Abstract
In recent years, nanomaterials have aroused extensive research interest in the world's material science community. Electrospinning has the advantages of wide range of available raw materials, simple process, small fiber diameter and high porosity. Electrospinning as a nanomaterial preparation technology with obvious advantages has been studied, such as its influencing parameters, physical models and computer simulation. In this review, the influencing parameters, simulation and models of electrospinning technology are summarized. In addition, the progresses in applications of the technology in biomedicine, energy and catalysis are reported. This technology has many applications in many fields, such as electrospun polymers in various aspects of biomedical engineering. The latest achievements in recent years are summarized, and the existing problems and development trends are analyzed and discussed.
Introduction
Nanowires, nanowhiskers, nanofibers, nanotubes and other one-dimensional nanostructured materials have excellent performance in improving the optical, electrical, thermal and mechanical properties of functional materials and composites. Dielectric and semiconductor nanomaterials made of these one-dimensional materials are widely used in the fields of photocatalysis, sensors, drug delivery, bifunctional materials and so on [1–10].
Many nanofiber fabrication techniques have been developed [11], such as splitting of bicomponent fibers [12], melt blowing [13], physical drawing [14], dry–wet spinning [15], phase separation [16], self-assembling [17], centrifugal spinning [18] and electrospinning [19].
Electrospinning is one of the most versatile, simplest and effective technologies compared with template polymerization and melt spraying [20–23]. It is also the only method for large-scale production of continuous nanofibers in industry [24, 25]
Electrospinning also has the advantages of wide range of available raw materials, simple process, small fiber diameter and high porosity. Although electrospinning technology originated in the early twentieth century, it was not widely used until around 2000. There are quite a few research results on the instrument development and the influence of process parameters.
History
Electrospinning technology can be traced back to 1897. Rayleigh et al. [26–29] studied the phenomenon of charged liquid changing from cylinder to bead. In 1900, Cooley [30] applied for the world's first patent for electrospinning and invented four types of indirectly charged spinning heads—a conventional head, a coaxial head, an air assisted model and a spinneret featuring a rotating distributor. It generally believes that this is the beginning of electrospinning industrialization. However, Morton's 1902 patent on electrospinning lacks some key details [31]. Then, Zeleny [32–37] mathematically simulated the behavior of a fluid under static electricity. Anton [38–44] applied for many patents in the USA, France and other countries between 1931 and 1944, contributing to the electrospinning technology. In 1936, Norton [45] applied for the patent of melt electrospinning. Cellulose acetate nanofibers were prepared by electrospinning with dichloroethane and ethanol as solvents in 1938 by N.D. Rozenblum and I.V. Petryanov Sokolov in 1938. The cellulose acetate nanofibers were applied to filter materials to enhance the toughness and durability of the materials. The materials were produced in a large quantity by Tver antivirus surface ware factory in 1939 [46]. From 1964 to 1966, Taylor [47–49] established the "leaky dielectric model" for electrospinning technology, which laid a theoretical foundation for the "Taylor cone." In 1966, Simons [50] invented a process for printing nonwoven fabrics using electrospinning technology. In 1971, Baumgarten [51] prepared acrylic fibers with DMF as solvent by electrospinning. In 1978, Annis et al. [52] published work examining electrospun polyurethane mats for use as vascular pros thesis. In 1981, Larrondo and St. John Manley [53–55] carried out electrospinning of polyethylene and polypropylene fibers from the melt. In 1985, Fisher et al. [56] studied electrospinning applications in arterial repair materials. In 1996, Reneker and Chun [57] successfully prepared more than 20 kinds of polymer nanofibers by electrospinning technology. In 2009, Jirsak et al. [58] invented a needleless electrospinning technology, and then, the Czech company Elmarco produced the world's first industrial electrospinning machine, Nanospider.
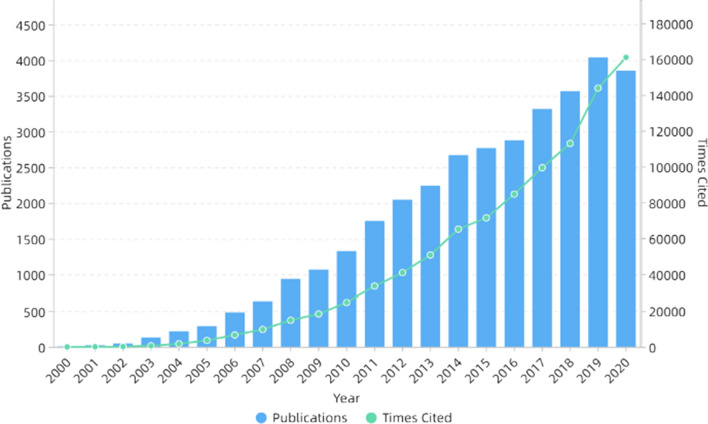
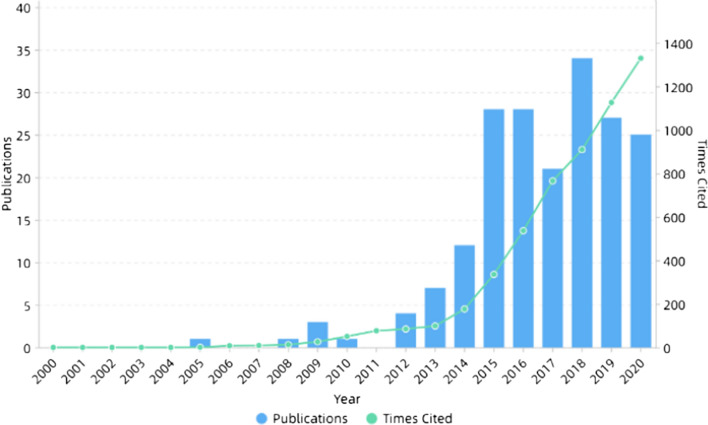
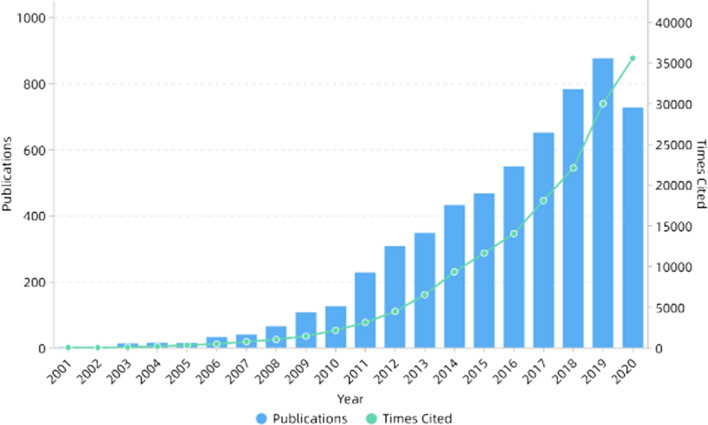
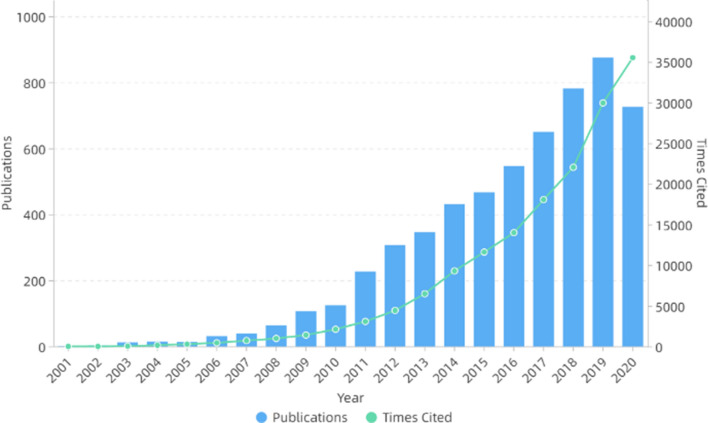
In the next decade or so, electrospinning technology was not only widely used in the biomedical field [59–61], but also widely used in energy [62–64], catalysis [65–67] and other fields because of its simple process and wide applicability. In addition, electrospinning can also be used to prepare self-assembled nanocomposites [68–72]. The number of publications and cited frequency of electrospinning increased year by year, as shown in Fig. 1. Figure 2 shows the number of publications on electrospinning in various countries. It can be seen that China published the most, followed by the USA and South Korea.
Figure 1.
Number of publications and times cited on electrospinning. All the data used are from Web of Science. The functions we used are analyze results and create citation report
Figure 2.
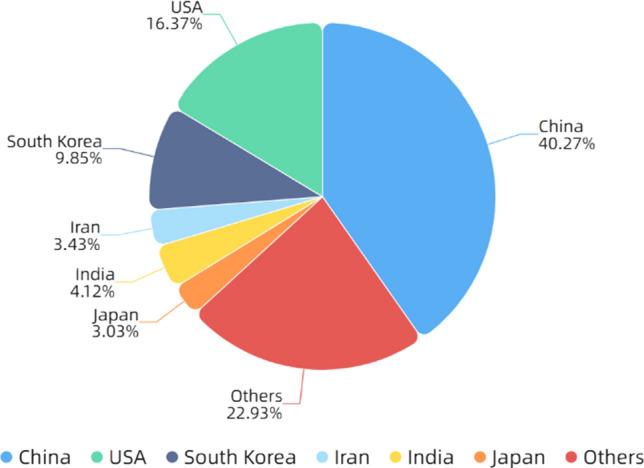
Number of publications on electrospinning in various countries. All the data used are from Web of Science. The functions we used are analyze results and create citation report
Process
The basic principle of electrospinning is that solution, suspension or melt is sprayed in a strong electric field to form continuous fibers. The basic electrospinning device consists of three parts (a) a high voltage power supply (b) a spinneret (c) a collector. Figure 3 shows the basic device of electrospinning. In the case of solution electrospinning, first, because of the surface tension of the solution, droplets are formed on the spinneret with induced charge on the surface [73]. When the electrostatic force is equivalent to the surface tension of the solution, the droplet changes from hemispheric shape to cone shape, which is called Taylor cone [49]. When the electrostatic force is greater than the surface tension, the solution can overcome the surface tension and form jets. In the process of reaching the collection device, the electrostatic force makes the jets stretched and the solvent evaporated, leaving only solid fibers. The collection device can collect the solid fibers with complex network structure [74]. The same is true for melt and suspension electrospinning.
Figure 3.
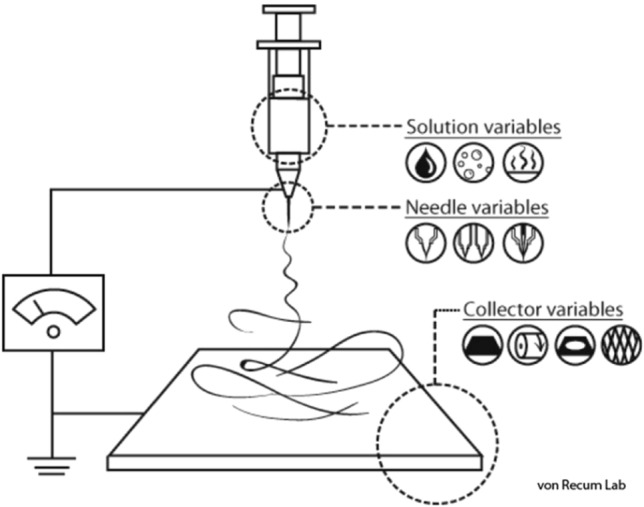
The basic equipment of electrospinning. https://en.wikipedia.org/wiki/Electrospinning. Accessed 3 August 2021
There are many types of electrospinning equipment on the market, most of which is innovative only in the jet device and collection device [75]. The traditional instrument uses electrode material as spinneret. In recent years, the needleless nozzle has been developed, which can be divided into two types: the rotary nozzle and the static nozzle. It can address the problems of needle clogging and low yield. It can be used in industrial production, but it is difficult to control the morphology and distribution of nanofibers [76]. The collection device is generally divided into vertical arrangement and horizontal arrangement [77, 78]. The difference between the two is the droplet formation power is different. When the spinneret and collecting plate are arranged horizontally, droplets are generated by electrostatic force, and gravity is also involved in the horizontal arrangement [79, 80].
Up to now, more than 100 kinds of nanofibers of polymers and blends have been successfully prepared by electrospinning, with diameters ranging from several nanometers to hundreds of microns [81]. Besides, if the same polymer is dissolved in different solvents, the morphology of nanofibers prepared will be different [82, 83].
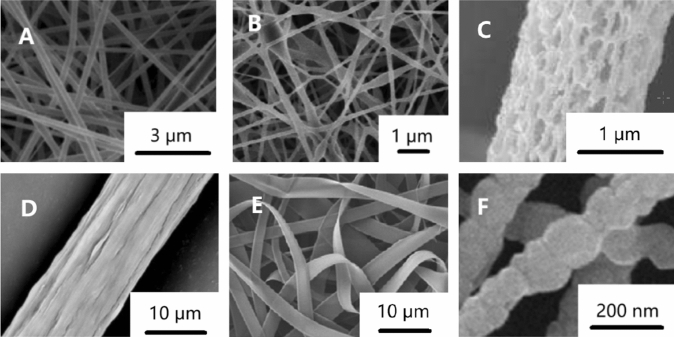
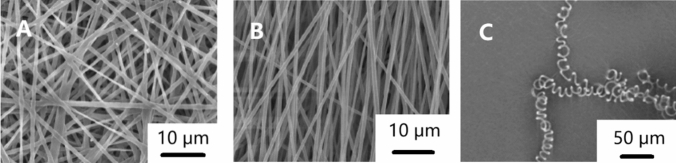
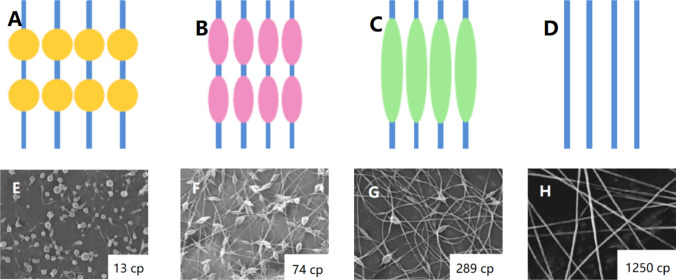
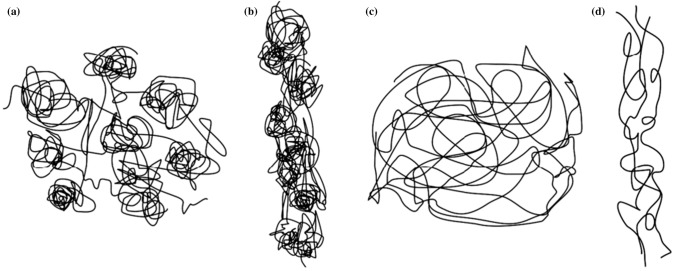
Electrospun nanofibers can be classified into many types according to different classification methods [11]. According to the chemical composition, they can be divided into inorganic nanofibers [84], organic nanofibers [85, 86], carbonaceous nanofibers [87, 88] and inorganic–organic hybrid nanofibers [89]. According to the morphology of nanofibers, as shown in Fig. 4, they can be divided into columnar nanofibers [90], beaded nanofibers [91], porous nanofibers [92], grooved nanofibers [93], nanograin nanofibers [94], nanobelt nanofibers [95] and so on. As shown in Fig. 5, according to the fiber orientation, it can be divided into random-distributed nanofibers [96], aligned nanofibers [96], crimped nanofibers [97] and so on.
Figure 4.
Morphology of electrospun nanofibers a columnar nanofibers [90] b beaded nanofibers [91] c porous nanofibers [92] d grooved nanofibers [93] e nanobelt nanofibers [95] f nanograin nanofibers [94].
Reproduced with permission from reference [90]. Copyright 2019, Elsevier B.V. Reproduced with permission from reference [91]. Copyright 2012, Elsevier B.V. Reproduced with permission from reference [92]. Copyright 2015, Elsevier Ltd. Reproduced with permission from reference [93]. Copyright 2016, Elsevier B.V. Reproduced with permission from reference [94]. Copyright 2013, Elsevier B.V. Reproduced with permission from reference [95]. Copyright 2011, Elsevier Ltd
Figure 5.
Orientation of electrospun nanofibers a random-distributed arrangement [96] b aligned nanofibers [96] c crimped nanofibers [97].
Reproduced with permission from reference [96]. Copyright 2015, Elsevier B.V. Reproduced with permission from reference [97]. Copyright 2014, American Chemical Society
Parameters
Besides the advantages of simple process and wide range of raw materials, electrospinning can control the morphology, orientation and pore size of nanofibers by adjusting the parameters [98, 99]. There are many parameters affecting electrospinning, some of which are uncontrollable. The controllable parameters can be divided into solution parameters, processing parameters and ambient parameters [100]. The solution parameters include concentration, viscosity, molecular weight, conductivity, surface tension and solvent type. The process parameters include applied voltage, flow rate and distance from jet device to collection device. The ambient parameters include temperature and humidity. However, if the parameters are adjusted appropriately, uniform and bead-free nanofibers with suitable diameter can be prepared. Although the National Science Foundation defines fibers with diameter less than 100 nm as nanofibers, in fact, submicron fibers are more widely used in many fields such as tissue engineering [101].
Solution parameters
Concentration
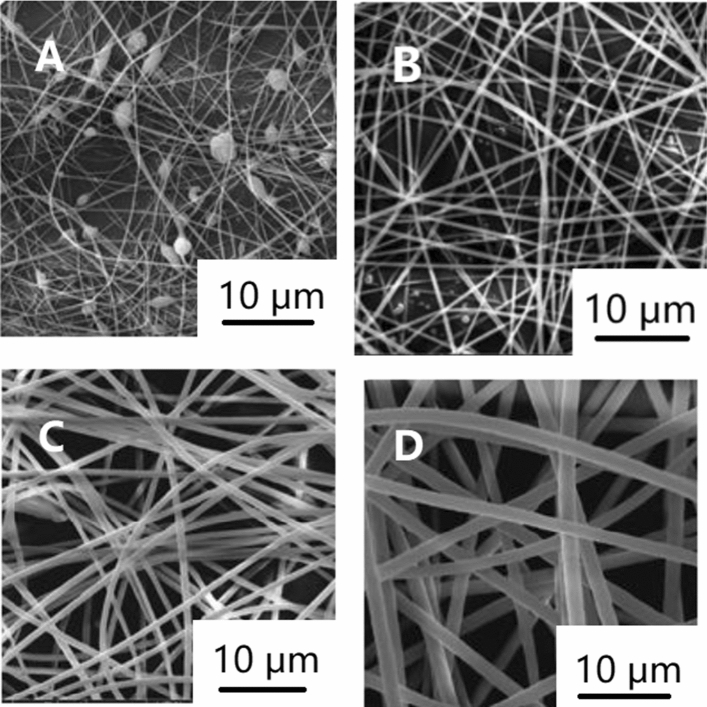
The concentration of the solution plays a decisive role in forming nanofibers. There is a minimum spinnability concentration. When the solution concentration is lower than this value, because of the low concentration and high surface tension, the interaction between the electric field force and surface tension will cause the entangled polymer to be disentangled before reaching the collection device [102, 103]. This produces many beads rather than fibers [104–106]. With the increase in the concentration and viscosity, the entanglement concentration of polymer increases and the surface tension decreases, resulting in more and more fibers, and finally, uniform and smooth nanofibers without beads are formed [107]. At this time, the concentration is the best. When the solution concentration is higher than the optimal concentration, the solution is easy to block the spinneret, resulting in coarse and uneven ribbon fiber [103, 108]. The optimal concentration is a range. As shown in Fig. 6, the nanofibers become coarser with the increase in the concentration in this range [105, 106, 109–111]. Some studies have also shown the solution viscosity can be improved by adding cosolvents at a certain polymer concentration. Increasing the concentration can usually improve the morphology of nanofibers or make it easier to electrospin polymers that are difficult. For example, sodium alginate can be electrospun into uniform and smooth nanofibers by adding glycerol or PEO [112, 113]. It has been found that if there are many kinds of polymers in the solution, even if the viscosity is the same, different polymer concentration ratio will change the diameter of nanofibers, which is caused by the interaction between polymers [113]. Some researchers have also changed the solution concentration and prepared microspheres instead of nanofibers by electrospinning, which provides a new idea [114].
Figure 6.
SEM images of electrospun polyimide nanofibers with different concentrations a 10%, b 15% c 20% d 25% [105].
Reproduced with permission from reference [105]. Copyright 2017, De Gruyter
Viscosity
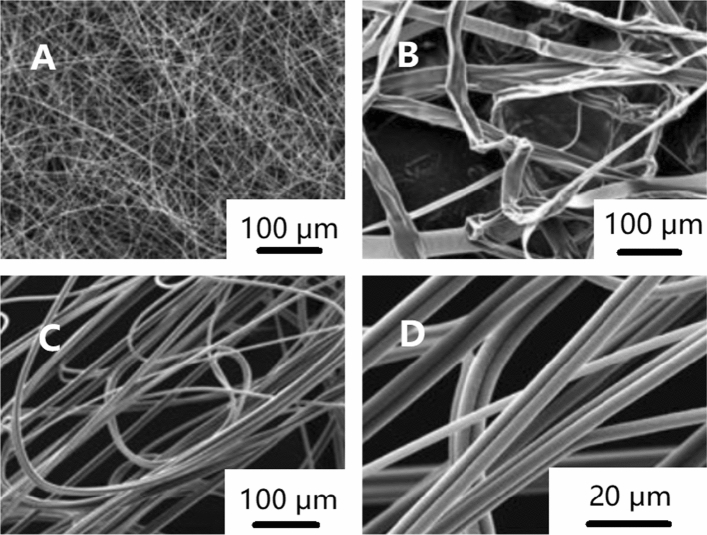
The viscosity of the solution is closely related to the concentration and molecular weight, which is one of the main parameters affecting the diameter and morphology of nanofibers [115]. If the viscosity is too low or too high, the bead structure will be formed [75]. If the viscosity is too low, it means the polymer entanglement is low and the surface tension is dominant, the droplets cannot connect into fibers and form spray [116]. With the increase in the viscosity, the stress relaxation time of the polymer becomes longer, which is conducive to forming nanofibers with larger and uniform diameter, as shown in Fig. 7 [117]. However, when the viscosity is too high, it is difficult for jets to form fiber [118]. Therefore, there is a best range of viscosity suitable for electrospinning nanofibers [102]. Some studies have shown the solution with viscosity of 1–20 P and surface tension of 35–55 dyn/cm2 is suitable for electrospinning nanofibers [119]. When the solution viscosity is too high to carry out electrospinning, some researchers propose using vibration technology to solve this problem [120]. For example, ultrasonic vibration can decrease the van der Waals force between polymer chains to achieve the purpose of temporarily reducing the viscosity of the solution, as shown in Fig. 8. At the same time, attention should be paid to avoid the rapid evaporation of the solvent during the vibration process [121]. The viscosity of the solution can be controlled by adjusting the polymer concentration. The viscosity of the solution can be increased with the increase in the solubility when the conductivity is high, but the change of the diameter of the nanofiber can be ignored, because the conductivity will also increase with the increase in the concentration. The increase in the conductivity will cause the diameter of the nanofiber to become smaller [110]. The solution concentration can also be increased by adding nanoparticles to the solution. Although the diameter of the nanofibers increases with the increase in the content of nanoparticles, the nanofibers will be rougher and more uneven because of the increase in the friction and viscosity between particles [122].
Figure 7.
Morphology of nanofibers with increasing viscosity a–d schematic diagram [123] and e–h SEM images of electrospun PEO nanofibers [104].
Reproduced with permission from reference [104]. Copyright 1999, Elsevier B.V. Reproduced with permission from reference [123]. Copyright 2013, MDPI (Basel, Switzerland)
Figure 8.
Influence of ultrasonic vibration on polymer molecular chain. a Entangled molecular chains in polymer solution without sonic vibration treatment, b entangled molecular chains in electrospun fiber without sonic vibration treatment, c molecular chains in polymer solution disentangled after sonic vibration treatment and d disentangled molecular chains in electrospun fiber with sonic vibration treatment [121].
Reproduced with permission from reference [121]. Copyright 2019, SAGE Publications Ltd
Molecular weight
The molecular weight of the polymer can affect the rheological and electrical properties of the solution such as viscosity, surface tension and conductivity [124]. When the molecular weight is large, the intermolecular force is large, and the polymer may also generate more hydrogen bonds between the solvents, making the polymer expand, thus increasing the viscosity value [125, 126]. Because of the inhomogeneity of polymer conductive system, the interchain conductivity is much lower than intrachain, and the larger the molecular weight, the smaller the degree of interchain discontinuity, the larger the interchain conductivity, and the larger the macroscopic conductivity [127–129]. However, some experiments have found the molecular weight has little relationship with the conductivity [130]. Similar to the effect of viscosity and concentration, the polymer with low molecular weight has insufficient entanglement degree, short chain length, small molecular friction force, difficult to resist unstable whipping, interrupted jet and difficult to form fibers [131]. In general, the diameter of nanofibers will also increase with the increase in the molecular weight. However, excessive molecular weight will produce ribbon fibers [132, 133]. Researchers have also found that intermolecular forces can be used to counter surface tension when the molecular weight is low [98].
