Abstract
Background
Aging affects immunity, potentially altering fever response to infection. We assess effects of biological variables on basal temperature, and during COVID-19 infection, proposing an updated temperature threshold for older adults ≥65 years.
Methods
Participants were from 4 cohorts: 1 089 unaffected adult TwinsUK volunteers; 520 adults with emergency admission to a London hospital with RT-PCR confirmed SARS-CoV-2 infection; 757 adults with emergency admission to a Birmingham hospital with RT-PCR confirmed SARS-CoV-2 infection and 3 972 adult community-based COVID Symptom Study participants self-reporting a positive RT-PCR test. Heritability was assessed using saturated and univariate ACE models; mixed-effect and multivariable linear regression examined associations between temperature, age, sex, and body mass index (BMI); multivariable logistic regression examined associations between fever (≥37.8°C) and age; receiver operating characteristic (ROC) analysis was used to identify temperature threshold for adults ≥ 65 years.
Results
Among unaffected volunteers, lower BMI (p = .001), and increasing age (p < .001) was associated with lower basal temperature. Basal temperature showed a heritability of 47% (95% confidence interval 18%–57%). In COVID-19+ participants, increasing age was associated with lower temperatures in Birmingham and community-based cohorts (p < .001). For each additional year of age, participants were 1% less likely to demonstrate a fever ≥37.8°C (OR 0.99; p < .001). Combining healthy and COVID-19+ participants, a temperature of 37.4°C in adults ≥65 years had similar sensitivity and specificity to 37.8°C in adults <65 years for discriminating infection.
Conclusions
Aging affects temperature in health and acute infection, with significant heritability, indicating genetic factors contribute to temperature regulation. Our observations suggest a lower threshold (37.4°C/97.3°F) for identifying fever in older adults ≥65 years.
Keywords: COVID-19, Fever, Immunesenescence, Infection, Thermoregulation
Background
Normal body temperature of older adults has been observed to be lower than that of younger people, with more limited tolerance of thermal extremes (1,2). Thermoregulation involves multiple systems, including cardiovascular, respiratory, and musculoskeletal. Decreasing basal temperature with age may reflect natural changes in these systems and reduced ability to maintain homeostasis in response to ambient temperature changes.
Normal human body temperature is approximately 37°C (98.6°F); however, variation is observed, with daily variations as much as 0.25–0.5°C (3). Individual biological factors, such as genetics, may account for some of the observed variation in human body temperatures as well as metabolic determinants. Commercially important species, such as cattle and poultry, show heritability of thermo-regulation under different heat conditions (4–6). However, human heritability data are more limited. A small study of 53 adult female twin-pairs (mean age 52) used continuous wrist temperature monitoring to demonstrate Circadian system heritability (7). Monozygotic pairs showed higher intra-pair correlation than dizygotic twins for most parameters, including temperature, with genetic factors responsible for 46%–70% of variance.
Fever is a physiological response to infection and is clinically important in identifying infection. There is no widespread consensus on the definition of fever, although a number of thresholds have been proposed. Infectious Diseases Society of America guidelines define fever as a single oral temperature >37.8°C (>100.0°F); repeated oral temperatures >37.2°C (>99.0°F) or rectal temperatures >37.5°C (>99.5°F); or an increase in temperature of >1.1°C (>2.0°F) above baseline (8). It has been observed that fever may be absent or blunted in older adults, as much as 20%–30% of the time (9). Many have therefore questioned applicability of these thresholds in older adults (10,11); indeed, lowering the threshold to 37.2°C (99.0°F) has been shown to increase fever detection in older people (8,12). A previous study found that only 20%–30% of patients aged ≥60 with infection present with a temperature of 37.8°C (100.0°F) or above (13). Many older adults display atypical presentations, which may be associated with worse outcomes (14,15). Age-related changes in immune function may affect pyrogen production and fever response. Altered immune function, or “immunesenescence,” is a well-established feature of physiological aging (16). Immunesenescence affects both innate and adaptive arms of the immune system and is thought to be responsible, at least in part, for an increased incidence and severity of infections in older adults (16). Proposed age-related changes in adaptive immunity include limited diversity in B- and T-cell receptor repertoire, decreased numbers of naive T and B cells and reduced antigen-specific antibody production (17). Neutrophil function is also compromised by age and frailty and has been proposed as a possible therapeutic target to enhance immune responses in frail, older adults during infection (18).
Coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Fever has been cited as a “typical” symptom of COVID-19, with reported prevalence of between 64% and 98% in hospitalized patients across a number of international studies (19–23). Current UK public health guidance proposes a temperature ≥37.8°C to define a possible COVID-19 case (24). As for other infections, atypical presentations of COVID-19 have been documented in older adults, with symptoms including weakness, headache, and delirium, often without fever (20,25,26). In work done by our research group on point-of-care testing for COVID-19, hypothermia (T < 36.0°C) was noted to be an early clinical sign in a minority of patients (27).
Demonstrating relationships between age and temperature in a healthy cohort and cohorts with confirmed SARS-CoV-2 infection is important in understanding effects of aging on temperature regulation and the fever response to infection. Identifying a more sensitive temperature threshold for older adults may help detect cases of infectious disease, including COVID-19. Case identification, especially in the case of COVID-19, is relevant for infection control.
In this study, we aim to:
Identify individual factors associated with baseline temperature in a sample of healthy volunteers, including age, sex, body mass index (BMI), and temperature heritability;
Determine if any identified associations are consistent with those observed during the acute temperature response to SARS-CoV-2 infection, in community-based and hospitalized cohorts;
Identify a more appropriate temperature threshold for discriminating SARS-CoV-2 infection in adults ≥ 65 years of age than 37.8°C (100.0°F)—the temperature proposed by UK public health guidance to screen for COVID-19 cases (24).
Method
Participants
This observational cohort study uses 4 distinct cohorts.
