicating that selective GHS-R1a upregulation in dCA1 inhibitory interneurons improves hippocampus-dependent memory. Our findings thus reveal, for the first time, that elevated GHS-R1a expression selectively in dCA1 excitatory/inhibitory neurons differentially regulates memory encoding. It will be interesting to know what kind of GHS-R1a activity, ligand-dependent or ligand-independent or both, mediates the differential effect of elevated GHS-R1a on memory. Additional studies are also needed to explore synaptic mechanisms and signaling cascades mediating these cell-type specific effects of GHS-R1a activation on memory.
The hippocampus is a complex network tightly regulated by interactions between excitatory pyramidal neurons and inhibitory interneurons. Although represent a minority in the hippocampus, interneurons play a critical role in shaping network activities [14]. However, no previous study has reported the physiological importance of ghrelin/GHS-R1a signaling in hippocampal interneurons. In this study, we uncovered its memory improvement effect by directly increasing GHS-R1a expression in dCA1 inhibitory neurons, as opposed to the memory impairment effect of GHS-R1a upregulation in excitatory neurons. Our current findings, together with on-going study based on conditional GHS-R1a knockout mice, will help to reveal causal association between hippocampal GHS-R1a expression and memory. In addition, accumulating evidence suggests a correlation between altered GHS-R1a expression and AD pathogenesis [15]. Therefore, it is necessary to test the direct impact of manipulating hippocampal GHS-R1a expression on AD memory impairment.
In conclusion, our findings reveal, for the first time, that elevated GHS-R1a expression selectively in dCA1 excitatory/inhibitory neurons differentially regulates memory encoding. It also suggests a causal relationship between hippocampal GHS-R1a expression and memory.
Supplementary Information
Additional file 1. Selectively increasing GHS-R1a expression in dCA1 excitatory/inhibitory neurons have opposite effects on memory encoding.
Acknowledgements
We thanks Ms. Jennifer Li for native language editing.
Abbreviations
- GHS-R1a
Growth hormone secretagogue receptor 1a
- hGhsr1a
Human Ghsr1a
- AG
Acylated ghrelin
- UAG
Unacylated ghrelin
- Aav
Adeno-associated virus
- GFP
Green fluorescent protein
- PCR
Polymerase chain reaction
- OPR
Object-place recognition
- αCaMKII
Ca2+/calmodulin-dependent protein kinase IIα
- AD
Alzheimer’s disease
- FISH
Fluorescence in situ hybridization
- hSyn
Human synapsin I
- Dlx5/6
Distal-less homeobox 5/6
- Vglut1
Vesicular glutamate transporter 1
- Gad1
Glutamate decarboxylase 1 (GAD1)
Authors’ contributions
YZ designed and supervised the experiments. NL, NL and FX performed behavioral experiments, NL and MY performed FISH and biochemical analyses. ZQ helped with data analyses. YZ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by NNSFC (Grant no. 32071141 and 91732110 to YZ), and NSFC of SD province (Grant no. ZR2019ZD34 and 2019GGX101045 to YZ).
Availability of data and materials
The detailed methods were described in Additional file 1.
Declarations
Ethics approval and consent to participate
The Chancellor’s Animal Research Committee at Qingdao University approved all the experiments according to National Institutes of Health guideline.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Perello M, Cabral A, Cornejo MP, De Francesco PN, Fernandez G, Uriarte M. Brain accessibility delineates the central effects of circulating ghrelin. J Neuroendocrinol. 2019;31:e12677. doi: 10.1111/jne.12677. [DOI] [PubMed] [Google Scholar]
- 3.Hornsby AKE, Buntwal L, Carisi MC, Santos VV, Johnston F, Roberts LD, et al. Unacylated-Ghrelin impairs hippocampal neurogenesis and memory in mice and is altered in Parkinson’s dementia in humans. Cell Rep Med. 2020;1:100120. doi: 10.1016/j.xcrm.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiimura Y, Horita S, Hamamoto A, Asada H, Hirata K, Tanaka M, et al. Structure of an antagonist-bound ghrelin receptor reveals possible ghrelin recognition mode. Nat Commun. 2020;11:4160. doi: 10.1038/s41467-020-17554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 2011;34:31–40. doi: 10.1016/j.tins.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Diano S, Farr SA, Benoit SC, McNay EC, Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 7.Song L, Zhu Q, Liu T, Yu M, Xiao K, Kong Q, et al. Ghrelin modulates lateral amygdala neuronal firing and blocks acquisition for conditioned taste aversion. PLoS ONE. 2013;8:e65422. doi: 10.1371/journal.pone.0065422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav. 2011;103:39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albarran-Zeckler RG, Brantley AF, Smith RG. Growth hormone secretagogue receptor (GHS-R1a) knockout mice exhibit improved spatial memory and deficits in contextual memory. Behav Brain Res. 2012;232:13–19. doi: 10.1016/j.bbr.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 11.Evron T, Peterson SM, Urs NM, Bai Y, Rochelle LK, Caron MG, et al. G Protein and beta-arrestin signaling bias at the ghrelin receptor. J Biol Chem. 2014;289:33442–33455. doi: 10.1074/jbc.M114.581397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Song G, Wang Y, Zhu Q, Han F, Zhang C, et al. Blocking constitutive activity of GHSR1a in the lateral amygdala facilitates acquisition of conditioned taste aversion. Neuropeptides. 2018;68:22–27. doi: 10.1016/j.npep.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Torz LJ, Osborne-Lawrence S, Rodriguez J, He Z, Cornejo MP, Mustafa ER, et al. Metabolic insights from a GHSR-A203E mutant mouse model. Mol Metab. 2020;39:101004. doi: 10.1016/j.molmet.2020.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat Commun. 2020;11:4395. doi: 10.1038/s41467-020-18074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian J, Guo L, Sui S, Driskill C, Phensy A, Wang Q, et al. Disrupted hippocampal growth hormone secretagogue receptor 1alpha interaction with dopamine receptor D1 plays a role in Alzheimer’s disease. Sci Transl Med. 2019;11:e6278. doi: 10.1126/scitranslmed.aav6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Selectively increasing GHS-R1a expression in dCA1 excitatory/inhibitory neurons have opposite effects on memory encoding.
Data Availability Statement
The detailed methods were described in Additional file 1.