Conductivity
The charged particles of polymer have great influence on jet formation. When the conductivity of the solution is too low, there will be beads and it is difficult to form uniform nanofibers. However, high conductivity may lead to bending whiplash and uneven diameter or formation of ribbon fibers [134]. The diameter of nanofibers decreases with the increase in the conductivity [113, 135]. Some researchers also found the conductivity has negligible effect on the fiber diameter [126]. It has been found that theoretically the jet radius is inversely proportional to the cube root of the solution conductivity [51]:
| 1 |
where r0 is jet or filament radius, Q is the mass flow rate, ε is the permittivity, σ is the electric conductivity, ρ is the density. The conductivity of the solution can be improved by adding some inorganic salts such as sodium chloride, lithium chloride, magnesium chloride and copper chloride to the solution. This helps forming beadless fibers with smaller diameters and increases uniformity [136]. Some researchers discovered the opposite [137]. Some organic compounds are also feasible, such as pyridinium formate [138], benzyl trialkylammonium chloride [139], dodecyltrimethyl ammonium bromide (DTAB) [140], tetrabutylammonium chloride (TBAC) [140], triethylbenzyl ammonium chloride (TEBAC) [141] and tetraethylammonium bromide (TEAB). However, their conclusions are not compatible. PH also affects the conductivity of the solution [106]. The electrospinning properties of polystyrene solutions with 18 kinds of common organic solvents were measured [142]. It was found that only 1,2-dichloroethane, DMF, ethylacetate, MEK and THF could meet the needs of electrospinning.
Surface tension
Surface tension is affected by many factors, including molecular weight, solution concentration, solvent type and temperature [126]. However, adding surfactant can also effectively decrease the surface tension, which only has to be removed in the subsequent process. When the surface tension is low, the beads are fewer and the nanofibers are finer and smoother [143, 144]. Some researchers believe the surface tension has little effect on the fiber diameter [145]. However, if the surface tension is too low, the jet will be unstable, the diameter distribution of nanofibers will be uneven [146] or even beads will be formed [147, 148]. In general, the surface tension of water is higher than that of ethanol. Ethanol can be added to the solution to decrease the surface tension [104]. A little surfactant can decrease the surface tension of the solution, such as sodium dodecyl benzene sulfonate (SDBS) [149], dodecyltrimethylammonium bromide (DTAB) [140], tetrabutylammonium chloride (TBAC) [140] and Tween 80 [110]. However, some surfactants, such as Triton X-405 [140], have little effect, and even more Triton X-100 (TX100) may increase the fiber diameter [150].
Solvent
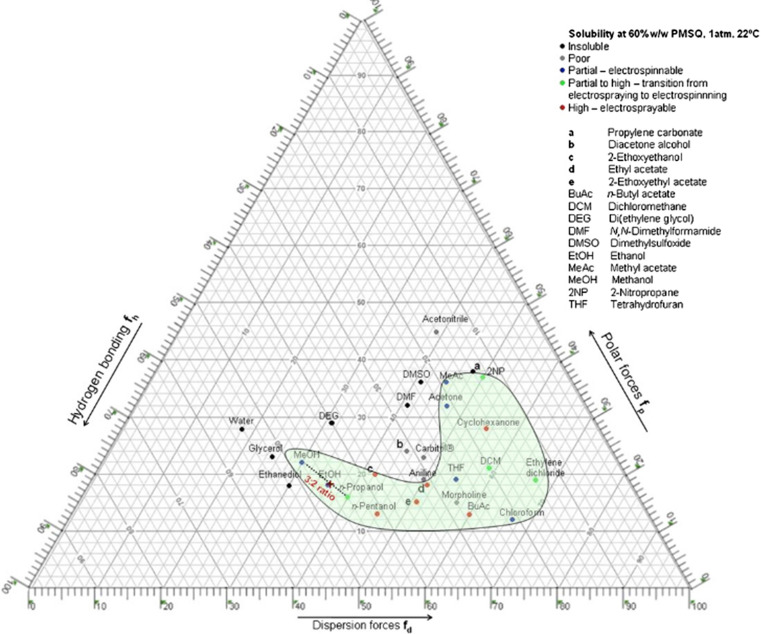
There is no doubt the choice of solvent is important. The properties of the solvent, including surface tension, dielectric constant, boiling point, density, as well as the interaction between solvent and solute, as shown in Fig. 9, will affect electrospinning [145]. For example, the volatility of the solvent is directly related to whether it can volatilize before reaching the collection device, and has a great impact on whether beads will appear or not [151]. The polarity and dielectric constant of the solvent will affect the conductivity of the solution [151]. Because of the toxicity of many organic solvents, if they cannot be completely removed, they would not be used in biological and food fields [152]. The influence of solvent types on electrospinning is complex. There is no clear theory to judge whether electrospinning can be carried out with a certain solvent [153–155]. According to Hansen solubility parameters, a ternary solubility diagram has been made by some researchers, as shown in Fig. 10. The three edges represent the dispersion component (δd), polar component (δp) and hydrogen bonding component (δh), respectively, which can be used for in-depth study of solvent solubility [156–160]. The solvents in the green region is expected to dissolve the polymer. For example, when MeOH and PrOH are mixed, a good solvent such as EtOH can be found near their connecting line. The volume ratio of the two solvents can be calculated by the length ratio from the closest point to EtOH to both ends. The effects of solubility of polycaprolactone [161], polyethylene terephthalate (PET) [162] and polymethylsilsesquioxane (PMSQ) [159] in different solvents on electrospinning have been studied. When the solubility is low, it is easy to form nanofibers, and high dielectric constant will make the diameter of nanofibers smaller [159]. Kohse et al. [105] found the electrospinning of polyimide in N,N-dimethylformamide (DMF) produced smooth and round shaped nanofibers, but only ribbon shaped fibers were formed in 1,1,1,3,3,3-jexafluoro-2propanol (HFIP) and smooth but stucked fibers in dimethylsulfoxide (DMSO).
Figure 9.
SEM of electrospun polyimide nanofibers in different solvents [105]. a DMF, b HFIP, c and d DMSO.
Reproduced with permission from reference [105]. Copyright 2017, De Gruyter
Figure 10.
Ternary solubility diagram of polymethylsilsesquioxane in different solvents. The solvent with good solubility is in the green region [159].
Reproduced with permission from reference [159]. Copyright 2010, Elsevier B.V
Processing parameters
Applied voltage
The applied voltage is also a key parameter in the electrospinning process. Only when the critical voltage is reached can the droplet be ejected and finally reach the collection device. The applied voltage also affects the morphology and diameter of nanofibers. If the applied voltage is too high or too low, it will lead to forming beads [163]. The diameter of nanofibers decreases with the increase in the applied voltage; because the electrostatic force is large, the droplets are stretched longer [164–169]. But in fact, according to previous studies, there is no consensus on the effect of applied voltage on nanofibers. Some researchers believe the diameter of nanofibers will increase with the increase in the voltage because of the increase in the jet velocity [170–172]. Kim et al. [173] also found the nanofibers first decreased and then increased with the increase in the applied voltage, and made some mechanical analysis. In addition, some researchers found that with the increase in the voltage, the diameter distribution of nanofibers is more uneven [168, 171]. The effect of applied voltage on the diameter of nanofibers may vary with the polymer solution concentration and the distance from jet device to collection device [174–176]. More research is needed on the effect of applied voltage on the diameter of nanofibers.
Flow rate
The flow rate also affects the diameter and morphology of nanofibers. The diameter of nanofibers increases with the increase in the flow rate [146, 168, 176, 177]. If the flow rate is too high, the solvent cannot completely evaporate before reaching the collection device, which will lead to forming beads [178] or ribbons [177]. Therefore, when the flow rate is moderate, the Taylor cone is more stable, and it is easier to generate smooth and uniform nanofibers [179]. Some researchers believe there is an ideal flow rate, and deviation from the optimal value will lead to the coarsening of nanofibers [180]. When the solution viscosity is low, the flow rate has little effect on the diameter of nanofibers [175, 176]. When the applied voltage is higher, the effect of flow rate is more significant.
Distance from jet device to collection device
The distance from jet device to collection device can also be used to control the diameter and morphology of electrospun nanofibers [181]. The distance from jet device to collection device needs to be large enough to allow the solvent to evaporate. If the distance is too small, there will be beads [177]. However, the distance should not be too large. If the distance is too large, the jet will not be stable enough, and there will be beads. In fact, the distance from jet device to collection device has less effect on the diameter of nanofibers than other parameters [170, 176, 182]. However, some researchers believe the distance has a great influence on the fiber diameter [183]. Generally speaking, the larger the distance, the greater the bending instability, resulting in the overlap of some nanofibers, resulting in larger fiber diameter [173, 184]. However, if the distance is small, the solvent evaporation time is not enough, and coarse nanofibers will be formed [116, 178, 185, 186].
Ambient parameters
Temperature
Generally speaking, the higher the temperature, the lower the viscosity and surface tension, the higher the solubility [147, 187, 188]. The increase in the temperature will lead to the decrease in the diameter of nanofibers and smoother surface [147, 187–189]. Yang et al. [189] studied the relationship between temperature and evaporation rate of PVP anhydrous ethanol solution using Knudsen layer theory. They found there was an inflection point in the relationship between temperature and nanofiber diameter, which was related to many properties of solution affected by temperature. However, De Vrieze et al. [147] also used the absolute ethanol solution of PVP and drew the opposite conclusion for the same temperature range.
Humidity
The influence of environmental humidity on electrospinning is also complex. When the humidity is low, the evaporation rate of solvent is faster, which will make the diameter of nanofibers larger [147, 190–193]. However, because of the different hydrophilicity of solvents, some organic solvents such as ethanol will absorb the moisture in the air, which makes the solvent more difficult to evaporate, and the electrostatic force is decreased, which makes the nanofibers thinner [147, 194]. Therefore, this should depend on the type and solubility of solvents [195]. Some researchers have also found there is no obvious relationship between ambient humidity and the thickness of nanofiber mats [106, 196, 197]. When the humidity is too high, the diameter distribution of nanofibers is more uneven, the surface is more rough, and the pores or fibers are less [106, 191, 197, 198]. Ding et al. [199] found that under the condition of high voltage and low humidity, the rapid separation of polymer and solvent can produce nanowebs with overlapping layers, uniform pore size and fine fiber.
Models and simulation
It is of great significance to simulate electrospinning by computer. Because the phenomena observed in many experiments are sometimes difficult to explain, we need to use theoretical models and computer simulation to help us better understand various problems. Although electrospinning technology has been widely concerned, there are few studies on simulation. In fact, because of the complex parameters affecting electrospinning, many researchers have put forward some empirical relationships. For example, Wang et al. [200, 201] obtained some empirical scaling laws of parameters and diameter through experiments, and Yousefi et al. [202] did some similar experiments. However, because of the lack of systematization and characterization, the applicability of the empirical model is limited [203]. By establishing a perfect mathematical and physical model for electrospinning simulation, the morphology and properties of nanofibers can be well predicted, which could improve the work efficiency of researchers and expand the electrospinning technology application.
Physical models
Models of electric field
Taylor [49] first studied the initial stage of the jet. In principle, when the electric field force and surface tension are equal, the viscous droplet will form a cone with the half vertex angle of 49.3° and the state is called the Rayleigh stability limit [28].
Taylor [48] gave the critical voltage of viscous fluid jet:
| 2 |
where Vk is the potential at breakdown, H is the distance between jet device and collection device, L is the injector length, R is the injector radius and T is the surface tension of the liquid.
Besides, they introduced the leaky dielectric model to explain the behavior of droplets deformed by a steady field, which laid the foundation for the later theoretical research.
Hendricks et al. [204] gave an empirical formula for the critical voltage of hemispheric suspension droplets:
| 3 |
where V is the critical voltage, γ is the surface tension, and a is the capillary radius.
Of course, Taylor's and Hendricks' studies assumed the droplet is in a stable state at the capillary port, and the model only applies to weakly conductive liquids, without considering the effects of liquid conductivity and viscosity.
Interestingly, Yarin et al. [205] confirmed theoretically and experimentally the half angle of Taylor cone should be 33.5° instead of 49.3°. This may be because of the existence of non-self-similar solutions for hyperboloid shape in equilibrium with its own electric field under the surface tension action.
Models of jet
When the applied voltage is higher than the critical voltage and the electric field force is greater than the surface tension, the jet will first go through the stable stage and then enter the unstable stage.
Stable stage
In the stable stage, the polymer jet does uniaxial stretching, and the jet shape does not change with time. In this process, the jet radius is the focus of research, because it directly affects the diameter of nanofibers.
Baumgarten [51] derived the jet radius from the relationship between transfer current and conduction current:
| 4 |
where r0 is jet or filament radius, Q is the mass flow rate, ε is the permittivity, σ is the electric conductivity and ρ is the density.
However, the axial voltage gradient in jet in units still needs to be obtained.
According to the equations of mass balance, electric charge balance and momentum balance, Spivak et al. [206, 207] established a simple one-dimensional model of nonlinear power-law fluid:
| 5 |
where z is the axial coordinate, R is the dimensionless jet radius, NW is the dimensionless Weber number, NE is the dimensionless parameter, NR is the effective Reynolds number and m is the flow index.
However, the one-dimensional linear symmetrical model is too simple, which is different from the actual.
Later, according to the leaky dielectric model and the slender body theory, Hohman et al. [208, 209] introduced the free charge and obtained the approximate model of jet dynamics:
| 6 |
where h is the radius of jet, v is the fluid velocity parallel to axis of jet, σ is the surface charge density, K* is the dimensionless conductivity, E is the electric field parallel to axis of jet, , ν* is the dimensionless viscosity, Χ is the aspect ratio and Ω0 is the dimensionless external field strength.
However, this model is not suitable for non-Newtonian fluid. After that, Feng [210] improved it and considered the non-Newtonian fluid:
| 7 |
where v is the axial velocity, Pe is the Electric Peclet number, Fr is the Froude number, Re is the Reynolds number, η is the viscosity, E is the axial field, z is the axial position, We is the Weber number, , is the dielectric constant of the ambient air, , Χ is the aspect ratio and E∞ is the external field.
Unstable Stage
The unstable stage is much more complicated than the stable stage, and researchers have made great efforts. The instability is caused by the charge repulsion in the jet. There are three kinds of instability in the unstable stage of electrospinning [211]: the classical Rayleigh mode (axisymmetric) instability, electric field-induced axisymmetric conducting mode (bending) instability and whipping conducting mode instabilities. These instabilities change with the applied voltage, the distance from jet device to collection device and the solution parameters, which affect the morphology and distribution of nanofibers [212].
Strutt and Rayleigh [26] first proposed and found the axisymmetric instability, and derived the relationship between the instability deviation distance and the potential and other factors. Unfortunately, there is no effective experimental support.
Huebner and Chu [213] analyzed the influence of surface tension and electrodynamic effect on jet radius on the basis of empirical formula, but also lacked experimental support.
Reneker et al. [214] established a three-point-like charges model and analyzed the cause of the bending instability:
| 8 |
where m is the mass, δ is the distance, l is the length and e is the charge.
They also proposed a viscoelastic model of a rectilinear electrified liquid jet. The linear Maxwell equations were used to describe the jet flow. The spinning process was simplified as a system of beads:
| 9 |
where σ is the stress, t is the time, l is the length, G is the elastic modulus, μ is the viscosity, , m is the mass, e is the charge, , V0 is the voltage, h is the distance from pendent drop to grounded collector, a is the radius and ki is the jet curvature.
| 10 |
According to Yarin et al. [215], the main cause of instability is the electric bending force, and the wave number Χ* and the growth rate γ of the fastest growing bending perturbation are derived:
| 11 |
| 12 |
where ρ is the density, a0 is the jet cross-sectional radius which does not change for small perturbations, μ is the viscosity, L is the cutoff length, a is the cross-sectional radius of the jet element and σ is the surface tension.
Hohman et al. [208, 209] developed the slender body theory and established a jet dynamic model to clarify the influence of the surrounding electric field on the jet charge:
| 13 |
where X is the oscillation of the centerline, s is the arc length, ν* is the dimensionless viscosity, is the air dielectric constant, , Ω0 is the dimensionless external field strength, P is the dipole density, σ0 is the dimensionless background free charge density, R is the radius, σD is the dipolar component of free charge density, Χ is the aspect ratio and ξ is the coordinate in the principal normal direction.
Shin et al. [216] also proposed the whipping instability model and gave the amplification factor for a perturbation convected a distance downstream:
| 14 |
where Γ is the amplification factor, E∞ is the applied electric field, Q is the flow rate, A is the amplitude of a perturbation, d is the distance, ω is the growth rates, E is the local electric field, h is the radius of the jet and σ is the surface charge distribution.
After that, Fridrikh et al. [217] established a mathematical model of the influence of parameters and obtained the equation of motion of the jet:
| 15 |
where ρ is the density, h is the jet diameter, x is the motion for normal displacements of the centerline of the jet, E∞ is the applied electric field, is the unit vectors normal to the centerline of the jet, γ is the surface tension, is the dielectric constant, is the unit vectors tangential to the centerline of the jet, Χ is the dimensionless wavelength of the instability responsible for the normal displacements and R is the radius of curvature.
Theron et al. [218] studied the parameters of electrospinning with the help of empirical formulas.
Carroll et al. [219] calculated the expected growth rate of the axisymmetric beads, as well as the expected bead wave number, and analyzed the instability mechanism from the perspective of energy. They found that electrical forces mainly drive the unstable axisymmetric mode for electrically driven, highly conducting jets:
| 16 |
where , , , , , , , , , , , , , , .
Using JETSPIN software package, Carroll and Joo [219] studied the effect of gas flow on electrospinning by using nonlinear Langevin-like approach and made experimental comparison:
| 17 |
where σi is the stress on the ith dumbbell which connects the bead i with the bead i + 1, li is the length of the element, G is the elastic modulus, μ is the viscosity of the fluid jet, t is the time, vi is the velocity of the ith bead, fel,i is the electric force, fc,i is the net coulomb force, fve,i is the viscoelastic force, fst,i is the force due to the surface tension, fg,i is the force due to the gravity, fdiss,i is the dissipative force, frand is the random force and ri is the position vector of the ith bead.
Yousefi et al. [220] used a physics-based computational model, mass–springdamper (MSD) approach to incorporate the mechanical properties of the fibers in predicting the formation and morphology of the electrospun fibers:
| 18 |
where ri is the position vector of bead i, mi is the mass of bead i, , , is the viscoelastic forces acting on bead i, is the surface tension force, is the electric attraction force and is the Columbic force.
Molecular simulation
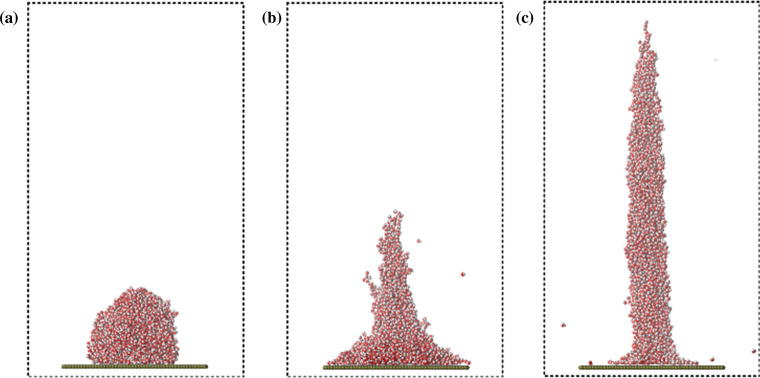
Molecular simulation technology includes Monte Carlo method and molecular dynamics method [83, 221]. Monte Carlo method is based on statistics and is suitable for equilibrium system. Molecular dynamics method is a deterministic method based on classical mechanics, which can solve the equations of motion of all particles in the system, and is suitable for studying time-dependent phenomena. In electrospinning, molecular dynamics method is commonly used to study the interaction of composite materials and the adsorption or surface interaction of nanofiber products with drugs or proteins. Jirsák et al. [222] used Monte Carlo method and molecular dynamics method to simulate the initial stage of the jet, as shown in Fig. 11. The results obtained by the two methods are qualitatively consistent with the experimental results and found the balance of ion concentration and field strength affects forming beads in nanofibers. Danwanichakul et al. [223] used Monte Carlo method to simulate the deposition stage of nanofibers, obtained the positive correlation between the concentration and the pore size of the fibers, and tested the filtration performance of polystyrene particles, which was consistent with the experimental results. Sarmadi et al. [224] simulated the protein adsorption capacity of electrospun PCL/PVA nanofibers by molecular dynamics method, which was consistent with the experimental results. In addition, Vao-soongnern [225] analyzed the effect of molecular weight and chain conformation of polymers on the structure and properties of electrospun nanofibers using Monte Carlo method. Steffens et al. [226] simulated the interaction between PVA nanofibers and drug-loaded dacarbazine using quantum mechanics calculations, molecular modeling techniques and molecular dynamics simulation, thus explaining the principle of controlled drug release.
Figure 11.
Sequence of snapshots showing the transformation of a droplet on the wall modeling the apex of the Taylor cone to a jet. a System before the field was applied, b the instant soon after the field was applied, and c a later instant when the droplet is transformed to the jet. Colored spheres represent atoms and ions as follows: red–oxygen, white–hydrogen, blue–sodium cation and cyan–chloride anion [222].
Reproduced with permission from reference [222]. Copyright 2014, American chemical society
Others
As well as the above models and simulation methods, there are some other interesting methods. For example, lattice Boltzmann method, which is an advanced computational fluid dynamics method, has the characteristics of mesoscopic model between micro-molecular dynamics model and macro-continuous model. Karra [227] modified the discrete bead model and proposed a hybrid numerical scheme that couples lattice Boltzmann method with the finite difference method to solve Oldroyd-B viscoelastic problem. Finite element model is equally a commonly used model in physics. Yin et al. [228] used representative volume element to analyze the relationship between the nanofiber membrane performance and single nanofiber performance, and verified the correctness of the conclusion through experiments. Neural network is a hot machine learning algorithm recently, but its application in electrospinning is still less. Sarkar et al. [229] used neural network algorithm, taking concentration, conductivity, flow rate and electric field strength as input variables, can well predict the change of fiber diameter, but only consider the influence of four variables, which is not suitable for complex electrospinning process.
Applications
Biomedicine
Tissue engineering
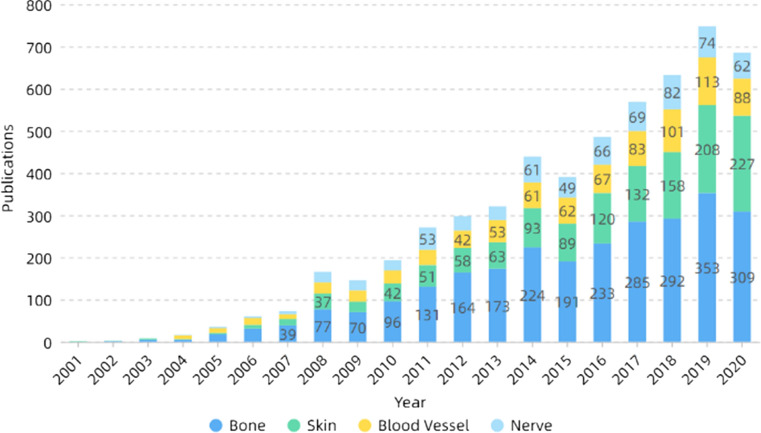
Tissue engineering is a major branch of biomedical engineering, whose main purpose is the repair and regeneration of organs or tissues [230–233]. Tissue engineering consists of three parts: scaffold materials, growth factors and cells. The nanofibers prepared by electrospinning have the characteristics of high porosity and large specific surface area, which are similar to the structure of extracellular matrix (ECM). They can provide sites for cell adhesion and facilitate the transport of nutrients and wastes [234–236]. Besides, the electrospinning parameters are mostly controllable, which can prepare nanofibers with ideal diameters and shapes, as well as nanoparticles or nanotubes and other structures, and have a wide application prospect in tissue engineering [214, 237, 238]. Figure 12 shows the number of publications on electrospinning in different directions of tissue engineering. It can be seen that researchers mainly focus on bones and skin, and less on blood vessels and nerves.
Figure 12.