(a) Unaffected twin volunteers: Measurements were collected between January 2017 and April 2018 on 1 089 adult twin volunteers, a convenience sample of the TwinsUK registry enrolled at the Department of Twin Research (DTR), King’s College London (KCL) (28). Demographic information recorded on twins in this cohort includes age, sex, zygosity, and BMI.
Hospital COVID-19+ cohorts: Data were collected as part of the international COVIDCollab study, led by the Geriatric Medicine Research Collaborative (29,30). All data are routinely collected and includes age, sex, BMI, and vital signs, including temperature. Full data collection information has been published previously (29). Only cases of COVID-19 infection confirmed by RT-PCR of nasopharyngeal swab were included. For patients readmitted during study period, data from index admission were used.
(b) London: First documented point-of-care temperature measurements were collected on all adult patients with COVID-19 infection confirmed by RT-PCR of nasopharyngeal swab, with unscheduled admission to St Thomas’ Hospital, London, UK between March 1, 2020 and May 4, 2020.
(c) Birmingham: First documented point-of-care temperature measurements were collected on all adult patients with confirmed COVID-19 infection, with unscheduled admission to Queen Elizabeth Hospital, Birmingham, UK between March 4, 2020 and April 13, 2020.
(d) Community-based “app” COVID + cohort: Data were obtained from UK-based participants enrolled in the COVID Symptom Study (CSS). CSS participants log using a free smartphone application “app,” which was developed by Zoe Limited (London, UK) with scientific input from researchers and clinicians at King’s College London (London, UK). The app was launched in the United Kingdom on March 24, 2020 and captures information related to COVID-19 symptoms, self-reported or reported-by-proxy. On first accessing the app, users record baseline demographic factors, for example, location, age, ethnicity, and health risk factors (31). Consent to sharing and analysis of data is obtained during signup for the app. Data were extracted for all UK participants self-reported to have tested positive for COVID-19 by RT-PCR between March 24, 2020 and June 9, 2020 who self-reported “fever or chills” and logged at least one recorded temperature using the app during the study period.
Measurements
(a) Unaffected volunteers: Three infrared tympanic temperature measurements were taken for each twin participant during a single routine research visit to the Guy’s and St Thomas’ Clinical Research Facility. A mean temperature for each participant was used for analysis.
(b) & (c) Hospital COVID-19+ cohorts: Electronic patient records were reviewed. Only COVID-19 cases confirmed by RT-PCR of nasopharyngeal swab were included. First documented oral or tympanic temperature on admission was used to reflect temperature during acute infection.
(d) Community “app” COVID-19+ cohort: Self-reported temperature measurements were extracted for all adult participants self-reported to have tested positive for COVID-19, who had recorded at least one temperature measurement using the app. Maximum temperature was used for analysis, to most likely reflect temperature during acute illness.
Analyses
Descriptive statistics were used to describe demographic and key clinical characteristics of the study cohorts.
Differences within the unaffected volunteer cohort (a) and the 2 hospitalized COVID-19+ cohorts (b&c) were compared using the Fisher exact test for categorical and Wilcoxon signed rank for continuous variables.
To analyze associations between age and temperature in the unaffected volunteer cohort (a), a linear mixed effect model was used, with age, gender, and BMI set as fixed factors and accounting for family and zygosity as random factors. In hospital COVID-19+ cohorts (b&c), multivariable linear regression was used to model the effects of age, sex, and BMI on temperature recorded on admission. In the community-based COVID-19+ cohort (d), multivariable linear regression was used to analyze associations between age, sex, and BMI and maximum self-recorded temperature in the context of confirmed COVID-19. For each cohort, 2 analyses were performed, the first including age and sex as predictor variables, the second including age, sex, and BMI. Participants with missing temperature data were excluded from analysis. Participants with missing BMI data were only excluded for analyses using BMI. Residuals were checked to confirm homoscedasticity in all samples. p-values were Bonferroni-adjusted for multiple testing.
In COVID-19+ hospital and community cohorts (b,c&d), logistic regression was performed to ascertain associations between recorded fever (defined as temperature≥ 37.8°C) and age, adjusted for sex and BMI. A complete case approach was used, as low levels of missing data were expected. p-values were not adjusted since multiple comparisons were not applied.
Receiver operating characteristic (ROC) analysis was performed on a combined data set of unaffected healthy volunteers (a) and hospitalized COVID-19+ participants (b&c) to identify a temperature threshold in older adults (≥ 65 years of age) with similar sensitivity and specificity for discriminating COVID-19 to a temperature ≥37.8°C in adults <65 years of age.
Genetic and environmental contributions of basal temperature in TwinsUK cohort was estimated using a univariate model (32). The model decomposes the observed variance of the basal temperature into additive genetic variance (A), shared environment variance (C), and non-shared environment variance (E), deducing the genetic influence of a trait and by comparison of the different nested models. The analysis was carried out using Open Mx (32) for R on residuals of linear regression adjusted for age and sex and BMI. Heritability of self-reported fever was carried out in 3099 COVID Symptom Study app participants who were also enrolled in TwinsUK (291 [9.4%] of whom were reporting fever), using biometric modeling for the liability threshold model, which, for a categorical trait, assumes an underlying continuous liability that follows a normal distribution (33).
Data analysis and graphics were performed in the R statistical environment (version 4.0) using the Tidyverse (34), Open Mx (32), and lme4 (35) packages, in Stata (version 15.0, StataCorp LLC, College Station, TX) and in Python (version 3.9).
Ethics
TwinsUK main ethics was reviewed and approved by the NHS London – London-Westminster Research Ethics Committee (REC reference EC/04/015), and by Guy’s and St Thomas’ NHS Foundation Trust Research and Development (R&D) in 2012. TwinsUK BioBank was approved by NHS North West - Liverpool East Research Ethics Committee (REC reference 19/NW/0187), IRAS ID 258513.
The Covid Symptom Study App Ethics has been approved by KCL ethics Committee REMAS ID 18210, review reference LRS-19/20-18210. All subscribers provided consent when signing up for the app.
