Abstract
Monoclonal antibodies that effectively neutralize HIV-1 have been widely sought, yet few have been isolated. Now, technological advances in sera evaluation, B cell stimulation, microneutralization and single cell antibody cloning have allowed Burton and colleagues to identify two broadly neutralizing monoclonal antibodies, PG9 and PG16, which provide new insights for HIV vaccine design.
In the struggle between human immunodeficiency virus type 1 (HIV-1) and the human immune system, the virus often seems to gain the upper hand. Antibodies to the viral envelope glycoproteins, gp120 and gp41, are diverted by conformational flexibility, and key epitopes are masked by variable loops and N-linked glycosylation [reviewed in (Pantophlet and Burton, 2006; Wyatt and Sodroski, 1998)]. Indeed, in the persistent infection that typifies HIV-1, the virus stays ahead of the immune system, with neutralizing antibodies against the predominant viral strain only elicited after several months, by which time the viral swarm has evolved sufficiently to escape (Richman et al., 2003; Wei et al., 2003).
Yet viral evasion is not perfect. After several years of infection, sera from a significant percentage of infected individuals (current estimates range from 15–25%) develop antibodies capable of neutralizing a diverse range of HIV-1 isolates [reviewed in (Stamatatos et al., 2009)]. A handful of monoclonal antibodies from infected individuals had been identified in the early 1990s (2F5, 2G12, 4E10 and b12), each of which could prevent infection in non-human primate animal models (Mascola, 2003). Because each of these monoclonal antibodies identifies a highly conserved target of HIV-1 neutralization, additional broadly neutralizing antibodies have been intensively sought. Despite repeated attempts, however, such antibodies have not been forthcoming suggesting that appropriate antibody-producing B cells might be rare.
To address this “needle in the haystack” problem, Burton and colleagues at the International AIDS Vaccine Initiative (IAVI), together with biotechnology collaborators, Monogram and Theraclone, developed three technological advances that allowed the definition of two new monoclonal antibodies, reported recently (Walker et al., 2009) (Fig. 1). First, it was necessary to identify optimal patient samples, a task that required collecting sera and lymphocyte samples on hundreds of HIV-1 infected individuals to identify a select group who have broadly neutralizing serum antibodies. Second, most isolated B cells do not actively secrete IgG. So the group developed methods to stimulate B cells to proliferate and to secrete IgG. Third, the group utilized a high-throughput and small scale or microneutralization assay that allowed analysis of neutralization activity against at least three different HIV-1 isolates. This analysis allowed identification of the rare B cells that synthesized broadly neutralizing antibodies (the long-sought needles!). After PCR amplification of IgG genes, and cloning and expression of antibodies from these B cell clones, the identity and activity of these selected IgGs could be confirmed and their properties characterized (Walker et al., 2009).