Number of publications on electrospinning in different directions of tissue engineering. All the data used are from Web of Science. The functions we used are analyze results and create citation report
Bones
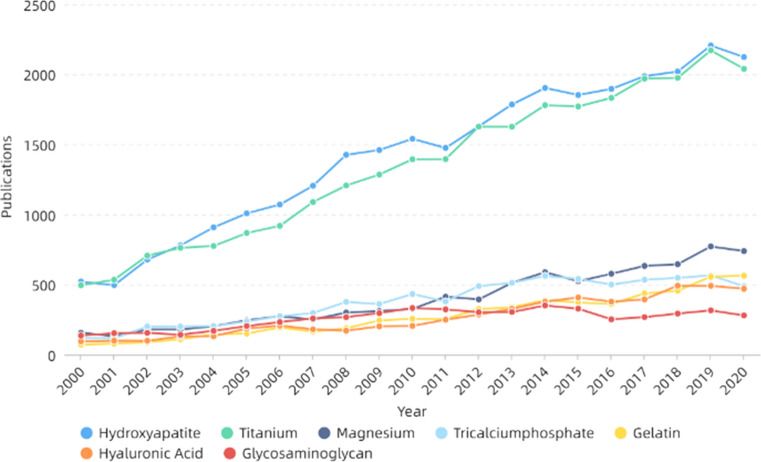
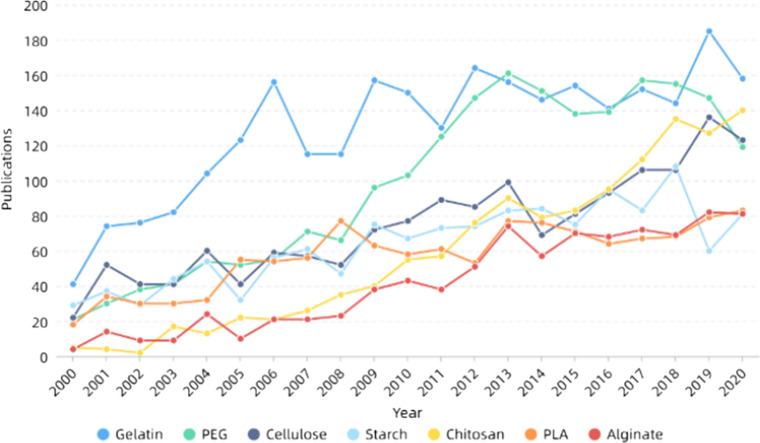
Bone is a complex nanocomposite comprising about 70% hydroxyapatite (HAP) and about 30% collagen fibers [239, 240]. When human bones are damaged by disease or accident, tissue engineering techniques can be utilized to repair or regenerate them. Electrospinning technology has great potential in bone repair [241, 242]. Bone repair materials can fall into three categories: metal, polymer and ceramic. Figure 13 and Table 1 both show that hydroxyapatite and titanium-related materials are widely used in bone repair, which are the two most important bone repair materials in metal and ceramics. Metal materials have excellent biocompatibility and mechanical properties, but they are generally non-biodegradable and have less effect on surrounding tissues. Polymers and ceramics have good biological activity, some with good mechanical properties are more suitable for bone tissue engineering [243]. Hydroxyapatite (HA), the main inorganic component of human bones, has good biocompatibility and is suitable for use as a bone repair material [244, 245]. Besides, HA can also promote growth and proliferation of osteoblasts and induce bone mineralization [246]. However, its mechanical properties are poor, brittle and easy to break, so it needs to be modified or compounded with other materials. In recent years, preparing hydroxyapatite-related materials by electrospinning has been widely studied. Many studies have shown that hydroxyapatite can be combined with gelatin [247], collagen [248], silk [249], PCL [250], chitosan [251] and so on, making it more suitable for bone repair. For example, Chen et al. [252] prepared gelatin–chitosan core–shell structure nanofibers using coaxial electrospinning technology. Hydroxyapatite was wet deposited on the surface of the fibers, which was conducive to the adhesion and proliferation of osteocytes, improved mineralization efficiency of hydroxyapatite, then cultured MG-63 cells for evaluation. It was noted the cell activity was significantly improved, indicating that its biocompatibility was good. Ko et al. [253] coated glycidoxypropyltrimethoxysilane (GPTMS) on the surface of hydroxyapatite to improve the dispersion in silk fibroin solution and added PEO to improve the stability of electrospinning. After electrospinning, polydopamine (PDA) and hydroxyapatite nanoparticles were coated on the surface, and a new nanofibrous scaffold material was obtained. Tests showed the coating improved the compressive resistance of the material. Then the human adipose-derived mesenchymal stem cells (hADMSCs) activated by TAZ transcription regulator were cultured on the scaffold material, which further improved the osteoconductivity of the material, developed osteogenic ability of the cells and promoted the formation of bone. At the same time, it was found the concentration of hydroxyapatite had a great impact on the osteogenic ability, and its importance is self-evident. Using hydroxyapatite–TSF composite nanoparticles as the inner layer and pure tussah fibroin as the outer layer, Shao et al. [254] prepared nanofibers with different core–shell mass ratio using coaxial electrospinning technology, which was conducive to the proliferation and adhesion of MG-63 cells. Alkaline phosphatase, osteocalcin and matrix mineralization at different times were also measured, indicating the materials made bone development and maturation faster.
Figure 13.
Number of publications of some bone repair materials in recent years. All the data used are from web of science. The functions we used are analyze results and create citation report
Table 1.
Some progress of electrospinning in bones in recent years
| Material | Solvent | Flow rate (mL/h) | Distance (cm) | Voltage (kV) | Cells | Reference |
|---|---|---|---|---|---|---|
| Chitosan/PEO/halloysites | Water | 1.2 | 15 | 19–26 | NHOst | [255] |
| PHB/keratin | Chloroform/DMF | 0.96 | 25 | 13 | MG-63 | [256] |
| PLGA/β-TCP particles | DCE/DMF | 1 | 25 | 18 | MC3T3 | [257] |
| Chitosan/PHB/alumina nanowires | TFA | 0.6 | 25 | 22 | MG-63 | [258] |
| PCL/nanomagnesium phosphate/nanohydroxyapatite/hyperbranched polyglycerol | DMF/CHCl3 | 0.5 | 11 | 9–13 | MG-63/hMSCs | [259] |
| Deacetylated cellulose acetate | Acetone/water | 2–4 | 12–15 | 20–22 | MC3T3 | [260] |
| Zein/trimethylolpropane triglycidyl ether (TMPGE) | Ethanol/water | 2.5 | 25 | 17–20 | MC3T3 | [261] |
| silk fibroin/PLCL/parathyroid hormone | HFIP | 1 | 12/15 | 12/15 | BMMSCs | [262] |
| PCL/amine-functionalized single-walled carbon nanotube | DCM/DMF | 2 | 23 | 17 | rMSCs | [263] |
| PHB/PCL/levofloxacin/sol–gel derived silica | Chloroform/DMF | 4 | 15 | 15 | MG-63 | [264] |
| PCL/chitosan or carboxymethyl chitosan | Acetic acid/formic acid | 0.1–0.7 | 16–20 | 18–30 | MG-63 | [265] |
| PCL/F18 bioactive glass | DCM/DMF | 3 | 18 | 17 | MG-63 | [266] |
| PLLA/synthetic glass-reinforced hydroxyapatite | DMC/DMF | 0.5 | 20 | 25 | MG-63 | [267] |
| PLLA/glass-reinforced hydroxyapatite microparticles | DMF/dichloromethane/DCM | 0.5 | 20 | 25 | MG-63 | [268] |
| PCL/gelatin | TFE/acetic acid/ethanol | 3 | 18 | 20 | MC3T3 | [269] |
| Cellulose/nanohydroxyapatite | LiCl/DMAc | 0.24 | 10 | 20 | HDFCs | [270] |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/silicate containing hydroxyapatite nanoparticles | Chloroform | 2 | 5 | 7 | hMSCs | [271] |
| Polyamide-6,6/chitosan | Acetic acid/HFIP | 0.5 | 17 | 20 | MC3T3 | [272] |
| PHB/hydroxyapatite nanoparticles | Chloroform | 1 | 20 | 15 | MSCs | [273] |
| Zein/calcium lactate/PCL | DMF/DCM | 1 | 18 | 17 | MC3T3 | [274] |
Skin
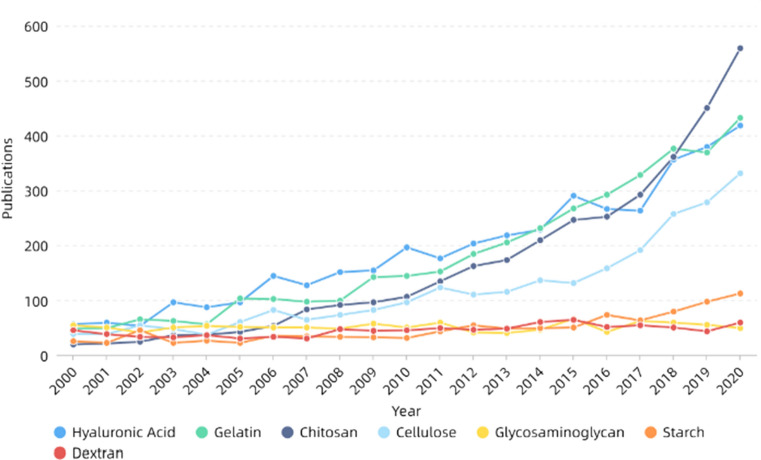
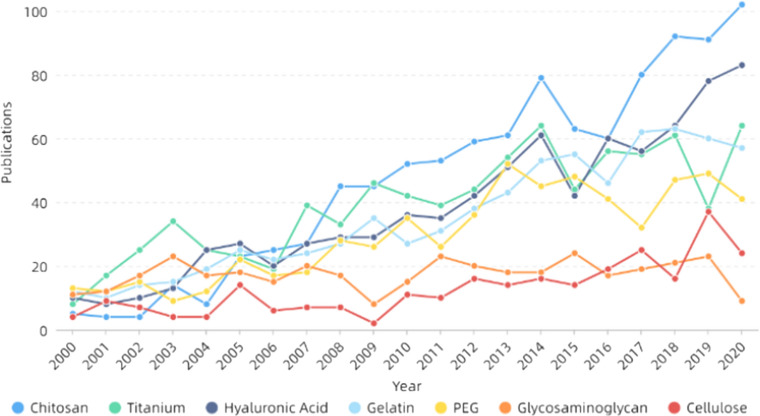
Skin is the largest organ of the human body, the first defense line of the matrix from dehydration, injury and infection, and a barrier to prevent from invading external microorganisms. The main functions are protection, sensation, regulation of body temperature, absorption, secretion and excretion, respiration, metabolism and so on [275, 276]. Skin tissue engineering is one of the most important components in skin repair. Ideal skin repair materials should have good biocompatibility, degradability, mechanical properties and so on and need to ensure the transport of gases and nutrients and prevent bacterial infections as well as the loss of body fluids and proteins [276, 277]. Electrospinning can prepare nanofibers with similar structure to extracellular matrix, which have the advantages of controllable pore size, large specific surface area and good permeability. It is conducive to cell attachment, proliferation and differentiation and is suitable for skin repair [278–281]. Table 2 and Fig. 14 show that gelatin, hyaluronic acid and cellulose are of high concern in skin repair. Gelatin, derived from partial hydrolysis of collagen, is one of the commonly used natural polymers. Its low molecular weight can make electrospinning more stable and the nanofibers have smoother and uniform morphology. It also has many binding sites that are beneficial to cell adhesion, proliferation and differentiation [282, 283]. However, its poor mechanical properties limit its application in skin repair and even tissue engineering. In recent years, researchers have combined gelatin with keratin [284], PCL [285], PHB [286], PU [287] and so on to improve the mechanical properties of the materials, which has a wider application in skin repair. For example, Baghersad et al. [288] prepared gelatin/aloe vera/PCL composite scaffolds using double-nozzle electrospinning technology, studied the effect of parameters on the performance and morphology of nanofibers. They found the materials had high antibacterial activity against both Gram-positive and Gram-negative bacteria, while improving the cell activity of NIH 3T3 fibroblast cells. In addition, Adeli-Sardou et al. [285] prepared nanofibers using coaxial electrospinning with PCL as shell polymer and gelatin–lawsone blend as core polymer, and found that they could significantly improve the biological activity of human gingiva fibroblast cells (HGF) and promote cell adhesion and proliferation. Then the in vitro gene expression of transforming growth factor β (TGF-B1), collagen (COL1) and epidermal growth factor (EGF) was monitored using RT-qPCR technique, and it was believed the material could effectively promote the regeneration of skin tissue. Shi et al. [289] prepared nanofiber membranes by mixing trimethoxysilylpropyl octadecyldimethyl ammonium chloride (QAS) with PCL/gelatin solution, and phase separation occurred. The nanofibers were mainly PCL inside and gelatin outside. The materials had broad-spectrum bactericidal effect and had no toxic side effects to L929 cells. They were suitable for use as skin antimicrobial materials.
Table 2.
Some progress of electrospinning in skin in recent years
| Material | Solvent | Flow rate (mL/h) | Distance (cm) | Voltage (kV) | Cells | Reference |
|---|---|---|---|---|---|---|
| Silk fibroin/PCL/PEO | Formic acid/TFE | 1/2.3 | 10–12 | 28 | NHDF | [290] |
| PCL/chitosan/PEO/aloe vera extract/keratin | Formic acid/acetic acid | 0.1 | 10–12 | 15/16 | L929 | [291] |
| PCL/gelatin/MgO particles | HFIP | 2 | 22 | 24 | hEnSCs | [292] |
| Chitosan/PVA/glyoxal/halloysite nanotubes | Acetic acid | 0.2 | 6 | 15 | Human normal fibroblast cells | [293] |
| Silk fibroin/PCL/PEO | Formic acid | 0.3 | 12 | 17.5 | Human BJ fibroblast cells | [294] |
| PCL/Althea Officinalis Extract/gelatin | Formic acid/acetic acid | 0.3 | 10 | 15–17 | L929 | [295] |
| PCL/N-(2-hydroxy)-propyl-3-trimethylammonium chitosan chloride | Formic acid/acetic acid | 0.9 | 15 | 25 | HDFn | [296] |
| PCL/chitosan/hyaluronic acid/PEO | Formic/acetone | 1.2/1/0.1 | 15/18/10 | 16/18–20/11 | Vero cells | [297] |
| PCL/zein/gum arabic | Formic acid/acetic acid | 0.2 | 15 | 18 | L929 | [298] |
| PEO/casein/silver nanoparticles | SDS | 0.3 | 15 | 20 | NIH-3T3 | [59] |
| Cellulose acetate/gelatin/nanohydroxyapatite | HFP | 0.8 | 13 | 18 | L929 | [299] |
| PCL/Maillard reaction products/glucose–arginine or fructosearginine | TFE | 2.5 | 14 | 25 | NHDF | [300] |
| PDLLA/PCL/Cu2S nanoparticles | DMF/THF | 0.9 | 15 | 8–12 | HDFs/HUVECs/murine B16F10 melanoma cells/human A375 melanoma | [301] |
| Chitosan/PEO/henna leaves extract | Acetic acid | 0.1–1.5 | 10–20 | 5–25 | NHF | [302] |
| Gelatin/PVA/keratin | Formic acid | 0.1 | 10 | 20 | L929 | [284] |
| PLA/hyperbranched polyglycerol/curcumin | Chloroform/methanol | 0.5 | 12 | 13–15 | 3T3 | [303] |
| Gelatin/sulfated hyaluronan/chondroitin sulfate | TFE | 0.75 | 13 | 20 | HaCaT/Hs27/hMSCs | [304] |
| PCL/chitosan | Chloroform/methanol/acetic acid | 0.18 | 10 | 22 | Human fibroblast/keratinocyte | [305] |
| PCL/cellulose acetate/chitosan/PEO | Chloroform/methanol/acetic acid | 2 | 15 | 20/25 | L929 | [306] |
| Gelatin/TEA/BDDGE | Acetic acid | 0.4 | 12 | 11 | hDNF | [307] |
| PCL/chitosan/PEO/aloe vera | TFE | 2.5/4 | 15/12 | 25/28 | NHDF | [308] |
| PLGA | DMF/THF | 0.8 | 15 | 28 | HDF/HaCat | [309] |
| Silk fibroin/PEO/NaCl | Water | 1.2 | 15 | 22 | HDF/HaCAT | [310] |
| Chitosan/bacterial cellulose/PEO/nanodiamond | Acetic acid | 0.3 | 10 | 20–22 | L929 | [311] |
| Cellulose acetate | Acetone/NaOH | 5 | 20 | 15 | L929 | [312] |
| PEUU/gelatin | HFIP | 1.5 | 15 | 12.5 | L929 | [313] |
| PHB/gelatin | HFIP | 1.5 | 12 | 18 | NIH 3T3/HaCaT | [286] |
| Silk fibroin/PEO/gold nanoparticle | Water | 0.8–2 | 13–23 | 12–19 | L929 | [314] |
| PLGA/collagen | HFIP | 1 | 17 | 28 | HDF/HaCat | [315] |
Figure 14.
Number of publications of some skin repair materials. All the data used are from web of science. The functions we used are analyze results and create citation report
Blood vessels
In recent years, cardiovascular disease has been the leading cause of death among non-communicable diseases. Atherosclerosis is one of the main causes of cardiovascular diseases [316]. The arteries are mainly composed of three layers: the tunica intima, the tunica media and the tunica adventitia. Among them, the tunica intima is the main part of vascular repair materials [317]. Electrospinning has been extensively used in vascular repair because of its controllable diameter and porosity of the prepared nanofibers [317–319]. Besides biocompatibility and mechanical properties, vascular repair materials also need to prevent thrombosis and vascular calcification [320, 321]. Some recent progress in electrospinning of blood vessels are listed in Table 3. It can be seen from Fig. 15 that the heat of gelatin in vascular repair has remained high, while PEG has been on the top in recent years and received extensive attention. PEG is a synthetic polymer with good biocompatibility. However, their biodegradability and mechanical strength are not ideal [322, 323]. But relative to blood vessels, PEG has excellent hemocompatibility, can inhibit hemolysis and has a certain anticoagulant capacity, can inhibit thrombosis, so it is suitable for use as a vascular repair material [324–327]. Yin et al. [328] used PEGylated chitosan and PLCL mixed solution as the inner layer and PLCL/PEG as the outer layer, and prepared nanofiber materials by coaxial electrospinning technology. They found the mechanical properties were good, the strength was higher than that of human blood vessels, the compliance was close to human blood vessels, and the degradation rate was appropriate. The patency of the material was studied by femoral artery replacement model, and the positive endothelial cells and smooth muscle cells grew well, with high levels of related protein expression and good calcification, and are suitable for vascular repair.
Table 3.
Some progress of electrospinning in blood vessels in recent years
| Material | Solvent | Flow rate (mL/h) | Distance (cm) | Voltage (kV) | Cells | Reference |
|---|---|---|---|---|---|---|
| PVA/alginate or chitosan | Acetic acid/DMSO | 4 | 8 | 8 | HMEC-1 | [329] |
| PCL/Pluronic P123/cilostazol | Chloroform/methanol | 2 | 15 | 10 | HUVECs/PASM | [330] |
| PU/gelatin/single-walled carbon nanotube | HFIP | 0.3 | 12 | 18 | myocardial myoblast/endothelial cells | [331] |
| PU/cedarwood oil/cobalt nitrate | DMF | 0.3 | 20 | 11 | HDF | [332] |
| PCL/PLCL | chloroform/ethanol/acetic acid | 1.5 | 20 | 15 | 3T3 | [333] |
| PCL/gelatin/cysteine-terminated REDV peptide | TFE/acetic acid | 0.4 | 15 | 10 | VEC/L929 | [334] |
| Acrylamide-terminated glycine–arginine–glycine–aspartic peptides modified PEUU | HFIP | 0.5–1 | 14 | 8.45–10 | HUVECs | [335] |
| Zinc oxide nanoparticles/poly(vinylidene fluoride-trifluoroethylene) | Acetone | 1.5 | 10 | 18 | hMSCs | [336] |
| PCU | DMF/THF | 1.5 | 13 | 16 | MEF | [337] |
| PCL/PU | THF/DMF/MEK | 1.1 | 20 | 15 | EA.hy926 | [338] |
| PCL/europium hydroxide nanorods | chloroform/methanol | 1 | 15 | 18 | HUVECs/EA.hy926 | [339] |
| PET | TFA/DCM | 1.5 | 15 | 18 | 3T3 | [340] |
| PU/PEG or PC | THF/DMF | 0.01 | 15 | 22–23 | HUVECs | [341] |
| PCL/gelatin | TFE/acetic acid | 1 | 20 | 20 | hMSCs | [342] |
| PU/PEG/cerium oxide nanoparticles/VEGF | THF/DMF | 0.8–1 | 10–15 | 8–10 | EPCs | [343] |
Figure 15.
Number of publications of some blood vessel repair materials. All the data used are from web of science. The functions we used are analyze results and create citation report
Nerves
Neural repair is an important component of tissue engineering, which is closely related to everyone's life [344–346]. The directionally aligned nanofibers prepared by electrospinning technology are conducive to the growth of nerve cells [347] and may have a good effect on cell synapses, which can be well applied in nerve tissue repair. Besides orientation, both morphology and surface coating of nanofibers affect nerve regeneration [348–350]. Some recent progress in electrospinning of nerves is listed in Table 4. Figure 16 shows that there are many kinds of nerve repair materials without obvious concentration, among which chitosan and cellulose have become the hot spots in recent years. Interestingly, these two materials are the two most abundant polymers in nature [351, 352]. Cellulose is a natural polymer produced by photosynthesis and is the most abundant polymer in nature. It can be converted into many derivatives, such as cellulose esters and cellulose ethers, which have good biocompatibility [353–355], and have better mechanical properties of other natural polymers [356], which are suitable for nerve conduit materials. Hou et al. [357] prepared PLGA outer conduit by electrospinning technology to bridge the sciatic nerve and then prepared oxidized bacterial cellulose-collagen sponge fillers by freeze-drying technology to facilitate nerve regeneration across the injured nerve gap. When combined into nerve conduit, it can increase the efficiency of gap bridge rebuilding and effectively promote the functional restoration of gastrocnemius muscle. Farzamfar et al. [358] mixed gelatin and cellulose for electrospinning and added gabapentin, a drug that can promote nerve remyelination, and found that it can improve the activity of Schwann cells and promote their proliferation. The regeneration effect of sciatic nerve was indirectly evaluated using rat gastrocnemius muscles and found the material can improve the regeneration ability of the injured site. However, the porosity of this material does not meet the requirements of ideal scaffolds.
Table 4.
Some progress of electrospinning in nerves in recent years
| Material | Solvent | Flow rate (mL/h) | Distance (cm) | Voltage (kV) | Cells | Reference |
|---|---|---|---|---|---|---|
| PLGA/Nimodipine | THF/DMF | 0.5–1 | 10–15 | 4.5–9 | SW10/RN33B/C8-D1A | [359] |
| PLLA | Chloroform | 2.08 | 5 | 15 | Dorsal root ganglia | [360] |
| PVA/poly(glycerol sebacate)/Kraft lignin nanoparticle | Water/DMF | 0.5 | 30 | 19 | PC12 | [361] |
| PDLLA/β-TCP/collagen | DCM/ethyl acetate | 0.8 | 15 | 3 | RSC96 | [362] |
| PCL | Methanol/chloroform | 5 | 20 | 12 | Schwann cells/dorsal root ganglions | [363] |
| PGS/PLLA | DMF/DCM | 0.5 | 15 | 25 | A59 | [364] |
| PLLA/NGF/VEGF | Dichloromethane/span-80/methanol/water | 0.8 | 12 | 16 | iPSCs-NCSCs | [365] |
| PLA/cellulose acetate | 1, 4-dioxane/acetone | 1 | 10 | 18 | Schwann cells | [366] |
| PCU | DMF/THF | 1 | 15 | 20 | DPSCs | [367] |
| PCL/collagen/nano bioglass | Acetic acid | 1 | 8 | 15–18 | hEnSCs | [368] |
| PVDF/gold colloidal nanoparticles | DMAC/acetone | 0.5 | 18 | 15 | PC-12 | [369] |
| Silk fibroin/PEO | Water | 1.2 | 14 | 12 | Schwann cells | [370] |
| PLLA/gum tragacanth | HFP | 1 | 14 | 13 | PC12 | [371] |
| PGS-poly(ethylene glycol) methyl ether methacrylate copolymers | HFP | 1 | 15 | 10 | PC12 | [372] |
| PS | THF/DMF | 0.2 | 4–5 | 5–14 | U373 | [373] |
| PELA-PPY | Trifluoroethanol | 1 | 15 | 20 | PC-12 | [374] |
Figure 16.