COVIDCollab project service evaluation approved by Guy’s and St Thomas’ NHS Trust audit leads. Reference number 10777.
The University Hospitals Birmingham (UHB) validation was performed as part of a service evaluation with a (CARMS-16005), after approval on routinely collected anonymized data set.
Results
Baseline characteristics and temperature measurements for all cohorts are given in Table 1. Six out of 520 patients in the London hospital cohort and 81/757 in the Birmingham hospital cohort had missing temperature data and were excluded from analysis. Of included participants, 28/514 in the London hospital cohort and a further 26/676 in the Birmingham hospital cohort had missing BMI data and were excluded from the analyses including BMI. Five out of 3 972 in the app cohort had sex “other” and were excluded from sex-adjusted analysis only.
Table 1.
Baseline Characteristics and Temperature Measurements of (a) Unaffected Volunteers; (b) London and (c) Birmingham Hospitalized Cohorts with RT-PCR-confirmed COVID-19 and (d) Community-based “app” Cohort with Self-reported Confirmed COVID-19
| (a) Unaffected Volunteers (n = 1089) | (b) London Hospitalized Patients (n = 514) |
(c) Birmingham Hospitalized Patients (n = 676) | (d) Community “app” Participants (n = 3 967) | |
|---|---|---|---|---|
| Unaffected Volunteers | COVID-19 + | COVID-19 + | COVID-19 + | |
| Age (years) | 56.6/16.8 | 61.9/17.1 | 67.8/17.0 | 42.9/12.8 |
| Sex (females) | 914 (84%) | 202 (39%) | 321 (43%) | 2 770 (70%) |
| BMI (kg/m2) | 25.8/5.2 | 28.5/6.8 (n = 486) | 29.2/7.2 (n = 646) | 28.2/6.2 |
| Temperature (°C) | 36.6/0.4* | 37.5/1.1† | 36.9/0.9† | 37.6/1.0‡ |
Notes: BMI = body mass index. Categorical variables presented as count (%) and continuous variables as mean (standard deviation). All presented as mean/standard deviation. *Mean value of 3 recordings of basal tympanic temperature; †first recorded temperature on hospital admission; ‡maximum temperature self-recorded on app.
Regression Analysis
Results of the linear mixed-effect model in the TwinsUK sample and linear regression models in the community-based and hospital cohorts are displayed in Table 2. The assumption of homoscedasticity of residuals was not violated for the cohorts used in regression analysis. Increasing age was associated with both lower basal temperature, and lower temperature during illness, while increasing BMI was associated with higher basal and illness temperature.
Table 2.
Results from Linear Mixed-effect Model for (a) TwinsUK Unaffected Volunteers; Multivariable Linear Regression for Hospitalized Cohorts from (b) London and (c) Birmingham with Confirmed COVID-19 Infection and (d) Community-based “app” Cohort with Self-reported Confirmed COVID-19 Infection
| (a) TwinsUK Cohort (n = 1 089) | (b) London Hospitalized Patients (n = 514) | (c) Birmingham Hospitalized Patients (n = 676) | (d) Community “app” (n = 3 967) | |||||
|---|---|---|---|---|---|---|---|---|
| Unaffected Volunteers | COVID-19 + | COVID-19 + | COVID-19 + | |||||
| Basal Temperature | First Recorded Temperature on Admission | First Recorded Temperature on Admission | Maximum Self-Reported Temperature | |||||
| Model 1 (age and sex) |
Model 2 (age, sex, and BMI) |
Model 1 (age and sex) |
Model 2 (age, sex, and BMI) |
Model 1 (age and sex) |
Model 2 (age, sex, and BMI) |
Model 1 (age and sex) |
Model 2 (age, sex, and BMI) |
|
| Age |
−0.214
(0.035) p < .001 |
−0.240
(0.036) p < .001 |
−0.007
(0.003) p = .040 |
−0.005
(0.003) p = .320 |
−0.007
(0.002) p < .001 |
−0.006
(0.002) p = .03 |
−0.006
(0.001) p < .001 |
−0.006
(0.001) p < .001 |
| Sex |
0.122
(0.035) p < .001 |
0.128
(0.035) p < .001 |
−0.028
(0.098) p = 1.000 |
−0.016
(0.101) p = 1.000 |
0.064
(0.072) p = 1.000 |
0.053
(0.070) p = 1.000 |
0.078
(0.034) p = .043 |
0.086
(0.034) p = .031 |
| BMI | --- |
0.100
(0.032) p = .001 |
--- |
0.0157
(0.007) p = .101 |
--- |
0.0087
(0.005) p = .49 |
--- |
0.0136
(0.003) p < .001 |
Notes: Beta coefficients are reported in bold and standard errors in brackets; p-values are Bonferroni adjusted at 5%. BMI = body mass index.
Logistic regression, exploring the relationship between age and temperature ≥37.8°C, demonstrated that for each additional year of age, participants with confirmed COVID-19 were 1% less likely to show a fever in both the app (OR 0.99; 95% confidence interval [CI] 0.98–0.99; p < .001) and the Birmingham hospital cohorts (OR 0.99; 95% CI 0.98–0.99; p = .040; Table 3).
Table 3.
Results of Logistic Regression for (b) London Hospital Cohort and (c) Birmingham Hospital Cohort, Adjusted for Sex and BMI, and (d) Community-based “app” Cohort
| (b) London Hospital (n = 514) | (c) Birmingham Hospital (n = 676) | (d) Community “app” (n = 3 967) | |
|---|---|---|---|
| COVID-19 PCR+ | COVID-19 PCR+ | Self-report COVID-19 PCR+ | |
| Temperature on Admission ≥ 37.8°C | Temperature on Admission ≥ 37.8°C | Self- reported Temperature ≥ 37.8°C | |
| Age OR (95% CI) |
0.99 (0.98–1.00) p = .45 |
0.99 (0.98–0.99) p = .040 |
0.99 (0.98–0.99) p < .001 |
Notes: Odds ratios (ORs) are reported in bold, confidence intervals (CI) in brackets. Fever is defined as (b) recorded temperature ≥37.8°C and (c) self-reported temperature ≥37.8C; p-values are not adjusted for multiple testing.