Figure 1. Identification of new broadly neutralizing antibodies is dependent on direct screening for neutralization.
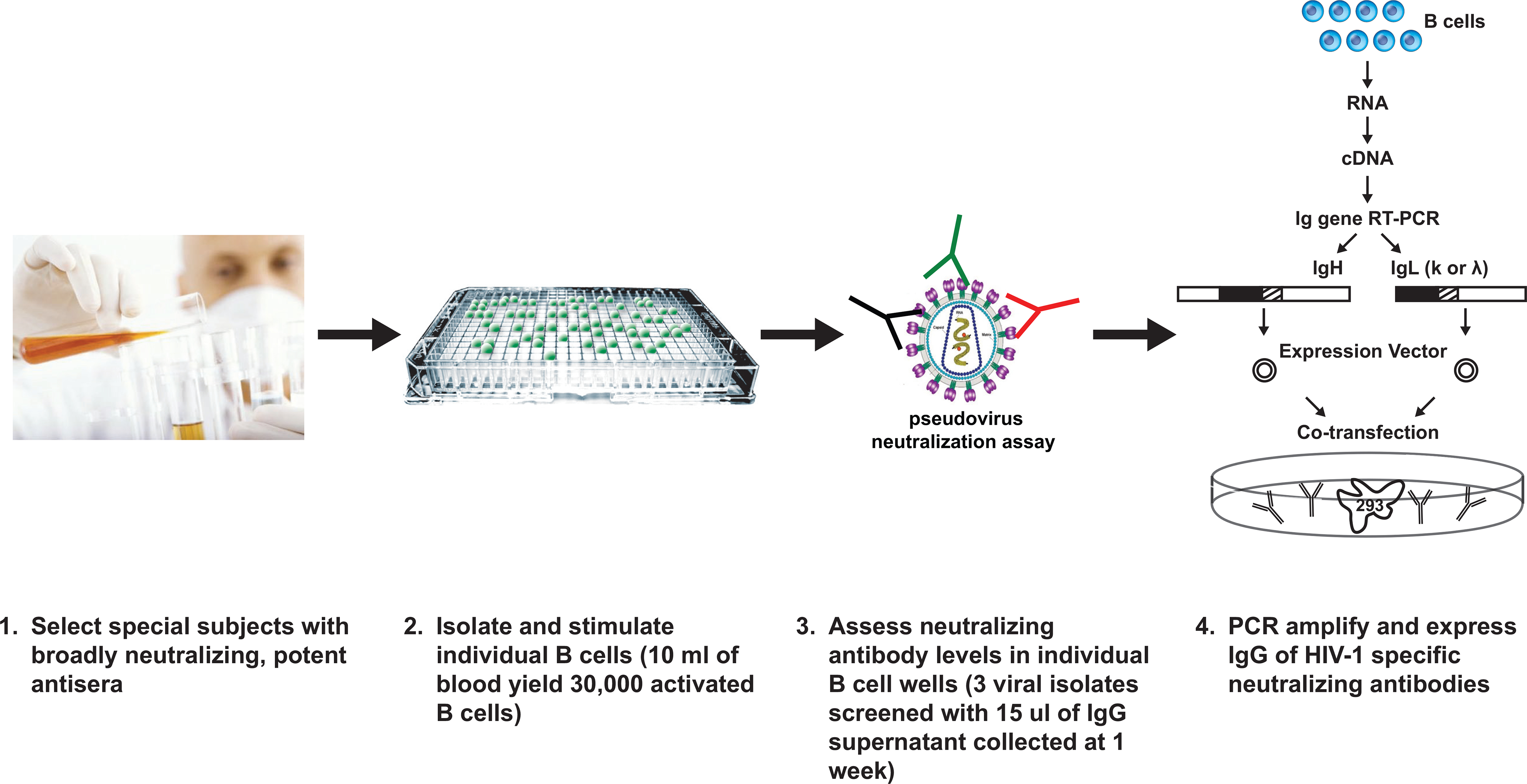
Steps involved in the isolation of new broadly neutralizing antibodies are outlined and involve identification of B cell clones with neutralizing antibody activity and direct cloning of immunoglobulins.
The most potent antibodies identified by the analysis, PG9 and PG16, were derived from the same germ line genes and appeared to be somatically related. Both PG9 and PG16 displayed only weak binding to soluble gp140s, but tight binding to cell-surface expressed oligomeric gp120/gp41, which presumably form trimers and give rise to functional virus spikes. The only previously described HIV-1-reactive antibody with a similar preference for the conformationally intact viral spike is antibody 2909 (Gorny et al., 2005). Like antibody 2909, antibodies PG9/PG16 recognize an epitope comprised of variable loops V2 and V3. Indeed, many of the key gp120 residues important for PG9/PG16 recognition are important for 2909 as well. These similarities suggest that antibodies PG9/PG16 and antibody 2909 recognize similar epitopes on the V2/V3 of gp120 (Fig. 2). However, PG9/PG16 recognize a common form of the epitope, which has an N-linked glycosylation at residue 160 in the V2 region of gp120, whereas antibody 2909 requires a rare form of the epitope, with a Lys at residue 160. Interestingly, antibodies PG9/PG16 and antibody 2909 all have a heavy chain derived from IgHV3, so similarities between these antibodies include not only the recognized epitope, but the antibody sequences themselves.
Figure 2. Neutralization of HIV-1 at a V2-V3 epitope.
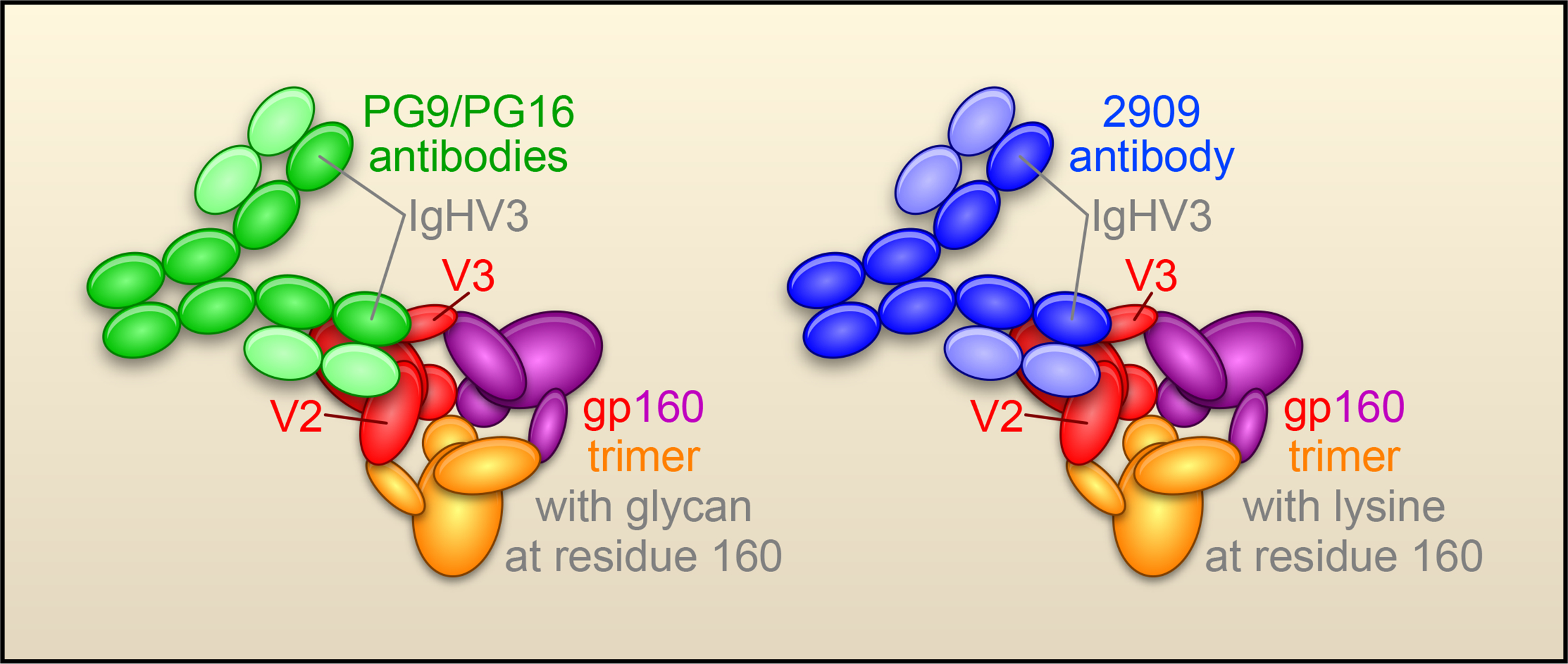
Recognition of trimeric HIV-1 Env by PG antibodies (green) requires both a V2-V3 epitope and an associated glycan, whereas the previously defined strain-specific 2909 antibody (blue) requires a V2-V3 epitope and a lysine. (The HIV-1 Env is shown with subunits in red, purple and yellow; gp41 is represented by the small ball at the center of the spike and gp120 as a larger oval with arms labeled V2 and V3.)
Perhaps the most important implications of the findings relate to vaccine development. PG9/PG16 neutralize a diverse spectrum of HIV-1 isolates at concentrations that were often 10 to 100-fold lower than the other previously identified broadly neutralizing antibodies. This raises the hope that protective levels of neutralizing antibodies might be achieved by vaccination. The findings also provide a glimpse into how the human immune system uses both VDJ recombination and somatic variation to increase the breadth of coverage. The importance of somatic variation to this process may be one reason why broad neutralization in infected individuals is only observed after a number of years, and suggests that a similar lengthy period of antigen exposure may be needed in vaccine settings to elicit a diverse neutralizing response. The new antibodies also highlight current limitations in the ability of vaccine immunogens, such