Number of publications of some nerve repair materials. All the data used are from web of science. The functions we used are analyze results and create citation report
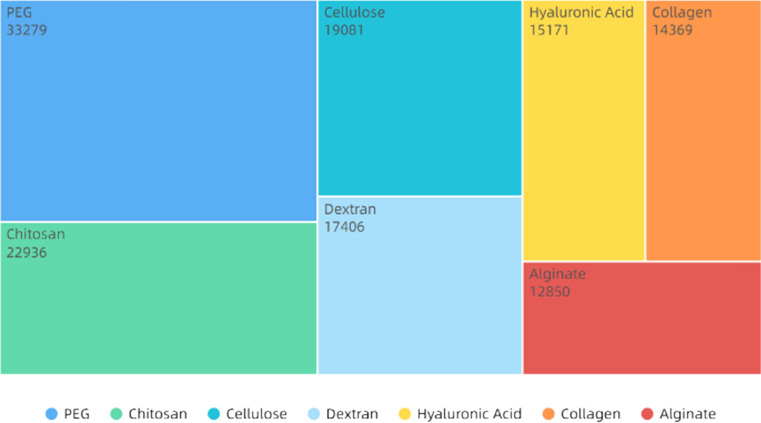
Drug delivery
Drug delivery refers to the use of carriers to transport drugs or biomolecules to certain areas, controllably release drugs and improve regional drug concentration [375–377]. Nanofibers have many advantages, such as large specific surface area, high drug loading, high porosity, high mechanical strength, easy surface modification and functionalization, and have been widely used to treat various diseases [378–380]. The diameter, porosity and drug binding mechanism of nanofibers all affect the drug release rate [102, 381–383]. There are two main methods, hybrid electrospinning and coaxial electrospinning, to combine drugs, as shown in Table 5 [384, 385]. As shown Fig. 17, PEG and chitosan are the main materials for drug delivery. Chitosan, a polysaccharide made by deacetylation of chitin, has been used in drug delivery in various forms such as films [386], microspheres [387], hydrogels [388] and nanofibers [389]. However, electrospinning of chitosan is difficult and needs to be compounded with other materials, such as PVA [390], pullulan [391] and xanthan gum [392]. Using electrospinning PVA/chitosan-loaded tetracycline hydrochloride (TCH), Alavarse et al. [393] found the drug could be evenly distributed and the material had good antibacterial property and cell compatibility. Balagangadharan et al. [394] developed a new approach by using ionic gelation technique to prepare sinapic acid/chitosan nanoparticles loaded on electrospun PCL nanofibers, which could promote the differentiation of osteoblasts, increase the expression levels of ALP, type Icollagen and osteocalcin. They further analyzed the effects of materials on the osteogenesis process and found that they actived various signaling pathways, such as TGF-β, BMP and FGF2. The calvarial bone defects in rats also showed the effect of materials to promote bone regeneration. In addition, Fazli et al. [395] mixed fumed silica nanoparticles with cefazolin and added them into chitosan–PEO solution for electrospinning to obtain nanofibrous mats. The results showed that drug molecules were coated by nanoparticles, and the nanofiber membranes had higher thermal stability, acid–base stability, tensile strength and better antimicrobial effect, and can promote skin healing.
Table 5.
Some progress of electrospinning in drug delivery in recent years
| Material | Solvent | Flow rate (mL/h) | Distance (cm) | Voltage (kV) | Type | Drug | Reference |
|---|---|---|---|---|---|---|---|
| Cellulose acetate/gliadin | HFIP/TFA | 0.1–0.5 | 20 | 15 | Coaxial | Ferulic acid | [396] |
| Hydroxypropyl-beta-cyclodextrin | Water | 0.5 | 15 | 15 | Blend | Ibuprofen | [397] |
| PLA/PVP/multiwall carbon nanotubes | DMF/THF | 0.3–1 | 22.5 | 15 | Blend | Tetracycline hydrochloride | [398] |
| PCL/PEO | DCM/DMF | 0.1–0.6 | 10–15 | 12/15 | Blend | Doxycycline | [399] |
| poly(glycolide-ε-caprolactone)/PLGA | DCM | 2 | 15 | 20 | Blend | papaverine hydrochloride | [400] |
| Poly(N-isopropylacrylamide)/Eudragit L100-55 | Ethanol | 1 | 20 | 16 | Blend | Ketoprofen | [401] |
| Carboxylated cellulose nanocrystals/cellulose acetate | acetone/DMAc | 1 | 15 | 25 | Blend | Tetracycline hydrochloride | [402] |
| PLA/PCL | DCM/DMF | 2 | 15 | 20/25 | Blend | Bovine serum albumin | [403] |
| PCL | Chloroform/methanol | 2 | 15 | 10 | Blend | Cilostazol | [404] |
| Silk fibroin/gelatin | Formic acid | 0.3 | 10 | 20 | Blend | Thyme essential oil/doxycycline monohydrate | [405] |
| Sodium carboxymethyl cellulose/methyl acrylate/PEO | DMF/chloroform/methanol | 3 | 18 | 18 | Coaxial | Tetracycline hydrochloride | [406] |
| PVA/chitosan/functionalized graphene oxide | Acetic acid | 0.6 | 14 | 22 | Blend | Curcumin | [407] |
| Ethyl hydroxy ethyl cellulose or hydrophobically modified ethyl hydroxy ethyl cellulose/PVA/citric acid | THF | 0.2 | 15 | 27 | Blend | Chlorhexidine Digluconate | [408] |
| PLA/PVA | DMF/chloroform | 1.5 | 9 | 15 | Blend | Dexamethasone | [409] |
| Sodium carboxymethyl cellulose/PEO/methyl acrylate | DMF/chloroform/methanol | 3/0.4 | 18 | 15 | Coaxial/Blend | Tetracycline hydrochloride | [406] |
| PCL-PEG-PCL | DCM/hexane | 6 | 12 | 18 | Blend | Magnetic iron oxide nanoparticles | [410] |
|
Poly(3- hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanocrystals |
DMF/chloroform/acetone | 1 | 18 | 15 | Blend | Tetracycline hydrochloride | [411] |
| PVA/montmorillonite | Acetic acid | 1–10 | 10–25 | 10–25 | Blend | Bovine serum albumin | [412] |
| Zein | Ethanol | 0.12 | 12 | 24 | Blend | Progesterone | [413] |
| Glutinous rice starch/PVA | Water | 0.3 | 15 | 18–20 | Blend | Chlorpheniramine maleate | [414] |
| PEG/PLGA/Au@Fe2O3 nanoparticles | DMF/DCM | 1 | 15 | 30 | Coaxial | Silibinin | [415] |
| PLLA | HFIP | 0.6 | 12 | 15 | Blend | Lovastatin | [416] |
| TPU | DMF | 1 | 20 | 13 | Blend | Naproxen | [417] |
| PCL/gum tragacanth | Acetic acid | 1 | 15 | 15 | Blend | Curcumin | [418] |
| Chitosan/phospholipids | TFA/DCM | 1.2 | 10 | 25 | Blend | Vitamin B12/curcumin/diclofenac | [419] |
| PCL | Chloroform/methanol | 0.5/0.6 | 9 | 12 | Coaxial | Ampicillin | [420] |
| PU/hydroxypropyl cellulose | DMF | 1 | 15 | 15 | Blend | Donepezil | [421] |
| PVA/collagen | Acetic acid | 0.4 | 15 | 18 | Blend | Salicylic acid | [422] |
| PVA/sodium alginate | Water | 0.3 | 10 | 14 | Blend | Moxifloxacin hydrochloride | [423] |
| Gelatin | Acetic acid | 0.3 | 10 | 12 | Blend | Piperine | [424] |
Figure 17.
Publications of drug delivery materials. All the data used are from web of science. The functions we used are analyze results and create citation report
Diagnosis
Medical diagnosis is of great importance in the early stage of disease. Medical diagnostic indicators are characterized by specificity and sensitivity. Biosensors are important in medical diagnosis. They have the advantages of low cost, fast detection speed, convenience and small side effects. The high specific surface area of nanofibers can improve the detection sensitivity. The biosensors prepared by electrospinning nanofibers mainly include electrochemical biosensors, organic gas sensors, fluorescent chemical sensors and immunosensors. As shown in Fig. 18, there are more researches on the application of electrospinning technology in medical diagnosis this year. Electrochemical sensors are mainly used for the molecular biological detection of glucose [425–427], proteins [428–430] and genes [431], which are generally composed of the working electrode, counter electrode and reference electrode [432]. Organic gas sensors mainly sense some specific volatile organic compounds, such as aromatic amines [433], acetaldehyde [434] and ammonia. They can be used for the diagnosis of lung cancer, kidney disease [435, 436] and so on. Cancer cells can also be diagnosed by detecting the oxygen concentration [437, 438]. Using tin dioxide and PEO as raw materials, Mehrabi et al. [439] developed a sensor for methanol and tetrahydrocannabinol (THC) by electrospinning, which can recognize smokers. Fluorescent chemical sensors are mainly used to detect amines in solution [440]. Immunosensors target antigens and antibodies [440]. In addition, Zhao et al. [441] developed a pulse measurement device that can be used to monitor heart rate using the piezoelectric characteristics of PLLA nanofibers along the fiber direction (d33). For cancer cells, targeting molecules include antibodies [442], DNA aptamers [443], E-selectins [444] and peptides, and modification methods include covalent coupling [445] and biotin–streptavidin-specific binding [446]. However, because targeting molecules mostly employed for only a single phenotype of cancer cells, the capture efficiency for multiple types of cancer cells is limited [446]. Pimentel et al. [447] developed a PLLA membrane-based microfluidic substrate for colorimetric detection of glucose, which has many advantages over conventional paper-based substrates. Xu et al. [448] developed a hyaluronic acid and PLGA nanofibers microfluidic chip that can selectively capture CD44 + carcinoma of various origins, especially for HeLa cancer cells and its effectiveness, which can be separated for further analysis.
Figure 18.
Number of publications and times cited on electrospinning in diagnosis. All the data used are from web of science. The functions we used are analyze results and create citation report
Energy
Nanomaterials are considered as good advanced materials in the energy field because of their good interfacial chemical reaction rate and easy conduction of charges because of nanosize effect [449–451]. In recent years, there have been many review articles on the application of electrospun nanofibers in energy, which have attracted much attention as shown in Fig. 19 [62, 452–456]. New devices in the energy field include solar cells, fuel cells, lithium-based batteries, sodium-ion batteries and supercapacitors. Lithium-ion batteries, lithium–sulfur batteries and lithium–oxygen batteries, collectively known as lithium-based batteries, are one of the most important energy storage devices. The advantages of nanofibers, such as electrochemical activity, mechanical strength, specific surface area and porosity, indicate that they can improve many shortages of traditional energy storage devices. Wu et al. [457] constructed a core–shell structure of silicon/honeycomb-like carbon framework composite fibers using coaxial electrospinning technology, which showed stable cycle performance and high specific capacity, and was a good anode material. Darbar et al. [458] compared the differences between oxalate decomposition method and electrospinning method for preparing magnesium cobalt oxide negative electrode material, and found the negative electrode material prepared by electrospinning method had better cycling performance. Fuel cells include proton exchange membrane fuel cells, methanol fuel cells, alkaline fuel cells, phosphoric acid fuel cells, solid oxide fuel cells and molten carbonate fuel cells. Park et al. [459] prepared perfluorosulfonic acid (PFSA)/polyphenylsulfone (PPSU) composite nanofiber membranes with high ion conductivity, which are good electrode materials for fuel cells. Supercapacitors can be divided into electrical double-layer supercapacitors and pseudocapacitors (Faradaic supercapacitors) [460]. Kabir et al. [461] studied Fe–N-C platinum group metal-free nanofiber electrodes and found the electric double-layer capacitance increased, the electrode proton transport reduced, and the bulk electrode gas transport properties improved significantly. Pant et al. [462] also studied the electrode materials of supercapacitors. They used titanium dioxide nanoparticles embedded in carbon nanofibers, and their performance was improved. Li et al. [463] prepared ordered mesoporous carbons fiber webs using low molecular resin as carbon precursors, F127 as structure directed agent and PVP as fiber forming agent. They can be directly used as electrode materials of supercapacitor without binders or conductive additives and has high specific capacitance. In addition, the characteristics of electrospun nanofibers make them widely used in the field of oil–water separation. The oil–water separation permeability of electrospinning nanofiber membrane is high, the separation efficiency is high and the energy consumption is low [464]. Obaid et al. [465] incorporated NaOH nanoparticles into PSF nanofibers and a layer of polyamide film on the surface of PSF nanofiber mats by interfacial polymerization of MPD and TMC. The results show that the contact angle of the modified membrane is obviously reduced, and the membrane has good hydrophilicity and permeability, high water flux and can be reused. Li et al. [466] prepared two smart membranes through solution-casting method and electrospinning technology, respectively, based on temperature-responsive copolymer PMMA-b-PNIPAAm and found that the electrospinning membrane has obvious advantages.
Figure 19.
Number of publications and times cited on electrospinning in energy. All the data used are from web of science. The functions we used are analyze results and create citation report
Catalysis
In recent years, there are more and more applications of electrospinning in catalysis, as shown in Fig. 20. Because the morphology, particle size and structure of the catalyst will affect its activity, the specific surface area, pore size, porosity and uniformity of electrospun nanofibers have great advantages. Some nanofibers can be directly used as catalysts after chemical treatment. In addition, catalyst immobilization on the surface of nanofibers is also widely used [467]. Nanofiber catalysts can be mainly divided into polymers, metals and oxides according to the materials. Nanofibers can immobilize enzymes [468], metals [469] and oxides [67, 470, 471]. Ternero-Hidalgo et al. [472] prepared V-Zr-O submicron fibers by electrospinning, which can be widely used in partial oxidation of alkanes. Lu et al. [473] prepared PAN/iron nitrate nanofibers and then converted them into carbon/iron nanofibers by one-pot carbonization process, which can be used as nanoadsorbents for Cr(VI). Xu et al. [474] prepared cellulose nanofibers and then deposited silver nanoparticles on the surface of the fibers, which was conducive to the reduction of p-nitrophenol. In addition, Hu et al. [475] used electrospun PVA/PEI nanofibers loaded with Au/Ag bimetallic nanoparticles to reduce 4-nitrophenol. Ma et al. [470] prepared PVP/[PW12 + Ti(OC4H9)4] composite nanofibers, which showed good desulfurization catalytic performance. NiO nanofibers were prepared by Hosseini et al. [476], which were effective for ethylene glycol oxidation after modification of carbon paste electrodes. Hosseini et al. [67] also prepared PVA/Cu(OAc)2-Ni(OAc)2 composite nanofibers to improve the electrocatalytic activity of the modified electrode. Interestingly, Chen et al. [477] prepared a kind of Fe3O4 nanofibers as peroxidase mimics with good catalytic activity for the oxidation of 3,3',5,5'-tetramethylbenzidine (TMB) by hydrogen peroxide.
Figure 20.
Number of publications and times cited on electrospinning in catalysis. All the data used are from web of science. The functions we used are analyze results and create citation report
Conclusion
Over the past years, because of the rapid development of nanotechnology, electrospinning as a nanomaterial processing technology has been widely used in various fields, especially in tissue engineering. The outbreak of COVID-19 brings opportunities for electrospinning nanofibers. Researchers have found that the nanofibers as active layers in face mask can protect people from the coronavirus disease (COVID-19)[478]. Some scholars summarized the advantages and prospects of the application of electrospun nanofibers in advanced face masks [479]. Electrospinning has the characteristics of wide source of raw materials, simple process, small fiber diameter, small pore size and high porosity, and can be used as the preferred method for researchers to prepare nanofibers. In addition, researchers can also control the morphology and diameter of nanofibers by adjusting parameters, including solution parameters, processing parameters and ambient parameters. However, the influence of some parameters on nanofibers is still controversial, such as the effect of applied voltage on fiber diameter. However, in general, electrospinning has made some progress and applications in biomedicine, energy and catalysis in recent years. Among these, researchers paid more attention to natural polymers, such as gelatin, chitosan and the composite of synthetic polymers, which can improve the biological and mechanical properties of materials at the same time. In addition, electrospinning has a set of physical theories to simulate the process of electrospinning and the influence of research parameters on nanofibers, not only in experiments, but also in theoretical research. With the development of computer technology, artificial intelligence and big data, it is believed the simulation method will be more applied in electrospinning technology to control and predict the morphology and performance for complex processes and parameters. However, although electrospinning has received more and more attention and research, this technology still has many shortcomings and development space, such as: (a) In principle, the influence of some parameters on electrospun nanofibers is not clear, such as the applied voltage, the optimal value of each parameter of each material is a complex but meaningful study. (b) As for equipment, the production speed of electrospinning is still a disadvantage to limit its large-scale production and application. Some researchers believed the productivity of nanofibers increases with the number of needles, but in fact, the space occupied by the nozzles will also increase, the cost will be higher, and there will be electric field interference between the nozzles. (c) The choice of solvent also made researchers distressed, because most of the organic solvents are biotoxic, although in the later process can be removed by various means, but there are still concerns for consumers. (d) Although electrospinning is widely used in many fields, it has not been applied to all aspects of life, such as magnetic properties of electrospun nanofibers, gradient scaffold materials and semiconductor-related applications. In this paper, the parameters, models, simulation and applications of electrospinning technology are discussed and summarized. It is hoped that these information can help researchers develop ideas and make progress in scientific research.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2017YFC1103800), the Major special projects of technological innovation of Hubei Province (No. 2017ACA168) and the Foshan Xianhu Laboratory of the Advanced EnergyScience and Technology Guangdong Laboratory (No. XHT2020-010).
Declarations
Conflict of interest
The authors have declared no conflicts of interest for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Law M, Goldberger J, Yang P. Semiconductor nanowires and nanotubes. Annu Rev Mater Res. 2004;34:83–122. [Google Scholar]
- 2.Bae C, Yoo H, Kim S, Lee K, Kim J, Sung MM, Shin H. Template-directed synthesis of oxide nanotubes: fabrication, characterization, and applications. Chem Mater. 2008;20:756–767. [Google Scholar]
- 3.Comini E, Baratto C, Faglia G, Ferroni M, Vomiero A, Sberveglieri G. Quasi-one dimensional metal oxide semiconductors: preparation, characterization and application as chemical sensors. Prog Mater Sci. 2009;54:1–67. [Google Scholar]
- 4.Rao C, Deepak FL, Gundiah G, Govindaraj A. Inorganic nanowires. Prog Solid State Chem. 2003;31:5–147. [Google Scholar]
- 5.Ge M, Cao C, Huang J, Li S, Chen Z, Zhang K-Q, Al-Deyab S, Lai Y. A review of one-dimensional TiO 2 nanostructured materials for environmental and energy applications. J Mater Chem A. 2016;4:6772–6801. [Google Scholar]
- 6.Ge M, Li Q, Cao C, Huang J, Li S, Zhang S, Chen Z, Zhang K, Al-Deyab SS, Lai Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv Sci. 2017;4:1600152. doi: 10.1002/advs.201600152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong S, Cheng W. One-dimensional nanomaterials for soft electronics. Adv Electron Mater. 2017;3:1600314. [Google Scholar]
- 8.Karnati P, Akbar S, Morris PA. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing: a review. Sens Actuat B: Chem. 2019;295:127–143. [Google Scholar]
- 9.Sayin S, Ozdemir E, Acar E, Ince GO (2019) Multifunctional one-dimensional polymeric nanostructures for drug delivery and biosensor applications. Nanotechnology, 30, 412001 [DOI] [PubMed]
- 10.Lu H, Wang J, Wang T, Zhong J, Bao Y, Hao H. Recent progress on nanostructures for drug delivery applications. J Nanomat. 2016 doi: 10.1155/2016/5762431. [DOI] [Google Scholar]
- 11.Wu T, Ding M, Shi C, Qiao Y, Wang P, Qiao R, Wang X, Zhong J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin Chem Lett. 2020;31:617–625. [Google Scholar]
- 12.Rajgarhia S, Jana SC. Influence of secondary stretching on diameter and morphology of bicomponent polymer nanofibers produced by gas jet fiber process. Polymer. 2017;123:219–231. [Google Scholar]
- 13.Ellison CJ, Phatak A, Giles DW, Macosko CW, Bates FS. Melt blown nanofibers: fiber diameter distributions and onset of fiber breakup. Polymer. 2007;48:3306–3316. [Google Scholar]
- 14.Wang P, Wang Y, Tong L. Functionalized polymer nanofibers: a versatile platform for manipulating light at the nanoscale. Light Sci Appl. 2013 doi: 10.1038/lsa.2013.58. [DOI] [Google Scholar]
- 15.Ma B, Qin A, Li X, He C. High tenacity regenerated chitosan fibers prepared by using the binary ionic liquid solvent (Gly· HCl)-[Bmim] Cl. Carbohyd Polym. 2013;97:300–305. doi: 10.1016/j.carbpol.2013.04.080. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Li P, Zhang C, Yu Y, Liu H, Zhang S, Jia X, Yang X, Xue Z, Ryu S. Inhomogeneous toughening of carbon fiber/epoxy composite using electrospun polysulfone nanofibrous membranes by in situ phase separation. Comp Sci Technol. 2008;68:987–994. [Google Scholar]
- 17.Yin H, Strunz F, Yan Z, Lu J, Brochhausen C, Kiderlen S, Clausen-Schaumann H, Wang X, Gomes ME, Alt V (2020) Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. Biomaterials, 236, 119802 [DOI] [PubMed]
- 18.Li Z, Mei S, Dong Y, She F, Kong L. High efficiency fabrication of chitosan composite nanofibers with uniform morphology via centrifugal spinning. Polymers. 2019;11:1550. doi: 10.3390/polym11101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Qiao R, Su J, Yan J, Xie Z, Qiao Y, Wang X, Zhong J. Recent advances of electrospun nanofibrous membranes in the development of chemosensors for heavy metal detection. Small. 2017;13:1604293. doi: 10.1002/smll.201604293. [DOI] [PubMed] [Google Scholar]
- 20.Xu M, Wang M, Xu H, Xue H, Pang H (2016) Electrospun‐technology‐derived high‐performance electrochemical energy storage devices. Chem Asian J, 11, 2967–2995 [DOI] [PubMed]
- 21.Cavaliere S. Electrospinning for advanced energy and environmental applications. USA: CRC Press; 2015. [Google Scholar]
- 22.Cavaliere S, Subianto S, Savych I, Jones DJ, Rozière J. Electrospinning: designed architectures for energy conversion and storage devices. Energy Environ Sci. 2011;4:4761–4785. [Google Scholar]
- 23.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:R89. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishna S (2005) An introduction to electrospinning and nanofibers; World Scientific
- 25.Sharma J, Lizu M, Stewart M, Zygula K, Lu Y, Chauhan R, Yan X, Guo Z, Wujcik EK, Wei S. Multifunctional nanofibers towards active biomedical therapeutics. Polymers. 2015;7:186–219. [Google Scholar]
- 26.Strutt JW, Rayleigh L. On the instability of jets. Proc London Math Soc. 1878 doi: 10.1112/plms/s1-10.1.4. [DOI] [Google Scholar]
- 27.Rayleigh L. On the capillary phenomena of jets. Proceed Royal Soc. 1879;29:71–97. [Google Scholar]
- 28.Rayleigh LXX. On the equilibrium of liquid conducting masses charged with electricity. Philosophic Mag Ser. 1882;14:184–186. [Google Scholar]
- 29.Strutt JWI. The influence of electricity on colliding water drops. Proc R Soc Lond. 1879;28:405–409. [Google Scholar]
- 30.Cooley JF. Improved methods of and apparatus for electrically separating the relatively volatile liquid component from the component of relatively fixed substances of composite fluids. United Kingdom Patent. 1900;6385:19. [Google Scholar]
- 31.Morton W (1902) Method of dispersing fluids US Patent Specification 705691
- 32.Zeleny J. Electrical discharges from pointed conductors. Phys Rev. 1920;16:102. [Google Scholar]