ROC Analysis
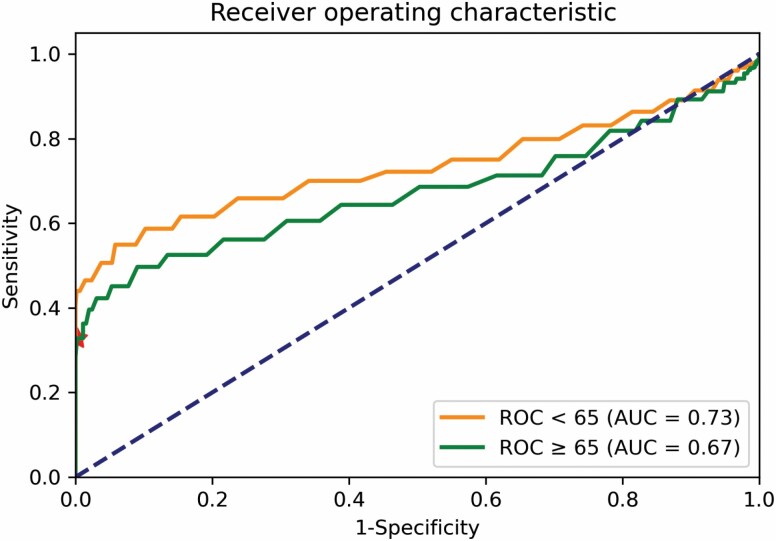
A temperature of 37.8°C had a sensitivity of 35.2% and specificity of 100% for discriminating between the unaffected volunteer cohort and hospitalized COVID-19+ patients aged <65 years of age, in a combined data set of unaffected healthy twin volunteers and hospitalized COVID-19+ participants. ROC analysis performed on a combined data set demonstrated that a temperature of 37.4°C in adults ≥65 years of age had the most closely matched sensitivity (36.3%) and specificity (98.9%) to that of 37.8°C in adults <65 years of age for discriminating COVID-19+ patients from unaffected volunteers. ROC curves for adults <65 and ≥65 years of age are shown in Figure 1, with sensitivity and specificity of different temperature thresholds in Supplementary Table S1. We note a slightly smaller area under the curve (AUC) in older versus younger adults which may point toward reduced discriminatory performance in older adults. AUC was similar in subanalyses of adults ≥75 and ≥85 years of age, although these numbers should be cautiously interpreted given small numbers of adults in older age groups (Supplementary Table S2).
Figure 1.
Receiver operating characteristic curves for adults <65 years of age (orange) and ≥ 65 years of age (green); the red star indicates the point characterized by a true positive rate of 0.36 and false-positive rate of 0.01.
Heritability Analysis
Results for heritability analysis are summarized in Table 4.
Table 4.
Results of Heritability Analysis for (i) Basal Temperature Adjusted for Age and Sex; (ii) Basal Temperature Adjusted for Age, Sex, and BMI; and (iii) Fever, Adjusted for Age, Sex, and BMI
| Variance Components | Model Fit | Model Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Model | A | C | E | es | –2LL | AIC | χ 2 | df | p | base |
| Temperature | Saturated | - | - | - | 10 | 921.88 | −1 236.111 | - | - | - | - |
| (Adjusted for Age and Sex) | ACE | 0.47 [0.18–0.57] | 0.03 [0–0.28] | 0.49 [0.42–0.57] | 4 | 923.73 | −1 246.262 | 1.85 | 6 | .933 | Saturated |
| AE | 0.50 [0.43–0.57] | - | 0.49 [0.42–0.57] | 3 | 923.79 | −1 248.205 | 0.06 | 1 | .810 | ACE | |
| CE | - | 0.42 [0.34–0.48] | 0.57 [0.51–0.65] | 3 | 934.40 | −1 237.598 | 10.61 | 1 | 1.092e−03 | ACE | |
| E | - | - | 1.00 [1.00–1.00] | 2 | 1 033.16 | −1 140.835 | 109.37 | 1 | 1.346e−25 | AE | |
| Temperature | Saturated | - | - | - | 10 | 913.39 | −1 244.602 | - | - | - | - |
| (Adjusted for Age, Sex and BMI) | ACE | 0.44 [0.15–0.57] | 0.06 [0–0.31] | 0.49 [0.42–0.57] | 4 | 915.40 | −1 254.594 | 2.01 | 6 | .918 | Saturated |
| AE | 0.50 [0.43–0.57 | - | 0.49 [0.42–0.57] | 3 | 915.61 | −1 256.384 | 0.21 | 1 | .646 | ACE | |
| CE | - | 0.24 [0.35–0.49] | 0.57 [0.50–0.64] | 3 | 924.83 | −1 247.169 | 9.22 | 1 | 2.139e−03 | ACE | |
| E | - | - | 1.00 [1.00–1.00] | 2 | 1 025.86 | −1 148.136 | 110.25 | 1 | 8.646e−26 | AE | |
| Fever | Saturated | - | - | - | 13 | 1 853.05 | −4 318.95 | - | - | - | - |
| (Adjusted for Age, Sex and BMI) | ACE | 0 [0–0] | 0.21 [0–0.38] | 0.79 [0.62–0.98] | 8 | 1 856.93 | −4 329.07 | 3.88 | 7 | .793 | Saturated |
| AE | 0.2 [0–0.21] | - | 0.8 [0.66–0.99] | 7 | 1 858.20 | −4 329.80 | 1.27 | 1 | .260 | ACE | |
| CE | - | 0.21 [0.02–0.38] | 0.79 [0.62–0.98] | 7 | 1 856.93 | −4 331.07 | 0.00 | 1 | 1.000 | ACE | |
| E | - | - | 1.00 [1.00–1.00] | 6 | 1 861.85 | −4 328.15 | 4.92 | 1 | .027 | CE | |
Note: BMI = body mass index.