as soluble Env gp120 and gp140, to mimic appropriately the viral spike. Hence, current Env vaccines are unable to elicit neutralizing antibodies that are similar to PG9/PG16. The flip side of this is that the ability of the PG9/PG16 antibodies to discriminate soluble gp140s from more native cell-surface oligomers provides a means to assess the spike mimicry of newly designed vaccine immunogens. Indeed, an accurate soluble mimic of the viral spike has long been sought as a vaccine immunogen.
The findings by Burton and colleagues represent the maiden voyage for their impressive B cell stimulation and microneutralization technologies, and it is likely that this success will be followed by others, using either the same approach or alternative methods of B cell enrichment, such as binding and enrichment with structurally designed ligands (Scheid et al., 2009). The top 1% of neutralizing sera identified by their screen of HIV-1 infected individuals display extraordinarily potent and broad neutralization of HIV-1 (Simek et al., 2009). The recent study presents a tantalizing preview of what may follow: additional monoclonal antibodies that broadly neutralize HIV-1. The new PG9/PG16 antibodies may herald a new era in B cell-immunogen design, where additional knowledge of the molecular targets of antibody neutralization and a greater understanding of B cell development and affinity maturation serve to guide future efforts in rational AIDS vaccine design.
Acknowledgments
We thank B. Hartman and J. Stuckey for assistance with figures and members of the Structural Biology Section, VRC for useful comments and discussion.
References
- Gorny MK, Stamatatos L, Volsky B, Revesz K, Williams C, Wang XH, Cohen S, Staudinger R, and Zolla-Pazner S (2005). Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J Virol 79, 5232–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR (2003). Defining the protective antibody response for HIV-1. Curr Mol Med 3, 209–216. [DOI] [PubMed] [Google Scholar]
- Pantophlet R, and Burton DR (2006). GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24, 739–769. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, and Petropoulos CJ (2003). Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A 100, 4144–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. (2009). Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640. [DOI] [PubMed] [Google Scholar]
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, et al. (2009). Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83, 7337–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Morris L, Burton DR, and Mascola JR (2009). Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15, 866–870. [DOI] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. (2009). Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. express on-line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. (2003). Antibody neutralization and escape by HIV-1. Nature 422, 307–312. [DOI] [PubMed] [Google Scholar]
- Wyatt R, and Sodroski J (1998). The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280, 1884–1888. [DOI] [PubMed] [Google Scholar]