- 33.Zeleny J. The discharge of electricity from pointed conductors differing in size. Phys Rev. 1907;25:305. [Google Scholar]
- 34.Zeleny J. The influence of humidity upon the electrical discharge from points in air. Phys Rev. 1908;26:448. [Google Scholar]
- 35.Zeleny J. The discharge of electricity from pointed conductors. Phys Rev. 1908;26:129. [Google Scholar]
- 36.Zeleny J. The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces. Phys Rev. 1914;3:69. [Google Scholar]
- 37.Zeleny J (1915) On the condition of instability of electrified drops, with applications to electrical discharge from liquid points. In Proceedings of the Proc. Camb. Phil. Soc., pp. 71–83
- 38.Anton F (1934) Process and apparatus for preparing artificial threads
- 39.Anton F (1937) Production of artificial fibers
- 40.Anton F (1938) Artificial fiber construction
- 41.Anton F (1938) Method and Apparatus for the Production of Fibers
- 42.Anton F (1939) Method of producing artificial fibers
- 43.Anton F (1939) Method and apparatus for the production of artificial fibers
- 44.Anton F (1943) Production of artificial fibers from fiber forming liquids
- 45.Norton CL (1936) Method of and apparatus for producing fibrous or filamentary material
- 46.Filatov Y, Budyka A, Kirichenko V. Electrospinning of micro-and nanofibers: fundamentals in separation and filtration processes. J Eng Fibers Fabrics. 2007;3:488. [Google Scholar]
- 47.Taylor GI. Disintegration of water drops in an electric field. Proc R Soc Lond. 1964;280:383–397. [Google Scholar]
- 48.Taylor, G.I.J.P.o.t.R.S.o.L.S.A.M. (1966) Sciences, P. The force exerted by an electric field on a long cylindrical conductor. Proceedings of the Royal Society of London, 291, 145–158
- 49.Taylor GI. Electrically driven jets. Proc R Soc Lond. 1969;313:453–475. [Google Scholar]
- 50.Simons HL (1966) Process and apparatus for producing patterned non-woven fabrics
- 51.Baumgarten PKJJOC. Electrostatic spinning of acrylic microfibers. J Coll Int Sci. 1971;36:71–79. [Google Scholar]
- 52.Annis D, Bornat A, Edwards R, Higham A, Loveday B, Wilson J. An elastomeric vascular prosthesis. ASAIO J. 1978;24:209–214. [PubMed] [Google Scholar]
- 53.Larrondo L, John Manley R. Electrostatic fiber spinning from polymer melts. I. experimental observations on fiber formation and properties. J Polym Sci Polym Phys Ed. 1981 doi: 10.1002/pol.1981.180190601. [DOI] [Google Scholar]
- 54.Larrondo L, John Manley R. Electrostatic fiber spinning from polymer melts II Examination of the flow field in an electrically driven jet. J Polym Sci Polym Phys Ed. 1981 doi: 10.1002/pol.1981.180190602. [DOI] [Google Scholar]
- 55.Larrondo L, John Manley R. Electrostatic fiber spinning from polymer melts Electrostatic deformation of a pendant drop of polymer melt. J Poly Sci Poly Phys Ed. 1981 doi: 10.1002/pol.1981.180190603. [DOI] [Google Scholar]
- 56.Fisher A, De Cossart L, How T, Annis D. Long term in-vivo performance of an electrostatically-spun small bore arterial prosthesis: the contribution of mechanical compliance and anti-platelet therapy. Life Supp Syst J Eur Soc Artif Org. 1985;3:462. [PubMed] [Google Scholar]
- 57.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216. [Google Scholar]
- 58.Jirsak O, Sanetrnik F, Lukas D, Kotek V, Martinova L, Chaloupek J (2009) Method of nanofibres production from a polymer solution using electrostatic spinning and a device for carrying out the method
- 59.Selvaraj S, Thangam R, Fathima NN. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int J Biol Macromol. 2018;120:1674–1681. doi: 10.1016/j.ijbiomac.2018.09.177. [DOI] [PubMed] [Google Scholar]
- 60.Ngadiman NHA, Yusof NM, Idris A, Misran E, Kurniawan D. Development of highly porous biodegradable γ-Fe2O3/polyvinyl alcohol nanofiber mats using electrospinning process for biomedical application. Mater Sci Eng C. 2017;70:520–534. doi: 10.1016/j.msec.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Hemamalini T, Dev VRG. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int J Biol Macromol. 2018;106:712–718. doi: 10.1016/j.ijbiomac.2017.08.079. [DOI] [PubMed] [Google Scholar]
- 62.Liu Q, Zhu J, Zhang L, Qiu Y. Recent advances in energy materials by electrospinning. Renew Sust Energy Rev. 2018;81:1825–1858. [Google Scholar]
- 63.Zhang Y, Liu X, Yu J, Fan M, Ji X, Sun B, Hu P (2019) Optimizing the dielectric energy storage performance in P (VDF-HFP) nanocomposite by modulating the diameter of PZT nanofibers prepared via electrospinning. Comp Sci Technol, 184, 107838
- 64.Chen W, Weng W. Ultrafine lauric–myristic acid eutectic/poly (meta-phenylene isophthalamide) form-stable phase change fibers for thermal energy storage by electrospinning. Appl Energy. 2016;173:168–176. [Google Scholar]
- 65.Zhang T, Li H, Yang Z, Cao F, Li L, Chen H, Liu H, Xiong K, Wu J, Hong Z. Electrospun YMn2O5 nanofibers: a highly catalytic activity for NO oxidation. Appl Catal B. 2019;247:133–141. [Google Scholar]
- 66.Guo Z, Ye W, Fang X, Wan J, Ye Y, Dong Y, Cao D, Yan D. Amorphous cobalt–iron hydroxides as high-efficiency oxygen-evolution catalysts based on a facile electrospinning process. Inorganic Chemistry Frontiers. 2019;6:687–693. [Google Scholar]
- 67.Hosseini SR, Ghasemi S, Kamali-Rousta M. Preparation of CuO/NiO composite nanofibers by electrospinning and their application for electro-catalytic oxidation of hydrazine. J Power Sources. 2017;343:467–476. [Google Scholar]
- 68.Huang X, Wang R, Jiao T, Zou G, Zhan F, Yin J, Zhang L, Zhou J, Peng Q. Facile preparation of hierarchical AgNP-loaded MXene/Fe3O4/polymer nanocomposites by electrospinning with enhanced catalytic performance for wastewater treatment. ACS Omega. 2019;4:1897–1906. doi: 10.1021/acsomega.8b03615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ge L, Yin J, Yan D, Hong W, Jiao T. Construction of nanocrystalline cellulose-based composite fiber films with excellent porosity performances via an electrospinning strategy. ACS Omega. 2021;6:4958–4967. doi: 10.1021/acsomega.0c06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C, Sun S, Zhang L, Yin J, Jiao T, Zhang L, Xu Y, Zhou J, Peng Q. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloids Surf A Physicochem Eng Asp. 2019;561:283–291. [Google Scholar]
- 71.Liu Y, Hou C, Jiao T, Song J, Zhang X, Xing R, Zhou J, Zhang L, Peng QJN. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials. 2018;8:35. doi: 10.3390/nano8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X, Jiao T, Liu Q, Zhang L, Zhou J, Li B, Peng Q (2019) Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2. 5 capture. Sci China Mater, 62, 423–436
- 73.Cleeton C, Keirouz A, Chen X, Radacsi N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater Sci Eng. 2019;5:4183–4205. doi: 10.1021/acsbiomaterials.9b00853. [DOI] [PubMed] [Google Scholar]
- 74.Ding C, Fang H, Duan G, Zou Y, Chen S, Hou H. Investigating the draw ratio and velocity of an electrically charged liquid jet during electrospinning. RSC Adv. 2019;9:13608–13613. doi: 10.1039/c9ra02024a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thenmozhi S, Dharmaraj N, Kadirvelu K, Kim HY. Electrospun nanofibers: new generation materials for advanced applications. Mater Sci Eng B. 2017;217:36–48. [Google Scholar]
- 76.Lagaron J, Solouk A, Castro S, Echegoyen Y (2017) Biomedical applications of electrospinning, innovations, and products. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Elsevier; pp. 57-72
- 77.Park JC (2011) Electric spinning apparatus for mass-production of nano-fiber
- 78.Li Y, Liu J, de Bruyn JR, Wan W. Optimization of the electrospinning process for core–shell fiber preparation. J Biomater Tissue Eng. 2014;4:973–980. [Google Scholar]
- 79.Yang C, Jia Z, Xu Z, Wang K, Guan Z, Wang L (2009) Comparisons of fibers properties between vertical and horizontal type electrospinning systems. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, pp. 204–207
- 80.Williams GR, Raimi-Abraham BT, Luo CJ (2018) Electrospinning fundamentals. In Nanofibres in Drug Delivery; UCL Press; pp. 24–59
- 81.Aliheidari N, Aliahmad N, Agarwal M, Dalir H. Electrospun nanofibers for label-free sensor applications. Sensors. 2019;19:3587. doi: 10.3390/s19163587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhattacharjee P, Rutledge G (2011) Electrospinning and polymer nanofibers: process fundamentals. In Comprehensive Biomaterials; Elsevier; pp. 497-512
- 83.Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Comp Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 84.Panda P, Ramakrishna S. Electrospinning of alumina nanofibers using different precursors. J Mater Sci. 2007;42:2189–2193. doi: 10.1007/s10853-007-1581-2. [DOI] [Google Scholar]
- 85.Feng Y, Xiong T, Jiang S, Liu S, Hou H. Mechanical properties and chemical resistance of electrospun polyterafluoroethylene fibres. RSC Adv. 2016;6:24250–24256. [Google Scholar]
- 86.Zhu M, Hua D, Pan H, Wang F, Manshian B, Soenen SJ, Xiong R, Huang C. Green electrospun and crosslinked poly (vinyl alcohol)/poly (acrylic acid) composite membranes for antibacterial effective air filtration. J Coll Interf Sci. 2018;511:411–423. doi: 10.1016/j.jcis.2017.09.101. [DOI] [PubMed] [Google Scholar]
- 87.Duan G, Fang H, Huang C, Jiang S, Hou H. Microstructures and mechanical properties of aligned electrospun carbon nanofibers from binary composites of polyacrylonitrile and polyamic acid. J Mater Sci. 2018;53:15096–15106. doi: 10.1007/s10853-018-2700-y. [DOI] [Google Scholar]
- 88.Zhou S, Zhou G, Jiang S, Fan P, Hou H. Flexible and refractory tantalum carbide-carbon electrospun nanofibers with high modulus and electric conductivity. Mater Lett. 2017;200:97–100. [Google Scholar]
- 89.Zhang P, Zhao X, Zhang X, Lai Y, Wang X, Li J, Wei G, Su Z. Electrospun doping of carbon nanotubes and platinum nanoparticles into the β-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS appl Mater Interf. 2014;6:7563–7571. doi: 10.1021/am500908v. [DOI] [PubMed] [Google Scholar]
- 90.Bortolassi ACC, Nagarajan S, de Araújo Lima B, Guerra VG, Aguiar ML, Huon V, Soussan L, Cornu D, Miele P, Bechelany M (2019) Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mater Sci Eng C, 102, 718–729 [DOI] [PubMed]
- 91.Lin Q, Li Y, Yang M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens Actuat B Chem. 2012;161:967–972. [Google Scholar]
- 92.Li Y, Lim CT, Kotaki M. Study on structural and mechanical properties of porous PLA nanofibers electrospun by channel-based electrospinning system. Polymer. 2015;56:572–580. [Google Scholar]
- 93.Wang X, Wu T, Wang W, Huang C, Jin X. Regenerated collagen fibers with grooved surface texture: physicochemical characterization and cytocompatibility. Mater Sci Eng C. 2016;58:750–756. doi: 10.1016/j.msec.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 94.Katoch A, Sun G-J, Choi S-W, Byun J-H, Kim SS. Competitive influence of grain size and crystallinity on gas sensing performances of ZnO nanofibers. Sens Actuat B Chem. 2013;185:411–416. [Google Scholar]
- 95.Fan H, Zhang T, Xu X, Lv N. Fabrication of N-type Fe2O3 and P-type LaFeO3 nanobelts by electrospinning and determination of gas-sensing properties. Sens Actuat B Chem. 2011;153:83–88. [Google Scholar]
- 96.Zhong J, Zhang H, Yan J, Gong X. Effect of nanofiber orientation of electrospun nanofibrous scaffolds on cell growth and elastin expression of muscle cells. Colloids Surf B Biointerf. 2015;136:772–778. doi: 10.1016/j.colsurfb.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 97.Jiang S, Duan G, Zussman E, Greiner A, Agarwal S. Highly flexible and tough concentric triaxial polystyrene fibers. ACS Appl Mater Interf. 2014;6:5918–5923. doi: 10.1021/am500837s. [DOI] [PubMed] [Google Scholar]
- 98.Burger C, Hsiao BS, Chu B. Nanofibrous materials and their applications. Annu Rev Mater Res. 2006;36:333–368. [Google Scholar]
- 99.Teo W-E, Ramakrishna S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos Sci Technol. 2009;69:1804–1817. [Google Scholar]
- 100.Thompson C, Chase GG, Yarin A, Reneker D. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer. 2007;48:6913–6922. [Google Scholar]
- 101.Bhat G. Advances in polymeric nanofiber manufacturing technologies. J Nanomater Mol Nanotechnol. 2016;5:2324. [Google Scholar]
- 102.Pillay V, Dott C, Choonara YE, Tyagi C, Tomar L, Kumar P, du Toit LC, Ndesendo VM. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater. 2013 doi: 10.1155/2013/789289. [DOI] [Google Scholar]
- 103.Haider S, Al-Zeghayer Y, Ali FAA, Haider A, Mahmood A, Al-Masry WA, Imran M, Aijaz MO. Highly aligned narrow diameter chitosan electrospun nanofibers. J Polym Res. 2013;20:105. [Google Scholar]
- 104.Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–4592. [Google Scholar]
- 105.Kohse S, Grabow N, Schmitz K-P, Eickner T. Electrospinning of polyimide nanofibres–effects of working parameters on morphology. Current Direct Biomed Eng. 2017;3:687–690. [Google Scholar]
- 106.Topuz F, Satilmis B, Uyar T (2019) Electrospinning of uniform nanofibers of polymers of intrinsic microporosity (PIM-1): The influence of solution conductivity and relative humidity. Polymer, 178, 121610
- 107.Kriegel C, Arrechi A, Kit K, McClements D, Weiss J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit Rev Food Sci Nutrit. 2008;48:775–797. doi: 10.1080/10408390802241325. [DOI] [PubMed] [Google Scholar]
- 108.Topuz F, Uyar T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater Sci Eng C. 2017;80:371–378. doi: 10.1016/j.msec.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Biber E, Gündüz G, Mavis B, Colak U. Effects of electrospinning process parameters on nanofibers obtained from Nylon 6 and poly (ethylene-n-butyl acrylate-maleic anhydride) elastomer blends using Johnson S B statistical distribution function. Appl Phys A. 2010;99:477–487. [Google Scholar]
- 110.Tam N, Oguz S, Aydogdu A, Sumnu G, Sahin S. Influence of solution properties and pH on the fabrication of electrospun lentil flour/HPMC blend nanofibers. Food Res Int. 2017;102:616–624. doi: 10.1016/j.foodres.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 111.Wei L, Qiu Q, Wang R, Qin X. Influence of the processing parameters on needleless electrospinning from double ring slits spinneret using response surface methodology. J Appl Polym Sci. 2018;135:46407. [Google Scholar]
- 112.Nie H, He A, Zheng J, Xu S, Li J, Han CC. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromol. 2008;9:1362–1365. doi: 10.1021/bm701349j. [DOI] [PubMed] [Google Scholar]
- 113.Mirtič J, Balažic H, Zupančič Š, Kristl J. Effect of solution composition variables on electrospun alginate nanofibers: response surface analysis. Polymers. 2019;11:692. doi: 10.3390/polym11040692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiep NT, Hai ND, Toi VV. Fabrication of core-shell PLGA-chitosan microparticles using electrospinning: effects of polymer concentration. Int J Polym Sci. 2017 doi: 10.1155/2017/9580209. [DOI] [Google Scholar]
- 115.Luzio A, Canesi EV, Bertarelli C, Caironi M. Electrospun polymer fibers for electronic applications. Materials. 2014;7:906–947. doi: 10.3390/ma7020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. J Electrostat. 1995;35:151–160. [Google Scholar]
- 117.Aydogdu A, Sumnu G, Sahin S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohyd Polym. 2018;181:234–246. doi: 10.1016/j.carbpol.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 118.Liu G, Gu Z, Hong Y, Cheng L, Li C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J Control Release. 2017;252:95–107. doi: 10.1016/j.jconrel.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 119.Amariei N, Manea L, Bertea A, Bertea A, Popa A (2017) The influence of polymer solution on the properties of electrospun 3D nanostructures. In Proceedings of the IOP Conference Series: Materials Science and Engineering; p. 012092.
- 120.Wan Y-Q, He J-H, Wu Y, Yu J-Y. Vibrorheological effect on electrospun polyacrylonitrile (PAN) nanofibers. Mater Lett. 2006;60:3296–3300. [Google Scholar]
- 121.Li X, Qiang J, Wan Y, Wang H, Gao W. The effect of sonic vibration on electrospun fiber mats. J Low Freq Noise Vib. 2019;38:1246–1251. [Google Scholar]
- 122.Yu G, Li TS, Xu M, Andersson M, Li B, Tang H, Parbey J, Shao J. Fabrication of nickel-YSZ cermet nanofibers via electrospinning. J Alloys Comp. 2017;693:1214–1219. [Google Scholar]
- 123.Zander NE. Hierarchically structured electrospun fibers. Polym. 2013;5:19–44. [Google Scholar]
- 124.Haghi A, Akbari M. Trends in electrospinning of natural nanofibers. Phys Status Solidi. 2007;204:1830–1834. [Google Scholar]
- 125.Koski A, Yim K, Shivkumar S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater Lett. 2004;58:493–497. [Google Scholar]
- 126.Nonato RC, Morales AR, Vieira AF, Nista SV, Mei LH, Bonse BC. Solution parameters in the manufacture of ceramic ZnO nanofibers made by electrospinning. Appl Phys A. 2016;122:244. [Google Scholar]
- 127.Pearson DS, Pincus PA, Heffner GW, Dahman SJ. Effect of molecular weight and orientation on the conductivity of conjugated polymers. Macromolecules. 1993;26:1570–1575. [Google Scholar]
- 128.Van Vooren A, Kim JS, Cornil J. Intrachain versus interchain electron transport in Poly (fluorene-alt-benzothiadiazole): a Quantum-Chemical Insight. ChemPhysChem. 2008;9:989–993. doi: 10.1002/cphc.200700847. [DOI] [PubMed] [Google Scholar]
- 129.Choi S, Lee HM, Kim HS. Effect of molecular weight on humidity-sensitive characteristics of electrospun polyethylene oxide. Sens Actuat A Phys. 2019;294:194–202. [Google Scholar]
- 130.Bordi F, Cametti C, Di Biasio A. Electrical conductivity behavior of poly (ethylene oxide) in aqueous electrolyte solutions. J Phys Chem. 1988;92:4772–4777. [Google Scholar]
- 131.Choi S, Kim HR, Jeong YK, Bang JY, Kim HS. Mechanism of electrospinning for poly (amic acid)/polyacrylonitrile fiber fabrication. J Macromol Sci Part B. 2018;57:222–230. [Google Scholar]
- 132.Abdullah NA, Ahmad Sekak K, Ahmad MR (2016) Effect of molecular weight on morphological structure of electrospun PVA nanofibre. In proceedings of the advanced materials research; pp. 203–208
- 133.Kuzelova Kostakova E, Meszaros L, Maskova G, Blazkova L, Turcsan T, Lukas D. Crystallinity of electrospun and centrifugal spun polycaprolactone fibers: a comparative study. J Nanomater. 2017 doi: 10.1155/2017/8952390. [DOI] [Google Scholar]
- 134.Hayati I, Bailey A, Tadros TF. Investigations into the mechanisms of electrohydrodynamic spraying of liquids: I. effect of electric field and the environment on pendant drops and factors affecting the formation of stable jets and atomization. J Colloid Interf Sci. 1987;117:205–221. [Google Scholar]
- 135.Lasprilla-Botero J, Álvarez-Láinez M, Lagaron J. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater Today Commun. 2018;14:1–9. [Google Scholar]
- 136.Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–4412. [Google Scholar]
- 137.Mit-uppatham C, Nithitanakul M, Supaphol P. Ultrafine electrospun polyamide-6 fibers: effect of solution conditions on morphology and average fiber diameter. Macromol Chem Phys. 2004;205:2327–2338. [Google Scholar]
- 138.Neamnark A, Rujiravanit R, Supaphol P. Electrospinning of hexanoyl chitosan. Carbohyd Polym. 2006;66:298–305. [Google Scholar]
- 139.Choi JS, Lee SW, Jeong L, Bae S-H, Min BC, Youk JH, Park WH. Effect of organosoluble salts on the nanofibrous structure of electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) Int J Biol Macromol. 2004;34:249–256. doi: 10.1016/j.ijbiomac.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 140.Lin T, Wang H, Wang H, Wang X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology. 2004;15:1375. [Google Scholar]
- 141.Seo JM, Arumugam GK, Khan S, Heiden PA. Comparison of the effects of an ionic liquid and triethylbenzylammonium chloride on the properties of electrospun fibers, 1–poly (lactic acid) Macromol Mater Eng. 2009;294:35–44. [Google Scholar]
- 142.Jarusuwannapoom T, Hongrojjanawiwat W, Jitjaicham S, Wannatong L, Nithitanakul M, Pattamaprom C, Koombhongse P, Rangkupan R, Supaphol P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polymer J. 2005;41:409–421. [Google Scholar]
- 143.Ray SS, Chen S-S, Nguyen NC, Hsu H-T, Nguyen HT, Chang C-T. Poly (vinyl alcohol) incorporated with surfactant based electrospun nanofibrous layer onto polypropylene mat for improved desalination by using membrane distillation. Desalination. 2017;414:18–27. [Google Scholar]
- 144.Rodrigues BV, Silva AS, Melo GF, Vasconscellos LM, Marciano FR, Lobo AO. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater Sci Eng C. 2016;59:782–791. doi: 10.1016/j.msec.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 145.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 146.Cadafalch Gazquez G, Smulders V, Veldhuis SA, Wieringa P, Moroni L, Boukamp BA, Ten Elshof JE. Influence of solution properties and process parameters on the formation and morphology of YSZ and NiO ceramic nanofibers by electrospinning. Nanomaterials. 2017 doi: 10.3390/nano7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Vrieze S, Van Camp T, Nelvig A, Hagström B, Westbroek P, De Clerck K. The effect of temperature and humidity on electrospinning. J Mater Sci. 2009;44:1357–1362. doi: 10.1007/s10853-008-3010-6. [DOI] [Google Scholar]
- 148.Yao T, Chen H, Samal P, Giselbrecht S, Baker MB, Moroni, L (2019) Self-assembly of electrospun nanofibers into gradient honeycomb structures. Mater Des, 168, 107614.
- 149.Zhao J, Sun Z, Shao Z, Xu L. Effect of surface-active agent on morphology and properties of electrospun PVA nanofibres. Fibers Polymers. 2016;17:896–901. [Google Scholar]
- 150.Arbeiter D, Kohse S, Eickner T, Grabow N, Schmitz K-P. Influence of additives on physico-chemical properties of electrospun poly (L-lactide) Current Direct Biomed Eng. 2018;4:493–496. [Google Scholar]
- 151.Son WK, Youk JH, Lee TS, Park WH (2004) The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly (ethylene oxide) fibers. Polymer, 45, 2959–2966
- 152.Vega-Lugo AC, Lim LT. Effects of poly (ethylene oxide) and pH on the electrospinning of whey protein isolate. J Polym Sci, Part B: Polym Phys. 2012;50:1188–1197. [Google Scholar]
- 153.Xu D, Sanchez-Romaguera V, Barbosa S, Travis W, de Wit J, Swan P, Yeates SG. Inkjet printing of polymer solutions and the role of chain entanglement. J Mater Chem. 2007;17:4902–4907. [Google Scholar]
- 154.Shenoy SL, Bates WD, Frisch HL, Wnek GE. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer–polymer interaction limit. Polymer. 2005;46:3372–3384. [Google Scholar]
- 155.Lu C, Chen P, Li J, Zhang Y (2006) Computer simulation of electrospinning. Part I. Effect of solvent in electrospinning. Polymer, 47, 915–921
- 156.Hansen CM. The three dimensional solubility parameter. Copenhagen: Danish Technical; 1967. p. 14. [Google Scholar]
- 157.Hansen CM. The universality of the solubility parameter. Ind Eng Chem Prod Res Develop. 1969;8:2–11. [Google Scholar]
- 158.Reneker D, Yarin A, Zussman E, Xu H. Electrospinning of nanofibers from polymer solutions and melts. Adv Appl Mech. 2007;41:43–346. [Google Scholar]
- 159.Luo C, Nangrejo M, Edirisinghe M. A novel method of selecting solvents for polymer electrospinning. Polymer. 2010;51:1654–1662. [Google Scholar]
- 160.Stanescu I, Achenie LE. A theoretical study of solvent effects on Kolbe-Schmitt reaction kinetics. Chem Eng Sci. 2006;61:6199–6212. [Google Scholar]
- 161.Luo C, Stride E, Edirisinghe M. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules. 2012;45:4669–4680. [Google Scholar]
- 162.Mahalingam S, Raimi-Abraham BT, Craig DQ, Edirisinghe M. Solubility–spinnability map and model for the preparation of fibres of polyethylene (terephthalate) using gyration and pressure. Chem Eng J. 2015;280:344–353. [Google Scholar]
- 163.Sill TJ, Von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 164.Larrondo, L.; St. John Manley, R.J.J.o.P.S.P.P.E. Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. 1981, 19, 909–920.