Analysis of monozygotic twin intra-pair correlation showed a clear heritability signal in comparison to dizygotic twin pairs for basal temperature. When adjusted for age and sex, genetic factors accounted for 47% (95% CI 18%–57%) of variance in mean temperature in the best model (ACE) when compared with the other models (Saturated, ACE, CE, E) (Table 4). When adjusted for age, sex, and BMI, genetic factors accounted for 44% (95% CI = 15%–57%) of variance in mean temperature in the best model (ACE) (Table 4).
Heritability of fever, using twins who are app participants, did not demonstrate a genetic component to temperature response to infection, which may be explained by predominantly environmental factors driving exposure to SARS-CoV-2 (Table 4).
Discussion
Sources of Variability in Temperature: Age, BMI, and Heritability
In this study, associations between age and temperature were replicated across healthy volunteer, hospitalized COVID-19 and community-based COVID-19 cohorts. The observed associations persisted after adjustment for confounding by sex and BMI in the healthy volunteer, the larger Birmingham hospital cohort, and community-based cohorts. Temperature regulation is affected by many physiological processes, including vasomotor sweating functions, skeletal muscle response, temperature perception, and physical behaviors, many of which have been described in previous studies and reviews (1,3,5). These processes change with aging, and our observation that increasing age reduces both basal temperature and temperature in response to SARS-CoV-2 infection supports this. Case reports and small cohort studies have previously shown that older adults, especially those with frailty or multiple chronic conditions, may not display a clinically significant fever response to infection (13,25). This may reflect both a lower baseline temperature in older adults, as well as a lower temperature change in response to infection. Age-related reductions in pro-inflammatory mediators in response to infection, such as interleukin-1 and tumor necrosis factor, may also have an effect.
The possible association between higher BMI, often considered a pro-inflammatory state, and an increased basal and fever temperature would appear to concord with this finding for age. The observed associations between BMI and temperature in unaffected volunteer and community-based COVID-19 cohorts were apparent after adjustment for age. Some earlier studies, often with smaller, specific samples, demonstrated a conflicting association between BMI and temperature (36). However, our observation is supported by the recent, large CoLaus study of 4 224 men and post-menopausal women (37). Here, other markers of obesity and insulin metabolism were also associated with higher temperature.
In this analysis, after adjustment for both age and BMI, we demonstrate a clear heritable component to basal temperature, pointing to other biological variables which may underlie temperature variation. Genetic factors may also play a crucial role in temperature variability among individuals of different ages and with different BMI. Physiological factors related to age and temperature control may also be heritable. In contrast to basal temperature, “fever,” defined here as a temperature of 37.8°C (100.0°F) or above, did not demonstrate a significant genetic component, suggesting that environmental factors (in this case exposure to the SARS-CoV-2 virus) may be the main driver of fever within this population.
Fever Threshold for Discriminating Infection in Older Adults
Results of ROC analysis, combining unaffected volunteers and hospitalized COVID-19 patients, supports observations from previous studies and concerns from frontline clinicians that current fever threshold definitions may be inappropriate for older adults (8,12). A lower temperature of 37.4°C (97.3°F) in older adults aged 65 years and over had a similar sensitivity for discriminating COVID-19 patients from healthy volunteers as the widely utilized threshold of 37.8°C in older adults. Our observation is supported by a previous study that demonstrated optimized sensitivity and specificity thresholds of 37.9°C for younger adults and 37.3°C for older adults for discriminating influenza infection (38). Our finding may have important utility for screening in clinical settings, with a lower threshold in older adults to promptly detect infections and implement effective containment measures. However, it should be noted that both in our study and the previous influenza study using a ROC analysis, AUCs of between 0.66 and 0.73 suggest that the discriminatory power of temperature alone for identifying infection is relatively poor. Furthermore, temperature thresholds identified in both studies for younger and older adults did not have a high sensitivity for discriminating infection. Using temperature alone to identify infection risks missing cases and we would advocate incorporating temperature measurements as part of a wider clinical assessment.
Implications and Generalizability
Associations observed in this study have important clinical implications for case detection and surveillance of infectious diseases, including COVID-19. Internationally, to our knowledge, there are currently no countries or organizations that have adopted age-related temperature thresholds for defining fever, highlighting the international relevance of this research. There is a risk of cases being missed in older adults if inappropriately high fever thresholds are used as a screening or diagnostic tool for COVID-19 infection. This may be compounded in those with lower BMI, for example as a result of sarcopenia, who have lower temperatures. While this study did not intend to analyze associations between temperature and mortality, we know that older adults experience a higher fatality rate from COVID-19 (20,21,39). Our findings support use of a lower temperature threshold for older adults to initiate appropriate testing and isolation precautions in hospital settings. This may be relevant for care homes and long-term care facilities, with older populations vulnerable to outbreaks of respiratory disease. Observations from our community-based cohort may have relevance when considering methods for community case detection and limitation of spread (such as quarantining or screening for travel). Frailty, when assessed by specialists, may be a better predictor of disease outcomes than age or comorbidity alone (39). However, an age threshold has utility for those in clinical settings lacking the specialist input required for accurate frailty assessment.
Strengths and Limitations
Our study combines data from 3 different types of research cohort: a classic research cohort (TwinsUK), routinely collected clinical data from hospital cohorts, and a cohort of community “citizen scientists.” We are not aware of any previous studies using an epidemiological approach across multiple large cohorts to analyze temperature at baseline and in febrile illness. The TwinsUK cohort benefits from a large number of observations collected using rigorous study procedures. The associations observed in this cohort were reassuringly recapitulated in the “real-life” hospital and community-based data. This concordance increases generalizability from the academic sphere to clinical and community-based settings.