- 165.Manandhar S, Vidhate S, D’Souza N. Water soluble levan polysaccharide biopolymer electrospun fibers. Carbohyd Polym. 2009;78:794–798. [Google Scholar]
- 166.Sencadas V, Correia DM, Areias A, Botelho G, Fonseca A, Neves I, Ribelles JG, Mendez SL. Determination of the parameters affecting electrospun chitosan fiber size distribution and morphology. Carbohyd Polym. 2012;87:1295–1301. [Google Scholar]
- 167.Zhou H, Shi Z, Wan X, Fang H, Yu D-G, Chen X, Liu P. The relationships between process parameters and polymeric nanofibers fabricated using a modified coaxial electrospinning. Nanomaterials. 2019;9:843. doi: 10.3390/nano9060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pezeshki-Modaress M, Mirzadeh H, Zandi M. Gelatin–GAG electrospun nanofibrous scaffold for skin tissue engineering: fabrication and modeling of process parameters. Mater Sci Eng C. 2015;48:704–712. doi: 10.1016/j.msec.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 169.Shao P, Niu B, Chen H, Sun P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int J Biol Macromol. 2018;107:1908–1914. doi: 10.1016/j.ijbiomac.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 170.Zhang C, Yuan X, Wu L, Han Y, Sheng J. Study on morphology of electrospun poly (vinyl alcohol) mats. Eur Polymer J. 2005;41:423–432. [Google Scholar]
- 171.Righi TM, Almeida RS, d'Ávila MA (2012) Electrospinning of gelatin/PEO blends: Influence of process parameters in the nanofiber properties. In Proceedings of the Macromolecular Symposia; pp. 230–234
- 172.Katsogiannis KAG, Vladisavljević GT, Georgiadou S. Porous electrospun polycaprolactone fibers: effect of process parameters. J Polym Sci, Part B: Polym Phys. 2016;54:1878–1888. [Google Scholar]
- 173.Kim J-H, Lee J-H, Kim J-Y, Kim SS. Synthesis of aligned TiO2 nanofibers using electrospinning. Appl Sci. 2018;8:309. [Google Scholar]
- 174.Yördem O, Papila M, Menceloğlu YZ. Effects of electrospinning parameters on polyacrylonitrile nanofiber diameter: An investigation by response surface methodology. Materials design. 2008;29:34–44. [Google Scholar]
- 175.Tan S-H, Inai R, Kotaki M, Ramakrishna S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 2005;46:6128–6134. [Google Scholar]
- 176.Drosou C, Krokida M, Biliaderis CG. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocolloids. 2018;77:726–735. [Google Scholar]
- 177.Megelski S, Stephens JS, Chase DB, Rabolt JF. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 2002;35:8456–8466. [Google Scholar]
- 178.Yuan X, Zhang Y, Dong C, Sheng J. Morphology of ultrafine polysulfone fibers prepared by electrospinning. Polym Int. 2004;53:1704–1710. [Google Scholar]
- 179.Xue J, Wu T, Dai Y, Xia Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem Rev. 2019;119:5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hossain MF, Gong RH, Rigout M. Optimization of the process variables for electrospinning of poly (ethylene oxide)-loaded hydroxypropyl-β-cyclodextrin nanofibres. J Text Inst. 2016;107:1–11. [Google Scholar]
- 181.Ki CS, Baek DH, Gang KD, Lee KH, Um IC, Park YH. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer. 2005;46:5094–5102. [Google Scholar]
- 182.Cruz KFN, Botelho EC, Cristovan FH, Guerrini LM. Electrospinning TPU/poly o-phenetidine (POEA) fibers: influence of POEA on fiber morphology. Polym Bull. 2017;74:2905–2919. [Google Scholar]
- 183.Matabola K, Moutloali R. The influence of electrospinning parameters on the morphology and diameter of poly (vinyledene fluoride) nanofibers-effect of sodium chloride. J Mater Sci. 2013;48:5475–5482. doi: 10.1007/s10853-013-7341-6. [DOI] [Google Scholar]
- 184.Pham QP, Sharma U, Mikos AG. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromol. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 185.Jaeger R, Bergshoef MM, Batlle CMI, Schönherr H, Julius Vancso G (1998) Electrospinning of ultra‐thin polymer fibers. In Proceedings of the Macromolecular symposia, pp. 141–150
- 186.Parajuli D, Koomsap P, Parkhi AA, Supaphol P. Experimental investigation on process parameters of near-field deposition of electrospinning-based rapid prototyping. Virtual Phys Prototyp. 2016;11:193–207. [Google Scholar]
- 187.Vinogradov G, Malkin AY. Temperature-independent viscosity characteristics of polymer systems. J Polym Sci Part A Gen Pap. 1964;2:2357–2372. [Google Scholar]
- 188.Yu D-G, Gao L-D, White K, Branford-White C, Lu W-Y, Zhu L-M. Multicomponent amorphous nanofibers electrospun from hot aqueous solutions of a poorly soluble drug. Pharm Res. 2010;27:2466–2477. doi: 10.1007/s11095-010-0239-y. [DOI] [PubMed] [Google Scholar]
- 189.Yang G-Z, Li H-P, Yang J-H, Wan J, Yu D-G. Influence of working temperature on the formation of electrospun polymer nanofibers. Nanoscale Res Lett. 2017;12:55. doi: 10.1186/s11671-016-1824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cai Y, Gevelber M. The effect of relative humidity and evaporation rate on electrospinning: fiber diameter and measurement for control implications. J Mater Sci. 2013;48:7812–7826. doi: 10.1007/s10853-013-7544-x. [DOI] [Google Scholar]
- 191.Pelipenko J, Kristl J, Janković B, Baumgartner S, Kocbek P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int J Pharm. 2013;456:125–134. doi: 10.1016/j.ijpharm.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 192.De Vrieze S, De Schoenmaker B, Ceylan Ö, Depuydt J, Van Landuyt L, Rahier H, Van Assche G, De Clerck K. Morphologic study of steady state electrospun polyamide 6 nanofibres. J Appl Polym Sci. 2011;119:2984–2990. [Google Scholar]
- 193.Bak SY, Yoon GJ, Lee SW, Kim HW. Effect of humidity and benign solvent composition on electrospinning of collagen nanofibrous sheets. Mater Lett. 2016;181:136–139. [Google Scholar]
- 194.Alnaqbi MA, Greish Y, Mohsin M, Elumalai E, Al Blooshi A. Morphological variations of micro-nanofibrous sorbents prepared by electrospinning and their effects on the sorption of crude oil. J Environ Chem Eng. 2016;4:1850–1861. [Google Scholar]
- 195.Nezarati RM, Eifert MB, Cosgriff-Hernandez E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C Methods. 2013;19:810–819. doi: 10.1089/ten.tec.2012.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Putti M, Simonet M, Solberg R, Peters GW. Electrospinning poly (ε-caprolactone) under controlled environmental conditions: influence on fiber morphology and orientation. Polymer. 2015;63:189–195. [Google Scholar]
- 197.Chen H, Huang X, Zhang M, Damanik F, Baker MB, Leferink A, Yuan H, Truckenmüller R, van Blitterswijk C, Moroni L. Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017;59:82–93. doi: 10.1016/j.actbio.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 198.Huang C, Thomas NL. Fabricating porous poly (lactic acid) fibres via electrospinning. Eur Polymer J. 2018;99:464–476. [Google Scholar]
- 199.Ding B, Li C, Miyauchi Y, Kuwaki O, Shiratori S. Formation of novel 2D polymer nanowebs via electrospinning. Nanotechnology. 2006;17:3685. [Google Scholar]
- 200.Wang C, Hsu C-H, Lin J-H. Scaling laws in electrospinning of polystyrene solutions. Macromolecules. 2006;39:7662–7672. [Google Scholar]
- 201.Wang C, Hsu C-H, Hwang I-H. Scaling laws and internal structure for characterizing electrospun poly [(R)-3-hydroxybutyrate] fibers. Polymer. 2008;49:4188–4195. [Google Scholar]
- 202.Yousefi S, Tang C, Tafreshi HV, Pourdeyhimi B. Empirical model to simulate morphology of electrospun polycaprolactone mats. J Appl Polym Sci. 2019;136:48242. [Google Scholar]
- 203.Ziabari M, Mottaghitalab V, Haghi AK. A new approach for optimization of electrospun nanofiber formation process. Korean J Chem Eng. 2010;27:340–354. [Google Scholar]
- 204.Hendricks JR CD, Carson RS, Hogan JJ, Schneider JM (1964) Photomicrography of electrically sprayed heavy particles. Aiaa J, 2, 733–737
- 205.Yarin AL, Koombhongse S, Reneker DH. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J Appl Phys. 2001;90:4836–4846. [Google Scholar]
- 206.Spivak A, Dzenis Y, Reneker D. Model of steady state jet in the electrospinning process. Mech Res Commun. 2000;27:37–42. [Google Scholar]
- 207.Spivak A, Dzenis YA. Asymptotic decay of radius of a weakly conductive viscous jet in an external electric field. Appl Phys Lett. 1998;73:3067–3069. [Google Scholar]
- 208.Hohman MM, Shin M, Rutledge G, Brenner MP. Electrospinning and electrically forced jets II applications. Phys Fluids. 2001;13:2221–2236. [Google Scholar]
- 209.Hohman MM, Shin M, Rutledge G, Brenner MP. Electrospinning and electrically forced jets I Stability theory. Phys Fluids. 2001;13:2201–2220. [Google Scholar]
- 210.Feng J. The stretching of an electrified non-Newtonian jet: a model for electrospinning. Phys Fluids. 2002;14:3912–3926. [Google Scholar]
- 211.Shin Y, Hohman M, Brenner M, Rutledge G. Experimental characterization of electrospinning: the electrically forced jet and instabilities. Polymer. 2001;42:09955–09967. [Google Scholar]
- 212.Baji A, Mai YW, Wong SC, Abtahi M, Chen PJCS. Electrospinning of polymer nanofibers: effects on oriented morphology, structures and tensile properties. Compos Sci Technol. 2010;70:703–718. [Google Scholar]
- 213.Huebner A, Chu H. Instability and breakup of charged liquid jets. J Fluid Mech. 1971;49:361–372. [Google Scholar]
- 214.Reneker DH, Yarin AL, Fong H, Koombhongse S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys. 2000;87:4531–4547. [Google Scholar]
- 215.Yarin AL, Koombhongse S, Reneker DH. Bending instability in electrospinning of nanofibers. J Appl Phys. 2001;89:3018–3026. [Google Scholar]
- 216.Shin Y, Hohman M, Brenner MP, Rutledge G. Electrospinning: a whipping fluid jet generates submicron polymer fibers. Appl Phys Lett. 2001;78:1149–1151. [Google Scholar]
- 217.Fridrikh, S.V.; Jian, H.Y.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Physical review letters2003, 90, 144502. [DOI] [PubMed]
- 218.Theron S, Zussman E, Yarin A. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer. 2004;45:2017–2030. [Google Scholar]
- 219.Carroll CP, Joo YL (2009) Axisymmetric instabilities in electrospinning of highly conducting, viscoelastic polymer solutions. Phys Fluids, 21, 103101
- 220.Yousefi S, Tafreshi HV (2020) Modeling electrospun fibrous structures with embedded spacer particles: application to aerosol filtration. Sep Purif Technol, 235, 116184
- 221.Allen MP, Tildesley DJ (2017) Computer simulation of liquids; Oxford university press
- 222.Jirsak J, Moucka F, Nezbeda I. Insight into electrospinning via molecular simulations. Indus Eng Chem Res. 2014;53:8257–8264. [Google Scholar]
- 223.Danwanichakul P, Danwanichakul D. Two-dimensional simulation of electrospun nanofibrous structures connection of experimental and simulated results. J Chem. 2014 doi: 10.1155/2014/479139. [DOI] [Google Scholar]
- 224.Sarmadi M, Shamloo A, Mohseni M (2017) Utilization of molecular dynamics simulation coupled with experimental assays to optimize biocompatibility of an electrospun PCL/PVA scaffold. PloS one, 12, e0169451 [DOI] [PMC free article] [PubMed]
- 225.Vao-soongnern V. Monte Carlo simulation of molecular and structural properties of mono-and bi-dispersed poly (ethylene oxide) nanofibers. J Polym Res. 2019;26:147. [Google Scholar]
- 226.Steffens L, Morás AM, Arantes PR, Masterson K, Cao Z, Nugent M, Moura DJ (2020) Electrospun PVA-Dacarbazine nanofibers as a novel nano brain-implant for treatment of glioblastoma: in silico and in vitro characterization. Europ J Pharmaceut Sci, 143, 105183 [DOI] [PubMed]
- 227.Karra S (2010) Modeling electrospinning process and a numerical scheme using Lattice Boltzmann method to simulate viscoelastic fluid flows. Texas A & M University
- 228.Yin Y, Pan Z, Xiong J. A tensile constitutive relationship and a finite element model of electrospun nanofibrous mats. Nanomaterials. 2018;8:29. doi: 10.3390/nano8010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sarkar K, Ghalia MB, Wu Z, Bose SC. A neural network model for the numerical prediction of the diameter of electro-spun polyethylene oxide nanofibers. J Mater Process Technol. 2009;209:3156–3165. [Google Scholar]
- 230.Cui W, Zhou Y, Chang J (2010) Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater, 11, 014108 [DOI] [PMC free article] [PubMed]
- 231.Walmsley GG, McArdle A, Tevlin R, Momeni A, Atashroo D, Hu MS, Feroze AH, Wong VW, Lorenz PH, Longaker MT. Nanotechnology in bone tissue engineering. Nanomed Nanotechnol Biol Med. 2015 doi: 10.1016/j.nano.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat Protoc. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 233.Wang Y, Wang X, Shi J, Zhu R, Zhang J, Zhang Z, Ma D, Hou Y, Lin F, Yang J. A biomimetic silk fibroin/sodium alginate composite scaffold for soft tissue engineering. Sci Rep. 2016;6:39477. doi: 10.1038/srep39477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603–5621. [Google Scholar]
- 235.Unnithan AR, Barakat NA, Pichiah PT, Gnanasekaran G, Nirmala R, Cha Y-S, Jung C-H, El-Newehy M, Kim HY. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohyd Polym. 2012;90:1786–1793. doi: 10.1016/j.carbpol.2012.07.071. [DOI] [PubMed] [Google Scholar]
- 236.Zou B, Liu Y, Luo X, Chen F, Guo X, Li X. Electrospun fibrous scaffolds with continuous gradations in mineral contents and biological cues for manipulating cellular behaviors. Acta Biomater. 2012;8:1576–1585. doi: 10.1016/j.actbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 237.Supaphol P, Suwantong O, Sangsanoh P, Srinivasan S, Jayakumar R, Nair SV. Electrospinning of biocompatible polymers and their potentials in biomedical applications. In Biomedical applications of polymeric nanofibers: Springer; 2011. pp. 213–239. [Google Scholar]
- 238.Gunn J, Zhang M. Polyblend nanofibers for biomedical applications: perspectives and challenges. Trends Biotechnol. 2010;28:189–197. doi: 10.1016/j.tibtech.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 239.Du C, Cui F, Zhang W, Feng Q, Zhu X, De Groot K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res. 2000;50:518–527. doi: 10.1002/(sici)1097-4636(20000615)50:4<518::aid-jbm7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 240.Chiara G, Letizia F, Lorenzo F, Edoardo S, Diego S, Stefano S, Eriberto B, Barbara Z. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int J Mol Sci. 2012;13:737–757. doi: 10.3390/ijms13010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 242.Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 243.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science: an introduction to materials in medicine; Elsevier
- 244.Pan Y, Xiong D. Friction properties of nano-hydroxyapatite reinforced poly (vinyl alcohol) gel composites as an articular cartilage. Wear. 2009;266:699–703. [Google Scholar]
- 245.Pandi K, Viswanathan N. In situ precipitation of nano-hydroxyapatite in gelatin polymatrix towards specific fluoride sorption. Int J Biol Macromol. 2015;74:351–359. doi: 10.1016/j.ijbiomac.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 246.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395:81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 247.Salifu AA, Lekakou C, Labeed FH. Electrospun oriented gelatin-hydroxyapatite fiber scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 2017;105:1911–1926. doi: 10.1002/jbm.a.36058. [DOI] [PubMed] [Google Scholar]
- 248.Yang X, Li Y, He W, Huang Q, Zhang R, Feng Q. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J Biomed Mater Res Part A. 2018;106:2863–2870. doi: 10.1002/jbm.a.36475. [DOI] [PubMed] [Google Scholar]
- 249.Niu B, Li B, Gu Y, Shen X, Liu Y, Chen L. In vitro evaluation of electrospun silk fibroin/nano-hydroxyapatite/BMP-2 scaffolds for bone regeneration. J Biomater Sci Polym Ed. 2017;28:257–270. doi: 10.1080/09205063.2016.1262163. [DOI] [PubMed] [Google Scholar]
- 250.Surmenev RA, Shkarina S, Syromotina DS, Melnik EV, Shkarin R, Selezneva II, Ermakov AM, Ivlev SI, Cecilia A, Weinhardt V. Characterization of biomimetic silicate-and strontium-containing hydroxyapatite microparticles embedded in biodegradable electrospun polycaprolactone scaffolds for bone regeneration. Eur Polymer J. 2019;113:67–77. [Google Scholar]
- 251.Nezafati N, Faridi-Majidi R, Pazouki M, Hesaraki S. Synthesis and characterization of a novel freeze-dried silanated chitosan bone tissue engineering scaffold reinforced with electrospun hydroxyapatite nanofiber. Polym Int. 2019;68:1420–1429. [Google Scholar]
- 252.Chen P, Liu L, Pan J, Mei J, Li C, Zheng Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater Sci Eng C. 2019;97:325–335. doi: 10.1016/j.msec.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 253.Ko E, Lee JS, Kim H, Yang SY, Yang D, Yang K, Lee J, Shin J, Yang HS, Ryu W. Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl Mater Interf. 2017;10:7614–7625. doi: 10.1021/acsami.7b03328. [DOI] [PubMed] [Google Scholar]
- 254.Shao W, He J, Sang F, Ding B, Chen L, Cui S, Li K, Han Q, Tan W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater Sci Eng C. 2016;58:342–351. doi: 10.1016/j.msec.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 255.Govindasamy K, Dahlan NA, Janarthanan P, Goh KL, Chai SP, Pasbakhsh P (2020) Electrospun chitosan/polyethylene-oxide (PEO)/halloysites (HAL) membranes for bone regeneration applications. Appl Clay Sci, 190, 105601
- 256.Naderi P, Zarei M, Karbasi S, Salehi H (2020) Evaluation of the effects of keratin on physical, mechanical and biological properties of poly (3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Europ Polym J, 124, 109502
- 257.Castro VO, Fredel MC, Aragones Á, de Oliveira Barra GM, Cesca K, Merlini C (2020) Electrospun fibrous membranes of poly (lactic-co-glycolic acid) with β-tricalcium phosphate for guided bone regeneration application. Polym Test, 106489
- 258.Toloue EB, Karbasi S, Salehi H, Rafienia M. Potential of an electrospun composite scaffold of poly (3-hydroxybutyrate)-chitosan/alumina nanowires in bone tissue engineering applications. Mater Sci Eng C. 2019;99:1075–1091. doi: 10.1016/j.msec.2019.02.062. [DOI] [PubMed] [Google Scholar]
- 259.Perumal G, Sivakumar PM, Nandkumar AM, Doble M (2020) Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater Sci Eng C, 109, 110527 [DOI] [PubMed]
- 260.Chakraborty PK, Adhikari J, Saha P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int J Biol Macromol. 2019;122:644–652. doi: 10.1016/j.ijbiomac.2018.10.216. [DOI] [PubMed] [Google Scholar]
- 261.Cardenas Turner J, Collins G, Blaber EA, Almeida EA, Arinzeh TL. Evaluating the cytocompatibility and differentiation of bone progenitors on electrospun zein scaffolds. J Tissue Eng Regener Med. 2020;14:173–185. doi: 10.1002/term.2984. [DOI] [PubMed] [Google Scholar]
- 262.Yin L, Zhu Y, He M, Chang Y, Xu F, Lai HC. Preparation and characteristics of electrospinning PTH-Fc/PLCL/SF membranes for bioengineering applications. J Biomed Mater Res Part A. 2020;108:157–165. doi: 10.1002/jbm.a.36801. [DOI] [PubMed] [Google Scholar]
- 263.Tohidlou H, Shafiei SS, Abbasi S, Asadi-Eydivand M, Fathi-Roudsari M. Amine-functionalized single-walled carbon nanotube/polycaprolactone electrospun scaffold for bone tissue engineering: in vitro study. Fibers Polym. 2019;20:1869–1882. [Google Scholar]
- 264.Ding Y, Li W, Correia A, Yang Y, Zheng K, Liu D, Schubert D, Boccaccini A, Santos H, Roether J. Electrospun phb/pcl/sol-gel derived silica hybrid scaffolds with drug releasing function for bone tissue engineering applications. ACS Appl Mater Interf. 2018;10:14540–14548. doi: 10.1021/acsami.8b02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sharifi F, Atyabi SM, Norouzian D, Zandi M, Irani S, Bakhshi H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018;115:243–248. doi: 10.1016/j.ijbiomac.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 266.Hidalgo Pitaluga L, Trevelin Souza M, Dutra Zanotto E, Santocildes Romero ME, Hatton PV. Electrospun F18 bioactive Glass/PCL—Poly (ε-caprolactone)—Membrane for guided tissue regeneration. Materials. 2018 doi: 10.3390/ma11030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Santos D, Silva DM, Gomes PS, Fernandes MH, Santos JD, Sencadas V. Multifunctional PLLA-ceramic fiber membranes for bone regeneration applications. J Colloid Interf Sci. 2017;504:101–110. doi: 10.1016/j.jcis.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 268.Santos D, Correia CO, Silva DM, Gomes PS, Fernandes MH, Santos JD, Sencadas V. Incorporation of glass-reinforced hydroxyapatite microparticles into poly (lactic acid) electrospun fibre mats for biomedical applications. Mater Sci Eng C. 2017;75:1184–1190. doi: 10.1016/j.msec.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 269.Ren K, Wang Y, Sun T, Yue W, Zhang H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater Sci Eng C. 2017;78:324–332. doi: 10.1016/j.msec.2017.04.084. [DOI] [PubMed] [Google Scholar]
- 270.Ao C, Niu Y, Zhang X, He X, Zhang W, Lu C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int J Biol Macromol. 2017;97:568–573. doi: 10.1016/j.ijbiomac.2016.12.091. [DOI] [PubMed] [Google Scholar]
- 271.Gorodzha SN, Muslimov AR, Syromotina DS, Timin AS, Tcvetkov NY, Lepik KV, Petrova AV, Surmeneva MA, Gorin DA, Sukhorukov GB. A comparison study between electrospun polycaprolactone and piezoelectric poly (3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloids Surf B Biointerfaces. 2017;160:48–59. doi: 10.1016/j.colsurfb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 272.Shrestha BK, Mousa HM, Tiwari AP, Ko SW, Park CH, Kim CS. Development of polyamide-6, 6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohyd Polym. 2016;148:107–114. doi: 10.1016/j.carbpol.2016.03.094. [DOI] [PubMed] [Google Scholar]
- 273.Chen Z, Song Y, Zhang J, Liu W, Cui J, Li H, Chen F. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater Sci Eng C. 2017;72:341–351. doi: 10.1016/j.msec.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 274.Liao N, Joshi MK, Tiwari AP, Park C-H, Kim CS. Fabrication, characterization and biomedical application of two-nozzle electrospun polycaprolactone/zein-calcium lactate composite nonwoven mat. J Mech Behav Biomed Mater. 2016;60:312–323. doi: 10.1016/j.jmbbm.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 275.Hay R, Augustin M, Griffiths C, Sterry W. The global challenge for skin health. Br J Dermatol. 2015;6:1469–1472. doi: 10.1111/bjd.13854. [DOI] [PubMed] [Google Scholar]
- 276.Vatankhah E, Prabhakaran MP, Jin G, Mobarakeh LG, Ramakrishna S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J Biomater Appl. 2014;28:909–921. doi: 10.1177/0885328213486527. [DOI] [PubMed] [Google Scholar]
- 277.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interf. 2007;4:413–437. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21:77–95. [Google Scholar]
- 279.Jun I, Han H-S, Edwards JR, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19:745. doi: 10.3390/ijms19030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Smith L, Ma P. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerf. 2004;39:125–131. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 281.Dias J, Granja P, Bártolo P. Advances in electrospun skin substitutes. Prog Mater Sci. 2016;84:314–334. [Google Scholar]