For historical reasons, the TwinsUK cohort is predominantly female. Females generally show greater individual temperature variability than males (40). The app cohort was also predominantly female. However, both hospital cohorts were predominantly male, reflecting the fact that men are more severely affected by COVID-19 (19–21). However, our models did not show any association with sex and recorded temperature, suggesting that these differences in cohort structure did not affect results. The TwinsUK cohort had a lower average BMI, possibly also reflecting that those with higher BMI are more likely to be unwell with COVID-19. However, we adjusted for BMI in our analyses, and the relationship between age and temperature was maintained in all of our analyses. Since the ROC method does not allow for adjustment for confounding variables, some of the difference between the COVID-positive and COVID-negative cohorts may be attributable to BMI. We used age in our ROC analysis as this can be used by clinicians as part of initial clinical assessment. BMI is not commonly or reliably assessed at triage. Importantly, the temperature threshold identified distinguishes between the 2 cohorts and is therefore relevant for clinical practice.
This study used data from a large population of individuals reporting on a mobile app. Information was self-reported and therefore there may be inaccuracies in logging of temperature and BMI. Although the app cohort only contained people with self-reported COVID-19 testing status, it is possible that maximal temperature logged in the app was not synchronous with COVID-19 infection or could be the result of another infection. Only those reporting “fever or chills” were prompted to enter a temperature measurement. Some may not have logged on the day of their maximal temperature. App users did under-represent older age groups (proportion ≥65 years 11.7%, compared to 18.3% of the UK population (41). 4.8% of app users with a confirmed positive COVID-19 test were ≥65 years of age, which may reflect shielding in this group. This is supported by the fact that UK Office for National Statistics data showed lower rates of antibody seropositivity in the older, non-patient facing population during the study period (42). Sampling using a mobile app will under-represent individuals without mobile devices, which often includes those from more deprived backgrounds and marginalized groups, known to be at greater risk for severe disease. Furthermore, community testing for COVID-19 was not universally available in the United Kingdom during the period of data collection. The method for temperature measurement was not documented, although oral thermometers are most widely available in the United Kingdom. However, these limitations would be most likely to diminish power to detect effects, but would not alter the key association seen between age and temperature, which is replicated across cohorts.
Hospitalized patients present at different stages of illness; hence, temperature on admission will not always capture the most febrile component of infection. Some patients may have received antibiotics, antipyretics such as paracetamol or corticosteroids prior to initial temperature recording. BMI data were missing for a small proportion (<5%) of patients—likely not missing at random, as there may have been difficulty ascertaining accurate heights and weights for example due to extremes of body habitus or severe frailty.
Conclusion
The significant associations observed between age and basal temperature are important in highlighting effects of aging on thermoregulation and the immune response. The observed heritability of basal temperature in TwinsUK unaffected participants suggests that biological factors underlie differences in temperature regulation. These require further elucidation. As well as demonstrating lower temperatures in older adults, we report lower temperature with decreasing BMI. These two factors may compound each other in older, frail patients with sarcopaenia.
Our findings support a lower temperature threshold of 37.4°C (97.3°F) for identifying possible COVID-19 infection in older adults aged 65 years and older. This has important implications for case detection, surveillance, and isolation and could be incorporated into vital signs assessments for older people.
Data Sharing
TwinsUK data used in this study are available upon reasonable request to TwinsUK.
COVIDCollab data are available upon application to the Geriatric Medicine Research Collaborative https://www.gemresearchuk.com/.
App data used in this study are available to bona fide researchers through UK Health Data Research using the following link https://healthdatagateway.org/detail/9b604483-9cdc-41b2-b82c-14ee3dd705f6
Supplementary Material
Acknowledgments
All authors listed were responsible for aspects of study design, data collection and analysis, and writing of the manuscript and meet criteria for authorship. All authors approved the final manuscript to be submitted.
Contributor Information
Rose S Penfold, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK; Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Maria Beatrice Zazzara, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK; Department of Gerontology, Neuroscience and Orthopedics, Sacred Heart Catholic University, Rome, Italy.
Marc F Österdahl, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Carly Welch, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK.
Mary Ni Lochlainn, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK.
Maxim B Freidin, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK.
Ruth C E Bowyer, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK.
Ellen Thompson, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK.
Michela Antonelli, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
Yu Xian Rachel Tan, Department of Medicine, Royal College of Surgeons in Ireland, Dublin 2, Ireland.
Carole H Sudre, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
Marc Modat, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
Benjamin Murray, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
Jonathan Wolf, Zoe Global Limited, London, UK.
Sebastien Ourselin, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
Tonny Veenith, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK.
Janet M Lord, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK.
Claire J Steves, Department of Twin Research and Genetic Epidemiology, School of Life Course Sciences, King’s College London, London, UK.