- 282.Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly (caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 283.Panzavolta S, Gioffrè M, Focarete ML, Gualandi C, Foroni L, Bigi A. Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 2011;7:1702–1709. doi: 10.1016/j.actbio.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 284.Yao C-H, Lee C-Y, Huang C-H, Chen Y-S, Chen K-Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater Sci Eng C. 2017;79:533–540. doi: 10.1016/j.msec.2017.05.076. [DOI] [PubMed] [Google Scholar]
- 285.Adeli-Sardou M, Yaghoobi MM, Torkzadeh-Mahani M, Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int J Biol Macromol. 2019;124:478–491. doi: 10.1016/j.ijbiomac.2018.11.237. [DOI] [PubMed] [Google Scholar]
- 286.Ramanathan G, Singaravelu S, Raja M, Nagiah N, Padmapriya P, Ruban K, Kaveri K, Natarajan TS, Sivagnanam UT, Perumal PT. Fabrication and characterization of a collagen coated electrospun poly (3-hydroxybutyric acid)–gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv. 2016;6:7914–7922. [Google Scholar]
- 287.Sheikholeslam M, Wright ME, Cheng N, Oh HH, Wang Y, Datu AK, Santerre JP, Amini-Nik S, Jeschke MG. Electrospun Polyurethane-Gelatin composite: a new tissue-engineered scaffold for application in skin regeneration and repair of complex wounds. ACS Biomater Sci Eng. 2019;6:505–516. doi: 10.1021/acsbiomaterials.9b00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Baghersad S, Bahrami SH, Mohammadi MR, Mojtahedi MRM, Milan PB. Development of biodegradable electrospun gelatin/aloe-vera/poly (ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater Sci Eng C. 2018;93:367–379. doi: 10.1016/j.msec.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 289.Shi R, Geng H, Gong M, Ye J, Wu C, Hu X, Zhang L. Long-acting and broad-spectrum antimicrobial electrospun poly (ε-caprolactone)/gelatin micro/nanofibers for wound dressing. J Colloid Interf Sci. 2018;509:275–284. doi: 10.1016/j.jcis.2017.08.092. [DOI] [PubMed] [Google Scholar]
- 290.Miguel SP, Simões D, Moreira AF, Sequeira RS, Correia IJ. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int J Biol Macromol. 2019;121:524–535. doi: 10.1016/j.ijbiomac.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 291.Zahedi E, Esmaeili A, Eslahi N, Shokrgozar MA, Simchi A. Fabrication and characterization of Core-Shell electrospun fibrous Mats containing medicinal herbs for wound healing and skin tissue engineering. Mar Drugs. 2019;17:27. doi: 10.3390/md17010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ababzadeh S, Farzin A, Goodarzi A, Karimi R, Sagharjoghi Farahani M, Eslami Farsani M, Gharibzad K, Zahiri M, Ai J. High porous electrospun poly (ε-caprolactone)/gelatin/MgO scaffolds preseeded with endometrial stem cells promote tissue regeneration in full-thickness skin wounds: An in vivo study. J Biomed Mater Res Part B Appl Biomater. 2020 doi: 10.1002/jbm.b.34626. [DOI] [PubMed] [Google Scholar]
- 293.Koosha M, Raoufi M, Moravvej H. One-pot reactive electrospinning of chitosan/PVA hydrogel nanofibers reinforced by halloysite nanotubes with enhanced fibroblast cell attachment for skin tissue regeneration. Colloids Surf B Biointerf. 2019;179:270–279. doi: 10.1016/j.colsurfb.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 294.Nazeer MA, Yilgor E, Yilgor I. Electrospun polycaprolactone/silk fibroin nanofibrous bioactive scaffolds for tissue engineering applications. Polymer. 2019;168:86–94. [Google Scholar]
- 295.Ghaseminezhad K, Zare M, Lashkarara S, Yousefzadeh M, Aghazadeh Mohandesi J. Fabrication of althea officinalis loaded electrospun nanofibrous scaffold for potential application of skin tissue engineering. J Appl Polym Sci. 2020;137:48587. [Google Scholar]
- 296.dos Santos DM, Leite IS, de Lacerda Bukzem A, de Oliveira Santos RP, Frollini E, Inada NM, Campana-Filho SP. Nanostructured electrospun nonwovens of poly (ε-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohyd Polym. 2018;186:110–121. doi: 10.1016/j.carbpol.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 297.Chanda A, Adhikari J, Ghosh A, Chowdhury SR, Thomas S, Datta P, Saha P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int J Biol Macromol. 2018;116:774–785. doi: 10.1016/j.ijbiomac.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 298.Rad ZP, Mokhtari J, Abbasi M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater Sci Eng C. 2018;93:356–366. doi: 10.1016/j.msec.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 299.Samadian H, Salehi M, Farzamfar S, Vaez A, Ehterami A, Sahrapeyma H, Goodarzi A, Ghorbani S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif Cells Nanomed Biotechnol. 2018;46:964–974. doi: 10.1080/21691401.2018.1439842. [DOI] [PubMed] [Google Scholar]
- 300.Simões D, Miguel SP, Correia IJ. Biofunctionalization of electrospun poly (caprolactone) fibers with Maillard reaction products for wound dressing applications. React Funct Polym. 2018;131:191–202. [Google Scholar]
- 301.Wang X, Lv F, Li T, Han Y, Yi Z, Liu M, Chang J, Wu C. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11:11337–11349. doi: 10.1021/acsnano.7b05858. [DOI] [PubMed] [Google Scholar]
- 302.Yousefi I, Pakravan M, Rahimi H, Bahador A, Farshadzadeh Z, Haririan I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater Sci Eng C. 2017;75:433–444. doi: 10.1016/j.msec.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 303.Perumal G, Pappuru S, Chakraborty D, Nandkumar AM, Chand DK, Doble M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater Sci Eng C. 2017;76:1196–1204. doi: 10.1016/j.msec.2017.03.200. [DOI] [PubMed] [Google Scholar]
- 304.Bhowmick S, Rother S, Zimmermann H, Lee PS, Moeller S, Schnabelrauch M, Koul V, Jordan R, Hintze V, Scharnweber D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application–The role of chondroitin sulfate and sulfated hyaluronan. Mater Sci Eng C. 2017;79:15–22. doi: 10.1016/j.msec.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 305.Pal P, Srivas PK, Dadhich P, Das B, Maulik D, Dhara S. Nano-/microfibrous cotton-wool-like 3D scaffold with core–shell architecture by emulsion electrospinning for skin tissue regeneration. ACS Biomater Sci Eng. 2017;3:3563–3575. doi: 10.1021/acsbiomaterials.7b00681. [DOI] [PubMed] [Google Scholar]
- 306.Trinca RB, Westin CB, da Silva JAF, Moraes ÂM. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur Polymer J. 2017;88:161–170. [Google Scholar]
- 307.Dias J, Baptista-Silva S, De Oliveira C, Sousa A, Oliveira AL, Bártolo P, Granja P. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur Polymer J. 2017;95:161–173. [Google Scholar]
- 308.Miguel SP, Ribeiro MP, Coutinho P, Correia IJ. Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers. 2017;9:183. doi: 10.3390/polym9050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sadeghi A, Nokhasteh S, Molavi A, Khorsand-Ghayeni M, Naderi-Meshkin H, Mahdizadeh A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater Sci Eng C. 2016;66:130–137. doi: 10.1016/j.msec.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 310.Park YR, Ju HW, Lee JM, Kim D-K, Lee OJ, Moon BM, Park HJ, Jeong JY, Yeon YK, Park CH. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int J Biol Macromol. 2016;93:1567–1574. doi: 10.1016/j.ijbiomac.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 311.Mahdavi M, Mahmoudi N, Rezaie Anaran F, Simchi A. Electrospinning of nanodiamond-modified polysaccharide nanofibers with physico-mechanical properties close to natural skins. Mar Drugs. 2016;14:128. doi: 10.3390/md14070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Nosar MN, Salehi M, Ghorbani S, Beiranvand SP, Goodarzi A, Azami M. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: influence of cellulose acetate concentration. Cellulose. 2016;23:3239–3248. [Google Scholar]
- 313.Yu K, Zhou X, Zhu T, Wu T, Wang J, Fang J, El-Aassar M, El-Hamshary H, El-Newehy M, Mo X. Fabrication of poly (ester-urethane) urea elastomer/gelatin electrospun nanofibrous membranes for potential applications in skin tissue engineering. RSC Adv. 2016;6:73636–73644. [Google Scholar]
- 314.Akturk O, Kismet K, Yasti AC, Kuru S, Duymus ME, Kaya F, Caydere M, Hucumenoglu S, Keskin DJRA. Wet electrospun silk fibroin/gold nanoparticle 3D matrices for wound healing applications. RSC Adv. 2016;6:13234–13250. doi: 10.1177/0885328216644536. [DOI] [PubMed] [Google Scholar]
- 315.Sadeghi-Avalshahr AR, Khorsand-Ghayeni M, Nokhasteh S, Molavi AM, Naderi-Meshkin H. Synthesis and characterization of PLGA/collagen composite scaffolds as skin substitute produced by electrospinning through two different approaches. J Mater Sci Mater Med. 2017;28:14. doi: 10.1007/s10856-016-5789-z. [DOI] [PubMed] [Google Scholar]
- 316.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 317.Melchiorri AJ, Hibino N, Fisher JP. Strategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular grafts. Tissue Eng Part B Rev. 2013;19:292–307. doi: 10.1089/ten.teb.2012.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Catto V, Farè S, Cattaneo I, Figliuzzi M, Alessandrino A, Freddi G, Remuzzi A, Tanzi MC. Small diameter electrospun silk fibroin vascular grafts: mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater Sci Eng C. 2015;54:101–111. doi: 10.1016/j.msec.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 320.Faries PL, LoGerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, Campbell DR, Pomposelli FB., Jr A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–1090. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- 321.Greisler HP. Interactions at the blood/material interface. Ann Vasc Surg. 1990;4:98–103. doi: 10.1007/BF02042699. [DOI] [PubMed] [Google Scholar]
- 322.Ulbricht J, Jordan R, Luxenhofer R. On the biodegradability of polyethylene glycol, polypeptoids and poly (2-oxazoline) s. Biomaterials. 2014;35:4848–4861. doi: 10.1016/j.biomaterials.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 323.Castillo R, Müller A. Crystallization and morphology of biodegradable or biostable single and double crystalline block copolymers. Prog Polym Sci. 2009;34:516–560. [Google Scholar]
- 324.Shinohara S, Kihara T, Sakai S, Matsusaki M, Akashi M, Taya M, Miyake J. Fabrication of in vitro three-dimensional multilayered blood vessel model using human endothelial and smooth muscle cells and high-strength PEG hydrogel. J Biosci Bioeng. 2013;116:231–234. doi: 10.1016/j.jbiosc.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 325.Wang H, Feng Y, Fang Z, Yuan W, Khan M. Co-electrospun blends of PU and PEG as potential biocompatible scaffolds for small-diameter vascular tissue engineering. Mater Sci Eng C. 2012;32:2306–2315. [Google Scholar]
- 326.Liu L, Guo S, Chang J, Ning C, Dong C, Yan D.J.J.o.B.M.R.P.B.A.B.A.O.J.o.T.S.f.B (2008) The Japanese Society for Biomaterials,; Biomaterials, T.A.S.f.; Biomaterials, t.K.S.f. Surface modification of polycaprolactone membrane via layer‐by‐layer deposition for promoting blood compatibility. J Biomed Mater Res Part B, 87, 244–250 [DOI] [PubMed]
- 327.Oriňaková R, Gorejová R, Králová ZO, Haverová L, Oriňak A, Maskaľová I, Kupková M, Džupon M, Baláž M, Hrubovčáková M (2020) Evaluation of mechanical properties and hemocompatibility of open cell iron foams with polyethylene glycol coating. Appl Surf Sci, 505, 144634
- 328.Yin A, Zhuang W, Liu G, Lan X, Tang Z, Deng Y, Wang Y (2020) Performance of PEGylated chitosan and poly (L-lactic acid-co-ε-caprolactone) bilayer vascular grafts in a canine femoral artery model. Colloids Surf B Biointerf, 188, 110806 [DOI] [PubMed]
- 329.Mulholland EJ, Ali A, Robson T, Dunne NJ, McCarthy HO. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nanofibres: An innovative angiogenic therapy for wound repair. J Control Release. 2019;316:53–65. doi: 10.1016/j.jconrel.2019.10.050. [DOI] [PubMed] [Google Scholar]
- 330.Rychter M, Milanowski B, Grześkowiak BF, Jarek M, Kempiński M, Coy EL, Borysiak S, Baranowska-Korczyc A, Lulek J. Cilostazol-loaded electrospun three-dimensional systems for potential cardiovascular application: Effect of fibers hydrophilization on drug release, and cytocompatibility. J Coll Interf Sci. 2019;536:310–327. doi: 10.1016/j.jcis.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 331.Tondnevis F, Keshvari H, Mohandesi JA. Fabrication, characterization, and in vitro evaluation of electrospun polyurethane-gelatin-carbon nanotube scaffolds for cardiovascular tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2020;108:2276–2293. doi: 10.1002/jbm.b.34564. [DOI] [PubMed] [Google Scholar]
- 332.Mani MP, Jaganathan SK, Prabhakaran P, Nageswaran G, Krishnasamy NP. Electrospun polyurethane patch in combination with cedarwood and cobalt nitrate for cardiac applications. J Appl Polym Sci. 2019;136:48226. [Google Scholar]
- 333.Horakova J, Mikes P, Saman A, Jencova V, Klapstova A, Svarcova T, Ackermann M, Novotny V, Suchy T, Lukas D. The effect of ethylene oxide sterilization on electrospun vascular grafts made from biodegradable polyesters. Mater Sci Eng C. 2018;92:132–142. doi: 10.1016/j.msec.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 334.Li Z, Zhou P, Zhou F, Zhao Y, Ren L, Yuan X. Antimicrobial eugenol-loaded electrospun membranes of poly (ε-caprolactone)/gelatin incorporated with REDV for vascular graft applications. Colloids Surf B Biointerf. 2018;162:335–344. doi: 10.1016/j.colsurfb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 335.Zhu T, Yu K, Bhutto MA, Guo X, Shen W, Wang J, Chen W, El-Hamshary H, Al-Deyab SS, Mo X. Synthesis of RGD-peptide modified poly (ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem Eng J. 2017;315:177–190. [Google Scholar]
- 336.Augustine R, Dan P, Sosnik A, Kalarikkal N, Tran N, Vincent B, Thomas S, Menu P, Rouxel D. Electrospun poly (vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017;10:3358–3376. [Google Scholar]
- 337.Li Q, Mu L, Zhang F, Mo Z, Jin C, Qi W. Manufacture and property research of heparin grafted electrospinning PCU artificial vascular scaffolds. Mater Sci Eng C. 2017;78:854–861. doi: 10.1016/j.msec.2017.04.148. [DOI] [PubMed] [Google Scholar]
- 338.Abdal-hay A, Bartnikowski M, Hamlet S, Ivanovski S. Electrospun biphasic tubular scaffold with enhanced mechanical properties for vascular tissue engineering. Mater Sci Eng C. 2018;82:10–18. doi: 10.1016/j.msec.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 339.Augustine R, Nethi SK, Kalarikkal N, Thomas S, Patra CR. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications. J Mater Chem B. 2017;5:4660–4672. doi: 10.1039/c7tb00518k. [DOI] [PubMed] [Google Scholar]
- 340.Pezzoli D, Cauli E, Chevallier P, Farè S, Mantovani D. Biomimetic coating of cross-linked gelatin to improve mechanical and biological properties of electrospun PET: A promising approach for small caliber vascular graft applications. J Biomed Mater Res Part A. 2017;105:2405–2415. doi: 10.1002/jbm.a.36098. [DOI] [PubMed] [Google Scholar]
- 341.Karahaliloğlu Z. Electrospun PU-PEG and PU-PC hybrid scaffolds for vascular tissue engineering. Fibers Polym. 2017;18:2135–2145. [Google Scholar]
- 342.Jiang Y-C, Jiang L, Huang A, Wang X-F, Li Q, Turng L-S. Electrospun polycaprolactone/gelatin composites with enhanced cell–matrix interactions as blood vessel endothelial layer scaffolds. Mater Sci Eng C. 2017;71:901–908. doi: 10.1016/j.msec.2016.10.083. [DOI] [PubMed] [Google Scholar]
- 343.Dai W-W, Guo H-F, Qian D-H, Qin Z-X, Lei Y, Hou X-Y, Wen C. Improving endothelialization by the combined application of polyethylene glycol coated cerium oxide nanoparticles and VEGF in electrospun polyurethane scaffolds. J Mater Chem B. 2017;5:1053–1061. doi: 10.1039/c6tb02391f. [DOI] [PubMed] [Google Scholar]
- 344.Yang F, Murugan R, Ramakrishna S, Wang X, Ma Y-X, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 345.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 346.Bellamkonda R, Aebischer PJB. Tissue engineering in the nervous system. Biotechnol Bioeng. 1994;43:543–554. doi: 10.1002/bit.260430703. [DOI] [PubMed] [Google Scholar]
- 347.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 348.Xie J, Liu W, MacEwan MR, Bridgman PC, Xia Y. Neurite outgrowth on electrospun nanofibers with uniaxial alignment: the effects of fiber density, surface coating, and supporting substrate. ACS Nano. 2014;8:1878–1885. doi: 10.1021/nn406363j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zuidema JM, Hyzinski-García MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials. 2014;35:1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Huang C, Ouyang Y, Niu H, He N, Ke Q, Jin X, Li D, Fang J, Liu W, Fan C. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl Mater Interf. 2015;7:7189–7196. doi: 10.1021/am509227t. [DOI] [PubMed] [Google Scholar]
- 351.Brigham C. Biopolymers: Biodegradable alternatives to traditional plastics. In Green chemistry: Elsevier; 2018. pp. 753–770. [Google Scholar]
- 352.Verma D, Fortunati E (2019) Biopolymer processing and its composites: An introduction. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier; pp. 3–23
- 353.Miyamoto T, Takahashi SI, Ito H, Inagaki H, Noishiki Y (1989) Tissue biocompatibility of cellulose and its derivatives. J Biomed Mater Res, 23, 125–133. [DOI] [PubMed]
- 354.Märtson M, Viljanto J, Hurme T, Saukko P. Biocompatibility of cellulose sponge with bone. Eur Surg Res. 1998;30:426–432. doi: 10.1159/000008609. [DOI] [PubMed] [Google Scholar]
- 355.Barbié C, Chauveaux D, Barthe X, Baquey C, Poustis J. Biological behaviour of cellulosic materials after bone implantation: Preliminary results. Clin Mater. 1990;5:251–258. doi: 10.1016/0267-6605(90)90023-o. [DOI] [PubMed] [Google Scholar]
- 356.Poustis J, Baquey C, Chauveaux D. Mechanical properties of cellulose in orthopaedic devices and related environments. Clin Mater. 1994;16:119–124. doi: 10.1016/0267-6605(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 357.Hou Y, Wang X, Zhang Z, Luo J, Cai Z, Wang Y, Li Y. Repairing transected peripheral nerve using a biomimetic nerve guidance conduit containing intraluminal sponge fillers. Adv Healthcare Mater. 2019;8:1900913. doi: 10.1002/adhm.201900913. [DOI] [PubMed] [Google Scholar]
- 358.Farzamfar S, Naseri-Nosar M, Vaez A, Esmaeilpour F, Ehterami A, Sahrapeyma H, Samadian H, Hamidieh A-A, Ghorbani S, Goodarzi A. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose. 2018;25:1229–1238. [Google Scholar]
- 359.Zech J, Leisz S, Göttel B, Syrowatka F, Greiner A, Strauss C, Knolle W, Scheller C, Mäder K (2020) Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Europ J Pharmaceut Biopharmaceut [DOI] [PubMed]
- 360.Ziemba AM, Fink TD, Crochiere MC, Puhl DL, Sapkota S, Gilbert RJ, Zha RH. Coating topologically complex electrospun fibers with nanothin silk fibroin enhances neurite outgrowth in vitro. ACS Biomater Sci Eng. 2020;6:1321–1332. doi: 10.1021/acsbiomaterials.9b01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Saudi A, Amini S, Amirpour N, Kazemi M, Kharazi AZ, Salehi H, Rafienia M (2019) Promoting neural cell proliferation and differentiation by incorporating lignin into electrospun poly (vinyl alcohol) and poly (glycerol sebacate) fibers. Mater Sci Eng C, 104, 110005 [DOI] [PubMed]
- 362.Lin F, Wang X, Wang Y, Yang Y, Li Y. Preparation and biocompatibility of electrospinning PDLLA/β-TCP/collagen for peripheral nerve regeneration. RSC Adv. 2017;7:41593–41602. [Google Scholar]
- 363.Zhu L, Wang K, Ma T, Huang L, Xia B, Zhu S, Yang Y, Liu Z, Quan X, Luo K. Noncovalent bonding of RGD and YIGSR to an electrospun poly (ε-caprolactone) conduit through peptide self-assembly to synergistically promote sciatic nerve regeneration in rats. Adv Healthcare Mater. 2017;6:1600860. doi: 10.1002/adhm.201600860. [DOI] [PubMed] [Google Scholar]
- 364.Yan Y, Sencadas V, Jin T, Huang X, Chen J, Wei D, Jiang Z. Tailoring the wettability and mechanical properties of electrospun poly (l-lactic acid)-poly (glycerol sebacate) core-shell membranes for biomedical applications. J Colloid Interf Sci. 2017;508:87–94. doi: 10.1016/j.jcis.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 365.Xia B, Lv Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater Sci Eng C. 2018;82:253–264. doi: 10.1016/j.msec.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 366.Naseri-Nosar M, Salehi M, Hojjati-Emami S. Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int J Biol Macromol. 2017;103:701–708. doi: 10.1016/j.ijbiomac.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 367.Lee SJ, Heo M, Lee D, Heo DN, Lim H-N, Kwon IK. Fabrication and design of bioactive agent coated, highly-aligned electrospun matrices for nerve tissue engineering: Preparation, characterization and application. Appl Surf Sci. 2017;424:359–367. [Google Scholar]
- 368.Mohamadi F, Ebrahimi-Barough S, Reza Nourani M, Ali Derakhshan M, Goodarzi V, Sadegh Nazockdast M, Farokhi M, Tajerian R, Faridi Majidi R, Ai J. Electrospun nerve guide scaffold of poly (ε-caprolactone)/collagen/nanobioglass: an in vitro study in peripheral nerve tissue engineering. J Biomed Mater Res Part A. 2017;105:1960–1972. doi: 10.1002/jbm.a.36068. [DOI] [PubMed] [Google Scholar]
- 369.Motamedi AS, Mirzadeh H, Hajiesmaeilbaigi F, Bagheri-Khoulenjani S, Shokrgozar MA. Piezoelectric electrospun nanocomposite comprising Au NPs/PVDF for nerve tissue engineering. J Biomed Mater Res Part A. 2017;105:1984–1993. doi: 10.1002/jbm.a.36050. [DOI] [PubMed] [Google Scholar]
- 370.Rajabi M, Firouzi M, Hassannejad Z, Haririan I, Zahedi P. Fabrication and characterization of electrospun laminin-functionalized silk fibroin/poly (ethylene oxide) nanofibrous scaffolds for peripheral nerve regeneration. J Biomed Mater Res B Appl Biomater. 2018;106:1595–1604. doi: 10.1002/jbm.b.33968. [DOI] [PubMed] [Google Scholar]
- 371.Ranjbar-Mohammadi M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Gum tragacanth/poly (l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohyd Polym. 2016;140:104–112. doi: 10.1016/j.carbpol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 372.Hu J, Kai D, Ye H, Tian L, Ding X, Ramakrishna S, Loh XJ. Electrospinning of poly (glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater Sci Eng C. 2017;70:1089–1094. doi: 10.1016/j.msec.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 373.Vimal SK, Ahamad N, Katti DS. A simple method for fabrication of electrospun fibers with controlled degree of alignment having potential for nerve regeneration applications. Mater Sci Eng C. 2016;63:616–627. doi: 10.1016/j.msec.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 374.Zhou Z-F, Zhang F, Wang J-G, Chen Q-C, Yang W-Z, He N, Jiang Y-Y, Chen F, Liu J-J. Electrospinning of PELA/PPY fibrous conduits: promoting peripheral nerve regeneration in rats by self-originated electrical stimulation. ACS Biomater Sci Eng. 2016;2:1572–1581. doi: 10.1021/acsbiomaterials.6b00335. [DOI] [PubMed] [Google Scholar]
- 375.Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core–shell structured fibers prepared by coaxial electrospinning. J Biomed Mater Res B Appl Biomater. 2006;79:50–57. doi: 10.1002/jbm.b.30510. [DOI] [PubMed] [Google Scholar]
- 376.Rujitanaroj P-O, Wang Y-C, Wang J, Chew SY. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 2011;32:5915–5923. doi: 10.1016/j.biomaterials.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 377.Sahoo S, Ang LT, Goh JCH, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res, Part A. 2010;93:1539–1550. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 378.Kamble P, Sadarani B, Majumdar A, Bhullar S. Nanofiber based drug delivery systems for skin: a promising therapeutic approach. J Drug Del Sci Technol. 2017;41:124–133. [Google Scholar]
- 379.Agrahari V, Agrahari V, Meng J, Mitra AK (2017) Electrospun nanofibers in drug delivery: fabrication, advances, and biomedical applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier; pp. 189-215
- 380.Khoshnevisan K, Maleki H, Samadian H, Shahsavari S, Sarrafzadeh MH, Larijani B, Dorkoosh FA, Haghpanah V, Khorramizadeh MR. Cellulose acetate electrospun nanofibers for drug delivery systems: applications and recent advances. Carbohyd Polym. 2018;198:131–141. doi: 10.1016/j.carbpol.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 381.Kuang G, Zhang Z, Liu S, Zhou D, Lu X, Jing X, Huang Y. Biphasic drug release from electrospun polyblend nanofibers for optimized local cancer treatment. Biomaterials Sci. 2018;6:324–331. doi: 10.1039/c7bm01018d. [DOI] [PubMed] [Google Scholar]
- 382.Yu H, Jia Y, Yao C, Lu Y. PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int J Pharm. 2014;469:17–22. doi: 10.1016/j.ijpharm.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 383.Nguyen TTT, Ghosh C, Hwang S-G, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int J Pharm. 2012;439:296–306. doi: 10.1016/j.ijpharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 384.Chew S, Wen Y, Dzenis Y, Leong KW. The role of electrospinning in the emerging field of nanomedicine. Curr Pharm Des. 2006;12:4751–4770. doi: 10.2174/138161206779026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Repanas A, Andriopoulou S, Glasmacher B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J Drug Deliv Sci Technol. 2016;31:137–146. [Google Scholar]
- 386.Vidyalakshmi K, Rashmi K, Pramod Kumar T, Siddaramaiah (2004) Studies on formulation and in vitro evaluation of PVA/chitosan blend films for drug delivery. J Macromol Sci Part A, 41, 1115–1122
- 387.Constantin M, Bucatariu SM, Doroftei F, Fundueanu G (2017) Smart composite materials based on chitosan microspheres embedded in thermosensitive hydrogel for controlled delivery of drugs. Carbohyd Polym, 157, 493-502 [DOI] [PubMed]
- 388.Xu J, Strandman S, Zhu JX, Barralet J, Cerruti M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials. 2015;37:395–404. doi: 10.1016/j.biomaterials.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 389.Fathollahipour S, Abouei Mehrizi A, Ghaee A, Koosha M. Electrospinning of PVA/chitosan nanocomposite nanofibers containing gelatin nanoparticles as a dual drug delivery system. J Biomed Mater Res Part A. 2015;103:3852–3862. doi: 10.1002/jbm.a.35529. [DOI] [PubMed] [Google Scholar]
- 390.Cui Z, Zheng Z, Lin L, Si J, Wang Q, Peng X, Chen W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv Polym Technol. 2018;37:1917–1928. [Google Scholar]
- 391.Qin Z-Y, Jia X-W, Liu Q, Kong B-H, Wang H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int J Biol Macromol. 2019;137:224–231. doi: 10.1016/j.ijbiomac.2019.06.224. [DOI] [PubMed] [Google Scholar]
- 392.Shekarforoush E, Ajalloueian F, Zeng G, Mendes AC, Chronakis IS. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater Lett. 2018;228:322–326. [Google Scholar]
- 393.Alavarse AC, de Oliveira Silva, FW, Colque JT, da Silva VM, Prieto T, Venancio EC, Bonvent JJJMS (2017) Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater Sci Eng C, 77, 271–281 [DOI] [PubMed]
- 394.Balagangadharan K, Trivedi R, Vairamani M, Selvamurugan N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohyd Polym. 2019;216:1–16. doi: 10.1016/j.carbpol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 395.Fazli Y, Shariatinia Z. Controlled release of cefazolin sodium antibiotic drug from electrospun chitosan-polyethylene oxide nanofibrous Mats. Mater Sci Eng C. 2017;71:641–652. doi: 10.1016/j.msec.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 396.Liu X, Yang Y, Yu D-G, Zhu M-J, Zhao M, Williams GR. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chem Eng J. 2019;356:886–894. [Google Scholar]
- 397.Celebioglu A, Uyar T. Fast dissolving oral drug delivery system based on electrospun nanofibrous webs of cyclodextrin/ibuprofen inclusion complex nanofibers. Mol Pharm. 2019;16:4387–4398. doi: 10.1021/acs.molpharmaceut.9b00798. [DOI] [PubMed] [Google Scholar]
- 398.Bulbul YE, Eskitoros-Togay ŞM, Demirtas-Korkmaz F, Dilsiz N (2019) Multi-walled carbon nanotube-incorporating electrospun composite fibrous mats for controlled drug release profile. Int J Pharmaceut, 568, 118513 [DOI] [PubMed]
- 399.Eskitoros-Togay ŞM, Bulbul YE, Tort S, Korkmaz FD, Acartürk F, Dilsiz N. Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int J Pharm. 2019;565:83–94. doi: 10.1016/j.ijpharm.2019.04.073. [DOI] [PubMed] [Google Scholar]
- 400.Jaworska J, Włodarczyk J, Karpeta-Jarząbek P, Janeczek H, Stojko M, Kasperczyk J. Electrospun, drug-enriched bioresorbable nonwovens based on poly (glycolide-ɛ-caprolactone) and poly (d, l-lactide-glycolide) for urological applications. Polym Degrad Stab. 2019;167:94–101. [Google Scholar]
- 401.Li H, Sang Q, Wu J, Williams GR, Wang H, Niu S, Wu J, Zhu L-M. Dual-responsive drug delivery systems prepared by blend electrospinning. Int J Pharm. 2018;543:1–7. doi: 10.1016/j.ijpharm.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 402.Hu S, Qin Z, Cheng M, Chen Y, Liu J, Zhang Y. Improved properties and drug delivery behaviors of electrospun cellulose acetate nanofibrous membranes by introducing carboxylated cellulose nanocrystals. Cellulose. 2018;25:1883–1898. [Google Scholar]
- 403.Herrero-Herrero M, Gómez-Tejedor J-A, Vallés-Lluch A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur Polymer J. 2018;99:445–455. [Google Scholar]
- 404.Rychter M, Baranowska-Korczyc A, Milanowski B, Jarek M, Maciejewska BM, Coy EL, Lulek J. Cilostazol-loaded poly (ε-Caprolactone) electrospun drug delivery system for cardiovascular applications. Pharm Res. 2018;35:32. doi: 10.1007/s11095-017-2314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Dadras Chomachayi M, Solouk A, Akbari S, Sadeghi D, Mirahmadi F, Mirzadeh H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: thyme essential oil and doxycycline monohydrate release study. J Biomed Mater Res Part A. 2018;106:1092–1103. doi: 10.1002/jbm.a.36303. [DOI] [PubMed] [Google Scholar]
- 406.Esmaeili A, Haseli M. Optimization, synthesis, and characterization of coaxial electrospun sodium carboxymethyl cellulose-graft-methyl acrylate/poly (ethylene oxide) nanofibers for potential drug-delivery applications. Carbohyd Polym. 2017;173:645–653. doi: 10.1016/j.carbpol.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 407.Sedghi R, Shaabani A, Mohammadi Z, Samadi FY, Isaei E. Biocompatible electrospinning chitosan nanofibers: a novel delivery system with superior local cancer therapy. Carbohyd Polym. 2017;159:1–10. doi: 10.1016/j.carbpol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 408.Wali A, Zhang Y, Sengupta P, Higaki Y, Takahara A, Badiger MV. Electrospinning of non-ionic cellulose ethers/polyvinyl alcohol nanofibers: characterization and applications. Carbohyd Polym. 2018;181:175–182. doi: 10.1016/j.carbpol.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 409.Bhattarai RS, Das A, Alzhrani RM, Kang D, Bhaduri SB, Boddu SH. Comparison of electrospun and solvent cast polylactic acid (PLA)/poly (vinyl alcohol)(PVA) inserts as potential ocular drug delivery vehicles. Mater Sci Eng C. 2017;77:895–903. doi: 10.1016/j.msec.2017.03.305. [DOI] [PubMed] [Google Scholar]
- 410.Zhang H, Xia J, Pang X, Zhao M, Wang B, Yang L, Wan H, Wu J, Fu S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater Sci Eng C. 2017;73:537–543. doi: 10.1016/j.msec.2016.12.116. [DOI] [PubMed] [Google Scholar]
- 411.Cheng M, Qin Z, Hu S, Dong S, Ren Z, Yu H. Achieving long-term sustained drug delivery for electrospun biopolyester nanofibrous membranes by introducing cellulose nanocrystals. ACS Biomater Sci Eng. 2017;3:1666–1676. doi: 10.1021/acsbiomaterials.7b00169. [DOI] [PubMed] [Google Scholar]
- 412.Ferrández-Rives M, Beltrán-Osuna ÁA, Gómez-Tejedor JA, Gomez Ribelles JL. Electrospun PVA/bentonite nanocomposites mats for drug delivery. Materials. 2017;10(12):1448. doi: 10.3390/ma10121448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Karuppannan C, Sivaraj M, Kumar JG, Seerangan R, Balasubramanian S, Gopal DR. Fabrication of progesterone-loaded nanofibers for the drug delivery applications in bovine. Nanoscale Res Lett. 2017;12:116. doi: 10.1186/s11671-016-1781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Jaiturong P, Sirithunyalug B, Eitsayeam S, Asawahame C, Tipduangta P, Sirithunyalug J. Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier. Asian J Pharm Sci. 2018;13:239–247. doi: 10.1016/j.ajps.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Spadaro S, Santoro M, Barreca F, Scala A, Grimato S, Neri F, Fazio E (2018) PEG-PLGA electrospun nanofibrous membranes loaded with Au@ Fe 2 O 3 nanoparticles for drug delivery applications. Front Phys, 13, 136201
- 416.Zhu Y, Pyda M, Cebe P. Electrospun fibers of poly (l-lactic acid) containing lovastatin with potential applications in drug delivery. J Appl Polym Sci. 2017;134:45287. [Google Scholar]
- 417.Akduman C, Özgüney I, Kumbasar EPAJMS (2016) Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater Sci Eng C, 64, 383–390 [DOI] [PubMed]
- 418.Ranjbar-Mohammadi M, Bahrami SH. Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int J Biol Macromol. 2016;84:448–456. doi: 10.1016/j.ijbiomac.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 419.Mendes AC, Gorzelanny C, Halter N, Schneider SW, Chronakis IS. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int J Pharm. 2016;510:48–56. doi: 10.1016/j.ijpharm.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 420.Sultanova Z, Kaleli G, Kabay G, Mutlu M. Controlled release of a hydrophilic drug from coaxially electrospun polycaprolactone nanofibers. Int J Pharm. 2016;505:133–138. doi: 10.1016/j.ijpharm.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 421.Gencturk A, Kahraman E, Güngör S, Özhan G, Özsoy Y, Sarac A. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: characterization studies and in vitro assays. Artif Cells Nanomed Biotechnol. 2017;45:655–664. doi: 10.3109/21691401.2016.1173047. [DOI] [PubMed] [Google Scholar]
- 422.Zhang X, Tang K, Zheng X. Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. J Bionic Eng. 2016;13:143–149. [Google Scholar]
- 423.Fu R, Li C, Yu C, Xie H, Shi S, Li Z, Wang Q, Lu L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly (vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Delivery. 2016;23:818–829. doi: 10.3109/10717544.2014.918676. [DOI] [PubMed] [Google Scholar]
- 424.Laha A, Yadav S, Majumdar S, Sharma CS. In-vitro release study of hydrophobic drug using electrospun cross-linked gelatin nanofibers. Biochem Eng J. 2016;105:481–488. [Google Scholar]
- 425.Ding Y, Wang Y, Su L, Bellagamba M, Zhang H, Lei Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosensors Bioelectronics. 2010;26:542–548. doi: 10.1016/j.bios.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 426.Vellayappan M, Venugopal J, Ramakrishna S, Ray S, Ismail A, Mandal M, Manikandan A, Seal S, Jaganathan S. Electrospinning applications from diagnosis to treatment of diabetes. RSC Adv. 2016;6:83638–83655. [Google Scholar]
- 427.Unal B, Yalcinkaya EE, Demirkol DO, Timur S. An electrospun nanofiber matrix based on organo-clay for biosensors: PVA/PAMAM-Montmorillonite. Appl Surf Sci. 2018;444:542–551. [Google Scholar]
- 428.Kim J, Hu J, Bezerra AB, Holtan MD, Brooks JC, Easley CJ. Protein quantification using controlled DNA melting transitions in bivalent probe assemblies. Anal Chem. 2015;87:9576–9579. doi: 10.1021/acs.analchem.5b03432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Davis BW, Niamnont N, Hare CD, Sukwattanasinitt M, Cheng Q. Nanofibers doped with dendritic fluorophores for protein detection. ACS Appl Mater Interf. 2010;2:1798–1803. doi: 10.1021/am100345g. [DOI] [PubMed] [Google Scholar]
- 430.Hu J, Yu Y, Brooks JC, Godwin LA, Somasundaram S, Torabinejad F, Kim J, Shannon C, Easley CJ. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J Am Chem Soc. 2014;136:8467–8474. doi: 10.1021/ja503679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang X, Wang X, Wang X, Chen F, Zhu K, Xu Q, Tang M. Novel electrochemical biosensor based on functional composite nanofibers for sensitive detection of p53 tumor suppressor gene. Anal Chim Acta. 2013;765:63–69. doi: 10.1016/j.aca.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 432.Rezaei Z, Mahmoudifard M. Pivotal role of electrospun nanofibers in microfluidic diagnostic systems–a review. J Mater Chem B. 2019;7:4602–4619. doi: 10.1039/c9tb00682f. [DOI] [PubMed] [Google Scholar]
- 433.Kosaki Y, Izawa H, Ishihara S, Kawakami K, Sumita M, Tateyama Y, Ji Q, Krishnan V, Hishita S, Yamauchi Y. Nanoporous carbon sensor with cage-in-fiber structure: Highly selective aniline adsorbent toward cancer risk management. ACS Appl Mater Interf. 2013;5:2930–2934. doi: 10.1021/am400940q. [DOI] [PubMed] [Google Scholar]
- 434.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 435.Röck F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]
- 436.Güntner AT, Abegg S, Königstein K, Gerber PA, Schmidt-Trucksäss A, Pratsinis SE (2019) Breath sensors for health monitoring. ACS Sens, 4, 268–280 [DOI] [PubMed]
- 437.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 438.Thomlinson R, Gray L. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Mehrabi P, Hui J, Janfaza S, O’Brien A, Tasnim N, Najjaran H, Hoorfar M. Fabrication of SnO2 composite nanofiber-based gas sensor using the electrospinning method for tetrahydrocannabinol (THC) detection. Micromachines. 2020;11:190. doi: 10.3390/mi11020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Chen Z, Chen Z, Zhang A, Hu J, Wang X, Yang Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater Sci. 2016;4:922–932. doi: 10.1039/c6bm00070c. [DOI] [PubMed] [Google Scholar]
- 441.Zhao G, Huang B, Zhang J, Wang A, Ren K, Wang ZL. Electrospun Poly (l-Lactic Acid) nanofibers for nanogenerator and diagnostic sensor applications. Macromol Mater Eng. 2017;302:1600476. [Google Scholar]
- 442.Hou S, Zhao L, Shen Q, Yu J, Ng C, Kong X, Wu D, Song M, Shi X, Xu X. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew Chem. 2013;125:3463–3467. doi: 10.1002/anie.201208452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Sun N, Liu M, Wang J, Wang Z, Li X, Jiang B, Pei R. Chitosan nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small. 2016;12:5090–5097. doi: 10.1002/smll.201600475. [DOI] [PubMed] [Google Scholar]
- 444.Ma K, Chan CK, Liao S, Hwang WY, Feng Q, Ramakrishna S. Electrospun nanofiber scaffolds for rapid and rich capture of bone marrow-derived hematopoietic stem cells. Biomaterials. 2008;29:2096–2103. doi: 10.1016/j.biomaterials.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 445.Zhao Y, Fan Z, Shen M, Shi X. Hyaluronic acid-functionalized electrospun polyvinyl alcohol/polyethyleneimine nanofibers for cancer cell capture applications. Adv Mater Interfaces. 2015;2:1500256. [Google Scholar]
- 446.Xiao Y, Shen M, Shi X. Design of functional electrospun nanofibers for cancer cell capture applications. J Mater Chem B. 2018;6:1420–1432. doi: 10.1039/c7tb03347h. [DOI] [PubMed] [Google Scholar]
- 447.Pimentel ES, Brito-Pereira R, Marques-Almeida T, Ribeiro C, Vaz F, Lanceros-Mendez S, Cardoso VF. Tailoring electrospun poly (L-lactic acid) nanofibers as substrates for microfluidic applications. ACS Appl Mater Interf. 2019;12:60–69. doi: 10.1021/acsami.9b12461. [DOI] [PubMed] [Google Scholar]
- 448.Xu G, Tan Y, Xu T, Yin D, Wang M, Shen M, Chen X, Shi X, Zhu X. Hyaluronic acid-functionalized electrospun PLGA nanofibers embedded in a microfluidic chip for cancer cell capture and culture. Biomater Sci. 2017;5:752–761. doi: 10.1039/c6bm00933f. [DOI] [PubMed] [Google Scholar]
- 449.Kalluri S, Seng KH, Guo Z, Liu HK, Dou SX. Electrospun lithium metal oxide cathode materials for lithium-ion batteries. RSC Adv. 2013;3:25576–25601. [Google Scholar]
- 450.Wang H-G, Yuan S, Ma D-L, Zhang X-B, Yan J-M. Electrospun materials for lithium and sodium rechargeable batteries: from structure evolution to electrochemical performance. Energy Environ Sci. 2015;8:1660–1681. [Google Scholar]
- 451.Srinivasu P, Islam A, Singh SP, Han L, Kantam ML, Bhargava SK. Highly efficient nanoporous graphitic carbon with tunable textural properties for dye-sensitized solar cells. J Mater Chem. 2012;22:20866–20869. [Google Scholar]
- 452.Zhang B, Kang F, Tarascon J-M, Kim J-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog Mater Sci. 2016;76:319–380. [Google Scholar]
- 453.Li X, Chen Y, Huang H, Mai Y-W, Zhou L. Electrospun carbon-based nanostructured electrodes for advanced energy storage–a review. Energy Storage Mater. 2016;5:58–92. [Google Scholar]
- 454.Jung J-W, Lee C-L, Yu S, Kim I-D. Electrospun nanofibers as a platform for advanced secondary batteries: a comprehensive review. J Mater Chem A. 2016;4:703–750. [Google Scholar]
- 455.Li Z, Zhang J-W, Yu L-G, Zhang J-W. Electrospun porous nanofibers for electrochemical energy storage. J Mater Sci. 2017;52:6173–6195. doi: 10.1007/s10853-017-0794-2. [DOI] [Google Scholar]
- 456.Sun G, Sun L, Xie H, Liu J. Electrospinning of nanofibers for energy applications. Nanomaterials. 2016;6:129. doi: 10.3390/nano6070129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wu J, Qin X, Miao C, He Y-B, Liang G, Zhou D, Liu M, Han C, Li B, Kang F. A honeycomb-cobweb inspired hierarchical core–shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon. 2016;98:582–591. [Google Scholar]
- 458.Darbar D, Reddy M, Sundarrajan S, Pattabiraman R, Ramakrishna S, Chowdari B. Anodic electrochemical performances of MgCo2O4 synthesized by oxalate decomposition method and electrospinning technique for Li-ion battery application. Mater Res Bull. 2016;73:369–376. [Google Scholar]
- 459.Park JW, Wycisk R, Pintauro PN, Yarlagadda V, Van Nguyen T. Electrospun Nafion®/Polyphenylsulfone composite membranes for regenerative Hydrogen bromine fuel cells. Materials. 2016;9:143. doi: 10.3390/ma9030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wang G, Zhang L, Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev. 2012;41:797–828. doi: 10.1039/c1cs15060j. [DOI] [PubMed] [Google Scholar]
- 461.Kabir S, Medina S, Wang G, Bender G, Pylypenko S, Neyerlin K (2020) Improving the bulk gas transport of Fe-NC platinum group metal-free nanofiber electrodes via electrospinning for fuel cell applications. Nano Energy, 104791
- 462.Pant B, Park M, Park S-J. TiO2 NPs assembled into a carbon nanofiber composite electrode by a one-step electrospinning process for supercapacitor applications. Polymers. 2019;11:899. doi: 10.3390/polym11050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Li P, Ma X, Liu F, Zhao YL, Ding Y, Yang J (2020) Synthesis of highly ordered mesoporous carbons nanofiber web based on electrospinning strategy for supercapacitor. Microporous Mesoporous Mater, 110283
- 464.Ma W, Zhang Q, Hua D, Xiong R, Zhao J, Rao W, Huang S, Zhan X, Chen F, Huang C. Electrospun fibers for oil–water separation. RSC Adv. 2016;6:12868–12884. [Google Scholar]
- 465.Obaid M, Barakat NA, Fadali O, Motlak M, Almajid AA, Khalil KA. Effective and reusable oil/water separation membranes based on modified polysulfone electrospun nanofiber mats. Chem Eng J. 2015;259:449–456. [Google Scholar]
- 466.Li J-J, Zhu L-T, Luo Z-H. Electrospun fibrous membrane with enhanced swithchable oil/water wettability for oily water separation. Chem Eng J. 2016;287:474–481. [Google Scholar]
- 467.Lu P, Murray S, Zhu M (2019) Electrospun nanofibers for catalysts. In Electrospinning: Nanofabrication and Applications; Elsevier; pp. 695–717
- 468.Shao L, Xing G, He L, Chen J, Xie H, Liang X, Qi C. Sulfonic groups functionalized preoxidated polyacrylonitrile nanofibers and its catalytic applications. Appl Catal A. 2012;443:133–137. [Google Scholar]
- 469.Kim J, Kang J, Jeong U, Kim H, Lee H. Catalytic, conductive, and transparent platinum nanofiber webs for FTO-Free dye-sensitized solar cells. ACS Appl Mater Interf. 2013;5:3176–3181. doi: 10.1021/am400179j. [DOI] [PubMed] [Google Scholar]
- 470.Ma W, Xu Y, Ma K, Zhang H. Electrospinning synthesis of H3PW12O40/TiO2 nanofiber catalytic materials and their application in ultra-deep desulfurization. Appl Catal A. 2016;526:147–154. [Google Scholar]
- 471.Pei CC, Leung WW-F. Photocatalytic oxidation of nitrogen monoxide and o-xylene by TiO2/ZnO/Bi2O3 nanofibers: Optimization, kinetic modeling and mechanisms. Appl Catal B. 2015;174:515–525. [Google Scholar]
- 472.Ternero-Hidalgo J, Torres-Liñán J, Guerrero-Pérez M, Rodríguez-Mirasol J, Cordero T (2019) Electrospun vanadium oxide based submicron diameter fiber catalysts. Part I: Preparation procedure and propane ODH application. Catal Today, 325, 131–143
- 473.Lu Y, Liu Z, You SW, McLoughlin L, Bridgers B, Hayes S, Wang X, Wang R, Guo Z, Wujcik EK. Electrospun carbon/iron nanofibers: the catalytic effects of iron and application in Cr (VI) removal. Carbon. 2020 doi: 10.1016/j.carbon.2020.05.031. [DOI] [Google Scholar]
- 474.Xu P, Cen C, Chen N, Lin H, Wang Q, Xu N, Tang J, Teng Z. Facile fabrication of silver nanoparticles deposited cellulose microfiber nanocomposites for catalytic application. J Colloid Interf Sci. 2018;526:194–200. doi: 10.1016/j.jcis.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 475.Hu D, Xiao Y, Liu H, Wang H, Li J, Zhou B, Liu P, Shen M, Shi X. Loading of Au/Ag bimetallic nanoparticles within electrospun PVA/PEI nanofibers for catalytic applications. Colloids Surf A Physicochem Eng Aspects. 2018;552:9–15. [Google Scholar]
- 476.Hosseini SR, Ghasemi S, Kamali-Rousta M, Nabavi SR. Preparation of NiO nanofibers by electrospinning and their application for electro-catalytic oxidation of ethylene glycol. Int J Hydrogen Energy. 2017;42:906–913. [Google Scholar]
- 477.Chen S, Chi M, Zhu Y, Gao M, Wang C, Lu X. A Facile synthesis of superparamagnetic Fe3O4 nanofibers with superior peroxidase-like catalytic activity for sensitive colorimetric detection of L-cysteine. Appl Surf Sci. 2018;440:237–244. [Google Scholar]
- 478.Tebyetekerwa M, Xu Z, Yang S, Ramakrishna S. Electrospun nanofibers-based face masks. Adv Fiber Mater. 2020;2:161–166. doi: 10.1007/s42765-020-00049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Zhang Z, Ji D, He H, Ramakrishna S (2021) Electrospun ultrafine fibers for advanced face masks. Mater Sci Eng R Rep, 143, 100594 [DOI] [PMC free article] [PubMed]