GSTT Covid Collaborative:
Rishi Iyer, Rachael Anders, Lindsay Hennah, Gitanjali Amaratunga, Abigail Hobill, Cassandra Fairhead, Amybel Taylor, Henry Maynard, Marc Osterdahl, Maria Dias, Taha Amir, Natalie Yeo, Jamie Mawhinney, Hamilton Morrin, Li Kok, Luca Scott, Aiden Haslam, Gavriella Levinson, Stephanie Mulhern, Stephanie Worrall, Thurkka Rajeswaran, Katherine Stamboullouian, Sophie McLachlan, Karla Griffith, Daniel Muller, Alice O’ Doherty, Baguiasri Mandane, Irem Islek, Alexander Emery, John Millwood-Hargrave, Andra Caracostea, Laura Bremner, Arjun Desai, Aneliya Kuzeva, Carolyn Akladious, Mettha Wimalasundera, Mairead Kelly, Sally Aziz, Sinead O’Dwyer, Rupini Perinpanathan, Anna Barnard, Nicole Hrouda, Ismini Panayotidis, Nirali Desai, Hannah Gerretson, Rebecca Lau, Zaynub Ghufoor, Hanna Nguyen, Torben Heinsohn, Jack Cullen, Eleanor Watkins, Vaishali Vyas, Daniel Curley, Niamh Cunningham, Vittoria Vergani, Kelvin Miu, Jack Stewart, Nicola Kelly, Lara Howells, Benyamin Deldar, Ross Sayers, Gracie Fisk, Sri Sivarajan, Tahmina Razzak, Helen Ye, Samiullah Dost, Nikhita Dattani, Catherine Wilcock, Gabriel Lee, Jodie Acott, Hannah Bridgwater, Antia Fernandez, Hesham Khalid, Katherine Hopkinson, Deirdre Green, Hejab Butt, Ayushi Gupta, Madeleine Garner, Hazel Sanghvi, Madeleine Daly, Emily Ross-Skinner, Shefali Patel, and Danielle Lis
Funding
Investigators received support from the Wellcome Trust, the Medical Research Council (MRC), BHF, European Union (EU), National Institute for Health Research (NIHR), Chronic Disease Research Foundation (CDRF), and the NIHR-funded BioResource, Alzheimer’s Society, Clinical Research Facility and NIHR Biomedical Research Centre (BRC) based at GSTT NHS Foundation Trust in partnership with KCL, NIHR Birmingham BRC, a partnership between University Hospital Birmingham NHS Trust and University of Birmingham. M.N.L. is supported by an NIHR Doctoral Fellowship (NIHR300159). The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care.
TwinsUK is funded by the Wellcome Trust, MRC, EU, CDRF, the NIHR-funded BioResource, Clinical Research Facility and BRC based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with KCL.
The app was developed by Zoe Limited with scientific input from King’s College London (KCL). Zoe provided in kind support for all aspects of building, running, and supporting the app and service to all users worldwide.
We would like to acknowledge the following GSTT COVIDCollab collaborators, responsible for London hospital data collection: Rishi Iyer, Rachael Anders, Lindsay Hennah, Gitanjali Amaratunga, Abigail Hobill, Cassandra Fairhead, Amybel Taylor, Henry Maynard, Marc Osterdahl, Maria Dias, Taha Amir, Natalie Yeo, Jamie Mawhinney, Hamilton Morrin, Li Kok, Luca Scott, Aiden Haslam, Gavriella Levinson, Stephanie Mulhern, Stephanie Worrall, Thurkka Rajeswaran, Katherine Stamboullouian, Sophie McLachlan, Karla Griffith, Daniel Muller, Alice O’ Doherty, Baguiasri Mandane, Irem Islek, Alexander Emery, John Millwood-Hargrave, Andra Caracostea, Laura Bremner, Arjun Desai, Aneliya Kuzeva, Carolyn Akladious, Mettha Wimalasundera, Mairead Kelly, Sally Aziz, Sinead O’Dwyer, Rupini Perinpanathan, Anna Barnard, Nicole Hrouda, Ismini Panayotidis, Nirali Desai, Hannah Gerretson, Rebecca Lau, Zaynub Ghufoor, Hanna Nguyen, Torben Heinsohn, Jack Cullen, Eleanor Watkins, Vaishali Vyas, Daniel Curley, Niamh Cunningham, Vittoria Vergani, Kelvin Miu, Jack Stewart, Nicola Kelly, Lara Howells, Benyamin Deldar, Ross Sayers, Gracie Fisk, Sri Sivarajan, Tahmina Razzak, Helen Ye, Samiullah Dost, Nikhita Dattani, Catherine Wilcock, Gabriel Lee, Jodie Acott, Hannah Bridgwater, Antia Fernandez, Hesham Khalid, Katherine Hopkinson, Deirdre Green, Hejab Butt, Ayushi Gupta, Madeleine Garner, Hazel Sanghvi, Madeleine Daly, Emily Ross-Skinner, Shefali Patel, Danielle Lis. (All affiliated with Guy’s and St Thomas’ Hospital NHS Foundation Trust).
Written permission was obtained to include the names of all individuals listed.
Conflict of Interest
None declared.
References
- 1. Blatteis CM. Age-dependent changes in temperature regulation - a mini review. Gerontology. 2012;58(4):289–295. doi: 10.1159/000333148 [DOI] [PubMed] [Google Scholar]
- 2. Geneva II, Cuzzo B, Fazili T, Javaid W. Normal body temperature: a systematic review. Open Forum Infect Dis. 2019;6(4):ofz032. doi: 10.1093/ofid/ofz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. VE DB. Temperature. In: Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990. https://www.ncbi.nlm.nih.gov/books/NBK331/. [PubMed] [Google Scholar]
- 4. Dikmen S, Wang XZ, Ortega MS, Cole JB, Null DJ, Hansen PJ. Single nucleotide polymorphisms associated with thermoregulation in lactating dairy cows exposed to heat stress. J Anim Breed Genet. 2015;132(6):409–419. doi: 10.1111/jbg.12176 [DOI] [PubMed] [Google Scholar]
- 5. Loyau T, Zerjal T, Rodenburg TB, et al. Heritability of body surface temperature in hens estimated by infrared thermography at normal or hot temperatures and genetic correlations with egg and feather quality. Animal. 2016;10(10):1594–1601. doi: 10.1017/S1751731116000616 [DOI] [PubMed] [Google Scholar]
- 6. Osei-Amponsah R, Chauhan SS, Leury BJ, et al. Genetic selection for thermotolerance in ruminants. Animals. 2019;9(11):948. doi: 10.3390/ani9110948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez-Minguez J, Ordoñana JR, Sánchez-Romera JF, Madrid JA, Garaulet M. Circadian system heritability as assessed by wrist temperature: a twin study. Chronobiol Int. 2015;32(1):71–80. doi: 10.3109/07420528.2014.955186 [DOI] [PubMed] [Google Scholar]
- 8. High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(2):149–171. doi: 10.1086/595683 [DOI] [PubMed] [Google Scholar]
- 9. Norman DC. Fever in the elderly. Clin Infect Dis. 2000;31(1):148–151. doi: 10.1086/313896 [DOI] [PubMed] [Google Scholar]
- 10. Sloane PD, Kistler C, Mitchell CM, et al. Role of body temperature in diagnosing bacterial infection in nursing home residents. J Am Geriatr Soc. 2014;62(1):135–140. doi: 10.1111/jgs.12596 [DOI] [PubMed] [Google Scholar]
- 11. Castle SC, Norman DC, Yeh M, Miller D, Yoshikawa TT. Fever response in elderly nursing home residents: are the older truly colder? J Am Geriatr Soc. 1991;39(9):853–857. doi: 10.1111/j.1532-5415.1991.tb04450.x [DOI] [PubMed] [Google Scholar]
- 12. McAlpine CH, Martin BJ, Lennox IM, Roberts MA. Pyrexia in infection in the elderly. Age Ageing. 1986;15(4):230–234. doi: 10.1093/ageing/15.4.230 [DOI] [PubMed] [Google Scholar]
- 13. Jung YJ, Yoon JL, Kim HS, Lee AY, Kim MY, Cho JJ. Atypical clinical presentation of geriatric syndrome in elderly patients with pneumonia or coronary artery disease. Ann Geriatr Med Res. 2017;21(4):158–163. doi: 10.4235/agmr.2017.21.4.158 [DOI] [Google Scholar]
- 14. Limpawattana P, Mitsungnern T, Phungoen P, Tansangworn N, Laosuangkoon W. A secondary analysis of atypical presentations of older patients with infection in the emergency department of a tertiary care hospital in Thailand. Asian Biomed. 2016;62:97–102. doi: 10.5372/1905-7415.1002.480 [DOI] [PubMed] [Google Scholar]
- 15. Hofman MR, van den Hanenberg F, Sierevelt IN, Tulner CR. Elderly patients with an atypical presentation of illness in the emergency department. Neth J Med. 2017;75(6):241–246. [PubMed] [Google Scholar]
- 16. Hazeldine J, Lord JM. Innate immunesenescence: underlying mechanisms and clinical relevance. Biogerontology. 2015;16(2):187–201. doi: 10.1007/s10522-014-9514-3 [DOI] [PubMed] [Google Scholar]
- 17. Oh SJ, Lee JK, Shin OS. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19(6):e37. doi: 10.4110/in.2019.19.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson D, Drew W, Jasper A, et al. Frailty is associated with neutrophil dysfunction which is correctable with phosphoinositol-3-kinase inhibitors. J Gerontol A Biol Sci Med Sci. 2020;75(12):2320–2325. doi: 10.1093/gerona/glaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. . Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Eurosurveillance. 2020;25(9):2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zayet S, Kadiane-Oussou NJ, Royer PY, Toko L, Gendrin V, Klopfenstein T. Coronavirus disease 2019: new things to know! J Med Virol. 2020;92(10):1767–1768. doi: 10.1002/jmv.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. COVID-19: Investigation and Initial Clinical Management of Possible Cases - GOV.UK . https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection. Updated 14 December, 2020. Accessed 8 July, 2021.
- 25. Holroyd-Leduc J, Gandell D, Miller A MPD. COVID-19 in Older Adults Resource.2020. https://www.rgptoronto.ca/wp-content/uploads/2020/04/COVID-19-Presentations-in-Frail-Older-Adults-U-of-C-and-U-fo-T.pdf.
- 26. Zazzara MB, Penfold RS, Roberts AL, et al. Probable delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. Age Ageing. 2021;50(1):40–48. doi: 10.1093/ageing/afaa223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Österdahl MF, Lee KA, Lochlainn MN, et al. Detecting SARS-CoV-2 at point of care: preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR). BMC Infect Dis. 2020;20(1):783. doi: 10.1186/s12879-020-05484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verdi S, Abbasian G, Bowyer RCE, et al. TwinsUK: The UK Adult Twin Registry Update. Twin Res Hum Genet. 2019;22(6):523–529. doi: 10.1017/thg.2019.65 [DOI] [PubMed] [Google Scholar]
- 29. Geriatric Medicine Research Collaborative. Innovations in geriatric medicine: inception and dissemination of a national trainee-led research collaborative. Future Healthc J. 2019;6(Suppl 1):118. doi: 10.7861/futurehosp.6-1-s118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geriatric Medicine Research Collaborative; Covid Collaborative, Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50(3):617–630. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neale MC, Hunter MD, Pritikin JN, et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 2016;81(2):535–549. doi: 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falconer DS, Mackay TFC.. Introduction to Quantitative Genetics. 4th ed. Longman Scientific & Technical; 1996. [Google Scholar]
- 34. Wickham et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 35. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 36. Sund-Levander M, Grodzinsky E, Loyd D, Wahren LK. Errors in body temperature assessment related to individual variation, measuring technique and equipment. Int J Nurs Pract. 2004;10(5):216–223. doi: 10.1111/j.1440-172X.2004.00483.x [DOI] [PubMed] [Google Scholar]
- 37. Bastardot F, Marques-Vidal P, Vollenweider P. Association of body temperature with obesity. The CoLaus study. Int J Obes (Lond). 2019;43(5):1026–1033. doi: 10.1038/s41366-018-0218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses. 2015;9 (Suppl 1):23–29. doi: 10.1111/irv.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewitt J, Carter B, Vilches-Moraga A, et al. ; COPE Study Collaborators. . The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaciuba-Uscilko H, Grucza R. Gender differences in thermoregulation. Curr Opin Clin Nutr Metab Care. 2001;4(6):533–536. doi: 10.1097/00075197-200111000-00012 [DOI] [PubMed] [Google Scholar]
- 41. Office for National Statistics. Overview of the Population. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/august2019. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/august2019. Published 2019.
- 42. ONS. Coronavirus (COVID-19) Infection Survey: characteristics of people testing positive for COVID-19 in England and antibody data for the UK: December 2020. Office for National Statistics; 2020; 1–12. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/november2020. Published 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.